Abstract
Objective(s)
Machine perfusion of donor hearts is a promising strategy to increase the donor pool. Antegrade perfusion is effective but can lead to aortic valve incompetence and non-nutrient flow. Experience with retrograde coronary sinus perfusion of donor hearts has been limited. We tested the hypothesis that retrograde perfusion could support myocardial metabolism over an extended donor ischemic interval.
Methods
Human hearts from donors rejected or not offered for transplantation were preserved for 12 hours in University of Wisconsin Machine Perfusion Solution by: 1. Static hypothermic storage 2. Hypothermic antegrade machine perfusion or 3. Hypothermic retrograde machine perfusion. Myocardial oxygen consumption (MVO2), and lactate accumulation were measured. Ventricular tissue was collected for proton (1H) and phosphorus-31 (31P) magnetic resonance spectroscopy (MRS) to evaluate the metabolic state of the myocardium. Myocardial water content was determined at end-experiment.
Results
Stable perfusion parameters were maintained throughout the perfusion period with both perfusion techniques. Lactate/alanine ratios were lower in perfused hearts compared to static hearts (p<.001). Lactate accumulation (Antegrade 2.0±.7, Retrograde 1.7±.1 mM) and MVO2 (Antegrade 0.25±.2, Retrograde 0.26±.3 mL O2/min/100g) were similar in machine perfused groups. High energy phosphates were better preserved in both perfused groups (p<.05). Left ventricular myocardial water content was increased in retrograde perfused (80.2±.8%) compared to both antegrade perfused (76.6±.8%, p=.02) and static storage hearts (76.7±1%, p=.02).
Conclusions
In conclusion, machine perfusion by either the antegrade or the retrograde technique can support myocardial metabolism over long intervals. Machine perfusion appears promising for long term preservation of human donor hearts.
Machine perfusion preservation of donor hearts has potential for improving heart preservation, extending the donor ischemic interval, retrieval of marginal donor organs and possibly utilization of non-heart beating donors.1-3 Most studies investigating machine perfusion for heart transplantation either by warm, beating heart perfusion or cold perfusion utilize antegrade perfusion of the coronary arteries by delivery of the perfusate into the ascending aorta.4-6 Research by our laboratory and others have confirmed that machine perfusion preservation is more effective than conventional static storage after standard and long term ischemic intervals.5-7 However, we have previously reported that under some conditions, aortic insufficiency and thus non nutrient flow could occur.8 In clinical practice, the likelihood of significant aortic valve incompetence would be even greater during conditions encountered when traveling to procure donor hearts. This is of minor consequence in the cold perfused heart over a standard ischemic interval since these hearts would essentially be undergoing static storage but may prove devastating during normothermic perfusion or over extended donor ischemic intervals.
Retrograde perfusion through the coronary sinus avoids the possibility of aortic insufficiency and is used routinely for cardioplegia delivery during cardiac surgery9,10 but its use for machine perfusion of donor hearts is rudimentary at best.11 Furthermore, we previously reported that right ventricular perfusion was reduced in canines when using retrograde perfusion, illustrating a potential limitation of this technique. 12
The ability of either technique for preserving human hearts over extended ischemic intervals has not been well characterized. We therefore decided to investigate myocardial metabolism of human hearts perfused by either antegrade or retrograde machine perfusion and compare this to conventional static storage. We hypothesized that machine perfusion is more effective than standard cold storage for long-term preservation of human donor hearts for transplantation.
Methods
This study was conducted in accordance with an organ procurement organization approved research protocol. Human donor hearts either not offered for transplantation or rejected for transplantation by all offered centers; and for tissue donation, were assigned to one of three preservation techniques: 1) Cold Storage 2) Antegrade machine perfusion 3) Retrograde machine perfusion. Tissue from additional hearts was procured immediately after cardiectomy to obtain baseline metabolic parameters.
Perfusion Device
Hearts assigned to the perfusion groups were perfused with a prototype perfusion device, the LifeCradle™ (Organ Transport Systems, Inc, Frisco, TX). The device delivers temperature regulated, oxygenated perfusate to the heart. Perfusate temperature, flow, and pressure were monitored continuously.
Procurement
Organ retrieval was performed through a median sternotomy. The heart was exposed and the donor was systemically heparinized with 30,000 units of heparin. After adequate time for heparin circulation had elapsed, the aorta was cross-clamped and one liter of ice-cold University of Wisconsin Machine Perfusion Solution (UWMPS) was used to arrest the heart. Hearts were decompressed through the inferior vena cava and the left atrial appendage. The donor cardiectomy was completed.
Preservation Techniques
Hearts were preserved for 12 hours by one of the three techniques. Conventional hypothermic storage hearts were immersed in 1L ice-cold UWMPS. Hearts in the antegrade perfusion group were perfused with UWMPS through the ascending aorta at a flow rate of 10mL/100g heart weight/min at 5 ±2°C. Donors hearts in the retrograde groups were perfused through a retrograde cardioplegia cannula (Medtronic, Inc, Minneapolis, MN) sewn into the coronary sinus with UWMPS at a flow rate of 13-20mL/100g/min, also at 5 ±2°C. Perfusion group flow rates were based on previously obtained large animal data and in some cases for the retrograde group, maximum achievable flow rate of the device.8,12 Temperature and perfusion pressure were measured continuously in machine perfused hearts. End perfusion myocardial oxygen consumption (MVO2), transmyocardial lactate and perfusate lactate accumulation were determined for the majority of perfused hearts using a commercial analyzer (Radiometer America, Inc, Westlake, OH). Separate MVO2 from right and left coronary artery effluent was calculated in retrograde perfused hearts. Myocardial water content was measured from tissue samples at end-experiment.
Magnetic Resonance Spectroscopy
The tissue samples were harvested, immediately freeze-clamped, and cooled in liquid nitrogen. The tissue was stored in an –80°C freezer and subsequently extracted with perchloric acid. Purified extracts were reconstituted in deuterium oxide, and pH was adjusted to 7.0–7.4 for magnetic resonance spectroscopy (MRS). Proton (1H) MR spectra were then acquired with a 14.1 Tesla Varian spectrometer operating at 600 MHz over a spectral width of 8000 Hz. Lactateto-alanine ratios were compared from 1H spectra as measures of cellular aerobic and anaerobic metabolism during storage.13,14 Proton decoupled phosphorus-31 (31P) spectra were obtained on the same spectrometer tuned to the 31P nucleus operating at 243 MHz over a spectral width of 36000 Hz. Phosphocreatine (PCr) – inorganic phosphate (Pi), gamma-adenosine triphosphate (γ-ATP) – inorganic phosphate and phosphocreatine – γ-ATP ratios were measured to determine preservation of high energy phosphates during the storage interval. Pi, PCr, and ATP standards were applied to selected samples to verify these signals.
Data Analysis
Data are reported as mean ± standard error of the mean (SEM). Statistical analysis was performed with SigmaPlot statistical software (SyStat Software, Inc, San Jose, CA) using a two-sided t-test or analysis of variance (ANOVA), as appropriate. A log10 function was applied to 31P MRS spectra to normalize the data. ANOVA on ranks using the Kruskal-Wallis test was performed on other variables that either demonstrated unequal variances or a non-normal distribution. When measurements were collected over multiple time points, data were compared with repeated-measures ANOVA. Adjustments for multiple comparisons were performed using Fisher's LSD or Dunn's Method, as appropriate. Relationships between variables were investigated with the Pearson correlation coefficient. For all statistical testing, a 2-sided p-value <.0.05 was considered statistically significant.
Results
Demographics
28 total donor hearts were studied with the 12 hour storage protocol: 10 using conventional cold storage, 8 using antegrade perfusion, and 7 using retrograde perfusion. 3 additional hearts were used to determine baseline metabolic parameters immediately after procurement. 21 hearts were procured using ground transportation and 7 hearts were procured by air transport. Donors were reasonably matched for age, ejection fraction, cause of death, height, and weight. Donor demographics are shown in Table 1. 7 of the 31 hearts in this study were offered for donation but rejected for transplantation.
Table 1.
Donor Demographics
| Control (n=3) | Static (n=10) | Antegrade Perfusion (n=8) | Retrograde Perfusion (n=7) | |
|---|---|---|---|---|
| Age | 48±10 | 39±6 | 51±5 | 43±6 |
| Sex (M/F) | 1/2 | 3/7 | 3/5 | 2/5 |
| Height (cm) | 161±7 | 174±3 | 171±3 | 169±3 |
| Weight (kg) | 78±6 | 72±3 | 76±5 | 91±12 |
| Cause of Death | ||||
| CVA | 2 | 7 | 6 | 3 |
| Trauma | 1 | 2 | ||
| Anoxia | 1 | 2 | 1 | 2 |
| Other | 1 (cardiac) | |||
| Ejection Fraction (%) | 60±0 (n=1) | 53±9 (n=6) | 43±9 (n=2) | 66±2 (n=4) |
| LVH | 1 | 2 | ||
| CAD | 2 | 2 | ||
Continuous data are reported as mean ± standard error of the mean. CVA – cerebrovascular accident; LVH: left ventricular hypertrophy; CAD – significant coronary artery disease in one or more coronary vessels.
Perfusion Characteristics
Both perfusion groups cooled rapidly within one hour to the set temperature and maintained a stable temperature over the 12 hour perfusion period. MVO2 was similar between the two perfused groups and did not differ between the right and left coronary artery distribution within the retrograde perfusion group. Lactate accumulation in both perfused groups was also low. Inflow and outflow lactate concentrations were not different across the right and left coronary distribution in retrograde perfused hearts (See Table 2, p>0.2). Perfusion pressures and coronary vascular resistance were higher in the retrograde group compared to the antegrade group at most time points. There appeared to be a decrease in both perfusion pressure and coronary vascular resistance in the antegrade group over time compared to retrograde perfused hearts (p=.04 for pressure, p=.008 for coronary vascular resistance), but this difference was not significant for individual time points.
Table 2.
Lactate Accumulation and MVO2 after 12 Hours Machine Perfusion Preservation
| Group | MVO2(mL/min/100g) | Lactate (mM) |
|---|---|---|
| Antegrade | 0.25±.02 | 2.0±.3 |
| Retrograde – right coronary artery | 0.28±.04 | 1.7±.03 |
| Retrograde – left coronary artery | 0.24±.02 | 1.7±.04 |
Data are reported as mean ± standard error of the mean. There were no important differences in MVO2 or lactate accumulation in the preservation solution between groups (p>0.20).
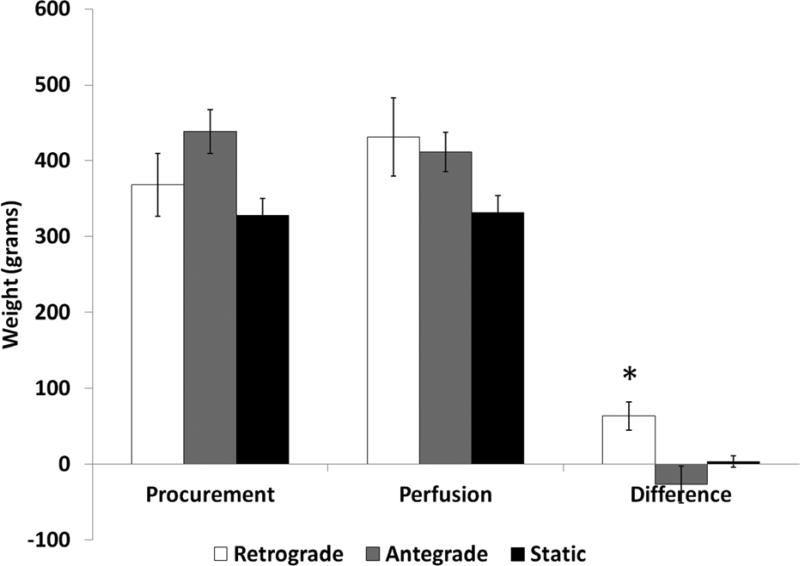
Heart Weight Gain and Myocardial Water Content
Potentially important changes in heart weights were noted over the storage interval. Retrograde perfused hearts demonstrated weight gain (17.6 ± 4.4%) compared to either antegrade perfused hearts (−4.9 ± 4.8%, p=0.002) or static storage hearts (1.3 ± 2.1%, p=0.016). See Figure 1, Online Table 3. This difference was primarily due to left ventricular edema as suggested by the myocardial water content. Left ventricular myocardial water content was higher in the retrograde perfused hearts (80.2±.8%) compared to both the antegrade perfused (76.6±.8%, p=.02) and the static storage hearts (76.7±1%, p=.02). Within the retrograde group, the left ventricular water content was also higher when compared to the right ventricular water content (75.7±2%, p=.066) (Online Table 4).
Figure 1. Heart Weight.
Heart weight increased in retrograde perfused hearts over the 12 hour preservation interval compared to all other groups (* − p<.05).
Metabolism and Energy State
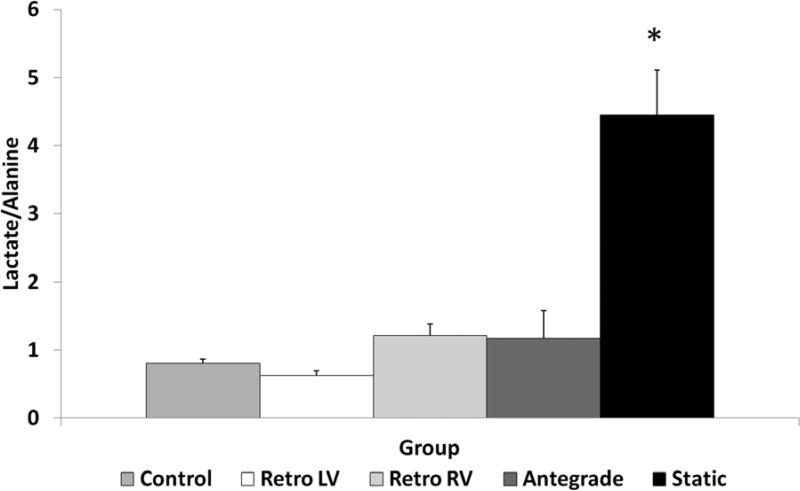
Lactate/Alanine ratios by 1H MRS were lower in all perfused hearts compared to the static storage group, indicative of ongoing oxidative metabolism and reduced intracellular lactate accumulation in the perfusion groups (p<.001). Within perfusion groups, the ratio was lowest in the retrograde left ventricular samples but this difference was minor compared to either the retrograde perfusion right ventricular samples or the antegrade group (p>0.20). See Figure 2, Online Table 5.
Figure 2. Lactate/Alanine Ratio.
The lactate/alanine ratio was increased in static storage hearts compared to all other groups (* − p<.001).
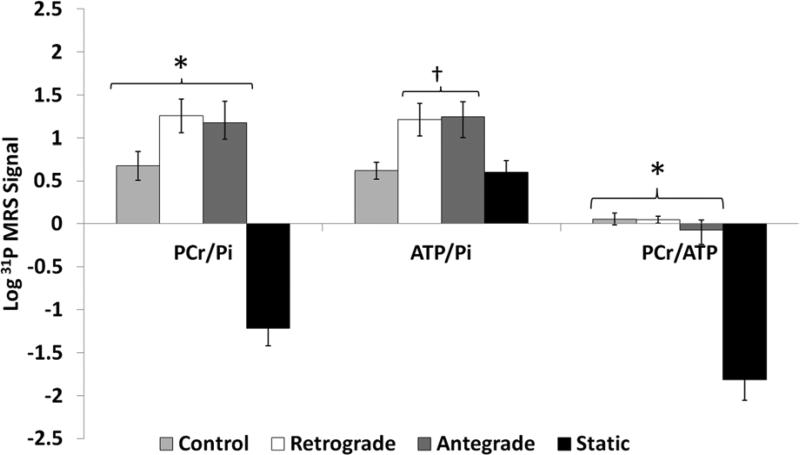
31P MRS also demonstrated that high energy phosphate to inorganic phosphate ratios were better maintained in the perfusion groups. Both perfusion groups demonstrated substantially higher PCr/Pi, γ-ATP/PI, and PCr/ γ-ATP ratios compared to the static storage group (p<.05). There were no important differences in the PCr/Pi, PCr/ γ-ATP ratios between the two perfused groups and control hearts. The γ-ATP/PI tended to be lower in control hearts compared to the two perfusion groups. See Figure 3. There was a strong inverse relationship between the lactate/alanine ratio and both the PCr/Pi (rho=−0.721, p<.001) and PCr/γ-ATP ratios (rho=−0.774, p<.001). No other parameter had a significant association with these indices of metabolism.
Figure 3. Phosphocreatine, ATP, and Inorganic Phosphate Ratios by 31P MRS.
The phosphocreatine/inorganic phosphate ratio and the phosphocreatine/ATP ratio were greater in control and both perfusion preservation groups compared to static storage hearts (* − p<.05). Both perfusion preservation groups demonstrated higher ATP/inorganic phosphate ratios compared to the static storage group († − p<.05). The difference in the ATP/inorganic phosphate ratio may also be important between both perfused groups and control hearts (p=.051 vs Antegrade, p=.07 vs Retrograde).
Discussion
Results of heart transplantation are superior to any other solid organ transplant and this therapy remains the standard of care for end-stage cardiac disease. Nevertheless, early graft failure, likely due to poor graft preservation is the major cause of recipient death within 30 days after transplantation.15 Also, heart transplantation remains limited by the number of suitable donors and the number transplants performed has not changed significantly in the last decade. Conventional wisdom suggests that most suitable donor hearts are recovered and transplanted. However, although the number of all organ donors has increased recently, the fraction of cardiac donors has actually decreased. Only 28% of hearts from all brain dead donors are utilized. The conversion rates of standard criteria cardiac donors between 18 and 50 years of age remains at less than 50%16 suggesting many potentially recoverable organs are not used. Reasons for this are not entirely clear but likely include lack of a suitable recipient within a reasonable procurement distance, particularly for smaller donors and less common blood types (ABO groups B and AB). These factors, in combination with concerns regarding the impact of projected ischemic times, may be important contributors leading to non-utilization of many of these otherwise acceptable donors.
Machine perfusion preservation is a promising technique for improving results of organ transplantation especially by increasing the acceptable donor ischemic interval.17 Its benefits have been clinically proven in kidney transplantation18 and in principle should potentially demonstrate an even greater benefit in heart transplantation because of the reduced ischemic tolerance of donor hearts. Perfusion preservation also offers the possibility for improved donor-recipient matching, such as by HLA, or extending the donor pool for highly sensitized patients by permitting for long distance procurements and prospective crossmatches.1 Finally, perfusion preservation may be useful to protect hearts from older donors, donors with left ventricular hypertrophy or coronary artery disease, donation after circulatory death, or donors with preexisting reversible ventricular dysfunction – organs that are otherwise either not considered for transplantation or at increased risk for primary graft failure.1,3
A number of studies from our laboratory and by others demonstrate that machine perfusion preservation is a promising technique for increasing the donor pool and improving the results of heart transplantation. A warm, beating heart machine perfusion preservation technique using the Organ Care System by Transmedics, Inc (Andover, MA) is currently undergoing clinical investigation in the United States and has obtained CE approval in Europe suggesting increasing enthusiasm for this technique.4,17
In the current study we evaluated metabolic indices of myocardial preservation in human hearts by two techniques of machine perfusion preservation and compared these two machine perfusion preservation techniques to conventional hypothermic static storage after a twelve hour storage interval. Our results suggest that perfused hearts from both perfusion groups continued to consume oxygen over the preservation period. Lactate accumulation in the perfusate was low in both perfused groups. Intracellular lactate/alanine ratios were also reduced suggesting ongoing oxidative metabolism in perfused groups. In contrast, this ratio was essentially reversed in conventional static storage hearts, consistent with anaerobic metabolism. 31P MRS demonstrated almost complete disappearance of phosphocreatine and a corresponding increase in inorganic phosphate in the static groups suggestive of substantial depletion of high energy phosphates. This was illustrated by the decreased phosphocreatine/Pi, γ-ATP/Pi, and phosphocreatine/γ-ATP ratios in the static storage hearts compared to perfused hearts. These ratios were not different between perfused hearts and immediately procured tissue from control hearts indicating that machine perfusion preservation is effective in maintaining myocardial high energy phosphates even over 12 hours of cold perfusion.
Comparing perfusion groups, no significant differences in indices of metabolism were noted over the perfusion interval. Perfusion pressures in the retrograde perfused hearts were higher, likely due to the increased flow rates used compared to antegrade perfused hearts. Coronary vascular resistance was also increased but this difference was not significant for any individual time point. Heart weight gain in the retrograde group and the corresponding slight weight loss in the antegrade group over the perfusion period may explain the difference in coronary vascular resistance noted between these groups. Myocardial water content was also increased in retrograde perfused LV tissue (but not RV tissue) suggestive of increased myocardial edema during preservation. Perfusion pressure and coronary vascular resistance in the retrograde group did not change after the first hour, indicating the development of edema was an early event that did not change over time. The significance of the increased water content is unclear. Flow rate dependent increases in heart weight are well characterized using these models. At least two previously published reperfusion experiments suggest that this weight increase is not necessarily associated with worse reperfusion cardiac function in organs that maintain satisfactory indices of oxidative metabolism.19,20 This may be because, as Amirhamzeh et al have suggested, hydrostatic edema resolves when systolic forces return interstitial fluid to the intravascular space after reperfusion,19 as long as endothelial integrity is maintained. Our own experience in a large animal model of machine perfusion preservation over shorter intervals also demonstrated that despite significant weight gain during the storage interval, myocardial function after implantation was not affected and myocardial water content after reperfusion was not increased.6,8
Both perfusion techniques have potential limitations. For antegrade perfusion, aortic valve incompetence is a possible concern based on experimental studies from our laboratory. Its significance is unclear. Non-coronary flow may result but aortic insufficiency with a competent mitral valve eventually would result in perfusion of the coronaries although this might be at the cost of some left ventricular distention during storage. As noted, both aortic and mitral valve incompetence might have devastating consequences over prolonged ischemic intervals or for recovery of marginal honors but its impact after a standard storage interval would likely be minor. In the current study, despite significant ground and air travel, no convincing evidence of impaired myocardial perfusion could be detected in any of the antegrade perfused hearts.
Retrograde perfusion preservation of human hearts has only rarely been described but this technique is used commonly for cardioplegia delivery during cardiac surgery. The major potential concerns with this technique include less efficient perfusate delivery21 and reduced nutrient flow to the right ventricle.22 In animal studies from our laboratory, higher flow rates were required with retrograde perfusion to achieve adequate myocardial perfusion. In these same experiments, right ventricular lactate/alanine ratios were increased and tissue flow was decreased by microsphere analysis suggesting reduced nutrient delivery to the right ventricle with this technique.12 The current study in human hearts, however, did not demonstrate any impairment in right ventricular perfusion. MVO2 calculated from right and left coronary effluent were not different. Also, lactate/alanine ratios were similar between retrograde right ventricular, retrograde left ventricular, and antegrade left ventricular tissue indicative of adequate tissue flow. Finally, arteriovenous pO2 differences in the retrograde group suggest that in humans, lower flow rates can be used with this technique which may reduce and potentially eliminate any differences in myocardial water content compared to the antegrade perfusion and static storage groups. These findings may in part be related to differences in cardiac venous anatomy between human and canine hearts as others have suggested.23
This study has several limitations. Donor characteristics and organ quality (such as donor age, ejection fraction, donor weight, donor height, or cardiac risk factors) were variable between and within groups. None of these factors, however, correlated with lactate/alanine ratios or high energy phosphate/Pi ratios. Organs were also not randomly assigned to groups mainly for logistical reasons. Perhaps most importantly, although these data indicate that myocardial metabolism and high energy phosphate levels are preserved after long-term cold perfusion of human donor hearts, we did not assess functional recovery of these organs. Transplantation of these organs was not performed for ethical reasons. We are currently investigating transplantation after long-term machine perfusion preservation in large animal model to evaluate post-transplant ventricular performance. Despite the noted limitations, the parameters measured in the current experiments have been used to predict human cardiac allograft function after implantation clinically. Rising lactate levels during warm, beating heart perfusion in the Organ Care System by Transmedics, Inc, are currently used to reject donor hearts procured for clinical transplantation.4 In studies by Caus et al, reduced PCr/Pi and ATP/Pi ratios were associated with poor graft outcomes and ratios similar to our experience were associated with improved functional recovery and reduced graft dysfunction after implantation,24 suggesting that transplantation of many of the machine perfused organs used in this study would have led to a satisfactory outcome. Other investigators in cardiac and other solid organ machine perfusion preservation transplantation models have demonstrated that the ability to maintain a stable MVO2 is perhaps the most important indicator of satisfactory graft preservation.1,25 The fact that even organs with known coronary disease, left ventricular hypertrophy, or reduced ejection fraction demonstrated preserved indices of oxidative metabolism and maintenance of myocardial energy stores suggests that some these organs could potentially be transplanted under the appropriate clinical conditions.
In summary, hypothermic machine perfusion preservation by either antegrade or retrograde perfusion can support myocardial metabolism over long intervals. Grafts preserved with these techniques continue to consume oxygen and maintain high energy phosphate levels. Our data suggest that with hypothermic machine perfusion preservation of donor hearts, storage intervals up to 12 hours can potentially yield acceptable hearts for transplantation. These findings will need to be validated in a comparable model of cardiac transplantation prior to clinical application.
Table 3.
Heart Weight Change After 12 Hour Preservation
| Group | Starting Heart Weight (g) | Heart Weight after 12 Hour Preservation (g) | Weight Gain (%) |
|---|---|---|---|
| Control | 355±65 | NA | NA |
| Static | 328±21 | 332±22 | 6.5±2 |
| Antegrade | 438±29 | 412±26 | −4.8±5 |
| Retrograde | 368±41 | 431±51 | 17.6±4* |
- p<.001 vs Antegrade, p=.005 vs Static
Table 4.
Myocardial Water Content
| Group | Water Content (%) |
|---|---|
| Static RV | 75.1±1 |
| Static LV | 76.7±1.4 |
| Retrograde RV | 75.7±2 |
| Retrograde LV | 80.2±.7* |
| Antegrade LV | 76.6±.8 |
- p<.05 vs All other groups; Antegrade RV results not available
Table 5.
Lactate/Alanine Ratios
| Group | Right Ventricle | Left Ventricle |
|---|---|---|
| Control | .58±.1 | .80±.1 |
| Static | 4.2±.5* | 4.4±.7* |
| Antegrade | Not Available | 1.2±.4 |
| Retrograde | 1.2±.2 | .62±.1 |
- p<.001 vs All other groups; Antegrade RV results not available
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in Part at the 91st Annual Meeting of the American Association for Thoracic Surgery, Philadelphia, PA, May 7-11, 2011
Disclosures: This research was funded in part by the Third Alfred Blalock Research Scholarship of the American Association for Thoracic Surgery.
References
- 1.Cobert ML, West LM, Jessen ME. Machine perfusion for cardiac allograft preservation. Curr Opin Organ Transplant. 2008;13:526–530. doi: 10.1097/MOT.0b013e32830fdf9a. [DOI] [PubMed] [Google Scholar]
- 2.Smulowitz PB, Serna DL, Beckham GE, Milliken JC. Ex vivo cardiac allograft preservation by continuous perfusion techniques. ASAIO J. 2000;46:389–96. doi: 10.1097/00002480-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Collins MJ, Moainie SL, Griffith BP, Poston RS. Preserving and evaluating hearts with ex vivo machine perfusion: an avenue to improve early graft performance and expand the donor pool. Europ J Cardio-thorac Surg. 2008;34:318–25. doi: 10.1016/j.ejcts.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeter R, Hübler M, Pasic M, Hetzer R, Knosalla C. Organ preservation with the organ care system. Appl Cardiopulm Pathophysiol. 2011;15:207–12. [Google Scholar]
- 5.Rosenbaum DH, Peltz M, DiMaio JM, Meyer DM, Wait MA, Merritt ME, Brown R, Chao RY, Ring WS, Jessen ME. Perfusion preservation versus static preservation for cardiac transplantation: effects on myocardial function and metabolism. J Heart Lung Transplant. 2008;27:93–9. doi: 10.1016/j.healun.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum DH, Peltz M, Merritt ME, Thatcher JE, Sasaki H, Jessen ME. Benefits of perfusion preservation in canine hearts stored for short intervals. J Surg Res. 2007;140:243–249. doi: 10.1016/j.jss.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Peltz M, He TT, Adams GA, Koshy S, Burgess SC, Chao Y, Meyer DM, Jessen ME. Perfusion preservation maintains myocardial ATP levels and reduces apoptosis in an ex vivo rat heart transplantation model. Surgery. 2005;138(4):795–805. doi: 10.1016/j.surg.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 8.Peltz M, Cobert ML, Rosenbaum DH, West LM, Jessen ME. Myocardial perfusion characteristics during machine perfusion for heart transplantation. Surgery. 2008;144:225–32. doi: 10.1016/j.surg.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Arom KV, Emery RW, Petersen RJ, Bero JW. Evaluation of 7,000+ patients with two different routes of cardioplegia. Ann Thorac Surg. 1997;63:1619–24. doi: 10.1016/s0003-4975(97)00359-7. [DOI] [PubMed] [Google Scholar]
- 10.Flameng WJ, Herijgers P, Dewilde S, Lesaffre E. Continuous retrograde blood cardioplegia is associated with lower hospital mortality after heart valve surgery. J Thorac Cardiovasc Surg. 2003;125:121–5. doi: 10.1067/mtc.2003.77. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki S, Sasaki H, Tomita E, et al. Twenty-four hour preservation of canine hearts by retrograde coronary sinus perfusion. J Heart Transplant. 1984;4:76–80. [Google Scholar]
- 12.Cobert ML, Merritt ME, West LM, Jessen ME, Peltz M. Differences in regional myocardial perfusion, metabolism, MVO2 and edema after coronary sinus machine perfusion preservation of canine hearts. ASAIO J. 2011;57(6):481–6. doi: 10.1097/MAT.0b013e31823769d5. [DOI] [PubMed] [Google Scholar]
- 13.Chatham JC, Forder JR. Metabolic compartmentalization of lactate in the glucose-perfused heart. Am J Physiol. 1996;270:H224–9. doi: 10.1152/ajpheart.1996.270.1.H224. [DOI] [PubMed] [Google Scholar]
- 14.Chatham JC, Des Rosiers C, Forder JR. Evidence of separate pathways for lactate uptake and release by the perfused rat heart. Am J Physiol Endocrinol Metab. 2001;281:E794–E802. doi: 10.1152/ajpendo.2001.281.4.E794. [DOI] [PubMed] [Google Scholar]
- 15.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, et al. The Registry of the International Society for Heart Transplantation and Lung Transplantation: 29th Official Adult Heart Transplant Report – 2012. J Heart Lung Transplant. 2012;31(10):1052–64. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Johnson MR, Meyer KH, Haft J, Kinder D, Webber SA, Dyke DB. Heart transplantation in the United States, 1999-2008. Am J Transplant. 2010;10:1035–1046. doi: 10.1111/j.1600-6143.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- 17.Taylor MJ, Baicu S. Current state of hypothermic machine perfusion preservation of organs: The clinical perspective. Cryobiol. 2010;60:S20–35. doi: 10.1016/j.cryobiol.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groen H, Moers C, Smits JM, Treckmann J, Monbaliu D, Rahmel A, et al. Cost-Effectiveness of hypothermic machine preservation versus static cold storage in renal transplantation. Am J Transplant. 2012;12:1824–30. doi: 10.1111/j.1600-6143.2012.04030.x. [DOI] [PubMed] [Google Scholar]
- 19.Amirhamzeh MMMR, Dean DA, Jia CX, Cabreriza SE, Starr JP, Sardo MJ, Chalik N, Dickstein ML, Spotnitz HM. Iatrogenic myocardial edema: increased diastolic compliance and time course of resolution in vivo. Ann Thorac Surg. 1996;62:737–43. doi: 10.1016/s0003-4975(96)00391-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhang F, Mo A, Wen Z, Zhou Y, Liang S, Lin H. Continuous perfusion of donor hearts with oxygenated blood cardioplegia improves graft function. Transplant International. 2010;23:1164–70. doi: 10.1111/j.1432-2277.2010.01112.x. [DOI] [PubMed] [Google Scholar]
- 21.Tian G, Xiang B, Dai G, Li G, Sun J, Summers R, et al. Retrograde cardioplegia. J Thorac Cardiovasc Surg. 2003;125:872–80. doi: 10.1067/mtc.2003.109. [DOI] [PubMed] [Google Scholar]
- 22.LeBoutillier M, Grossi EA, Steinberg BM, Baumann FG, Colvin SB, Spencer FC, et al. Effect of retrograde warm continuous cardioplegia on right ventricular function. Circulation. 1994;90(2):306–9. [PubMed] [Google Scholar]
- 23.Ardehali A, Laks H, Drinkwater DC, Gates RN, Kaczer E. Ventricular effluent of retrograde cardioplegia in human hearts has traversed capillary beds. Ann Thorac Surg. 1995;60:78–82. [PubMed] [Google Scholar]
- 24.Caus T, Kober F, Mouly-Bandini A, Riberi A, Métras DR, Cozzone PJ, Bernard M. 31P MRS of heart grafts provides metabolic markers of early dysfunction. Europ J Cardiothorac Surg. 2005;28:576–80. doi: 10.1016/j.ejcts.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Weegman BP, Kirchner VA, Scott WE, III, Avgoustiniatos ES, Suszynski TM, Ferrer-Fabrega JF, et al. Continuous real-time viability assessment of kidneys based on oxygen consumption. Transplant Proc. 2010;42:2020–3. doi: 10.1016/j.transproceed.2010.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]