Abstract
Background
Aminopeptidase P (APP) plays an important role in the catabolism of kinins in human plasma, mostly for des-Arg9-bradykinin. Impaired degradation of this active bradykinin metabolite was found to be associated with a decreased APP activity in hypertensive patients who experienced angioedema while being treated with angiotensin I–converting enzyme inhibitors. The pathophysiology of hereditary angioedema is presently attributed only to a quantitative/qualitative C1 inhibitor (CI-INH) defect with increased bradykinin release.
Objectives
In the context of androgen prophylaxis, increased CI-INH function cannot fully explain protection from angioedema attacks alone because of the limited reversion of the CI-INH defects. Therefore we hypothesized that androgen prophylaxis could enhance plasma APP activity.
Methods
Patients with hereditary angioedema were investigated for plasma metallopeptidase activities responsible for kinin catabolism (APP, angiotensin I-converting enzyme, and carboxypeptidase N) and for CI-INH function in treated and untreated patients.
Results
APP activity was asymmetrically distributed in untreated patients (n = 147): the mean value was significantly lower than the value in a reference healthy and unmedicated population (n = 116; P ≤ .001). Prophylaxis with androgen induced a significant increase in APP activity (P ≤ .001), whereas it did not affect the other metallopeptidase activities. In both patient groups, APP activity showed a significant inverse relationship to disease severity (P ≤ .001).
Conclusion
In addition to the effect on circulating CI-INH levels, the increase in APP levels brought on by androgens could contribute to a more effective control of the kinin accumulation considered to be responsible for the symptoms of angioedema.
Keywords: Hereditary angioedema, aminopeptidase P, C1 inhibitor, androgen, danazol, stanozolol, bradykinin, des-Arg9-bradykinin
Hereditary angioedema (HAE) with C1 inhibitor (C1-INH) deficiency (Online Mendelian Inheritance of Man 106100) manifests clinically with acute recurrent skin swelling, painful abdominal attacks, and potentially life-threatening laryngeal edema. The release of vasoactive bradykinin was found to increase in the plasma of patients with HAE during attacks of angioedema1,2 and has been recognized as being probably the sole mediator responsible for the increased vascular permeability in this pathophysiology.3 Bradykinin is rapidly metabolized, mainly by angiotensin I–converting enzyme (ACE) and aminopeptidase P (APP), whereas carboxypeptidase N (CPN) transforms bradykinin into its active metabolite, des-Arg9-brady-kinin (des-Arg9-BK), a plasma metabolic pathway the importance of which is demonstrated by the observation of angioedema in cases of CPN deficiency or in situations in which ACE is inhibited.4,5 The biologic activities of des-Arg9-BK, which are similar to those of bradykinin, are short lived by ACE and APP.6 Although the carboxytruncated metabolites of bradykinin have a poor affinity for the B2 receptors, they are the agonists of the strongly regulated B1 receptors, the expression of which is increased in inflammation processes. Although no anomaly was found in the metabolism of bradykinin, a decreased plasma APP activity was associated with a significantly slower degradation of des-Arg9-BK in vitro on samples from patients with a history of ACE-associated angioedema.7–9 The accumulation of des-Arg9-BK was shown to cause proinflammatory effects in vivo,7,10 at least when B1 receptors were expressed. This underlines the importance of low APP activity as a susceptibility factor for angioedema.
Androgens such as danazol and stanozolol were introduced for HAE prophylaxis 30 years ago, and their efficacy in preventing the development of HAE attacks has been shown.11 It has been suggested that androgen increases the expression of the SERP-ING1/C1NH gene.12 However, the increased C1-INH function alone cannot fully explain its protective effect. In fact, androgen induces only a limited reversion of the C1-INH function defect in patients with HAE.12
The aim of this prospective study was to explore APP, ACE, and CPN activity in a group of patients with HAE and to compare the effect of androgen prophylaxis on these plasma activities and their relationship to the severity of the HAE disease, as well as its effect on C1-INH function.
METHODS
Patients
Fifty-nine patients with HAE (41 female and 18 male patients, 18–81 years old, 5 with type II HAE) receiving long-term androgen prophylaxis (200 mg of danazol or 4 mg of stanozolol per 1–4 days) and 157 patients with HAE (105 female and 52 male patients, 4–87 years old, 18 with type II HAE) with no prophylaxis for the last 2 years were enrolled. The diagnosis of angioedema was made according to the criteria defined by Cicardi and Zingale.13 The mutations in the SERPING1/C1NH gene were systematically established in the families participating in this study by using previously described techniques,14 and whenever possible, the patients were included in the European HAE register (http://www.haeregister.org). Data obtained from patients were compared with those from a reference group of healthy individuals. The ethical committees of Grenoble (France) and Madrid (Spain) approved the study.
Enzymatic assays
ACE, APP, and CPN activity was measured by using the methods described previously. ACE activity was determined by using the Bühlmann ACE radioenzymatic assay (ACE direct; American Laboratory Products Company, Windham, NH), according to the manufacturer’s instructions. The CPN and APP activity was assessed by using the fluorimetric assay, as described previously.5,15 Metallopeptidase activity data were expressed in nanomoles of hydrolyzed substrate per minute per milliliter (reference values median [25th–75th percentiles]: ACE, 42.2 nmol · min−1 · mL−1 [36.7–51.2 nmol · min−1 · mL−1]; CPN, 68.3 nmol · min−1 · mL−1 [60.7–76.0 nmol · min−1 · mL−1]; APP, 21.2 nmol · min−1 · mL−1 [14.9–27.1 nmol · min−1 · mL−1], n = 116). C1-INH function was quantified from the residual esterase activity of the protease C1s after incubation with citrate plasma samples: C1-INH function was defined as the volume of diluted plasma required to produce 50% residual C1s activity; it is expressed as a percentage of the normal control (reference values: 100.0% [80.4% to 118.6%], n = 69).16
Statistical analysis
Discrete data are reported as numbers and frequencies, and continuous data are reported as medians (25th–75th percentiles) when distribution was not normal (descriptions, comparisons, and correlations). Continuous parameters were tested for distribution normality by using the Shapiro-Wilk test. Medians between 2 groups were compared by using the unpaired Mann-Whitney test when not normally distributed, and otherwise, the unpaired Student t test was used. The relationship between biologic parameters (including all metallopeptidase activities and C1-INH function) and the clinical severity groups was tested by using the stepwise forward and backward logistic regression models. Statistical analyses were performed with Stata 9.2 (Stata Corp LP, College Station, Tex) software. A P value of less than .05 was considered significant. All data were analyzed in the Clinical Research Center of the Grenoble University Hospital.
RESULTS
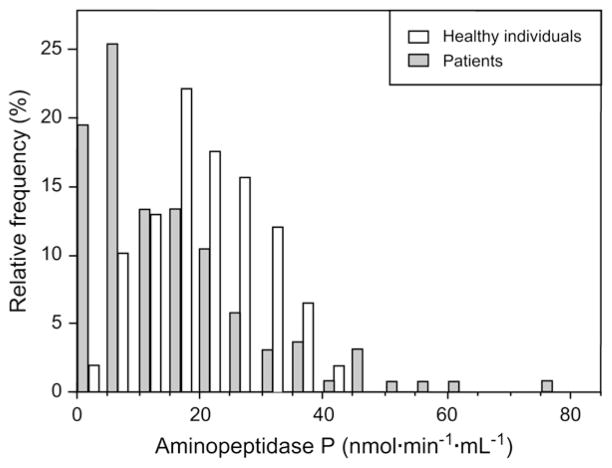
As expected, plasma C1-INH function was found to be at low levels in the patient population tested, irrespective of whether they were receiving prophylaxis (19.0% [25th–75th percentiles, 10.5% to 28.0%], n = 216) compared with that of the reference group. Fig 1 represents the distribution of APP activity measured in untreated patients with HAE compared with that seen in the reference group. The median value of this asymmetric distribution (12 nmol · min−1 · mL−1 [6–22 nmol · min−1 · mL−1], n = 147) was significantly lower than the median value of the reference group (21.2 nmol · min−1 · mL−1 [15–27 nmol · min−1 · mL−1], n = 116; P ≤.001). ACE and CPN activities were not significantly different between the 2 groups (P = .38 and P = .91, respectively).
FIG 1.
Distribution of APP activity in patients with HAE with no prophylaxis (solid columns, n = 147) and in healthy individuals used as the reference group (open columns, n = 116).
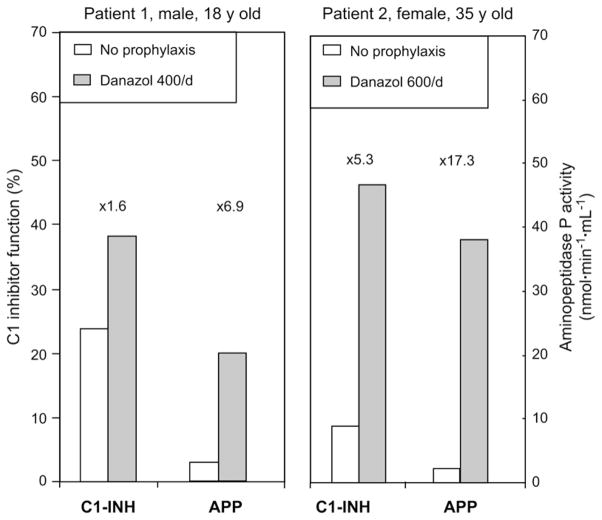
Patients with HAE receiving androgen prophylaxis showed a significantly higher plasma APP activity (P ≤.001; ×2.7 median increase) when compared with untreated patients. This effect was greater than that observed for C1-INH function (P ≤ .001; ×1.3 median increase; Figure 2). Androgen prophylaxis did not significantly change the plasma activity of ACE (43 [34–57; n = 100] vs 43 [21–58; n =36] for untreated and treated patients, respectively; P =.79) and CPN (62 [55–70; n = 98] vs 65 [57–70; n = 36] for untreated and treated patients, respectively; P = .54). To determine the individual effect of androgen treatment on plasma APP activity, we considered a female and a male patient with HAE with short-term danazol prophylaxis before surgery. Fig 3 shows that both APP activity and C1-INH function markedly increased after a 10-day period of 400 to 600 mg of danazol per day.
FIG 2.
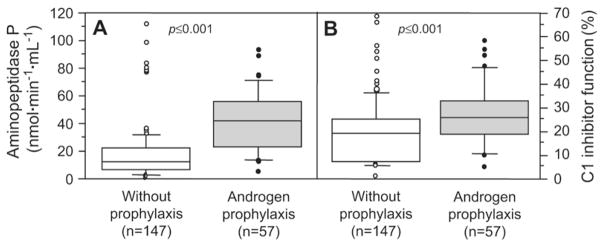
Plasma APP activity (A) and CI-INH serpin function (B) in patients treated with long-term androgen prophylaxis (200 mg of danazol or 4 mg of stanozolol per 1–4 days; solid symbols) or not treated (open symbols). Data are presented in box whisker plots showing the median (horizontal bars within boxes), the inter-quartile range (boxes), and the 5th to 95th percentiles (vertical bars). Values greater than and less than these levels were dotted separately. Data comparisons between groups of patients taking androgen or not are presented according to the Mann-Whitney or Student nonparametric tests.
FIG 3.
Plasma APP activity and CI-INH serpin function of patients 1 and 2 with HAE undergoing short-term prophylaxis with danazol (400 and 600 mg/d, respectively, for 10 days). Differences between values observed in the context of danazol treatment (solid columns) or no treatment (open columns) are indicated as increasing factors.
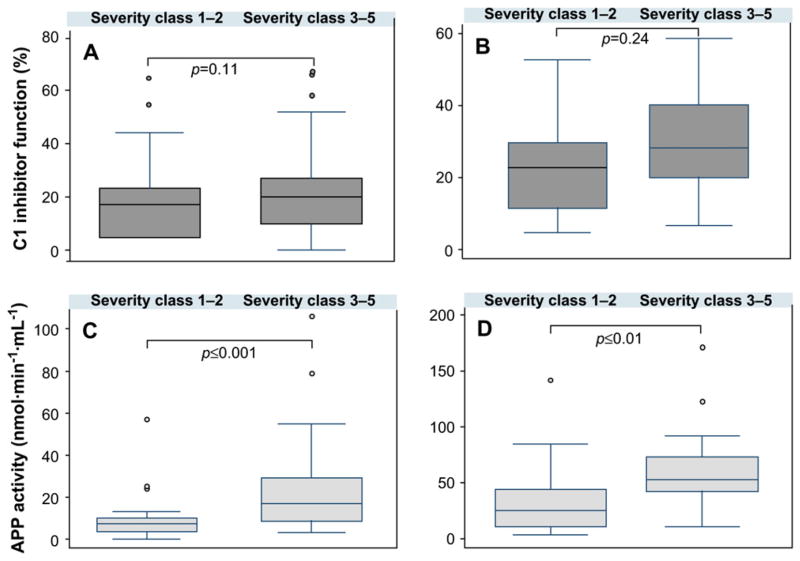
We sought to determine whether metallopeptidase activities or C1-INH function was related to disease severity. One hundred fifty-seven untreated patients and 59 treated patients were separated into 2 groups of disease severity: (1) moderate-to-severe disease (n = 65 and 27 for untreated and treated patients, respectively; classes 1 and 2 according to Cicardi and Zingale13) and (2) asymptomatic to mild disease (n =92 and 32 for untreated and treated patients, respectively; classes 3–5). The equality of means across both pathologic groups was tested by using the Student t test; the groups were also compared by using the Mann-Whitney rank sum test when distribution was not normal. The differences between the 2 groups were not significant for C1-INH function (P =.11 and P =.24 for untreated and treated patients, respectively) but were found to be significant for APP activity (P ≤ .001 and P ≤ .01 for untreated and treated patients, respectively). Diagrams of the severity profiles show that in both groups of patients disease severity was inversely correlated with C1-INH function (Fig 4, A and B) and APP activity (Fig 4, C and D) but not with CPN and ACE activity (data not shown).
FIG 4.
CI-INH function or APP activity versus disease severity. Box whisker plots of CI-INH function (A and B) or APP activity (C and D) represent the median (horizontal bars within boxes) and the interquartile range (boxes) with the 5th to 95th percentiles (vertical bars). Data were expressed for each disease severity class12 (1–2, severe disease; 3–5, mild to asymptomatic disease) in patients with no treatment (n = 157; Fig 4, A and C) and those with androgen prophylaxis (n = 59; Fig 4, B and D). Data comparisons for severity of disease between groups follow the equality of means with the Student t test (Fig 4, B) or the Mann-Whitney rank sum test (Fig 4, A, C, and D).
Considering the relative importance of the assays developed for HAE diagnosis, we next sought a possible risk factor as a disease severity parameter. The backward regression logistic model was used in both series of patients to compare the severity classes in patients with and without androgen prophylaxis. Fig 5 shows that for untreated patients, the odds ratio was lower than 1, was significant for APP activity in both the untreated and treated patients, and was significant for CPN only in the untreated patients. The values for C1-INH function and ACE were not retained. The model is acceptable to good for group A (n = 98; no prophylaxis; area under the receiver operating characteristic curve, 0.800 [95% CI, 0.711–0.889]) and good for group B (n = 36; patients taking an androgen; area under the receiver operating characteristic curve, 0.846 [95% CI, 0.719–0.972]). Therefore the risk of severe disease decreased by 10.7% for each increase of 1 nmol · min−1 · mL−1 in APP activity (by 67.7% for an increase of 10 nmol · min−1 · mL−1) and by 4.5% for each increase of 1 nmol · min−1 · mL−1 in CPN activity. This suggests that high plasma APP activity (and CPN for the untreated patient group) could be associated with protecting patients from a severe form of angioedema.
FIG 5.
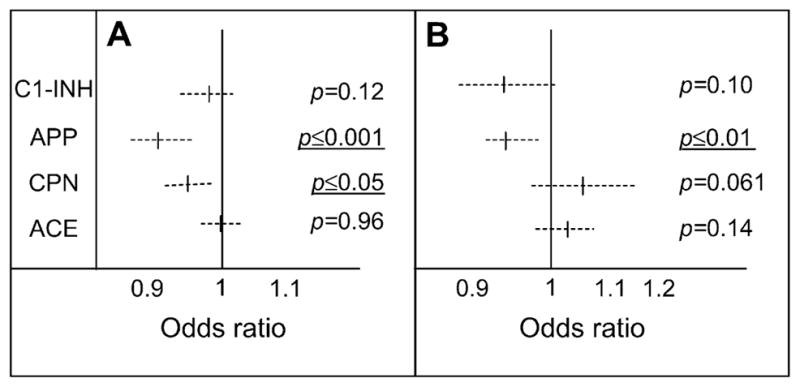
Risk estimate for the association of the biologic parameters with disease severity. Odds ratios were calculated from plasma CI-INH function and APP, CPN, and ACE activity. A, Untreated patients (n = 98). B, Treated patients (n = 36). Dashed lines represent the 95% Cis, and vertical bars represent the median values.
DISCUSSION
These results support the observation that a majority of patients with HAE exhibit low circulating APP activity, the main metabolic pathway of des-Arg9-BK in human plasma.6,7,17 This low level of activity could be associated with an enhanced susceptibility to angioedema attacks, in particular for individuals in the left part of the distribution panel, which is in agreement with previous observations concerning ACE inhibitor–associated angioedema.8 In addition, the wide distribution of APP activity, partially regulated by genetic factors, such as the presence of a single nucleotide polymorphism within the gene encoding APP,18 could contribute to the substantial variability in disease expression.
These results obtained at the plasma level raised some questions on their pathophysiologic meaning at the local edema reaction site. The same question also emerged for the measurement of plasma C1-INH, whereas in this case experiments from C1INH knockout animals have highlighted this question.19 Concerning APP, some experiments in human subjects provided evidence for its role in the regulation of the local inflammatory reaction. In fact, the coinjection of apstatin, a specific inhibitor of APP, potentiated the local reaction induced by subcutaneous injection of exogenous bradykinin.20 Also in vivo, inhibition of APP exhibited the same effect as ACE inhibition in the inflammatory response to bradykinin.20 However, because B2 receptors were rapidly desensitized,21 the B1 receptors, with the des-Arg9-BK as ligand, took over and were responsible for the inflammatory symptoms, as shown in experimental models.22–24 Because APP is the main degrading pathway of des-Arg9-BK, the proinflammatory effect of the B1 agonist is still more potentiated.
The high increase in plasma APP activity with androgen use that we observed could be interpreted as challenging its possible participation in the protection against angioedema attacks. Further investigations on membrane APP activity using cells in the presence of androgen, with an estimation of the ligand–receptor interaction, could help to explain this.
It was tempting to associate the increased APP activity on androgen prophylaxis with possible androgen inducibility of the APP gene. The XPNPEP2 gene encoding the membrane form of APP does not display an androgen response element–like sequence motif, such as 5′-AGAAGAnxTGTACA-3′ (x varying from 0 to 8 nucleotides), as defined by Zilliacus et al.25 Therefore it could be hypothesized that the effect that androgens have on the circulating APP activity results from a potentiating effect on a glycosylphosphatidylinositol hydrolase activity. This includes metalloprotease and phospholipase mechanisms, with subsequent release of glycosylphosphatidylinositol–anchored proteins,26 which are responsible for the shedding of APP from the endothelial membrane.27
In conclusion, for the first time, this study provides evidence that APP, a major degrading metallopeptidase involved in the inactivation of kinins, mainly des-Arg9-BK, could play a role in the pathophysiology of HAE. The potentiating effect of androgens on this peptidase could open a new area of investigation into the protective mechanism of androgens. In conjunction with the increase in circulating C1-INH levels and the restoration of C4 antigenic levels that has already been observed,11 these data indicate that androgens contribute to a more effective control of the pathologic development of kinin-dependent activities, with subsequent repair of both production and catabolism of kinins. Moreover, considering that high plasma APP activity might protect against severe disease, its assay in patient samples could be recommended as a predictive parameter of disease severity.
Key messages.
Plasma activity of APP, a major metallopeptidase involved in kinin inactivation, is increased during androgen treatment.
Its assay could be recommended as a predictive parameter of angioedema severity.
Acknowledgments
Supported by grants from the thematic action 5 of the 2002 Programme Hospitalier de Recherche Clinique PHRC and Ministerio de Ciencia y Tecnología (SAF 2003-03485). The work of A.A. is funded by the Canadian Institutes of Health Research (grant MOP-14077) and the National Institutes of Health (grant 1-R01-HL079184).
We thank F. Csopaki for skilful technical assistance, Professor J.-Y. Cesbron (Grenoble) for continuous encouragement, Dr C. Dumestre-Pérard (Grenoble) for advice, and the physicians treating the patients: Drs M. Abbal (Toulouse), M. Bouvier (Lyon), T. Caballero-Molina (Madrid), L. Faivre (Dijon), S. Gayet (Marseille), L. Fernández Pereira (Cáceres), M. Guilarte (Barcelona), J. R. Harlé (Marseille), I. Heliot (Bordeaux), C. Jacquot (Grenoble), D. Launay (Lille), A. Leimgruber (Lausanne), M. Lifermann (Dax), H. Maillard (Le Mans), L. Marqués (Lérida), I. Sánchez-Machín (Santa Cruz de Tenerife), D. Vervloet, and P. J. Weiller (Marseille). Above all, we thank the families who cooperated in this study for their invaluable help.
Abbreviations used
- ACE
Angiotensin I–converting enzyme
- APP
Aminopeptidase P
- C1-INH
C1 inhibitor
- CPN
Carboxypeptidase N
- des-Arg9-BK
Des-Arg9-bradykinin
- HAE
Hereditary angioedema
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
References
- 1.Nussberger J, Cugno M, Amstutz C, Cicardi M, Pellacani A, Agostoni A. Plasma bradykinin in angio-oedema. Lancet. 1998;351:1693–7. doi: 10.1016/S0140-6736(97)09137-X. [DOI] [PubMed] [Google Scholar]
- 2.Cugno M, Nussberger J, Cicardi M, Agostoni A. Bradykinin and the pathophysiology of angioedema. Int Immunopharmacol. 2003;3:311–7. doi: 10.1016/S1567-5769(02)00162-5. [DOI] [PubMed] [Google Scholar]
- 3.Davis AE., III The pathophysiology of hereditary angioedema. Clin Immunol. 2005;114:3–9. doi: 10.1016/j.clim.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Matthews KW, Mueller-Ortiz SL, Wetsel RA. Carboxypeptidase N: a pleiotropic regulator of inflammation. Mol Immunol. 2004;40:785–93. doi: 10.1016/j.molimm.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Cyr M, Lepage Y, Blais C, Jr, Gervais N, Cugno M, Rouleau JL, et al. Bradykinin and des-Arg(9)-bradykinin metabolic pathways and kinetics of activation of human plasma. Am J Physiol Heart Circ Physiol. 2001;281:H275–83. doi: 10.1152/ajpheart.2001.281.1.H275. [DOI] [PubMed] [Google Scholar]
- 6.Décarie A, Raymond P, Gervais N, Couture R, Adam A. Serum interspecies differences in metabolic pathways of bradykinin and [des-Arg9]BK: influence of enalaprilat. Am J Physiol Heart Circ Physiol. 1996;271:H1340–7. doi: 10.1152/ajpheart.1996.271.4.H1340. [DOI] [PubMed] [Google Scholar]
- 7.Blais C, Jr, Marc-Aurèle J, Simmons WH, Loute G, Thibault P, Skidgel RA, et al. Des-Arg9-bradykinin metabolism in patients who presented hypersensitivity reactions during hemodialysis: role of serum ACE and aminopeptidase P. Peptides. 1999;20:421–30. doi: 10.1016/s0196-9781(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 8.Adam A, Cugno M, Molinaro G, Perez M, Lepage Y, Agostoni A. Aminopeptidase P in individuals with a history of angio-oedema on ACE inhibitors. Lancet. 2002;359:2088–9. doi: 10.1016/S0140-6736(02)08914-6. [DOI] [PubMed] [Google Scholar]
- 9.Molinaro G, Cugno M, Perez M, Lepage Y, Gervais N, Agostoni A, et al. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther. 2002;303:232–7. doi: 10.1124/jpet.102.038067. [DOI] [PubMed] [Google Scholar]
- 10.Blais C, Jr, Couture R, Drapeau G, Colman RW, Adam A. Involvement of endogenous kinins in the pathogenesis of peptidoglycan-induced arthritis in the Lewis rat. Arthritis Rheum. 1997;40:1327–33. doi: 10.1002/1529-0131(199707)40:7<1327::AID-ART18>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 11.Gelfand JA, Sherins RJ, Alling DW, Frank MM. Treatment of hereditary angioedema with danazol. Reversal of clinical and biochemical abnormalities. N Engl J Med. 1976;295:1444–8. doi: 10.1056/NEJM197612232952602. [DOI] [PubMed] [Google Scholar]
- 12.Pappalardo E, Zingale LC, Cicardi M. Increased expression of C1-inhibitor mRNA in patients with hereditary angioedema treated with Danazol. Immunol Lett. 2003;6:271–6. doi: 10.1016/s0165-2478(03)00029-4. [DOI] [PubMed] [Google Scholar]
- 13.Cicardi M, Zingale L. Clinical manifestations of hereditary angioedema. J Allergy Clin Immunol. 2004;114(suppl):S55–8. doi: 10.1016/j.jaci.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Drouet C, Blanch A, Roche O, Monnier N, Duponchel C, Kalmár L, et al. Mutation analysis of the C1NH gene. J Allergy Clin Immunol. 2004;114(suppl):S66–74. [Google Scholar]
- 15.Molinaro G, Carmona A, Juliano MA, Juliano L, Malitskaya E, Yessine M-A, et al. Human recombinant membrane-bound aminopeptidase P: production of a soluble form and characterization using novel, internally quenched fluorescent substrates. Biochem J. 1995;385:389–97. doi: 10.1042/BJ20040849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drouet C, Alibeu C, Ponard D, Arlaud GJ, Colomb MG. A sensitive method to assay blood complement C1 Inhibitor activity. Clin Chim Acta. 1988;174:121–30. doi: 10.1016/0009-8981(88)90379-8. [DOI] [PubMed] [Google Scholar]
- 17.Blais C, Jr, Marceau F, Rouleau JL, Adam A. The kallikrein-kininogen system: lessons from the quantification of endogenous kinins. Peptides. 2000;21:1903–40. doi: 10.1016/s0196-9781(00)00348-x. [DOI] [PubMed] [Google Scholar]
- 18.Duan QL, Nikpoor B, Dubé M-P, Molinaro G, Meijer IA, Dion P, et al. A variant in XPNPEP2 is associated with angioedema induced by angiotensin converting enzyme inhibitors. Am J Hum Genet. 2005;77:617–26. doi: 10.1086/496899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han ED, MacFarlane RC, Mulligan AN, Scafidi J, Davis AE., III Increased vascular permeability in C1 inhibitor-deficient mice mediated by the bradykinin type 2 receptor. J Clin Invest. 2002;109:1057–63. doi: 10.1172/JCI14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K-S, Kumar S, Simmons WH, Brown NJ. Inhibition of aminopeptidase P potentiates wheal response to bradykinin in angiotensin-converting enzyme inhibitor-treated humans. J Pharmacol Exp Ther. 2000;292:295–8. [PubMed] [Google Scholar]
- 21.Leeb-Lundberg LMF, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:22–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- 22.Raymond P, Décarie A, Lantin F, Raut R, Morais R, Couture R, et al. A role for B1 and B2 kinin receptors in the modulation of T-kininogen during the acute phase response of inflammation. Peptides. 1996;17:1163–70. doi: 10.1016/s0196-9781(96)00174-x. [DOI] [PubMed] [Google Scholar]
- 23.Décarie A, Adam A, Couture R. Effects of captopril and Icatibant on bradykinin (BK) and des [Arg9] BK in carrageenan-induced edema. Peptides. 1996;17:1009–15. doi: 10.1016/0196-9781(96)00145-3. [DOI] [PubMed] [Google Scholar]
- 24.Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99:6–38. doi: 10.1254/jphs.srj05001x. [DOI] [PubMed] [Google Scholar]
- 25.Zilliacus J, Wright APH, Carlstedt-Duke J, Gustafsson J-A. Structural determinants of DNA-binding specificity by steroid receptors. Mol Endocrinol. 1995;9:389–400. doi: 10.1210/mend.9.4.7659083. [DOI] [PubMed] [Google Scholar]
- 26.Parkin ET, Watt NT, Turner AJ, Hooper NM. Dual mechanisms for shedding of the cellular prion protein. J Biol Chem. 2004;279:11170–8. doi: 10.1074/jbc.M312105200. [DOI] [PubMed] [Google Scholar]
- 27.Ryan JW, Papetropoulos A, Ju H, Denslow ND, Antonov A, Virmani R, et al. Aminopeptidase P is disposed on human endothelial cells. Immunopharmacology. 1996;32:149–52. doi: 10.1016/0162-3109(95)00078-x. [DOI] [PubMed] [Google Scholar]