Abstract
Throughout its adult life, the colonial urochordate, Botryllus schlosseri, produces its entire body, every week, from endogenous stem cells. In this developmental process, all of the body organs including heart, digestive system, branchial sac, endostyle nerve cells and others are created de novo. Here we discover a stem cell niche in these animals- an organ called the endostyle, which also produces and exports hormones. In this study using a combination of in vivo cell labeling, cell engraftments, and chimeric fusion techniques, all followed by automated time lapse microscopy we identifies the endostyle as a stem cell niche that harbors and export adult stem cells to developing organs, wherein they participate in tissue regeneration.
Introduction
Adult stem-cells are defined by their ability to self renew and to generate differentiated cell populations that maintain tissues. New studies provide an increasing support for the notion that stem cells in vivo require input from particularly defined microenvironments known as “niches” (review in Morrison and Spradling, 2008). Since the hypothesis of stem cell niche was first proposed, 30 years ago (Schofield, 1978), a few adult stem cells niches have been identified in invertebrate models (gonads, intestine of Drosophila and germ line organization region of Caenorhanditis elegans; Crittenden et al., 2002; Gilboa and Lehmann, 2004; Kiger et al., 2001; Kimble and White, 1981; Lin and Spradling, 1993; Nystul and Spradling, 2007; Ohlstein and Spradling, 2007; Tulina and Matunis, 2001; Wieschaus and Szabad, 1979; Xie and Spradling, 2000). Several somatic niches have been implicated in mammals (hematopoietic, skin/hair follicle, mammary gland, incisor teeth, neural and intestine; Barker et al., 2007; Calvi et al., 2003; Cotsarelis et al., 1990; Doetsch et al., 1999; Harada et al., 1999; Palmer et al., 1997; Potten et al., 2002; Shen et al., 2004; Tumbar et al., 2004; Villadsen et al., 2007; Zhang et al., 2003). Here we report about the identification of a novel somatic adult stem cell niche in a simple chordate, Botryllus schlosseri (Tunicata). Various developmental and regeneration processes in this colonial organism are mediated by adult stem cells. In a highly coordinated asexual budding process which occurs every week, B. schlosseri adult individuals (zooids) generate new zooids. This renewal process includes the generation of all somatic organs (a heart, endostyle, branchial sac, neural complex, oral and atrial siphons, digestive tract, Fig 1) and the germline (review in Manni and Burighel, 2006). Genetically distinct B. schlosseri colonies can form natural parabionts by vascular fusions. Following their anastomosis, cells transmigrate between colonies (SFig 1; Svideo 1) and often join with host cells and can even replace the germline and somatic tissues of the host (Oka and Watanabe, 1960; Pancer et al., 1995; Sabbadin and Zaniolo, 1979; Stoner and Weissman, 1996; Stoner et al., 1999; termed cell parasitism by Burnet, 1971). We have shown that cell parasitism is determined genetically and is an inherent property of stem cells (Laird et al., 2005; Pancer et al., 1995; Stoner and Weissman, 1996; Stoner et al., 1999). Under certain conditions colonial tunicates can even regenerate themselves from the vasculature alone (e.g. Rinkevich et al., 2007; Sabbadin et al., 1975; Voskoboynik et al., 2007). B. schlosseri thus offers a unique chordate model organism for studying diverse activities mediated by adult stem cells. Although we could prospectively isolate germline and somatic stem cells from the bodies of these organisms (Laird et al., 2005), the location of these cells remained unknown. Our study investigates the site of stem cells in the adult zooid, and shows that one such site, termed the endostyle niche (Fig 1), harbors somatic stem cells.
Fig 1. The endostyle niche (EN).
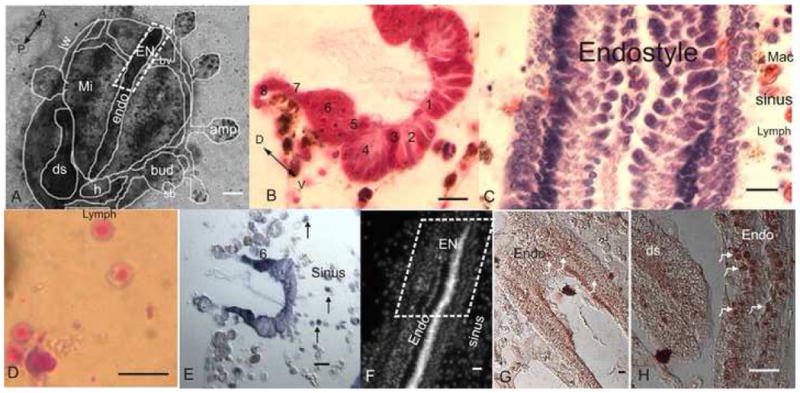
A. A microscopic ventral view of zooid buds and vasculature embedded in a tunic. The zooid endostyle bathes by cells that flow through its sinuses, with macrophages organized in islands next to it (role of macrophages in reutilization of materials in a new bud formation has been shown; Lauzon et al., 2002), digestive system and heart are located near its posterior end. EN is outlined at its anterior end. B. A cross section of the endostyle zones (1–8; azan heidenhain). C. A longitudinal section of the endostyle, lymphocyte cells and macrophages can be seen in its sinus (hematoxylin eosin). D. Potential stem cells, lymphocyte like cells which were drawn from the EN for the transplantation and labeling experiments, stained in May Grunwald Giemsa. E. Expression of Raldh (in dark purple) in the endostyle epithelial (higher at zone 6). Cells in the subendostylar sinus also express it (arrows) F. Nucleus location in the endostyle epithelial and sinus (Hoechst). G. Expression of PCNA in the endostyle (brown/arrows). H. Higher magnification of PCNA staining in the endostyle epithelial nucleus (arrows) andthe digestive system epithelial. endo-endostyle, EN-endostyle niche, sb-secondary bud, h-heart, ds-digestive system, amp-ampulla, mi-macrophage islands, bv-blood vessel, lw-lateral wall, A-anterior, P-posterior, D-dorsal, V-ventral, mac-macrophage, lymph-lymphocyte, numbers-zone number. scale bar A-100μm, B-H-10μm.
Results
Identifying a somatic stem cell niche in Botryllus
We tested the ability of cells from different sites: 1). to participate in bud generation, 2). to contribute to vasculature regeneration and 3). to participate in bud generation of host colonies, following engraftment of cells or fusion between distinct colonies. We used several approaches to evaluate the involvement of stem cells in these developmental and regeneration processes.
To identify potential stem cells that contribute to bud development and vasculature regeneration, we first labeled hundreds of cells in situ in 10 colonies and tracked their location and migration using automated time lapse microscopy. A massive recruitment of labeled cells to developing or regenerating tissues was observed in two colonies in which their endostyle anterior ventral area included labeled cells (Fig 2). Then, we further labeled 10–40 cells at various sites of the colony and tracked their proliferation and location. Cells were labeled in: a) the anterior ventral region of the zooid endostyle (cells from the subendostylar sinus adjacent to the endostyle epithelium; termed endostyle niche EN; Fig 1), b) the anterior ventral region of the primary bud endostyle c) primary bud lateral wall, d) zooid lateral wall, digestive system and e) in the colony vasculature (STable 1; n=40 colonies). Time lapse images taken every 30–60 minutes over ~120 hours, and tracking number, location, expansion and fluorescent intensity of labeled cells revealed that only the cells from the EN proliferated and migrated to developing or regenerating tissues (secondary buds, primary buds and regenerating vasculature). Cells from other sites (zooid lateral wall, digestive system, primary bud lateral wall or vasculature) did not increase in number and did not home to developing or regenerating organs even when transplanted into the EN (Supplemental material; Fig 2, 3; SFig1; SVideos 2A, 2B, 2C; STable 1).
Fig 2. In vivo labeling and tracing of cells distribution in Botryllus colonies.
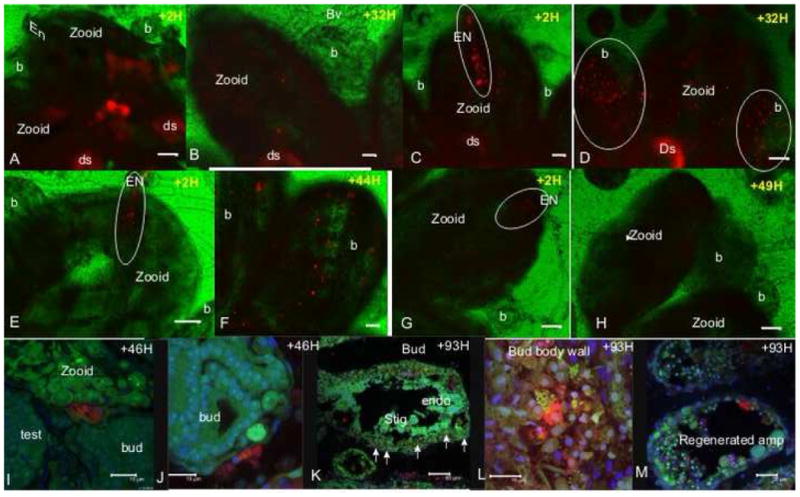
A. A zooid with hundreds of in situ labeled cells (red) in its body wall, 2 hours post labeling. B. Same zooid, 32 hours post labeling, labeled cells are observed in the zooid but not in its buds. C. Labeled cells in the zooid body wall and the EN (outlined), 2 hours post labeling. D. Homing of labeled cells from the endostyle in panel C to remote buds in the colony, 32 hours following labeling. Labeled cells are detected in the buds (outlined). E. A few labeled cells in the zooid EN (outlined), 2 hours post labeling. F. Labeled cells in the buds 44 hours post labeling. These buds were directly connected to the EN labeled zooid shown in panel E. G. Labeled cells which were transplanted from the vasculature into the zooid EN (outlined), 2 hours post labeling. H. Same zooid 49 hours post labeling, labeled cells taken from vasculature, did not increase in number and did not home to developing buds. I. and J. A few labeled cells in 2 different buds (red), 46 hours post EN labeling (confocal images). K. 93 hours following EN labeling, labeled cells are detected in the bud’s body wall and stigmata (arrows; confocal image). L. Higher magnification confocal image of labeled cells in the bud’s body wall from panel K. M. Labeled cells in the regenerating vasculature epithelial 93 hours following EN labeling and vasculature removal (confocal image). Vybrant Did labeling -red, Hoechst nucleus stain-blue, natural autofluorescence (501nm emissions spectra) –green. EN-endostyle niche, endo-endostyle, b-bud, ds-digestive system, st-stigmata, amp-ampulla, bv-blood vessel, H-hour. scale bar A-H=100μm, I, J, L =15 μm, K-50 μm, M=25μm.
Fig 3. Fluorescent intensity of labeled cells.
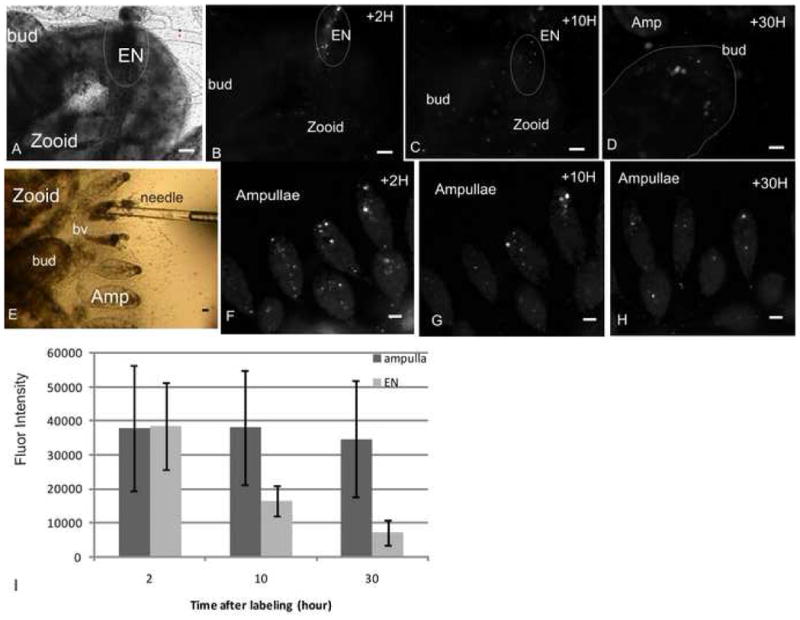
A. EN (outlined) in a zooid (phase contrast image) and ~14 labeled cells within (B, fluorescent image), 2 hours post labeling. C. Same EN 10 hours post labeling, fluorescent intensity of the cells from the EN decreased and expansion of labeled cells in the zooid (~20 labeled cells) is observed. D. 30 hours post labeling ~14 labeled cells detected in the bud which was directly connected to the EN of the labeled zooid (panel A). At that time ~40 labeled cells were tracked in other buds of the colony. E. Injection of labeled cells into an ampulla by a glass needle. F. 2 hours post labeling, ~50 labeled cells detected in ampullae near the injected site. G. 10 hours post labeling ~ 17 labeled cells remained at this site, their fluorescence intensity did not decrease. H. 30 hours following labeling, ~12 cells detected in the labeling site, their fluorescence intensity remained unchanged. I. The average fluorescence intensity in labeled cells from ampullae versus cells from the EN as a function of time. EN-endostyle niche, H-hour, amp-ampulla, bv-blood vessel, scale bar=50μm.
Migration of cells from the EN was not restricted to budding regions of the same zooid. EN labeled cells were also detected in remote buds of other zooids in the colony. Confocal microscopy analysis demonstrated that labeled cells which migrated from the EN were incorporated in the developing tissues of the buds (body wall, stigmata; Fig 2) and within the epithelial cells of the vasculature (regenerated vasculature, following vasculature removal; Fig 2). To test the capability of EN cells to cross genotypic boundaries in chimeras, we labeled several cells in one of the chimera partners. Within 14–20 hours, labeled cells from the EN of the labeled partner appeared at the buds of the other partner (n=3 chimeras). By contrast, labeled cells from other sites did not reach the other partner buds (STable 1; n=8 chimeras).
Establishing a Botryllus stemness assay
Further support for the identification of stem cell potential in the EN cells was provided by transplantation experiments. Transferring cells between compatible colonies can lead to chimerism and cell parasitism (Laird et al., 2005; Oka and Watanabe, 1960, Pancer et al., 1995; Sabbadin and Zaniolo, 1979; Stoner and Weissman, 1996; Stoner et al., 1999). The long term contribution of a few transplanted cells to tissues in the recipient colony is an evidence for multipotency and self renewal capacities of the engrafted cells. Indeed, single cell transplantation and serial engraftment assays showed that adult stem cells are the cells responsible for a stable long term chimerism in B. schlosseri (Laird et al., 2005).
To evaluate the stemness of cells from the EN and to compare them with cells from other sites, we tested their ability to induce a long term chimerism in genetically distinct but compatible partners. Donor cells were drawn from the EN, digestive system, or the vasculature (STable 2). Small amounts of these cells (5–20; Fig 1G) were subsequently microinjected into the EN or the bud of a compatible recipient colony (58 colonies, STable 1). Somatic and germline tissues were harvested every month during the first 6 months following injection. Chimerism was assayed by two independent methods: (i) amplified fragment length polymorphism (AFLP’s), which identifies donor genetic markers in recipient tissues and (ii) differential pigmentation of the donor and recipient colonies, in which the chimeras are visible by eye (Fig 4).
Fig 4. Genotype takeover and multilineage differentiation of cells from the EN.
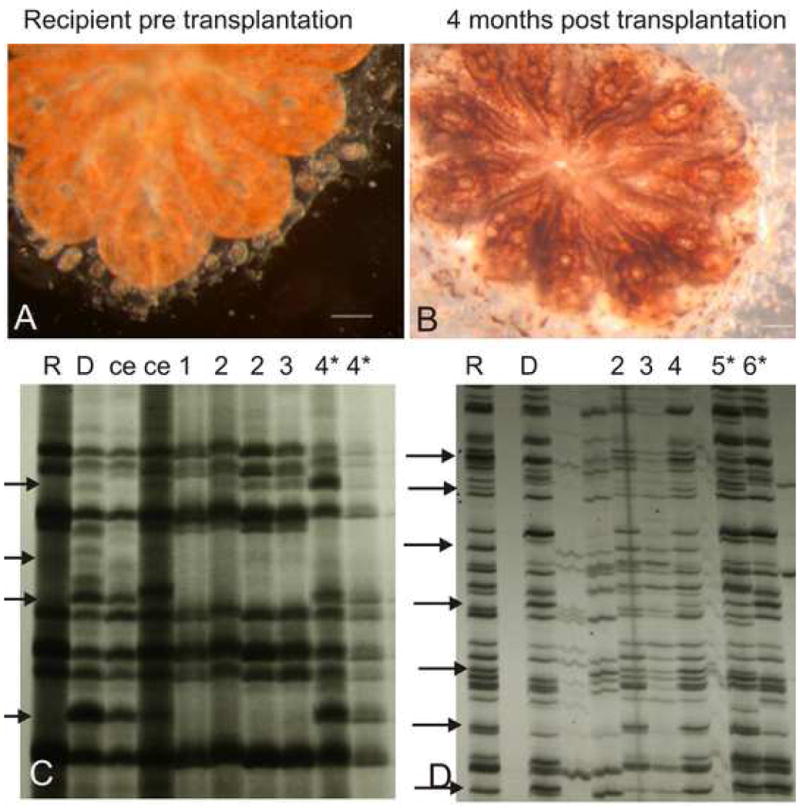
In this transplantation experiment, the recipient colony was orange (Panel A) and the donor colony was black, 4 months following transplantation of few cells from the donor colony EN, the recipient colony changed its pigmentation from orange to a mixed black and orange pigmentation (Panel B). The transplanted colony AFLPs genetic fingerprints at that time reflected the donor genotype (Panel C). D. AFLPs genetic fingerprints of recipient colony (R), donor colony (D) and the genetic fingerprints of the recipient colony 2–6 months following transplantation of few cells from the donor colony EN. Five months post transplantation the recipient colony became a chimera. ce-known chimera, numbers indicate number of months post transplantation, *-chimeric colony, arrows indicate AFLP’s markers which differ between the recipient and the donor colonies. Scale bar = 1mm
We tested 259 DNA samples by AFLP (STable 2). Chimerism was determined when the genetic fingerprints of the donor were identified in the host, either exclusively or with host fingerprints (STable 2; Fig 4). Engraftment of donor EN cells led to somatic chimerism in 50% of the recipient colonies (8 out of 19 transplants, 22 out of 69 DNA samples; STable 2), whereas cells from two other sites (digestive system, vasculature), showed no sign of somatic chimerism (0 out of 20 different transplants, 75 somatic DNA samples; STable 2). Germline chimerism was not observed, irrespective of donor cell origin (0 out of 17 transplants, 31 DNA samples; STable 2).
Cells from the donor EN survived, proliferated, migrated and integrated in the somatic organs of recipient colonies. Chimerism was detected, 1–2 months following transplantation and lasted up to 6 months after transplantation (last sampling date; up to ~25 generations of new buds; STable 2). The capacity of cells from the EN to differentiate into multiple lineages in the recipient colony was confirmed by a pigmentation-based assay, where 5–20 cells from the EN of a black-pigmented donor colony were injected into a compatible, orange-pigmented recipient colony. Within 4 months from injection, recipient colonies changed pigmentation from orange to black and orange mix. This shift was also reflected by the AFLP markers (STable 2, n=4; Fig 4). Pigmentation chimeras were not observed when the donor cells were drawn from ampullae or the stomach (STable 2, n=4).
Discussion
Stem cells are rare cells, uniquely capable of both reproducing themselves (self renewing) and differentiating into a diverse range of specialized cell types. The rarity of stem cells and the lack of specific markers to definitive identification of stem cells in vivo make it difficult to confidently isolate stem cell niches (Morrison and Spradling, 2008). In Botryllus colonies, the unique weekly budding process of somatic self renewal (review in Manni and Burighel, 2006) and the high vasculature regeneration capacities (e.g. Sabbadin et al., 1975, Tiozzo et al., 2008, Voskoboynik et al., 2007), demonstrate a model organism that maintain high capacity of stem cell activity throughout life. To isolate stem cell niches’ locations in this organism we developed several in vivo whole colonial assays. These include a long term tracking of cell proliferation and migration towards developing organs, surgical manipulations to induce regeneration activity and various engraftment protocols to evaluate self renewing ability and developmental potential of engrafted cells. Therefore, our discovery of the first urochordate stem cell niche is based on direct visualization of cells that exhibit stem cells functionalities.
The endostyle niche harbors and exports stem cells
In Botryllus colonies an old generation of zooids is replaced every week by a new generation of asexual developing buds, which outgrow from the zooid’s lateral body wall (review in Manni and Burighel, 2006). Based on morphological observations, it has been hypothesized that zooids asexual development in tunicates is mediated by stem cells (Ermak, 1982; Freeman, 1964). Morphological studies proposed that lymphocyte-like cells (hemoblasts) differentiate into various tissues and organs (e.g. Freeman, 1964; Oka and Watanabe, 1957; Rinkevich et al., 1995; Rinkevich et al., 2007; Voskoboynik et al., 2007; Nunzi et al., 1979; Kawamura and Nakauchi, 1986; Brown and Swalla, 2007; Sunanaga et al., 2006). Furthermore, transfer of hand-picked ‘lymphocyte’ cells from Perophora colonies was shown to restore budding in irradiated colonies (Freeman, 1964). In parabionts B. schlosseri colonies, cells from one genotype (vascular fused or experimentally transplanted), can replace the germline and somatic tissues of the host (e.g. Laird et al., 2005; Sabbadin and Zaniolo, 1979). In a set of single cell transplantation experiments and serial engraftments, Laird et al., (2005) have further demonstrated precursor-progeny relationships and proved that the cells responsible for stable long term chimerism or parasitism in B. schlosseri are indeed adult stem cells, which as a population include both the somatic and germline lineages. The location of these cells however, remained unknown. Here, three different experimental approaches independently revealed that cells with the properties of somatic stem cells reside in the anterior ventral region of the endostyle (the EN). As few as 5 to 20 engrafted cells drawn from a donor EN sufficed to generate a somatic chimerism and contributed to asexual development of zooids for ~25 asexual generations, demonstrating long term self-renewal potential. However, these cells do not contribute to the germline.
The endostyle: a niche which governs high demand stem cells activity
Upon demand, adult stem cells are divided throughout life to produce new progeny that undergoes programs of differentiation and maturation, required to replace old or damaged tissue cells (Weissman, 2000). In solitary organisms like humans and mice, the demand usually occurs at low levels and occasionally at high levels; tissue stem cells (for example blood-forming) are mainly quiescent in cell cycle G0 stage and enter cell cycle when tissue turnover demands new input (Bradford et al., 1997; Cheshier et al., 1999). In contrast, Botryllus does not maintain its genome in long-lived individuals, but in colonies where individuals have a lifespan of ~21 days (14 days as developing buds and 7 days as zooids), while the entire colony has a long lifespan. To support a complete turnover of the individuals in the colony every week, the Botryllus stem cell niche must maintain high levels of stem cell proliferation and outward migration. Indeed, calculation of proliferation ratios and decline of fluorescent intensity in labeled cells with time revealed higher levels of proliferation in cells from the EN (versus other sites). Staining for proliferating cell nuclear antigen (PCNA) revealed that the endostyle is a highly proliferating organ, and that many of its epithelial cells are constantly proliferating throughout the colony life cycle as well as circulating cells in its subendostylar sinus (Fig 1). Likewise, the intense migration of cells out of the EN (compared to other sites) may reflect the export of precursors to developing tissues. BrdU (5-bromo-2′-deoxyuridine) labeling experiments in Botryllus primigenus also revealed high proliferation activity in the zooids endostyle and sinuses (Kawamura et al., 2008).
A stem cell niche is an interactive structural unit, organized to facilitate cell-fate decisions in a proper spatiotemporal manner (Morrison and Spradling, 2008; Scadden, 2006). Indeed, the endostyle of tunicates exhibits a special anatomic structure; it is a long glandular groove extending medially at the ventral face of the zooid branchial sac, along its anterior posterior axis (Burighel and Cloney, 1997). It consists of eight distinct anatomical zones and is immersed by blood flow through the large subendostylar sinus and other sinuses (Burighel and Brunetti, 1971). The endostyle is considered as the invertebrate chordate homologue of the vertebrate thyroid gland. It exhibits unique spatial and temporal features with site-specific factors that were previously implicated in developmental regulation and stem cell maintenance in other systems. These include thyroid hormones, serotonin, Otx, Hox1, Pax 2/5/8, PL10 and Cadherin (Canestro et al., 2008; Dunn, 1980; Hiruta et al., 2005; Nilsson et al., 1988; Pennati et al., 2001; Rosner et al., 2006; Rosner et al., 2007). The canonical Wnt pathway was shown to be involved in stem cell regulation and proliferation in various stem cells niches (reviewed in Morrison and Spradling, 2008; Scadden, 2006). Two Wnt genes are expressed in the Amphioxus (Cephalochordata) endostyle during embryogenesis and in larvae (Schubert et al., 2000). Previous enrichment of stem cells in B. schlosseri was based on the high enzymatic activity of aldehyde dehydrogenase (Laird et al., 2005) which delineates stem and progenitor cell in multiple lineages (Corti et al., 2005; Fallon et al., 2003;; Storms et al., 1999). As might be expected from Botryllus stem cells, we found that the cells of the EN (epithelial and blood cells) also exhibit high levels of aldehyde dehydrogenase activity as indicated by the high expression of retinaldehyde dehydrogenase (Raldh). Thus, the architecture and molecular features of the endostyle are also consistent with its role as a stem cell niche, but a direct role for these factors has not yet been shown in the generation and maintenance of the closely associated somatic stem cells.
Our study establishes the anterior ventral region of the endostyle, which includes adhering blood cells from the subendostylar sinus, as a special stem cell niche. The EN harbored stem cells participate in organogenesis by migrating out of the niche and homing to developing and regenerating sites. Botryllus belong to a taxonomic group which is considered the closest living invertebrate relative of the vertebrates (Delsuc et al., 2006). Throughout life this model organism exhibits a natural high demand and high activity of adult stem cells; activity that can be visualized, measured and manipulated in vivo. Characterization of the various endostyle subsets, and the types and stages of stem cells with which they interact will contribute to our understanding of how stem cell niches support extensive demand and utilization of stem cells.
Experimental Procedures
Animals
Colonies of Botryllus schlosseri were maintained as described (Boyd et al., 1986). Colonies were chosen for the experiments as described in supplementary.
In vivo fluorescent cell labeling assay
Cell drawing, labeling and transplanting were performed under a microscope (Diaphot 200, Nikon, NY, USA). We used an air compressed microinjector (PLI-188, Nikon, NY, USA) and a glass needle micropipette (50–60μm diameter sharp tip) to draw cells from one of the tested sites. Cells were drawn into a glass micropipette which contained 1μl Vybrant DiD dye solution (emission-665nm; Molecular Probes, Eugene OR USA) diluted in a tunicate saline buffer (TS; Negm et al., 1991; details in supplemental). The cells in the micropipette were counted under a microscope, incubated for 5 minutes (room temperature), and then 10–40 cells (~0.5μl), were injected back into the relevant tested sites (STable 1). As a control, 0.5μl of the dilution solution (TS) was injected. In addition we performed heterotypic transplantation experiments where ~40 cells taken from the vasculature or zooid lateral wall, were labeled and injected into the EN (STable 1). In several colonies, including the control groups, the vasculature (marginal vessel and ampullae) was dissected away from the colonies following cell labeling (n=13, STable 1).
Imaging
Time lapse imaging was performed by an automated microscopy (ImageXpress, Molecular devices Corp., Palo Alto, CA) as described (Voskoboynik et al., 2007). Phase contrast images and fluorescent images (cy5, maximum emission at 670nm; Fig 3) at varying magnifications were performed every 30–90 minutes during the first 5 days following labeling and twice a day thereafter (days 6–8). Time-lapse sequences were generated from consecutive images of same location.
Proliferation/migration and fluorescent intensity measurements
Labeled cells in organs (zooid, bud, vasculature) were counted using Image J software, version 1.32j (NIH, USA). Proliferation migration ratios and fluorescent intensity were calculated on fluorescent images that were taken from the labeling sites, 2, 10 and 30 hours following labeling (Fig 3; detailed description in supplemental).
Transplantation experiments
Cells and hemolymph from each of the tested organs (EN, stomach and ampullae; STable 2) were drawn into a glass micropipette as described above. 5–20 cells were microinjected into the EN or the bud of the recipient colony (STable 2). Cells were counted under the microscope while being transplanted into the colonies semitransparent body (Diaphot 200, Nikon, NY, USA).
Genotyping
Somatic and germ tissues were collected every month, 1–6 months following transplantation. Samples were dissected and flash frozen in liquid nitrogen. 259 DNA samples were extracted using a modified version of the Hoss and Pabbo protocol (1993) as described (De Tomaso et al., 1998). The samples were screened for polymorphism using amplified fragment length polymorphism (AFLP’s). AFLP was preformed as described (Vos et al., 1995; Rinkevich et al., 1998; details in supplementary).
Histology
Colonies were fixed and sections from paraplast embedded tissues were made, cross and longitudinal sections (5μm) were stained with Azan Heidenhain or Hematoxyline Eosin as described (Moiseeva et al., 2004).
PCNA immunohistochemistry staining and Raldh in situ hybridization were preformed as described (Rinkevich et al., 2007).
Confocal analysis
Vybrant DiD labeled colonies (n=7) were fixed in 4% paraformaldehyde at 4°C (6–8 hours). Colonies were washed with PBS, cryoprotected overnight in 30% sucrose, and quick-frozen in optimum cutting temperature (OCT) compound. Frozen sections (5–7um) were cut at −20°C from OCT-embeded tissues using a microtome (Bright Instruments, Huntington, UK). Nuclei were stained with Hoechst 33342 (Molecular Probes; 1uM for 2 min) and washed with PBS. Samples were analyzed by laser scanning confocal microscopy (Lecia SP2 AOBS confocal laser scanning microscope with 5 lasers lines).
Supplementary Material
Summary.
Stem-cell populations exist in ‘niches’ that hold and regulate their fate decisions. Identification and characterization of these niches are essential for understanding stem cell maintenance and tissue regeneration. Here we report about the identification of a novel stem cell niche in Botryllus schlosseri, a colonial urochordate with high stem cell mediated developmental activities. Using in vivo cell labeling, cell engraftment, confocal microscopy and time-lapse imaging we ascertained the Botryllus endostyle’s anterior ventral region as an adult somatic stem cell niche. Transplanted cells from this niche contributed to somatic tissue development and induced long term chimerism in allogeneic tissues. Labeled cells in the endostyle niche proliferated, transmigrated, and expanded in regenerating organs and developing buds including buds of chimeric partners but did not contribute to germline. Cells from other tested tissues did not show any of above stemness capabilities. Cumulatively, results illustrate the endostyle region as a stem cell niche that harbors functionally adult somatic stem cells.
Acknowledgments
We thank Amir Voskoboynik, T. Raveh and A. De Tomaso for critical review. P. Brown, R. Marinelli, R. Pesich L. Jerabek, C. Patton K. Lee and R. Will, for generous support and helpful assistance. This study was supported by USPHS grant -DK54762 and to a minor extent by HL58770 by a grant from the US-Israel Binational Science Foundation (2003-010) and by the Israel Science Foundation (project 550-06).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker N, vanes JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving G, Begthel H, Peters PJ, Clevers H. Identification of stem cell in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Boyd HC, Brown SK, Harp JA, Weissman IL. Growth and sexual maturation of laboratory-cultured Monterey Botryllus schlosseri. Biol Bull. 1986;170:91–109. [Google Scholar]
- Bradford GB, Williams B, Rossi R, Bertoncello I. Quiescence, cycling and turnover in the primitive hematopoietic stem cell compartment. Exp Hematol. 1997;25:445–453. [PubMed] [Google Scholar]
- Brown FD, Swalla BJ. Vasa expression in a colonial ascidian, Botrylloides violaceus. Evolution and Development. 2007;9:165–177. doi: 10.1111/j.1525-142X.2007.00147.x. [DOI] [PubMed] [Google Scholar]
- Burighel P, Brunetti R. The circulatory system in the blastozooid of the colonial ascidian Botryllus schlosseri (Pallas) Boll Zool. 1971;38:278–289. [Google Scholar]
- Burighel P, Cloney RA. Urochordata: Ascidiacea. In: Harrison FW, Ruppert EE, editors. Microscopic anatomy of invertebrates. Wiley-Liss, Inc; NY: 1997. pp. 221–347. [Google Scholar]
- Burnet FM. “Self-recognition” in colonial marine forms and flowering plants in relation to the evolution of immunity. Nature. 1971;232:230–235. doi: 10.1038/232230a0. [DOI] [PubMed] [Google Scholar]
- Canestro C, Bassham S, Postlethwait JH. Evolution of the thyroid: Anterior-posterior reorganization of the Oikopleura endostyle revealed by Otx, Pax 2/5/8, and Hox1 expression. Dev Dyn. 2008 doi: 10.1002/dvdy.21525. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Cheshier SH, Morrison SJ, Liao X, Weissman IL. In vivo proliferation and cell cycle kinetics of long-term hematopoietic stem cells. Proc Natl Acad Sci USA. 1999;96:3120–3125. doi: 10.1073/pnas.96.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Crittenden SL, Bernstein DS, Bachorik JL, Thompson BE, Gallegos M, Petcherski AG, Moulder G, Barstead R, Wickens M, Kimble J. A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature. 2002;417:660–663. doi: 10.1038/nature754. [DOI] [PubMed] [Google Scholar]
- Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicate and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–968. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- De Tomaso AW, Saito Y, Ishuzuka KJ, Palmeri KJ, Weissman IL. Mapping the genome of a model protochordate. I. A low resolution genetic map encompassing the fusion/histocompatibility (Fu/HC) locus of Botryllus schlosseri. Genetics. 1998;149:277–287. doi: 10.1093/genetics/149.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Dunn AD. Properties of an iodinating enzyme in the ascidian endostyle. Gen Comp Endocrinol. 1980;40:484–493. doi: 10.1016/0016-6480(80)90012-x. [DOI] [PubMed] [Google Scholar]
- Ermak TH. The renewing cell population of Ascidians. Amer Zool. 1982;22:795–805. [Google Scholar]
- Freeman G. The role of blood cells in the process of asexual reproduction in the tunicate Perophora virdis. J Exp Zool. 1964;156:157–183. doi: 10.1002/jez.1401560204. [DOI] [PubMed] [Google Scholar]
- Gilboa L, Lehmann R. Soma-germline interactions coordinate homeostasis and growth in the Drosophila gonad. Nature. 2004;443:97–100. doi: 10.1038/nature05068. [DOI] [PubMed] [Google Scholar]
- Harada H, Kettunen P, Jung HS, Mustonen T, Wang YA, et al. Localization of putative stem cells in dental epithelium and their association with notch and FGF signaling. J Cell Biol. 1999;147:105–120. doi: 10.1083/jcb.147.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruta J, Mazet F, Yasui K, Zhang P, Ogasawara M. Comparative expression analysis of transcription factor genes in the endostyle of invertebrate chordates. Dev Dyn. 2005;233:1031–1037. doi: 10.1002/dvdy.20401. [DOI] [PubMed] [Google Scholar]
- Hoss M, Paabo S. DNA extraction from Pleistocene bones by a silica-based purification method. Nucleic acids research. 1993;21:3913–3914. doi: 10.1093/nar/21.16.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Nakauchi M. Development of spatial organization in palleal buds of the compound ascidian, Symplegma reptans. Biol Bull. 1986;171:580–597. doi: 10.2307/1541621. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Tachibana M, Sunanaga T. Cell proliferation dynamics of somatic and germline tissues during zooidal life span in the colonial tunicates Botryllus primigenus. Dev Dyn. 2008;233:1812–1825. doi: 10.1002/dvdy.21592. [DOI] [PubMed] [Google Scholar]
- Kiger AA, Jones DL, Schulz C, Rogers MB, Fuller MT. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science (New York, NY. 2001;294:2542–2545. doi: 10.1126/science.1066707. [DOI] [PubMed] [Google Scholar]
- Kimble JE, White JG. On the control of germ cell development in Caenorhabditis elegans. Developmental biology. 1981;81:208–219. doi: 10.1016/0012-1606(81)90284-0. [DOI] [PubMed] [Google Scholar]
- Laird DJ, De Tomaso AW, Weissman IL. Stem cells are units of natural selection in a colonial ascidian. Cell. 2005;123:1351–1360. doi: 10.1016/j.cell.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Lauzon RJ, Ishizuka KJ, Weissman IL. Cyclical generation and degeneration of organs in a colonial urochordate involves crosstalk between old and new. A model for development and regeneration. Dev Biol. 2002;249:333–348. doi: 10.1006/dbio.2002.0772. [DOI] [PubMed] [Google Scholar]
- Lin H, Spradling AC. Germline stem cell division and egg chamber development in transplanted Drosophila germaria. Developmental biology. 1993;159:140–152. doi: 10.1006/dbio.1993.1228. [DOI] [PubMed] [Google Scholar]
- Manni L, Burighel P. Common and divergent pathways in alternative developmental processes of ascidians. BioEssays. 2006;28:902–912. doi: 10.1002/bies.20462. [DOI] [PubMed] [Google Scholar]
- Moiseeva E, Rabinowitz C, Yankelevich I, Rinkevich B. ‘Cup cell disease’ in the colonial tunicate Botryllus schlosseri. Diseases of aquatic organisms. 2004;60:77–84. doi: 10.3354/dao060077. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negm HI, Mansour MH, Cooper EL. Identification and structural characterization of Lyt-1 glycoproteins from tunicate hemocytes and mouse thymocytes. Comp Biochem Physiol B. 1991;99:741–749. doi: 10.1016/0305-0491(91)90137-3. [DOI] [PubMed] [Google Scholar]
- Nilsson O, Fredriksson G, Ofverholm T, Ericson LE. Electron-microscopic immunocytochemistry of 5-hydroxytryptamine in the ascidian endostyle. Cell Tissue Res. 1988;253:137–143. doi: 10.1007/BF00221748. [DOI] [PubMed] [Google Scholar]
- Nunzi MG, Burighel P, Schiaffino S. Muscle cell differentiation in the ascidian heart. Developmental biology. 1979;68:371–380. doi: 10.1016/0012-1606(79)90211-2. [DOI] [PubMed] [Google Scholar]
- Nystul T, Spradling A. An epithelial niche in the Drosophila Ovary undergoes long-range stem cell replacement. Cell Stem Cell. 2007;1:277–285. doi: 10.1016/j.stem.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential Notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Oka H, Watanabe H. Vascular budding, a new type of budding in Botryllus. Biol Bull. 1957;112:225–240. [Google Scholar]
- Oka H, Watanabe H. Problems of colony specificity in compound ascidians. Bull Mar Biol Stat Asamushi, Tohoku Univ. 1960;10:153–155. [Google Scholar]
- Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Molecular and cellular neurosciences. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- Pancer Z, Gershon H, Rinkevich B. Coexistence and possible parasitism of somatic and germ cell lines in chimeras of the colonial urochordate Botryllus schlosseri. Biol Bull. 1995;189:106–112. doi: 10.2307/1542460. [DOI] [PubMed] [Google Scholar]
- Pennati R, Groppelli S, Sotgia C, Candiani S, Pestarino M, De Bernardi F. Serotonin localization in Phallusia mammillata larvae and effects of 5-HT antagonists during larval development. Develop Growth Differ. 2001;43:647–656. doi: 10.1046/j.1440-169x.2001.00608.x. [DOI] [PubMed] [Google Scholar]
- Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. Journal of cell science. 2002;115:2381–2388. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- Rinkevich B, Weissman IL. A long-term study of fused subclones of a compound ascidian. The resorption phenomenon. J of Zool. 1987;213:717–733. [Google Scholar]
- Rinkevich B, Shlemberg Z, Fishelson L. Whole-body protochordate regeneration from totipotent blood cells. Proc Natl Acad Sci USA. 1995;92:7695–7699. doi: 10.1073/pnas.92.17.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich B, Weissman IL, De Tomaso AW. Transplantation of Fu/HC-incompatible zooids in Botryllus schlosseri results in chimerism. The Biological bulletin. 1998;195:98–106. doi: 10.2307/1542816. [DOI] [PubMed] [Google Scholar]
- Rinkevich Y, Paz G, Rinkevich B, Reshef R. Systemic Bud Induction and Retinoic Acid Signaling Underlie Whole Body Regeneration in the Urochordate Botrylloides leachi. PLoS biology. 2007;5:e71. doi: 10.1371/journal.pbio.0050071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner A, Paz G, Rinkevich B. Divergent roles of the DEAD-box protein BS-PL10, the urochordate homologue of human DDX3 and DDX3Y proteins, in colony astogeny and ontogeny. Dev Dyn. 2006;235:1508–1521. doi: 10.1002/dvdy.20728. [DOI] [PubMed] [Google Scholar]
- Rosner A, Rabinowitz C, Moiseeva E, Voskoboynik A, Rinkevich B. BS-Cadherin in the colonial urochordate Botryllus schlosseri: One protein, many functions. Developmental biology. 2007;304:687–700. doi: 10.1016/j.ydbio.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Sabbadin A, Zaniolo G, Majone F. Determination of polarity and bilateral asymmetry in palleal and vascular buds of the ascidian Botryllus schlosseri. Developmental biology. 1975;46:79–87. doi: 10.1016/0012-1606(75)90088-3. [DOI] [PubMed] [Google Scholar]
- Sabbadin A, Zaniolo G. Sexual differentiation and germ cell transfer in the colonial ascidian Botryllus schlosseri. J Exp Zool. 1979;207:289–304. [Google Scholar]
- Schofield R. The relationship between the spleen colony forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Schubert M, Holland LZ, Holland ND. Characterization of two amphioxus Wnt genes (AmphiWnt4 and AmphiWnt7b) with early expression in the developing central nervous system. Dev Dyn. 2000a;217:205–215. doi: 10.1002/(SICI)1097-0177(200002)217:2<205::AID-DVDY7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science (New York, NY. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- Stoner DS, Weissman IL. Somatic and germ cell parasitism in a colonial ascidian: possible role for a highly polymorphic allorecognition system. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:15254–15259. doi: 10.1073/pnas.93.26.15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner DS, Rinkevich B, Weissman IL. Heritable germ and somatic cell lineage competitions in chimeric colonial protochordates. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9148–9153. doi: 10.1073/pnas.96.16.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunanaga T, Saito Y, Kawamura K. Postembryonic epigenesis of Vasa-positive germ cells from aggregated hemoblasts in the colonial ascidian, Botryllus primigenus. Development, growth & differentiation. 2006;48:87–100. doi: 10.1111/j.1440-169X.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- Tiozzo S, Voskoboynik A, Brown FD, De Tomaso AW. A conserved role of the VEGF pathway in angiogenesis of an ectodermally-derived vasculature. Dev Biol. 2008;315:243–255. doi: 10.1016/j.ydbio.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulina N, Matunis E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science (New York, NY. 2001;294:2546–2549. doi: 10.1126/science.1066700. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science (New York, NY. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadsen R, Fridriksdottir AJ, Ronnov-Jessen L, Gudjonsson T, Rank F, LaBarge MA, Bissell MJ, Peterson OW. Evidence for a stem cell hierarchy in the adult human breast. J Cell Biol. 2007;177:87–101. doi: 10.1083/jcb.200611114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos P, Hogers R, Bleeker M, Reijans M, van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, et al. AFLP: a new technique for DNA fingerprinting. Nucleic acids research. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskoboynik A, Simon-Blecher N, Soen Y, Rinkevich B, De Tomaso AW, Ishizuka KJ, Weissman IL. Striving for normality: whole body regeneration through a series of abnormal generations. Faseb J. 2007;21:1335–1344. doi: 10.1096/fj.06-7337com. [DOI] [PubMed] [Google Scholar]
- Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- Wieschaus E, Szabad J. The development and function of the female germ line in Drosophila melanogaster: a cell lineage study. Developmental biology. 1979;68:29–46. doi: 10.1016/0012-1606(79)90241-0. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science (New York, NY. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


