Abstract
Under normal physiologic conditions, cellular homeostasis is partly regulated by balancing pro- and anti-phagocytic signals. CD47 is highly expressed on several human cancers including acute myeloid leukemia, non-Hodgkin lymphoma, and bladder cancer, allowing cancer cells to evade phagocytosis by the innate immune system. Blockade of CD47 with a monoclonal antibody enables phagocytosis of cancer cells and leads to in vivo tumor elimination, but leaves most normal cells unaffected. In order for target cells to be phagocytosed upon blockade of an anti-phagocytic signal, we postulate that the cells must also display a potent pro-phagocytic signal. Here we identify calreticulin as a pro-phagocytic signal highly expressed on the surface of several human cancers including acute myeloid and lymphoblastic leukemias, chronic myeloid leukemia, non-Hodgkin lymphoma (NHL), bladder cancer, glioblastoma, and ovarian cancer, but minimally expressed on most normal cells. Increased CD47 expression correlated with high calreticulin levels on cancer cells, and was necessary for protection from calreticulin-mediated phagocytosis. Phagocytosis induced by anti-CD47 antibody required the interaction of target cell calreticulin with its receptor low density lipoprotein-receptor related protein (LRP) on phagocytic cells, as blockade of the calreticulin/LRP interaction prevented anti-CD47 antibody mediated phagocytosis. Lastly, increased calreticulin expression was an adverse prognostic factor in diverse tumors including neuroblastoma, bladder cancer, and NHL. These findings identify calreticulin as the dominant pro-phagocytic signal on several human cancers, provide an explanation for the selective targeting of tumor cells by anti-CD47 antibody, and highlight the balance between pro- and anti-phagocytic signals in the immune evasion of cancer.
INTRODUCTION
Malignant cellular transformation occurs through a progression of genetic mutations and epigenetic reprogramming that activate oncogenes and inactivate tumor suppressor pathways leading to inheritance of several hallmarks shared by most cancer cells including: self-sufficiency in growth signals, insensitivity to anti-growth signals, tissue invasion and metastasis, poorly regulated replicative potential, sustained angiogenesis, and evasion of cell death by a variety of pathways, including apoptosis (1). In addition to these cell intrinsic properties, recent evidence suggests that many cancers are also able to evade the immune system through several distinct mechanisms (2–4).
Recently, we showed that evasion of phagocytosis through upregulation of the anti-phagocytic signal CD47 is another mechanism by which tumor cells escape immunosurveillance (5–9). CD47 is a pentaspanin cell surface protein that serves as a signal inhibiting phagocytosis through ligation of its receptor SIRPα on phagocytic cells (10–12). Disruption of the CD47-SIRPα interaction can be therapeutically targeted with a monoclonal blocking antibody against CD47, which enabled phagocytosis of acute myeloid leukemia (AML), bladder cancer, and non-Hodgkin lymphoma (NHL) cells in vitro and in vivo (6, 8, 9). In contrast, administration of anti-mouse CD47 antibody caused minimal toxicity (6, 9), despite broad expression of CD47 on normal tissues (13). In order for target cells to be phagocytosed upon blockade of an anti-phagocytic signal, these cells must also display a potent pro-phagocytic signal. CD47 has also been implicated in the regulation of phagocytosis of apoptotic cells, as these cells become phagocytosed due to loss of CD47 expression and coordinate upregulation of cell surface calreticulin (14). During apoptosis, cell surface calreticulin serves as a pro-phagocytic signal by binding to its macrophage receptor, low density lipoprotein-related protein (LRP), which leads to engulfment of the target cell (14, 15). We hypothesized that the selective targeting of tumor cells with anti-CD47 antibody was due to the presence of a pro-phagocytic stimulus on tumor cells, but not on most normal cells, that becomes unopposed after CD47 blockade. Here, we identify cell surface calreticulin (CRT) as this pro-phagocytic stimulus, whose differential expression helps to explain the lack of anti-CD47 antibody-mediated toxicity against most normal cells. We propose that calreticulin expression of newly arising neoplasms may be an early event, and only those tumor clones that upregulate CD47 can escape the phagocytic consequences of cell surface calreticulin expression.
RESULTS
Cell surface calreticulin is expressed on cancer, but not most normal, stem and progenitor cells
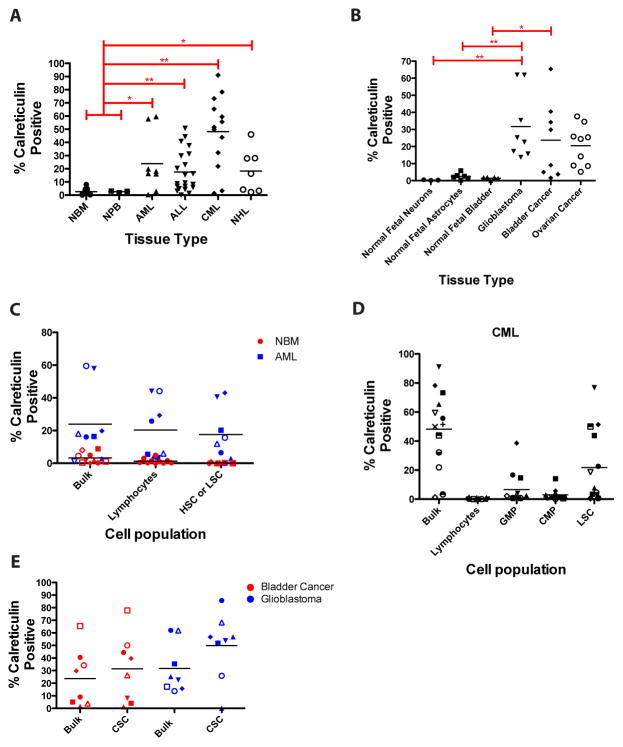
Cell surface calreticulin expression was determined on a variety of primary human cancer cells and their normal cell counterparts by flow cytometry. In hematologic malignancies, cell surface calreticulin was expressed on a greater percentage of bulk cells in AML (average=23.9%), acute lymphocytic leukemia (ALL, 17.6%), chronic phase chronic myeloid leukemia (CML, 47.6%), and NHL (18.3%) when compared to normal bone marrow (2.6%) and normal peripheral blood cells (2.6%) (Fig. 1A). In solid tumors, cell surface calreticulin was also expressed on a greater percentage of bulk cells in ovarian cancer (average=20.5%), glioblastoma (31.7%), and bladder cancer (23.7%) when compared to normal fetal neurons (0.3%), astrocytes, (2.5%) and normal fetal bladder cells (1.41%) (Fig. 1B). In this analysis, annexin V-positive cells were excluded, indicating that calreticulin-positive cancer cells were just in the apoptotic subset. In addition, calreticulin positive-cancer cells (from AML and bladder cancer patients) formed tumors when engrafted into immunodeficient mice similarly to CRT-negative cancer cells, indicating that CRT-positive cancer cells were functionally viable and possess tumorigenic potential in vivo (fig. S1).
Figure 1. Cell surface calreticulin is expressed on cancer, but not normal, stem and progenitor cells.
(A) Cell surface calreticulin expression was determined by flow cytometry on primary human patient samples from several hematologic cancer types and normal cell counterparts including normal bone marrow (NBM, n=9), normal peripheral blood (NPB, n=3), acute myeloid leukemia (AML, n=8), acute lymphoblastic leukemia (ALL, n=21), chronic myeloid leukemia (CML, n=13), and non-Hodgkin lymphoma (NHL, n=7). (B) A similar analysis as in A was performed for solid tumors (glioblastoma, n=9; transitional cell bladder carcinoma, n=8; serous papillary ovarian carcinoma, n=9) and normal human fetal tissues (neurons, n=3; astrocytes, n=6, bladder cells, n=6). ESA+ urothelium was analyzed for normal fetal bladder. Primary human bladder cancer patient samples and samples that had been passaged once in mice were used for profiling. (C and D) Cell surface calreticulin expression was determined on normal stem and progenitor cells, lymphocytes, and cancer stem and progenitor cells. Each symbol represents a different patient sample. Patient samples tested: NBM=10, AML=8, CML=13, bladder cancer=8, glioblastoma=8. NBM HSC=CD34+CD38-CD90+Lin-, AML LSC=CD34+CD38-CD90-Lin-, GMP=CD34+CD38+IL3rα+CD45RA+, CMP=CD34+CD38+IL3rα+CD45RA-. (E) Calreticulin expression did not differ between bulk and cancer stem cell populations for either bladder cancer (p=0.54) or glioblastoma (p=0.14). Bladder cancer CSC=CD44+Lin- (8), glioblastoma CSC=CD133+Lin- (22, 23). Annexin V-positive cells were excluded in the analysis of all samples.
Previous studies have identified that the endoplasmic reticulum (ER) protein ERp57 co-translocates with CRT to the cell surface and is required for CRT cell surface exposure under conditions of apoptosis (16, 17). Accordingly, we assessed the relationship between cell surface CRT and ERp57 expression on tumor cells. On non-apoptotic (annexin V negative) tumor cells, cell surface ERp57 expression was associated with cell surface CRT expression (fig. S2A). Furthermore, across several different tumor types (including primary human tumor samples and cancer cell lines), ERp57 was expressed on a higher percentage of CRT+ cells compared to CRT− counterparts (fig. S2B,C).
Given that primary human tumors are heterogeneous and contain a subpopulation of tumor-initiating cells (reviewed in (18)), we next investigated whether cell surface calreticulin was present on the cancer stem cell (CSC) population of each tumor type in which the immunophenotype of functional CSC is known. In AML and chronic phase CML, cell surface calreticulin was expressed on CD34+CD38-CD90-Lin- AML (19, 20) and CD34+CD38-CD90+ chronic phase CML (21) leukemia stem cells (LSC), as well as downstream progenitor populations, while normal bone marrow hematopoietic stem and progenitor populations expressed minimal cell surface calreticulin (Fig. 1C,D). For AML, similar levels of cell surface calreticulin expression were observed for LSC compared to other cellular subsets (Fig. 1C). In contrast, CML LSC expressed higher levels of cell surface calreticulin compared to downstream CMP and GMP populations (Fig. 1D). Cell surface calreticulin was also expressed on CSC of solid tumors including CD44+Lin- bladder CSC (8) and CD133+Lin- glioblastoma CSC (22, 23) (Fig. 1E).
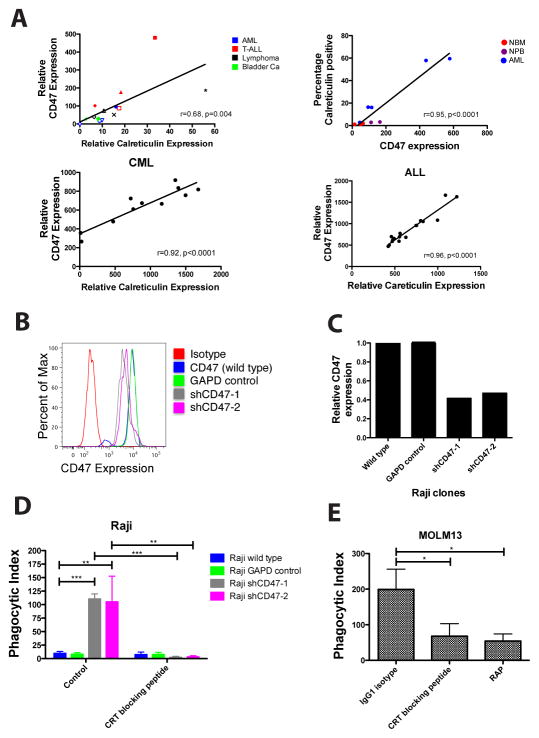
We next determined whether there was a correlation between calreticulin (CRT) and CD47 expression in human tissues, postulating that a balance between pro- (CRT) and anti- (CD47) phagocytic signals may be maintained as a homeostatic mechanism. CRT and CD47 cell surface expression were profiled in a variety of human cancer cell lines, primary cancers, and normal cells. CD47 expression correlated with CRT expression in a variety of hematologic and solid tumor cell lines as well as in primary human AML, CML, and ALL patient samples (Fig. 2A). Notably, normal cells expressed minimal levels of both CRT and CD47 (Fig. 2A, top panels). In normal human bone marrow and fetal bladder, those cells that were CRT positive expressed higher levels of CD47 compared to CRT negative cellular counterparts (fig. S3). Thus, in both normal and cancer cells, there is a strong positive correlation between CRT and CD47 expression.
Figure 2. Increased CD47 Expression on Cancer Cells Protects Them from Calreticulin- Mediated Phagocytosis.
(A) Correlation between cell surface calreticulin and CD47 expression was determined for human cancer cell lines (top left) and primary human normal and cancer samples (top right, bottom panels). Expression was calculated as mean fluorescence intensity normalized over isotype control and for cell size. Pearson correlation (r) and p-value is shown for each correlation. Top left panel: blue solid circle=HL60, blue open circle=Kasumi1, blue open inverted triangle=MOLM13, blue open diamond=KG-1, red triangle=Jurkat, red solid square=CCRF-CEM, red open square=CCRF-HSB2, red diamond=MOLT4, black star=Raji, black open diamond=SUDHL6, black open triangle=Daudi, black x=U937, green plus=639V, green open diamond=HT1197, green inverted triangle=UMUC3. (B) CD47 protein expression was determined by flow cytometry on Raji cells transduced with lentiviruses encoding shRNA CD47-knockdown constructs (shCD47) or controls. (C) Relative CD47 expression levels were quantified by comparing MFI to wild type Raji cells. (D) Raji cell clones were incubated with human macrophages in media alone or with CRT blocking peptide for 2 hours, after which phagocytosis was analyzed by fluorescence microscopy. Knockdown of CD47 in Raji cells (shCD47-1,-2) resulted in increased phagocytosis compared to untransduced Raji cells. No difference in phagocytosis was observed between untransduced and GAPD control-transduced Raji cells (p=0.45) Blockade of calreticulin on CD47-knockdown Raji cells completely abrogated phagocytosis. (E) MOLM-13 cells, a CD47-deficient human AML cell line, were incubated with human macrophages for two hours with the indicated peptides and monitored for phagocytosis as above. Significant phagocytosis was observed with IgG1 isotype control, while blockade of calreticulin or LRP reduced levels of phagocytosis (p=0.03 and p=0.01, respectively). Conditions were performed in triplicate; data presented as mean ± SD. *p<0.05, **p<0.005, ***p<0.0005 (2-tailed Student’s t-test).
Increased CD47 on cancer cells protects them from calreticulin-mediated phagocytosis
We observed increased cell surface calreticulin and CD47 on human cancer cells leading us to hypothesize that increased CD47 protects these cells from calreticulin-mediated phagocytosis. To investigate this hypothesis, we performed in vitro phagocytosis assays on two different CRT-expressing cancer cell lines: one expressing high CD47 levels (Raji) and one deficient in CD47 expression (MOLM13). First, Raji cells, a Burkitt’s NHL cell line that expresses high levels of CD47 and calreticulin (Fig. 2B and fig. S4), were incubated with human macrophages under conditions where CD47 expression was knocked down to various levels by lentiviral transduction of shRNAs (Fig. 2B,C). Cell surface calreticulin expression was unaffected by shRNA-mediated CD47 knockdown (fig. S4). Upon incubation with human macrophages, Raji cells with approximately 2 fold knockdown of CD47 expression (shCD47-1 and shCD47-2) were more robustly phagocytosed by human macrophages than were wild type and GAPD control transduced Raji cells which were the minimally phagocytosed (Fig. 2D). Phagocytosis of shCD47-1 and shCD47-2 Raji cells was dependent on the calreticulin-LRP interaction as the observed phagocytosis was completely abrogated in the presence of a CRT blocking peptide (Fig. 2D). In the second experiment, MOLM13 cells, a human AML cell line that is deficient in CD47 expression (5) but expresses calreticulin (fig. S4), were incubated with human macrophages. As expected, MOLM13 cells were robustly phagocytosed at baseline, while phagocytosis was significantly reduced when the CRT-LRP interaction was blocked (Fig. 2E). These findings demonstrate that overexpression of CD47 in cancers counterbalances calreticulin-mediated phagocytosis.
Calreticulin is the dominant pro-phagocytic signal on several human cancers and is required for anti-CD47 antibody-mediated phagocytosis
In prior studies, we demonstrated that in several human cancers overexpression of CD47 contributes to evasion of macrophage phagocytosis, and furthermore that monoclonal antibody-mediated blockade of CD47 can enable phagocytosis and elimination of tumors in vitro and in mouse xenografts (5, 6, 8). Although anti-CD47 antibody is effective in enabling phagocytic elimination of tumors and is an attractive therapeutic anti-cancer agent, its potential off-target effects represent a potential concern, given that CD47 is expressed at low levels on most normal tissues (13). However, we also showed that normal hematopoietic progenitor cells, which express CD47, were not phagocytosed when coated with anti-CD47 antibody (6). Additionally, administration of a blocking anti-mouse CD47 antibody to wild type mice caused minimal tissue toxicity (6). The lack of antibody toxicity is not likely exclusively due to overexpression of CD47 on cancer cells compared to normal counterparts given that both normal and cancer cells are coated with anti-CD47 antibody at therapeutic doses. Instead, it is likely a result of the fact that, in order for target cells to be phagocytosed upon blockade of an anti-phagocytic signal (CD47), the cells must also display a potent pro-phagocytic signal, which is absent on normal cells.
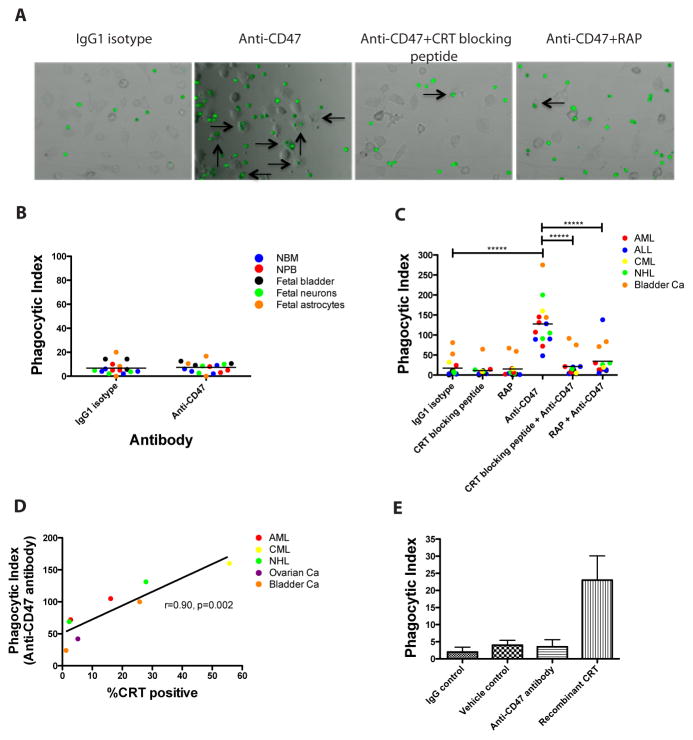
Given the known role of CRT as a pro-phagocytic signal, its correlation with CD47 expression (Fig. 2A), and its ability to be counteracted by CD47 (Fig. 2), we investigated whether the expression of cell surface CRT on cancer but not normal cells could explain the selective targeting of tumor cells by a blocking anti-CD47 antibody. In vitro phagocytosis assays were performed by incubating primary human normal cells or cancer cells with human macrophages in the presence of anti-CD47 antibody. CD47 was expressed on all normal and cancer cells profiled (Fig. 2A and fig. S3,5), but expression of calreticulin was primarily restricted to tumor cells (Fig. 1A,B). No phagocytosis of cells from a variety of normal human tissue types was observed with anti-CD47 antibody (Fig. 3B), while primary cancer cells from a variety of tumor types were robustly phagocytosed (Fig. 3A,C). Significantly, anti-CD47 antibody-mediated phagocytosis of cancer cells was completely abrogated in most cases when cells were simultaneously incubated with peptides that inhibit the CRT-LRP interaction, including a calreticulin blocking peptide and receptor-associated protein (RAP), an inhibitor of LRP (14) (Fig. 3C). Increasing concentrations of a calreticulin blocking peptide lead to a dose-dependent reduction in anti-CD47 antibody mediated phagocytosis (fig. S6). Notably, additional blockade of other pro-phagocytic signals was not required to abolish anti-CD47 antibody-mediated phagocytosis, as cells incubated with anti-CD47 antibody under CRT-LRP blockade were phagocytosed at levels similar to baseline controls (Fig. 3C). However, two bladder cancer samples exhibited higher baseline levels of phagocytosis with IgG1 isotype control compared to other cancer cell types which may be due to expression of other pro-phagocytic signals on these specific cells. Nevertheless, blockade of CRT or LRP in the presence of anti-CD47 antibody abrogated phagocytosis of these bladder cancer cells to levels similar to IgG1 isotype controls. Blockade of the calreticulin-LRP interaction alone by CRT blocking peptide or LRP had no effect on phagocytosis when compared to IgG control (Fig. 3C). Next, the relationship between the level of tumor cell surface CRT expression and level of phagocytosis by anti-CD47 antibody was investigated. Cell surface CRT expression on tumor cells positively correlated with the degree of anti-CD47 antibody mediated phagocytosis, regardless of tumor cell type (Fig. 3D). Finally, given that normal cells express minimal levels of cell surface CRT, we investigated whether the addition of CRT to the surface of these cells could enable phagocytosis. An in vitro phagocytosis assay was performed on NBM cells incubated with exogenous recombinant calreticulin protein, previously demonstrated to adsorb onto the cellular surface allowing them to bind to macrophage LRP (14). In contrast to vehicle control, incubation with exogenous CRT enabled phagocytosis of NBM cells while anti-CD47 antibody did not (Fig. 3E). Collectively, these results demonstrate that anti-CD47 antibody-mediated phagocytosis requires the presence of cell surface calreticulin.
Figure 3. Cell Surface Calreticulin is the Dominant Pro-Phagocytic Signal on Several Human Cancers and is Required for Anti-CD47 Antibody-Mediated Phagocytosis.
(A) Primary human AML cells were fluorescently-labeled with CFSE and incubated with human macrophages in the presence of the indicated antibodies/peptides for 2 hours, after which phagocytosis was analyzed by fluorescence microscopy. Arrows indicate phagocytosis. (B) Cells from several normal human tissue types were incubated with human macrophages in the presence of the indicated antibodies and monitored for phagocytosis. No difference in phagocytosis was detected between IgG1 isotype control and anti-CD47 antibody incubation (p=0.77). (C) Primary human cancer cells were incubated with human macrophages in the presence of the indicated antibodies/peptides for two hours and monitored for phagocytosis. Each data point represents a different patient sample. Compared to IgG1 isotype control, incubation with anti-CD47 antibody enabled phagocytosis of cancer cells (p<0.0001) while incubation with calreticulin blocking peptide (p=0.37) or RAP, an LRP inhibitor (p=0.67), did not enable phagocytosis. In the presence of anti-CD47 antibody, incubation of cancer cells with either calreticulin blocking peptide or RAP completely abrogated anti-CD47 antibody-mediated phagocytosis (p=0.77 and p=0.16, respectively compared to IgG1 isotype control). *****p<0.00001 (2-sided Student’s t-test). (D) A positive correlation was observed between cell surface CRT expression and degree of anti-CD47 antibody mediated phagocytosis (Pearson’s correlation coefficient is shown). Each point represents a distinct patient sample that was incubated in the same in vitro phagocytosis assay. (E) Human NBM cells were incubated with human macrophages in the presence of the indicated antibodies or protein. Exogenous CRT enabled increased phagocytosis of NBM cells compared to vehicle control (p=0.05). No difference in phagocytosis was observed between IgG1 isotype control and anti-CD47 antibody (p=0.49). Conditions were performed in triplicate; data presented as mean ± SD.
Increased calreticulin expression confers a worse clinical prognosis in multiple human malignancies
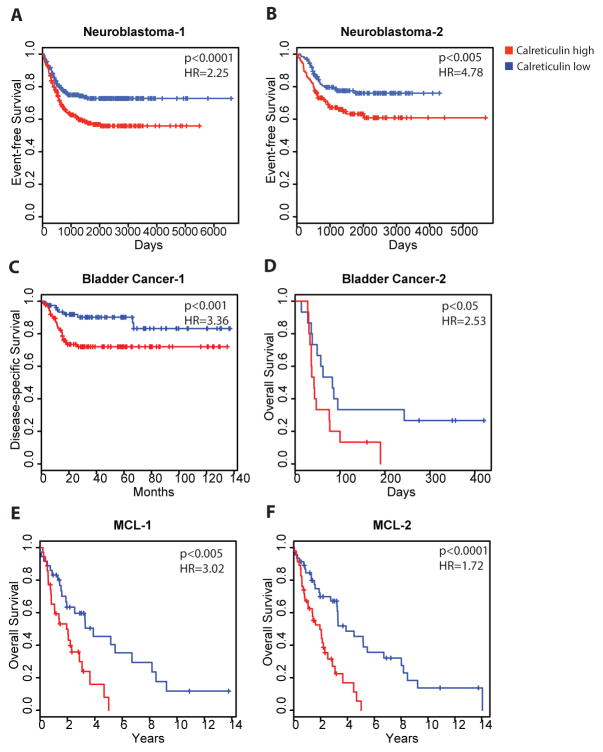
Lastly, we sought to investigate the clinical relevance of these findings by investigating the association between CRT expression and clinical outcomes. We analyzed calreticulin mRNA levels in patients with human malignancies of distinct tumor types and investigated their correlation with tumor progression and clinical outcome. Utilizing previously published gene profiling datasets with associated clinical outcome data, we determined calreticulin expression in both hematologic and solid tumor malignancies, including non-Hodgkin lymphoma (mantle cell lymphoma (MCL)), superficial and invasive bladder cancer, and neuroblastoma. Patients were stratified into calreticulin high and low-expressing cohorts relative to the median value and analyzed for clinical outcomes. For each tumor type, correlations between calreticulin expression and event-free, disease-specific, or overall survival were measured in two independent datasets to test and validate significant associations. Regardless of tumor type, higher calreticulin expression predicted a worse clinical outcome in all malignancies analyzed: neuroblastoma (Fig. 4A,B), bladder cancer (Fig. 4C,D), and NHL (MCL, Fig. 4E,F). These associations were significant when calreticulin expression was considered either as a dichotomous variable (relative to the median) or as a continuous variable (table S1). The prognostic power of CRT was independent from type of therapy as patients with the various tumors received disparate treatments including observation, surgery, or chemotherapy (table S1). Additionally, the prognostic power of CRT was preserved in both early and late stage tumors as increased calreticulin levels correlated with worse survival in both superficial and invasive bladder cancer (Fig. 4C.D, table S1). Thus, calreticulin expression is associated with tumor progression and worse clinical outcome across several tumor types. It should, however, be cautioned that only calreticulin mRNA was analyzed here, and thus the specific role of cell surface CRT in tumor progression cannot be inferred from this study.
Figure 4. Increased calreticulin expression confers a worse clinical prognosis in multiple human malignancies.
Stratification of clinical outcomes based on the level of expression of calreticulin mRNA is shown in previously described cohorts (50–55) of patients with diverse malignancies including neuroblastoma (A,B), superficial or invasive bladder cancer (C,D), and mantle cell lymphoma (E,F). Patients were divided into calreticulin high and low expression groups based on median calreticulin expression with Kaplan-Meier analyses of patient outcome shown. Hazard ratios (HR) and log-rank p values are shown for the relationship of outcomes to continuous expression of calreticulin using a univariate Cox regression model. HR, 95% confidence intervals, and log-rank p values for calreticulin expression as a dichotomous variable are shown in table S1. Description of clinical datasets is shown in table S1.
DISCUSSION
In this report, we identify calreticulin as a pro-phagocytic signal highly expressed on the surface of several human cancers, but minimally expressed on normal cell counterparts, and demonstrate that CRT expression is required for anti-CD47 antibody-mediated phagocytosis.
Anti-CD47 antibody preferentially eliminates tumor cells because of differential expression of cell surface calreticulin
We recently demonstrated that several cancers overexpress CD47 and that a blocking anti-CD47 monoclonal antibody can eliminate tumor cells in vitro and in vivo (6, 8, 9). These pre-clinical findings provide a strong rationale for the use of an anti-CD47 antibody in the treatment of human cancers. However, given the broad low level expression of CD47 on both hematopoietic and most other normal tissues, antibody toxicity could be a significant barrier to clinical translation. To investigate this issue, we previously injected a blocking anti-mouse CD47 antibody into wild type mice at a dose that coated >98% of bone marrow cells but observed no overt toxicity, with the exception of isolated neutropenia (6). Moreover, a recent report demonstrated that inhibition of CD47 with either an antibody or morpholino could confer radioprotective effects to normal tissues (24). Here, we demonstrate that, despite low level CD47 expression, normal human cells from several tissues are not phagocytosed by human macrophages when coated with anti-CD47 antibody (Fig. 3B). We speculate that the selective phagocytosis of tumor cells is not simply dictated by CD47 expression level, but is also governed by the presence of the pro-phagocytic signal calreticulin, which is present on tumor cells but not on normal cells. Several lines of evidence support this hypothesis. First, normal cells that express CD47 but not calreticulin are not phagocytosed with an anti-CD47 antibody despite being coated with the antibody (Fig. 3B). Second, tumor cells that express CD47 and calreticulin are phagocytosed when coated with anti-CD47 antibody (Fig. 3A,C). Third, phagocytosis of tumor cells with anti-CD47 antibody is completely abrogated when the calreticulin-LRP interaction is blocked (Fig. 3A,C). Fourth, adsorption of exogenous CRT onto the surface of NBM cells, which express minimal CRT (Fig. 1A), enabled increased phagocytosis compared to vehicle control or anti-CD47 antibody administration (Fig. 3E). Collectively, these findings demonstrate that calreticulin is necessary for anti-CD47 antibody-mediated phagocytosis, and that surface expression of this protein is primarily restricted to tumor cells.
This study indicates that the therapeutic window for anti-CD47 antibody therapy is not just a consequence of CD47 level on target cells, but that it also depends on the surface expression of pro-phagocytic calreticulin. On the basis of our findings, we propose a model in which the overall contribution of pro (CRT)- and anti (CD47)-phagocytic signals determines whether normal or tumor cells are phagocytosed at steady state, or by anti-CD47 antibody therapy (fig. S7). At steady state, tumor cells express calreticulin, but evade phagocytosis through overexpression of CD47, indicating the dominance of the ”don’t eat me” anti-phagocytic signal (fig. S7A,B). Normal cells express low levels of CD47, and avoid phagocytosis because of a lack of CRT expression. In contrast, cells undergoing DNA damage or apoptosis express calreticulin on their cell surface (14, 25), which is dominant over low CD47 expression and leads to phagocytosis. In the context of anti-CD47 antibody therapy, the anti-phagocytic signal (CD47) is blocked, unmasking the pro-phagocytic signal (CRT) on tumor cells, leading to phagocytosis (fig. S7C,D). In contrast, blockade of CD47 on normal cells does not lead to phagocytosis since the pro-phagocytic “eat me” signal (CRT) is absent.
Although calreticulin appears to be primarily expressed on the surface of apoptotic or malignant cells, prior reports detected surface calreticulin on some human normal cells including activated peripheral blood T cells (26) and circulating neutrophils (27). In addition, a blocking monoclonal anti-CD47 antibody enhances phagocytosis of apoptotic neutrophils (28, 29). Interestingly, in our mouse toxicity studies, administration of a blocking anti-mouse CD47 antibody led to selective depletion of neutrophils, while other hematopoietic cells were unaffected (6). Similar to tumor cells, this selective neutropenic toxicity may be due to unmasking of calreticulin on neutrophils when the “don’t eat me” signal (CD47) is blocked by anti-CD47 antibody. Although most normal cells do not express cell surface calreticulin, normal cells may upregulate calreticulin under certain conditions, including radiation and anthracycline-based chemotherapy as has been shown in some tumor types (25, 30). Our findings provide a cautionary note that normal cells might upregulate calreticulin as a consequence of radiation and chemotherapy-based cancer therapy, and thus combination chemoradiation and anti-CD47 antibody therapy must be tested for potential increased toxicity to normal cells.
Calreticulin is the dominant pro-phagocytic signal on several human cancers
We demonstrate that several human cancers, including both hematopoietic and solid tumor malignancies, express the pro-phagocytic signal calreticulin. Known physiologic pro-phagocytic signals have previously been identified in several cancers including phosphatidylserine (31–35) and annexin-1 (reviewed in (36)). However, most of these studies were not performed on primary human patient samples as in this study. Additionally, ligand expression appears to be mixed across tumor types (36) with the functional role of these ligands in cancer not known. A complete survey of human tumors for cell surface calreticulin expression will be required to determine whether the regulation of the CD47-CRT phagocytic axis is a universal trait of cancers. Although CRT appears to be the dominant pro-phagocytic signal on the cancer samples profiled, it is possible that these and other tumor types might express other pro-phagocytic signals that similarly regulate phagocytosis. A number of pro-phagocytic signals have been identified on apoptotic cells (reviewed in (37)), thus warranting an investigation of the potential pro-phagocytic roles of these ligands in cancer.
One key question is raised by these studies: Why do cancers express cell surface calreticulin, a pro-phagocytic signal? We have demonstrated that certain cancers evade the innate immune system by upregulating anti-phagocytic signals, specifically CD47 (5, 6, 8). One might expect cancers to simultaneously downregulate pro-phagocytic signals to further increase their ability to evade macrophage phagocytosis. We propose two possible explanations. First, expression of cell surface calreticulin may be an unwanted consequence of cellular stress, whereby CD47 expression is upregulated to compensate and enable phagocytic evasion. In normal physiology, cell surface calreticulin is induced on cells undergoing DNA damage (14, 25, 30), marking these damaged cells for homeostatic phagocytosis. It is possible that a small fraction of these cells may selectively avoid phagocytic clearance due to higher levels or upregulation of CD47, which allows these damaged cells to survive and acquire additional mutations, eventually transforming into fully malignant cells. Several lines of evidence support this hypothesis. First, CD47 and CRT expression are highly correlated in several human tumors (Fig. 2A). Second, the small percentage of live cells that are calreticulin positive in some normal human tissue types (bone marrow and bladder) express higher CD47 levels than their calreticulin negative counterparts (fig. S3). Third, this increase in CD47 expression appears to protect against calreticulin-mediated phagocytosis as knockdown of CD47 to 50% of wild type levels enabled calreticulin-dependent phagocytosis (Fig. 2).
In a second hypothesis, expression of cell surface calreticulin may confer some unknown pro-tumorigenic phenotype to cancer cells that is independent of phagocytosis. This hypothesis is supported by the finding that increased calreticulin expression in human tumors confers a worse clinical outcome across disparate tumor types, tumor stage, and tumor-specific therapies (Fig. 4). One possibility is that cell surface calreticulin may allow more invasion and angiogenesis, as its ligand, LRP, is expressed on several vascular cell types (reviewed in (38)). In two reports, overexpression of calreticulin or calreticulin fragments in tumor cell lines enhanced in vitro migration and invasion (39, 40); however, other studies have reported alternative roles for calreticulin (41–43). In all of these studies the function of cell surface calreticulin was not distinguished from its intracellular roles. Other possible tumorigenic roles include cell adhesion (44) and immune escape through reduction of MHC class I antigen presentation (45).
In summary, we have identified cell surface calreticulin as the dominant pro-phagocytic signal on several human cancers, which is absent on normal cell counterparts and is required for anti-CD47 antibody-mediated phagocytosis. These findings support the rationale for the development of an anti-CD47 antibody therapy for human malignancies and highlight the dynamic relationship between pro- and anti-phagocytic signals in human cancer.
MATERIALS AND METHODS
Cell Lines and Human Samples
MOLT4 and Daudi cell lines were obtained from the lab of Ronald Levy. 639V was obtained from the DSMZ. All other cell lines were obtained from the American Type Culture Association (ATCC). Normal human bone marrow mononuclear cells were purchased from AllCells Inc. Normal peripheral blood and human cancer samples were obtained from patients at the Stanford Medical Center with informed consent according to IRB-approved protocols: AML, ALL, and NHL human samples from Stanford IRB# 76935, 6453, and 13500, bladder cancer samples from Stanford IRB #1512, glioblastoma samples from Stanford IRB# 9363, and ovarian cancer samples from Stanford IRB #13939. Normal fetal bladder and brain cells were purchased from ScienCell Research Laboratories.
Flow Cytometry Analysis
For analysis of normal peripheral blood cells, normal bone marrow cells, AML, CML, ALL, bladder cancer, ovarian cancer, and brain cancer, the following antibodies were used: CD34, CD38, CD90, CD45, CD31, CD3, CD4, CD7, CD11b, CD14, CD19, CD20, CD56, Glycophorin A (Invitrogen and BD Biosciences). Lineage negative (Lin-) was defined as CD3-CD19-CD20- for AML LSC and CD45-CD31- for GBM and bladder cancer CSC. Lin- was defined as CD3-CD4-CD7-CD8-CD11b-CD14-CD19-CD20-CD56-Glycophorin A- for NBM HSC, chronic phase CML GMP, CML CMP, and CML LSC. Analysis of CD47 expression was performed using an anti-human CD47 FITC antibody (clone B6H12.2, BD Biosciences). Analysis of human cell surface calreticulin expression was performed using mouse anti-human calreticulin conjugated to PE or FITC (clone FMC 75, Abcam). Human ERp57 expression was performed using a polyclonal rabbit anti-ERp57 antibody (Abcam) and then staining with a donkey anti-rabbit secondary antibody conjugated to PE (Ebioscience).
In vitro phagocytosis assay
Generation of human macrophages and in vitro phagocytosis assays were performed as previously described (6). Primary human samples or cell lines were incubated with 10μg/ml IgG1 isotype control (Ebiosciences), 10μg/ml anti-CD47 antibody (clone B6H12.2, ATCC), 4μg/ml calreticulin blocking peptide (MBL International Coorporation), 10μg/ml RAP (Fitzgerald Industries International), or 125μg/ml recombinant CRT human protein (Thermo Scientific). Per MBL, confirmation of blocking activity was performed by Western blot analysis, verified by incubation of an anti-CRT antibody with 5 times higher concentration of the peptide and performing a Western blot analysis to determine if the specific band had been diminished. Characterization of the LRP-antagonizing effects of RAP are detailed in (46–48). Cells were then analyzed by fluorescence microscopy to determine the phagocytic index (number of cells ingested per 100 macrophages).
shRNA knockdown of Raji cells
shRNA constructs targeting knockdown of human CD47 or a GAPD control packaged in the SMARTvector 2.0 lentiviral vector containing a turbo GFP reporter were purchased from Dharmacon, Inc. (Lafayette, CO). Viral titers for each shRNA construct were greater than 10>8 TU/ml. Raji cells were transduced with these lentiviral constructs, analyzed and sorted for GFP expression, expanded, and sorted again for GFP expression for stable propagation of lentivirally-transduced cells. Knockdown of CD47 protein levels was assessed by flow cytometry with anti-CD47 antibody (B6H12.2) with fold knockdown calculated by reduction in MFI normalized over isotype control.
Xeontransplantation of primary human cancer cells into mice
For engraftment of human AML cells, AML LSC (CD34+CD38-CD90-Lin-) were sorted by fluorescence-activated cell sorting (FACS) and transplanted into the facial vein of newborn NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) mice, sublethally-irradiated with 200 rads. Leukemic engraftment was analyzed 8 weeks later in the bone marrow of transplanted mice. For engraftment of human bladder cancer, bulk bladder cancer cells were resuspended in 25% matrigel (BD Biosciences) and transplanted subcutaneously into the flanks of adult NSG mice. Tumor volume was serially monitored post-transplantation by analyzing weights of excised tumors.
Analysis of the prognostic value of calreticulin expression in human malignancies
Gene expression and clinical data were analyzed for six previously described cohorts of neuroblastoma, superficial and invasive urothelial carcinoma of the bladder, and mantle cell lymphoma (see table S1 for dataset descriptions). Patients were stratified into high and low calreticulin expression groups based on the median expression level within each cohort and analyzed for event-free, disease-specific, or overall survival by Kaplan-Meier analysis. Subsequent dichotomous hazard ratios, 95% confidence intervals, and log-rank p-values were analyzed reflecting estimates within Kaplan-Meier analyses (table S1). Additionally, analyses were performed based on continuous expression of calreticulin and clinical outcome as measured by log-likelihood p-values within a univariate Cox regression model (table S1). Affymetrix microarray data were processed starting with CEL files, with Entrez Gene probeset summarization using CustomCDF version 12 (49), and normalization using MAS 5.0 linear scaling method. Overlapping samples from related studies (Fig. 4A,B and 4E,F), have not been removed.
Supplementary Material
Fig. S1. Live calreticulin positive cancer cells form tumors in vivo
Figure S2: Cell surface CRT correlates with ERp57 expression on tumor cells
Fig. S3. Live calreticulin positive cells from normal human tissues have higher levels of CD47 compared to calreticulin negative cells
Fig. S4. Calreticulin expression is unaffected by CD47 shRNA knockdown in Raji cells
Fig. S5. CD47 is expressed on normal human cells
Fig. S6. Abrogation of anti-CD47 antibody-mediated phagocytosis is dose dependent on calreticulin blockade
Fig. S7. Model for the integration of pro (CRT)- and anti (CD47)-phagocytic signals on normal and tumor cells at steady state and during anti-CD47 antibody therapy
Table S1. Analysis of the prognostic value of calreticulin in human malignancies
Acknowledgments
We thank Dr. Ronald Levy for providing clinical samples and support, Adriane Mosley for animal husbandry, and Libuse Jerabek, Theresa Storm, and Feifei Zhao for excellent lab management. M.P.C. is supported by a medical fellowship from the Howard Hughes Medical Institute and the Stanford Cancer Biology Program. R.M. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund. This work was supported in part by a grant from the American Association for Cancer Research to R.M., NIH grant P01CA139490 to I.L.W., and funding from the Ludwig Foundation. M.P.C., R.M., and I.L.W. wrote the manuscript. M.P.C., R.M., and I.L.W. designed the experiments. M.P.C., R.W.T., A.A.A., A.J.G, S.J., J.V., and K.W. performed experiments and analyzed data. A.A.A., J.V., S.B.W., T.R., and C.Y.P. provided clinical samples. The authors declare no competing interests. S.J., M.P.C., R.M., and I.L.W. filed US Patent Application Serial No. 12/321,215 entitled “Methods For Manipulating Phagocytosis Mediated by CD47.”
Footnotes
The authors do not have any competing interests and fully endorse all contents of this study.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Stagg J, Johnstone RW, Smyth MJ. From cancer immunosurveillance to cancer immunotherapy. Immunol Rev. 2007;220:82–101. doi: 10.1111/j.1600-065X.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 3.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 5.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaiswal S, Chao MP, Majeti R, Weissman IL. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. 2010;31:212–219. doi: 10.1016/j.it.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, Gill H, Presti J, Jr, Chang HY, van de Rijn M, Shortliffe L, Weissman IL. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, Jan M, Cha AC, Chan CK, Tan BT, Park CY, Zhao F, Kohrt HE, Malumbres R, Briones J, Gascoyne RD, Lossos IS, Levy R, Weissman IL, Majeti R. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai RK, Rodriguez PL, Discher DE. Self inhibition of phagocytosis: The affinity of ‘marker of self’ CD47 for SIRPalpha dictates potency of inhibition but only at low expression levels. Blood Cells Mol Dis. 2010;45:67–74. doi: 10.1016/j.bcmd.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barclay AN, Brown MH. The SIRP family of receptors and immune regulation. Nat Rev Immunol. 2006;6:457–464. doi: 10.1038/nri1859. [DOI] [PubMed] [Google Scholar]
- 12.Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem. 1999;274:559–562. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- 13.Reinhold MI, Lindberg FP, Plas D, Reynolds S, Peters MG, Brown EJ. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47) J Cell Sci. 1995;108(Pt 11):3419–3425. doi: 10.1242/jcs.108.11.3419. [DOI] [PubMed] [Google Scholar]
- 14.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 15.Orr AW, Pedraza CE, Pallero MA, Elzie CA, Goicoechea S, Strickland DK, Murphy-Ullrich JE. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J Cell Biol. 2003;161:1179–1189. doi: 10.1083/jcb.200302069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, Fimia GM, Kepp O, Piacentini M, Froehlich KU, van Endert P, Zitvogel L, Madeo F, Kroemer G. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 2008;15:1499–1509. doi: 10.1038/cdd.2008.67. [DOI] [PubMed] [Google Scholar]
- 17.Obeid M. ERP57 membrane translocation dictates the immunogenicity of tumor cell death by controlling the membrane translocation of calreticulin. J Immunol. 2008;181:2533–2543. doi: 10.4049/jimmunol.181.4.2533. [DOI] [PubMed] [Google Scholar]
- 18.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 19.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 20.Blair A, Hogge DE, Ailles LE, Lansdorp PM, Sutherland HJ. Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood. 1997;89:3104–3112. [PubMed] [Google Scholar]
- 21.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, Sawyers CL, Weissman IL. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 22.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 23.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 24.Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M, Wink DA, Isenberg JS, Roberts DD. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med. 2009;1:3ra7. doi: 10.1126/scitranslmed.3000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, Zitvogel L, Kroemer G. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–1850. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 26.Arosa FA, de Jesus O, Porto G, Carmo AM, de Sousa M. Calreticulin is expressed on the cell surface of activated human peripheral blood T lymphocytes in association with major histocompatibility complex class I molecules. J Biol Chem. 1999;274:16917–16922. doi: 10.1074/jbc.274.24.16917. [DOI] [PubMed] [Google Scholar]
- 27.Ghiran I, Klickstein LB, Nicholson-Weller A. Calreticulin is at the surface of circulating neutrophils and uses CD59 as an adaptor molecule. J Biol Chem. 2003;278:21024–21031. doi: 10.1074/jbc.M302306200. [DOI] [PubMed] [Google Scholar]
- 28.Park YJ, Liu G, Lorne EF, Zhao X, Wang J, Tsuruta Y, Zmijewski J, Abraham E. PAI-1 inhibits neutrophil efferocytosis. Proc Natl Acad Sci U S A. 2008;105:11784–11789. doi: 10.1073/pnas.0801394105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrence DW, King SB, Frazier WA, Koenig JM. Decreased CD47 expression during spontaneous apoptosis targets neutrophils for phagocytosis by monocyte-derived macrophages. Early Hum Dev. 2009;85:659–663. doi: 10.1016/j.earlhumdev.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 31.Connor J, Bucana C, Fidler IJ, Schroit AJ. Differentiation-dependent expression of phosphatidylserine in mammalian plasma membranes: quantitative assessment of outer-leaflet lipid by prothrombinase complex formation. Proc Natl Acad Sci U S A. 1989;86:3184–3188. doi: 10.1073/pnas.86.9.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Utsugi T, Schroit AJ, Connor J, Bucana CD, Fidler IJ. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991;51:3062–3066. [PubMed] [Google Scholar]
- 33.Rao LV, Tait JF, Hoang AD. Binding of annexin V to a human ovarian carcinoma cell line (OC-2008). Contrasting effects on cell surface factor VIIa/tissue factor activity and prothrombinase activity. Thromb Res. 1992;67:517–531. doi: 10.1016/0049-3848(92)90013-z. [DOI] [PubMed] [Google Scholar]
- 34.Woehlecke H, Pohl A, Alder-Baerens N, Lage H, Herrmann A. Enhanced exposure of phosphatidylserine in human gastric carcinoma cells overexpressing the half-size ABC transporter BCRP (ABCG2) Biochem J. 2003;376:489–495. doi: 10.1042/BJ20030886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zwaal RF, Comfurius P, Bevers EM. Surface exposure of phosphatidylserine in pathological cells. Cell Mol Life Sci. 2005;62:971–988. doi: 10.1007/s00018-005-4527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim LH, Pervaiz S. Annexin 1: the new face of an old molecule. Faseb J. 2007;21:968–975. doi: 10.1096/fj.06-7464rev. [DOI] [PubMed] [Google Scholar]
- 37.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 38.Strickland DK, Kounnas MZ, Argraves WS. LDL receptor-related protein: a multiligand receptor for lipoprotein and proteinase catabolism. Faseb J. 1995;9:890–898. doi: 10.1096/fasebj.9.10.7615159. [DOI] [PubMed] [Google Scholar]
- 39.Liu M, Imam H, Oberg K, Zhou Y. Gene transfer of vasostatin, a calreticulin fragment, into neuroendocrine tumor cells results in enhanced malignant behavior. Neuroendocrinology. 2005;82:1–10. doi: 10.1159/000089749. [DOI] [PubMed] [Google Scholar]
- 40.Chen CN, Chang CC, Su TE, Hsu WM, Jeng YM, Ho MC, Hsieh FJ, Lee PH, Kuo ML, Lee H, Chang KJ. Identification of calreticulin as a prognosis marker and angiogenic regulator in human gastric cancer. Ann Surg Oncol. 2009;16:524–533. doi: 10.1245/s10434-008-0243-1. [DOI] [PubMed] [Google Scholar]
- 41.Pike SE, Yao L, Setsuda J, Jones KD, Cherney B, Appella E, Sakaguchi K, Nakhasi H, Atreya CD, Teruya-Feldstein J, Wirth P, Gupta G, Tosato G. Calreticulin and calreticulin fragments are endothelial cell inhibitors that suppress tumor growth. Blood. 1999;94:2461–2468. [PubMed] [Google Scholar]
- 42.Pike SE, Yao L, Jones KD, Cherney B, Appella E, Sakaguchi K, Nakhasi H, Teruya-Feldstein J, Wirth P, Gupta G, Tosato G. Vasostatin, a calreticulin fragment, inhibits angiogenesis and suppresses tumor growth. J Exp Med. 1998;188:2349–2356. doi: 10.1084/jem.188.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu WM, Hsieh FJ, Jeng YM, Kuo ML, Chen CN, Lai DM, Hsieh LJ, Wang BT, Tsao PN, Lee H, Lin MT, Lai HS, Chen WJ. Calreticulin expression in neuroblastoma--a novel independent prognostic factor. Ann Oncol. 2005;16:314–321. doi: 10.1093/annonc/mdi062. [DOI] [PubMed] [Google Scholar]
- 44.Coppolino MG, Woodside MJ, Demaurex N, Grinstein S, St-Arnaud R, Dedhar S. Calreticulin is essential for integrin-mediated calcium signalling and cell adhesion. Nature. 1997;386:843–847. doi: 10.1038/386843a0. [DOI] [PubMed] [Google Scholar]
- 45.Howe C, Garstka M, Al-Balushi M, Ghanem E, Antoniou AN, Fritzsche S, Jankevicius G, Kontouli N, Schneeweiss C, Williams A, Elliott T, Springer S. Calreticulin-dependent recycling in the early secretory pathway mediates optimal peptide loading of MHC class I molecules. Embo J. 2009;28:3730–3744. doi: 10.1038/emboj.2009.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bu G. The roles of receptor-associated protein (RAP) as a molecular chaperone for members of the LDL receptor family. Int Rev Cytol. 2001;209:79–116. doi: 10.1016/s0074-7696(01)09011-8. [DOI] [PubMed] [Google Scholar]
- 47.Obermoeller-McCormick LM, Li Y, Osaka H, FitzGerald DJ, Schwartz AL, Bu G. Dissection of receptor folding and ligand-binding property with functional minireceptors of LDL receptor-related protein. J Cell Sci. 2001;114:899–908. doi: 10.1242/jcs.114.5.899. [DOI] [PubMed] [Google Scholar]
- 48.Orr AW, Elzie CA, Kucik DF, Murphy-Ullrich JE. Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration. J Cell Sci. 2003;116:2917–2927. doi: 10.1242/jcs.00600. [DOI] [PubMed] [Google Scholar]
- 49.Dai M, Wang P, Boyd AD, Kostov G, Athey B, Jones EG, Bunney WE, Myers RM, Speed TP, Akil H, Watson SJ, Meng F. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res. 2005;33:e175. doi: 10.1093/nar/gni179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oberthuer A, Juraeva D, Li L, Kahlert Y, Westermann F, Eils R, Berthold F, Shi L, Wolfinger RD, Fischer M, Brors B. Comparison of performance of one-color and two-color gene-expression analyses in predicting clinical endpoints of neuroblastoma patients. Pharmacogenomics J. 2010;10:258–266. doi: 10.1038/tpj.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Preter K, Vermeulen J, Brors B, Delattre O, Eggert A, Fischer M, Janoueix-Lerosey I, Lavarino C, Maris JM, Mora J, Nakagawara A, Oberthuer A, Ohira M, Schleiermacher G, Schramm A, Schulte JH, Wang Q, Westermann F, Speleman F, Vandesompele J. Accurate outcome prediction in neuroblastoma across independent data sets using a multigene signature. Clin Cancer Res. 2010;16:1532–1541. doi: 10.1158/1078-0432.CCR-09-2607. [DOI] [PubMed] [Google Scholar]
- 52.Kim WJ, Kim EJ, Kim SK, Kim YJ, Ha YS, Jeong P, Kim MJ, Yun SJ, Lee KM, Moon SK, Lee SC, Cha EJ, Bae SC. Predictive value of progression-related gene classifier in primary non-muscle invasive bladder cancer. Mol Cancer. 2010;9:3. doi: 10.1186/1476-4598-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Als AB, Dyrskjot L, von der Maase H, Koed K, Mansilla F, Toldbod HE, Jensen JL, Ulhoi BP, Sengelov L, Jensen KM, Orntoft TF. Emmprin and survivin predict response and survival following cisplatin-containing chemotherapy in patients with advanced bladder cancer. Clin Cancer Res. 2007;13:4407–4414. doi: 10.1158/1078-0432.CCR-07-0109. [DOI] [PubMed] [Google Scholar]
- 54.Blenk S, Engelmann JC, Pinkert S, Weniger M, Schultz J, Rosenwald A, Muller-Hermelink HK, Muller T, Dandekar T. Explorative data analysis of MCL reveals gene expression networks implicated in survival and prognosis supported by explorative CGH analysis. BMC Cancer. 2008;8:106. doi: 10.1186/1471-2407-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenwald A, Wright G, Wiestner A, Chan WC, Connors JM, Campo E, Gascoyne RD, Grogan TM, Muller-Hermelink HK, Smeland EB, Chiorazzi M, Giltnane JM, Hurt EM, Zhao H, Averett L, Henrickson S, Yang L, Powell J, Wilson WH, Jaffe ES, Simon R, Klausner RD, Montserrat E, Bosch F, Greiner TC, Weisenburger DD, Sanger WG, Dave BJ, Lynch JC, Vose J, Armitage JO, Fisher RI, Miller TP, LeBlanc M, Ott G, Kvaloy S, Holte H, Delabie J, Staudt LM. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3:185–197. doi: 10.1016/s1535-6108(03)00028-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Live calreticulin positive cancer cells form tumors in vivo
Figure S2: Cell surface CRT correlates with ERp57 expression on tumor cells
Fig. S3. Live calreticulin positive cells from normal human tissues have higher levels of CD47 compared to calreticulin negative cells
Fig. S4. Calreticulin expression is unaffected by CD47 shRNA knockdown in Raji cells
Fig. S5. CD47 is expressed on normal human cells
Fig. S6. Abrogation of anti-CD47 antibody-mediated phagocytosis is dose dependent on calreticulin blockade
Fig. S7. Model for the integration of pro (CRT)- and anti (CD47)-phagocytic signals on normal and tumor cells at steady state and during anti-CD47 antibody therapy
Table S1. Analysis of the prognostic value of calreticulin in human malignancies