Abstract
Oral leiomyoma are rare neoplasms of the oral cavity. Ossification within leiomyoma is not unusual but is mostly reported in leiomyoma of the deep soft tissue. Ossifying leiomyoma is extremely rare in the head and neck. We identified a total of three cases of extensively ossified leiomyoma in the head and neck in the literature including lesions in the lateral pterygoid muscle and orbit. To the best of our knowledge, only one case of extensively calcified leiomyoma has been reported in the oral cavity. We present two such rare cases of oral leiomyoma with extensive intratumoral calcifications and ossification. Ossified leiomyoma should be considered in the differential diagnosis of calcified or hard/firm soft tissue masses in the oral cavity.
Keywords: Leiomyoma, Oral cavity, Ossification, Calcification
Introduction
Leiomyoma are benign tumors of smooth muscle that are rare in the head and neck and oral cavity. These tumors may occur anywhere in the body where smooth muscle is present but most commonly occur in the genitourinary tract, gastrointestinal tract and skin [1, 2]. Based on histology, leiomyoma are generally classified into three variants: solid, vascular or epithelioid. Leiomyoma of the head and neck region are most commonly either the solid or vascular variants. Within the oral cavity, 75 % of oral leiomyoma are of the vascular subtype [3]. Degenerative changes, such as edema, hyalinization, hemorrhage, calcification and cystic degeneration are often found in leiomyoma [4–7]. Calcification has been reported to occur commonly in the leiomyoma of the deep soft tissues of the extremities but is a rare degenerative change in leiomyoma in other locations [8–12]. Extensive calcification or ossification within leiomyoma of the head and neck is a rare finding, and a thorough search of the literature revealed only one case of oral leiomyoma with extensive calcification [13]. We present the first two cases of leiomyoma of the oral cavity with extensive ossification. We also review and discuss the clinical characteristics and histologic differential diagnoses of these lesions.
Case Reports
Case 1
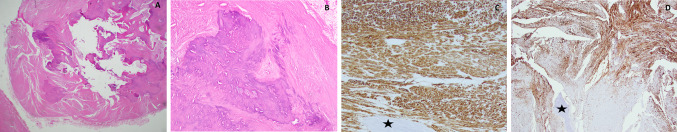
A 51 year-old Caucasian female presented to her oral surgeon for evaluation of an anterior maxillary lesion. The lesion had slowly increased in size. A computed tomography scan revealed a well circumscribed radiopacity in the soft tissue apical to the right maxillary central incisor and labial to the maxillary alveolus, causing resorption of the bone (Fig. 1). The patient’s prior medical history was non-contributory. The tissue was submitted for decalcification due to presence of hard tissue felt during grossing. Microscopic examination of an incisional biopsy revealed a circumscribed proliferation of smooth muscle intermixed with dense fibrous connective tissue and large zones of significant dystrophic calcifications and bone. Normal appearing bony trabeculae were noted within the lesion, which demonstrated significant resorption. The tumor was discrete and partially encapsulated. The lesion appeared to erode the underlying bone. Areas of the lesion were composed of interlacing fascicles of spindle shaped smooth muscle cells with minimal nuclear pleomorphism. No mitoses were identified. The smooth muscle was heavily collagenized and large zones of calcifications including dense viable bone were seen in the deeper areas of the specimen (Fig. 2a, b). Immunohistochemical stains for smooth muscle actin (SMA) (Fig. 2c), smooth muscle myosin heavy chain (SMMS-1) (Fig. 2d) and desmin were performed. The neoplastic cells were strongly reactive for SMA and SMMS-1 and negative for desmin with adequate controls. A diagnosis of leiomyoma with prominent ossification was rendered. The lesion was completely excised and the patient was seen for routine follow-up 6 months later. A nodular growth was noted in the site of previous surgery and was excised completely. Microscopic examination of the recurrent tissue revealed fibrous connective tissue scar with skeletal muscle and accessory salivary glands. No muscle tissue or calcifications were identified.
Fig. 1.
Sections from a computed tomography scan of Case 1 showing a well circumscribed radiopaque mass causing cupping resorption of the anterior maxillary alveolus near the apical region of the maxillary incisor
Fig. 2.
Photomicrograph panel from Case 1. a Hematoxylin and eosin stained section (magnification ×5) demonstrating well circumscribed soft tissue mass interspersed with large islands of calcifications. b Higher magnification (×20) exhibits a large area of irregularly calcified tissue within the soft tissue mass. c Immunohistochemical stain for smooth muscle actin demonstrates diffuse strong reactivity throughout the soft tissue areas. An area of bone is noted at the base (asterisk) (×10 magnification). d Immunohistochemical stain for smooth muscle myosin heavy chain shows strong reactivity with lesional cells. Area of bone marked with asterisk (×10 magnification)
Case 2
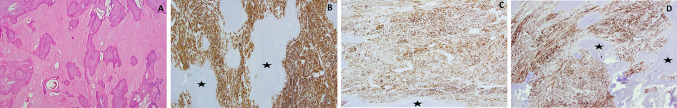
An 18 year-old Caucasian female was referred to an oral surgeon by her general dentist for extraction of tooth number 32 and biopsy of a vestibular lesion. The patient reported that the lesion had been present for as long as she could remember with no change in size. She denied any pain, swelling or paresthesia. Her medical history was unremarkable. Clinically, a yellow–brown soft tissue nodule was noted in the right mandibular vestibule. The tissue was submitted for decalcification due to obvious hard tissue felt during grossing. The microscopic examination revealed a benign neoplastic proliferation of spindle shaped smooth muscle, fibrous connective tissue, and extensive areas of calcification and ossification (Fig. 3a). Extensive areas of dystrophic eosinophilic to basophilic amorphous calcifications were noted. Multinucleated giant cells surrounded the calcifications in several areas. The surrounding soft tissue was composed of poorly oriented fascicles of spindle shaped cells with elongated spindle shaped nuclei with rounded edges and vacuolated and abundant eosinophilic cytoplasm. Immunohistochemical stains for SMA (Fig. 3b), muscle specific actin (MSA) (Fig. 3c) and SMMS-1 (Fig. 3d) were strongly reactive for these spindle shaped cells. A diagnosis of leiomyoma with extensive ossification was rendered. The patient was lost to follow-up.
Fig. 3.
Photomicrograph panel from Case 2. a Hematoxylin and eosin stained section (magnification ×5) demonstrating numerous irregularly mineralized islands of calcifications distributed in a stroma composed of spindle cells. b Immunohistochemical stain for smooth muscle actin demonstrates diffuse strong reactivity throughout the soft tissue areas. The areas of bone are not stained (asterisk) (×10 magnification). c Immunohistochemical stain for muscle specific actin with diffuse strong reactivity throughout the soft tissue areas. Calcified area is marked with an asterisk (×10 magnification). d Immunohistochemical stain for smooth muscle myosin heavy chain demonstrating strong reactivity with spindled, lesional cells. Bone marked with asterisks (×10 magnification)
Discussion
Leiomyoma are uncommon tumors of the oral cavity. Less than 1 % of all leiomyoma occur within the oral cavity, due the paucity of smooth muscle [1, 3]. Most leiomyoma in the oral cavity arise from the smooth muscle of the vasculature [3, 14]. Uncommon histologic variations, including epithelioid, clear cell, granular cell and myxoid changes, have been reported in leiomyoma of the head and neck and oral cavity [15–19]. Secondary degenerative changes in leiomyoma such as hyaline degeneration, cystic change, myxoid degeneration, infection, necrosis, calcification and rarely ossification are not uncommon in leiomyoma of any location. These changes are thought to be a result of inadequate blood supply resulting in replacement of muscle fibers by hyaline material, collagen, calcium, mucopolysaccharide or a combination of these factors [6, 7]. While degenerative changes are common (over 60 %) in uterine leiomyomas, calcification has only been reported 4–10 % of cases [6, 7, 20]. Within the somatic soft tissue of the extremities, calcification within leiomyoma occurs frequently [4, 21]. Small calcifications have been reported in oral cavity leiomyoma, but this remains a rare finding [22, 23]. Microscopically, calcifications in leiomyoma of any site may appear as psammomatous spherules to large irregular masses [2, 9, 24]. These deposits may present with several radiographic patterns including amorphous mottled mass, multiple small, spotty calcifications or rarely, a curvilinear ring-like rim [7, 10, 20, 25]. Ossification is a rarely reported phenomenon that may be a degenerative change or may be the result of osseous metaplasia of mesenchymal cells [26]. Cases of extensively calcified or ossified leiomyoma are uncommon but have been reported in the genitourinary tract, gastrointestinal tract [27], soft tissues of the extremities [4, 9, 10, 12, 21, 28, 29] and the skin [8].
A search of the literature revealed 3 reports of leiomyoma with extensive calcification or ossification in the head and neck (not including the esophagus). See Table 1 (references cited with table). Only one case of leiomyoma with extensive calcification occurring in the dorsal tongue in a 38 year-old male was identified [13]. To our knowledge, these are the first 2 cases of leiomyoma of the oral cavity with extensive ossification.
Table 1.
Extensively ossified or calcified leiomyoma of the head and neck
| Author | Location | Age and sex (M) | Size (cm) | Calcification | Ossification | SMA | Desmin | S-100 | Treatment | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| Manojlovic et al. [24] | Lateral pterygoid | 8 | 4 | Psammoma-like to large masses | Small foci within calcification | + | + | N/P | Resection | None, 7 years follow-up |
| Wiechens et al. [34] | Orbit | 25 | Large | Spotty to bone fragments | Foci within large calcifications | + | N/P | N/P | Resection | None, 3 years follow-up |
| Nonaka et al. [13] | Tongue | 38 | 2 | Granular to masses | Not reported | + | N/P | − | Excision | None, 1 year follow-up |
M male, N/P not performed, SMA smooth muscle actin, + positive, − negative
The clinical differential diagnosis for leiomyoma with or without ossification is broad. Most reported leiomyoma of the oral cavity are located in a relatively superficial submucosal location and are found in middle aged or older patients [30]. Oral leiomyoma are most commonly found in the lips, followed by the tongue, cheeks, palate and gingiva [3, 22]. These lesions have the potential to grow up to several centimeters in size [2, 22]. A clinical differential diagnosis for oral leiomyoma may include other soft tissue neoplasms, both benign or malignant, reactive submucosal lesions such as fibroma, calcified vascular anomalies or salivary gland lesions. The microscopic differential diagnoses of soft tissue masses with calcifications include calcified vascular malformation (calcified thrombus), sialolith, peripheral ossifying fibroma, ossifying fibromyxoid tumor, osseous choristoma and benign neural lesions with calcification resulting from degenerative changes. Although these lesions have calcifications in common with calcified or ossified leiomyoma, they have specific histologic features and patterns, which allow differentiation. Calcified vascular malformations contain numerous, variably sized, endothelial lined blood vessels in addition to calcified thrombi. Sialoliths present with concentric ring-like calcifications and are often located within salivary ducts or near accessory salivary gland tissue. Peripheral ossifying fibromas may contain basophilic calcifications or areas of ossification, but the fibroblasts do not appear spindled and do not stain with SMA. Peripheral ossifying fibromas are frequently ulcerated and demonstrate inflammation. Another rare benign neoplasm reported in the oral cavity, the ossifying fibromyxoid tumor, often present with a peripheral rim of calcification and may exhibit calcifications between the lobules of the lesion [31, 32]. However, ossifying fibromyxoid tumors are typically lobular with a fibrous capsule or pseudo capsule and are characterized by cells arranged in cords and nests distributed in a myxoid background, a pattern not seen in leiomyoma [31, 32]. Osseous choristoma, also known as soft tissue osteomas, are composed of well circumscribed masses of bone, often with well-developed haversian canals and fatty or hematopoietic marrow spaces. The calcified mass is surrounded by fibrous tissue but lacks the spindle cells typically seen in leiomyoma. Schwannomas present for long duration may exhibit calcifications, but the typical Antoni A and/or Antoni B patterns, along with Verocay bodies can usually be identified. Radiographically, ossified leiomyoma of the soft tissues are occasionally misdiagnosed as calcifying schwannoma, myositis ossificans (rare in the oral cavity) or even cartilaginous entities [2].
Microscopically, leiomyoma is characterized by bundles of elongated spindle cells intersecting at right angles with perinuclear vacuoles and eosinophilic cytoplasm [9]. Masson trichrome special stain highlights the smooth muscle, which stains fuchsia [30]. They can usually be differentiated from most other spindle cell tumors by their positive expression of smooth muscle markers (SMA, MSA and SMMS-1) and may rarely be positive for desmin [21]. Other spindle cell tumors that may express smooth muscle markers and contain calcifications [2] include myofibroma, which present a different microscopic appearance due to their hybrid characterization between fibroblastic and smooth muscle cells. Additionally, the lesional cells of myofibroma display less cytoplasm and more spindled and narrower nuclei than leiomyoma [30]. Importantly, myofibroma also present with mitotic figures, a “tram track” pattern of staining to SMA but lack immunoreactivity to SMMS-1 [30, 33]. The strong positive staining of lesional cells for SMMS-1 in our cases helped confirm smooth muscle, rather than myofibroblastic origin. Differentiation between leiomyoma and well-differentiated leiomyosarcoma may be problematic but the latter exhibits cellular pleomorphism or nuclear atypia, necrosis and mitoses (occasionally abnormal) numbering more than 4 per 10 high power fields [30]. The histologic differential diagnosis also includes a smooth muscle hamartoma, which is generally seen in early life and occurs in the lumbar region. It is histologically distinct with compact bundles of mature smooth muscle separated by dermal collagen [21, 30].
Leiomyoma and leiomyoma with ossification are generally treated by excision. Few recurrences have been reported for leiomyoma in the oral cavity [2, 3]. The limited reports available on behavior of ossified leiomyoma similarly suggest a very low to negligible recurrence rate [26].
Conclusion
In conclusion, ossification within leiomyoma is a phenomenon most often seen in deeper soft tissue lesions and is rarely reported in the head, neck and oral cavity. Ossified leiomyoma may mimic a variety of lesions clinically and microscopically, hence awareness of the existence of this entity in the oral cavity may be useful in developing a broad differential diagnosis, especially when evaluating a hard or apparently calcified submucosal lesion. Pathologists and clinicians should be cognizant of the histologic features of leiomyoma with ossification when reviewing soft tissue lesions of the oral cavity, especially those which are associated with a radiopacity on imaging.
References
- 1.Farman AG. Benign smooth muscle tumours. S Afr Med J. 1975;49(33):1333–1340. [PubMed] [Google Scholar]
- 2.Weiss SW, Goldblum JR. Benign tumors of smooth muscle. In: Weiss SW, Goldblum JR, editors. Engineer and weiss’s soft tissue tumors. 5. Philadelphia: Mosby; 2008. [Google Scholar]
- 3.Baden E, Doyle JL, Lederman DA. Leiomyoma of the oral cavity: a light microscopic and immunohistochemical study with review of the literature from 1884 to 1992. Eur J Cancer B Oral Oncol. 1994;30B(1):1–7. doi: 10.1016/0964-1955(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 4.Billings SD, Folpe AL, Weiss SW. Do leiomyomas of deep soft tissue exist? An analysis of highly differentiated smooth muscle tumors of deep soft tissue supporting two distinct subtypes. Am J Surg Pathol. 2001;25(9):1134–1142. doi: 10.1097/00000478-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Liu JY, Liao SL, Zheng J. Cutaneous epithelioid angioleiomyoma with clear-cell change. Am J Dermatopathol. 2007;29(2):190–193. doi: 10.1097/DAD.0b013e3180332462. [DOI] [PubMed] [Google Scholar]
- 6.Persaud V, Arjoon PD. Uterine leiomyoma: incidence of degenerative change and a correlation of associated symptoms. Obstet Gynecol. 1970;35(3):432–436. [PubMed] [Google Scholar]
- 7.Ueda H, Togashi K, Konishi I, Kataoka ML, Koyama T, Fujiwara T, et al. Unusual appearances of uterine leiomyomas: MR imaging findings and their histopathologic backgrounds. Radiographics: a review publication of the Radiological Society of North America, Inc. 1999;19:S131–45. doi: 10.1148/radiographics.19.suppl_1.g99oc04s131. [DOI] [PubMed] [Google Scholar]
- 8.Kacerovska D, Michal M, Kreuzberg B, Mukensnabl P, Kazakov DV. Acral calcified vascular leiomyoma of the skin: a rare clinicopathological variant of cutaneous vascular leiomyomas: report of 3 cases. J Am Acad Dermatol. 2008;59(6):1000–1004. doi: 10.1016/j.jaad.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Barea F, Rodriguez-Peralto JL, Burgos E, Gonzalez-Lopez J. Calcified leiomyoma of deep soft tissue: report of a case in childhood. Virchows Arch. 1994;425(2):217–220. doi: 10.1007/BF00230360. [DOI] [PubMed] [Google Scholar]
- 10.Miki Y, Abe S, Tokizaki T, Harasawa A, Imamura T, Matsushita T. Imaging characteristics of calcified leiomyoma of deep soft tissue. J Orthop Sci. 2007;12(6):601–605. doi: 10.1007/s00776-007-1167-5. [DOI] [PubMed] [Google Scholar]
- 11.Mutrie CJ, Donahue DM, Wain JC, Wright CD, Gaissert HA, Grillo HC, et al. Esophageal leiomyoma: a 40-year experience. Ann Thorac Surg. 2005;79(4):1122–1125. doi: 10.1016/j.athoracsur.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Yamato M, Nishimura G, Koguchi Y, Saotome K. Calcified leiomyoma of deep soft tissue in a child. Pediatr Radiol. 1999;29(2):135–137. doi: 10.1007/s002470050557. [DOI] [PubMed] [Google Scholar]
- 13.Nonaka CF, Pereira KM, Miguel MC. Oral vascular leiomyoma with extensive calcification areas. Braz J Otorhinolaryngol. 2010;76(4):539. doi: 10.1590/S1808-86942010000400022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherrick HM, Dunlap CL, King OH., Jr Leiomyomas of the oral cavity: review of the literature and clinicopathologic study of seven new cases. Oral Surg Oral Med Oral Pathol. 1973;35(1):54–66. doi: 10.1016/0030-4220(73)90094-7. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharyya I, Summerlin DJ, Cohen DM, Ellis GL, Bavitz JB, Gillham LL. Granular cell leiomyoma of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(3):353–359. doi: 10.1016/j.tripleo.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Dobashi Y, Iwabuchi K, Nakahata J, Yanagimoto K, Kameya T. Combined clear and granular cell leiomyoma of soft tissue: evidence of transformation to a histiocytic phenotype. Histopathology. 1999;34(6):526–531. doi: 10.1111/j.1365-2559.1999.00680.x. [DOI] [PubMed] [Google Scholar]
- 17.Koutlas IG, Manivel JC. Epithelioid leiomyoma of the oral mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82(6):670–673. doi: 10.1016/S1079-2104(96)80442-2. [DOI] [PubMed] [Google Scholar]
- 18.Mentzel T, Wadden C, Fletcher CD. Granular cell change in smooth muscle tumours of skin and soft tissue. Histopathology. 1994;24(3):223–231. doi: 10.1111/j.1365-2559.1994.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 19.Vincenzi A, Rossi G, Monzani D, Longo L, Rivasi F. Atypical (bizarre) leiomyoma of the nasal cavity with prominent myxoid change. J Clin Pathol. 2002;55(11):872–875. doi: 10.1136/jcp.55.11.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casillas J, Joseph RC, Guerra JJ., Jr CT appearance of uterine leiomyomas. Radiographics. 1990;10(6):999–1007. doi: 10.1148/radiographics.10.6.2259770. [DOI] [PubMed] [Google Scholar]
- 21.Weiss SW. Smooth muscle tumors of soft tissue. Adv Anatomic Pathol. 2002;9(6):351–359. doi: 10.1097/00125480-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Brooks JK, Nikitakis NG, Goodman NJ, Levy BA. Clinicopathologic characterization of oral angioleiomyomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(2):221–227. doi: 10.1067/moe.2002.125276. [DOI] [PubMed] [Google Scholar]
- 23.Lloria-Benet M, Bagan JV, Lloria de Miguel E, Borja-Morant A, Alonso S. Oral leiomyoma: a case report. Medicina oral: organo oficial de la Sociedad Espanola de Medicina Oral y de la Academia Iberoamericana de Patologia y Medicina Bucal 2003;8(3):215–9. [PubMed]
- 24.Manojlovic S, Aljinovic-Ratkovic N, Kruslin B. Calcified leiomyoma of the lateral pterygoid muscle in an 8-year-old boy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(2):199–203. doi: 10.1067/moe.2000.102600. [DOI] [PubMed] [Google Scholar]
- 25.Ghahremani GG, Meyers MA, Port RB. Calcified primary tumors of the gastrointestinal tract. Gastrointest Radiol. 1978;2(4):331–339. doi: 10.1007/BF02256516. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Lacouture M, Petronic-Rosic V, Soltani K, Shea CR. Ossified soft tissue leiomyoma in a patient with sickle cell anemia. J Cutan Pathol. 2005;32(10):696–699. doi: 10.1111/j.0303-6987.2005.00435.x. [DOI] [PubMed] [Google Scholar]
- 27.Gupta AK, Berry M, Mitra DK. Ossified gastric leiomyoma in a child: a case report. Pediatr Radiol. 1995;25(1):48–49. doi: 10.1007/BF02020845. [DOI] [PubMed] [Google Scholar]
- 28.Kilpatrick SE, Mentzel T, Fletcher CD. Leiomyoma of deep soft tissue. Clinicopathologic analysis of a series. Am J Surg Pathol. 1994;18(6):576–582. doi: 10.1097/00000478-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Lubbers PR, Chandra R, Markle BM, Downey EF, Jr, Malawer M. Case report 421: calcified leiomyoma of the soft tissues of the right buttock. Skeletal Radiol. 1987;16(3):252–256. doi: 10.1007/BF00356963. [DOI] [PubMed] [Google Scholar]
- 30.Fanburg-Smith JC, Lasota J, Auerbach A, Foss RD, Laskin WB, Murphey MD. Tumors and tumor-like lesions of the soft tissues. In: Barnes L, editor. Surgical pathology of the head and neck. 3. New York: Informal Healthcare; 2009. [Google Scholar]
- 31.Nonaka CF, Pacheco DF, Nunes RP, Freitas Rde A, Miguel MC. Ossifying fibromyxoid tumor in the mandibular gingiva: case report and review of the literature. J Periodontol. 2009;80(4):687–692. doi: 10.1902/jop.2009.080535. [DOI] [PubMed] [Google Scholar]
- 32.Williams SB, Ellis GL, Meis JM, Heffner DK. Ossifying fibromyxoid tumour (of soft parts) of the head and neck: a clinicopathological and immunohistochemical study of nine cases. J Laryngol Otol. 1993;107(1):75–80. doi: 10.1017/S0022215100122200. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Montiel MD, Plaza JA, Dominguez-Malagon H, Suster S. Differential expression of smooth muscle myosin, smooth muscle actin, h-caldesmon, and calponin in the diagnosis of myofibroblastic and smooth muscle lesions of skin and soft tissue. Am J Dermatopathol. 2006;28(2):105–111. doi: 10.1097/01.dad.0000200009.02939.cc. [DOI] [PubMed] [Google Scholar]
- 34.Wiechens B, Werner JA, Luttges J, Rudert H, Rochels R. Primary orbital leiomyoma and leiomyosarcoma. Ophthalmologica. Journal international d’ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1999;213(3):159–64. doi: 10.1159/000027412. [DOI] [PubMed] [Google Scholar]