Abstract
High risk human papillomavirus (HPV) is firmly established as an important cause of oropharyngeal carcinoma. Recent studies have also implicated HPV as a cause of mucoepidermoid carcinoma (MEC)—a tumor of salivary gland origin that frequently harbors MAML2 translocations. The purpose of this study was to determine the prevalence of transcriptionally active HPV in a large group of MECs and to determine whether HPV infection and the MAML2 translocation are mutually exclusive events. Break-apart fluorescence in situ hybridization for MAML2 was performed on a tissue microarray containing 92 MECs. HPV testing was performed using RNA in situ hybridization targeting high risk HPV mRNA E6/E7 transcripts. Of the 71 MECs that could be evaluated by FISH, 57 (80 %) harbored the MAML2 rearrangement. HPV was not detected in any of the 57 MECs that contained a MAML2 rearrangement, in any of the 14 MECs that did not contain the rearrangement, or in any of the 21 MECs where MAML2 status was unknown. High risk HPV does not appear to play any significant role in the development of MEC. It neither complements nor replaces MAML2 translocation in the tumorigenesis of MEC.
Keywords: Mucoepidermoid carcinoma, Human papillomavirus, MAML2
Introduction
Human papillomavirus (HPV) is now well established as an important cause of head and neck cancer [1–3], but its distribution is highly restricted by anatomic site and tumor type. It is detected in 50–80 % of oropharyngeal cancers where it tracks with the non-keratinizing squamous cell phenotype, but it is not frequently detected in head and neck squamous cell carcinomas arising outside of the oropharynx [4–7]. In oropharyngeal carcinomas, the detection of HPV is of great clinical significance, as HPV positivity is associated with improved clinical outcomes in ways that modulate therapeutic management [8, 9]. In view of its profound clinical relevance as a biomarker for patients with oropharyngeal squamous cell carcinoma, there is considerable interest in identifying high risk HPV in other types of head and neck cancer.
Mucoepidermoid carcinoma (MEC) is the most common type of salivary gland carcinoma. Up to 75 % of MECs harbor chromosomal rearrangements involving MAML2 [10–12]. Some have suggested that the presence of a MAML2 rearrangement identifies a biologically distinct group of MEC with a less aggressive clinical behavior, but other molecular genetic factors that act in concert with or independent of the MAML2 rearrangement are not yet well defined. Recently, high risk HPV has been identified in a significant subset of MECs [13]. We performed RNA in situ hybridization for E6/E7 mRNA transcripts both to confirm the high prevalence of transcriptionally active HPV in MECs and to determine the relationship between HPV infection and MAML2 translocation.
Methods
Cases
This study was approved by Institutional Review Board of The Johns Hopkins Medical Institutions. The surgical pathology files of The Johns Hopkins Hospital were searched for cases of MEC diagnosed from 1984 to 2012. Hematoxylin and eosin-stained sections were reviewed to confirm the diagnosis, and the tumors were graded using the grading scheme advocated by the World Health Organization [14].
Tissue Microarray
A tissue microarray (TMA) was constructed from the formalin-fixed paraffin-embedded (FFPE) tissue blocks of 92 MECs. The MECs consisted of 45 low grade, 29 intermediate grade, and 18 high grade carcinomas. The primary sites were the parotid gland (n = 43), oral cavity (n = 39), submandibular gland (n = 6) and sinonasal tract (n = 4). Three cores, each 1 mm in diameter, were taken from each donor block to address tumor heterogeneity.
MAML2 Fluorescence In Situ Hybridization
Fluorescence in situ hybridization (FISH) was performed on FFPE section using a commercially available MAML2 dual color break apart probe (Z-2014-200, Zytovision, Germany). Prior to hybridization, the slides were deparaffinized using a VP 2000 processor (Abbott Molecular, Des Plains, IL, USA) in which pretreatment with protease I was used. Following deparaffinization, the slides and the MAML2 probe were co-denatured at 80 °C for 7 min and allowed to hybridize for 22 h at 37 °C in humidified atmosphere. At the end of the incubation, the slides were washed in 2 × SSC/0.3 % NP-40 for 2 min at 72 °C and for 2 min at room temperature, with agitation. Traces of detergent were removed by washing the slides in 2 × SSC at room temperature. The slides were counterstained with DAPI, and a cover slip was applied using Vectashield mounting medium (H-1000, Vector Laboratories, Inc.). A fluorescence microscope was used to evaluate the probe pattern. Cells with two fusion signals of one orange and one green fluorochrome were scored as normal. Cells with rearrangements for MAML2 gene had one normal fusion signal and one orange and one green signal at a distance from each other. A mucoepidermoid carcinoma known to harbor the MAML2 rearrangement served as a positive control, while normal salivary tissue served as a negative control.
HPV RNA In Situ Hybridization
HPV status was determined using an RNA in situ hybridization approach. In this study, p16 immunohistochemical staining was not used because p16 staining has been found to be a very poor surrogate marker for the presence of high risk HPV when dealing with salivary gland tumors [15, 16]. In situ hybridization for HPV E6/E7 mRNA was performed manually using the RNAscope kit (Advanced Cell Diagnostics Inc., Hayward, CA, USA). The HPV HR (18) probe containing a cocktail of 18 high-risk HPV genotypes (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) was used for HPV detection. A probe to the housekeeping gene PPiB (Peptidyl-prolyl cis–trans isomerase B) was used as a positive control to ensure presence of an intact RNA target in the specimen, and a known HPV-related squamous cell carcinoma included on the TMA to serve as a positive control (Fig. 1). A probe to the bacterial gene dapB was used as a negative control. The staining protocol was carried out according to the manufacturer’s instructions. The incubations/hybridization steps were performed in HybEZ™ Oven, which provides a gasket-sealed, temperature-controlled humidifying chamber (Advanced Cell Diagnostics Inc., Hayward, CA). Briefly, 4-mm-thick FFPE tissue sections were pretreated with heat (slow-boiling in P2 solution for 10 min) and protease (P3, 1:5 dilution, at 40 °C in the humid chamber) before probe hybridization. The preamplifier, amplifier, and horseradish peroxidase-labeled probes were then hybridized sequentially, followed by color development with diaminobenzidine. Specific staining signals were identified as brown, punctate dots present in the cytoplasm and/or nucleus.
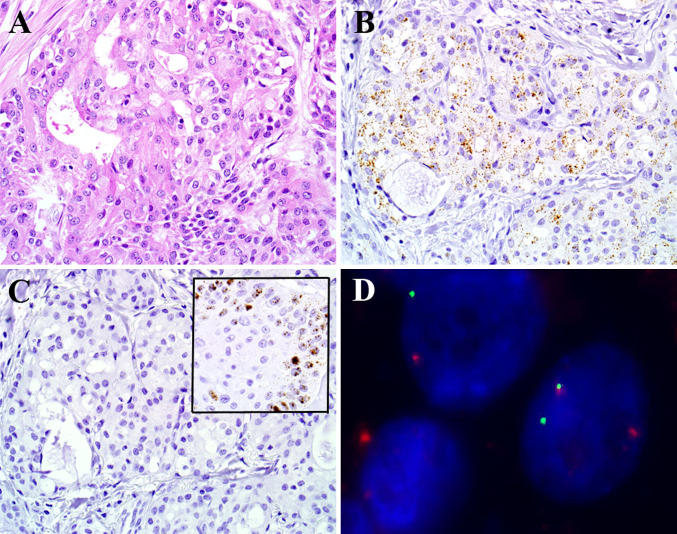
Fig. 1.
By RNA in situ hybridization, this example of mucoepidermoid carcinoma (a, hematoxylin and eosin stain) was positive for transcripts of the housekeeping gene peptidyl-prolyl isomerase B (b, RNA in situ hybridization) but completely negative for the E6 and E7 viral transcripts of high risk HPV (c, RNA in situ hybridization; inset showing positive hybridization signals in an HPV-positive oropharyngeal carcinoma serving as a positive control). Break apart fluorescent in situ hybridization for MAML2 was positive (separate green and red signals) (d, fluorescent in situ hybridization)
Results
The status of the MECs for the presence of the MAML2 rearrangement and high risk HPV infection as a function of tumor grade and tumor site are summarized in Table 1. Break-apart FISH for the MAML2 rearrangement was successfully performed on 71 of 92 (77 %) MECs. 57 of 71 (80 %) were positive for the rearrangement including 37 of 42 (88 %) low grade, 17 of 20 (85 %) intermediate grade, and 3 of 9 (33 %) high grade MECs. Low and intermediate grade MECs were statistically more likely to harbor the MAML2 rearrangement than high grade MECs (p = 0.001, Fisher’s exact test, 2-sided). By anatomic site, the MAML2 rearrangement was detected in 31 of 39 (79 %) parotid tumors, 22 of 26 (85 %) oral cavity tumors, 3 of 4 (75 %) submandibular tumors, and 1 of 2 (50 %) sinonasal tumors. High risk HPV was not detected in any of the 92 MECs by RNA in situ hybridization (Fig. 1).
Table 1.
MAML2 translocation status and HPV status in mucoepidermoid carcinomas as a function of tumor grade and site
| Mucoepidermoid carcinoma | MAML2 translocation (n = 71) | High risk HPV (n = 92) | ||
|---|---|---|---|---|
| Present (%) | Absent (%) | Positive (%) | Negative (%) | |
| Grade | ||||
| Low | 37 (88) | 5 (12) | 0 (0) | 45 (100) |
| Intermediate | 17 (77) | 3 (23) | 0 (0) | 29 (100) |
| High | 3 (33) | 6 (67) | 0 (0) | 18 (100) |
| Site | ||||
| Parotid gland | 31 (79) | 8 (21) | 0 (0) | 43 (100) |
| Oral cavity | 22 (85) | 4 (15) | 0 (0) | 39 (100) |
| Submandibular gland | 3 (75) | 1 (25) | 0 (0) | 6 (100) |
| Sinonasal tract | 1 (50) | 1 (50) | 0 (0) | 4 (100) |
| Total | 57 (80) | 14 (20) | 0 (0) | 92 (100) |
Discussion
In the oropharynx, the recognition of an HPV-related form of oropharyngeal cancer has opened the door to a new understanding of tumorigenesis that is translating into effective prevention measures (e.g. preventive vaccines) and novel therapeutic strategies (e.g. therapeutic vaccines and de-intensification therapy). Recent studies have suggested that, like the oropharynx, the salivary glands may also be targeted by oncogenic HPV [13, 16–18]. These HPV-related forms of salivary gland cancer warrant considerable attention as they could provide new insights into a type of head and neck cancer in which little is known about the biologic mechanisms underlying tumorigenesis.
In light of potential clinical repercussions, claims regarding the identification of a new form of HPV-related head and neck cancer warrant careful corroboration. For example, the existence of a newly identified HPV-related adenoid cystic carcinoma either has been unconfirmed in subsequent studies or has been redefined as non-salivary gland carcinoma [5, 16, 19, 20]. Recently, Isayeva et al. [13]. reported that about half of all MECs are infected with transcriptionally active high risk HPV, an incidence that rivals its occurrence in the oropharynx.
In an effort to substantiate this finding, Jour et al. [15] were not able to detect HPV in any of their MECs, but their number of cases was limited (n = 14) and HPV detection was restricted to a DNA in situ hybridization approach. Our study was designed to help resolve the uncertainty surrounding the potential role of HPV in MECs. First, we analyzed a large group of MECs across all histologic grades. With MECs in particular, the genetic profile may modulate tumor grade in ways where certain alterations may elude detection if case selection is too restricted. As one example, the MAML2 rearrangement is known to be much more common in low grade than high grade MECs [12, 21, 22]. Second, our analysis included documentation of MAML2 status. As MAML2 translocation resulting in NOTCH pathway activation is believed to drive oncogenic transformation [23], its presence could preclude HPV-induced oncogenesis in much the same way that p53 inactivation and HPV infection are inversely proportional in head and neck squamous cell carcinomas [24–26]. Awareness of MAML2 status could potentially uncover bias based on overrepresentation of translocation positive tumors. Third, this study takes advantage of recently developed RNA in situ hybridization probes complementary to E6/E7 mRNA that permit direct visualization of viral transcripts in routinely processed tissues. In formalin-fixed and paraffin-embedded oropharyngeal carcinomas, the sensitivity of this method has been shown to exceed that of HPV DNA in situ hybridization [6, 27–29].
Using the RNA in situ hybridization approach, we found that transcriptionally active HPV is not commonly encountered in MECs. Indeed, E6/E7 mRNA viral transcripts were not detected in any of the 92 MECs evaluated including those 14 tumors that were known not to harbor a driver translocation involving MAML2. The absence of high risk HPV using an RNA in situ hybridization approach concurs with its reported absence in those studies using a DNA in situ hybridization approach [15, 19]. Outlier studies may reflect, in part, differences in the ability to distinguish between biologically relevant and irrelevant HPV based on methodologies and test interpretation. For example, Isayeva et al. [13]. used highly sensitive PCR-based methods to detect HPV E6/E7 mRNA transcripts in 43 % of MECs, but the presence of these transcripts did not correlate with overexpression of P16INK4a—a marker that is now widely used to confirm both the presence and biologic activity of HPV in oropharyngeal carcinomas [29]. HPV DNA in situ hybridization was also used by the Isayeva group to confirm the presence of integrated virus, but the interpretation of cytoplasmic hybridization signals as evidence of transcriptionally active HPV represents a deviation from the standard practice where only nuclear signals are regarded as positive (http://www.uclad.com/newsletters/HPV_ISH_Tissue-Probe-Interpretation_Guide.pdf).
Based on our findings, oncogenic forms of HPV do not appear to be a relevant cause of MEC, either as a primary agent or as a substitutionary agent in those MECs lacking a driver MAML2 translocation. This observation may appropriately temper recent enthusiasm for exploiting HPV detection as a relevant biomarker when dealing with MECs. Indeed, the finding that the MAML2 translocation correlates with tumor grade supports the view that MAML2 status, not HPV status, may prove to be a much more promising prognostic marker for patients with MEC [12, 21, 22].
Acknowledgments
This study was funded in part by the National Institutes of Health/National Institute of Dental and Craniofacial Research (NIH/NIDCR) Head and Neck SPORE Grant P50 DE019032.
References
- 1.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 2.D’Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, Koch WM, et al. Case–control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 3.Adelstein DJ, Ridge JA, Gillison ML, Chaturvedi AK, D’Souza G, Gravitt PE, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9–10, 2008, Washington, DC. Head Neck. 2009;31:1393–1422. doi: 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- 4.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 5.Bishop JA, Guo TW, Smith DF, Wang H, Ogawa T, Pai SI, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37:185–192. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop JA, Ma XJ, Wang H, Luo Y, Illei PB, Begum S, et al. Detection of transcriptionally active high-risk HPV in patients with head and neck squamous cell carcinoma as visualized by a novel E6/E7 mRNA in situ hybridization method. Am J Surg Pathol. 2012;36:1874–1882. doi: 10.1097/PAS.0b013e318265fb2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis JS, Jr, Ukpo OC, Ma XJ, Flanagan JJ, Luo Y, Thorstad WL, et al. Transcriptionally-active high-risk human papillomavirus is rare in oral cavity and laryngeal/hypopharyngeal squamous cell carcinomas—a tissue microarray study utilizing E6/E7 mRNA in situ hybridization. Histopathology. 2012;60:982–991. doi: 10.1111/j.1365-2559.2011.04169.x. [DOI] [PubMed] [Google Scholar]
- 8.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, et al. Molecular classification identifies a subset of human papillomavirus—associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 10.Behboudi A, Enlund F, Winnes M, Andren Y, Nordkvist A, Leivo I, et al. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1–MAML2 fusion oncogene. Genes Chromosomes Cancer. 2006;45:470–481. doi: 10.1002/gcc.20306. [DOI] [PubMed] [Google Scholar]
- 11.Chiosea SI, Dacic S, Nikiforova MN, Seethala RR. Prospective testing of mucoepidermoid carcinoma for the MAML2 translocation: clinical implications. Laryngoscope. 2012;122:1690–1694. doi: 10.1002/lary.22419. [DOI] [PubMed] [Google Scholar]
- 12.Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34:1106–1121. doi: 10.1097/PAS.0b013e3181de3021. [DOI] [PubMed] [Google Scholar]
- 13.Isayeva T, Said-Al-Naief N, Ren Z, Li R, Gnepp D, Brandwein-Gensler M. Salivary mucoepidermoid carcinoma: demonstration of transcriptionally active human papillomavirus 16/18. Head Neck Pathol. 2013;7:135–148. doi: 10.1007/s12105-012-0411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goode RK, El-Naggar AK. Mucoepidermoid carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidranksy D, editors. World health organization classification of tumours. Pathology and genetics of head and neck tumors. Lyon: IARC Press; 2005. pp. 219–220. [Google Scholar]
- 15.Jour G, West K, Ghali V, Shank D, Ephrem G, Wenig BM. Differential expression of p16(INK4A) and cyclin D1 in benign and malignant salivary gland tumors: a study of 44 Cases. Head Neck Pathol. 2013;7:224–231. doi: 10.1007/s12105-012-0417-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boland JM, McPhail ED, Garcia JJ, Lewis JE, Schembri-Wismayer DJ. Detection of human papilloma virus and p16 expression in high-grade adenoid cystic carcinoma of the head and neck. Mod Pathol. 2012;25:529–536. doi: 10.1038/modpathol.2011.186. [DOI] [PubMed] [Google Scholar]
- 17.Hafed L, Farag H, Shaker O, El-Rouby D. Is human papilloma virus associated with salivary gland neoplasms? An in situ-hybridization study. Arch Oral Biol. 2012;57:1194–1199. doi: 10.1016/j.archoralbio.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Vageli D, Sourvinos G, Ioannou M, Koukoulis GK, Spandidos DA. High-risk human papillomavirus (HPV) in parotid lesions. Int J Biol Markers. 2007;22:239–244. doi: 10.1177/172460080702200401. [DOI] [PubMed] [Google Scholar]
- 19.Bishop JA, Ogawa T, Stelow EB, Moskaluk CA, Koch WM, Pai SI, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol. 2013;37:836–844. doi: 10.1097/PAS.0b013e31827b1cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson LD, Penner C, Ho NJ, Foss RD, Miettinen M, Wieneke JA et al. Sinonasal tract and nasopharyngeal adenoid cystic carcinoma: a clinicopathologic and immunophenotypic study of 86 cases. Head Neck Pathol. 2013 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- 21.Okabe M, Miyabe S, Nagatsuka H, Terada A, Hanai N, Yokoi M, et al. MECT1–MAML2 fusion transcript defines a favorable subset of mucoepidermoid carcinoma. Clin Cancer Res. 2006;12:3902–3907. doi: 10.1158/1078-0432.CCR-05-2376. [DOI] [PubMed] [Google Scholar]
- 22.Miyabe S, Okabe M, Nagatsuka H, Hasegawa Y, Inagaki A, Ijichi K, et al. Prognostic significance of p27Kip1, Ki-67, and CRTC1-MAML2 fusion transcript in mucoepidermoid carcinoma: a molecular and clinicopathologic study of 101 cases. J Oral Maxillofac Surg. 2009;67:1432–1441. doi: 10.1016/j.joms.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Komiya T, Park Y, Modi S, Coxon AB, Oh H, Kaye FJ. Sustained expression of Mect1-Maml2 is essential for tumor cell growth in salivary gland cancers carrying the t(11;19) translocation. Oncogene. 2006;25:6128–6132. doi: 10.1038/sj.onc.1209627. [DOI] [PubMed] [Google Scholar]
- 24.Snijders PJ, Steenbergen RD, Top B, Scott SD, Meijer CJ, Walboomers JM. Analysis of p53 status in tonsillar carcinomas associated with human papillomavirus. J Gen Virol. 1994;75:2769–2775. doi: 10.1099/0022-1317-75-10-2769. [DOI] [PubMed] [Google Scholar]
- 25.Scholes AG, Liloglou T, Snijders PJ, Hart CA, Jones AS, Woolgar JA, et al. p53 mutations in relation to human papillomavirus type 16 infection in squamous cell carcinomas of the head and neck. Int J Cancer. 1997;71:796–799. doi: 10.1002/(SICI)1097-0215(19970529)71:5<796::AID-IJC17>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Westra WH, Taube JM, Poeta ML, Begum S, Sidransky D, Koch WM. Inverse relationship between human papillomavirus-16 infection and disruptive p53 gene mutations in squamous cell carcinoma of the head and neck. Clin Cancer Res. 2008;14:366–369. doi: 10.1158/1078-0432.CCR-07-1402. [DOI] [PubMed] [Google Scholar]
- 27.Schache AG, Liloglou T, Risk JM, Jones TM, Ma XJ, Wang H, et al. Validation of a novel diagnostic standard in HPV-positive oropharyngeal squamous cell carcinoma. Br J Cancer. 2013;108:1332–1339. doi: 10.1038/bjc.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ukpo OC, Flanagan JJ, Ma XJ, Luo Y, Thorstad WL, Lewis JS., Jr High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–1350. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]