Abstract
Intraoral basal cell carcinoma (IOBCC) is an extremely rare entity that bears close microscopic resemblance to and is often confused with the peripheral ameloblastoma (PA). Basal cell carcinomas are thought to arise from pluripotential basal cells present within surface epithelium and adnexal structures, so theoretically they can arise within the oral cavity. Many of the early cases reported as IOBCC actually represent PA. Most of the well documented cases arise from the gingiva. The histologic features of basal cell carcinoma that help separate it from a PA include: tumor arising from surface epithelium, scattered mitotic figures and apoptotic cells, presence of mucoid ground substance and tumor infiltrating widely throughout the connective tissue and often exhibiting a prominent retraction artifact. Clinically IOBCC resemble carcinomas, compared to the benign and innocuous appearance of the PA and typically presents as surface ulcerations varying from rodent ulcer to an ulcerated erythroplakia appearance. This contrasts with the classic “bump on the gum” appearance of PAs with usually intact surface and appearing as small discrete, sessile, exophytic lesions. Importantly, the proliferative basaloid epithelium demonstrates positive immunoreactivity for the anti-epithelial antibody, Ber-EP4, a cell surface glycoprotein. The IOBCC has the potential for local recurrence and aggressive behavior and should be treated with wide surgical excision and close clinical follow up. We present 3 rare cases of IOBCC and discuss the salient histologic, immunohistochemical and clinical features.
Keywords: Intraoral basal cell carcinoma, Peripheral ameloblastoma, Ber-EP4, EMA, Calretinin
Introduction
Basal cell carcinoma (BCC) is considered to be the most common malignancy in humans and occurs primarily on the skin especially in the head and neck and accounts for approximately 75–80 % of all malignant diseases of skin [1]. Due to their high incidence rate, epidemiological studies to ascertain exact numbers are sparse [1]. The most recent estimate of non-melanoma skin cancers in the US for 2013 is 3.5 million, with the great majority of these being BCC [2]. The incidence of skin cancer is exponentially increasing worldwide, with some estimates reporting up to a 10 % annual increase in incidence [3].
Intraoral basal cell carcinoma (IOBCC) is a controversial entity due to its rarity and histologic similarity to the peripheral ameloblastoma (PA). We describe three rare cases of IOBCC and discuss the salient histologic and clinical characteristics with an immunohistochemical staining profile. We review the reported cases of IOBCC in literature and differentiate this unusual entity from the peripheral ameloblastoma.
Case Report #1
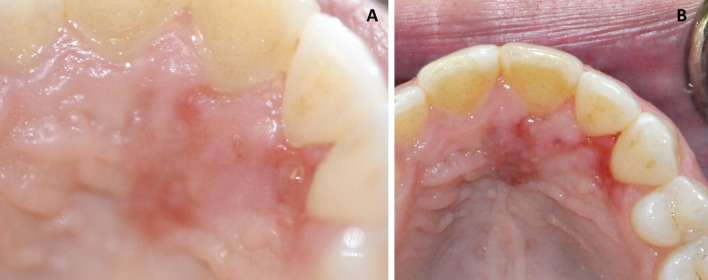
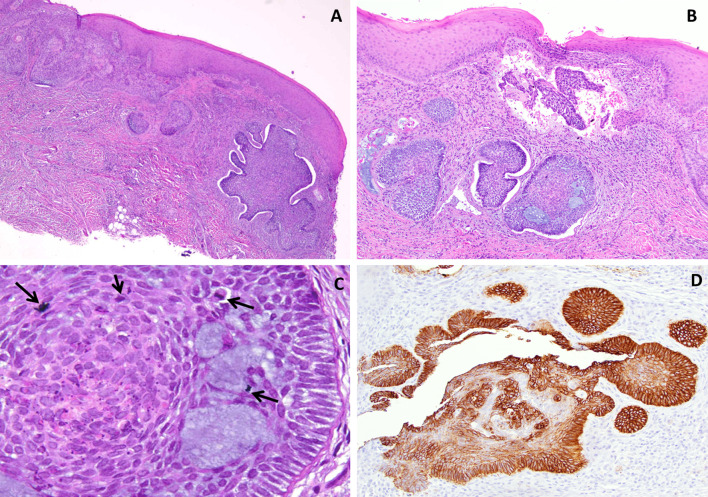
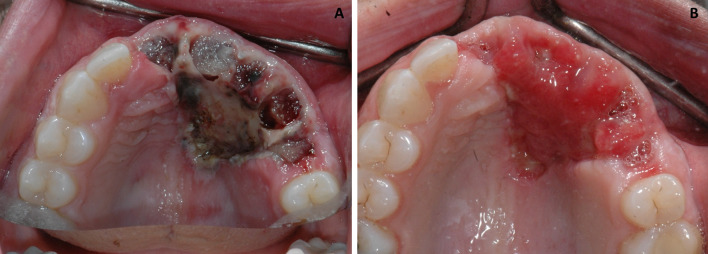
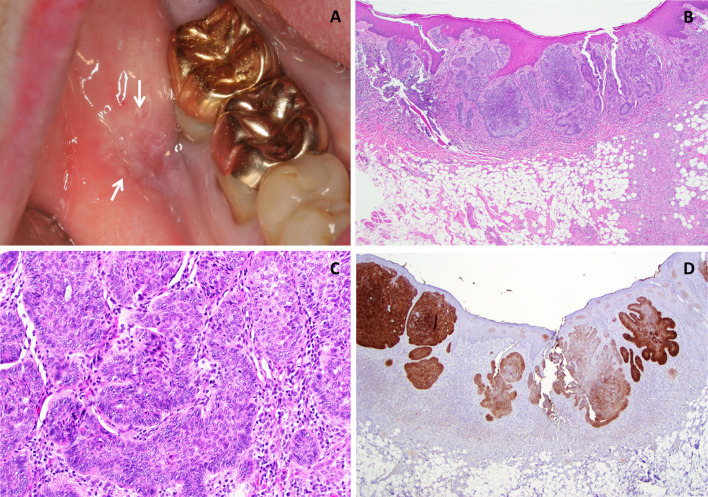
A 39-year-old female presented to an oral and maxillofacial surgeon with a non-healing, painful erythematous growth on the anterior hard palatal mucosa, present for approximately 1 year. Clinical examination revealed an irregularly shaped, red to purple swelling extending from the mesiolingual papilla of tooth #4 to the lingual margin of tooth #9, measuring approximately 2.2 × 1.7 × 0. 2 cm (Fig. 1a). The interproximal papilla between teeth #6 and #7 revealed a slight redness on the buccal aspect. An excisional biopsy was performed and diagnosed as a peripheral ameloblastoma. This diagnosis was rendered by another pathology service, and information on margins was not available in their report. The patient returned 3 weeks later with progressive erythema and swelling in the area of biopsy. The lesional area appeared to have enlarged and involved the papillary and palatal gingiva of several contiguous teeth (Fig. 1b). At this time, an incisional biopsy was performed revealing multiple sections of a malignant neoplasm arising from the basal cell layer of surface epithelium invading into dense, desmoplastic appearing fibrous connective tissue (Fig. 2a). The surface epithelium adjacent to the ulcerated and eroded zones did not demonstrate any dysplastic or atypical features. Islands of basaloid cells were seen budding off from the basal cell layer of the epithelium from the rete ridges over almost the entire length of the specimen. These islands contained numerous apoptotic cells, mitotic figures, and abundant mucoid stroma (Fig. 2b). The peripheral cells of the islands displayed a palisaded arrangement with prominent retraction from the surrounding stroma (Fig. 2c). The tumor was multifocal with areas exhibiting squamous differentiation with formation of occasional small keratin pearls. Immunohistochemical (IHC) stains (Table 1) for epithelial membrane antigen (EMA) and calretinin (to rule out a PA) were negative. Importantly, an IHC stain for Ber-EP4 was positive for the lesional cells in the invading tumor islands (Fig. 2d). Based on the histologic features and IHC profile, a diagnosis of IOBCC was rendered. The lesion was excised with wide assured margins, and involved teeth numbers 5 through 9 were removed (Fig. 3a). The mucosa was essentially stripped completely in the area using a carbon dioxide laser. Microscopic examination of the final excision revealed uninvolved margins and neoplastic proliferation very similar to those noted in the incisional biopsy. At 3 weeks’ follow up, the patient was healing well but still in significant pain (Fig. 3b). Five months later, an erythematous, linear area over the anterior palatal tissue was noted, and this lesion failed to resolve with antifungal therapy. An incisional biopsy to rule out recurrent IOBCC revealed non-specific inflammation, with no evidence of malignancy. The patient continues to be monitored for the last 3.5 years with no recurrence but does report atypical neuralgia secondary to the surgery.
Fig. 1.
Case 1. a Clinical presentation at initial visit demonstrating areas of swelling and erythroplakia of anterior palatal mucosa. b Three weeks post-biopsy, recurrent and progressive swelling with erythema, deepening in color was noted
Fig. 2.
Case 1. a Photomicrograph from the excisional biopsy sample demonstrating a basaloid epithelial proliferation arising from the basal surface of the epithelium and invading into the underlying connective tissue (H & E ×4 magnification). b The peripheral cells of the invasive islands displaying a palisaded arrangement with prominent retraction from the surrounding stroma. Small pools of mucoid material are noted as well. (H & E ×10 magnification). c Apoptotic cells and abnormal and increased mitoses were observed scattered amidst pools of basophilic mucoid material (arrows) (H & E x 40 magnification). d Immunohistochemical stain for Ber EP4 exhibiting strong positivity in tumor cells (H & E ×20 magnification)
Table 1.
Immunohistochemical reactivity
| Ber-EP4 (Dako) |
EMA (Cellmarque) |
Calretinin (Invitrogen) | |
|---|---|---|---|
| Case 1 | Positive | Negative | Negative |
| Case 2 | Positive | Negative | Negative |
| Case 3 | Positive | Negative | Negative |
Fig. 3.
Case 1. a Post surgical view demonstrating wide local excision of lesion with removal of multiple teeth. b Healing surgical site 3 weeks later
Case Report #2
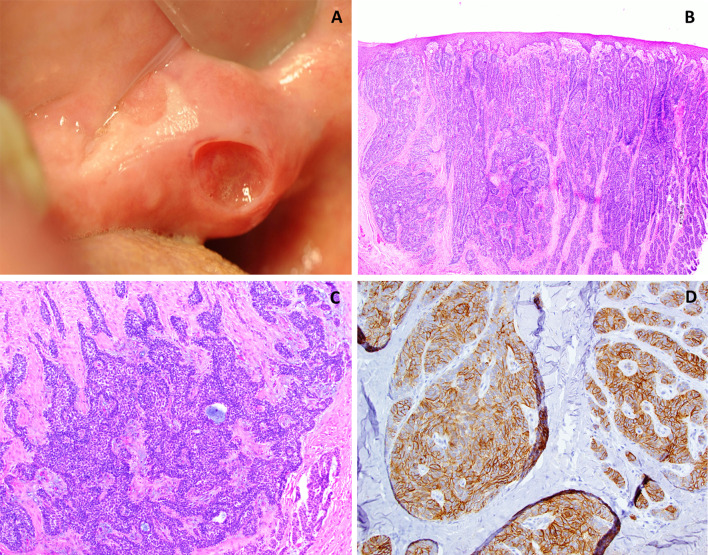
A 75 years old male presented to an oral and maxillofacial surgeon with an asymptomatic, indurated, slightly ulcerated swelling of 3 months’ duration in the right retromolar pad, measuring approximately 1.0 × 0.6 × 0.4 cm. An incisional biopsy was performed and submitted for histopathologic examination. This incisional biopsy was diagnosed as a peripheral ameloblastoma. At the time of excisional biopsy (6 weeks later), the lesion had enlarged significantly, had remained ulcerated and the previous biopsy site had failed to heal (Fig. 4a). The excisional biopsy revealed keratinized surface epithelium giving rise to a basaloid neoplasm exhibiting arborization and infiltrative rete ridges (Fig. 4b). Multiple tumor islands consisting of hyperchromatic basophilic neoplastic cells were noted within the superficial lamina propria. The tumor islands exhibited a prominent palisading of peripheral basal cells, widespread extension into the underlying desmoplastic stroma, scattered mitotic figures, and mucoid ground substance (Fig. 4c). IHC staining was performed (Table 1) with tumor islands exhibiting strong reactivity with Ber-EP4 and negative labeling for calretinin and EMA (Fig. 4d).
Fig. 4.
Case 2. a Clinical photograph demonstrating a raised mass (non-healing biopsy site clearly visible) on the right retromolar pad. b Photomicrograph depicting an invading, infiltrative neoplasm composed of strands and clusters of basaloid appearing epithelial cells arising from the keratinized surface epithelium (H & E ×10 magnification). c Higher magnification demonstrates basophilic tumor islands distributed throughout the connective tissue exhibiting prominent peripheral palisading, and mucoid material (H & E ×20 magnification). d Immunohistochemical stain for Ber EP4 exhibiting positivity within most of the tumor islands (H & E ×20 magnification)
A diagnosis of intraoral basal cell carcinoma was rendered. No recurrence was noted at the 1 year follow up.
Case Report #3
A 74-year-old female presented to an oral and maxillofacial surgeon with a painless 1.0 × 1.0 × 0.5 cm erythroleukoplakic area on the right buccal mucosa of approximately 6–12 months duration (Fig. 5a). A past incisional biopsy in this area had been interpreted as “ameloblastoma versus basal cell carcinoma”. An excisional biopsy was then performed. Histologic examination revealed multi-focal proliferations of basaloid epithelial cells that invaded the underlying fibrous connective tissue which displayed prominent desmoplasia (Fig. 5b). The proliferative islands of neoplastic epithelium were contiguous with the basal layer of the surface epithelium. However, no atypia nor dysplasia of the surface epithelium was noted. The invasive nests demonstrated prominent, palisading layers of columnar cells with a basaloid appearance and displayed irregularly arranged, polygonal cells with relatively large, oval nuclei with vesicular chromatin (Fig. 5c). Frequent mitotic figures, apoptotic cells, foci of squamous differentiation with some tumor nests forming small, pseudoductal structures containing a pale, mucoid product were observed. Margins were clear of tumor islands. Positive IHC labeling (Table 1) with Ber-EP4 was noted in the tumor islands (Fig. 5d), which were negative for calretinin and EMA. A final diagnosis of mucosal basal cell carcinoma was rendered. Five months after the excisional biopsy, the patient remains free of any recurrent lesion.
Fig. 5.
Case 3. a A painless non-healing somewhat depressed ulceration present for 6–12 month duration in the buccal mucosa adjacent to teeth #30–#31. b Photomicrograph depicting an invasive basaloid epithelial cell proliferation arising from keratinized surface epithelium. mucosa (H & E ×4 magnification). c Palisading of the peripheral cells as well as occasional apoptotic cells are noted within the invasive islands which are surrounded by chronic inflammatory cells. Retraction from the adjacent stroma is noted (H & E ×20 magnification). d Immunohistochemical stain for Ber EP4 exhibiting strong positivity in tumor cells (H & E ×20 magnification)
Discussion
Basal cell carcinoma (BCC) of the oral cavity has been controversial entity for over half a century, dating back to the first published case of Saint in 1951 when he described basaloid lesions in the mid-dorsum of the tongue and soft palate in two patients [4]. However, many of the reports of IOBCC in literature have not been confirmed utilizing immunohistochemistry (Table 2). In 2001, Del Rosario et al. [5] reported the first well documented case of IOBCC on the buccal mucosa and confirmed their diagnosis utilizing Ber-EP4 antibody which produced positive labeling of the neoplastic basal cells, supporting an origin from the basal cell layer of the stratified squamous mucosa. Shumway et al. [6] also reported an additional case of IOBCC and reviewed past reports of IOBCC. They stressed the importance of accurate histologic diagnosis with positive Ber-EP4 labeling in confirming a diagnosis of IOBCC [6]. Ber-EP4 is a monoclonal antibody against cell surface glycoproteins (34 and 39 kDa) of most epithelial cells that reacts with epithelial tumors, with the exception of superficial layers of squamous epithelia [7]. Therefore, it reliably discriminates BCC from squamous cell carcinoma (SCC) and mesothelioma from adenocarcinoma [8, 9]. A recent study by Winters et al. [10] reported Ber-EP4 positivity in 82 % of basaloid SCC; however, BCC can be distinguished histologically from basaloid SCC. Ber-EP4 has been proven to be negative in peripheral ameloblastomas [11, 12]. Additionally, epithelial membrane antigen (EMA) a mucin-like glycoprotein often used as a pan-epithelial marker has been utilized to differentiate BCC from SCC, especially since some variants of SCC such as basaloid SCC may resemble BCC [13, 14].
Table 2.
Reported cases of intraoral basal cell carcinoma
| Reference | Age | Sex Race |
Site | Clinical Presentation |
Immunohistochemical Staining | Comments | Our interpretation |
|---|---|---|---|---|---|---|---|
| Saint [4] | n/a | n/a | Mid-dorsum of tongue and soft palate | Diffuse, flat, non-ulcerated growth with deep invasion | None | Called a “basal-celled carcinoma” | May represent a basaloid SCC |
| Thoma [30] | 62 | M, C | R anterior mandible | Soft swelling, involving #27–29 | None | History of epidermoid carcinoma in floor of mouth | May represent a basaloid SCCA |
| Keen [32] | 66 | M, C | Inner mucosal surface of lower lip | Firm, white, indurated lesion, involving buccal gingiva of #23–26 | None | Patient had prior history of BCCA (cheek, neck, upper lip) | Cutaneous BCC |
| Lawson et al. [24] | 60 | M, C | Maxillary L buccal gingiva, around #10 & 11 | Red, pebbly, rough, ovoid lesion with irregular borders | None | Recurrence in 6 mos.; Previous BCCA lesion of nose removed 6 years Prior | Inadequate documentation |
| Williamson et al. [31] | 74 | M, C | Mandibular gingiva | Slightly raised, ovoid, sessile swelling of mandibular buccal gingiva of #22–25 | None | Previous history of BCCA (eyelids, temple) | Ameloblastic Carcinoma |
| Mori et al. [25] |
41 66 |
F M |
L mandibular ridge L mandibular gingiva |
Large swelling in molar region Painless swelling in #17 area for 15 years duration, now involving posterior alveolar bone of maxilla |
None | None available | Inadequate documentation |
| Liroff [27] | 47 | M, C | L maxilla, lingual and posterior to #15 | Red, round ulceration with raised, rolled borders | None | Previous history of BCCA of eyelid and psoriasis | May represent a PA |
| Peters, et al. [26] |
72 55 |
F F, C |
R posterior maxilla L posterior maxilla |
Red, tender ulceration large red ulcer of attached gingiva involving teeth #14 & 15 | None | No recurrence recurrence 5 years later | Inadequate documentation |
| Simpson, et al. [29] | 28 | M, C | L mandibular ridge | Nodular mass, distal to #18 | None | May represent a PA | |
| Samit [28] | 44 | M, C | L anterior tonsillar pillar, extending into soft palate and retromolar pad | Ulceration and soreness | None | Recurrence 7.5 years later, as a painful ulcerated, nodular buccal mass; red, cratered depression in lingual cortex of body and ramus | May represent a basaloid SCC |
| Edmonson et al. [34] | 49 | M | L retromolar pad | Ulcer under denture | None | Recurrence 3 years later | Ameloblastic Carcinoma or IOBCC |
| Blinder et al. [33] | 78 | F | R mandibular ridge | Diffuse ulceration | None | Recurrence 2 years later; history of BCCA on shoulder | Metastatic lesion in patient with known cutaneous BCC |
| Del Rosario et al. [5] | 69 | M | R buccal mucosa | Ulcerated plaque | Ber-EP4+ | Previous “cancer” in site 10 years prior | IOBCC |
| Koutlas et al. [22] | 67 | F | L buccal gingiva distal to #18- premolar region; oropharynx | Unknown | Ber-EP4 + calretinin, vimentin, actin, desmin, S100 (only few cells positive) | Recurrence 8 times in 20 years | IOBCC |
| Shumway et al. [6] | 73 | F | L anterior buccal mucosa | Non-tender mass | Ber-EP4 and p63 positive, calretinin (weak) cytokeratin focal & mildly positive | IOBCC | |
| Our cases |
39 75 74 |
F M F |
Hard palatal mucosa R Retromolar pad R posterior buccal mucosa |
Erythematous growth Ulcerated swelling Erythroleukoplakia |
Ber-EP4 positive, EMA and calretinin negative for all 3 cases | Case 1 had post-surgical neuralgia |
IOBCC IOBCC IOBCC |
C caucasian, M male, F female, R right, L left, PA peripheral ameloblastoma, BCCA basal cell carcinoma, EMA epithelial membrane antigen, n/a not available
The histopathologic features of IOBCC are similar to those of cutaneous BCC [2, 15, 16]. IOBCC most closely resembles the peripheral ameloblastoma (PA) microscopically and is often misdiagnosed as PA, as noted in all three cases we present here. We contrast and compare the histologic features of IOBCC and PA in (Table 3). In 1911, Kuru reported an intraosseous ameloblastoma which had penetrated through the alveolar bone as a PA [17]. This cannot be considered a true PA since this case simply represented an extra-osseous presentation of an intra-osseous lesion. In 1959, Stanley and Krogh published the first well documented report of PA with detailed clinical and pathological characteristics [18]. PAs are uncommon odontogenic tumors, accounting for approximately 1 % of all ameloblastomas. It has an innocuous clinical behavior, presenting as a slow-growing, firm, pink, painless mass with a sessile or pedunculated base with a smooth surface [19]. These are thought to arise from extraosseous rests of dental lamina (rests of Serres) beneath the oral mucosa or from surface epithelium having potential to differentiate into odontogenic epithelium, and usually occur in the mandible, particularly in the lingual premolar region or posterior gingival and alveolar mucosa [19]. Histopathologically, islands of ameloblastic epithelium with peripheral palisading, reverse polarity, and apical vacuolization of the nuclei are noted beneath the surface epithelium, with approximately 50 % of cases demonstrating connection of the tumor with the basal layer of the surface [11]. Immunohistochemically, ameloblastoma and PA stain with calretinin, also called calbindin-2 which is a 29-kDa calcium-binding protein [12]. It specifically labels the stellate reticulum cells of ameloblastoma and is considered an important IHC aid for detecting neoplastic ameloblastic epithelium. In addition, ameloblastomas react with anti-cytokeratin antibodies but not the anti-epithelial antibody Ber-EP4 [12, 20]. In contrast, tumor cells of BCC are positive for cytokeratin and Ber-EP4, while negative for EMA [15]. Vigneswaran et al. [21] reported positive peanut agglutinin staining in both PA and cutaneous BCC, speculating that PA and BCC may have same tissue of origin. However, other reports could not support this finding [22, 23]. Importantly, IOBCC is an invasive malignant neoplasm with potential of recurrence whereas PA are benign lesions with minimal destructive potential and low recurrence, therefore similarities between the two are difficult to establish.
Table 3.
Comparison between Intraoral basal cell carcinoma (IOBCC) and peripheral ameloblastoma (PA)
| IOBCC | PA | |
|---|---|---|
| Clinical | Appears malignant- resembles carcinoma; mixed red/white, maybe ulcerated | Appears benign: “bump on the gum” |
| Origin | Pluripotent basal cells in surface epithelium and adnexal structures | Basal cells in surface epithelium or dental lamina rests |
| Microscopic features |
Budding of epithelial islands into lamina propria Prominent retraction artifact Scattered mitotic figures Apoptotic cells Mucoid ground substance Widely infiltrative throughout connective tissue Peripheral palisading but lacking apical vacuolization & reverse polarity |
May or may not have connection to surface epithelium Little or no retraction artifact Few to absent mitotic figures Mucoid ground substance Not significantly infiltrative Islands of hyperchromatic basaloid cells with peripheral palisading, areas of stellate reticulum, prominent reverse polarity & apical vacuolization |
| Immunohistochemistry | Positive Ber-EP4; Negative for EMA and Calretinin | Negative Ber-EP4; Positive EMA and Calretinin |
EMA epithelial membrane antigen
Based on a careful review of the literature, we identified six cases of IOBCC supported by IHC (Table 2). A female predilection with a ratio of 2:1 over males was found. The average age of presentation was 66, and the most common clinical presentation was a non-healing ulceration. The most common location is the buccal mucosa (4 of 6 cases, or 67 %), followed by the retromolar pad and hard palatal mucosa. In an excellent review, Shumway et al. [6] critically reviewed the cases listed in Table 2 and concluded that 6 of these cases were inadequately documented and did not support a diagnosis of BCC. They reviewed the photomicrographs available on other case reports and concluded that only one case may have represented a true BCC while the other cases may have been misdiagnosed. Clinically, IOBCC usually resembles a malignancy, specifically a carcinoma that may or not have associated leukoplakia and/or erythroplakia. We reviewed these cases as well and concur with Shumway et al. Our assessment of these reports concluded that 3 of the cases were inadequately documented with unclear photomicrographs [24–26]. We believe 6 of the cases have been diagnosed incorrectly and many of these would be better classified as PA or basaloid SCC [4, 27–31]. One case was a BCC of the skin [32] and another represented a metastatic BCC [33]. One case by Edmondson et al. [34] may have represented an IOBCC, but the lesion invaded into bone and no retraction artifact was noted. This case was interpreted as ameloblastic carcinoma by Shumway et al. [6].
A significant aspect in all three of our cases was the distinct lack of surface epithelial dysplasia. The neoplastic tissue appeared to arise from the basal cell layer with no evidence of atypical alterations of the rest of the epithelial layers. Moreover, the epithelium adjacent to the neoplastic proliferation also failed to display any dysplastic changes. This finding is also supported by recent literature [5, 6, 22]. This obvious lack of surface epithelial dysplasia and the fact that the neoplasm arises from the basal cell layer further supports origin from pluripotent basal cells [5, 6].
A definitive mode of therapy has not yet been determined; however, according to the literature, the most common treatment consisted of wide local excision followed by close clinical follow up for at least a few years. IOBCC may exhibit signs of local recurrence and enlargement after conservative excision as in the case reported by Koutlas where multiple recurrences were noted [22]. The small series of confirmed IOBCC cases provide us with a 17 % recurrence rate (Table 2).
Conclusions
Intraoral basal cell carcinoma (IOBCC) is a rare entity with histologic similarity to the peripheral ameloblastoma (PA). While the two lesions share many similar features, they are two distinct and separate entities. Ber-EP4 is considered to be a consistent and reliable marker in distinguishing between the two immunohistochemically. IOBCC appears to arise from pluripotent cells in the basal layer of stratified squamous mucosa with no evidence of surface epithelial dysplasia. Several of the prior reports of IOBCC have been potentially misdiagnosed and may actually represent other entities. This is compounded by the lack of adequate photomicrographs and unavailability of immunohistochemical stains. Furthermore, we believe that IOBCC may be under reported. IOBCC can be locally aggressive and may recur if inadequately or conservatively excised. Wide local excision or resection appears to be the treatment of choice based on the limited number of cases in the literature.
Acknowledgments
We would like to thank Dr. Samuel L. Bobek, M.D. D.M.D of Portland, OR for his case contribution.
References
- 1.Wu S, Han J, Li WQ, Li T, Qureshi AA. Basal-cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178(6):890. doi: 10.1093/aje/kwt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luzar B, Bastian BC, Calonje E: Mckee’s Pathology of the Skin. Mckee PH editors. Amsterdam: Elsevier; 2012.
- 3.Bath-Hextall F, Leonardi-Bee J, Smith C, Meal A, Hubbard R. Trends in incidence of skin basal cell carcinoma. Additional evidence from a UK primary care database study. Int J Cancer. 2007;121:2105–2108. doi: 10.1002/ijc.22952. [DOI] [PubMed] [Google Scholar]
- 4.Saint CF. The embryology of the stomodeum and its bearing on the pathology of tumours of the tongue and the salivary glands. S Afr J Clin Sci. 1951;2:1–17. [PubMed] [Google Scholar]
- 5.Del Rosario RN, Barr RJ, Jensen JL, Cantos KA. Basal cell carcinoma of the buccal mucosa. Am J Dermatopathol. 2001;23:203–205. doi: 10.1097/00000372-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Shumway BS, Kalmar JR, Allen CM, Rawal YB. Basal cell carcinoma of the buccal mucosa in a patient with nevoid basal cell carcinoma syndrome. Int J Surg Pathol. 2011;19:348–354. doi: 10.1177/1066896908329596. [DOI] [PubMed] [Google Scholar]
- 7.Latza U, Niedobitek G, Schwarting R, Nekarda H, Stein H. Ber-EP4: new monoclonal antibody which distinguishes epithelia from mesothelial. J Clin Pathol. 1990;43:213–219. doi: 10.1136/jcp.43.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tellechea O, Reis JP, Domingues JC, Baptista AP. Monoclonal antibody Ber EP4 distinguishes basal-cell carcinoma from squamous-cell carcinoma of the skin. Am J Dermatopathol. 1993;15:452–455. doi: 10.1097/00000372-199310000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Yu L, Galan A, McNiff JM. Caveats in BerEP4 staining to differentiate basal and squamous cell carcinoma. J Cutan Pathol. 2009;36:1074–1176. doi: 10.1111/j.1600-0560.2008.01223.x. [DOI] [PubMed] [Google Scholar]
- 10.Winters R, Naud S, Evans MF, Trotman W, Kasznica P, Elhosseiny A. Ber-EP4, CK1, CK7 and CK14 are useful markers for basaloid squamous carcinoma: a study of 45 cases. Head Neck Pathol. 2008;2:265–271. doi: 10.1007/s12105-008-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.el-Mofty SK, Gerard NO, Farish SE, Rodu B. Peripheral ameloblastoma: a clinical and histologic study of 11 cases. J Oral Maxillofac Surg. 1991;49:970–974. doi: 10.1016/0278-2391(91)90061-P. [DOI] [PubMed] [Google Scholar]
- 12.Ide F, Mishima K, Miyazaki Y, Saito I, Kusama K. Peripheral ameloblastoma in situ: an evidential fact of surface epithelium origin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:763–767. doi: 10.1016/j.tripleo.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Beer TW, Shepherd P, Theaker JM. Ber EP4 and epithelial membrane antigen aid distinction of basal cell, squamous cell and basosquamous carcinomas of the skin. Histopathology. 2000;37:218–223. doi: 10.1046/j.1365-2559.2000.00999.x. [DOI] [PubMed] [Google Scholar]
- 14.Fan YS, Carr RA, Sanders DS, Smith AP, Lazar AJ, Calonje E. Characteristic Ber-EP4 and EMA expression in sebaceoma is immunohistochemically distinct from basal cell carcinoma. Histopathology. 2007;51:80–86. doi: 10.1111/j.1365-2559.2007.02722.x. [DOI] [PubMed] [Google Scholar]
- 15.Weedon D Tumours of the epidermis. In: Weedon D editors. Edinburgh, Churchill Livingstone Elsevier, 2010; p. 685–686.
- 16.Barnhill RL Tumors of the epidermis. In: Crowson AN, editors. New York, McGraw HIll, 2004; p. 599–608.
- 17.Kuru H. Uber das Adamantinoma. Zentralbl allg path u Path Anatomie (Iena) 1911;22:291–295. [Google Scholar]
- 18.Stanley HR, Jr, Krogh HW. Peripheral ameloblastoma; report of a case. Oral Surg Oral Med Oral Pathol. 1959;12:760–765. doi: 10.1016/0030-4220(59)90124-0. [DOI] [PubMed] [Google Scholar]
- 19.LeCorn DW, Bhattacharyya I, Vertucci FJ. Peripheral ameloblastoma: a case report and review of the literature. J Endod. 2006;32:152–154. doi: 10.1016/j.joen.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Kato H, Ota Y, Sasaki M, Karakida K, Kaneko A, Sekido Y, Tsukinoki K. Peripheral ameloblastoma of the lower molar gingiva: a case report and immunohistochemical study. Tokai J Exp Clin Med. 2012;37:30–34. [PubMed] [Google Scholar]
- 21.Vigneswaran N, Whitaker SB, Budnick SD, Waldron CA. Expression patterns of epithelial differentiation antigens and lectin-binding sites in ameloblastomas: a comparison with basal cell carcinomas. Hum Pathol. 1993;24:49–57. doi: 10.1016/0046-8177(93)90062-L. [DOI] [PubMed] [Google Scholar]
- 22.Koutlas IG, Koch CA, Vickers RA, Brouwers FM, Vortmeyer AO. An unusual ostensible example of intraoral basal cell carcinoma. J Cutan Pathol. 2009;36:464–470. doi: 10.1111/j.1600-0560.2008.01059.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Ueta E, Yoneda K, Osaki T. Peripheral ameloblastoma: case report with immunohistochemical investigation. J Oral Maxillofac Surg. 1990;48:197–200. doi: 10.1016/S0278-2391(10)80210-1. [DOI] [PubMed] [Google Scholar]
- 24.Lawson BF, Griffin JW, Waldron CA. Basal-cell carcinoma of the gingiva. Report of a case. Oral Surg Oral Med Oral Pathol. 1967;24:648–653. doi: 10.1016/0030-4220(67)90210-1. [DOI] [PubMed] [Google Scholar]
- 25.Mori M, Morimoto Y, Yoshimura Y, Kawamura H. Enzymatic histochemical demonstration of basal-cell carcinoma in the oral cavity. Oral Surg Oral Med Oral Pathol. 1968;25:746–755. doi: 10.1016/0030-4220(68)90044-3. [DOI] [PubMed] [Google Scholar]
- 26.Peters RA, Gingrass RP, Reyes CN, Hintz CS. Basal cell carcinoma of the oral cavity: report of case. J Oral Surg. 1972;30:63–66. [PubMed] [Google Scholar]
- 27.Liroff KP, Zeff S. Basal cell carcinoma of the palatal mucosa. J Oral Surg. 1972;30:730–733. [PubMed] [Google Scholar]
- 28.Samit AM. Intraoral basal cell carcinoma. J Surg Oncol. 1978;10:27–32. doi: 10.1002/jso.2930100105. [DOI] [PubMed] [Google Scholar]
- 29.Simpson HE. Basal-cell carcinoma and peripheral ameloblastoma. Oral Surg Oral Med Oral Pathol. 1974;38:233–240. doi: 10.1016/0030-4220(74)90062-0. [DOI] [PubMed] [Google Scholar]
- 30.Thoma KH, Goldman HM Oral pathology. Edited by St. Louis, MO, CV Mosby, 1960.
- 31.Williamson JJ, Cohney BC, Henderson BM. Basal cell carcinoma of the mandibular gingiva. Arch Dermatol. 1967;95:76–80. doi: 10.1001/archderm.1967.01600310082019. [DOI] [PubMed] [Google Scholar]
- 32.Keen RR, Elzay RP. Basal cell carcinoma from mucosal surface of lower lip: report of case. J Oral Surg Anesth Hosp Dent Serv. 1964;22:453–455. [PubMed] [Google Scholar]
- 33.Blinder D, Taicher S. Metastatic basal cell carcinoma presenting in the oral cavity and auditory meatus. A case report and review of literature. Int J Oral Maxillofac Surg. 1992;21:31–34. doi: 10.1016/s0901-5027(05)80449-7. [DOI] [PubMed] [Google Scholar]
- 34.Edmondson HD, Browne RM, Potts AJ. Intra-oral basal cell carcinoma. Br J Oral Surg. 1982;20:239–247. doi: 10.1016/S0007-117X(82)80018-8. [DOI] [PubMed] [Google Scholar]