Abstract
Schneiderian papilloma (SP) are uncommon tumors with malignant transformation even less common. The histologic criteria to define malignant transformation are not well developed nor is the immunohistochemical profile reported in a large series of carcinomas. 20 cases of malignant transformation of SP included 7 females and 13 males, aged 38–86 years (mean 60.7 years). Patients presented most frequently with a mass (n = 11) and obstructive symptoms (n = 7), present for 38.7 months (mean). Most patients had no previous history of SP (n = 13); metachronous carcinoma was identified in 7 patients an average of 34.4 months after the first diagnosis of SP, with 1–4 recurrences of SP. With a mean size of 4.1 cm, the majority of tumors involved a combination of more than one anatomic site (n = 10), followed by the maxillary sinus only (n = 5) or nasal cavity only (n = 3). Histologically, 17 were inverted and 3 exophytic type SP. There were 17 squamous cell carcinomas, 2 mucoepidermoid carcinomas and 1 sinonasal undifferentiated carcinoma, comprising from 10 to 95 % of the tumor volume. Malignant histologic features included atypical mitoses, necrosis, bone invasion, lymphovascular invasion, decreased transmigrating neutrophils, paradoxical maturation, dyskeratosis and/or perineural invasion (n = 3). Patients tended to present with advanced stage (n = 14, Stage III and IV). Immunohistochemical studies showed positive reactions in the malignancies for CK5/6 (86 %), p63 (86 %), CK7 (luminal, 50 %), p53 (83 %), and p16 (25 %). In situ hybridization detected human papillomavirus in 26 %. Surgery was often accompanied by radiation therapy (n = 13), with a mean of 2.4 years of follow-up. Five patients developed a recurrence between 0.8 and 3.3 years. Carcinomas ex-SP are less common and are associated with better outcome than previously reported. Patients tend to present with a synchronous carcinoma, developing in an inverted type SP, with squamous cell carcinoma the most common malignancy. Development of metachronous carcinomas ex-SP was always preceded by SP recurrence in this series.
Keywords: Sinonasal tract, Schneiderian papilloma, Malignant transformation, Review, Immunohistochemistry, HPV
Introduction
Schneiderian papilloma (SP) are uncommon benign sinonasal tract tumors. Malignant transformation is reported to be around 27 %, although in daily practice the transformation rate seems much lower. The criteria used to establish malignant transformation are not well defined. While there have been a few series in the English literature that have reported malignant transformation in SP (Table 1), the information is shrouded within data about SP in general, about human papillomavirus (HPV) findings, significance of squamous dysplasia in SP, or about malignancies in general (Table 2). Therefore, there is no single, large, comprehensive evaluation of primary malignant transformation of SP with respect to their clinical findings, histomorphology, immunohistochemical reactivity, HPV findings, and management. The purposes of this study was to attempt to identify any specific histologic and immunophenotypic features which can be used to aid in the diagnosis of malignant transformation of SP.
Table 1.
| Characteristics | Total: N = 59 |
|---|---|
| Gendera | |
| Women | 11 |
| Men | 48 |
| Age (in years)a | |
| Range | 32–83 |
| Mean | 61.3 |
| Women (mean) | 57.6 |
| Men (mean) | 62.1 |
| Symptom duration (in months)a | |
| Range | 0.5–540 |
| Mean | 62.6 |
| Women (mean) | 204.0 |
| Men (mean) | 48.6 |
| Symptoms at presentationa,b | |
| Mass, facial swelling | 17 |
| Obstructive symptoms | 15 |
| Epistaxis | 8 |
| Pain and/or headache | 8 |
| Optic symptoms (proptosis, ptosis, diplopia) | 8 |
| Drainage, discharge, crusting | 6 |
| Nerve changes (paralysis, palsy, paresthesias, numbness, dysphagia, trismus, tingling) | 2 |
| Otic symptoms | 1 |
| Locationa | |
| Mixed (more than one anatomic site) | 38 |
| Maxillary sinus only | 8 |
| Nasal cavity only | 8 |
| Lateralitya | |
| Right | 15 |
| Left | 19 |
| Bilateral | 1 |
| Papilloma type | |
| Inverted | 43 |
| Exophytic | 3 |
| Oncocytic | 13 |
| Carcinoma type | |
| Squamous cell carcinoma (including in situ) | 49 |
| Mucoepidermoid carcinoma | 6 |
| Sinonasal undifferentiated carcinoma | 2 |
| Carcinoma, not otherwise specified | 2 |
| All patients with follow-up (n = 54) (mean years of survival) | |
| Follow-up range | 0.3–15.9 |
| Alive, no evidence of disease (n = 25) | 5.9 |
| Alive, with disease (n = 3) | 2.5 |
| Dead, no evidence of disease (n = 3) | 3.0 |
| Dead, with local disease (n = 14) | 3.5 |
| Dead, with disseminated disease (n = 9) | 1.2 |
| Patients with recurrence (n = 18) | 4.3 |
| Patients without recurrence (n = 36) | 4.1 |
This table does not include the series reported in this study
aParameter was not stated in all cases
bPatients may have experienced more than one symptom
Table 2.
Incidence and percentage of malignant transformation [2, 4, 6, 7, 9, 11–13, 16, 18–24, 26, 27, 29–31, 33–35, 37–40]
| References | Total no. of papillomas | No. of carcinomas | Synchronous | Metachronous | % Carcinoma |
|---|---|---|---|---|---|
| Norris [11] | 28 | 1 | 1 | 0 | 4 |
| Norris [12] | 29 | 2 | 1 | 1 | 7 |
| Skolnick [33] | 33 | 5 | 1 | 4 | 15 |
| Hyams [23] | 149 | 19 | n/r | n/r | 13 |
| Snyder [13] | 39 | 8 | 7 | 1 | 21 |
| Lasser [24] | 17 | 4 | 2 | 2 | 24 |
| Ridolfi [30] | 30 | 1 | 0 | 1 | 3 |
| Abildgaard-Jensen [20] | 21 | 3 | 2 | 1 | 14 |
| Kristensen [38] | 83 | 7 | 4 | 3 | 8 |
| Woodson [35] | 90 | 4 | 1 | 3 | 4 |
| Christensen [21] | 39 | 7 | 6 | 1 | 18 |
| Segal [31] | 30 | 3 | 0 | 3 | 10 |
| Weissler [21] | 223 | 11 | 8 | 3 | 5 |
| Klemi [7] | 19 | 3 | 2 | 1 | 16 |
| Myers [26] | 33 | 7 | 5 | 2 | 21 |
| Phillips [29] | 112 | 8 | 8 | 0 | 7 |
| Furuta [22] | 26 | 7 | 5 | 2 | 27 |
| Outzen [27] | 67 | 1 | 0 | 1 | 2 |
| Dolgin [4] | 42 | 5 | 4 | 1 | 12 |
| Bielamowicz [19] | 61 | 10 | 5 | 5 | 16 |
| Kapadia [6] | 800 | 56 | n/r | n/r | 7 |
| Vrabec [16] | 101 | 8 | 7 | 1 | 8 |
| Beck [18] | 39 | 10 | 3 | 7 | 26 |
| Lawson [39] | 112 | 6 | 4 | 2 | 5 |
| Lesperance [9] | 51 | 14 | 6 | 8 | 27 |
| Buchwald [2] | 57 | 5 | 3 | 2 | 9 |
| Nachtigal [40] | 72 | 8 | n/r | n/r | 11 |
| Kaufman [37] | 34 | 3 | 2 | 1 | 9 |
| Current series | 740 | 14 | 9 | 5 | 1.9 |
| Total | 3,177 | 240 | 100 | 63 | 7.6 |
Materials and Methods
The records of 20 patients with tumors diagnosed with “Schneiderian papilloma” and “carcinoma” (any type) were selected. The primary sites included the nasal cavity and paranasal sinuses, nasopharynx, and ear and temporal bone. In all cases, focal areas of residual SP were present within the material that showed carcinoma. However, review of previous material, when available, was also performed as a point of comparison and documentation of metachronous versus synchronous histologic malignant development. Fourteen of the cases were identified from a review of all SP diagnosed during a 10 consecutive-year period of cases within the Southern California Permanente Medical Group (including 10 different medical centers). An additional 6 cases were obtained from the University of Pittsburgh Medical Center (IRB approval #991206), between 2008 and 2013.
Materials within the files were supplemented by a review of the patient demographics (gender, age, race); symptoms and physical findings and duration of symptoms, and past surgical history specifically related to SP. We reviewed imaging and operative reports and obtained follow-up information by direct communication with the referring pathologist, patient’s physician, or the patient. Follow-up data, available for all patients, included information regarding presence of locally recurrent or metastatic disease, treatment modalities used, and the current patient status. The fragmented nature of most samples precluded confident evaluation for the adequacy of excision. Preoperative imaging studies were personally reviewed in 14 cases, including computed tomography and magnetic resonance imaging studies, establishing size, exact site of origin, and tumor extent. All patients were staged according to the 2010 American Joint Commission on Cancer Staging [1]. This clinical investigation was conducted in accordance and compliance with all statutes, directives, and guidelines of the Code of Federal Regulations, Title 45, Part 46, and with an IRB approval #5216 from Southern California Permanente Medical Group with respect to human subjects in research.
The macroscopic pathology observations were gathered from the gross descriptions, including exact tumor location, lateralization, and tumor size (in centimeters). Hematoxylin and eosin-stained slides from all cases were reviewed, with a range of 1–42 slides reviewed per case (mean 11 slides/case). Specific histologic features were recorded, including cellular pleomorphism (mild, moderate, severe [anaplastic]); epithelium to stroma ratio (divided into quartiles); mitotic figures (number of mitotic figures per 10 high power fields (HPF) [magnification at 40× with a 10× objective lens using an Olympus BX41 microscope]); atypical mitotic figures (present or absent, and defined by abnormal chromosome spread, tripolar or quadripolar forms, circular forms, or indescribably bizarre). Lymph-vascular invasion (LVI) required the presence of neoplastic cells within an endothelial lined space, with or without thrombus and whether free floating or attached to the endothelium. Stromal-epithelial retraction was carefully excluded, seeking endothelial cells before definitive LVI was noted, but without performing immunohistochemistry for a vascular marker. Bone needed to be destroyed by the neoplastic cells to qualify for bone invasion.
Immunophenotypic analysis was performed in all cases with suitable material by a standardized Envision™ method employing 4 μm-thick, formalin fixed, paraffin embedded sections. Table 3 documents the pertinent, commercially available immunohistochemical antibodies used. The analysis was performed on a single representative block for each tumor. Epitope retrieval was performed, as required by the manufacturer guidelines. Standard positive controls were used throughout, with serum used as the negative control. The antibody reactions were graded as absent to weak (0 to 1+), moderate (2+ to 3+) and strong (4+) staining, and the fraction of positive cells was determined by separating them into diffuse, focal, or surface; nuclear and/or cytoplasmic or membrane; for the proliferation markers and p53 into four groups: <10 %, 11–50 %, 51–90 %, and >90 % nuclear reactivity. In situ hybridization (ISH) for HPV was performed using probes targeting a wide spectrum of HPV strains including 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, and 52 (Y1404; Dako, Carpinteria, CA). Five-micrometer tissue sections were deparaffinized and digested with proteinase K (Roche Diagnostics, Indianapolis, IN). Cases with punctate or diffuse nuclear signal were considered positive.
Table 3.
Immunohistochemical panel
| Antigen/antibody/clone | Company | Dilution | Antigen recovery | Number of cases with positive reactions | Reaction pattern |
|---|---|---|---|---|---|
| Cytokeratin (AE1/AE3:M3515) | Dako, Carpinteria, CA | 1:40 | CC1, 30 min | 14/14 (100 %) | S, D, C |
| CK7 (OV-TL-12/30) | Dako | 1:200 | CC1, 30 min | 7/14 (50 %) | S, D-F, C |
| CK5/6 (D5/16 B4) | Dako | 1:25 | E2, 20 min | 12/14 (86 %) | S, D, C |
| Epithelial membrane antigen (EMA)(E29) | Ventana Medical Systems, Tucson, AZ | Neat | CC1, 30 min | 13/14 (93 %) | S, F, C, L |
| CK903 (34ßE12; 1/5/10/14) | Enzo Life Sciences, Farmingdale, NY | Neat | n/a | 11/14 (86 %) | S, D, C |
| CEAm (CLO1-cea ab-1) | Lab Vision/NeoMarkers, Fremont, CA | 1:250 | CC1, 30 min | 13/14 (93 %) | S, L, C |
| p63 (7jul) | Leica Microsystems, Buffalo Grove, IL | 1:40 | E2, 30 min | 12/14 (86 %) | S, D, N |
| p53 (DO-7) | Dako | Neat | CC1, 30 min | 15/18 (83 %) | S, N, 1–90 % |
| p16INK4a (E6H4) | MTM Laboratories | Neat | CC1, 30 min | 5/20 (25 %) | S, F-D, N&Ca |
| CD56 (123C3.D5) | Lab Vision/NeoMarkers, Fremont, CA | Neat | CC1, 30 min | 2/14 (14 %) | S, D, C |
| Synaptophysin | Ventana | Neat | CC1, 30 min | 0/14 (0 %) | n/a |
| EBER (EBER1 DNP) | Ventana (BenchMark) | n/a | n/a | 0/14 (0 %) | n/a |
| Ki-67 (MIB-1) | Dako | 1:100 | CC1, 30 min | 2–90 % | S, N |
| HPV (ISH)(Y1404) | Dako | n/a | n/a | 5/19 (26 %) | N, dot/diffuse |
mm mouse monoclonal, rp rabbit polyclonal, S Strong, D Diffuse, F Focal, C Cytoplasmic, N Nuclear, L Luminal, n/a not applicable
aReactions ranged from 20 % to all cells. Only >70 % strong, diffuse, nuclear and cytoplasmic reactions were considered positive
A review of publications in English (MEDLINE 1966–2013) was performed, with all cases reported as carcinomas within SP included in the review [2–17]. However, cases were excluded if the information was too generalized and non-specific to make a meaningful interpretation of the demographics, histologic features or patient outcome. Several publications were included to document the frequency of malignant transformation [2–4, 6–35].
Results
Prevalence of Malignant Transformation in SP
Seven hundred and forty SP were diagnosed in a 10 consecutive year period, within a population of approximately 3,221,390 patients (mid-year patient count, 10-year average), yielding approximately 2.3 cases per 100,000 population per year (Southern California Permanente Medical Group incidence). Within this group, there were 14 cases that underwent malignant transformation, giving a 1.9 % malignant transformation prevalence for the whole population of SP cases (or 1.4 cases per year; 0.04 cases per 100,000 population).
Clinical
The patients included 13 men and 7 women (Table 4), with a mean age at presentation of 60.7 years (range 38–86 years). The patients were white (n = 15), black (n = 3) or unknown (n = 2) race. Most patients presented with a mass, but more than one symptom was usually experienced (obstruction, sinusitis, epistaxis, discharge, pain, polyps), for a mean duration of 38.7 months (range 0.5–300 months). Otic symptoms included otitis, hearing loss, tinnitus or vertigo, while ophthalmologic symptoms included diplopia and periorbital swelling. No patients were asymptomatic. On average, men presented at a younger age (57.5 vs. 66.6 years) and with a longer symptom duration (53 vs. 13 months) than women (p = 0.256). When separated by anatomic site, the mean duration of symptoms was quite different: nasal cavity alone: 6.0 months; maxillary sinus alone: 5.8 months; combination of nasal cavity and sinuses: 83.4 months (p = 0.019). Patients with epistaxis, a symptom which dictates immediate clinical assessment, had an average of 5.4 months of symptoms, much shorter than patients with other symptoms at an average of 50.5 months (p = 0.249). Twelve patients were ever smokers, while 7 patients were ever drinkers (available in 18 patients).
Table 4.
Clinical characteristics for current patient series
| # | Age, race, sex | Side and site | Previous history | Duration and symptoms | Size; stage | Diagnosis, grade, HPV | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 38 B F | L; NC, E, P | N | 70 mo; sinusitis, vertigo and migraine | 3.5 cm; III | Inverted, SCC, grade 3, HPV− | Resection; 60 Gy | A, NED, no recurrences, 6.3 year |
| 2 | 62 W M | L; NC | N | 4 mo; sinusitis, obstruction | 5 cm; III | Inverted, SNUC, grade 3, HPV− | Wide resection; 70 Gy and chemotherapy | A, NED, no recurrences, 6.5 year |
| 3 | 72 B F | R; Ma | Y: 17 mo before | 5 mo; mass, obstruction, discharge and serous otitis | 7.5 cm; III | Inverted, SCC, grade 3, HPV− | Medial maxillectomy | A, NED, no recurrences, 4.4 year |
| 4 | 46 B M | Bi; NC, NP | Y: 7 mo before | 6 mo; mass, obstruction, epistaxis, hearing loss | 4 cm; IVA | Inverted, SCC, grade 3, HPV−, LN+ | FESS; 70 Gy; chemotherapy | A, NED, no recurrences, 4.3 year |
| 5 | 55 W M | L; NC, NP, Mastoid | Y: 4 recurrences, with 42 mo from 1st diagnosis to carcinoma | 300 mo; mass and hearing loss | 4 cm; IVA | Inverted, SCC, grade 2, HPV+ | Exenteration; 66 Gy; chemotherapy | A; L, local recurrence with surgery, 3.5 year |
| 6 | 72 W M | R; Ma | N | 10 mo; mass, pain, discharge, epistaxis, polyps, sinusitis, transient diplopia and periorbital swelling | 2.9 cm; II | Inverted, Mucoepidermoid carcinoma, grade 3, HPV− | Wide resection (including Caldwell Luc), 60 Gy | D, L, local recurrence to skull base, with surgery, 2.4 year |
| 7 | 50 W F | L; NC (septum) | N | 8 mo; mass, occasional sore throat and hoarseness | 3 cm; I | Exophytic, SCC, grade 2, HPV− | FESS; radiation (unknown total dose) | A, NED, no recurrences; 1.9 year |
| 8 | 58 W M | R; NC; S; skull base | N | 27; discharge, obstruction and sinusitis | 5 cm; IVA | Inverted; SCC, grade 3, HPV− | FESS; 66 Gy | A, NED, no recurrences; 1.1 year |
| 9 | 70 W F | Bi; Ma | Y: 2 recurrences, with 62 mo from 1st diagnosis to carcinoma | 5 mo; mass, epistaxis | 2 cm; 0 | Inverted; carcinoma in situ, grade 1, HPV− | FESS | A, L, recurrence 8 months after primary, treated by surgery, 1 year |
| 10 | 54 W M | R; NC (septum) | N | 6 mo; obstruction | 3.0 cm; II | Exophytic, SCC, grade 3, HPV+ | FESS | A, L, incomplete excision with local disease remaining; 1.0 year |
| 11 | 46 W M | R; NC; Ma; E, NP | N | 4 mo; mass, pain, epistaxis, difficulty breathing, weight loss | 3.0 cm; IVA | Inverted, SCC, grade 2, HPV+ | Wide excision; radiation on-going; chemotherapy | A, L, disease is still present; 0.3 year |
| 12 | 50 W M | Bi; NC; S, ear | N | 120 mo; mass, sinusitis, hearing loss, tinnitus | 5.4 cm; IVA | Inverted, SCC, grade 2, HPV+ | Wide resection; 60 Gy; chemotherapy | A, NED, no recurrences, 0.4 year |
| 13 | 76 W F | R; NC; E; S | N | 2 mo; mass, epistaxis, sinusitis, obstruction | 3.7 cm; III | Inverted; SCC in situ, grade I, HPV−, LN+ | FESS; lymph node dissection | A, NED, but only 0.3 year |
| 14 | 79 W M | R; Ma | Y: 9.8 mo before | 8 mo; mass, obstruction | 5.3 cm; III | Inverted, SCC, grade 2, HPV− | Wide resection | A, NED, no recurrences, but only 0.3 years |
| 15 | 74 F | R; Ma | N | 1 mo; mass | 5.5 cm; IVA | Inverted, SCC, grade 1, HPV− | Wide resection | Lost to follow-up |
| 16 | 86 F | L; E | N | 2 weeks; discharge and diplopia | 6 cm; III | Inverted, SCC, grade 1, HPV− | Resection; radiation and chemotherapy | A, NED, no recurrences, 1.6 year |
| 17 | 63 W M | Bi; NC; E | N | 120 mo; mass, polyps and headache | 5 cm; IVA | Inverted, SCC, grade 3, HPV− | Wide resection; radiation and chemotherapy | A, NED, no recurrence, but only 0.3 year |
| 18 | 60 W M | L; Ma; E | N | unknown symptoms | 3.5 cm; III | Inverted, SCC, grade 1, HPV− | Wide resection | A, NED, no recurrences, 5.2 year |
| 19 | 50 W M | Bi; NC; Ma | Y: 73 mo before | 24 mo; polyps | 2.7 cm; II | Exophytic, mucoepidermoid carcinoma, grade 3, HPV− | Wide resection; radiation | A, NED, no recurrences, 1.2 year |
| 20 | 52 W M | L; S | Y: 29 mo before | 14 mo; mass | 1.0 cm; I | Inverted, SCC, grade 1, HPV− | Wide resection | A, NED, no recurrences, 2.5 year |
HPV human papillomavirus, F Female, M Male, B Black, W White, L Left, R Right, Bi Bilateral, NC Nasal cavity, F Frontal, S Sphenoid, Ma Maxillary sinus, E Ethmoid, NP Nasopharynx, mo months, SCC Squamous cell carcinoma, Gy Gray, A Alive, D Dead, NED no evidence of disease, LD local disease, yr years, SNUC Sinonasal undifferentiated carcinoma, FESS Functional endoscopic sinus surgery, LN lymph node metastasis
Seven patients had prior history of SP (i.e., metachronous malignant transformation in 6 inverted and 1 exophytic SP). On average these patients first presented 34.4 months before the diagnosis of carcinoma ex SP (range 7.3–73.2 months). All of these patients had 1–4 recurrences before the development of carcinoma. This cohort showed a mean age at presentation of 60.6 years, involved 5 men and 2 women, 5 whites and 2 blacks, and showed bilateral disease in 5 patients (without septal destruction). The tumors involved the sphenoid only (n = 1), maxillary sinus only (n = 3) or mixed sites (n = 3). The average size was 3.8 cm. There were no statistically significant differences between patients with synchronous and metachronous presentation.
Pathologic Features
Macroscopic
Most tumors affected mixed anatomic sites (n = 10), with maxillary sinus only (n = 5), nasal cavity only (n = 3), sphenoid and ethmoid sinus only (1 each, respectively). Within the nasal cavity, the septum was specifically involved in 2 cases by an exophytic type SP. The four patients with nasopharyngeal involvement also had disease in other sites. There were no tumors that secondarily involved the sinuses from the ear/temporal bone or lacrimal gland. Tumors were right (n = 8), left (n = 7), or bilateral (n = 5: 4 inverted, 1 exophytic). Tumors were on average 4.1 cm (range 1–7.5 cm); recurrent tumors were not larger than those of the patients without a recurrence (mean 3.8 cm). The tumors were described as papillary, polypoid, sessile, pale to white-tan, and frequently gritty, characteristics undoubtedly related to fragments of bony tissue normally present in turbinate tissue or samples removed via curettage of the sinuses. Most patients presented with high stage disease: Stage 0: 1; Stage I: 2; Stage II: 3; Stage III: 8; Stage IV: 6.
Microscopic
In all of the cases, areas of benign SP were present (i.e., not just a clinical history of SP) (Fig. 1a). In general, the portion of the sample that represented malignant transformation ranged from 10 to 95 % of the sample. However, without processing all of the submitted tissue, the residual SP may not be identified or the tumor may not be classified accurately. Inverted (n = 17) and exophytic type (n = 3) were the SP types represented (no oncocytic type in this series). In this series, there was no well established arc of development from benign to malignant with successive recurrences of the SP. However, subtle changes of increased pleomorphism, increased mitoses and decreased neutrophils could suggest a transformation (Table 5).
Fig. 1.
a This composite image demonstrates several zones of transition. A The left shows ciliated respiratory epithelium that abruptly transitions to carcinoma in situ. B The left shows a Schneiderian papilloma that abruptly transitions into an area of carcinoma. C This inverted papilloma contains areas of Pagetoid spread of the carcinoma (SNUC was noted below). D Paradoxical maturation is noted at the base of this dysplastic area, with a small area suggestive of early invasion. b This composite highlights areas of transition between benign and malignant. A The left portion shows a malignant transformation that blends with the right sided benign component. B Three different fields show unremarkable ciliated epithelium, dysplasia, and carcinoma in situ. C The upper portion of the field shows a Schneiderian papilloma, while the lower field shows malignant transformation. D The upper field demonstrates koilocytic atypia of a benign papilloma, while the lower field shows full thickness carcinoma in situ
Table 5.
Pathology findings
| Microscopic feature | Number (n = 20) |
|---|---|
| Invasion | |
| Bone invasion by histology | 9 |
| Lymphovascular invasion | 6 |
| Perineural invasion | 3 |
| Desmoplastic response to invasion | 12 |
| Architecture disorder | 20 |
| Keratin pearls | 12 |
| Paradoxical maturation | 14 |
| Dyskeratosis | 17 |
| Mucocytes or microabscess | 6 |
| Epithelial to stroma ratio (quartile) | |
| 1st | 2 |
| 2nd | 1 |
| 3rd | 5 |
| 4th | 12 |
| Pleomorphism | |
| Mild | 2 |
| Moderate | 6 |
| Severe | 12 |
| Necrosis | 13 |
| Mitotic figures | |
| Mean (per 10 HPF) | 53 |
| Range | 3–280 |
| Atypical figures (present) | 19 |
| Carcinoma type | |
| Squamous cell carcinoma | 17 |
| Mucoepidermoid carcinoma | 2 |
| Sinonasal undifferentiated carcinoma | 1 |
| Carcinoma grade | |
| Low grade (grade 1) | 6 |
| Intermediate grade (grade 2) | 5 |
| High grade (grade 3) | 9 |
HPF High power field
Benign areas of inverted SP showed an endophytic growth of thickened squamous epithelium (8–12 cell layers thick) broadly expanding into the stroma. The epithelium was bland, showing squamous to focally transitional epithelium, covered with columnar cells admixed with mucocytes (goblet cells) and intraepithelial mucous cysts. Transepithelial neutrophils were easy to identify in nearly all cases, forming small abscesses in many. The cells are bland in appearance with uniform nuclei, no piling up or overlapping and pleomorphism limited to isolated single cells. Atypical mitoses are not seen, but basal and parabasal mitoses (up to 24/10 HPFs) were identified. Focal areas of surface keratinization were noted.
Dysplasia was difficult to define, primarily due to inverted growth, tangential sectioning, and technical issues. Dyskeratosis, surface keratinization and increased epithelial pleomorphism suggest dysplasia, but there is such a lack of reproducibility both intra- and inter-observer, that it is better to think in terms of carcinoma in situ (severe dysplasia) as the only clinically significant parameter. The SP were in most cases intimately associated with the areas of malignant transformation (Fig. 1a, b). The carcinoma types included squamous cell carcinoma (SCC; n = 17), mucoepidermoid carcinoma (MEC, n = 2, Fig. 2), and one sinonasal undifferentiated carcinoma (SNUC; Fig. 2). Two of the cases showed only carcinoma in situ, but were associated with lymph node metastasis, suggesting non-representative surgery sampling may have accounted for this discrepancy (5 and 9 slides were reviewed, respectively, well within the range for these types of cases). One exophytic case was associated with a mucoepidermoid carcinoma (Fig. 2), while the other two exophytic cases were associated with a SCC. There were 4 well differentiated, keratinizing SCC; 5 moderately differentiated, keratinizing SCC; and 6 poorly differentiated SCC, three keratinizing and three non-keratinizing (Fig. 2). Overall, the tumors were separated into low (n = 6), intermediate (n = 5), and high grade (n = 9; the MEC and SNUC were all high grade).
Fig. 2.
a This mucoepidermoid carcinoma shows an epidermoid and transitional epithelium, with mucocytes within the tumor. There are no cilia to suggest they may be surface epithelium. b A non-keratinizing squamous cell carcinoma showing lymphoepithelial-like features in this poorly differentiated non-keratinizing squamous cell carcinoma. c A sinonasal undifferentiated carcinoma was the malignancy noted in one case
The following features, when identified, were associated with malignant transformation: architecture disorder with a lack of well developed maturation towards the surface (Table 5; Fig. 1a, b). This feature was associated with paradoxical maturation [abnormal keratinization or keratin pearl formation in the basal zone (Fig. 1a)], abnormal keratinization, and keratin pearl formation. These are also features of dysplasia, although the number of features in combination was sufficient for a diagnosis of carcinoma. Any single feature may be seen in a benign SP, but it is the aggregate of findings that is diagnostic. Nuclear molding was noted (n = 3; Fig. 3a), but was not a prominent finding. The nuclear to cytoplasmic ratio was intermediate (n = 3) to high (n = 17), showing mild (n = 2), moderate (n = 6) to severe (profound) nuclear pleomorphism (n = 12; Fig. 3A). There was an increased epithelium to stroma ratio, with a >90 % epithelial component in most cases. There was a high epithelial to stroma ratio in the majority of the cases (3rd and 4th quartiles), with the exception of the carcinoma in situ cases, where a “stroma” ratio was not present. If the entire sample is represented by epithelium only with limited stroma, additional features to support malignancy should be identified. There was a decrease in the extent of transepithelial migration (elimination) of neutrophils (Fig. 3b), although difficult to quantify. Neutrophils, often in microabscesses and mucus, are a characteristic feature of benign SP, especially the inverted (and oncocytic) type. Foamy histiocytes were noted within the stroma of areas of malignant epithelium (n = 4). Mucocytes were seen in four cases, especially in the two cases of MEC. There was a remarkable increase in mitoses (Fig. 3a), but with a range of 3–280/10 HPFs, with a mean of 53/10 HPFs in the areas of malignant transformation. This is well above the average in the benign areas (8.7/10 HPFs). Atypical mitoses were not identified in areas of benign SP.
Fig. 3.
a A composite of various cytologic features in malignant transformation. A Koilocytic atypia, pleomorphism and architectural disorganization. B Focal nuclear molding with cells that have a high nuclear to cytoplasmic ratio. Cell borders are prominent. C Increased mitoses (15 in this field), including atypical forms (off center), seen in carcinoma. D Dyskeratosis and keratin pearl formation. b A A benign Schneiderian papilloma with well developed transepithelial migration of neutrophils. B Neutrophils were greatly reduced or absent in areas of carcinoma. c A A complex inverted and destructive architecture, but the spicules of bone are native, and not destroyed. B The spicule of bone is being destroyed by highly atypical squamous epithelium, associated with a desmoplastic stroma. Note osteoblasts and osteoclasts. C Vascular invasion by a tumor thrombus. d A Central comedonecrosis with keratin debris is noted in this area of carcinoma. B Ghost cell outlines along with degenerated and necrotic debris are intermixed with the viable neoplastic cells in this area of necrosis
Various types of invasion were noted. A desmoplastic stromal response was seen in 12 cases. In general, benign inverted type SP have a broad, pushing, endophytic pattern of growth, with an intact, very thin basement membrane and no desmoplasia. However, a desmoplastic stromal reaction with loss of the already quite thin basement membrane was noted in areas of invasion. Single cell infiltration, small islands, and irregular bulbous projections without intact basement membrane were seen. A rich inflammatory infiltrate at the advance edge was also seen in several cases. Exophytic SP also have an intact basement membrane in benign areas, while a desmoplastic stroma was noted in all three cases. Destructive bone and/or cartilage invasion was present in nine cases (Fig. 3c). There was an osteoblastic and/or osteoclastic response associated with the epithelium. This is not just bone remodeling due to pressure, but is a genuine destructive bone and/or cartilage invasion (Fig. 3c). Perineural invasion (PNI) was only seen in three cases. LVI was present in six cases (Fig. 3c).
Tumor necrosis was present in 13 cases (Fig. 3d): tumor necrosis and comedonecrosis (n = 9), tumor necrosis alone (n = 2) or comedonecrosis alone (n = 2) was present in areas of carcinoma. Ghost-tumor cell outlines within degenerated material were required to qualify as tumor necrosis. Mucocytes with inflammatory cells are normally part of a SP, and so it is important that a microabscess not be included as evidence of necrosis. Genuine, tumor necrosis is a feature seen in malignancy that is not seen in benign SP.
The diagnosis of MEC versus adenosquamous carcinoma in sinonasal tract is controversial. With a bias towards MEC, these tumors showed mucocytes, transitional cells, and epidermoid cells (Fig. 2). Focal dyskeratosis was present, but keratin pearl formation was absent. The tumors showed bone invasion and desmoplasia, but only one case had LVI. Carcinoma in situ or surface dysplasia was not seen in these tumors. They had 19 and 26 mitoses/10 HPFs, with atypical mitoses noted in both, along with areas of necrosis. There was no well developed cyst formation.
A meningothelial-pattern was seen in two cases (Fig. 3a); one case each had a small cell pattern and a syncytial architecture (Fig. 2); Pagetoid spread was noted in the SNUC case (Fig. 1a). The SNUC was arranged in a destructive pattern of undifferentiated cells. However, the carcinoma only accounted for ~40 % of the tumor volume. A background of concurrent sinonasal inflammatory polyps was seen in one case.
Immunohistochemistry
The neoplastic cells were immunoreactive with pan-keratin in all cases tested (Table 3). Further, most of the cases showed strong diffuse to focal reactivity with CK5/6, EMA, p63 and CK903. There was a topographic distribution to the reactivity, with the various reactions highlighted in the malignant areas more intensely than in benign areas. The CK7 was only identified in 50 % of cases, with a strong, diffuse to focal reactivity in the cytoplasm. Two of the 14 p63-tested carcinomas ex-SP were p63 negative—SNUC and a poorly differentiated, but keratinizing, HPV-positive SCC.
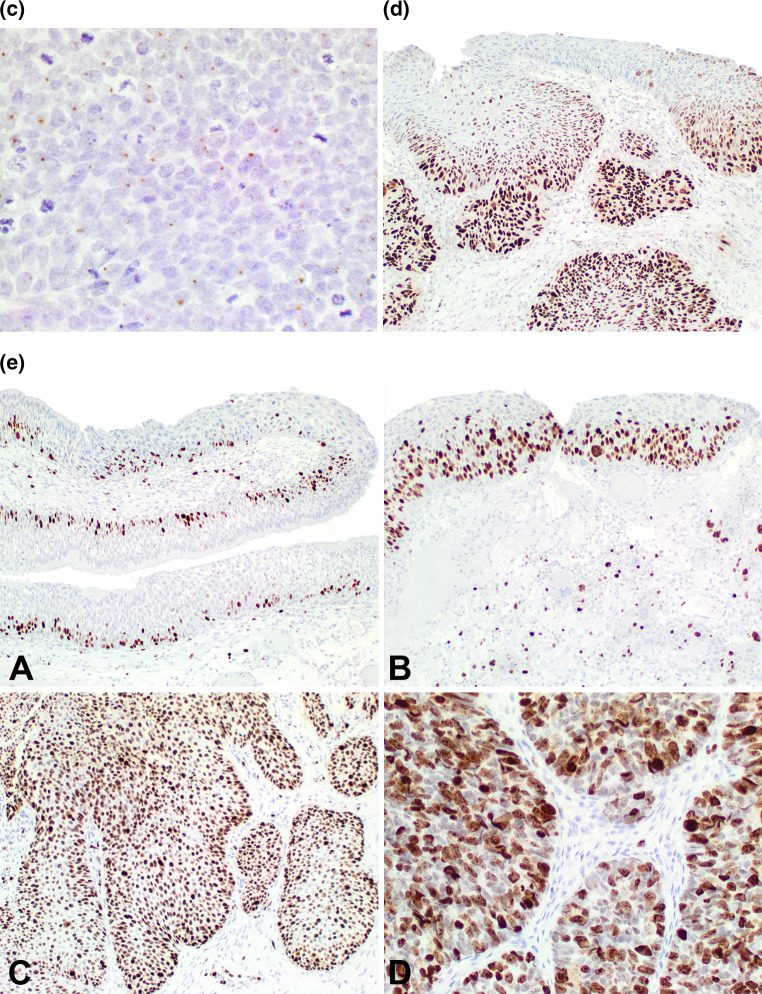
The p16 was positive in 8 of 20 (40 %) cases with a strong, diffuse, nuclear and cytoplasmic reaction in >70 % of cells in 5 cases (only 3 of which were HPV ISH positive). Three cases showed strong, nuclear and cytoplasmic reactivity in 20–40 % of cells (Fig. 4a, b). The expression was in both benign and malignant areas, often with a gradient of reactivity, more intense in areas of carcinoma (Fig. 4a, b). Further, p16 was reactive in the nuclei and cytoplasm of stromal cells (Fig. 4a), but not specifically in direct contact with the epithelium.
Fig. 4.
a A composite image of p16 immunoreactivity. A Strong, diffuse, nuclear and cytoplasmic reaction in the benign and malignant areas of this case. B Strong nuclear reaction (left sided) in an area of malignant transformation, mixed nuclear and cytoplasmic reactivity in the same area, while the area of benign papilloma showed a different pattern (lower). C Strong, diffuse, nuclear reaction of the whole epithelium in an area of carcinoma. D No reaction in an area of carcinoma, but the stroma shows a strong and diffuse nuclear and cytoplasmic reaction in the fibroblastic cells. b There was quite variable reactivity with p16. A: In an area of early invasion, there is nuclear and cytoplasmic strong reactivity, while the remaining area is negative. B This carcinoma showed strong and diffuse nuclear and cytoplasmic reactivity. c A delicate, single nuclear dot reaction with HPV ISH in an area of carcinoma. d p53 showed a gradient of reactivity, with a greater percentage of the nuclei showing a positive reaction in areas of carcinoma compared to benign areas. There was a greater intensity of the stain in areas of malignancy. e Ki-67 immunoreactivity. A: Benign Schneiderian papilloma with a limited, basal reaction. B Increased number of nuclei positive and above the basal zone in an area of severe dysplasia. C Most of the nuclei are positive in this area of carcinoma. D Very strong, heavy nuclear reaction was present in areas of carcinoma
p53 was present in 15 of 18 cases (83 %), but ranged from 1 to 90 % of the nuclei (the HPV ISH positive cases showed only focal, <5 % nuclear reactions, mimicking the recently described lack of p53 abnormalities in HPV-positive oropharyngeal carcinomas). The reaction was stronger, with a heavier chromogen deposition in carcinoma areas, with a much weaker reaction in benign areas (Fig. 4d). This finding was paralleled by the Ki-67 reaction, in which there was a greater degree of nuclear reaction in areas of carcinoma than in benign areas (Fig. 4e). There was a stronger and greater percentage of nuclear reaction noted in areas of carcinoma (Fig. 4e). Synaptophysin and EBER were negative in all cases. CD56 was expressed in the SNUC, while also focally positive in a single case of poorly differentiated SCC.
The HPV by ISH showed a dot-like (integrated) nuclear reaction in 3 of 19 (16 %) cases (Fig. 4c), present in benign and malignant areas, while a diffuse (episomal) nuclear reaction was seen in two additional cases (26 % total) (although a diffuse pattern was seen in 4 total cases). Two cases with dot-like HPV ISH were p16 IHC positive; 2 cases with diffuse HPV ISH pattern were p16 IHC positive; while 1 diffuse HPV ISH case and 1 dot-like and diffuse case were p16 negative.
Treatment and Follow-up
All patients were treated by surgery. The type of procedure varied based on the specific anatomic site of involvement. Excision, wide local excision, maxillectomy, Caldwell Luc procedure, Weber-Ferguson procedure, functional endoscopic sinus surgery (FESS), degloving procedure, and exenteration were employed to remove the primary tumor. Thirteen patients were further managed with radiation. Patients received from 600 to 700 cGy. Seven of the radiation patients received follow-up chemotherapy (agents included carboplatin, cisplatin, taxotere, 5FU, and/or docetaxel). Two patients had lymph node metastasis at the time of presentation of the carcinoma ex-SP, without prior history of SP, out of 14 with selected or modified radical neck dissections. Local recurrences developed in 3 patients, within 3–18 months.
Follow-up was available in 19 patients (Table 6). One patient had died with local disease 2.4 years after initial treatment. The patient had a pT2N0 Group II high grade mucoepidermoid carcinoma arising from an inverted SP. All of the remaining 18 patients remain alive (average follow-up, 2.6 years), although 4 patients have local recurrences (n = 3) or regional lymph node metastasis (n = 1) (average follow-up, 1.5 years). Follow-up is of limited duration, and so definitive comparisons about criteria which may predict better survival would be unreliable.
Table 6.
Summary of patient outcome (in 19 available patients)
| All patients | A, NED | A, D | D, D | |
|---|---|---|---|---|
| All patients with follow-up (mean years) | 19 (2.3) | 14 (2.6) | 4 (1.5) | 1 (2.4) |
| Follow-up range (years) | 0.3–6.5 | 0.3–6.5 | 0.2–3.3 | 2.4 |
| Gender | ||||
| Females | 6 (2.6) | 5 (2.9) | 1 (1.0) | n/a |
| Males | 13 (2.2) | 9 (2.4) | 3 (1.6) | 1 (2.4) |
| Age | ||||
| <60 years | 10 (2.2) | 7 (2.5) | 3 (1.6) | n/a |
| ≥60 years | 9 (2.4) | 7 (2.7) | 1 (1.0) | 1 (2.4) |
| Size | ||||
| <4.0 cm | 10 (2.1) | 6 (2.9) | 3 (0.8) | 1 (2.4) |
| ≥4.0 cm | 9 (2.5) | 8 (2.4) | 1 (3.5) | n/a |
| Anatomic site | ||||
| Nasal cavity alone | 3 (3.1) | 2 (4.2) | 1 (1.0) | n/a |
| Single sinus alone (maxillary, ethmoid or sphenoid) | 6 (2.0) | 4 (2.2) | 1 (1.0) | 1 (2.4) |
| Mixed | 10 (2.3) | 8 (2.4) | 2 (1.9) | n/a |
| Patients with a recurrence | 3 (2.3) | n/a | 2 (2.5) | 1 (2.4) |
| Patients without a recurrence | 16 (2.4) | 14 (2.6) | 2 (0.6) | n/a |
| Grade | ||||
| 1 (Low) | 5 (2.1) | 4 (2.4) | 1 (1.0) | n/a |
| 2 (Intermediate) | 5 (1.3) | 3 (0.9) | 2 (1.9) | n/a |
| 3 (High) | 9 (3.1) | 7 (3.5) | 1 (1.0) | 1 (2.4) |
| Stage | ||||
| 0 | 1 (1.0) | n/a | 1 (1.0) | n/a |
| I | 2 (2.2) | 2 (2.2) | n/a | n/a |
| II | 3 (1.5) | 1 (1.2) | 1 (1.0) | 1 (2.4) |
| III | 7 (3.5) | 7 (3.5) | n/a | n/a |
| IV | 6 (1.7) | 4 (1.5) | 2 (1.9) | n/a |
A, NED Alive, no evidence of disease, D, NED Dead, no evidence of disease, D, D Dead with disease
Discussion
SP are a well described, although uncommon neoplasm affecting the upper aerodigestive tract. Carcinomas arising from SP are rare, with more carcinomas identified in the inverted type and oncocytic types, with only isolated reports describing carcinomas arising from the exophytic type. Although there were no oncocytic SP in this series, it is well known they also hold a potential risk for malignant transformation [6, 36]. Carcinoma ex-SP ranges from 2 to 27 % in the literature, but in this series, without referral or academic institution bias, the 1.9 % rate may be a more accurate rate. This current series is derived from a review of all cases identified during a consecutive 10-year period, without any selection or patient bias, except perhaps as dictated by being a resident in southern California. During this period, 740 SP were identified, of which 14 were associated with malignant transformation. Nine patients presented with SP and a synchronous carcinoma, while five patients had presented with SP followed by metachronous development of the carcinoma. Within this cohort, there were 12 inverted type SPs, two exophytic types, and no oncocytic types. The overall progression of 1.9 % of SP to carcinoma may be a more representative incidence of malignant transformation than previously reported in the literature [2, 4, 6, 7, 9, 11–13, 16, 18–24, 26, 27, 29–31, 33–35, 37–40].
Clinical
In general, the male to female ratio of patients with carcinoma ranges from 1.2 up to 6.7, with an overall average of about 3.4:1. Patients range in age from 32 to 86 years, with an overall mean around 61 years. Patients presented with a variety of symptoms and most patients experienced more than one symptom. The Eustachian tube may be a route for nasal or nasopharyngeal SP to expand into the middle ear, yielding hearing changes or vestibulo-cochlear findings, although primary otic SP are reported [41]. Similarly, lacrimal gland SP could expand into the nasal cavity. Patients are usually symptomatic for a long time (mean 39 months), with a longer symptom duration if more than one anatomic site was involved (6.1 vs. 83.4 months), suggesting an extension of the disease from just a single site to multiple sites with time.
Most patients experienced a mixed anatomic site presentation: nasal cavity combined with maxillary, ethmoid, sphenoid, and/or frontal sinus, with possible involvement of the nasopharynx and ear. Similar to the reports from the literature, squamous cell carcinoma is the most common carcinoma type, with other carcinoma types less frequent. There is an approximately even distribution between tumor grades (1, 2, and 3).
Extrapolating from the patients reported in the literature, 42.6 % of patients died with local or disseminated disease (n = 23 of 54 with data), an average of 2.6 years after initial carcinoma diagnosis, with 5.5 % of patients alive with local disease (n = 3; mean 2.5 years follow-up). By contrast, 51.9 % of patients were alive or had died without evidence of disease (n = 28), with an average follow-up of 5.6 years. By contrast, 5.3 % (n = 1) of patients in this cohort died with disease, 21 % (n = 4) are alive with disease (mean 1.5 years), while the remaining 73.7 % (n = 14) are alive with no evidence of disease (mean 2.6 years). However, in this series and in the literature, the follow-up periods are short. Local recurrences and metastatic disease seem to develop within 2 years of the carcinoma diagnosis, suggesting very close early clinical follow-up and imaging evaluation to exclude further progression or recurrence.
Most patients presented with high stage disease: 65 % with stage III or IV disease. The majority of the patients reported in the literature did not have a recurrence (66.7 %); but, with limited follow-up data, there was no difference in survival time between those with a recurrence versus those without a recurrence (mean 4.3 vs. 4.1 years). Similarly, 15.8 % in this series developed a recurrence, but these patients all still have local disease or have died of disease (mean 2.3 years), with the remaining 84.2 % without a recurrence, alive (n = 14, mean 2.6 years) without disease or have persistent disease (n = 2, mean 0.6 years) that has not been cleared.
In this clinical series, the tumors were usually removed piecemeal, making margin assessment unreliable, except for the surgical team being able to estimate the adequacy of excision. Radiation therapy was employed in the majority of patients (68.4 %), a finding similar to that reported in the literature (59.3 %). As noted, SCC is usually managed with surgery followed by radiation, and since SCC is the most common malignancy, radiation is empirically employed in these patients, even though there is a different pathway of development.
Pathology Features
Although most sinonasal SCC arise de novo, a subset of sinonasal SCC is preceded by SP, especially of inverted type. The majority of the tumors were synchronous (carcinoma present at primary presentation) with 36 % metachronous (carcinoma developing after initial treatment of SP). The vast majority of these carcinomas are SCC (83 and 85 %, literature and this series, respectively). However, mucoepidermoid, sinonasal undifferentiated carcinoma, and carcinoma, NOS have been reported [6, 10, 12, 13, 15, 17]. The percentage of tumor represented by the carcinoma is quite variable, from 10 to 95 % of tumor volume, although not specifically correlated to outcome.
An arc of development from mild, moderate, to severe dysplasia before invasive SCC is noted in many cases of carcinoma ex-SP reported in the literature. General limitations to interpretation include: (1) no residual SP in the current material that shows carcinoma; (2) a history of “dysplasia”, but only carcinoma in the current sample, and (3) carcinoma only without known history. It is for this reason that all submitted tissue should be processed when SP is present or suspected. Our results indicate that squamous dysplasia in SP preceding carcinomas is practically undetectable. By extension, MEC does not have a specific precursor lesion, so the presence or absence of dysplasia may not be related to the development of MEC.
The histologic features most closely associated with carcinoma are: LVI, PNI, atypical mitoses, desmoplastic stromal reaction, bone invasion, architectural disorder, paradoxical maturation and keratin pearl formation, high epithelial to stroma ratio, profound pleomorphism, tumor necrosis, increased mitoses (>25/10 HPFs), and decreased transepithelial neutrophil elimination (microabscess formation). These are listed in order of sensitivity (i.e., if present, the diagnosis of carcinoma can be made) versus order of frequency (i.e., all cases had architectural disorder). For instance, transepithelial neutrophil loss may be present in some benign SP, so its absence may be difficult to use as a criterion for malignancy. As they have been identified in the literature, a few specific points of clarification are proposed [40, 42–46].
LVI, PNI, and atypical mitoses are, when present, diagnostic of carcinoma. However, PNI is only identified in 15 % of cases. When interpreting bone invasion, it is best interpreted when there is an osteoblastic or osteoclastic reaction in direct association with the epithelial proliferation. Small trabeculae of bone are part of the turbinate tissue and sometimes these fragments of bone can be caught up in the proliferation, while not truly representing a destructive growth (Fig. 3c). When bone is seen directly destroyed by the neoplasm, then the diagnosis of a carcinoma can be confirmed. If this is the only feature present, additional levels are recommended in order to document areas of invasion or other features of malignancy. A high epithelial to stroma ratio is of limited value, as it is high even in benign SP. Finally, increased mitoses in general is a feature of malignant transformation. However, benign SP evaluated as part of this series had up to 25 mitoses/10 HPFs and did not have any other histologic features of malignancy. Therefore, while increased mitoses can be helpful in reaching a diagnosis of carcinoma, it is important to have a threshold of >25/10 HPFs when this feature is seen in isolation. Having said this, it is most unlikely that increased mitoses would be the sole feature used in making a diagnosis of carcinoma.
Immunohistochemistry and In Situ Hybridization
There is usually a remarkable increase in Ki-67 proliferation index, compared to SP without dysplasia or invasion [44, 47]. If the Ki-67 labeling is greater than one half of the epithelial thickness affected, the diagnosis of severe dysplasia or carcinoma is likely. Interestingly, the intensity of the reaction pattern is often much stronger than the reaction seen in benign SP. p53 overexpression may be correlated to the Ki-67 index, especially when looking at severe dysplasia and invasive SCC [44]. While a 5 % cutoff is used for oropharyngeal carcinoma, using >25 % positive tumors cells as a cut-off for aberrant p53 protein expression may help to distinguish a carcinoma ex-SP presumably associated with p53 abnormalities from carcinoma ex-SP associated with HPV. HPV positive cases were p53 negative. Otherwise, the only thing we can comfortably say about HPV is that it is uncommon and can be identified in both pre-existing SP and carcinoma ex-SP.
Summary
Based on the results of this series, it would seem that carcinomas ex-SP are less common and are associated with better outcome than originally reported. Most patients seem to present with synchronous SCC carcinoma, with inverted papilloma being the most common type of pre-existing SP.
SP that may undergo malignant transformation are nearly impossible to identify based on morphologic examination (very few appear to be caught at the stage with squamous dysplasia only). Furthermore, such attempts appear to be of uncertain clinical value as development of metachronous carcinomas ex-SP was always preceded by SP recurrence in this series. The diagnosis of carcinoma ex-SP is straightforward, while the value of recognizing squamous dysplasia in otherwise benign SP has to be studied in larger series.
Acknowledgments
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of Southern California Permanente Medical Group or the University of Pittsburgh.
References
- 1.AJCC Cancer Staging Manual, 7th ed. New York: Springer; 2009.
- 2.Buchwald C, Franzmann MB, Jacobsen GK, Juhl BR, Lindeberg H. Carcinomas occurring in papillomas of the nasal septum associated with human papilloma virus (HPV) Rhinology. 1997;35:74–78. [PubMed] [Google Scholar]
- 3.Dingle I, Stachiw N, Bartlett A, Lambert P. Bilateral inverted papilloma of the middle ear with intracranial involvement and malignant transformation: first reported case. Laryngoscope. 2012;122:1615–1619. doi: 10.1002/lary.23247. [DOI] [PubMed] [Google Scholar]
- 4.Dolgin SR, Zaveri VD, Casiano RR, Maniglia AJ. Different options for treatment of inverting papilloma of the nose and paranasal sinuses: a report of 41 cases. Laryngoscope. 1992;102:231–236. doi: 10.1288/00005537-199203000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Halimi M, Aghbali A, Emamverdizadeh P, Talesh KT. Inverted papilloma of the palate with malignant transformation. J Oral Maxillofac Pathol. 2012;16:291–293. doi: 10.4103/0973-029X.99093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapadia SB, Barnes L, Pelzman K, Mirani N, Heffner DK, Bedetti C. Carcinoma ex oncocytic Schneiderian (cylindrical cell) papilloma. Am J Otolaryngol. 1993;14:332–338. doi: 10.1016/0196-0709(93)90091-K. [DOI] [PubMed] [Google Scholar]
- 7.Klemi PJ, Joensuu H, Siivonen L, Virolainen E, Syrjanen S, Syrjanen K. Association of DNA aneuploidy with human papillomavirus-induced malignant transformation of sinonasal transitional papillomas. Otolaryngol Head Neck Surg. 1989;100:563–567. doi: 10.1177/019459988910000607. [DOI] [PubMed] [Google Scholar]
- 8.Kobylecki C, Gnanalingham KK, Soh C, Du Plessis D, Hamdalla HH. Malignant transformation of a Schneiderian papilloma presenting with isolated sixth nerve palsy. Br J Neurosurg. 2013;27:262–263. doi: 10.3109/02688697.2012.717982. [DOI] [PubMed] [Google Scholar]
- 9.Lesperance MM, Esclamado RM. Squamous cell carcinoma arising in inverted papilloma. Laryngoscope. 1995;105:178–183. doi: 10.1288/00005537-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Maitra A, Baskin LB, Lee EL. Malignancies arising in oncocytic Schneiderian papillomas: a report of 2 cases and review of the literature. Arch Pathol Lab Med. 2001;125:1365–1367. doi: 10.5858/2001-125-1365-MAIOSP. [DOI] [PubMed] [Google Scholar]
- 11.Norris HJ. Papillary lesions of the nasal cavity and paranasal sinuses. I. Exophytic (squamous) papillomas. A study of 28 cases. Laryngoscope. 1962;72:1784–1797. doi: 10.1288/00005537-196212000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Norris HJ. Papillary lesions of the nasal cavity and paranasal sinuses. II. Inverting papillomas. A study of 29 cases. Laryngoscope. 1963;73:1–17. doi: 10.1288/00005537-196301000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Snyder RN, Perzin KH. Papillomatosis of nasal cavity and paranasal sinuses (inverted papilloma, squamous papilloma). A clinicopathologic study. Cancer. 1972;30:668–690. doi: 10.1002/1097-0142(197209)30:3<668::AID-CNCR2820300315>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 14.Terada T. Malignant transformation of exophytic Schneiderian papilloma of the nasal cavity. Pathol Int. 2012;62:199–203. doi: 10.1111/j.1440-1827.2012.02795.x. [DOI] [PubMed] [Google Scholar]
- 15.Vayisoglu Y, Unal M, Apa DD, Gucluturk MT. Schneiderian carcinoma developing in an inverted papilloma of the palatine tonsil: an unusual case. Ear Nose Throat J. 2011;90:E32–E34. doi: 10.1177/014556131109000516. [DOI] [PubMed] [Google Scholar]
- 16.Vrabec DP. The inverted Schneiderian papilloma: a 25-year study. Laryngoscope. 1994;104:582–605. doi: 10.1002/lary.5541040513. [DOI] [PubMed] [Google Scholar]
- 17.Ward BE, Fechner RE, Mills SE. Carcinoma arising in oncocytic Schneiderian papilloma. Am J Surg Pathol. 1990;14:364–369. doi: 10.1097/00000478-199004000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Beck JC, McClatchey KD, Lesperance MM, Esclamado RM, Carey TE, Bradford CR. Human papillomavirus types important in progression of inverted papilloma. Otolaryngol Head Neck Surg. 1995;113:558–563. doi: 10.1016/S0194-5998(05)80668-0. [DOI] [PubMed] [Google Scholar]
- 19.Bielamowicz S, Calcaterra TC, Watson D. Inverting papilloma of the head and neck: the UCLA update. Otolaryngol Head Neck Surg. 1993;109:71–76. doi: 10.1177/019459989310900113. [DOI] [PubMed] [Google Scholar]
- 20.Abildgaard-Jensen J, Greisen O. Inverted papillomas of the nose and the paranasal sinuses. Clin Otolaryngol Allied Sci. 1985;10:135–143. doi: 10.1111/j.1365-2273.1985.tb01182.x. [DOI] [PubMed] [Google Scholar]
- 21.Christensen WN, Smith RR. Schneiderian papillomas: a clinicopathologic study of 67 cases. Hum Pathol. 1986;17:393–400. doi: 10.1016/S0046-8177(86)80463-4. [DOI] [PubMed] [Google Scholar]
- 22.Furuta Y, Shinohara T, Sano K, Nagashima K, Inoue K, Tanaka K, et al. Molecular pathologic study of human papillomavirus infection in inverted papilloma and squamous cell carcinoma of the nasal cavities and paranasal sinuses. Laryngoscope. 1991;101:79–85. doi: 10.1288/00005537-199101000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Hyams VJ. Papillomas of the nasal cavity and paranasal sinuses. A clinicopathological study of 315 cases. Ann Otol Rhinol Laryngol. 1971;80:192–206. doi: 10.1177/000348947108000205. [DOI] [PubMed] [Google Scholar]
- 24.Lasser A, Rothfeld PR, Shapiro RS. Epithelial papilloma and squamous cell carcinoma of the nasal cavity and paranasal sinuses: a clinicopathological study. Cancer. 1976;38:2503–2510. doi: 10.1002/1097-0142(197612)38:6<2503::AID-CNCR2820380640>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 25.Lawson W, Kaufman MR, Biller HF. Treatment outcomes in the management of inverted papilloma: an analysis of 160 cases. Laryngoscope. 2003;113:1548–1556. doi: 10.1097/00005537-200309000-00026. [DOI] [PubMed] [Google Scholar]
- 26.Myers EN, Fernau JL, Johnson JT, Tabet JC, Barnes EL. Management of inverted papilloma. Laryngoscope. 1990;100:481–490. doi: 10.1288/00005537-199005000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Outzen KE, Grontved A, Jorgensen K, Clausen PP. Inverted papilloma of the nose and paranasal sinuses: a study of 67 patients. Clin Otolaryngol Allied Sci. 1991;16:309–312. doi: 10.1111/j.1365-2273.1991.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 28.Pelausa EO, Fortier MA. Schneiderian papilloma of the nose and paranasal sinuses: the University of Ottawa experience. J Otolaryngol. 1992;21:9–15. [PubMed] [Google Scholar]
- 29.Phillips PP, Gustafson RO, Facer GW. The clinical behavior of inverting papilloma of the nose and paranasal sinuses: report of 112 cases and review of the literature. Laryngoscope. 1990;100:463–469. doi: 10.1288/00005537-199005000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Ridolfi RL, Lieberman PH, Erlandson RA, Moore OS. Schneiderian papillomas: a clinicopathologic study of 30 cases. Am J Surg Pathol. 1977;1:43–53. doi: 10.1097/00000478-197701010-00005. [DOI] [PubMed] [Google Scholar]
- 31.Segal K, Atar E, Mor C, Har-El G, Sidi J. Inverting papilloma of the nose and paranasal sinuses. Laryngoscope. 1986;96:394–398. doi: 10.1288/00005537-198604000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Shah AA, Evans MF, Adamson CS, Peng Z, Rajendran V, Cooper K. HPV DNA is associated with a subset of Schneiderian papillomas but does not correlate with p16(INK4a) immunoreactivity. Head Neck Pathol. 2010;4:106–112. doi: 10.1007/s12105-010-0176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skolnik EM, Loewy A, Friedman JE. Inverted papilloma of the nasal cavity. Arch Otolaryngol. 1966;84:61–67. doi: 10.1001/archotol.1966.00760030063005. [DOI] [PubMed] [Google Scholar]
- 34.Weissler MC, Montgomery WW, Turner PA, Montgomery SK, Joseph MP. Inverted papilloma. Ann Otol Rhinol Laryngol. 1986;95:215–221. doi: 10.1177/000348948609500301. [DOI] [PubMed] [Google Scholar]
- 35.Woodson GE, Robbins KT, Michaels L. Inverted papilloma. Considerations in treatment. Arch Otolaryngol. 1985;111:806–811. doi: 10.1001/archotol.1985.00800140050009. [DOI] [PubMed] [Google Scholar]
- 36.Barnes L. Schneiderian papillomas and nonsalivary glandular neoplasms of the head and neck. Mod Pathol. 2002;15:279–297. doi: 10.1038/modpathol.3880524. [DOI] [PubMed] [Google Scholar]
- 37.Kaufman MR, Brandwein MS, Lawson W. Sinonasal papillomas: clinicopathologic review of 40 patients with inverted and oncocytic Schneiderian papillomas. Laryngoscope. 2002;112:1372–1377. doi: 10.1097/00005537-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Kristensen S, Vorre P, Elbrond O, Sogaard H. Nasal Schneiderian papillomas: a study of 83 cases. Clin Otolaryngol Allied Sci. 1985;10:125–134. doi: 10.1111/j.1365-2273.1985.tb01181.x. [DOI] [PubMed] [Google Scholar]
- 39.Lawson W, Ho BT, Shaari CM, Biller HF. Inverted papilloma: a report of 112 cases. Laryngoscope. 1995;105:282–288. doi: 10.1288/00005537-199503000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Nachtigal D, Yoskovitch A, Frenkiel S, Braverman I, Rochon L. Unique characteristics of malignant Schneiderian papilloma. Otolaryngol Head Neck Surg. 1999;121:766–771. doi: 10.1053/hn.1999.v121.a98734. [DOI] [PubMed] [Google Scholar]
- 41.Wenig BM. Schneiderian-type mucosal papillomas of the middle ear and mastoid. Ann Otol Rhinol Laryngol. 1996;105:226–233. doi: 10.1177/000348949610500310. [DOI] [PubMed] [Google Scholar]
- 42.Eggers G, Eggers H, Sander N, Kossling F, Chilla R. Histological features and malignant transformation of inverted papilloma. Eur Arch Otorhinolaryngol. 2005;262:263–268. doi: 10.1007/s00405-004-0818-9. [DOI] [PubMed] [Google Scholar]
- 43.Katori H, Nozawat A, Tsukuda M. Relationship between p21 and p53 expression, human papilloma virus infection and malignant transformation in sinonasal-inverted papilloma. Clin Oncol (R Coll Radiol) 2006;18:300–305. doi: 10.1016/j.clon.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Fan GK, Imanaka M, Yang B, Takenaka H. Characteristics of nasal inverted papilloma and its malignant transformation: a study of cell proliferation and programmed cell death. Am J Rhinol. 2006;20:360–363. doi: 10.2500/ajr.2006.20.2851. [DOI] [PubMed] [Google Scholar]
- 45.Katori H, Nozawa A, Tsukuda M. Markers of malignant transformation of sinonasal inverted papilloma. Eur J Surg Oncol. 2005;31:905–911. doi: 10.1016/j.ejso.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Tufano RP, Mokadam NA, Montone KT, Weinstein GS, Chalian AA, Wolf PF, et al. Malignant tumors of the nose and paranasal sinuses: hospital of the University of Pennsylvania experience 1990–1997. Am J Rhinol. 1999;13:117–123. doi: 10.2500/105065899782106698. [DOI] [PubMed] [Google Scholar]
- 47.Katori H, Nozawa A, Tsukuda M. Cell proliferation, apoptosis, and apoptosis inhibition in malignant transformation of sinonasal inverted papilloma. Acta Otolaryngol. 2007;127:540–546. doi: 10.1080/00016480600951400. [DOI] [PubMed] [Google Scholar]