Abstract
Metastasis from basal cell carcinoma of the skin is very rare with cases being documented in the lymph nodes, lung, bone and parotid gland. The main histopathological differential diagnosis is the locally arising basal cell adenocarcinoma from which it is difficult to distinguish by morphology and routine immunohistochemistry. Approximately 85 % of all reported metastatic basal cell carcinomas arise in the head and neck region. Here we present a case of basal cell carcinoma of the skin of the left lateral canthus of the eye which metastasized to the intraparotid lymph nodes with infiltration of the adjacent parotid parenchyma. More awareness and vigilance is required on the part of the reporting pathologist to consider metastasis in the presence of a parotid tumour. Features favouring metastasis include history of primary cutaneous basal cell carcinoma, histological similarity to the primary lesion and absence of any demonstrable direct extension from the skin lesion. We also review the literature on metastatic basal cell carcinoma and discuss the need for adequate follow up in high risk patients.
Keywords: Cutaneous, Basal cell carcinoma, Metastasis, Parotid gland
Introduction
Basal cell carcinoma of the skin is well known for its locally aggressive behaviour. Metastasis from basal cell carcinoma is very rare with the reported incidence ranging from 0.0028 to 0.55 %. Metastasis has been documented in sites such as lymph nodes, lung, bone and parotid gland. Here we present a case of basal cell carcinoma of the left lateral canthus of the eye which metastasized to the intraparotid lymph nodes with infiltration of adjacent parotid parenchyma.
Case Report
A 68 year old man presented to the accident and emergency department after a fall, which was attributed to a left ptosis which he had had for 3 years. Clinical examination revealed a large ulcerated skin lesion in the left lateral canthus of the eye which was the cause for the ptosis (Fig. 1). A CT scan of the orbit revealed a 3.1 × 2 cm mass in the left lateral canthus with bony erosion of the lateral orbital rim and involving the left nasolacrimal duct. An incisional biopsy was performed which confirmed an infiltrative type basal cell carcinoma (Fig. 2). The patient underwent orbital exenteration with en bloc excision of the lateral wall and medial maxilla. However the excision was incomplete with positive medial margin. Further management by radiotherapy was considered but was thought to carry a high risk of morbidity with respect to the surviving right eye. Re-excision was considered to be too difficult and imprecise to reliably remove the residual tumour. Therefore a watch and wait policy was adopted.
Fig. 1.
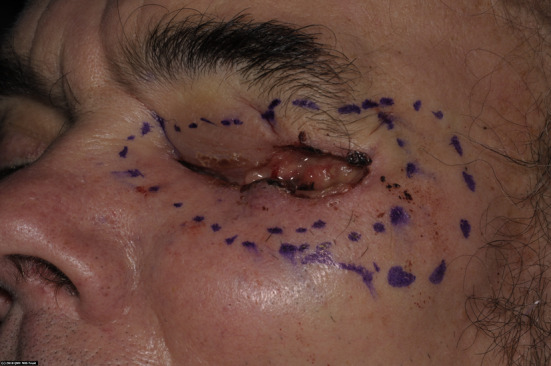
Ulcerated lesion in the skin of left lateral canthus of eye
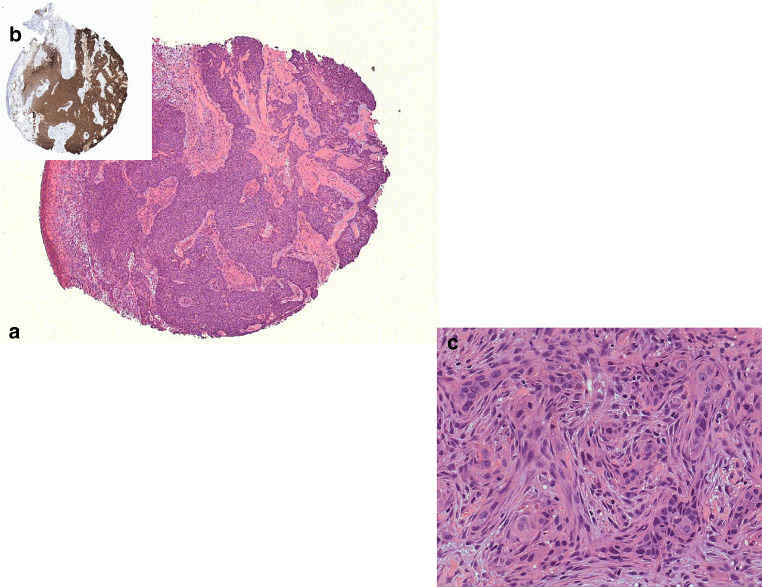
Fig. 2.
Photomicrograph of incisional biopsy from the lesion in the left lateral canthus of eye. a Scanning magnification of basal cell carcinoma showing peripheral palisading. b Primary tumour demonstrating strong BerEP4 positivity. c Higher magnification demonstrating infiltrative pattern of growth
Thirteen months after the initial surgery, the patient developed left ipsilateral facial nerve palsy. A Magnetic resonance imaging and ultrasound scan revealed a necrotic mass in the left parotid gland. A fine needle aspiration biopsy was attempted which was inconclusive. However an incisional biopsy of the parotid gland demonstrated salivary gland and fibroadipose tissue infiltrated by a carcinoma showing peripheral palisading. Immunohistochemistry revealed positive staining with BER-EP4 and cytokeratin 7. Cytokeratin 20 and S100 were negative. Given the clinical history, the morphological and immunohistochemical features were interpreted as consistent with metastatic basal cell carcinoma.
Three months later he underwent a left total parotidectomy with level I–III left neck lymph node dissection. Histopathological examination yielded 45 lymph nodes with one intraparotid lymph node showing metastatic basal cell carcinoma with extensive involvement of the parotid parenchyma and prominent perineural invasion (Fig. 3). This metastatic deposit measured 22.2 mm and was clear of the resection margins by 1.9 mm. Comparison between the primary basal cell carcinoma of the left lateral canthus and parotid tumour revealed similar features. The overlying skin was uninvolved by tumour. Subsequently he was given radiotherapy (50 Gy, 20 fractions) to the left parotid area and neck. He has been disease free for 1 year, and is currently awaiting a left eyebrow lift to correct descent as a result of wound contraction, thus allowing him to wear a more discreet ocular prosthesis. He remains under biannual followup.
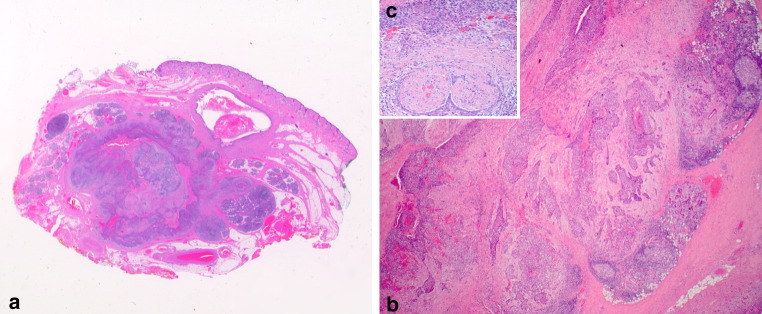
Fig. 3.
Photomicrograph of metastatic basal cell carcinoma in an intraparotid lymph node. a Scanning magnification demonstrating infiltration of adjacent parotid parenchyma. b Infiltration of the parotid gland and lymph node (note the germinal centre) by nodular and infiltrative type BCC similar to Fig. 2. c Photomicrograph demonstrating perineural invasion
Discussion
Basal cell carcinoma is the most common cutaneous malignancy in Caucasians. It is known to be a locally aggressive tumour, but metastasis from basal cell carcinomas have been reported in literature [1–8, 11]. The incidence of metastasis ranges from 0.0028 to 0.55 % [5], although this may not be a true representation due to the rarity of diagnosis. Many of the cases of metastasis reported in the literature follow local recurrence at the primary site. The time to metastasis ranges from 2.5 to 16 years (Table 1). This is in contrast to squamous cell carcinomas which metastasize early. Metastasis of basal cell carcinoma occurs by lymphatic spread in 70 % of cases, although it can be haematogenous with cases being reported in the lung and bone [5]. Primary basal cell carcinomas of the head and neck area metastasize to the neck lymph nodes and parotid gland [1–5, 7] while cases in the anterior chest and back spread to the axillary lymph nodes [6, 8]. Less common sites of metastasis include liver, spleen, spinal cord, adrenal glands, brain and dura mater, heart and kidney [5].
Table 1.
Literature review summarising age and sex incidence of metastatic basal cell carcinoma, site of primary and metastatic tumour, histological subtype, time to metastasis and survival after metastasis
| Serial No: | Authors | Age | Sex | Primary site | Histological subtype(s) | Time to develop metastasis from primary/local recurrence | Site(s) of metastasis | Survival after metastasis |
|---|---|---|---|---|---|---|---|---|
| 1 | Gropper AB [1] | 63 | M | Posterior neck | Adenoid and infiltrative | 3 years | Parotid and neck lymph nodes | Free of disease for 32 months |
| 2 | Eray Copcu [2] | 62 | F | Right inner canthus of eye | Adenoid | 10 years | Parotid, lung and neck lymph nodes | Free of disease for 6 months |
| 3 | James P Malone [3] | 68 | M | Multiple on face scalp and trunk | Infiltrative and nodular | 16 years | Parotid | Free of disease for 36 months |
| 4 | Akhil Wadhera [4] | 55 | M | Right anterior helix | Nodular | 6 years | Parotid | Free of disease for 24 months |
| 5 | Anthony Wu [5] | 52 | Left side of nose | 12 years | Submandibular gland and bone | Died of disease shortly | ||
| 6 | Joshua M Berlin [6] | 59 | M | Back | Infiltrative | 6 years | Axillary lymph nodes | Free of disease for18 months |
| 7 | Yadranko Ducic [7] | Median age 63 | M:F 5:4 | Head and Neck area | Median 2.5 years |
Lungs-4 Parotid-5 |
Free of disease for 55 months | |
| 8 | Doruk Ozgediz [8] | 52 | M | Left anterior chest and left shoulder | Nodular | 6 years | Left axillary lymph nodes | Free of disease for 24 months. |
| 9 | Kurian et al. (current case) | 68 | M | Left lateral canthus of eye | Infiltrative | 13 months | Parotid | Free of disease for 12 months |
The differential diagnosis of basal cell carcinoma at the primary site are the anaplastic variant of sebaceous gland carcinoma and basaloid variant of adenoid cystic carcinoma extending to the eyelid. The former resemble BCC of morphoeic type but shows pagetoid spread in the overlying epidermis and occasional vacuolated cells may be identified by Oil Red O stain. In addition sebaceous gland carcinoma is EMA positive and Ber EP4 negative. Basaloid variant of adenoid cystic carcinoma would show focal cribriform areas and comedo necrosis. In the parotid gland the main histopathological differential diagnoses are the locally arising primary basal cell adenocarcinoma, basaloid squamous cell carcinoma, adenoid cystic carcinoma, basal cell adenoma and cellular pleomorphic adenoma. Basaloid squamous cell carcinoma is primarily a tumour of the upper aerodigestive tract, and usually shows areas of obvious squamous differentiation and squamous cell carcinoma in situ [13]. It is usually negative for BER-EP4, by contrast to basal cell carcinoma. Adenoid cystic carcinoma has a solid pattern reminiscent of basal cell carcinoma, but can be distinguished by the presence of well formed cribriform islands, with genuine glandular cysts filled with mucin and pseudocystic spaces containing basement membrane material [9]. Basal cell adenomas and cellular pleomorphic adenomas lack the infiltrative growth pattern, vascular and capsular invasion. The most difficult differential diagnosis is between primary basal cell adenocarcinoma of the parotid gland and cutaneous basal cell carcinoma metastatic to the parotid gland. Primary basal cell adenocarcinoma of the parotid gland is defined as an infiltrative neoplasm dominated by basaloid epithelial cells in which there are four subtypes which include solid, membraneous, trabecular and tubular [14]. Cutaneous Basal cell carcinoma is defined as a group of malignant tumours characterized by the presence of lobules, columns, bands or cords of basaloid (germinative) cells [15]. Therefore the real differential diagnosis of metastatic cutaneous basal cell carcinoma is with the solid type of primary basal cell adenocarcinoma of the parotid gland. Immunohistochemistry is not helpful as basal cell adenocarcinomas of salivary gland origin demonstrate variable BER-EP4 expression [10]. But the clinical information of previous recurrent BCC in the skin of the head and neck area is a helpful guide to diagnosis.
In 1951 Lattes and Kessler [11] proposed the following criteria for diagnosis of metastatic basal cell carcinoma:
Primary tumour originating from skin and not from mucous membrane or other glands.
Metastatic nodules must be in lymph nodes or distinct from the primary tumour.
Both primary and metastatic tumours have identical histomorphology.
Features of squamous cell carcinoma must be absent.
Our case meets three of the above criteria. There was a previous history of basal cell carcinoma of the left eyelid, metastasis occurred to the parotid gland which is a site different from the primary and both primary and metastatic tumours were morphologically similar. The primary tumour showed focal squamous differentiation, but the strong and diffuse BerEP4 positivity (Fig. 2b) confirms that the tumour is a basal cell carcinoma. Areas of squamous differentiation are reported in less than 15 % of both primary and metastatic lesions of metastasizing basal cell carcinoma [20].
Several risk factors have been proposed for the development of metastasis. These include size of the primary tumour, location, aggressive subtype, local recurrence, history of radiation, immunosuppression and perineural invasion. The incidence of metastasis is reported to be 2 % for tumours larger than 3 cm, 25 % for tumours larger than 5 cm and 50 % for those more than 10 cm [5, 8]. Between 67 and 85 % of all examples of metastatic basal cell carcinomas arise from primary tumours in the facial region, especially the ear and scalp regions [1]. The high vascularity of the area has been speculated to contribute to the reason for increased metastatic risk [16]. Aggressive variants of basal cell carcinoma include morpheaform, infiltrative, micronodular and basosquamous/metatypical type [1, 19]. Although the most common histological subtype associated with metastasis is infiltrative type, there have been case reports of metastasis of the more common nodular subtype [3, 4, 8] and adenoid basal cell carcinoma [1, 2]. A previous history of radiation therapy and tumour recurrence refractory to treatment has been found to lead to an increased incidence of metastatic basal cell carcinoma [3, 17]. Sitz K.V et al. [18] has suggested that immunosuppression and impaired cell mediated immunity (including AIDS) may predispose to basal cell carcinoma metastasis. A review of literature reveals that several cases of metastatic basal cell carcinomas demonstrated perineural invasion in the primary lesion and it has been suggested as one of the risk factors for metastasis [4]. Our patient had several of these risk factors for metastasis including size more than 3 cm, location in the facial region and an infiltrative morphology of the primary lesion, which may be responsible for the very short (13 months) interval time between the primary and the parotid metastasis in the case here reported.
Metastatic basal cell carcinomas occur predominantly in males with male to female ratio being 2:1 [4] similar to our case. The median age reported in literature is 59 years [Table 1], our case being slightly older.
Treatment of primary cutaneous basal cell carcinoma has traditionally been surgical and no metastatic work up has been felt to be necessary. However the increasing awareness regarding its metastatic potential and the risk factors for metastasis warrant adequate follow up, especially in patients with high risk criteria. Malone et al. [3] suggested that high risk patients should be followed up with periodic chest X-rays and thorough clinical examination of the primary tumour site and sites of regional lymphatic drainage. Adjuvant radiotherapy can be offered to those with deep seated lesions, prominent perineural invasion and positive margins. Our patient had an incompletely excised primary lesion warranting radiotherapy but this was not given because of the risk of morbidity to the other eye.
Therapy for metastatic basal cell carcinomas include local excision of metastasis, with adjunctive radiotherapy used at the discretion of the treating physician [1]. However there is only limited evidence-based data regarding these treatment modalities due to the rarity in diagnosis and poor survival of patients with metastatic basal cell carcinoma. Our patient was managed by a superficial parotidectomy with selective lymph node dissection followed by radiotherapy to the left neck and parotid area. Chemotherapy with Cisplatin [12] and radiotherapy are the options for haematogenous metastasis especially in the lungs and bone. Targeted therapy with anti—EGFR monoclonal antibodies have been found to offer new options in the treatment of metastatic basal cell carcinoma [1].
The prognosis of metastatic basal cell carcinoma is poor with a high mortality rate of 50 % in 8 months [6]. In general, prognosis of localised metastasis tends to be better compared to haematogenous spread. Our patient has been disease free for 1 year, but will remain under regular review for the foreseeable future.
Conclusions
The metastatic potential of basal cell carcinomas of the skin has been increasingly recognised and they can metastasize years after excision of the primary lesion.
The main histopathological differential diagnosis in the parotid gland is primary basal cell adenocarcinoma and care must be taken to elicit the history of primary cutaneous basal cell carcinoma when encountered with a basaloid neoplasm in the parotid gland.
Adequate clinical and radiological follow up is recommended after excision of primary cutaneous basal cell carcinoma in those patients with high risk factors.
References
- 1.Gropper AB, et al. Metastatic basal cell carcinoma of the posterior neck: case report and review of the literature. J Cutan Pathol. 2012;39:526–534. doi: 10.1111/j.1600-0560.2012.01871.x. [DOI] [PubMed] [Google Scholar]
- 2.Copcu Eray, Aktas Alper. Simultaneous two organ metastases of the giant basal cell carcinoma of the skin. Int Semin Surg Oncol. 2005;2(1):1. doi: 10.1186/1477-7800-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malone James P, et al. Basal cell carcinoma metastatic to the parotid: report of a new case and review of the literature. Ear Nose Throat J. 2000;79(7):511–515. [PubMed] [Google Scholar]
- 4.Wadhera Akhil, et al. Metastatic basal cell carcinoma: a case report and literature review. How accurate is our incidence data? Dermatol Online J. 2006;12(5):7. [PubMed] [Google Scholar]
- 5.Anthony Wu, Laub Donald. Metastatic basal cell carcinoma: a case report and review of the literature. Eplasty. 2011;11:ic8. [PMC free article] [PubMed] [Google Scholar]
- 6.Berlin Joshua M, et al. Metastatic basal cell carcinoma presenting as unilateral axillary lymphadenopathy: report of a case and review of the literature. Dermatol Surg. 2002;28(11):1082–1084. doi: 10.1046/j.1524-4725.2002.02090.x. [DOI] [PubMed] [Google Scholar]
- 7.Ducic Yadranko, Marra Diego E. Metastatic basal cell carcinoma. Am J Otolaryngol. 2011;32(6):455–458. doi: 10.1016/j.amjoto.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Ozgediz Doruk, et al. Basal cell carcinoma does metastasize. Dermatol Online J. 2008;14(8):5. [PubMed] [Google Scholar]
- 9.Mills Stacey E. Salivary glands: basal cell adenocarcinoma. In: Stacey E. Mills et al., editors. Sternberg’s diagnostic surgical pathology. Philadelphia; 2010. p. 848.
- 10.Williams SB, Ellis GL, Auclair PL. Immunohistochemical analysis of basal cell adenocarcinoma. Oral Surg Oral Med Oral Pathol. 1993;75(1):64–69. doi: 10.1016/0030-4220(93)90408-V. [DOI] [PubMed] [Google Scholar]
- 11.Lattes R, Kessler RW. Metastasizing basal cell epithelioma of the skin. Cancer. 1951;4:866–878. doi: 10.1002/1097-0142(195107)4:4<866::AID-CNCR2820040424>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 12.Pfeiffer P, Hansen O, Rose C. Systemic cytotoxic therapy of basal cell carcinoma: a review of literature. Eur J Cancer. 1990;26(1):73–77. doi: 10.1016/0277-5379(90)90262-R. [DOI] [PubMed] [Google Scholar]
- 13.Cardesa A, Zidar N, Ereno C. Basaloid Squamous cell carcinoma. In: Barnes L, Eveson J, Reichart P, Sidransky D, editors. World Health Organisation classification of tumours. Pathology and genetics head and neck tumours. Lyon: IARC Press; 2005. pp. 124–125. [Google Scholar]
- 14.Ellis G. Basal cell adenocarcinoma. In: Barnes L, Eveson J, Reichart P, Sidransky D, editors. World Health Organisation classification of tumours. Pathology and genetics head and neck tumours. Lyon: IARC Press; 2005. pp. 229–230. [Google Scholar]
- 15.Kossard S, Epstein EH, Jr, Cerio R, Yu LL, Weedon D. Basal cell carcinoma. In: Leboit Philip E, Burg Gunter, Weedon David, Sarasin Alain., editors. World Health Organisation classification of tumours. Pathology and genetics of skin tumours. Lyon: IARC Press; 2006. pp. 13–19. [Google Scholar]
- 16.Ducic Y, Marra DE. Metastatic basal cell carcinoma. Am J Otolaryngol. 2010;32:455. doi: 10.1016/j.amjoto.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Snow S, Sahl W, Lo J, et al. Metastatic basal cell carcinoma. Cancer. 1994;73:328–335. doi: 10.1002/1097-0142(19940115)73:2<328::AID-CNCR2820730216>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 18.Sitz KV, Keppen M, Johnson DF. Metastatic basal cell carcinoma in acquired immunodeficiency syndrome-related complex. JAMA. 1987;257:340–343. doi: 10.1001/jama.1987.03390030070024. [DOI] [PubMed] [Google Scholar]
- 19.Ting PT, Kasper R, Arlette JP. Metastatic basal cell carcinoma: report of two cases and literature review. J Cutan Med Surg. 2005;9(1):10–15. doi: 10.1007/s10227-005-0027-1. [DOI] [PubMed] [Google Scholar]
- 20.von Domarus H, Stevens PJ. Metastatic basal cell carcinoma. Report of five cases and review of 170 cases in the literature. J Am Acad Dermatol. 1984;10(6):1043–1060. doi: 10.1016/S0190-9622(84)80334-5. [DOI] [PubMed] [Google Scholar]