Abstract
Over the past several decades, it has become clear that human papillomavirus (HPV) is important for the development and progression of many head and neck squamous cell carcinomas, particularly those arising in the oropharyngeal tonsillar crypts. Yet, our understanding of HPV’s role in premalignant squamous lesions remains relatively poor. This is in part because premalignant lesions of the oropharyngeal tonsillar crypt tissue, where most HPV-related carcinomas arise, are difficult if not impossible to identify. Recent evidence does suggest a role for HPV in a subset of premalignant lesions of the surface epithelium, especially the oral cavity, despite the rarity of HPV-related invasive squamous cell carcinomas at this site. Furthermore, these HPV-related oral cavity dysplasias appear to have unique, bowenoid histologic features described as ‘basaloid’ with full-thickness loss of squamous maturation, mitotic figures and apoptosis throughout. Here, we present a unique case of an HPV-related premalignant lesion (squamous cell carcinoma in situ) extensively involving the surface epithelium of the oral cavity, oropharynx and larynx that had ‘nonkeratinizing’ histologic features typical of HPV-related invasive squamous cell carcinoma. This case was strongly p16 positive by immunohistochemistry and harbored transcriptionally active HPV as demonstrated by E6/E7 RNA in situ hybridization. Furthermore, the patient had an excellent response to radiation treatment.
Keywords: HPV, E6/E7 RNA ISH, Carcinoma in situ, Dysplasia, Oral, Nonkeratinizing squamous cell carcinoma
Introduction
The role of human papillomavirus (HPV) in invasive squamous cell carcinoma of the oropharynx (particularly the tonsils) is well established. Meta-analysis demonstrates the presence of HPV in over half of tonsillar tumors and in some studies the rates are much higher, while HPV-related squamous cell carcinoma is rare in the oral cavity and larynx [1–3]. Furthermore, the virus is transcriptionally active indicating a key role in tumor pathogenesis. Interestingly, HPV-related squamous cell carcinomas have a particular predilection for tonsillar crypt tissue with the majority occurring in either the palatine tonsils or base of tongue [4]. When arising in tonsillar tissue, they usually have a unique ‘nonkeratinizing’ morphology [5, 6]. Identification of HPV-related oropharyngeal squamous cell carcinoma is important because of its unique clinical features including younger patient age, less strong linkage to tobacco and alcohol use and better patient outcomes compared to HPV-unrelated cases [7, 8].
Unlike in cervical carcinoma where progression from HPV-related dysplasia to invasive carcinoma is well characterized, little is known about HPV in premalignant squamous lesions of the head and neck and their progression to invasive carcinoma. In the cervix, HPV infection usually occurs in the transformation zone where the epithelial cells initially may show viral cytopathic effect (‘koilocytic atypia’) which then may develop into low grade dysplasia and eventually high grade dysplasia or invasive carcinoma, if the infection is not cleared. The Pap smear can be used to detect exfoliated cells from the preinvasive lesions so that they can be treated before an invasive carcinoma develops. The success of the Pap smear is well known. It is estimated that cervical cancer incidence and deaths declined by more than 60 % in the United States after its introduction (report.nih.gov/nihfactsheets/Pdfs/CervicalCancer(NCI).pdf).
A better understanding of preinvasive HPV-related lesions in the head and neck is desirable in order to develop better preventative measures or early treatment strategies, as has been done for cervical cancer. This is especially important because the incidence of HPV-related oropharyngeal cancer appears to be increasing [9]. However, because most HPV-related squamous cell carcinomas in the head and neck arise deep within oropharyngeal tonsillar crypts, preinvasive lesions are usually not clinically visible or cytologically detectable in the surface mucosa. A recent study documented the lack of feasibility of a “Pap-test equivalent” in the oropharynx due to lack of abnormal exfoliated surface cells in the absence of a clinically obvious mass [10]. As a result, tumors are almost always already invasive at the time of diagnosis—most patients present with metastatic disease in the neck and/or with an oropharyngeal mass.
Recent evidence suggests that a subset of oral cavity severe dysplasia cases may be HPV-related and have unique morphologic features that resemble Bowen’s disease of the skin [11–13]. Here, we present an unusual case of extensive HPV-related carcinoma in situ of the upper aerodigestive tract that occurred in a tobacco user and had ‘nonkeratinizing’ histologic features. The presence of transcriptionally active virus was confirmed by HPV E6/E7 RNA ISH and p16 immunohistochemistry. The morphologic features of the presented case are distinct from the bowenoid HPV-related dysplasias described in the literature and expand the spectrum of HPV-related disease in the head and neck.
Methods
Immunohistochemistry for CDKN2A(p16)
Immunohistochemistry was performed on formalin-fixed paraffin-embedded, 4 μm tissue sections using an antibody to CDKN2A(p16) (MTM Laboratories, Inc, Westborough, MA; mouse monoclonal; 1:1 dilution). Immunostaining was performed on a Ventana Benchmark automated immunostainer (Ventana Medical Systems, Inc., Tucson, AZ) according to standard protocols with appropriate positive controls. Antigen retrieval, standard on the machine, utilized the Ventana CC1, EDTA-Tris, pH 8.0 solution.
HPV RNA In Situ Hybridization
In situ hybridization for high risk HPV E6/E7 RNA was performed by hand using the RNAscope™ HPV kit (Advanced Cell Diagnostics, Inc., Hayward, CA) according to the manufacturer’s instructions as previously described [14]. Probes targeted the high risk HPV genotypes 16, 18, 31, 33, 35, 52, and 58 (performed as a cocktail and HPV 16 performed separately). Control probes for the bacterial gene DapB (negative control) and for the housekeeping gene ubiquitin C (positive control) were also included.
Case Report
The patient was a 60 year old man who presented in 2009 with ‘sore spots’ on the right palate for 2 years irritated by spicy food. He had a 28-year tobacco chewing history, predominately on the right side, and was status post tonsillectomy for chronic tonsillitis in 2005. It is unknown whether the tonsils were examined pathologically. Physical exam showed an extensive, well-demarcated area of erythroplakia involving predominately the right side of the oral cavity and oropharynx, corresponding to the preferred side of tobacco use (Fig. 1). Specifically, the posterior third of the right hard palate and right soft palate (extending to the left soft palate but sparing the uvula), the entire right retromolar trigone, right anterior tonsillar pillar and the prior tonsillectomy site were affected. The erythroplakia also extended on to the posterior floor of mouth, lateral tongue and up onto the lingual surface of the mandible posteriorly, all on the right side. Laryngoscopy identified another area of erythroplakia in the supraglottic larynx centered on the right posterior arytenoid. Abnormal mucosa was additionally seen on the posterior oropharyngeal wall. No suspicious lymphadenopathy was identified on physical exam or on CT scan of the neck.
Fig. 1.
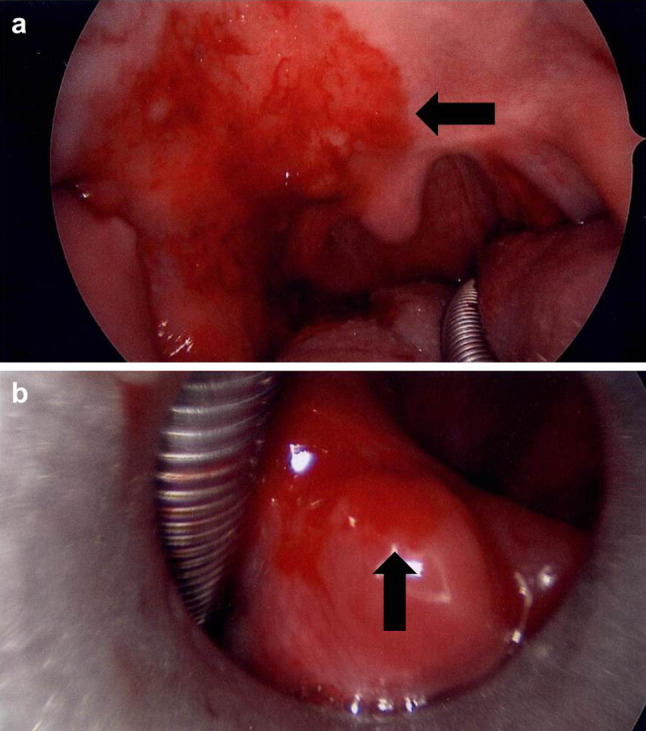
Intraoperative digital photographs of the erythroplakia extensively involving the oral cavity, oropharynx and larynx. a A fiery red patch can be seen involving the right hard and soft palate (arrow) and prior tonsillectomy site. b A separate area of erythroplakia is present in the larynx, centered on the right arytenoid (arrow)
Partial excision of the right hard and soft palate and biopsies from the right retromolar trigone, right arytenoid and posterior oropharyngeal wall were performed. These all showed identical appearing squamous cell carcinoma in situ in the surface mucosa with minimal maturation, imparting a ‘nonkeratinizing’ appearance reminiscent of HPV-related tonsillar squamous cell carcinoma (Figs. 2, 3). Specifically, the dysplastic cells had oval to spindled, hyperchromatic nuclei with indistinct cell borders. Brisk mitotic activity and apoptotic debris was observed throughout the thickness of the epithelium. A dense lymphoplasmacytic infiltrate was also present in the submucosa. No areas of stromal invasion were identified. A case of conventional severe dysplasia that lacks ‘nonkeratinizing’ histologic features is shown for morphologic comparison (Fig. 2c). While the clinical presentation was strongly consistent with field cancerization secondary to tobacco use, the ‘nonkeratinizing’ histologic appearance raised the possibility of an HPV-related lesion. A p16 immunostain was performed on the hard and soft palate specimen as a surrogate marker for HPV and was strongly and diffusely positive in the carcinoma in situ (Fig. 4a). HPV E6/E7 RNA ISH testing was also strongly positive for HPV type 16 (Fig. 4b). Taken together, the findings were of extensive HPV-related ‘nonkeratinizing’ carcinoma in situ of the upper aerodigestive tract.
Fig. 2.

Carcinoma in situ with ‘nonkeratinizing’ histologic features compared to conventional severe dysplasia. a Low power (×100) hematoxylin and eosin stained section showing extensive ‘nonkeratinizing’ carcinoma in situ with a dense lymphoplasmocytic inflammatory infiltrate in the submucosa. b High power (×400) hematoxylin and eosin stained section of ‘nonkeratinizing’ carcinoma in situ showing complete loss of maturation with replacement of the epithelium by dysplastic cells that have oval to spindled, hyperchromatic nuclei and indistinct cell borders. Mitotic activity is brisk and apoptotic debris is present. c Conventional severe dysplasia showing polygonal cells with pleomorphic nuclei, abundant pink cytoplasm and distinct cell borders (×200)
Fig. 3.
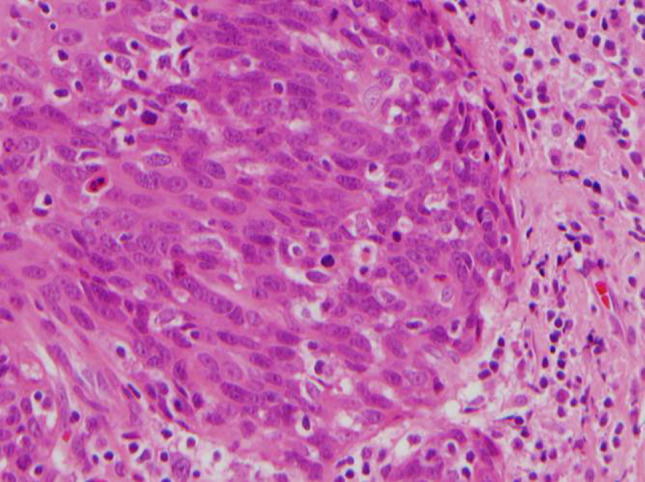
Hematoxylin and eosin stained section of HPV-related ‘nonkeratinizing’ squamous cell carcinoma of the tonsillar crypts showing cells with oval to spindled, hyperchromatic nuclei and indistinct cell borders, an appearance similar to the current case (×600)
Fig. 4.
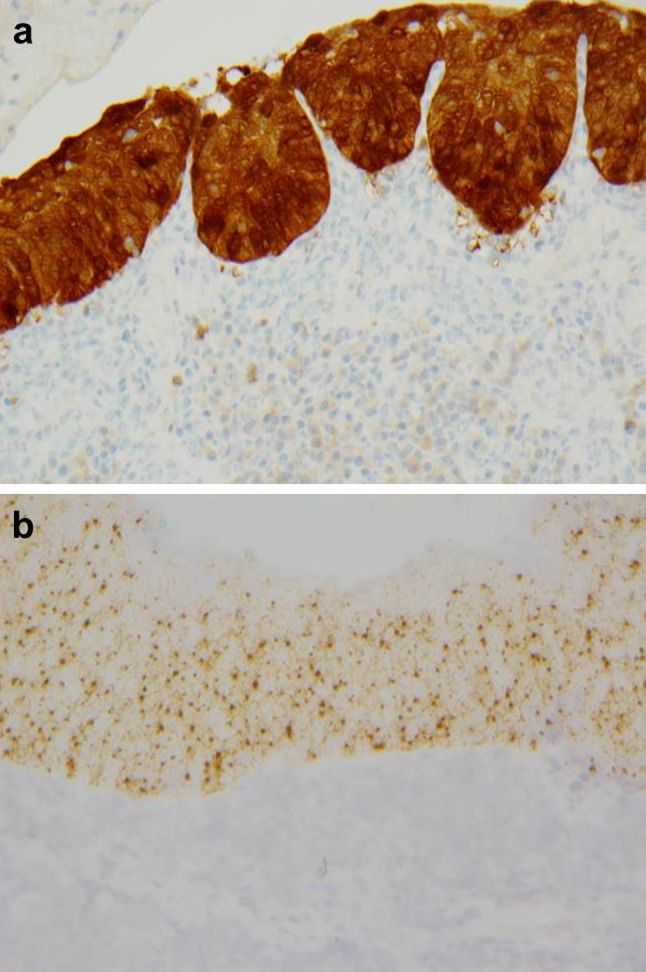
p16 immunohistochemistry (a) and HPV type 16 E6/E7 RNA in situ hybridization (ISH) (b) in the ‘nonkeratinizing’ carcinoma in situ (×200)
Due to the confluent nature collectively covering an extensive area of mucosa and making the erythroplakia unresectable, the patient was treated with a trial of high dose retinoic acid and discontinuation of tobacco products. Although the erythroplakia initially showed some improvement, 6 months after diagnosis, the patient developed progressive erythroplakia on the right lateral tongue and right arytenoid. High dose retinoic acid was discontinued due to toxicity. The patient deferred biopsy for another 9 months, at which time multiple suspicious areas were biopsied including the right posterior pharyngeal wall, right retromolar trigone, right tonsil bed, right pyriform sinus and left aryepiglottic fold—all of which showed squamous cell carcinoma in situ, again with a ‘nonkeratinizing’ appearance throughout. A biopsy of a suspicious area on the right epiglottis, however, showed invasive poorly differentiated keratinizing squamous cell carcinoma that was clinically staged as T1N0M0 (Fig. 5a). Synaptophysin and chromogranin immunostains were negative, excluding neuroendocrine differentiation. A p16 immunostain was strongly and diffusely positive, as was HPV HR RNA ISH, consistent with an HPV-related tumor (Fig. 5b, c).
Fig. 5.
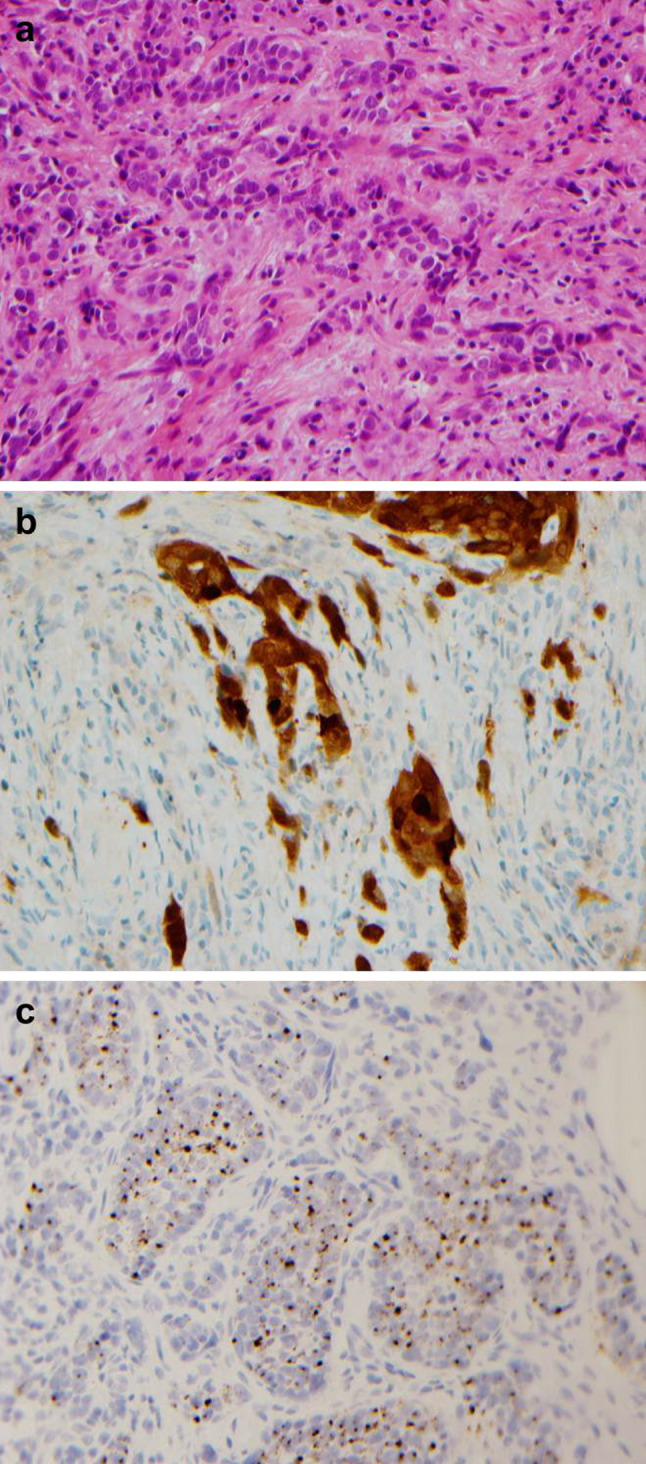
Subsequent invasive poorly differentiated keratinizing squamous cell carcinoma arising in the larynx (a, hematoxylin and eosin stained section) which was also p16 positive (b) by immunohistochemistry and HPV high risk RNA ISH positive (c) (×400)
The patient underwent definitive radiation treatment to the supraglottic larynx, as well as to the multiple areas of carcinoma in situ, with complete resolution of disease. The decision was made to include the areas of carcinoma in situ in the radiation field due to the lack of other treatment options as well as existing evidence in the literature that carcinoma in situ (particularly of the vocal cords) can be successfully treated with definitive radiation [15]. One and a half years after completion of radiation treatment (and 3 years after initial presentation), the patient developed a recurrence of the erythroplakia on the right floor of mouth measuring 3 × 2 cm. Biopsy again demonstrated squamous cell carcinoma in situ that was strongly and diffusely positive for p16 by immunohistochemistry. This was completely excised with negative margins. The patient is currently free of disease.
Discussion
HPV-related premalignant lesions of the head and neck are poorly characterized. The majority of invasive HPV-related squamous cell carcinomas arise in the tonsillar crypts rather than the surface epithelium and, thus, tonsillar ‘crypt dysplasia’ as a precursor to these tumors should theoretically exist. However, electron microscopy studies have shown that the tonsillar crypt reticulated epithelium itself contains numerous small blood vessels and has a discontinuous basement membrane. Thus, invasion and even metastasis may occur at a very early stage in tumor development, even in lesions that histologically appear ‘in situ’ [16]. It is not an uncommon occurrence clinically for very small or occult stage T1 tumors to be associated with metastases to regional neck lymph nodes. For these reasons, some consider all HPV-related neoplasia of the tonsillar crypts as potentially malignant regardless of whether or not there is clear-cut histologic evidence of stromal invasion [17].
Numerous studies have also evaluated squamous dysplasia/carcinoma in situ of the surface mucosa of the upper aerodigestive tract (mainly oral cavity but also larynx) for the presence of HPV [18–25]. However, the rates of HPV detection are widely variable between studies. The variability (ranging from 0 to 100 %) may be attributed to differences in inclusion criteria and detection methods, small sample sizes and differing patient populations [26, 27]. For example, some studies evaluated clinically defined ‘oral leukoplakias’ without further characterization histologically as to presence or degree of dysplasia. Other studies fail to distinguish oral cavity from oropharynx subsites. This is important because it is possible that some cases classified as oropharyngeal ‘dysplasias’ arising in the tonsillar crypts may truly be invasive carcinomas, since precursor lesions are difficult to identify in the tonsillar crypts (as discussed above). HPV detection method is important as some DNA detection methods (particularly sensitive and non-quantitative PCR) may not correlate with the presence of transcriptionally active virus. Only a few studies have used DNA ISH combined with p16 immunohistochemistry, which does serve as a good surrogate indicator of the presence of transcriptionally active virus. A search of the literature did not yield any studies that directly evaluated premalignant squamous lesions of the head and neck for transcriptionally active HPV (i.e. presence of viral E6/E7 RNA).
Despite these limitations, a recent meta-analysis by Syrjanen et al. [19] did find an increased risk of HPV detection in oral cavity potential premalignant lesions and dysplasia relative to controls in biopsies (odds ratio of 3.87, 95 % CI 2.22–6.13 and 5.10, 95 % CI 2.03–12.80, respectively). Another meta-analysis including 15 studies of oral cavity dysplastic lesions found an HPV prevalence of 25.3 % [18]. Several studies evaluated premalignant squamous lesions by HPV DNA ISH but very few studies used HPV DNA ISH combined with p16 immunohistochemistry. A recent study of a relatively large number of oral cavity dysplasias by McCord et al. [12] did utilize both HPV DNA ISH and p16 immunohistochemistry. They found that a significant subset of high grade dysplasias, but not low grade dysplasias were HPV DNA ISH positive and showed strong and diffuse p16 expression (7 of 40 high grade dysplasias or 17.5 %) [12]. The floor of mouth was the most common site of HPV-related dysplasia. There were a few cases (4 high grade and 1 low grade dysplasia) that were strongly and diffusely p16 positive but HPV negative. Overall, this data is good evidence that a subset of oral cavity dysplasias do harbor transcriptionally active HPV. However, further studies utilizing HPV E6/E7 RNA testing (as in the case presented here) are desirable to actually directly confirm the presence of transcriptionally active HPV in these lesions. Furthermore, the clinical significance, including the rates of malignant progression, has not been determined.
Much less data exists for laryngeal premalignant lesions. Only a few studies evaluated laryngeal dysplasias for HPV by ISH with variable results ranging from 0 positive cases to greater than 50 % [20–25]. Although Laco et al. [21] found 67 % of laryngeal dysplasias to be HPV positive by DNA ISH, none were strongly and diffusely p16 positive, suggesting that the virus was not transcriptionally active.
There is little description in the literature of histologic features associated with HPV-related dysplasia. Fornatora et al. [28] found a high rate of HPV by DNA ISH in oral cavity/oropharynx dysplasias that had ‘koilocytic’ features compared to dysplasias without ‘koilocytic’ features (80.6 vs. 0 %, respectively). Daley et al. [11] described a high rate of HPV by DNA ISH in several cases of oral cavity dysplasia that had bowenoid histologic features (numerous mitoses and apoptotic debris throughout the thickness of the epithelium). More recently, McCord et al. [12] noted that the HPV DNA ISH and p16 positive oral cavity dysplasias in their study were histologically distinct from the HPV-negative cases as well. The HPV-related dysplasias were described as having a more ‘basaloid’ appearance with loss of maturation, mitotic activity and apoptosis throughout the thickness of the epithelium. This description is similar to the bowenoid dysplasia described by Daley et al. [11]. Another recent study by Woo et al. [13] examined only oral cavity high grade dysplasias with ‘marked apoptosis’ and found all 20 cases to harbor high risk HPV DNA by ISH. Focal, but not diffuse, koilocytes were noted in all of these HPV-related cases and all tested cases showed strong and continuous p16 positivity, as well. Most occurred on the oral tongue or floor of mouth. Matched control cases of conventional dysplasia were all HPV negative. The described bowenoid histology of the HPV-related dysplasias in these studies is nearly identical and suggests that there may be a subset of HPV-related oral cavity dysplasias with unique histologic features [11–13].
The morphology of the case presented here has features that are similar to those described by Daley et al., McCord et al., and Woo et al. [11–13] in that it has full-thickness loss of maturation and a high mitotic rate, but it is not identical. The present case is more akin to HPV-related ‘nonkeratinizing’ squamous cell carcinoma of the tonsillar crypts in that it had oval to spindled nuclei and indistinct borders—features that were not described or presented in the previous studies [11–13]. Nonetheless, it appears that HPV-related premalignant lesions may have histologic features that are distinct from HPV-unrelated lesions.
If recent evidence bears out that transcriptionally active HPV is more common in oral cavity severe dysplasia than in invasive squamous cell carcinoma (~20 % compared to 5 % or less)—what is the role of the virus in oncogenesis of these tumors? There are several possible explanations. One is a ‘hit and run’ theory in which the virus plays a role in early oncogenesis (dysplasia) but is lost, or becomes inactive, in some cases as the tumor progresses and becomes invasive, perhaps because it is only a cofactor in tumor development. However, in the case presented, transcriptionally active HPV was also found in the invasive squamous cell carcinoma that the patient subsequently developed negating the ‘hit and run’ hypothesis in this case. Another possibility is that HPV-related dysplasias progress to invasive carcinoma less frequently than HPV-unrelated dysplasias and thus HPV is less commonly detected in invasive oral cavity carcinomas than in situ lesions. It is well known that HPV-related dysplasia of the cervix does not always progress to invasive carcinoma and in some cases actually regresses [29, 30].
Clearly, additional evaluation of dysplasias for transcriptionally active HPV with clinical follow-up, particularly for those arising in the oral cavity, is necessary to determine the frequency and significance of HPV-related premalignant squamous lesions. In the presented case, the patient appeared to have an excellent response to radiation treatment but it is not possible to know if the treatment response was related to the presence of HPV. Attention should also be paid to the histologic features of HPV-related dysplasias, as they may be different from HPV-unrelated cases. Although currently there is no indication for routine clinical HPV testing for dysplasia or carcinoma in situ of the upper aerodigestive tract, recognition and characterization of HPV-related premalignant lesions may have important implications for disease prevention, particularly in this emerging era of HPV vaccination.
Acknowledgments
The authors would like to acknowledge Neha Dahiya, MD, MBA and the Anatomic and Molecular Pathology Core Laboratory for their expert technical assistance with immunohistochemistry.
References
- 1.Syrjanen S. Human papillomavirus (HPV) in head and neck cancer. J Clin Virol. 2005;32S:S59–S66. doi: 10.1016/j.jcv.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Chernock R, Wang X, Gao G, Lewis J, Jr, Zhang Q, Thorstad W, et al. Detection and significance of human papillomavirus, CDKN2A(p16) and CDKN1A(p21) expression in squamous cell carcinoma of the larynx. Mod Pathol. 2013;26:223–231. doi: 10.1038/modpathol.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis J, Ukpo O, Ma X, Flanagan J, Luo Y, Thorstad W, et al. Transcriptionally-active high-risk human papillomavirus is rare in oral cavity and laryngeal/hypopharyngeal squamous cell carcinomas–a tissue microarray study utilizing E6/E7 mRNA in situ hybridization. Histopathol. 2012;60(6):982–991. doi: 10.1111/j.1365-2559.2011.04169.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim S, Koo B, Kang S, Park K, Kim H, Lee K, et al. HPV integration begins in the tonsillar crypt and leads to alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer. 2007;120:1418–1425. doi: 10.1002/ijc.22464. [DOI] [PubMed] [Google Scholar]
- 5.El-Mofty S, Patil S. Human papillomavirus (HPV)-related oropharyngeal nonkeratinizing squamous cell carcinoma: characterization of a distinct phenotype. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):339–345. doi: 10.1016/j.tripleo.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Chernock R, El-Mofty S, Thorstad W, Parvin C, Lewis J. HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3(3):186–194. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillison M, D’Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100(6):407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 8.D’Souza G, Kreimer A, Viscidi R, Pawlita M, Fakhry C, Koch W, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 9.Nasman A, Attner P, Hammarstedt L, Du J, Eriksson M, Giraud G, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125(2):362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 10.Fakhry C, Rosenthal BT, Clark DP, Gillison ML. Associations between oral HPV16 infection and cytopathology: evaluation of an oropharyngeal “Pap-test equivalent” in high-risk populations. Cancer Prev Res. 2011;4:1378–1384. doi: 10.1158/1940-6207.CAPR-11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daley T, Birek C, Wysocki GP. Oral bowenoid lesions: differential diagnosis and pathologic insights. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:466–473. doi: 10.1067/moe.2000.107975. [DOI] [PubMed] [Google Scholar]
- 12.McCord C, Xu J, Xu W, Qiu X, McComb R, Perez-Ordonez B, et al. Association of high-risk human papillomavirus infection with oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:541–549. doi: 10.1016/j.oooo.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Woo S, Cashman E, Lerman M. Human papillomavirus-associated oral intraepithelial neoplasia. Mod Pathol. 2013. (Epub ahead of print). [DOI] [PubMed]
- 14.Ukpo O, Flanagan J, Ma X, Luo Y, Thorstad W, Lewis J. High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35:1343–1350. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 15.Sengupta N, Morris CG, Kirwan J, Amdur RJ, Mendenhall WM. Definitive radiotherapy for carcinoma in situ of the true vocal cords. Am J Clin Oncol. 2010;33:94–95. doi: 10.1097/COC.0b013e3181a3194f. [DOI] [PubMed] [Google Scholar]
- 16.Perry ME. The specialized structure of crypt epithelium in the human palatine tonsil and its functional significance. J Anat. 1994;185:111–127. [PMC free article] [PubMed] [Google Scholar]
- 17.Westra WH. The morphologic profile of HPV-related head and neck squamous carcinoma: implications for diagnosis, prognosis, and clinical management. Head Neck Pathol. 2012;6:S48–S54. doi: 10.1007/s12105-012-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayaprakash V, Reid M, Hatton E, Merzianu M, Rigual N, Marshall J, et al. Human papillomavirus types 16 and 18 in epithelial dysplasia of oral cavity and oropharynx: a meta-analysis, 1985–2010. Oral Oncol. 2011;47:1048–1054. doi: 10.1016/j.oraloncology.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Syrjanen S, Lodi G, Bultzingslowen I, Aliko A, Arduino P, Campisi G, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: a systematic review. Oral Dis. 2011;17(Suppl. 1):58–72. doi: 10.1111/j.1601-0825.2011.01792.x. [DOI] [PubMed] [Google Scholar]
- 20.Brito H, Vassallo J, Altemani A. Detection of human papillomavirus in laryngeal squamous dysplasia and carcinoma. An in situ hybridization and signal amplification study. Acta Otolaryngol. 2000;120:540–544. doi: 10.1080/000164800750046072. [DOI] [PubMed] [Google Scholar]
- 21.Laco J, Slaninka I, Jirasek M, Celakovsky P, Vosmikova H, Ryska A. High-risk human papillomavirus infection and p16INK4a protein expression in laryngeal lesions. Pathol Res Pract. 2008;204(8):545–552. doi: 10.1016/j.prp.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Gorgoulis V, Rassidakis G, Karameris A, Giatromanolaki A, Barbatis C, Kittas C. Expression of p53 protein in laryngeal squamous cell carcinoma and dysplasia: possible correlation with human papillomavirus infection and clinicopathological findings. Virchows Arch. 1994;425:481–489. doi: 10.1007/BF00197551. [DOI] [PubMed] [Google Scholar]
- 23.Azzimonti B, Hertel L, Aluffi P, Pia F, Monga G, Zocchi M, et al. Demonstration of multiple HPV types in laryngeal premalignant lesions using polymerase chain reaction and immunohistochemistry. J Med Virol. 1999;59:110–116. doi: 10.1002/(SICI)1096-9071(199909)59:1<110::AID-JMV18>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 24.Gallo A, Degener A, Pagliuca G, Pierangeli A, Bizzoni F, Greco A, et al. Detection of human papillomavirus and adenovirus in benign and malignant lesions of the larynx. Otolaryngol Head Neck Surg. 2009;141(2):276–281. doi: 10.1016/j.otohns.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 25.Fouret P, Dabit D, Sibony M, Alili D, Commo F, Saint-Guily J, et al. Expression of p53 protein related to the presence of human papillomavirus infection in precaner lesions of the larynx. Am J Pathol. 1995;146(3):599–604. [PMC free article] [PubMed] [Google Scholar]
- 26.Hennessey P, Westra W, Califano J. Human papillomavirus and head and neck squamous cell carcinoma: recent evidence and clinical implications. J Dent Res. 2009;88(4):300–306. doi: 10.1177/0022034509333371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ha P, Califano J. The role of human papillomavirus in oral carcinogenesis. Crit Rev Oral Biol Med. 2004;15(4):188–196. doi: 10.1177/154411130401500402. [DOI] [PubMed] [Google Scholar]
- 28.Fornatora M, Jones A, Kerpel S, Freedman P. Human papillomavirus-associated oral epithelial dysplasia (koilocytic dysplasia): an entity of unknown biologic potential. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:47–56. doi: 10.1016/S1079-2104(96)80377-5. [DOI] [PubMed] [Google Scholar]
- 29.Chan JK, Monk BJ, Brewer C, Keefe KA, Osann K, McMeekin S, et al. HPV infection and number of lifetime sexual partners are strong predictors for ‘natural’ regression of CIN 2 and 3. Br J Cancer. 2003;89:1062–1066. doi: 10.1038/sj.bjc.6601196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trimble CL, Piantadosi S, Gravitt P, Ronnett B, Pizer E, Elko A, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLV phenotype. Clin Cancer Res. 2005;11:4717–4723. doi: 10.1158/1078-0432.CCR-04-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]


