Abstract
Research in the past two decades has generated unequivocal evidence that host genetic variations substantially account for the heterogeneous outcomes following human immunodeficiency virus type 1 (HIV-1) infection. In particular, genes encoding human leukocyte antigens (HLA) have various alleles, haplotypes, or specific motifs that can dictate the set-point (a relatively steady state) of plasma viral load (VL), although rapid viral evolution driven by innate and acquired immune responses can obscure the long-term relationships between HLA genotypes and HIV-1-related outcomes. In our analyses of VL data from 521 recent HIV-1 seroconverters enrolled from eastern and southern Africa, HLA-A*03:01 was strongly and persistently associated with low VL in women (frequency = 11.3 %, P < 0.0001) but not in men (frequency = 7.7 %, P = 0.66). This novel sex by HLA interaction (P = 0.003, q = 0.090) did not extend to other frequent HLA class I alleles (n = 34), although HLA-C*18:01 also showed a weak association with low VL in women only (frequency = 9.3 %, P = 0.042, q > 0.50). In a reduced multivariable model, age, sex, geography (clinical sites), previously identified HLA factors (HLA-B*18, B*45, B*53, and B*57), and the interaction term for female sex and HLA-A*03:01 collectively explained 17.0 % of the overall variance in geometric mean VL over a 3-year follow-up period (P < 0.0001). Multiple sensitivity analyses of longitudinal and cross-sectional VL data yielded consistent results. These findings can serve as a proof of principle that the gap of “missing heritability” in quantitative genetics can be partially bridged by a systematic evaluation of sex-specific associations.
Introduction
In the era of genome-wide association studies (GWAS) on human traits and diseases, one overwhelming issue is “missing heritability,” as thousands of GWAS (http://www.genome.gov/gwastudies/) have readily identified and confirmed quantitative trait loci (QTLs) based on statistical significance, but these QTLs typically explain little or rather limited phenotypic variance (Brookfield 2013). Proponents of quantitative genetics have called for close attention to study design (Putter et al. 2011), phenotypic robustness (Queitsch et al. 2012), and the effects of rare (including de novo) variants, haplotypes (combinations of variants that are inherited as a single unit), gene by gene interaction (epistasis), gene by environment interaction, as well as epigenetics (Eichler et al. 2010; Gianola et al. 2013; Keller et al. 2012; Lee et al. 2011; Mahachie John et al. 2011). For complex traits with evolving and multifactorial mechanisms, the journey ahead for finding the missing heritability can be long and bumpy.
During the natural course of human immunodeficiency virus type 1 (HIV-1) infection, viremia and time from infection to development of severe immunodeficiency or AIDS are often used as quantitative traits to gauge HIV-1 pathogenesis and/or rates of disease progression. In particular, plasma viral load (VL) set-point during chronic HIV-1 infection offers a relatively steady and widely available outcome measure with both clinical and epidemiological implications (Fideli et al. 2001; Lyles et al. 2000; Mellors et al. 1995; Quinn et al. 2000; Saag et al. 1996). Predictors of set-point VL range from viral characteristics (e.g., subtypes and replicative capacity) (Prentice et al. 2014a; Prince et al. 2012; Yue et al. 2013) to host genotypes (QTLs) that govern innate and adaptive immune responses (Apps et al. 2013; Fellay et al. 2009; Leslie et al. 2010; Prentice and Tang 2012). Depending on the study population and definition of set-point VL (single or multiple measurements), the proportion of VL variance explained by any single host or viral factor is often less than 4 % (Fellay et al. 2007; Prentice et al. 2014a; Yue et al. 2013). The most promising model that incorporates genetic and non-genetic features of epidemiologically linked HIV-1 transmission pairs (source and recipient partners) can account for nearly 37 % of early set-point VL variance (Yue et al. 2013).
Our recent data from a large cohort of HIV-1 seroconverters (SCs) suggest that host and viral factors associated with set-point VL can evolve as the infection progresses (Prentice et al. 2014a), even during the early chronic phase when complications by coinfections and comorbidities are infrequent. The correlates of longitudinal and cross-sectional VL in this cohort include four HLA-B variants (B*18, B*45, B*53, and B*57) that encode polymorphic cell surface glycoproteins specializing in antigen presentation (Prentice et al. 2014). While these observations are consistent with the well-documented hypothesis that viral epitopes bound to HLA-B molecules can dominate the induction of HIV-1-specific, cytotoxic T-lymphocyte responses (Kiepiela et al. 2004, 2007; Rajapaksa et al. 2012) and further dictate viral evolution or adaptation (Goulder and Walker 2012; Kawashima et al. 2009; Leslie et al. 2004; Moore et al. 2002; Rolland et al. 2010), the VL variance explained by individual HLA-B variants is also limited (ranging from 0.7 to 1.6 %). Our new objective is to refine the analytical approaches and to identify potential interaction terms between sex and HLA variants.
Subjects and methods
Study population
Recent HIV-1 seroconverters (SCs) were enrolled from Kenya, Rwanda, Uganda, and Zambia between 2005 and 2011 (Table 1), under a uniform study protocol sponsored by the International AIDS Vaccine Initiative (IAVI) (Amornkul et al. 2013; Price et al. 2011). The procedures for written informed consent and multidisciplinary research activities were approved by institutional review boards at all clinical research centers and participating institutions.
Table 1.
Characteristics of HIV-1 seroconverters stratified by sex: demographic features, viral subtypes, outcome measures, and major HLA variants of interest
| Characteristicsa | Men | Women | P b |
|---|---|---|---|
| No. of subjects | 327 | 194 | NA |
| Age: mean ± SD (year) | 32.5 ± 8.8 | 29.5 ± 7.3 | <0.0001 |
| Age ≥40: no. (%) | 59 (18.0) | 21 (10.8) | 0.027 |
| Enrolment site | <0.0001 | ||
| Kenya | 89 (27.2) | 14 (7.2) | <0.0001 |
| Rwanda | 50 (15.3) | 35 (18.0) | 0.412 |
| Uganda | 69 (21.1) | 58 (29.9) | 0.024 |
| Zambia | 119 (36.4) | 87 (44.9) | 0.057 |
| EDIs | |||
| Earliest | 3/15/2005 | 2/4/2005 | NA |
| Latest | 10/12/2011 | 6/29/2011 | NA |
| Length of follow-up (months): median (IQR) | 29 (21–31) | 29 (20–31) | 0.376 |
| Eligible visits per person: median (IQR) | 10 (8–11) | 9 (7–11) | 0.370 |
| HIV-1 subtypes | 0.040 | ||
| Subtype A1 | 123 (37.6) | 52 (26.8) | 0.012 |
| Subtype C | 118 (36.1) | 80 (41.2) | 0.242 |
| Others (B, D, recombinants, or unknown) | 86 (26.3) | 62 (32.0) | 0.167 |
| Person-visits with eligible viral load (2–36 months) | 3,002 | 1,732 | NA |
| Person-visits with eligible CD4+ T-cell counts | 3,000 | 1,777 | NA |
| First eligible viral load (log10)c: mean ± SD | 4.49 ± 1.03 | 4.30 ± 1.09 | 0.048 |
| HLA-A*03: no. (%) | 25 (7.7) | 22 (11.3) | 0.155 |
| Kenya | 5 (5.6) | 1 (7.1) | – |
| Rwanda | 5 (10.0) | 5 (14.3) | – |
| Uganda | 5 (7.3) | 9 (15.5) | 0.140 |
| Zambia | 10 (8.4) | 7 (8.1) | – |
| HLA-B*18: no. (%) | 23 (7.0) | 11 (5.7) | – |
| HLA-B*45: no. (%) | 58 (17.7) | 25 (12.9) | 0.144 |
| HLA-B*53: no. (%) | 60 (18.4) | 41 (21.1) | 0.437 |
| HLA-B*57: no. (%) | 30 (9.2) | 18 (9.3) | – |
| HLA-C*18: no. (%) | 26 (8.0) | 18 (9.3) | – |
aNon-standard abbreviations: IQR interquartile range (25th to 75 % percentile), SD standard deviation of the mean, NA not applicable
bThe P values >0.50 are omitted (−)
cFirst eligible outcome beyond the acute phase of infection (>9 weeks after EDI)
Follow-up strategies, genotyping, and outcome measures
SCs in this study were identified by frequent (monthly to quarterly) testing of HIV-1 seronegative subjects at high risk of HIV-1 infection through heterosexual and homosexual exposure, with the majority being seronegative partners in HIV-1 discordant couples and/or individuals reporting multiple heterosexual partners or diagnosed with sexually transmitted infections (85 % of the SC cohort). The subjects included for this study were SCs with sufficient longitudinal data, and the visit intervals were expanded from 3 to 24 months (Prentice et al. 2014a) to 2 to 36 months beyond estimated dates of infection (EDI). All study visits considered were before the initiation of antiretroviral therapy under national guidelines (Ngongo et al. 2012). Viral sequencing, molecular HLA genotyping, and quantification of plasma VL followed procedures described in detail elsewhere (Amornkul et al. 2013; Prentice et al. 2014a; Price et al. 2011; Tang et al. 2011). Identification of HLA-B*18 (unfavorable), B*45 (unfavorable), B*53 (unfavorable), and B*57 (favorable) as independent correlates of longitudinal or cross-sectional VL in this heterogeneous cohort (Prentice et al. 2014a) was highly consistent with results concerning Africans and African Americans (Apps et al. 2013; Lazaryan et al. 2011; Leslie et al. 2010; Tang et al. 2010).
Descriptive statistics
HIV-1-infected men and women were compared for their overall baseline characteristics, including (a) Wilcoxon’s rank-sum test for quantitative variables lacking a normal distribution, (b) t test for quantitative variables with a normal distribution, and (c) χ 2 and Fisher exact tests for categorical variables (Table 1). These and other analytical procedures (summarized below) were done using SAS, version 9.3 (SAS Institute, Cary, NC, USA).
Central hypothesis and analytical procedures
Our study aimed to test a central hypothesis that gene (HLA class I) by sex (viral microenvironment) interaction can be uncovered by separate analyses of men and women, especially when longitudinal VL measurements (with log10-transformation) are evaluated in mixed models. Data analyses began with the screening of potential interaction terms, with a focus on common HLA variants (population frequencies ≥4 %). The timing and magnitude of sex-specific effects on VL were further assessed by local regression (LOESS) curves (longitudinal data) and generalized linear models for geometric mean (cross-sectional) VL. Association signals with false discovery rate (FDR) below 0.20 were entered into a series of sensitivity analyses using subsets of data corresponding to (1) the 3- to 24-month follow-up period with densely distributed visits (Prentice et al. 2014a), (2) stepwise elimination of subjects representing individual countries or geographic regions (e.g., eastern versus southern Africa), and (3) elimination of subjects infected with rare or unknown HIV-1 subtypes. In the final multivariable models, age, sex, duration of infection (DOI, measured quarterly), and previously identified (generalizable) HLA variants (B*18, B*45, B*53, and B*57) (Prentice et al. 2014) were treated as covariates. The performance of individual statistical models was gauged by their overall R 2 values (corresponding to variance explained by factors in the model), while the impact of individual factors was measured by the regression beta (adjusted mean beta difference, Δβ, and standard error, SE). Associations with borderline statistical significance (P ≤ 0.050, FDR = 0.20–0.50) were exempt from multivariable models or sensitivity analyses.
Refinement through evaluation of linkage disequilibrium (LD) profiles and extended haplotypes
Using SAS Genetics (SAS Institute, Cary, NC, USA), HLA genotyping data for eastern and southern African SCs were analyzed separately for LD and extended haplotypes, with additional reference to fully resolved haplotypes in other populations (Cao et al. 2001). Association analyses based on 2- and 3-locus haplotypes were deemed informative if the adjusted effect sizes improved over those attributable to the component alleles.
Bioinformatics
Several public databases were surveyed for existing evidence of function mechanisms pertinent to HLA/MHC gene expression and immune surveillance. First, HLA-restricted HIV-1 epitopes were retrieved from the HIV Molecular Immunology Database (http://www.hiv.lanl.gov/content/immunology/ctl_search, last accessed on May 18, 2014). Second, MHC SNPs known to tag-specific HLA class I alleles in Africans (de Bakker et al. 2006) were queried in HaploReg (Ward and Kellis 2012) for additional LD information uncovered by The 1000 Genomes Project or functional properties annotated by the ENCODE project (Encode Project Consortium et al. 2012; Rosenbloom et al. 2010). Third, previous associations with immune disorders and/or gene expression QTLs (eQTLs) (Fairfax et al. 2012) were checked in the NCBI Global Cross-database (http://www.ncbi.nlm.nih.gov/) and the SCAN database (http://www.scandb.org/newinterface/index.html, last accessed on May 20, 2014), with close attention to cis- and trans-acting eQTLs (Nicolae et al. 2010).
Results
Characteristics of men and women in the study population
A total of 521 subjects had sufficient prospective data (three or more visits) during the 2- to 36-month interval after EDI (Table 1). The overall baseline data differed between 327 men and 194 women in terms of (1) age (P < 0.0001), country of origin (P < 0.0001), HIV-1 subtype (P = 0.040), and first available VL (P = 0.048). HLA alleles of interest had similar distribution in men and women (P = 0.14–0.97) (Table 1).
Screening for interaction terms between sex and HLA factors
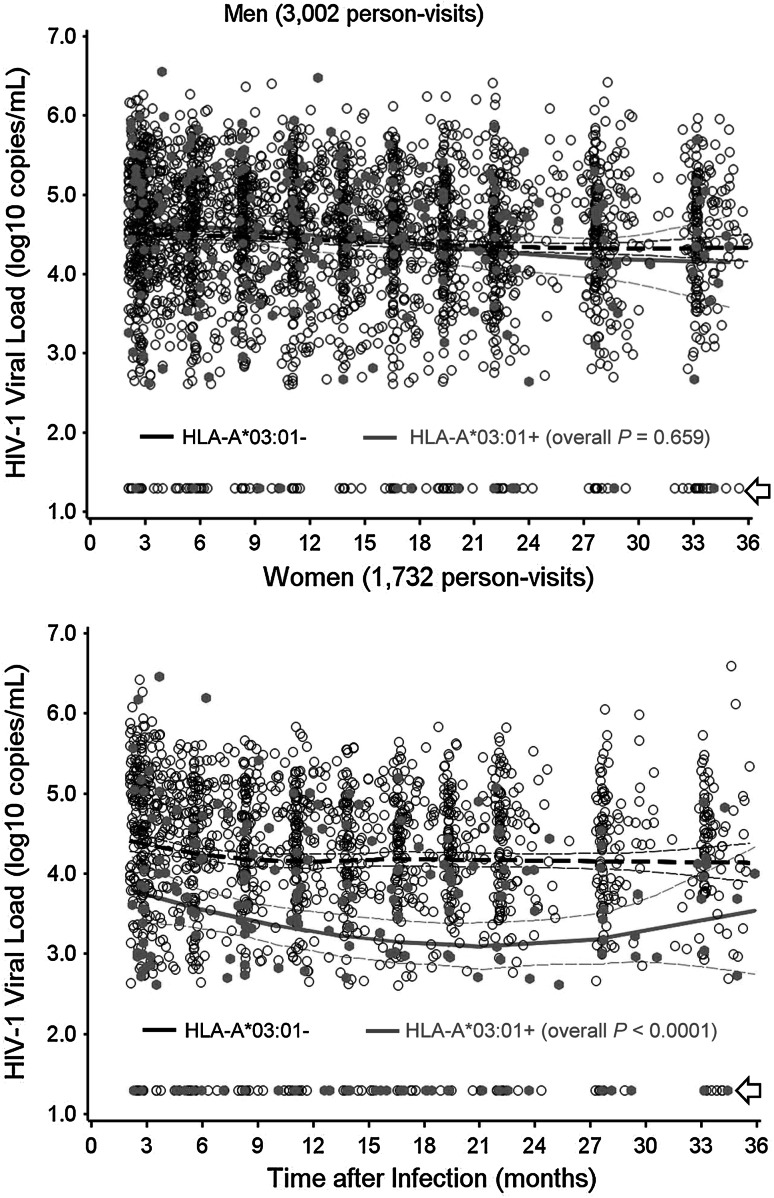
When 35 common HLA variants (2- or 4-digit resolution levels, whenever possible) were screened in mixed models with adjustment for demographic factors (age and geography), only HLA-A*03:01 showed a clear interaction with sex (P = 0.003, FDR = 0.09). LOESS curves supported this finding, as women with (+) and without (−) HLA-A*03:01 persistently differed in VL over the study intervals (1,732 person-visits, P < 0.0001) (Fig. 1). In contrast, HLA-A*03:01+ and A*03:01− men (3,002 person-visits) had highly comparable VL trajectories (P = 0.66).
Fig. 1.
Longitudinal viral loads in HIV-1-infected men and women stratified by HLA-A*03:01. Viral load measurements at various intervals (2 to 36 months after infection) are plotted for HLA-A*03:01-positive and HLA-A*03:01-negative subjects. The thick and thin lines correspond to the expected mean value and 95 % confidence intervals for each stratum (see Table 2 for summary statistics based on mixed models). Arrows indicate plasma viral load measurements that are <400 RNA copies/ml (routinely transformed to 1.30 log10)
Multivariable models for longitudinal VL data
For the 2- to 36-month intervals, the interaction term between female sex and HLA-A*03:01 was independent of other known factors pertinent to the study population (Table 2), with an adjusted P value of 0.005. On average, VL differed by −0.67 ± 0.24 log10 between HLA-A*03:01+ and A*03:01− women after adjusting for other known factors. Analyses of data over the 3–24 months intervals yielded almost identical results (−0.71 ± 0.25 log10, P = 0.005 for the interaction term) (Table 2).
Table 2.
Multivariable models for longitudinal viral load (VL) at two overlapping intervals of early HIV-1 infection
| Factors in model | Analyses of VLa in the 2–36 months interval | Analyses of VLa in the 3–24 months intervalb | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Δβ | SE | Adjusted P | n | Δβ | SE | Adjusted P | |
| Age (if >40)c | 80 | −0.06 | 0.10 | 0.536 | 75 | −0.10 | 0.10 | 0.309 |
| Region (if Zambia) | 206 | 0.36 | 0.07 | <0.0001 | 199 | 0.38 | 0.07 | <0.0001 |
| DOI (every 3 months) | 521 | −0.02 | 0.01 | <0.0001 | 503 | −0.02 | 0.01 | 0.007 |
| HLA-B*18 | 34 | 0.34 | 0.14 | 0.015 | 34 | 0.33 | 0.14 | 0.023 |
| HLA-B*45 | 83 | 0.25 | 0.09 | 0.009 | 82 | 0.22 | 0.10 | 0.027 |
| HLA-B*53 | 101 | 0.22 | 0.09 | 0.013 | 93 | 0.19 | 0.09 | 0.047 |
| HLA-B*57 | 48 | −0.48 | 0.12 | <0.0001 | 46 | −0.48 | 0.12 | 0.0001 |
| Female sex | 194 | −0.27 | 0.07 | <0.001 | 187 | −0.29 | 0.08 | <0.001 |
| Female sex × HLA-A*03 | 22 | −0.67 | 0.24 | 0.005 | 22 | −0.71 | 0.25 | 0.005 |
aRepeated measurements, with log10-transformation before analysis. Summary statistics: Δβ regression beta (mean deviation, Δ, from the reference group), SE standard error of the mean Δβ
bAs part of the sensitivity analyses (see text)
cFor consistency with earlier work, age is retained as a covariate regardless of its statistical significance
Sensitivity analyses
In separate analyses of subjects representing individual countries or major geographic regions (eastern versus southern Africa), the interaction term for female sex and HLA-A*03:01 was persistently favorable, with adjusted effect sizes (Δβ) ranging from −0.30 ± 0.35 log10 (P = 0.378) to −0.90 ± 0.32 log10 (P = 0.005) (Table 3), well within the 95 % confidence intervals established by the overall cohort. Data analyses restricted to subjects with different infecting HIV-1 subtypes led to similar observations as well (Table 3).
Table 3.
Alternative models for evaluating the interaction term between female sex and HLA-A*03
| Six alternative modelsa | n | Impact on longitudinal VLb | |
|---|---|---|---|
| Δβ ± SE | Adjusted P | ||
| a) Removing Kenyan subjects | |||
| Female sex | 180 | −0.29 ± 0.08 | <0.001 |
| Female sex × HLA-A*03 | 21 | −0.79 ± 0.25 | 0.002 |
| b) Removing Ugandan subjects | |||
| Female sex | 136 | −0.31 ± 0.09 | <0.001 |
| Female sex × HLA-A*03 | 13 | −0.39 ± 0.28 | 0.175 |
| c) Removing Zambian subjectsc | |||
| Female sex | 107 | −0.22 ± 0.10 | 0.039 |
| Female sex × HLA-A*03 | 15 | −0.90 ± 0.32 | 0.005 |
| d) Zambian subjects onlyd | |||
| Female sex | 87 | −0.34 ± 0.10 | 0.001 |
| Female sex × HLA-A*03 | 7 | −0.30 ± 0.35 | 0.378 |
| e) Removing rare HIV-1 subtypese | |||
| Female sex | 132 | −0.27 ± 0.08 | 0.001 |
| Female sex × HLA-A*03 | 13 | −0.76 ± 0.28 | 0.006 |
| f) Removing HIV-1 subtype A1 | |||
| Female sex | 142 | −0.33 ± 0.09 | 0.0001 |
| Female sex × HLA-A*03 | 15 | −0.53 ± 0.29 | 0.064 |
aPart of the sensitivity analyses
bRepeated measurements in the 2–36 months interval, with log10-transformation before analysis. The summary statistics are adjustment for other factors shown in Table 2. β regression beta (mean deviation, Δ, from the reference group), SE standard error of the mean (Δ)
cThe remaining subjects correspond to eastern Africans
dCorresponding to southern Africans
eDefined as others (not A1 and not C) in Table 1
Alternative multivariable models for cross-sectional VL data
In a reduced multivariable model, age, sex, geography (clinical sites), previously identified HLA variants (HLA-B*18, B*45, B*53, and B*57), and the interaction term for female sex and HLA-A*03:01 collectively explained 17.0 % of the total variance in the overall geometric mean VL during the 2- to 36-month period (P < 0.0001) (Table 4). Statistical adjustments for the number of eligible visits or the length of follow-up for each subject did affect the model (data not shown). The summary statistics remained unchanged in analysis of geometric mean VL during the 3- to 24-month period (Table 4). In this case, the joint model explained 16.2 % of the overall VL variance (Fig. 1).
Table 4.
Multivariable models for geometric mean viral load (VL) at two overlapping intervals of early HIV-1 infection
| Factors in model | Analyses of VLa in the 2–36 months interval | Analyses of VLa in the 3–24 months intervalb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Δβ | SE | Adjusted P | R 2 | n | Δβ | SE | Adjusted P | R 2 | |
| Age (if >40)c | 80 | −0.05 | 0.10 | 0.585 | 0.000 | 75 | −0.11 | 0.10 | 0.276 | 0.002 |
| Southern Africa (Zambia) | 206 | 0.35 | 0.07 | <0.0001 | 0.038 | 199 | 0.36 | 0.08 | <0.0001 | 0.040 |
| HLA-B*18 | 34 | 0.35 | 0.14 | 0.015 | 0.010 | 34 | 0.35 | 0.15 | 0.018 | 0.010 |
| HLA-B*45 | 83 | 0.29 | 0.10 | <0.001 | 0.014 | 82 | 0.27 | 0.10 | 0.008 | 0.012 |
| HLA-B*53 | 101 | 0.27 | 0.09 | 0.003 | 0.014 | 93 | 0.22 | 0.10 | 0.021 | 0.009 |
| HLA-B*57 | 48 | −0.49 | 0.12 | <0.0001 | 0.026 | 46 | −0.46 | 0.13 | <0.001 | 0.022 |
| Female sex | 194 | −0.28 | 0.08 | <0.001 | NA | 187 | −0.29 | 0.08 | <0.001 | NA |
| Female sex × HLA-A*03 | 22 | −0.72 | 0.25 | 0.004 | 0.014 | 22 | −0.77 | 0.26 | 0.003 | 0.016 |
| Overall | 521 | NA | NA | <0.0001 | 0.170 | 503 | NA | NA | <0.0001 | 0.162 |
aWith log10-transformation before analysis. Summary statistics: β regression beta (mean deviation, Δ, from the reference group), SE standard error of the mean (Δ); R 2 proportion of VL variance attributable to each factor
bAs part of the sensitivity analyses
cFor consistency with earlier work, age is retained as a covariate regardless of its statistical significance
Other HLA variants of interest
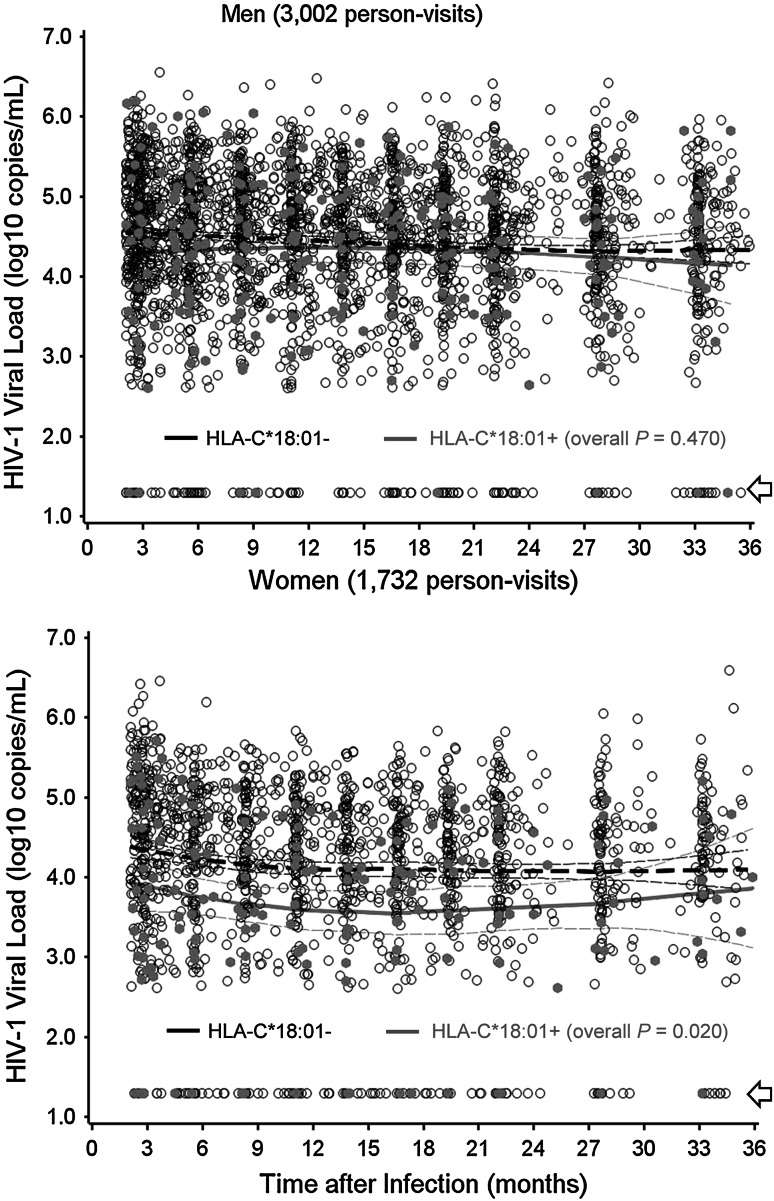
In addition to the main observations on HLA-A*03:01, HLA-C*18:01 showed a trend for favorable interaction with female sex (P = 0.042, FDR > 0.50) (Fig. 2). Lack of LD between A*03:01 and C*18:01 (D′ = −0.06, r 2 < 0.001, P = 0.90) ruled out the possibility of mutual tagging. Stratification by country did not reveal LD between A*03:01 and C*18:01 either (P = 0.34–0.99). Meanwhile, a previously reported, sex-specific effect for HLA-A*74:01 and HIV-1 VL (Koehler et al. 2010) could not be substantiated (P > 0.50 for the interaction term), although HLA-A*74:01+ and A*74:01− men did differ slightly in longitudinal VL (Δβ = −0.22 ± 0.13 log10 for HLA-A*74:01+ men, P = 0.131).
Fig. 2.
Additional evidence for gene × sex interactions. Prospective viral load measurements are plotted for HLA-C*18:01-positive and HLA-C*18:01-negative subjects. The thick and thin lines correspond to the expected mean value and 95 % confidence intervals for each stratum. Arrows indicate plasma viral load measurements that are <400 RNA copies/ml (transformed to 1.30 log10)
HLA-A*03:01-related haplotypes
In contrast to earlier observations based on five North American populations (Cao et al. 2001), HLA-A*03:01 is not in strong LD with B*07:02-C*07:02 in our study cohort. The only statistically meaningful LD profiles for A*03:01 (P ≤ 0.02) involved two haplotypes (B*49:01-C*07:01 and B*58-C*06) present in the eastern African SCs. Neither B*49:01-C*07:01 nor B*58-C*06 had differential impact on VL in women (adjusted P = 0.17 and 0.64, respectively). The observed 3-locus haplotypes containing A*03:01 were too diverse (9–11 per geographic region) to allow separate testing.
HLA-A*03-restricted HIV-1 epitopes
In the context of antigen presentation and CTL responses, multiple studies have identified HLA-A*03-restricted HIV-1 epitopes, especially a conserved epitope (KK9/RK9) in Gag (p17) (Balamurugan et al. 2010; Brumme et al. 2008; Dinges et al. 2010; Goulder et al. 1997; Heath et al. 2011; Peretz et al. 2011; Schneidewind et al. 2009). Other HLA-A*03-restricted CTL epitopes have been mapped to Env/gp120 (TW9 and VE12) (McKinnon et al. 2007; Peretz et al. 2011; Schneidewind et al. 2009), Nef (AK9, GL9, QK10, RK9, and RK10) (Almeida et al. 2011; Balamurugan et al. 2010; Brumme et al. 2008; Peretz et al. 2011; Schneidewind et al. 2009), Pol (AK11, ATK9, DI11, KA9, and SK11) (Balamurugan et al. 2010; Brumme et al. 2008; Chen et al. 2009; Peretz et al. 2011; Turnbull et al. 2009), Rev (TY9) (Yang et al. 2005), and a cryptic antigen (RR9) encoded by an alternative open reading frame (Berger et al. 2010). None of these existing immunologic data have been stratified by sex.
Further findings from bioinformatics
In populations of African ancestry (e.g., Yoruba), HLA-A*03:01 is tagged by one intergenic SNP (rs2524024), which is in strong LD (r 2 = 0.81–1.0) with 63 other intergenic SNPs distributed along a 45.1 kb region (5.9–51 kb upstream of HLA-A). The rs2524024 SNP is also a known eQTL for the integral membrane protein 2A gene (ITM2A) at Xq13.3-Xq21.2. When ranked by P values, rs2524024 (8.0 × 10−6) falls out of the top 20 eQTLs (SNPs) associated with ITM2A gene expression in lymphoblastoid cell lines (Nicolae et al. 2010).
Discussion
By focusing on generalizable findings that are applicable to eastern and southern Africa with multiple circulating HIV-1 subtypes, our analyses yielded clear evidence that female sex can be an important environmental factor to facilitate HLA class I-mediated immune control of HIV-1 infection. Because women typically have lower VL than men after acquiring HIV-1 (Fideli et al. 2001; Prentice and Tang 2012; Tang et al. 2002), our hypothesis about gene by sex interaction may offer some explanation.
In the context of HIV-1 infection, at least two earlier studies have alluded to sex-specific findings with HLA-A*74:01 and HLA-DRB1*11 (Hendel et al. 1999; Koehler et al. 2010). In our analysis, HLA-A*74:01 (a frequent allele) was weakly associated with relatively low VL in men. However, there was no evidence for interaction between HLA-A*74:01 and sex. The second hypothesis about HLA-DRB1*11 being unfavorable in women was derived from a French cohort (Hendel et al. 1999), but analyses of HIV-1-infected Zambians did not replicate that finding (Tang et al. 2010). Unlike earlier studies that did not account for potential false discoveries from random, multiple testing, the interaction term seen here for female sex and HLA-A*03:01 was accompanied by a low FDR (<0.10). A series of sensitivity analyses established that other potential confounders, including age, geography, and viral subtypes, did not obscure or compromise our analytical approaches. Data from the Multicenter AIDS Cohort Study may provide anecdotal evidence to support our key findings, as analyses of viral load and disease progression have never detected differential effects for HLA-A*03 in HIV-1-infected men (Kaslow et al. 1996; Mann et al. 1998).
Statistical significance aside, the threshold for a biologically significant difference in HIV-1 VL is around 0.30 log10 after accounting for intra- and inter-assay variability (Modjarrad et al. 2008; Saag et al. 1996). By our estimates, female sex by HLA-A*03:01 interaction was independently associated with ~0.70 log10 reduction in VL (Tables 2, 3, 4), which should impact disease progression and vertical or horizontal HIV-1 transmission.
The condition for analyzing gene by sex interactions in our study population was somewhat suboptimal. First, men and women eligible for analyses differed in several non-genetic (and potentially confounding) features (Table 1), which mandates the application of multivariable models and sensitivity analyses. As such, the effect sizes (regression beta and R 2 values) attributable to specific interaction terms often differed by statistical models and complicated the interpretation process. Second, HLA profile and genetic backgrounds can differ by country and geographic region, suggesting that our emphasis on generalizable findings might have come at the expense of country-specific phenomena. Third, sample size was not equal between men and women, so the statistical power was somewhat compromised in analyses of female-specific associations. As such, the modest trend seen with HLA-C*18:01 in women (Fig. 2) is still worth noting. In the long term, statistical models for gene by sex interactions should continue to improve when homogeneous cohorts with unbiased sex ratios are available for follow-up studies.
HLA alleles that have early influences on HIV-1 viral load tend to impose a strong selection pressure for viral immune escape mutations, as often seen in individuals with HLA-B*57 and related alleles (Bansal et al. 2007; Crawford et al. 2009; Leslie et al. 2004; Novitsky et al. 2010; Wang et al. 2009). In HIV-1-infected African women, the VL trajectory associated with HLA-A*03:01 was relatively steady in the first 3 years of follow-up (Fig. 1). Further evaluation of immune responses and HIV-1 immune escape mutations in HIV-1-infected women with HLA-A*03:01 may provide new insights about durable immune protection against a broad spectrum of HIV-1 subtypes.
Although HLA-A*03:01 itself can play an important role in inducing immune responses to a variety of CTL epitopes, it is also possible that the interaction term seen with A*03:01 actually reflects the function of other variants that operate in a sex-specific fashion. Such genetic variations can be either upstream (telomeric) or downstream (centromeric) from the HLA-A locus (Vandiedonck and Knight 2009). The LD profiles in our study cohort strongly suggested that genes downstream from the HLA-A locus, including HLA-C and HLA-B, could not explain the A*03:01 effect. Two alternative hypotheses can relate to other genomic regions. First, through strong LD with rs2524024, a trans-acting eQTLs for the ITM2A gene at Xq13.3-Xq21.2, HLA-A*03:01 can tag various functionally relevant SNPs. The ITM2A product has been shown to regulate CD8 T-cell selection and activation in mice (Kirchner and Bevan 1999). This biological connection can offer a probable mechanism for the observed interaction between HLA-A*03:01 and female sex. The other alternative hypothesis points to a long-range (~4 Mb) LD between A*03 and the C282Y mutation in HFE, which is a recessive causal variant for hereditary haemochromatosis (iron overload) in Caucasians (Cardoso and de Sousa 2003; de Bakker et al. 2006; Hanson et al. 2001). However, this is an unlikely explanation as fine mapping using the ImmunoChip array (Illumina, San Diego, CA, USA) has confirmed that haplotype blocks in the MHC region are relatively short in Africans (Prentice et al. 2014b).
Potential interactions between HLA alleles and sex have been reported for several autoimmune disorders and human malignancies (Davis and Dorak 2010; Dorak et al. 1999; Morrison et al. 2010). For HLA-A variants alone, evidence of sex-specific effect further points to a short sequence motif corresponding to polymorphic amino acid residues 161, 163, and 165 of the HLA-A protein product (Song et al. 2009). This particular sequence motif does not match the ones highlighted in a recent fine-mapping of HLA class I amino acid sequences in HIV-1-infected African Americans (in the absence of stratification by sex) (McLaren et al. 2012). Nonetheless, the HLA-A locus is often over-shadowed by HLA-B and HLA-C in studies of HIV/AIDS (Apps et al. 2013; Fellay et al. 2009; Leslie et al. 2010; Prentice and Tang 2012). If environmental factors indeed dictate how HLA-A alleles are expressed or regulated, close attention to gene × environment or gene × sex interaction should provide a deeper understanding of “missing heritability” in quantitative genetics.
Acknowledgments
This work was funded in part by IAVI and made possible by the support from many donors, including: the Bill & Melinda Gates Foundation, the Ministry of Foreign Affairs of Denmark, Irish Aid, the Ministry of Finance of Japan, the Ministry of Foreign Affairs of the Netherlands, the Norwegian Agency for Development Cooperation (NORAD), the United Kingdom Department for International Development (DFID), and the United States Agency for International Development (USAID). The full list of IAVI donors is available at http://www.iavi.org. Additional funding for this work came from (i) the United States National Institute of Allergy and Infectious Diseases (NIAID), through two R01 grants (AI071906 to R.A.K./J.T. and AI064060 to E.H.), (ii) the Fogarty AIDS International Training and Research Program (AITRP) (grant FIC 2D43 TW001042 to S.L.), and (iii) the KEMRI-Wellcome Trust Research Programme at the Centre for Geographical Medicine Research-Kilifi (Wellcome Trust award #077092). Submission of this study for publication required approval by representatives of the Kenya Medical Research Institute (KEMRI) and IAVI, but the contents are the responsibility of the study authors and do not necessarily reflect the views of IAVI, NIAID, USAID or the United States government. We thank all members of the IAVI Africa HIV Prevention Partnership for their valuable contributions to cohort assembly and collection of prospective data. We are also grateful to several associates, especially Travis R. Porter, Heather A. Prentice, and Wei Song, for assistance with genotyping and biostatistics.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Almeida CA, Bronke C, Roberts SG, McKinnon E, Keane NM, Chopra A, Kadie C, Carlson J, Haas DW, Riddler SA, Haubrich R, Heckerman D, Mallal S, John M. Translation of HLA-HIV associations to the cellular level: HIV adapts to inflate CD8 T cell responses against Nef and HLA-adapted variant epitopes. J Immunol. 2011;187:2502–2513. doi: 10.4049/jimmunol.1100691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amornkul PN, Karita E, Kamali A, Rida WN, Sanders EJ, Lakhi S, Price MA, Kilembe W, Cormier E, Anzala O, Latka MH, Bekker LG, Allen SA, Gilmour J, Fast PE, Partnership IAHP. Disease progression by infecting HIV-1 subtype in a seroconverter cohort in sub-Saharan Africa. AIDS. 2013;27:2775–2786. doi: 10.1097/QAD.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Qi Y, Carlson JM, Chen H, Gao X, Thomas R, Yuki Y, Del Prete GQ, Goulder P, Brumme ZL, Brumme CJ, John M, Mallal S, Nelson G, Bosch R, Heckerman D, Stein JL, Soderberg KA, Moody MA, Denny TN, Zeng X, Fang J, Moffett A, Lifson JD, Goedert JJ, Buchbinder S, Kirk GD, Fellay J, McLaren P, Deeks SG, Pereyra F, Walker B, Michael NL, Weintrob A, Wolinsky S, Liao W, Carrington M. Influence of HLA-C expression level on HIV control. Science. 2013;340:87–91. doi: 10.1126/science.1232685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamurugan A, Ng HL, Yang OO. Rapid T cell receptor delineation reveals clonal expansion limitation of the magnitude of the HIV-1-specific CD8+ T cell response. J Immunol. 2010;185:5935–5942. doi: 10.4049/jimmunol.1002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Yue L, Conway J, Yusim K, Tang J, Kappes J, Kaslow RA, Wilson CM, Goepfert PA. Immunological control of chronic HIV-1 infection: HLA-mediated immune function and viral evolution in adolescents. AIDS. 2007;21:2387–2397. doi: 10.1097/QAD.0b013e3282f13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger CT, Carlson JM, Brumme CJ, Hartman KL, Brumme ZL, Henry LM, Rosato PC, Piechocka-Trocha A, Brockman MA, Harrigan PR, Heckerman D, Kaufmann DE, Brander C. Viral adaptation to immune selection pressure by HLA class I-restricted CTL responses targeting epitopes in HIV frameshift sequences. J Exp Med. 2010;207:61–75. doi: 10.1084/jem.20091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookfield JF. Quantitative genetics: heritability is not always missing. Curr Biol. 2013;23:R276–R278. doi: 10.1016/j.cub.2013.02.040. [DOI] [PubMed] [Google Scholar]
- Brumme ZL, Brumme CJ, Carlson J, Streeck H, John M, Eichbaum Q, Block BL, Baker B, Kadie C, Markowitz M, Jessen H, Kelleher AD, Rosenberg E, Kaldor J, Yuki Y, Carrington M, Allen TM, Mallal S, Altfeld M, Heckerman D, Walker BD. Marked epitope- and allele-specific differences in rates of mutation in human immunodeficiency type 1 (HIV-1) Gag, Pol, and Nef cytotoxic T-lymphocyte epitopes in acute/early HIV-1 infection. J Virol. 2008;82:9216–9227. doi: 10.1128/JVI.01041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao K, Hollenbach J, Shi X, Shi W, Chopek M, Fernandez-Vina MA. Analysis of the frequencies of HLA-A, B, and C alleles and haplotypes in the five major ethnic groups of the United States reveals high levels of diversity in these loci and contrasting distribution patterns in these populations. Hum Immunol. 2001;62:1009–1030. doi: 10.1016/S0198-8859(01)00298-1. [DOI] [PubMed] [Google Scholar]
- Cardoso CS, de Sousa M. HFE, the MHC and hemochromatosis: paradigm for an extended function for MHC class I. Tissue Antigens. 2003;61:263–275. doi: 10.1034/j.1399-0039.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- Chen H, Piechocka-Trocha A, Miura T, Brockman MA, Julg BD, Baker BM, Rothchild AC, Block BL, Schneidewind A, Koibuchi T, Pereyra F, Allen TM, Walker BD. Differential neutralization of human immunodeficiency virus (HIV) replication in autologous CD4 T cells by HIV-specific cytotoxic T lymphocytes. J Virol. 2009;83:3138–3149. doi: 10.1128/JVI.02073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford H, Lumm W, Leslie A, Schaefer M, Boeras D, Prado JG, Tang J, Farmer P, Ndung’u T, Lakhi S, Gilmour J, Goepfert P, Walker BD, Kaslow R, Mulenga J, Allen S, Goulder PJ, Hunter E. Evolution of HLA-B*5703 HIV-1 escape mutations in HLA-B*5703-positive individuals and their transmission recipients. J Exp Med. 2009;206:909–921. doi: 10.1084/jem.20081984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CF, Dorak MT. An extensive analysis of the hereditary hemochromatosis gene HFE and neighboring histone genes: associations with childhood leukemia. Ann Hematol. 2010;89:375–384. doi: 10.1007/s00277-009-0839-y. [DOI] [PubMed] [Google Scholar]
- de Bakker PI, McVean G, Sabeti PC, Miretti MM, Green T, Marchini J, Ke X, Monsuur AJ, Whittaker P, Delgado M, Morrison J, Richardson A, Walsh EC, Gao X, Galver L, Hart J, Hafler DA, Pericak-Vance M, Todd JA, Daly MJ, Trowsdale J, Wijmenga C, Vyse TJ, Beck S, Murray SS, Carrington M, Gregory S, Deloukas P, Rioux JD. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat Genet. 2006;38:1166–1172. doi: 10.1038/ng1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges WL, Richardt J, Friedrich D, Jalbert E, Liu Y, Stevens CE, Maenza J, Collier AC, Geraghty DE, Smith J, Moodie Z, Mullins JI, McElrath MJ, Horton H. Virus-specific CD8+ T-cell responses better define HIV disease progression than HLA genotype. J Virol. 2010;84:4461–4468. doi: 10.1128/JVI.02438-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorak MT, Lawson T, Machulla HK, Darke C, Mills KI, Burnett AK. Unravelling an HLA-DR association in childhood acute lymphoblastic leukemia. Blood. 1999;94:694–700. [PubMed] [Google Scholar]
- Eichler EE, Flint J, Gibson G, Kong A, Leal SM, Moore JH, Nadeau JH. Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet. 2010;11:446–450. doi: 10.1038/nrg2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium. Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfax BP, Makino S, Radhakrishnan J, Plant K, Leslie S, Dilthey A, Ellis P, Langford C, Vannberg FO, Knight JC. Genetics of gene expression in primary immune cells identifies cell type-specific master regulators and roles of HLA alleles. Nat Genet. 2012;44:502–510. doi: 10.1038/ng.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, Zhang K, Gumbs C, Castagna A, Cossarizza A, Cozzi-Lepri A, De Luca A, Easterbrook P, Francioli P, Mallal S, Martinez-Picado J, Miro JM, Obel N, Smith JP, Wyniger J, Descombes P, Antonarakis SE, Letvin NL, McMichael AJ, Haynes BF, Telenti A, Goldstein DB. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay J, Ge D, Shianna KV, Colombo S, Ledergerber B, Cirulli ET, Urban TJ, Zhang K, Gumbs CE, Smith JP, Castagna A, Cozzi-Lepri A, De Luca A, Easterbrook P, Gunthard HF, Mallal S, Mussini C, Dalmau J, Martinez-Picado J, Miro JM, Obel N, Wolinsky SM, Martinson JJ, Detels R, Margolick JB, Jacobson LP, Descombes P, Antonarakis SE, Beckmann JS, O’Brien SJ, Letvin NL, McMichael AJ, Haynes BF, Carrington M, Feng S, Telenti A, Goldstein DB. Common genetic variation and the control of HIV-1 in humans. PLoS Genet. 2009;5:e1000791. doi: 10.1371/journal.pgen.1000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fideli US, Allen SA, Musonda R, Trask S, Hahn BH, Weiss H, Mulenga J, Kasolo F, Vermund SH, Aldrovandi GM. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retrovir. 2001;17:901–910. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianola D, Hospital F, Verrier E. Contribution of an additive locus to genetic variance when inheritance is multi-factorial with implications on interpretation of GWAS. Theor Appl Genet. 2013;126:1457–1472. doi: 10.1007/s00122-013-2064-2. [DOI] [PubMed] [Google Scholar]
- Goulder PJ, Walker BD. HIV and HLA class I: an evolving relationship. Immunity. 2012;37:426–440. doi: 10.1016/j.immuni.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder PJ, Sewell AK, Lalloo DG, Price DA, Whelan JA, Evans J, Taylor GP, Luzzi G, Giangrande P, Phillips RE, McMichael AJ. Patterns of immunodominance in HIV-1-specific cytotoxic T lymphocyte responses in two human histocompatibility leukocyte antigens (HLA)-identical siblings with HLA-A*0201 are influenced by epitope mutation. J Exp Med. 1997;185:1423–1433. doi: 10.1084/jem.185.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson EH, Imperatore G, Burke W. HFE gene and hereditary hemochromatosis: a HuGE review. Am J Epidemiol. 2001;154:193–206. doi: 10.1093/aje/154.3.193. [DOI] [PubMed] [Google Scholar]
- Heath SL, Sabbaj S, Bansal A, Kilby JM, Goepfert PA. CD8 T-cell proliferative capacity is compromised in primary HIV-1 infection. J Acquir Immune Defic Syndr. 2011;56:213–221. doi: 10.1097/QAI.0b013e3181ff2aba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendel H, Caillat-Zucman S, Lebuanec H, Carrington M, O’Brien S, Andrieu JM, Schachter F, Zagury D, Rappaport J, Winkler C, Nelson GW, Zagury JF. New class I and II HLA alleles strongly associated with opposite patterns of progression to AIDS. J Immunol. 1999;162:6942–6946. [PubMed] [Google Scholar]
- Kaslow RA, Carrington M, Apple R, Park L, Munoz A, Saah AJ, Goedert JJ, Winkler C, O’Brien SJ, Rinaldo C, Detels R, Blattner W, Phair J, Erlich H, Mann DL. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med. 1996;2:405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- Kawashima Y, Pfafferott K, Frater J, Matthews P, Payne R, Addo M, Gatanaga H, Fujiwara M, Hachiya A, Koizumi H, Kuse N, Oka S, Duda A, Prendergast A, Crawford H, Leslie A, Brumme Z, Brumme C, Allen T, Brander C, Kaslow R, Tang J, Hunter E, Allen S, Mulenga J, Branch S, Roach T, John M, Mallal S, Ogwu A, Shapiro R, Prado JG, Fidler S, Weber J, Pybus OG, Klenerman P, Ndung’u T, Phillips R, Heckerman D, Harrigan PR, Walker BD, Takiguchi M, Goulder P. Adaptation of HIV-1 to human leukocyte antigen class I. Nature. 2009;458:641–645. doi: 10.1038/nature07746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MF, Saad M, Bras J, Bettella F, Nicolaou N, Simon-Sanchez J, Mittag F, Buchel F, Sharma M, Gibbs JR, Schulte C, Moskvina V, Durr A, Holmans P, Kilarski LL, Guerreiro R, Hernandez DG, Brice A, Ylikotila P, Stefansson H, Majamaa K, Morris HR, Williams N, Gasser T, Heutink P, Wood NW, Hardy J, Martinez M, Singleton AB, Nalls MA, International Parkinson’s Disease Genomics C. Wellcome Trust Case Control C Using genome-wide complex trait analysis to quantify ‘missing heritability’ in Parkinson’s disease. Hum Mol Genet. 2012;21:4996–5009. doi: 10.1093/hmg/dds335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiepiela P, Leslie AJ, Honeyborne I, Ramduth D, Thobakgale C, Chetty S, Rathnavalu P, Moore C, Pfafferott KJ, Hilton L, Zimbwa P, Moore S, Allen T, Brander C, Addo MM, Altfeld M, James I, Mallal S, Bunce M, Barber LD, Szinger J, Day C, Klenerman P, Mullins J, Korber B, Coovadia HM, Walker BD, Goulder PJ. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature. 2004;432:769–775. doi: 10.1038/nature03113. [DOI] [PubMed] [Google Scholar]
- Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- Kirchner J, Bevan MJ. ITM2A is induced during thymocyte selection and T cell activation and causes downregulation of CD8 when overexpressed in CD4+CD8+ double positive thymocytes. J Exp Med. 1999;190:217–228. doi: 10.1084/jem.190.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler RN, Walsh AM, Saathoff E, Tovanabutra S, Arroyo MA, Currier JR, Maboko L, Hoelscher M, Robb ML, Michael NL, McCutchan FE, Kim JH, Kijak GH. Class I HLA-A*7401 is associated with protection from HIV-1 acquisition and disease progression in Mbeya, Tanzania. J Infect Dis. 2010;202:1562–1566. doi: 10.1086/656913. [DOI] [PubMed] [Google Scholar]
- Lazaryan A, Song W, Lobashevsky E, Tang J, Shrestha S, Zhang K, McNicholl JM, Gardner LI, Wilson CM, Klein RS, Rompalo A, Mayer K, Sobel J, Kaslow RA. The influence of human leukocyte antigen class I alleles and their population frequencies on human immunodeficiency virus type 1 control among African Americans. Hum Immunol. 2011;72:312–318. doi: 10.1016/j.humimm.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Wray NR, Goddard ME, Visscher PM. Estimating missing heritability for disease from genome-wide association studies. Am J Hum Genet. 2011;88:294–305. doi: 10.1016/j.ajhg.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie AJ, Pfafferott KJ, Chetty P, Draenert R, Addo MM, Feeney M, Tang Y, Holmes EC, Allen T, Prado JG, Altfeld M, Brander C, Dixon C, Ramduth D, Jeena P, Thomas SA, St John A, Roach TA, Kupfer B, Luzzi G, Edwards A, Taylor G, Lyall H, Tudor-Williams G, Novelli V, Martinez-Picado J, Kiepiela P, Walker BD, Goulder PJ. HIV evolution: CTL escape mutation and reversion after transmission. Nat Med. 2004;10:282–289. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- Leslie A, Matthews PC, Listgarten J, Carlson JM, Kadie C, Ndung’u T, Brander C, Coovadia H, Walker BD, Heckerman D, Goulder PJ. Additive contribution of HLA class I alleles in the immune control of HIV-1 infection. J Virol. 2010;84:9879–9888. doi: 10.1128/JVI.00320-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles RH, Munoz A, Yamashita TE, Bazmi H, Detels R, Rinaldo CR, Margolick JB, Phair JP, Mellors JW. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J Infect Dis. 2000;181:872–880. doi: 10.1086/315339. [DOI] [PubMed] [Google Scholar]
- Mahachie John JM, Van Lishout F, Van Steen K. Model-based multifactor dimensionality reduction to detect epistasis for quantitative traits in the presence of error-free and noisy data. Eur J Hum Genet. 2011;19:696–703. doi: 10.1038/ejhg.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DL, Garner RP, Dayhoff DE, Cao K, Fernandez-Vina MA, Davis C, Aronson N, Ruiz N, Birx DL, Michael NL. Major histocompatibility complex genotype is associated with disease progression and virus load levels in a cohort of human immunodeficiency virus type 1-infected Caucasians and African Americans. J Infect Dis. 1998;178:1799–1802. doi: 10.1086/314519. [DOI] [PubMed] [Google Scholar]
- McKinnon LR, Ball TB, Wachihi C, McLaren PJ, Waruk JL, Mao X, Ramdahin S, Anzala AO, Kamene J, Luo M, Fowke KR, Plummer FA. Epitope cross-reactivity frequently differs between central and effector memory HIV-specific CD8+ T cells. J Immunol. 2007;178:3750–3756. doi: 10.4049/jimmunol.178.6.3750. [DOI] [PubMed] [Google Scholar]
- McLaren PJ, Ripke S, Pelak K, Weintrob AC, Patsopoulos NA, Jia X, Erlich RL, Lennon NJ, Kadie CM, Heckerman D, Gupta N, Haas DW, Deeks SG, Pereyra F, Walker BD, de Bakker PI. Fine-mapping classical HLA variation associated with durable host control of HIV-1 infection in African Americans. Hum Mol Genet. 2012;21:4334–4347. doi: 10.1093/hmg/dds226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors JW, Kingsley LA, Rinaldo CR, Jr, Todd JA, Hoo BS, Kokka RP, Gupta P. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Internal Med. 1995;122:573–579. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS. 2008;22:2179–2185. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science. 2002;296:1439–1443. doi: 10.1126/science.1069660. [DOI] [PubMed] [Google Scholar]
- Morrison BA, Ucisik-Akkaya E, Flores H, Alaez C, Gorodezky C, Dorak MT. Multiple sclerosis risk markers in HLA-DRA, HLA-C, and IFNG genes are associated with sex-specific childhood leukemia risk. Autoimmunity. 2010;43:690–697. doi: 10.3109/08916930903567492. [DOI] [PubMed] [Google Scholar]
- Ngongo PB, Priddy F, Park H, Becker J, Bender B, Fast P, Anzala O, Mutua G, Ruzagira E, Kamali A, Karita E, Mugo P, Chomba E, Bekker LG, Roux S, Nanvubya A, Mebrahtu T. Developing standards of care for HIV prevention research in developing countries—a case study of 10 research centers in Eastern and Southern Africa. AIDS Care. 2012;24:1277–1289. doi: 10.1080/09540121.2012.656572. [DOI] [PubMed] [Google Scholar]
- Nicolae DL, Gamazon E, Zhang W, Duan S, Dolan ME, Cox NJ. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet. 2010;6:e1000888. doi: 10.1371/journal.pgen.1000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitsky V, Wang R, Margolin L, Baca J, Moyo S, Musonda R, Essex M. Dynamics and timing of in vivo mutations at Gag residue 242 during primary HIV-1 subtype C infection. Virology. 2010;403:37–46. doi: 10.1016/j.virol.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz Y, Marra O, Thomas R, Legault D, Cote P, Boulassel MR, Rouleau D, Routy JP, Sekaly RP, Tsoukas CM, Tremblay C, Bernard NF. Relative contribution of HIV-specific functional lymphocyte subsets restricted by protective and non-protective HLA alleles. Viral Immunol. 2011;24:189–198. doi: 10.1089/vim.2010.0117. [DOI] [PubMed] [Google Scholar]
- Prentice HA, Tang J. HIV-1 dynamics: a reappraisal of host and viral factors, as well as methodological issues. Viruses. 2012;4:2080–2096. doi: 10.3390/v4102080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice HA, Price MA, Porter TR, Cormier E, Mugavero MJ, Kamali A, Karita E, Lakhi S, Sanders EJ, Anzala O, Amornkul PN, Allen S, Hunter E, Kaslow RA, Gilmour J, Tang J, IAVI Africa HIV Prevention Partnership Dynamics of viremia in primary HIV-1 infection in Africans: insights from analyses of host and viral correlates. Virology. 2014;449:254–262. doi: 10.1016/j.virol.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice HA, Pajewski NM, He D, Zhang K, Brown EE, Kilembe W, Allen S, Hunter E, Kaslow RA, Tang J (2014b) Host genetics and immune control of HIV-1 infection: fine mapping for the extended human MHC region in an African cohort. Genes Immun. doi:10.1038/gene.2014.16 [DOI] [PMC free article] [PubMed]
- Price MA, Wallis CL, Lakhi S, Karita E, Kamali A, Anzala O, Sanders EJ, Bekker LG, Twesigye R, Hunter E, Kaleebu P, Kayitenkore K, Allen S, Ruzagira E, Mwangome M, Mutua G, Amornkul PN, Stevens G, Pond SL, Schaefer M, Papathanasopoulos MA, Stevens W, Gilmour J. Transmitted HIV type 1 drug resistance among individuals with recent HIV infection in East and Southern Africa. AIDS Res Hum Retrovir. 2011;27:5–12. doi: 10.1089/aid.2010.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince JL, Claiborne DT, Carlson JM, Schaefer M, Yu T, Lahki S, Prentice HA, Yue L, Vishwanathan SA, Kilembe W, Goepfert P, Price MA, Gilmour J, Mulenga J, Farmer P, Derdeyn CA, Tang J, Heckerman D, Kaslow RA, Allen SA, Hunter E. Role of transmitted Gag CTL polymorphisms in defining replicative capacity and early HIV-1 pathogenesis. PLoS Pathog. 2012;8:e1003041. doi: 10.1371/journal.ppat.1003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putter C, Pechlivanis S, Nothen MM, Jockel KH, Wichmann HE, Scherag A. Missing heritability in the tails of quantitative traits? A simulation study on the impact of slightly altered true genetic models. Hum Hered. 2011;72:173–181. doi: 10.1159/000332824. [DOI] [PubMed] [Google Scholar]
- Queitsch C, Carlson KD, Girirajan S. Lessons from model organisms: phenotypic robustness and missing heritability in complex disease. PLoS Genet. 2012;8:e1003041. doi: 10.1371/journal.pgen.1003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- Rajapaksa US, Li D, Peng YC, McMichael AJ, Dong T, Xu XN. HLA-B may be more protective against HIV-1 than HLA-A because it resists negative regulatory factor (Nef) mediated down-regulation. Proc Natl Acad Sci USA. 2012;109:13353–13358. doi: 10.1073/pnas.1204199109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland M, Carlson JM, Manocheewa S, Swain JV, Lanxon-Cookson E, Deng W, Rousseau CM, Raugi DN, Learn GH, Maust BS, Coovadia H, Ndung’u T, Goulder PJ, Walker BD, Brander C, Heckerman DE, Mullins JI. Amino-acid co-variation in HIV-1 Gag subtype C: HLA-mediated selection pressure and compensatory dynamics. PLoS ONE. 2010;5:e12463. doi: 10.1371/journal.pone.0012463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom KR, Dreszer TR, Pheasant M, Barber GP, Meyer LR, Pohl A, Raney BJ, Wang T, Hinrichs AS, Zweig AS, Fujita PA, Learned K, Rhead B, Smith KE, Kuhn RM, Karolchik D, Haussler D, Kent WJ. ENCODE whole-genome data in the UCSC Genome Browser. Nucleic Acids Res. 2010;38:D620–D625. doi: 10.1093/nar/gkp961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saag MS, Holodniy M, Kuritzkes DR, O’Brien WA, Coombs R, Poscher ME, Jacobsen DM, Shaw GM, Richman DD, Volberding PA. HIV viral load markers in clinical practice. Nat Med. 1996;2:625–629. doi: 10.1038/nm0696-625. [DOI] [PubMed] [Google Scholar]
- Schneidewind A, Tang Y, Brockman MA, Ryland EG, Dunkley-Thompson J, Steel-Duncan JC, St John MA, Conrad JA, Kalams SA, Noel F, Allen TM, Christie CD, Feeney ME. Maternal transmission of human immunodeficiency virus escape mutations subverts HLA-B57 immunodominance but facilitates viral control in the haploidentical infant. J Virol. 2009;83:8616–8627. doi: 10.1128/JVI.00730-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Ruder AM, Hu L, Li Y, Ni R, Shao W, Kaslow RA, Butler M, Tang J. Genetic epidemiology of glioblastoma multiforme: confirmatory and new findings from analyses of human leukocyte antigen alleles and motifs. PLoS ONE. 2009;4:e7157. doi: 10.1371/journal.pone.0007157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Tang S, Lobashevsky E, Myracle AD, Fideli U, Aldrovandi G, Allen S, Musonda R, Kaslow RA. Favorable and unfavorable HLA class I alleles and haplotypes in Zambians predominantly infected with clade C human immunodeficiency virus type 1. J Virol. 2002;76:8276–8284. doi: 10.1128/JVI.76.16.8276-8284.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Malhotra R, Song W, Brill I, Hu L, Farmer PK, Mulenga J, Allen S, Hunter E, Kaslow RA. Human leukocyte antigens and HIV type 1 viral load in early and chronic infection: predominance of evolving relationships. PLoS ONE. 2010;5:e9629. doi: 10.1371/journal.pone.0009629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Cormier E, Gilmour J, Price MA, Prentice HA, Song W, Kamali A, Karita E, Lakhi S, Sanders EJ, Anzala O, Amornkul PN, Allen S, Hunter E, Kaslow RA. Human leukocyte antigen variants B*44 and B*57 are consistently favorable during two distinct phases of primary HIV-1 infection in sub-Saharan Africans with several viral subtypes. J Virol. 2011;85:8894–8902. doi: 10.1128/JVI.00439-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull EL, Wong M, Wang S, Wei X, Jones NA, Conrod KE, Aldam D, Turner J, Pellegrino P, Keele BF, Williams I, Shaw GM, Borrow P. Kinetics of expansion of epitope-specific T cell responses during primary HIV-1 infection. J Immunol. 2009;182:7131–7145. doi: 10.4049/jimmunol.0803658. [DOI] [PubMed] [Google Scholar]
- Vandiedonck C, Knight JC. The human major histocompatibility complex as a paradigm in genomics research. Brief Funct Genomic Proteomic. 2009;8:379–394. doi: 10.1093/bfgp/elp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YE, Li B, Carlson JM, Streeck H, Gladden AD, Goodman R, Schneidewind A, Power KA, Toth I, Frahm N, Alter G, Brander C, Carrington M, Walker BD, Altfeld M, Heckerman D, Allen TM. Protective HLA class I alleles that restrict acute-phase CD8+ T-cell responses are associated with viral escape mutations located in highly conserved regions of human immunodeficiency virus type 1. J Virol. 2009;83:1845–1855. doi: 10.1128/JVI.01061-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–D934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang OO, Daar ES, Jamieson BD, Balamurugan A, Smith DM, Pitt JA, Petropoulos CJ, Richman DD, Little SJ, Brown AJ. Human immunodeficiency virus type 1 clade B superinfection: evidence for differential immune containment of distinct clade B strains. J Virol. 2005;79:860–868. doi: 10.1128/JVI.79.2.860-868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Prentice HA, Farmer P, Song W, He D, Lakhi S, Goepfert P, Gilmour J, Allen S, Tang J, Kaslow RA, Hunter E. Cumulative impact of host and viral factors on HIV-1 viral load control during early infection. J Virol. 2013;87:708–715. doi: 10.1128/JVI.02118-12. [DOI] [PMC free article] [PubMed] [Google Scholar]