Abstract
Disengagement of emotion regulation circuits was previously shown in depressed mothers and was hypothesized to underlie the impaired maternal–infant sensitivity described in postpartum depression (PPD). We hypothesized similarly reduced resting-state functional connectivity in default mode network (DMN) regions involved in social cognition in PPD. Resting-state functional MRI, clinical and mother–infant attachment data were obtained from 14 unmedicated postpartum women with major depression and 23 healthy postpartum women. Posterior cingulate cortex (PCC) time series were extracted, filtered between 0.007 and 0.08 Hz and used as regressors in a whole brain general linear model analysis. PCC–right amygdala connectivity was significantly disrupted in depressed compared to healthy mothers for low-frequency neural activity, showing a negative (inverse) coupling in the depressed group but not in the controls. PCC–right amygdala connectivity was positively correlated with PCC–parahippocampus connectivity. Resting connectivity patterns of positive co-activations in postpartum women mirrored the canonical DMN. These findings of reduced PCC–amygdala coupling raise the possibility that PPD might involve the disruption of outward, preventative aspects of self-relevant thought and theory of mind/empathy processes. Further integrated studies of neural connectivity and these cognitive/behavioral dimensions are warranted.
Keywords: postpartum depression, default mode network, posterior cingulate cortex, fMRI
INTRODUCTION
Postpartum depression (PPD) accounts for significant disability of reproductive-aged women given the 19% period prevalence of PDD in developed countries during the 3-month postpartum period (Gavin et al., 2005) and nearly 60% prevalence rates in inner-city (Hobfoll et al., 1995) and adolescent populations (Trad, 1995). PPD strikes precisely at a time when sensitivity and synchrony of maternal–infant interactions are required to positively shape the child’s long-term behavioral, cognitive and socioemotional development (Feldman, 2007; Field, 2010). Depressed mothers’ diminished ability to recognize and respond sensitively to their infant’s affective cues and their decreased ability to recognize their infant as separate beings with unique desires (mind-mindedness) consequently places offspring at risk for poor emotion regulation, insecure attachment problems and developmental delays. Although awareness and detection of PPD and its adverse effects on children has increased (Wichman et al., 2010), treatment trials reveal disappointing PPD remission rates of 40–50% (O'Hara et al., 2000; Wisner et al., 2006) and, furthermore, extant PPD treatments may not uniformly modify the negative developmental impact of PPD on the child (Weinberg and Tronick, 1998; Weissman et al., 2006).
To enhance the understanding of maternal neural processing of emotional stimuli in order to guide preventative treatments for PPD, we have undertaken emotional processing studies of depressed and healthy mothers within 2–3 months postpartum. In our study of implicit negative emotional face processing (Moses-Kolko et al., 2010), face-related amygdala activity did not distinguish postpartum healthy from depressed women; however, within the PPD group, infant-related hostility was associated with lower right amygdala activity to faces. This suggested that a disengagement of amygdala to negative emotions in PPD could be a neural mechanism for deficient maternal detection of salient infant cues and impaired maternal sensitivity reported in PPD. We also reported that dorsomedial prefrontal (dmPFC) activity and preceding dmPFC–amygdala connectivity to fearful and threatening faces were significantly reduced in PPD, further suggesting disengagement in PPD of a cortico-limbic circuit critical for emotional reappraisal and empathic/self-other relational processes.
In the present study, we build upon our earlier findings of neural circuitry disruptions in PPD through examination of resting-state neural connectivity, in which the spontaneous blood oxygen-level-dependent (BOLD) activity has the potential to shed light on intrinsic functional organization of the postpartum healthy vs depressed brain. We focus on the default mode network (DMN), which encompasses most prominently the posterior (PCC) and anterior (ACC) cingulate cortices, inferior parietal cortex and precuneus (Buckner et al., 2008). The DMN is a temporally coherent network found to be more active during passive than active cognitive tasks, as shown in analyses of pooled neuroimaging data from diverse positron emission tomography studies (Shulman et al., 1997; Mazoyer et al., 2001) and later confirmed using functional magnetic resonance imaging (fMRI: Greicius et al., 2003). As post-scan interviews identified that subjects often thought about autobiographical material, past experiences, making plans and personal thoughts and experiences during resting-state paradigms, the DMN is commonly considered a network that subserves self-referential processing [reviewed by Buckner et al. (2008)]. The self-referential role of DMN has been further validated by studies comparing spontaneous/internal cognition to external attention and monitoring (Andrews-Hanna et al., 2010) and studies designed to specifically elicit self-referent ideation (Johnson et al., 2006; Northoff et al., 2006). Although DMN circuitry is detected during different states of consciousness, including sleep, coupling of PCC and ACC is less apparent during sleep relative to resting wakefulness (Larson-Prior et al., 2011; Samann et al., 2011). We were particularly interested in PCC connectivity with whole brain given the centrality of the PCC within the default mode brain circuit, reports of altered PCC connectivity to limbic regions in major depressive disorder (MDD) (Greicius et al., 2007; Berman et al., 2011), involvement of PCC in more outward, preventative aspects of self-relevant thought, including duties and responsibilities to others (Johnson et al., 2006) as well as theory of mind mental functions (Mars et al., 2012). As successful maternal role attainment involves identity transformation from being self to other focused (Mercer, 2004), and in line with our prior findings of disengagement of circuits of empathy and social cognition in PPD, we hypothesized that depressed mothers would have decreased PCC connectivity to other limbic regions relative to healthy mothers, in whom socially focused reflective ideation would be more intact during resting states.
METHODS
Subjects
Subjects provided written informed consent as approved by the University of Pittsburgh, Biomedical Institutional Review Board. Subjects delivered a healthy, term infant in the preceding 12 weeks, were medication free, multiparous and breastfeeding or bottlefeeding. Depression was defined by DSM IV criteria for unipolar major depression (First et al., 1998) and a 25-item Hamilton depression scale score ≥15. Both prevalent (beginning antenatally) and incident (new onset postpartum) cases of PPD were included to maximize generalizability, since the disorder commonly begins antenatally (Stowe et al., 2005; Wisner et al., 2013). Women with bipolar illness were excluded. Healthy subjects had no personal or family history of an Axis I affective disorder. Subjects were excluded if they had medical or neurological illnesses likely to affect cerebral physiology or anatomy, gross abnormalities of brain structure evident by magnetic resonance images, suicidal intent, substance abuse within 1 year, lifetime history of substance dependence (other than nicotine), eating disorders, use of hormonal contraception or exposure to medications likely to alter cerebral physiology within 3 weeks.
Sixteen depressed and 28 healthy mothers were enrolled and imaged. Clinical variables collected on the scan day included the Hamilton depression scale, the Edinburgh Postnatal Depression Scale [a well-validated, 10-item self-report measure of perinatal depression, anxiety and function (Cox et al., 1987)] and the parent-to-infant attachment questionnaire [a reliable and valid self-report of attachment quality, hostility and pleasure in interaction during the first postpartum year (Condon and Corkindale, 1998)]. Statistical tests of group differences in demographic, reproductive, psychiatric and behavioral data were performed with Pearson chi-square for categorical and Mann–Whitney U-exact tests for continuous variables.
fMRI data acquisition and analysis
Imaging was performed using a 3 T Siemens Trio scanner (Erlangen, Germany) at the UPMC MRRC. High-resolution, T1-weighted structural images were acquired using a magnetization prepared rapid gradient-echo sequence [repetition time (TR) = 2300 ms, echo time (TE) = 3.29 ms, flip angle = 9°, field of view (FOV) 208 × 256 mm2, slice thickness = 1 mm, matrix = 208 × 256, 176 continuous slices]. Subjects were then instructed to rest and not think of anything in particular, with their eyes closed for the duration of 5 min. They were asked to remain awake during the 5 min acquisition. Resting blood oxygenation level dependent (BOLD) images were acquired using a gradient-echo echoplanar image sequence [150 whole-brain volumes, TR = 2000 ms, TE = 31 ms, flip angle = 90°, FOV = 224 × 224 mm2, slice thickness = 3 mm, matrix = 64 × 64, covering 36 axial slices].
Data from seven subjects were excluded for positive Tetrahydrocannabinol (THC) on urine drug screen (n = 2), brain anomaly (n = 1) and movement >2 mm (n = 4), resulting in 14 depressed and 23 healthy mothers with usable functional data. Neuroimaging data were preprocessed and analyzed using Statistical Parametric Mapping software (SPM8, www.fil.ion.ucl.ac.uk/spm). Images were realigned and unwarped, spatially normalized to a standardized stereotactic space (Montreal Neurological Institute) via the segmented structural image (the ‘Unified Segmentation’ method), resampled to 3 × 3 × 3 mm3 voxels and smoothed using an 8 mm full-width half-maximum Gaussian kernel.
In order to reduce the confounding effects of physiological noise, a variation of the component-based CompCor algorithm was performed. The CompCor algorithm assumes that BOLD signal fluctuations in non-gray matter tissue [i.e. white matter, cerebrospinal fluid (CSF)] or in voxels with high variability largely reflect physiological (e.g. cardiac, respiratory) noise, such that time series from these regions are effective noise regressors in subsequent analyses (Behzadi et al., 2007). Time series were extracted from voxels in a white matter/CSF mask or from voxels with a high standard deviation (the top 2% within a whole-brain mask), and then band-pass filtered between 0.007–0.15 Hz. Using singular value decomposition, the five principal components that explained the majority of variance within this matrix were extracted.
PCC was defined by the anatomical boundaries (cluster 9) established in a prior study, which divided the cingulate cortex into distinct clusters based upon diffusion tractography and connectivity-based parcellation techniques (Beckmann et al., 2009). The PCC region of interest (ROI) overlapped Brodmann area 31 and other similar ROIs in resting BOLD studies of MDD. Next, we averaged the time series of all voxels within the PCC ROI. Nuisance regressors, including a mean whole-brain average time series high-pass filtered at 0.15 Hz, the five physiological regressors described above, six motion parameters and a linear trend were included as independent measures in a multiple regression analysis, with the average PCC time series as the dependent measure. The resulting residuals were band-pass filtered twice at low (0.007–0.08 Hz) and mid (0.08–0.15 Hz) frequencies (Salvador et al., 2008), and each were mean and standard deviation corrected. The two resulting time series were then used as covariates of interest in a whole-brain linear regression (SPM8) for each subject (first-level analysis). The nuisance regressors (five physiological/six motion/one whole brain) were also included in the model. A high-pass filter of 143 s/0.007 Hz was included in the SPM first-level model. Autoregressive (AR(1)) modeling was also applied.
As the majority of resting-state fMRI studies focus on low frequencies, contrast images for low-frequency regressors for each subject were entered in the second-level analysis. A secondary analysis on the mid frequencies was also performed to explore connections among subcortical structures at this frequency range (Salvador et al., 2008). Analysis was conducted using a series of planned contrasts. Results of this initial analysis focused our attention on PCC–right amygdala connectivity as an important circuit that was disrupted in PPD. We therefore performed several follow-up tests, including examining bivariate relationships between clinical variables and PCC–amygdala-extracted connectivity values from the whole sample. An additional ROI analysis was performed to clarify the involvement of different amygdala subregions using analytic techniques (3 mm voxel size, 8 mm smoothing kernel) akin to Hurlemann et al. (2008), suited for resolving larger nuclei of the amygdala. Additionally, because direct anatomical connections between PCC and amygdala are thought to be weak or absent (Stein et al., 2007; Robinson et al., 2010), we examined the overlap of networks connected to the PCC and networks connected to the right amygdala to elucidate, which intermediate brain regions displayed significant connectivity with both the PCC and right amygdala.
RESULTS
Subject characteristics
Healthy and depressed mothers did not differ on demographic and medical/obstetric characteristics with the exception that depressed mothers were scanned on average 2 weeks closer to delivery (8.1 ± 2.2 weeks postpartum) relative to healthy mothers (10.4 ± 1.9) (P < 0.05). Subjects were free from hormonal contraceptives except for a single control subject who had a levonorgestrel intrauterine device inserted 6 days prior to the scan (Table 1). Depressed mothers were medication free for at least 2 years prior to the date of the scan. As expected, depressed mothers were significantly more depressed and anxious, had more reported nighttime awakenings, were more likely to have taken antidepressants in the past and were less well attached to their infants relative to healthy mothers.
Table 1.
Sample characteristics, mean (s.d.)
| Healthy mothers, N = 23 | Depressed mothers, N = 14 | |
|---|---|---|
| Demographic characteristics | ||
| Age | 27.7 (5.2) | 26.4 (5.1) |
| Education (years) | 15.8 (2.9) | 15.1 (2.3) |
| Caucasian, n | 18 (78.3%) | 10 (71.4%) |
| Medical/reproductive characteristics | ||
| Right handed | ||
| Body mass index | 26.8 (4.0) | 30.0 (5.9) |
| Smoker, n | 2 (8.7%) | 3 (21.4%) |
| Primiparous, n | 12 (52.2%) | 7 (50.0%) |
| Breastfeeding, n | 14 (60.9%) | 12 (85.7%) |
| Time since childbirth (weeks)* | 10.4 (1.9) | 8.1 (2.2) |
| Psychiatric characteristics | ||
| Antidepressant naïve*, n | 23 (100%) | 11 (78.6%) |
| Hamilton depression rating scale score (25-item)** | 3.3 (2.6) | 21.6 (6.9) |
| Hamilton anxiety rating scale score** | 1.4 (2.0) | 13.9 (6.2) |
| Edinburgh postnatal scale for depression score** | 1.6 (1.3) | 14.0 (4.8) |
| Quality of mother–infant attachmenta,** | 43.3 (1.9) | 35.7 (7.1) |
| Absence of maternal–infant hostilitya,** | 22.2 (1.9) | 15.9 (4.1) |
| Pleasure in maternal–infant interactiona,** | 22.7 (1.6) | 18.7 (5.8) |
| Average no. of awakenings per night in the past 2 weeks* | 1.7 (1.0) | 3.0 (1.3) |
| Average no. of minutes awake per night in past 2 weeks | 53.9 (42.1) | 50.8 (33.6) |
Values are expressed as mean (s.d.) unless otherwise specified.
aCondon scale for parent–infant attachment (Condon and Corkindale, 1998).
*P < 0.05; **P < 0.001.
Functional connectivity analysis
Across all subjects, the low-frequency (0.007–0.08 Hz) filtered PCC time series was positively coupled to a set of regions reflecting the canonical ‘DMN’, including the medial prefrontal cortex, lateral parietal cortex, thalamus, parahippocampal gyrus and middle temporal gyrus at a whole brain, family wise error (FWE) rate corrected threshold of p < 0.05 (Figure 1). The same analysis conducted separately within the depressed and healthy groups revealed similar patterns of findings. Regions negatively coupled with the PCC seed included a variety of canonical ‘task positive’ regions at corrected significance levels, including the insula, lateral prefrontal cortex, sensorimotor association cortex, parietal cortex and visual cortex (Figure 1). The PCC was similarly anticorrelated with these regions across each group. For the mid-frequency (0.08–0.15 Hz) filtered PCC time series, activity within the PCC was also positively associated with similar default network regions and likewise was negatively associated with the anterior insula and visual cortex across all participants and within each subgroup.
Fig. 1.
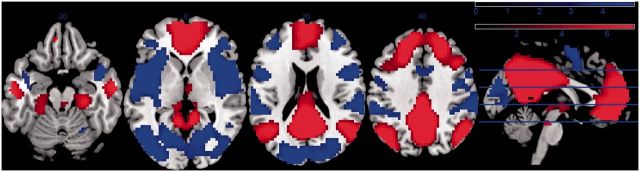
Statistical map of PCC/whole-brain functional connectivity at low frequencies (0.007–0.08 Hz) across all subjects (n = 37: threshold set at uncorrected P < 0.001). Regions in red represent areas that are positively coupled and blue regions represent areas that are negatively coupled with PCC time series. The scale at the top right represents the color coding of the range of t-statistics.
Direct comparison of depressed and healthy mothers revealed a region overlapping with the right amygdala, which was reduced in depressed compared with healthy mothers (peak voxel: 33 5 −20; T = 5.45, P = 0.043 FWE corrected) for low but not mid-frequency neural activity (Figure 2). Within the whole cluster (74 voxels, thresholded at P < 0.001, uncorrected), a negative coupling between the amygdala region and the PCC was observed in the patient group [activation significantly less than zero: t(13) = −3.97, P = 0.002], whereas the PCC–amygdala coupling did not differ from zero in the control group [t(22)<1]. Using the template defined by the SPM anatomy toolbox, we investigated this difference in more detail. The peak voxel itself lay just anterior to the basolateral region of the amygdala. The cluster overlapped with the right superficial (12.7% of cluster within region) and basolateral (6.8%) nuclei of the amygdala, as well as extending in a lateral and rostral direction into the anterior temporal lobe. We then performed an ROI analysis within six amygdala subregions (bilateral superficial, basolateral and centromedian), Bonferroni correcting for the six tests. Connectivity group differences between the PCC and the right superficial [t(35) = 2.80, P = 0.008, partial η2 = 0.18] and basolateral [t(35) = 3.014, P = 0.005, partial η2 = 0.21] subregions reached corrected significance, whereas differences between the right centromedian [t(35) = 2.34, P = 0.025, partial η2 = 0.14] and left basolateral [t(35) = 2.64, P = 0.012, partial η2 = 0.17] subregions reached uncorrected significance. Across all analyses (whole brain and ROI), no regions were found to show significantly greater PCC connectivity in depressed compared with healthy mothers.
Fig. 2.
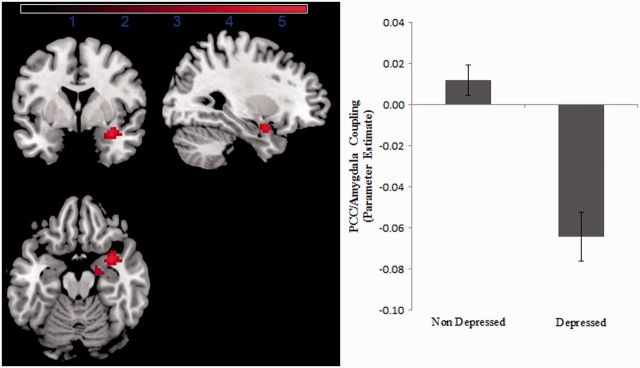
Figure describing the right amygdala region which displayed reduced PCC–amygdala connectivity in depressed compared with healthy mothers (peak voxel: 33 5 −20; T = 5.45, P = 0.043, FWE corrected). The region (displayed at a threshold of P < 0.001, uncorrected) overlapped with the right basolateral and superficial nuclei of the amygdala. Parameter estimates of the PCC connectivity within the significant region displayed on the right hand side (error bars reflect standard error of the mean).
We investigated whether variation in clinical symptoms was associated with PCC–right amygdala coupling within the sample as a whole or the depressed and healthy subgroups individually. No association between connectivity and mother–infant attachment was observed. Hamilton depression scale scores were inversely correlated with PCC–right amygdala coupling, as would be expected based upon the group difference (see Supplementary data). Although these relationships were not significant in the depressed and healthy subgroups individually, a signal for a dose–response relationship (Supplementary Figure S1) might be strengthened with a larger sample. Taking into consideration the significantly greater reports of nighttime awakenings in depressed relative to healthy mothers (see Table 1), we examined whether the altered PCC–amygdala coupling in PPD could be explained by greater sleepiness during scan acquisition. We found no significant correlation between sleep variables and PCC–right amygdala connectivity in the full sample or depressed and healthy subsamples. When we examined the potentially confounding effect of scan acquisition closer to delivery in depressed relative to healthy mothers, we found that duration postpartum at time of scan was significantly correlated with PCC–right amygdala coupling (r = 0.40, n = 37, P = 0.014); however, when entered as a covariate with depression status in a linear regression of PCC–amygdala connectivity, duration postpartum had no impact on the main effect of depression status on disrupted PCC–right amygdala coupling, and duration postpartum was not a significant covariate in the model (F < 1) and [F(1,34) = 22.21, P < 0.001]. Including duration postpartum in the whole-brain analysis prevented the right amygdala group difference cluster reaching a whole-brain corrected significance threshold, but the same peak voxel remained significant at uncorrected thresholds (T = 4.62, P < 0.001).
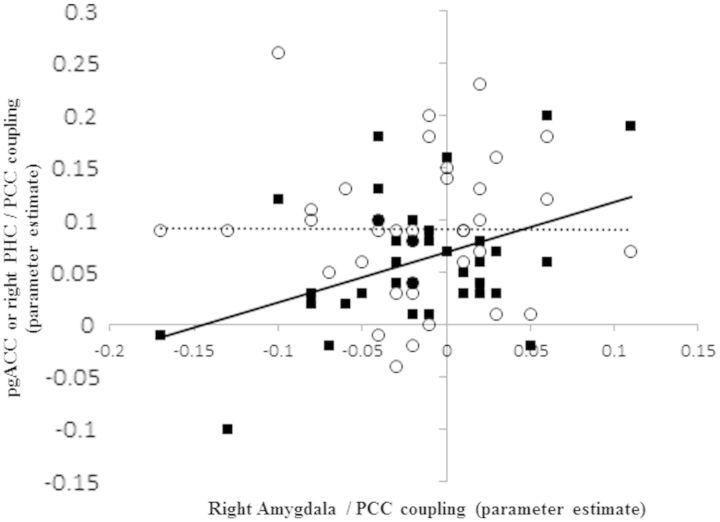
To define the overlap between networks connected to the PCC and networks connected to the right amygdala, we first created a functional mask by seeding the right amygdala region identified in the group contrast, thresholded at P < 0.001 uncorrected. Across both groups, we observed a network including bilateral amygdala, insula, parahippocampal gyrus, temporal lobe and a region of the medial frontal cortex (dorsal/mid ACC). A negative coupling between this seed region and the precuneus and lateral prefrontal cortex was observed. We then examined PCC connectivity within this functional mask, thresholded voxelwise at P < 0.001, uncorrected. As expected, given anatomical evidence the most prominent regions of PCC positive connectivity were within the bilateral parahippocampal gyrus/subiculum (21 −19 −20; −24 −19 −23) and a region of pregenual ACC (6 26 10) (Figure 3). We also noted PCC positive connectivity with several small brainstem clusters (e.g. 9 −13 14) and a temporal lobe cluster (48 −7 −23). PCC–ACC [t(35)<1] and PCC–bilateral parahippocampal gyrus/subiculum connectivity (t’s < 1.46, P’s > 0.15) did not differ between depressed and healthy mothers. However, within the full sample, PCC–right amygdala connectivity was correlated with PCC–parahippocampus gyrus/subiculum connectivity [left PHC (r = 0.33, n = 37, P = 0.044); right PHC (r = 0.42, n = 37, P = 0.011)] but not PCC–ACC connectivity (r = −0.002, n = 37, P = 0.99) [hypothesis of equality of correlations coefficients was rejected [t(34) = 2.00, P = 0.054]] (Figure 4). That the ACC did not appear to play any role in our findings was supported by a further analysis in which we seeded the ACC, using a mask specified by regions 2 and 3 of the Beckmann study (2009). Although activity in this seed was strongly coupled to the DMN regions identified by the PCC across all participants, as well as other regions such as the striatum and amygdala (particularly the superficial subregion), significant group differences in ACC connectivity were neither observed within amygdala subregions [t(35) < 1.1 in all cases] nor at the whole-brain level. Together, these results raise the possibility that the intermediate parahippocampal gyrus/subiculum region may play a role in the disrupted amygdala–PCC resting connectivity in PPD, and that this disruption is not a consequence of a generalized abnormality of default mode activation.
Fig. 3.
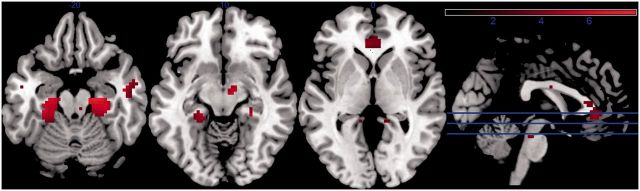
Figure representing most prominent regions of PCC connectivity within a mask constrained to amygdala–whole-brain connectivity (P < 0.001, uncorrected). This included the bilateral parahippocampal gyrus/subiculum (21 −19 −20; −24 −19 −23) and a region of pregenual ACC (6 26 10). There were no significant between-group differences in PCC connectivity within these regions.
Fig. 4.
Association of PCC–right amygdala connectivity with PCC–parahippocampal and PCC–pregenualACC connectivity: PCC–right amygdala coupling correlated positively and significantly with PCC–right parahippocampus/subiculum coupling (black squares) but not PCC–pgACC coupling (open circles).
DISCUSSION
The present study is the first to our knowledge to examine resting-state functional neural connectivity in postpartum women. An advantage of resting-state fMRI is that it provides data informing neural circuitry dysfunction without the need for more complex fMRI challenge tasks. A strength of the study is that it was performed in unmedicated, carefully characterized depressed and healthy mothers in the early puerperium. In this study, we focused on the ‘DMN’, by examining functional correlations between the PCC and the rest of the brain. The canonical network of coactivated regions was replicated, as well as an anticorrelation between the PCC and a network of ‘task positive’ regions. We also report the novel finding of a clear disruption of PCC connectivity with the right amygdala in depressed but not healthy mothers.
Anatomical evidence suggests that a direct connection between the PCC and the amygdala, if present, is somewhat limited (Stein et al., 2007; Robinson et al., 2010). However, there are at least two plausible alternative pathways connecting the two regions: one via the anterior cingulate/medial prefrontal cortex, and the second via the retrosplenial cortex and parahippocampal gyrus (Stefanacci et al., 1996; Stein et al., 2007). Our post hoc analyses provided evidence for the presence of both of these connections, insofar as positive connectivity with both PCC and the right amygdala region was observed in these regions. Since the strength of PCC/amygdala coupling covaried across individuals with PCC/parahippocampus coupling, but not PCC/ACC coupling, it will be important in future studies to perform mediation analyses to further examine the role of a parahippocampal circuit in the disrupted PCC/amygdala network in PPD. Additionally, two studies employing meta-analytic connectivity modeling have suggested that amygdala, the basolateral subregion in particular, is often activated by similar tasks or contrasts that activate the DMN (e.g. pregenual cingulate and the PCC) (Robinson et al., 2010; Bzdok et al., 2012). It is therefore likely that the amygdala has an important functional relationship with the posterior cingulate, even if this is mediated via a polysynaptic pathway.
The interpretation of the negative coupling between PCC and amygdala in the depressed group, seen in light of an ongoing controversy within the resting fMRI literature (Fox et al., 2009; Murphy et al., 2009; Scholvinck et al., 2010; Chai et al., 2012), may not be straightforward. We consider broadly two possible accounts of the finding. First, there is a progressively weaker pattern of positive, functional connectivity between PCC and parahippocampus/subiculum and then amygdala. The result is that the PCC and amygdala are essentially uncorrelated in the depressed group, but that our method of physiological noise correction overcompensates, leading to an apparent negative correlation (Murphy et al., 2009) A second interpretation is that there is an overactive inhibitory connection in the depressed group compared with the non-depressed group. This would constitute an unusual finding when seen in the context of previous studies examining DMN activation in major depression. Prominent observations, within a highly heterogeneous literature (Wang et al., 2012), have included enhanced cross-network associations with the DMN via the dorsal nexus (Sheline et al., 2010), stronger coupling between DMN and limbic structures (Greicius et al., 2007) or the attenuation of a positive coupling present in healthy individuals (Anand et al., 2005). Verifying the presence of a specific inhibitory connection remains a topic for future study and may have nosological implications for the status of PPD among other mood disorders.
The study findings of the disrupted PCC connectivity with the right amygdala suggest potentially important alterations in default mode processing in PPD. Default mode processing is understood to encompass self-relevant thought (Buckner et al., 2008) and well as theory of mind functions (Mars et al., 2012). Indeed, the PCC extending into precuneus is increasingly recognized for its role in self-reflection and social cognition (Mars et al., 2012), although the PCC does serve other diverse functions including visuospatial orientation and memory and processing of emotional and non-emotional information (Vogt et al., 2006). Johnson and colleagues’ study of subtypes of self-relevant thought noted the anteroinferior PCC having a more outward, preventative focus on duties and responsibilities and ventromedial ACC having a more inward, promotional focus on self-related hopes and aspirations and (Johnson et al., 2006). Mothers who have adapted well to their new role as infant caregivers would be expected to have a stronger focus on infant-related responsibilities as well as being more involved in thinking about the intentions of others, particularly the newborn, during resting states. Since PPD has been associated with impaired quality of mother–infant attachment in the broader literature as well as this sample, we speculate that disrupted amygdala–PCC connectivity might be related to how cognitively oriented the mother is to others in her social network, particularly the infant, as well as her adaptation to the responsibilities of motherhood. Our measure of mother–infant attachment did not show a relationship to resting-state neural connectivity. Future studies with more rigorous dimensional ratings of mother–infant attachment and self-reflective function are needed to substantiate these speculations. Additionally, because pre-existing social cognition impairments may contribute to the pathogenesis of depression, theory of mind and other social cognition processes also deserve further elucidation with respect to neural connectivity in PPD.
It is possible that the PCC–amygdala disconnectivity may stem more from cognitive differences in attention and memory in the PPD group, a dimensional characteristic highlighted in a recent factor analysis of newly postpartum women (Marrs et al., 2009). Since PCC–parahippocampal cortical connections provide access to autobiographical and other memories involved in self/other-relevant thought, women with PPD may be less apt to access these cognitive functions. Based on the current study design, it is also unknown to what extent pre-existing depression history and neural vulnerability traits for depression contributed to circuit disruption and possibly impaired social cognition (Barrett and Fleming, 2011).
Although published data on inter-regional connectivity in healthy mothers are sparse, engagement of maternal amygdala activity to infant stimuli is a consistent finding among healthy mothers (Seifritz et al., 2003; Leibenluft et al., 2004; Ranote et al., 2004; Swain et al., 2008; Lenzi et al., 2009), and echoes the broader literature describing the role of amygdala in social and attachment behaviors (Numan et al., 2003). Furthermore, amygdala activity to infant stimuli was associated with heightened oxytocin exposures through vaginal relative to surgical delivery (Swain et al., 2008) and breast relative to bottle feeding (Kim et al., 2011) and was correlated with maternal sensitivity within the first 3 months postpartum (Kim et al., 2011). In contrast, mothers characterized as having intrusive infant-interactional patterns displayed more disorganized patterns of amygdala–whole-brain connectivity while watching mother–infant interactional videos (Atzil et al., 2011) and depressed postpartum women displayed reduced amygdala activity (Silverman et al., 2007) and amygdala–mPFC connectivity to emotional stimuli (Moses-Kolko et al., 2010). These collective findings highlight a putative mechanistic role of reduced amygdala reactivity and amygdala–cortical connectivity to infant and emotional stimuli and during rest in PPD and impaired maternal caregiving. Whether altered amygdala activity/connectivity in depressed or poorly attached mothers relates to unique brain sensitivities to neurosteroids or neuropeptides or alterations in stress-reactivity would be a fruitful direction for additional research. Though our findings were strongest in the basolateral and superficial nuclei of the right amygdala, high-resolution neuroimaging of the amygdala (e.g. Prevost et al., 2012) would be required both to confirm the precise anatomical coordinates of the location of the largest effect and to demonstrate a selective role for these nuclei.
Although alterations in functional connectivity may be a common feature of PPD based upon these and our prior findings, altered functional interactions of the amygdala may be context specific. We previously found that amygdala connectivity with the ACC during the presentation of fearful faces was disrupted in PPD, without evidence for amygdala–PCC disconnectivity (Moses-Kolko et al., 2010). In contrast, the present resting-state study provided no evidence for disrupted amygdala–ACC connectivity. The relationship between these regions, therefore, may depend on different task conditions (Mennes et al., 2012), which suggests that a fuller description of the disrupted connectivity between the PCC and amygdala in PPD requires further examination under different cognitive states.
An important direction for future research will be to more clearly map patterns of resting neural connectivity onto specific phenotypes of PPD and styles of maternal–infant attachment as well as to consider the roles of social attachment-related reproductive hormones (i.e. oxytocin), past psychiatric history and cognitive function. Additional attention should also be paid to wakefulness in the scanner and thought content during acquisition of resting BOLD data in future studies; however, it is noteworthy that descent into light and deeper stages of sleep reflected by a decoupling between the PCC and ACC (Larson-Prior et al., 2011; Samann et al., 2011) was neither observed in either group; nor was there any group difference in PCC/pregenual ACC coupling. Additionally, PCC retained a clear robust inverse functional connectivity with anterior insula, the salience network and the task positive network across both groups, which is opposite to what would be expected in sleep deprivation (De Havas et al., 2012). Although it has been argued that some limbic regions communicate information at frequencies higher than typically investigated in resting fMRI studies (Salvador et al., 2008), our observations suggest that PPD is associated with disrupted connectivity within the low-frequency bands which tend to dominate the resting fMRI signal. Despite the complexity of studying the neural mechanisms of PPD, it is an area ripe for investigation. Improved mechanistic understanding of the illness cannot only benefit mothers, children and families, through guiding development of neural circuit-specific treatments, but it may also shed light on fundamental mechanisms of social attachment and role transition in the pathogenesis of depression.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
We thank all of the research participants and their families for their time dedicated to this study. We thank the University of Pittsburgh, Magnetic Resonance Research Center (MRRC) staff for performing the MR acquisition. We would like to acknowledge Michael Hallquist for providing comments on the manuscript. We also appreciate the efforts of A. Tova Saul, who coordinated this study. We thank Medela, Inc for donation of a multiuse breast pump. We thank the Clinical and Translational Science Institute of the University of Pittsburgh, School of Medicine for assistance with recruitment. This research was supported by National Institute of Health R01 MH079164, R01 HD067185 and a NARSAD Young Investigator Award to Dr E.L.M.-K. Dr H.W.C.’s effort was supported by U01MH092221 to Dr M.L.P. C.Z. and Dr M.L.P.s’ contribution to this work was supported by R01 HD067185. Dr K.L.W.’s contribution was supported by R01 MH079164 to Dr E.L.M.-K. Drs H.W.C., E.L.M.-K., K.L.W. and M.L.P. and Mr C.Z. report no competing interests. Dr K.L.W. receives additional grant support from the National Institute of Mental Health. Dr M.L.P. receives grant support from the National Institute of Mental Health, The United States Army Research Office and The Pennsylvania Department of Health.
References
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstetrics Gynecology. 2005;106:1071–83. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Hobfoll SE, Ritter C, Lavin J, Hulsizer MR, Cameron RP. Depression prevalence and incidence among inner-city pregnant and postpartum women. Journal of Consulting and Clinical Psychology. 1995;63:445–53. doi: 10.1037//0022-006x.63.3.445. [DOI] [PubMed] [Google Scholar]
- Trad PV. Mental health of adolescent mothers. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:130–42. doi: 10.1097/00004583-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Field T. Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behavior and Development. 2010;33:1–6. doi: 10.1016/j.infbeh.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry. 2007;48:329–54. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- Wichman CL, Angstman KB, Lynch B, Whalen D, Jacobson N. Postpartum Depression Screening. Journal of Primary Care & Community Health. 2010;1:158–63. doi: 10.1177/2150131910380055. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Hanusa BH, Perel JM, et al. Postpartum Depression: A randomized trial of Sertraline vs. Nortriptyline. Journal of Clinical Psychopharmacology. 2006;26:353–60. doi: 10.1097/01.jcp.0000227706.56870.dd. [DOI] [PubMed] [Google Scholar]
- O'Hara MW, Stuart S, Gorman LL, Wenzel A. Efficacy of interpersonal psychotherapy for postpartum depression. Archives of General Psychiatry. 2000;57:1039–45. doi: 10.1001/archpsyc.57.11.1039. [DOI] [PubMed] [Google Scholar]
- Weinberg MK, Tronick EZ. Emotional characteristics of infants associated with maternal depression and anxiety. Pediatrics. 1998;102:1298–304. [PubMed] [Google Scholar]
- Weissman MM, Pilowsky DJ, Wickramaratne PJ, et al. Remissions in maternal depression and child psychopathology: a STAR*D-child report. JAMA. 2006;295:1389–98. doi: 10.1001/jama.295.12.1389. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. American Journal of Psychiatry. 2010;167:1373–80. doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–63. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Research Bulletin. 2001;54:287–98. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Science USA. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network's role in spontaneous cognition. Journal of Neurophysiology. 2010;104:322–35. doi: 10.1152/jn.00830.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen-Hoeksema S. Dissociating medial frontal and posterior cingulate activity during self-reflection. Social Cognitive Affective Neuroscience. 2006;1:56–64. doi: 10.1093/scan/nsl004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain–a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Samann PG, Wehrle R, Hoehn D, et al. Development of the brain's default mode network from wakefulness to slow wave sleep. Cerebral Cortex. 2011;21:2082–93. doi: 10.1093/cercor/bhq295. [DOI] [PubMed] [Google Scholar]
- Larson-Prior LJ, Power JD, Vincent JL, et al. Modulation of the brain's functional network architecture in the transition from wake to sleep. Progress in Brain Research. 2011;193:277–94. doi: 10.1016/B978-0-444-53839-0.00018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MG, Peltier S, Nee DE, Kross E, Deldin PJ, Jonides J. Depression, rumination and the default network. Social Cognitive Affective Neuroscience. 2011;6:548–55. doi: 10.1093/scan/nsq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Neubert FX, Noonan MP, Sallet J, Toni I, Rushworth MF. On the relationship between the “default mode network” and the “social brain". Frontiers in Human Neuroscience. 2012;6:189. doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RT. Becoming a mother versus maternal role attainment. Journal of Nursing Scholarship. 2004;36:226–32. doi: 10.1111/j.1547-5069.2004.04042.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition. New York: New York State Psychiatric Institute, Biometrics Research Department; 1998. [Google Scholar]
- Stowe ZN, Hostetter AL, Newport DJ. The onset of postpartum depression: Implications for clinical screening in obstetrical and primary care. Americal Journal of Obstetrics and Gynecology. 2005;192:522–6. doi: 10.1016/j.ajog.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Sit DKY, McShea MC, et al. Onset timing, thoughts of self-harm and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013;70:490–8. doi: 10.1001/jamapsychiatry.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Holden J, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Condon JT, Corkindale CJ. The assessment of parent-to-infant attachment: development of a self-report questionnaire instrument. Journal of Reproductive and Infant Psychology. 1998;16:57–76. [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. Journal of Neuroscience. 2009;29:1175–90. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Martinez A, Pomarol-Clotet E, et al. A simple view of the brain through a frequency-specific functional connectivity measure. NeuroImage. 2008;39:279–89. doi: 10.1016/j.neuroimage.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Rehme AK, Diessel M, et al. Segregating intra-amygdalar responses to dynamic facial emotion with cytoarchitectonic maximum probability maps. Journal of Neuroscience Methods. 2008;172:13–20. doi: 10.1016/j.jneumeth.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, et al. A validated network of effective amygdala connectivity. NeuroImage. 2007;36:736–45. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Metaanalytic connectivity modeling: delineating the functional connectivity of the human amygdala. Human Brain Mapping. 2010;31:173–84. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanacci L, Suzuki WA, Amaral DG. Organization of connections between the amygdaloid complex and the perirhinal and parahippocampal cortices in macaque monkeys. Journal of Comparative Neurology. 1996;375:552–82. doi: 10.1002/(SICI)1096-9861(19961125)375:4<552::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Human Brain Mapping. 2012 doi: 10.1002/hbm.22138. doi: 10.1002/hbm.22138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2012;59:1420–8. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proceedings of the National Academy of Sciences USA. 2010;107:10238–43. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology. 2009;101:3270–83. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hermens DF, Hickie IB, Lagopoulos J. A systematic review of resting-state functional-MRI studies in major depression. Journal of Affective Disorders. 2012;142:6–12. doi: 10.1016/j.jad.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences USA. 2010;107:11020–5. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biological Psychiatry. 2005;57:1079–88. doi: 10.1016/j.biopsych.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. NeuroImage. 2006;29:452–66. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs CR, Durette RT, Ferraro DP, Cross CL. Dimensions of postpartum psychiatric distress: preliminary evidence for broadening clinical scope. Journal of Affective Disorder. 2009;115:100–11. doi: 10.1016/j.jad.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Barrett J, Fleming AS. Annual research review: all mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry. 2011;52:368–97. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers' neural activation in reponse to pictures of their children and other children. Biological Psychiatry. 2004;56:225–32. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, et al. Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cerebral Cortex. 2009;19:1124–33. doi: 10.1093/cercor/bhn153. [DOI] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, et al. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biological Psychiatry. 2003;54:1367–75. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF. Maternal brain response to own baby-cry is affected by cesarean section delivery. Journal of Child Psychology and Psychiatry. 2008;49:1042–52. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JFW, Appleby L. The neural basis of maternal responsiveness to infants: an fMRI study. NeuroReport. 2004;15:1825–9. doi: 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. Neurochemistry and molecular biology of maternal behavior. In: Numan M, Insel TR, editors. The Neurobiology of Parental Behavior. New York: Springer; 2003. pp. 190–245. [Google Scholar]
- Kim P, Feldman R, Mayes LC, et al. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of Child Psychology and Psychiatry. 2011;52:907–15. doi: 10.1111/j.1469-7610.2011.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Feldman R. Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology. 2011;36:2603–15. doi: 10.1038/npp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman ME, Loudon H, Safier M, et al. Neural dysfunction in postpartum depression: an fMRI pilot study. CNS Spectrums. 2007;12:853–62. doi: 10.1017/s1092852900015595. [DOI] [PubMed] [Google Scholar]
- Prevost C, Liljeholm M, Tyszka JM, O'Doherty JP. Neural correlates of specific and general Pavlovian-to-Instrumental Transfer within human amygdalar subregions: a high-resolution fMRI study. Journal of Neuroscience. 2012;32:8383–90. doi: 10.1523/JNEUROSCI.6237-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Colcombe S, Castellanos FX, Milham MP. The extrinsic and intrinsic functional architectures of the human brain are not equivalent. Cerebral Cortex. 2012;23:223–9. doi: 10.1093/cercor/bhs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Havas JA, Parimal S, Soon CS, Chee MW. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. NeuroImage. 2012;59:1745–51. doi: 10.1016/j.neuroimage.2011.08.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.