Abstract
Joint actions require the integration of simultaneous self- and other-related behaviour. Here, we investigated whether this function is underpinned by motor simulation, that is the capacity to represent a perceived action in terms of the neural resources required to execute it. This was tested in a music performance experiment wherein on-line brain stimulation (double-pulse transcranial magnetic stimulation, dTMS) was employed to interfere with motor simulation. Pianists played the right-hand part of piano pieces in synchrony with a recording of the left-hand part, which had (Trained) or had not (Untrained) been practiced beforehand. Training was assumed to enhance motor simulation. The task required adaptation to tempo changes in the left-hand part that, in critical conditions, were preceded by dTMS delivered over the right primary motor cortex. Accuracy of tempo adaptation following dTMS or sham stimulations was compared across Trained and Untrained conditions. Results indicate that dTMS impaired tempo adaptation accuracy only during the perception of trained actions. The magnitude of this interference was greater in empathic individuals possessing a strong tendency to adopt others’ perspectives. These findings suggest that motor simulation provides a functional resource for the temporal coordination of one’s own behaviour with others in dynamic social contexts.
Keywords: motor simulation, TMS, music, joint action, temporal coordination, empathy
INTRODUCTION
The coordination of one’s own movements with those of other individuals is a basic form of social interaction that represents a critical step in human phylogenetic and ontogenetic development (Tomasello et al., 2005). However, the brain mechanisms and strategies adopted to achieve successful joint actions remain poorly understood (see Bekkering et al., 2009 for a review).
One of the key components of successful interaction with another individual is the capacity to predict and adapt to incoming perceptual information about another’s behaviour and, eventually, to form integrated representations of self- and other-related actions in real time. Although the former capacity relies on sensorimotor mechanisms that control action timing (which can be studied in the context of interactions with computer-controlled pacing signals, see e.g. Repp and Keller, 2008; Fairhurst et al., 2012), the latter requires dedicated forms of neural representations that connect self and other. Indeed, these are not compatible prima facie because, on the one hand, self-initiated actions are generated internally, whereas others’ actions are exogenous and, as such, can only be accessed via perceptual input.
‘Common coding’ of perception and action (Prinz, 1997; Schütz-Bosbach and Prinz, 2007) has been suggested to support fast and efficient integration of multiple individuals’ actions (Sebanz and Knoblich 2009; Knoblich et al., 2011). In terms of neural mechanisms, this function might be underpinned by ‘motor simulation’ (Jeannerod, 2001) referring to the brain’s capacity to represent a perceived action in terms of the neural resources necessary to execute it (Rizzolatti and Senigaglia, 2010). Thus, motor simulation is a ‘covert’ stage of action representation that does not ultimately result in execution of a represented action. Motor simulation is hypothesized to play a special role in facilitating social interactions, as it is enhanced in empathic individuals who have a strong tendency to adopt others’ perspectives (Gazzola et al., 2006; Kaplan and Iacoboni 2006; see also Decety and Jackson, 2004 for a review).
It has been claimed that motor simulation is particularly important for joint actions requiring temporal coordination (Sebanz and Knoblich, 2009; Knoblich et al., 2011). On-line motor simulation is suited to this task because it anticipates others’ actions in the observer’s brain (Kilner et al., 2004; Borroni et al., 2005) by means of forward models (Wolpert et al., 2003) that anticipate sensory events and thereby assist perceptual processes (see Kokal and Keysers, 2010; Schipper and Keysers, 2011). However, while behavioural evidence supports this notion indirectly (Knoblich and Jordan, 2003; Keller et al., 2007), it is unknown whether the neural processes associated with motor simulation are a specific requirement for the temporal regulation of inter-individual behaviour.
Here, we sought to test directly whether interfering (by means of transcranial magnetic stimulation, TMS) with the motor simulation of an action impairs the ability to coordinate with it.
We adopted a musical coordination task where pianists were required to perform the right-hand melody part of a musical piece in synchrony with audible renditions of the complementary left-hand bassline part (Figure 1), which either had (Trained) or had not (Untrained) been practiced beforehand. An earlier TMS study using a similar task (Novembre et al., 2012) showed that corticospinal excitability associated with the (resting) left arm increased when pianists listened to a part that they had previously rehearsed (see also D’Ausilio et al., 2006). Moreover, this effect was enhanced in individuals who had a strong tendency to adopt others’ perspectives. Therefore, the manipulation employed here was intended to call upon action-specific simulation processes that occur when a perceived action sequence exists in an individual’s motor repertoire, that is in the Trained condition (cf. Haueisen and Knösche, 2001; D’Ausilio et al., 2006, see also Lahav et al., 2007; Chen et al., 2012).
Fig. 1.
(A). Schematic illustration of the experimental paradigm. Participants performed the melody part (the highest in pitch) with the right hand in synchrony with audible left-hand bassline part (which was not performed). dTMS (or sham) was delivered over right primary motor cortex (M1) prior to the tempo changes in the bassline part. Participants were asked to adapt to the new tempo as quickly and accurately as possible. The brace signs indicate the analysed inter-keystroke intervals. The tempo changed at the interval marked with the grey brace sign (this interval provided pre-adaptation indices) and continued regularly from that point onwards. The black brace sign indicates the first predictable interval in the bassline part (which provided the tempo adaptation indices). (B) Illustration of the 2 × 2 factorial design. Participants either had (Trained) or had not (Untrained) practiced the bassline part (with their left hand) before the experimental session.
In the present study, we implemented this paradigm as a tempo adaptation task to investigate to what extent motor simulation of others’ actions supports the temporal coordination of self-initiated actions. One would expect that the greater the role of motor simulation, the more interpersonal coordination would deteriorate when simulation is obstructed. To this aim, the recording of the left-hand part contained tempo changes, which the pianists were required to adapt to. In order to interfere with the motor simulation of the left-hand part, double-pulse TMS (dTMS) or control sham TMS pulses (Chen et al., 1997; Oshio et al., 2010) were delivered over the right primary motor cortex (M1) prior to each tempo change. After that, pianists’ accuracy in adapting to the new tempo was quantified using standard behavioural measures from studies of sensorimotor synchronization (Repp, 2005). To the extent that motor simulation underpins temporal coordination, we expected that tempo adaptation accuracy would decrease only when dTMS was delivered in the Trained condition. In addition, as more empathic individuals have been shown to rely on motor simulation to a stronger degree, we also expected the magnitude of the dTMS interference to be particularly enhanced in these individuals.
MATERIALS AND METHODS
Participants
Ten right-handed amateur pianists (three males) participated in the study. Pianists’ mean age was 24.81 years (s.d. = 3.18) and the average age at which they commenced piano studies was 7.09 years (s.d. = 2.62). On average, participants played the piano for 3.04 h/week (s.d. = 3.79) and had received 13.81 years (s.d. = 3.86) of formal training. The local ethics committee approved the experiment. Informed written consent was obtained from each participant, though the specific aims of the study were not divulged.
Musical materials and pre-experimental training
Four chorales by J.S. Bach (originally scored for soprano, alto, tenor and bass voices) were adapted for use in this study. The length of the chorales ranged from 13 to 15 bars (mean = 14.25, s.d. = 0.95), with 4 beats per bar. Performance duration (at a constant tempo of 120 bpm) ranged from 26 to 30 s (mean = 28.5, s.d. = 1.91).
The melody (the part highest in pitch) and the bassline (the lowest part) of the chorales were arranged in such a way to be performed on the piano with the right and left hands, respectively. To ensure that musical complexity and the number of keystrokes were comparable across chorales, embellishments such as ‘passing’ notes between main melodic notes and notated pauses (except for those separating musical phrases) were omitted.
Notated scores comprising the edited melody and bassline were created and sent to the participants 1 week prior to the experiment. Two of the scores depicted only the melody part, which had to be practiced with the right hand, while the bassline part was not shown (these scores were used in the Untrained conditions). The other two scores depicted both the melody and the bassline part, which had to be practiced with the right and left hand, respectively (in the Trained conditions). The chorales used for the Trained and Untrained conditions were counterbalanced across two groups of participants: group A (n = 5) practised both parts (i.e. both the melody and the bassline) of chorales 1 and 2, and only the melody part of chorales 3 and 4. Group B (n = 5) did the opposite. The pianists had never played this musical material before participating in this study, as assessed by self-report.
Experimental procedure
Participants, who were tested individually, sat on a chair in front of an electronic piano keyboard (Yamaha Clavinova CLP130), which recorded their performances in Musical Instrument Digital Interface (MIDI) format. The pianist’s left arm rested comfortably on a supportive surface fixed to the armrest of the chair, while the right hand was free to move on the keyboard. Participants could hear (through Sony MDR-EX35LP headphones) the sound of their performance (of the melody) as well as the sounds of the bassline produced by the computer controlling the experiment (via Presentation Software 14.2, Neurobehavioral Systems, Inc.).
Before commencing the experiment, we made sure that each participant was able to perform all the pieces fluently, without any pitch or rhythmic error, at the regular tempo they had practiced (120 bpm). These test trials were not recorded.
The experiment consisted of 16 performances of each of the 4 chorales, resulting in 64 experimental randomly ordered trials. Prior to each trial, participants were presented with a notated score of the melody part to be played. This remained visible throughout the trial, which was initiated by pressing the rightmost key on the piano keyboard. Consequently, the participant was presented with a metronome (sampled mechanical metronome sound, tempo = 120 bpm) playing for two bars and, after that, the initial two bars of the chorale played by the computer, which signalled when to start performing (Figure 1). Waveform audio file format (WAV) files of the bassline parts were created with music software (LogicPro8.0.2, Apple Inc.). Participants were instructed to synchronize their performance of the right-hand melody with the sounds of the bassline part and to continue playing the entire melody part in synchrony with it. The time interval between note onsets in the bassline parts was regular prior to and following the tempo changes (described below).
Two tempo changes occurred within each trial at locations that were constant across musical pieces. These locations were chosen in such a way that (i) they occurred approximately one-third and two-thirds of the way through each piece, (ii) they were followed by at least 10 consecutive quarter notes and (iii) they were preceded by a musical pause (either a long note or a pause symbol) between phrases. The direction of the tempo change (i.e. acceleration vs deceleration) and its magnitude (either ± 8 or ± 16 bpm) were balanced and their presentation was randomized. Within a trial, the tempo included transitions between the following tempi: 104, 112, 120, 128 and 136 bpm. In total, participants were required to adapt to 128 tempo changes (i.e. 32 per Training and TMS condition, with equal occurrence of directions and magnitudes in each condition).
Before the actual experiment, participants were familiarized with the experimental task by means of a practice session in which they rehearsed all the trained right-hand parts and all the tempo changes (i.e. 2 directions × 2 magnitudes) in randomized order. This training session permitted participants to learn at which point of each musical piece the tempo would change.
dTMS (Magstim200, Whitland, UK; 70 mm figure-of-eight stimulation coil) was delivered over the hand representation in the right primary motor cortex (M1), which was defined as the scalp position from which motor-evoked potentials (MEPs) with maximal amplitude were elicited in resting left first dorsal interosseous (FDI) muscle.
The coil was positioned tangentially over M1 with the handle pointing backwards and laterally 45° away from the midline. Sham TMS was administered by tilting the coil 90° off the scalp, with one or two wings of the coil touching the vertex. The intensity of both the TMS pulses was set at 110% of each participant’s resting motor threshold, which ranged from 28% to 52% (mean = 39.00, s.d. = 6.79) of the maximum stimulator output. Resting motor threshold was defined as the lowest stimulator output that evoked in the left FDI muscle at least 5 out of 10 successive MEPs with amplitude >50 µV. Muscular contraction (Electromyography signal) in the target muscle was visually monitored and full muscular relaxation was obtained.
dTMS pulses were delivered on-line during the musical pauses preceding the tempo changes. The inter-pulse interval for each delivery was set at 100 ms (Chen et al., 1997; Oshio et al., 2010). The two pulses were locked to the presentation of the first tone belonging to the new musical phrase—which was itself not deviant in terms of tempo—with the first pulse anticipating this tone by 100 ms and the second coincident with it. The following tone (i.e. the second of the musical phrase) was irregular in terms of timing, that is it was either relatively early or late depending on whether the tempo change was an acceleration or deceleration. Subsequent tones continued at the new (regular) tempo and were thus predictable in their timing.
At the end of the experiment, each participant’s ‘perspective taking’ tendencies (i.e. how spontaneously they adopt others’ perspectives) were assessed via an empathy questionnaire (Paulus, 2009) based on the Interpersonal Reactivity Index (Davis, 1980).
Data analysis
Pianists’ performances were examined off-line to evaluate keystroke identity (MIDI note number) and keystroke timing accuracy for the four keystrokes (constituting one musical bar, see figure 1) prior to, and the first four keystrokes following, each tempo change. Timing accuracy was quantified for these keystrokes by measuring asynchronies, that is the time difference between a keystroke and the onset of its target bassline tone. Keystroke timing was considered to be erroneous if asynchronies exceeded ±250 ms. This criterion was adopted because values outside this range indicate that a keystroke was closer to the tone preceding or following the one with which it was supposed to be synchronized at the average tempo (mean inter-beat interval = 500 ms) used throughout the experiment.
Accuracy at tempo adaptation for the first two keystrokes following each tempo change was quantified on the basis of the relationship between produced keystroke inter-onset intervals and target inter-onset intervals in recorded bassline parts. Adaptation indices were computed for correct keystrokes (as defined above) by dividing each keystroke inter-onset interval by the appropriate target inter-onset interval, yielding a ratio (cf. Repp, 2005). Adaptation indices of 1 indicate perfect adaptation to the new tempo, while values above or below 1 indicate that performance tempo was too slow or too fast, respectively.
Note that the two keystrokes that were analysed correspond to one keystroke inter-onset interval, and therefore provide a single adaptation index. We report results for only the first keystroke inter-onset interval initiated following each tempo change (and dTMS or sham stimulation) because this is the keystroke inter-onset interval that is produced in time with the first predictable interval in the recorded bassline part (see the black brace sign in Figure 1). Subsequent keystrokes were analysed, but the results are not reported here as synchronization recovered quickly after the tempo change and none of our experimental manipulations produced significant effects upon adaptation accuracy beyond the first keystroke inter-onset interval at the new tempo.
In addition to adaptation indices for the first keystroke inter-onset interval following each tempo change, we also report ‘pre-adaptation’ accuracy indices, that is from the position preceding the first predictable inter-onset interval (i.e. see the grey brace sign in Figure 1). Note that, at this position, dTMS has already been delivered, and the participant is playing in synchrony with the initial interval at the new tempo. Thus, the accuracy of this interval permits us to gauge general effects of dTMS that are not associated specifically with the adaptation task (see below).
Adaptation indices deviating >3 s.d. from the mean were excluded from analyses (0.83% of data per participant, s.d. = 0.41). Remaining data for initial keystroke inter-onset intervals following tempo changes were entered into a 2 × 2 repeated-measures analysis of variance (ANOVA) with within-participant factors Training (Trained and Untrained) and TMS (dTMS and Sham). One-sample t-tests were used to compare adaptation indices in each condition against 1 (i.e. perfect adaptation). An additional ANOVA examined the effects of tempo change Direction (acceleration and deceleration) and Magnitude (small and large) to test whether these factors modulated the effects of Training and TMS (the same analysis was run with the pre-adaptation indices).
Individual differences related to empathy were examined by estimating the correlation between adaptation indices obtained in the Trained-dTMS condition and self-reported perspective-taking scores (cf. Gazzola et al., 2006; Novembre et al., 2012). Prior to running this correlation analysis, individual participant’s mean adaptation indices and perspective-taking scores were screened to check that they (i) did not fall >2 s.d. from the sample mean and (ii) did not yield a Cook’s distance value exceeding 1 (Cook and Weisberg, 1982).
RESULTS
Performance accuracy
Analysis of keystroke identity and timing revealed that performance accuracy ranged from 85.94% to 98.44% across participants (mean = 92.75, s.d. = 5.52). The asynchronies associated with correct keystrokes (collapsed across conditions) ranged between −32.43 and 20.36 ms before the tempo change (mean = −7.83, s.d. = 9.44) and between −33.25 and 17.16 ms (mean = −4.07, s.d. = 10.64) after the tempo change. These mean asynchronies were not significantly different from one another [t(9) = −2.030, P > 0.05].
Effect of dTMS on tempo adaptation
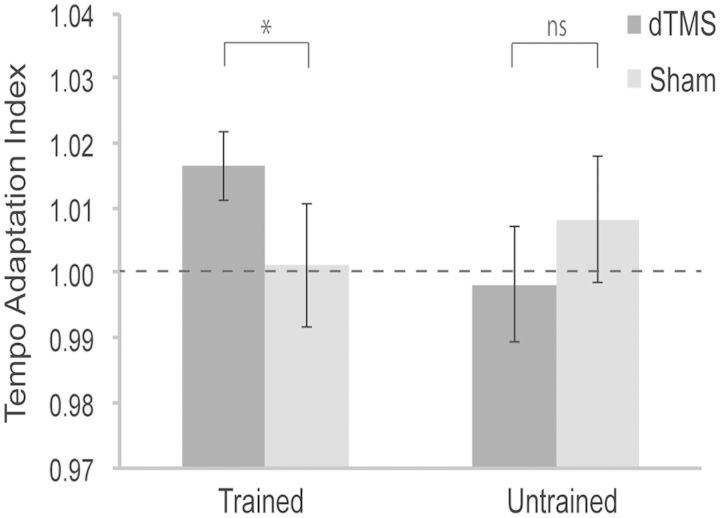
Average tempo adaptation indices for the first keystroke inter-onset interval following tempo changes in each Training and TMS condition are shown in Figure 2.
Fig. 2.
Average tempo adaptation indices for the first keystroke inter-onset interval following tempo changes in Training (Trained vs Untrained) and TMS (dTMS vs Sham) conditions (collapsed across tempo change directions and magnitudes). The dashed horizontal line indicates 1 (perfect adaptation), while values above or below 1 indicate that performance tempo was too slow or too fast, respectively. Error bars represent 1 s.e.m. *P < 0.05, ns P > 0.05.
The ANOVA on these data revealed that the main effects of Training [F(1,9) = 0.382, P > 0.05] and TMS [F(1,9) = 0.405, P > 0.05] were not statistically significant, implying that neither Training nor dTMS had an effect on the temporal adaptation task per se. There was, however, a significant interaction between Training and TMS [F(1,9) = 11.288, P < 0.01]. This interaction reflected the fact that adaptation indices were significantly higher (i.e. performance tempo was slower) with dTMS than with sham stimulation in the Trained condition (P < 0.05), but not in the Untrained condition (P > 0.05). Furthermore, one-sample t-tests confirmed that adaptation indices in the Trained-TMS condition differed significantly from 1 (indicating perfect adaptation) [t(9) = 3.094, P < 0.05], while those in the other conditions did not (all t’s < 1.0 and P’s > 0.05). These results indicated that dTMS impaired tempo adaptation accuracy only when pianists had practiced the left-hand bassline part beforehand.
A noteworthy feature of this effect is that the impaired tempo adaptation accuracy observed in the Trained-TMS condition was associated with deceleration irrespective of the actual tempo change direction. This was assessed by means of a separate ANOVA including the variables tempo change Direction (acceleration vs deceleration) and Magnitude (large vs small changes). The ANOVA replicated the above interaction between Training and TMS [F(1,9) = 9.844, P < 0.05] and, importantly, showed that the three-way interactions between TMS, Training and either Direction [F(1,9) = 0.039, P > 0.05] or Magnitude [F(1,9) = 2.012, P > 0.05] were not significant. These results suggest that the differential effects of dTMS on untrained and trained parts generalized across the different tempo conditions. The ANOVA also yielded a significant main effect of Magnitude [F(1,9) = 5.558, P < 0.05] and an interaction between Magnitude and Training [F(1,9) = 8.932, P < 0.05]: Adaptation indices were higher (i.e. performance tempo was slower) for large than small tempo changes, and this effect was most pronounced in the Untrained condition (Table 1). No other main effects or interactions were significant (all P > 0.05).
Table 1.
Adaptation index values and s.d. for the first keystroke inter-onset interval following tempo changes in different direction (deceleration and acceleration) and magnitude (small and large) conditions
| Condition | Deceleration |
Acceleration |
||
|---|---|---|---|---|
| Small | Large | Small | Large | |
| Trained-TMS | 1.0129 ± 0.0223 | 1.0248 ± 0.0323 | 1.0165 ± 0.0358 | 1.0123 ± 0.0448 |
| Trained-Sham | 1.0080 ± 0.0250 | 1.0121 ± 0.0447 | 0.9990 ± 0.0538 | 0.9868 ± 0.0435 |
| Untrained-TMS | 0.9875 ± 0.0240 | 1.0043 ± 0.0434 | 0.9953 ± 0.0442 | 1.0064 ± 0.0516 |
| Untrained-Sham | 0.9938 ± 0.0395 | 1.0284 ± 0.0560 | 0.9942 ± 0.0363 | 1.0178 ± 0.0358 |
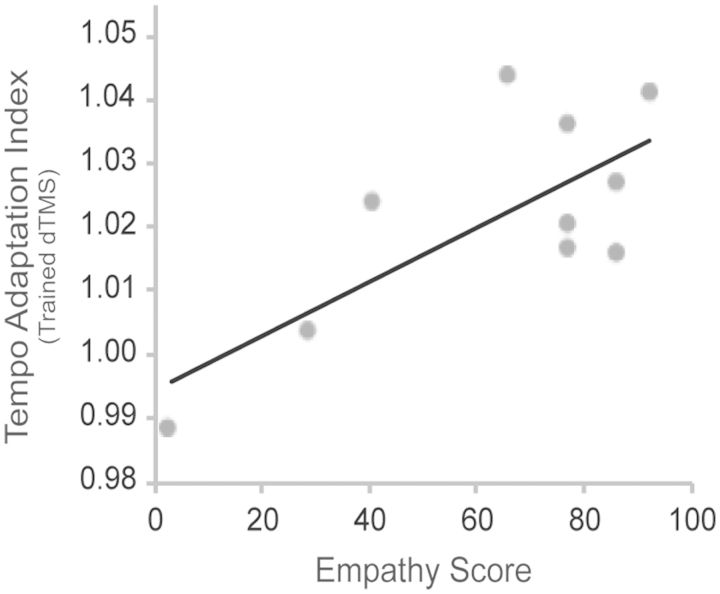
Finally, our analysis of empathy-related individual differences revealed that there was a positive correlation between adaptation indices in the Trained-TMS condition and individual participants’ perspective taking scores [r(8) = 0.734, P < 0.05]. Thus, the decelerating effect of dTMS on performance tempo was greater in individuals who possessed a stronger tendency to adopt others’ perspectives (Figure 3).
Fig. 3.
Scatter plot displaying the positive correlation between the adaptation indices from the Trained-TMS condition (quantifying the TMS-interference on motor simulation) and the perspective-taking (empathy) scores of the participants.
Specificity of dTMS effect
To test whether dTMS had general effects on motor performance that were independent from the adaptation task, we also analysed the pre-adaptation accuracy indices from the position preceding the first predictable inter-onset interval (i.e. the interval indicated by the grey brace sign in Figure 1). Note that, at this position, dTMS has already been delivered, and the participant is playing in synchrony with the initial interval at the new tempo, which could not be predicted at this stage (due to the randomized ordering of trials in terms of the magnitude and direction of tempo changes). Hence, this analysis permits us to control for general TMS-related interference on the motor system that is not related specifically to action simulation or temporal adaptation.
A repeated-measures ANOVA (with factors: TMS, Training, Magnitude and Direction) was run on pre-adaptation accuracy indices (Table 2). This analysis yielded a significant main effect of Direction [F(1,9) = 1307.228, P < 0.000] showing that the pre-adaptation indexes were larger for accelerations compared with decelerations of the bassline parts, and an interaction between Direction and Magnitude [F(1,9) = 158.521, P < 0.000] implying that this difference was stronger for large compared with small tempo change magnitudes. The main effect of TMS [F(1,9) = 0.234, P > 0.05], the interaction between TMS and Training [F(1,9) = 0.044, P > 0.05] and all other main effects and interactions were not significant (all P > 0.05). Moreover, there was no significant correlation between the mean indices of the Trained-TMS conditions and the empathic scores of the participants [r(8) = 0.315, P > 0.05).
Table 2.
Pre-adaptation index values and s.d. associated with the keystroke inter-onset interval coincident with the new tempo (which was unknown at this position) in different direction (deceleration and acceleration) and magnitude (small and large) conditions
| Condition | Deceleration |
Acceleration |
||
|---|---|---|---|---|
| Small | Large | Small | Large | |
| Trained-TMS | 0.9332 ± 0.0556 | 0.8624 ± 0.0241 | 1.0863 ± 0.0310 | 1.1251 ± 0.0314 |
| Trained-Sham | 0.9479 ± 0.0494 | 0.8632 ± .0569 | 1.0549 ± 0.0534 | 1.1304 ± 0.0550 |
| Untrained-TMS | 0.9320 ± 0.0571 | 0.8762 ± 0.0537 | 1.0728 ± 0.0633 | 1.1544 ± 0.0702 |
| Untrained-Sham | 0.9380 ± 0.0535 | 0.8579 ± 0.0620 | 1.0730 ± 0.0550 | 1.1486 ± 0.0669 |
These data clearly indicate that the decrease in temporal coordination accuracy was observed at the time point when the magnitude and direction of the tempo change were already known rather than during the earlier interval that immediately followed TMS stimulation. This result suggests that dTMS did not interfere directly with motor execution, but more likely disrupted motor prediction processes associated with the simulation of the left-hand part—which in turn assisted temporal adaptation.
DISCUSSION
The present study combined behavioural and brain stimulation methods in order to investigate the role of motor simulation in the temporal coordination of self-initiated and other-related actions. In a musical paradigm, pianists were required to perform right-hand melodies in synchrony with audio recordings of bassline parts that they either had or had not practiced earlier with the left hand. dTMS applied over the right motor cortex just prior to tempo changes in the bassline parts was found to disrupt tempo adaptation accuracy (compared with sham stimulations) only when the bassline part had been previously trained. These results suggest that dTMS interfered with motor simulation processes associated with trained left-hand parts, and thereby impaired pianists’ ability to coordinate with the recordings by slowing down their performance tempo with respect to the perceived tempo (of the bassline). Thus, these data imply that motor simulation is a functional resource for successfully coordinating in real time with another agent, and therefore it might be one of the key mechanisms supporting joint actions (see Sebanz et al., 2006; Sebanz and Knoblich, 2009; Knoblich et al., 2011). This finding has broader implications for the fields of action observation, prediction and interaction.
In the field of action observation, it has been shown that the perception of actions that already exist in an individual’s motor repertoire, such as listening to rehearsed music (Haueisen and Knösche, 2001; D’Ausilio et al., 2006; Lahav et al., 2007; Chen et al., 2012, see also Ticini et al., 2011) or watching a well-practiced dance move (Calvo-Merino et al., 2005, 2006; Cross et al., 2006; Orgs et al., 2008), modulate the activation of cortical motor regions including inferior parietal, premotor and M1 (see also Caetano et al., 2007; Kilner and Frith 2007; Aglioti et al., 2008). Similar modulations have also been disclosed when observing another individual making an action error (Koelewijn et al., 2008; van Schie, 2004), including pianists watching (without listening to) piano performance errors (Novembre and Keller, 2011; Candidi et al., 2012; Sammler et al., 2013). But what is the behavioural relevance of these activations? What function do they serve? By making a further step with respect to previous studies advocating predictive accounts (Kilner et al., 2004; Borroni et al., 2005, see also Kilner et al., 2007), our study explored the relevance of motor simulation for inter-individual behaviour. The present data suggest that simulating others’ actions facilitates interaction with them, such as when jointly playing music or dancing, by allowing subtle real-time adaptations (at the millisecond timescale) with respect to action-related auditory information. From this perspective, motor simulation can be seen as a neural mechanism supporting the ‘common coding’ of perception and action in dynamic social contexts (Prinz, 1997; Schütz-Bosbach and Prinz, 2007).
Motor coordination with external events requires precise temporal prediction (see Wolpert and Ghahramani, 2000; Kilner et al., 2007; Schubotz, 2007, see also Brown and Brüne 2012). In our task, prediction was necessary in order to make temporal estimates of upcoming tones, which in turn support detection of a discrepancy between predicted and actual tone onsets (tempo change), and allow adaptation accordingly. Through (learned) associations between sensory and motor processes, internal models of sensorimotor transformations can compute the causal relationship between actions and their consequences (Wolpert and Ghahramani, 2000), functioning as simulations of motor commands that generate predictions of their effects. Our data are consistent with this view because, when dTMS was delivered in the Trained condition, accuracy in adaptation dropped (performance tempo decelerated) equally across different tempo change directions and magnitudes. This general slowing suggests that ‘reactive’ error correction mechanisms governed adaptation after the stimulations in lieu of predictive mechanisms associated with motor simulation (Kilner et al., 2004; Borroni et al., 2005), which were interfered with by dTMS over M1. This is a remarkable finding in that it implies that internal knowledge (or models) about how to perform an action may assist motor control during not only action prediction but also action adaptation, a key requirement of social interactions.
Our results thus point to an interesting link between neuroimaging research on action observation (see above) and motor control studies dealing with sensorimotor synchronization (Repp, 2005 for a review). It is possible, for example, that TMS interfered with the brain network underpinning period correction—a mechanism that enables adaptation to tempo changes, for example, in paced finger tapping studies (see Repp, 2005)—which can be applied in anticipation of a tempo change (Repp, 2006). Nevertheless, the comparison between the brain networks implicated our task and paced finger-tapping tasks is not straightforward: our study focused on the representation of trained action sequences, which, compared with other sounds, are known to elicit additional activations that reflect the specific motor resources necessary to perform the same actions (Rizzolatti and Sinigaglia, 2010). This does not imply that adaptation to actions that are not trained relies solely on brain networks outside the motor system (see Schubotz, 2007), but rather that the two are achieved by means of different neural networks depending on whether or not a perceived action is present in an individual’s motor repertoire. From this perspective, the present study extends previous TMS investigations of the effects of perturbations to motor areas on synchronization with simple pacing signals (see e.g. Kornysheva and Schubotz, 2011; Ruspantini et al., 2011; Giovannelli et al., 2012) to synchronization with naturalistic signals that resemble human action effects.
The above interpretation is consistent with the finding that dTMS over M1 interfered with temporal coordination for trained actions but not for untrained actions. It should be noted that this account is not intended to imply that motor simulation is confined to M1, or that M1 itself is directly engaged in simulation. Rather, we claim to have shown that interfering with a network of motor areas underpinning simulation (including, e.g. the premotor cortices, supplementary motor area and the cerebellum) via stimulation of M1 (cf. Haueisen and Knösche, 2001; D’Ausilio et al., 2006; Novembre et al., 2012) led to a decrease in coordination accuracy.
We also found that the magnitude of TMS interference was enhanced in individuals with stronger tendency to adopt others’ perspectives (as assessed by an empathy questionnaire). This is noteworthy given that a previous study using a comparable musical task showed that participants with high scores on this scale display relatively high cortico-spinal excitability associated with the resting left forearm (Novembre et al., 2012). More generally, the current finding is consistent with the evidence showing that individuals with such tendencies rely strongly on motor simulation during action observation (Gazzola et al., 2006; see also Babiloni et al., 2012 for further evidence of the neural mechanisms mediating the observation of musical actions and empathy) and extend it by implying that individuals rely on perspective-taking for inter-individual coordination in complex contexts requiring temporal adaptation.
The above interpretation is also supported by previous accounts concerning the predictive function of internal models in the context of social interactions (Wolpert et al., 2003), including joint musical performance. Research in this field suggests that successful coordination between musicians is mediated by ‘socially endowed’ internal models that serve to simulate co-performers’ actions (Wolpert et al., 2003) and to generate predictions (see Lee and Noppeney, 2011) about the overall ensemble sound (Keller, 2008, 2012). Furthermore, behavioural investigations have shown that individuals are influenced by properties of their own motor systems when predicting the timing of others’ actions. For example, pianists synchronize better with recordings of pieces previously performed by themselves (Keller et al., 2007) or with other pianists that best resemble their own preferred performance tempo (Loehr and Palmer, 2011). These pieces of evidence fit very well with our finding.
Also note that, although our task did not involve interaction between mutually adaptive individuals, it did address a key component of joint action to the extent that it entailed coordination with an externally controlled sequence. In interactions involving two mutually adaptive individuals, each individual is required to coordinate with an externally controlled sequence of events generated by another (see Tognoli et al., 2007; Konvalinka et al., 2010; Noy et al., 2011; Pecenka and Keller, 2011; D’Ausilio et al., 2012; Sacheli et al., 2012; Sänger et al., 2012). Mutual adaptation raises the non-trivial issue of determining who is adapting to whom and by how much (Repp and Keller, 2008). Our paradigm focused on the process of one individual adapting to tempo changes in a non-adaptive pacing sequence, which afforded a degree of experimental control that served our initial purpose.
Finally, it is necessary to discuss an alternative account of our findings, one that is based on the notion that dTMS interfered with ‘bimanual motor representations’—associated with the Trained, but not Untrained conditions—(Levin et al., 2004; Stinear et al., 2006) instead of motor simulation processes per se. Although we cannot exclude the possibility that interference with bimanual representations provides a partial account of our results, this interpretation does not provide a full account for two reasons. First, the role of motor simulation in the context of this task appears to be social in nature, as evidenced by the correlation with empathy. This claim is also supported by a related study (Novembre et al., 2012), where multiple conditions implying bimanual learning were compared, and only those presented in an interactive context lead to motor simulation (which was measured in terms of cortico-spinal excitability) of the left-hand part, and correlated with the same scale of empathy used in the current investigation. Second, dTMS interfered specifically with the adaptation task, rather than the motor task alone. In fact, temporal coordination at the interval immediately following the stimulations (i.e. pre-adaptation indices) was not affected by our experimental manipulations, while it was at the subsequent interval when the participants were able to perceive the new tempo and adapt to it. Nevertheless, it should be pointed out that the physiological effect of dTMS on the motor system is likely to change with time, and therefore the comparison between adaptation and pre-adaptation indices is not straightforward.
In conclusion, the present study showed that interfering (by means of dTMS) with the brain processes associated with the motor simulation of an action impairs the capacity for temporal coordination with that action. This finding provides a direct link between neural representations of observed actions and motor control and is therefore relevant for understanding the mechanisms that underpin action observation, prediction, adaptation and their relevance for joint action. Hence, motor simulation should not be seen solely as a multimodal representation of an observed action, but also as a functional resource that can be used to coordinate self-initiated (goal-directed) behaviour with others.
Acknowledgments
The authors wish to thank all the pianists who participated in this study and Felix Haiduk for his help with data acquisition. This work was supported by the Max Planck Society.
REFERENCES
- Aglioti SM, Cesari P, Romani M, Urgesi C. Action anticipation and motor resonance in elite basketball players. Nature Neuroscience. 2008;11:1109–16. doi: 10.1038/nn.2182. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Buffo P, Vecchio F, et al. Brains “in concert”: frontal oscillatory alpha rhythms and empathy in professional musicians. Neuroimage. 2012;60:105–16. doi: 10.1016/j.neuroimage.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Bekkering H, de Bruijn ERA, Cuijpers RH, Newman-Norlund R, van Schie HT, Meulenbroek R. Joint action: neurocognitive mechanisms supporting human interaction. Topics in Cognitive Science. 2009;1:340–52. doi: 10.1111/j.1756-8765.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- Borroni P, Montagna M, Cerri G, Baldissera F. Cyclic time course of motor excitability modulation during the observation of a cyclic hand movement. Brain Research. 2005;1065:115–24. doi: 10.1016/j.brainres.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Brown EC, Brüne M. The role of prediction in social neuroscience. Frontiers in Human Neuroscience. 2012;6:147. doi: 10.3389/fnhum.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano G, Jousmäki V, Hari R. Actor’s and observer’s primary motor corticies stabilize similarly after seen or heard motor actions. PNAS. 2007;104:9058–62. doi: 10.1073/pnas.0702453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Merino B, Glaser DE, Grèzes J, Passingham RE, Haggard P. Action observation and acquired motor skills: an fMRI study with expert dancers. Cerebral Cortex. 2005;15:1243–9. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- Calvo-Merino B, Grèzes J, Glaser DE, Passingham RE, Haggard P. Seeing or doing? Influence of visual and motor familiarity in action observation. Current Biology. 2006;16:1905–10. doi: 10.1016/j.cub.2006.07.065. [DOI] [PubMed] [Google Scholar]
- Candidi M, Sacheli LM, Mega I, Aglioti SM. Somatotopic mapping of piano fingering errors in sensorimotor experts: TMS studies in pianists and visually trained musically naives. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs325. doi:10.1093/cercor/bhs325. [DOI] [PubMed] [Google Scholar]
- Chen JL, Rae C, Watkins KE. Learning to play a melody: an fMRI study examining the formation of auditory-motor associations. NeuroImage. 2012;59:1200–8. doi: 10.1016/j.neuroimage.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Chen R, Wassermann EM, Caños M, Hallett M. Impaired inhibition in writer's cramp during voluntary muscle activation. Neurology. 1997;49:1054–9. doi: 10.1212/wnl.49.4.1054. [DOI] [PubMed] [Google Scholar]
- Cook RD, Weisberg S. Residuals and Influence in Regression. New York, NY: Chapman & Hall; 1982. [Google Scholar]
- Cross ES, Hamilton AF, Grafton ST. Building a motor simulation de novo: observation of dance by dancers. NeuroImage. 2006;31:1257–67. doi: 10.1016/j.neuroimage.2006.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ausilio A, Altenmüller E, Olivetti Belardinelli M, Lotze M. Cross-modal plasticity of the motor cortex while listening to a rehearsed musical piece. European Journal of Neuroscience. 2006;24:955–8. doi: 10.1111/j.1460-9568.2006.04960.x. [DOI] [PubMed] [Google Scholar]
- D’Ausilio A, Badino L, Li Y, et al. Leadership in orchestra emerges from causal relationship of movement kinematics. PloS ONE. 2012;7:e35757. doi: 10.1371/journal.pone.0035757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology. 1980;10:85. [Google Scholar]
- Decety J, Jackson PL. The functional architecture of human empathy. Behavioural and Cognitive Neuroscience Reviews. 2004;2:71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- Fairhurst MT, Janata P, Keller PE. Being and feeling in synch with an adaptive virtual partenr: brain mechanisms underlying dynamic cooperativity. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs243. doi:10.1093/cercor/bhs243. [DOI] [PubMed] [Google Scholar]
- Gazzola V, Aziz-Zadeh L, Keysers C. Empathy and the somatotopic auditory mirror system in humans. Current Biology. 2006;16:1824–9. doi: 10.1016/j.cub.2006.07.072. [DOI] [PubMed] [Google Scholar]
- Giovannelli F, Innocenti I, Rossi S, et al. Role of the dorsal premotor cortex in rhythmic auditory-motor entrainment: a perturbation approach by rTMS. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs386. doi:10.1093/cercor/bhs386. [DOI] [PubMed] [Google Scholar]
- Haueisen J, Knösche TR. Involuntary motor activity in pianists evoked by music perception. Journal of Cognitive Neuroscience. 2001;13:786–92. doi: 10.1162/08989290152541449. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. NeuroImage. 2001;14:S103–9. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- Kaplan JT, Iacoboni M. Getting a grip on other minds: mirror neurons, intention understanding, and cognitive empathy. Social Neuroscience. 2006;1:175–83. doi: 10.1080/17470910600985605. [DOI] [PubMed] [Google Scholar]
- Keller PE. Joint action in music performance. In: Morganti F, Carassa A, Riva G, editors. Enacting Intersubjectivity: A Cognitive and Social Perspective on the Study of Interactions. Amsterdam: IOS Press; 2008. pp. 205–21. [Google Scholar]
- Keller PE. Mental imagery in music performance: underlying mechanisms and potential benefits. Annals New York Academy of Sciences. 2012;1252:206–13. doi: 10.1111/j.1749-6632.2011.06439.x. [DOI] [PubMed] [Google Scholar]
- Keller PE, Knoblich G, Repp BH. Pianists duet better when they play with themselves: on the possible role of action simulation in synchronization. Consciousness and Cognition. 2007;16:102–11. doi: 10.1016/j.concog.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Frith CD. A possible role for primary motor cortex during action observation. PNAS. 2007;104:8683–4. doi: 10.1073/pnas.0702937104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Friston KJ, Frith CD. Predictive coding: an account of the mirror neuron system. Cognitive Processing. 2007;8:159–66. doi: 10.1007/s10339-007-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Vargas C, Duval S, Blakemore SJ, Sirigu A. Motor activation prior to observation of a predicted movement. Nature Neuroscience. 2004;7:1299–301. doi: 10.1038/nn1355. [DOI] [PubMed] [Google Scholar]
- Knoblich G, Butterfill S, Sebanz N. Psychological research on joint action: theory and data. In: Ross B, editor. The Psychology of Learning and Motivation. Burlington: Academic Press; 2011. pp. 59–101. [Google Scholar]
- Knoblich G, Jordan JS. Action coordination in groups and individuals: learning anticipatory control. Journal of Experimental Psychology Learning. 2003;29:1006–16. doi: 10.1037/0278-7393.29.5.1006. [DOI] [PubMed] [Google Scholar]
- Koelewijn T, van Schie HT, Bekkering H, Oostenveld R, Jensen O. Motor-cortical beta oscillations are modulated by correctness of observed action. Neuroimage. 2008;40:767–75. doi: 10.1016/j.neuroimage.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Kokal I, Keysers C. Granger causality mapping during joint actions reveals evidence for forward models that could overcome sensory-motor delays. PLoS ONE. 2010;5:e13507. doi: 10.1371/journal.pone.0013507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konvalinka I, Vuust P, Roepstorff A, Frith CD. Follow you, follow me: continuous mutual prediction and adaptation in joint tapping. The Quarterly Journal of Experimental Psychology. 2010;93:2220–30. doi: 10.1080/17470218.2010.497843. [DOI] [PubMed] [Google Scholar]
- Kornysheva K, Schubotz RI. Impairment of auditory-motor timing and compensatory reorganization after ventral premotor cortex stimulation. PLoS ONE. 2011;6:e21421. doi: 10.1371/journal.pone.0021421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav A, Saltzman E, Schlaug G. Action representation of sound: audiomotor recognition network while listening to newly acquired actions. Journal of Neuroscience. 2007;27:308–14. doi: 10.1523/JNEUROSCI.4822-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Noppeney U. Long-term music training tunes how the brain temporally binds signals from multiple senses. PNAS. 2011;108:E1441–50. doi: 10.1073/pnas.1115267108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin O, Steyvers M, Wenderoth N, Li Y, Swinnen SP. Dynamical changes in corticospinal excitability during imagery of unimanual and bimanual wrist movements in humans: A transcranial magnetic stimulation study. Neuroscience Letters. 2004;359:185–9. doi: 10.1016/j.neulet.2004.01.070. [DOI] [PubMed] [Google Scholar]
- Loehr JD, Palmer C. Temporal coordination between performing musicians. Quarterly Journal of Experimental Psychology. 2011;64:2153–67. doi: 10.1080/17470218.2011.603427. [DOI] [PubMed] [Google Scholar]
- Noy L, Dekel E, Alon U. The mirror game as a paradigm for studying the dynamics of two people improvising motion together. PNAS. 2011;108:20947–52. doi: 10.1073/pnas.1108155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novembre G, Keller PE. A grammar of action generates predictions in skilled musicians. Consciousness and Cognition. 2011;20:1232–43. doi: 10.1016/j.concog.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Novembre G, Ticini LF, Schütz-Bosbach S, Keller PE. Distinguishing self and other in joint action. Evidence from a musical paradigm. Cerebral Cortex. 2012;22:2894–903. doi: 10.1093/cercor/bhr364. [DOI] [PubMed] [Google Scholar]
- Orgs G, Dombrowski JH, Heil M, Jansen-Osmann P. Expertise in dance modulates alpha/beta event-related desynchronization during action observation. European Journal of Neuroscience. 2008;27:3380–4. doi: 10.1111/j.1460-9568.2008.06271.x. [DOI] [PubMed] [Google Scholar]
- Oshio R, Tanaka S, Sadato N, Sokabe M, Hanakawa T, Honda M. Differential effect of double-pulse TMS applied to dorsal premotor cortex and precuneus during internal operation of visuospatial information. NeuroImage. 2010;49:1108–15. doi: 10.1016/j.neuroimage.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Paulus C. The Saarbruecken Personality Questionnaire on Empathy: Psychometric Evaluation of the German Version of the Interpersonal Reactivity Index. 2009 http://bildungswissenschaften.uni-saarland.de/personal/paulus/empathy/SPF.html (6 June 2013, date last accessed) [Google Scholar]
- Pecenka N, Keller PE. The role of temporal prediction abilities in interpersonal sensorimotor synchronization. Experimental Brain Research. 2011;211:505–15. doi: 10.1007/s00221-011-2616-0. [DOI] [PubMed] [Google Scholar]
- Prinz W. Perception and action planning. European Journal of Cognitive Psychology. 1997;9:129–54. [Google Scholar]
- Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nature Neuroscience. 2010;11:264–74. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Repp BH. Sensorimotor synchronization: a review of the tapping literature. Psychonomic Bulletin and Review. 2005;12:969–92. doi: 10.3758/bf03206433. [DOI] [PubMed] [Google Scholar]
- Repp BH. In: Music, Motor Control, and the Brain. Oxford, UK: Oxford University Press; 2006. Musical synchronization; pp. 55–76. [Google Scholar]
- Repp BH, Keller PE. Sensorimotor synchronization with adaptively timed sequences. Human Movement Science. 2008;27:423–56. doi: 10.1016/j.humov.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Ruspantini I, Maeki H, Korhonen R, D’Ausilio A, Ilnoniemi RJ. The functional role of the ventral premotor cortex in a visually paced finger tapping task: a TMS study. Behavioral Brain Research. 2011;220:325–30. doi: 10.1016/j.bbr.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Sacheli LM, Candidi M, Pavone EF, Tidoni E, Aglioti SM. And yet they act together: interpersonal perception modulates visuo-motor interference and mutual adjustments during a joint-grasping task. PLoS ONE. 2012;7:e50223. doi: 10.1371/journal.pone.0050223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammler D, Novembre G, Koelsch S, Keller PE. Syntax in a pianist’s hand: ERP signatures of “embodied” syntax processing in music. Cortex. 2013;49:1325–39. doi: 10.1016/j.cortex.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Sänger J, Müller V, Lindenberger U. Intra- and interbrain synchronoization and network properties when playing guitar in duets. Frontiers in Human Neuroscience. 2012;6:312. doi: 10.3389/fnhum.2012.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers MB, Keysers C. Mapping the flow of information within the putative mirror neuron system during gesture observation. Neuroimage. 2011;57:37–44. doi: 10.1016/j.neuroimage.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Schubotz RI. Prediction of external events with our motor system: towards a new framework. Trends in Cognitive Science. 2007;11:211–8. doi: 10.1016/j.tics.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Schütz-Bosbach S, Prinz W. Perceptual resonance: action-induced modulation of perception. Trends in Cognitive Science. 2007;11:349–55. doi: 10.1016/j.tics.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Sebanz N, Bekkering H, Knoblich G. Joint action: bodies and minds moving together. Trends Cogn Science. 2006;10:70–6. doi: 10.1016/j.tics.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Sebanz N, Knoblich G. Prediction in joint action: what, when, and where. Topics in Cognitive Science. 2009;1:353–67. doi: 10.1111/j.1756-8765.2009.01024.x. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Fleming MK, Byblow WD. Lateralization of unimanual and bimanual motor imagery. Brain Research. 2006;1095:139–47. doi: 10.1016/j.brainres.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Ticini LF, Schütz-Bosbach S, Weiss C, Casile A, Waszak F. When sounds become actions: higher-order representation of newly learnt action sounds in the human motor system. Journal of Cognitive Neuroscience. 2011;24:464–74. doi: 10.1162/jocn_a_00134. [DOI] [PubMed] [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: the origins of cultural cognition. Behavioural Brain Science. 2005;28:675–91. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- Tognoli E, Lagarde J, DeGuzman GC, Kelso JAS. The phi complex as a neuromarker of human social coordination. PNAS. 2007;104:8190–5. doi: 10.1073/pnas.0611453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schie HT, Mars RB, Coles MGH, Bekkering H. Modulation of activity in medial frontal and motor cortices during error observation. Nature Neuroscience. 2004;7:549–54. doi: 10.1038/nn1239. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nature Neuroscience. 2000;3:1212–7. doi: 10.1038/81497. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Doya K, Kawato M. A unifying computational framework for motor control and social interaction. Philosophical Transactions of the Royal Society B. 2003;358:593–602. doi: 10.1098/rstb.2002.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]