Abstract
Social anxiety is the intense fear of negative evaluation by others, and it emerges uniquely from a social situation. Given its social origin, we asked whether an anxiety-inducing social situation could enhance the processing of faces linked to the situational threat. While past research has focused on how individual differences in social anxiety relate to face processing, we tested the effect of manipulated social anxiety in the context of anxiety about appearing racially prejudiced in front of a peer. Visual processing of faces was indexed by the N170 component of the event-related potential. Participants viewed faces of Black and White males, along with nonfaces, either in private or while being monitored by the experimenter for signs of prejudice in a ‘public’ condition. Results revealed a difference in the N170 response to Black and Whites faces that emerged only in the public condition and only among participants high in dispositional social anxiety. These results provide new evidence that anxiety arising from the social situation modulates the earliest stages of face processing in a way that is specific to a social threat, and they shed new light on how anxiety effects on perception may contribute to the regulation of intergroup responses.
Keywords: face, anxiety, social, event-related potential (ERP), N170, race
Social disapproval ranks among humans’ most potent fears. At moderate levels, anxiety about social evaluation is motivating, compelling individuals to abide by social norms and to conform to others’ expectations (Schlenker and Leary, 1982). At extremes, social anxiety is debilitating (Marcin and Nemeroff, 2003); the fear of social interaction can keep a person from engaging in social and professional activities, as well as everyday personal tasks. Most people experience some degree of social anxiety in their daily lives, and social anxiety disorder is among the most common psychiatric disorders in the USA (Kessler et al., 2005). Given the pervasiveness and impact of social anxiety, research on its etiology has been a priority in psychological and neuroscience research.
Since social anxiety arises from social situations, much research on its psychological underpinnings has focused on how social anxiety affects the processing of faces. A common hypothesis is that social anxiety may affect the way faces are processed, such that anxiety may amplify and potentially bias one’s perception of a face so that it appears more threatening than it would under low-anxiety conditions. To date, most research on this question has asked whether dispositional social anxiety tendencies are associated with greater neural processing of socially threatening faces (e.g. faces with anger expressions), as compared with nonthreatening faces (e.g. Kolassa et al., 2007; Muhlberger et al., 2009). Although this approach has yielded several important findings, we suggest that it has neglected a critical element of social anxiety: the social situation. Hence, our goal in the present research was to test the interactive effect of trait social anxiety and a socially threatening situation on the neural processing of faces.
SOCIAL ANXIETY AND NEURAL MECHANISMS OF FACE PROCESSING
Does social anxiety enhance the neural processing of faces? Past investigations of this question have focused on the N170 component of the event-related potential (ERP) as an index of early face processing. The N170 is a negative polarity electrical signal that peaks approximately 170 ms after a face is presented and emerges most strongly over lateral occipital brain regions. N170 amplitudes are typically larger in response to faces compared with nonface stimuli, and accumulating evidence indicates that the N170 reflects the structural encoding of a face in visual processing (Bentin et al., 1996; Carmel and Bentin, 2002). Although faces are known to elicit greater attention in comparison with most other visual stimuli (Ro et al., 2001; Theeuwes and Van der Stigchel, 2006), research has shown that the N170 response is specifically related to face-encoding processes and not merely attentional processing (e.g. Cauquil et al., 2000; although attention away from a face stimulus will reduce the N170 amplitude; Jacques and Rossion, 2007).
Prior research on social anxiety and the N170 has tested whether highly socially anxious participants show different N170 responses to images of angry and neutral faces, presented privately, in a nonsocial context, in comparison to low-anxiety participants. The rationale is that highly anxious participants would interpret the angry faces as more socially threatening. However, findings from these studies have been somewhat mixed: some studies reported no effect of dispositional social anxiety on N170 responses to angry faces (Kolassa et al., 2007, 2008; Muhlberger et al., 2009), whereas other studies found that highly socially anxious participants exhibited larger N170 amplitudes to angry faces than low-anxiety participants (Kolassa and Miltner 2006; see also Mueller et al., 2008). Several factors may contribute to the different results found across studies, such as the use of different stimuli (e.g. schematic drawings of faces vs natural photographs) and different tasks (e.g. emotional categorization vs passive viewing).
However, there is a critical limitation to previous research on social anxiety and face processing: social anxiety arises from a social situation in which one fears scrutiny from another person, yet past research has focused on how individual differences in socially anxious tendencies predict responses to pictures of faces presented in nonsocial situations, in the absence of social-evaluative threat. That is, an angry face may be threatening (e.g. Ohman, 1986; Schupp et al., 2004), but it does not necessarily signal disapproval situated in a social context. As such, this methodology may not directly capture the essence of social anxiety processing. Therefore, the goal of the present research was to examine the interactive effects of situation-based social anxiety and trait social anxiety on the neural processing of faces.
SOCIAL ANXIETY FROM THE SOCIAL SITUATION: THE CASE OF INTERGROUP ANXIETY
Anxiety in response to social evaluation has been studied extensively in the field of social psychology (Leary and Kowalski, 1995). Although this work typically examines anxiety in normally functioning individuals, as opposed to those suffering from anxiety disorders, the situations that elicit social anxiety are similar for both populations. For example, being evaluated by others as incompetent or socially deviant can elicit strong emotional and physiological responses associated with anxiety (Baumeister and Tice, 1990; Williams, 2007; Dickerson and Kemeny, 2004). Research on intergroup prejudice, in particular, has revealed that people experience strong social anxiety about appearing prejudiced in front of others, given the strong social norms against the expressions of racial bias (Stephan and Stephan, 1985; Plant and Devine, 1998). This form of social anxiety, referred to as intergroup anxiety, may emerge in interracial interactions, as well as in same-race interactions in which an individual fears social sanctions from ingroup members for expressing prejudice toward a disadvantaged outgroup (e.g. when a White American fears disapproval from other Whites for expressing prejudice toward African Americans).1 Both situations can elicit strong feelings of social anxiety.
The intergroup anxiety literature is especially relevant to the present concerns about face processing: individuals worried about appearing prejudiced are particularly vigilant to racial cues, such as an outgroup member’s face (Amodio et al., 2003; Monteith et al., 2002), and this concern is amplified in situations where responses to in- and outgroup faces are observed publicly (Plant et al., 2003; Amodio et al., 2006). Prior research has shown that individuals high in trait intergroup anxiety, as measured by Plant and Devine’s (1998) external motivation scale, attend more strongly to Black than White faces in the context of a dot-probe task or as assessed using eye-tracking measures (Richeson and Trawalter, 2008; Bean et al., 2012). However, research has not yet examined this response in the context of anxiety arising from a manipulated social situation. Furthermore, in the perception literature, research has not tested whether situational elicitors of social anxiety modulate the perceptual process of face encoding, as opposed to attention. Given the strong social concerns associated with intergroup responses and the direct relevance of these concerns to faces, research on racial prejudice suggests an effective paradigm for studying the effects of situation-based social anxiety on face processing. We adopted this paradigm in the present work.
STUDY OVERVIEW
The present research examined the effects of an anxiety-eliciting social situation and trait social anxiety on the neural processing of faces. Unlike previous research, in which social anxiety was manipulated by the emotional expression of face stimuli, we manipulated social anxiety by varying whether participants’ responses to neutral faces were publicly evaluated. White American participants viewed faces of Black and White males with neutral expressions, as part of a computer task purported to measure their degree of racial prejudice, while electroencephalography (EEG) was recorded. Participants, who had completed a measure of dispositional social anxiety earlier in the semester, completed this task in either a ‘private’ condition, in which their responses were private and confidential, or a ‘public’ condition, in which their responses were ostensibly observed and evaluated for signs of prejudice by the experimenter. In private, neither White nor Black faces would be associated with a potential social threat, but in public, Black faces would be associated with the threat of negative evaluation from the experimenter. This design allowed us to test whether situationally elicited social anxiety affected the visual processing of faces. Since the public response condition was expected to elicit greater anxiety among participants predisposed to social anxiety concerns, we examined the effect of response condition as a function of participants’ scores on a social anxiety inventory.
Our main prediction was that social anxiety about appearing prejudiced in public would be associated with greater processing of Black than White faces, because the Black faces are directly relevant to participants’ social anxiety concerns. Hence, we predicted a larger N170 race effect among participants in the public condition who were highly prone to social anxiety. Following previous work, low-anxiety participants and/or those responding in private were not expected to exhibit a race effect on the N170 (He et al., 2009; Wiese et al., 2009; Ofan et al., 2011). This pattern would provide evidence that situation-based social anxiety enhances the visual processing of faces that are specifically relevant to one’s socio-evaluative concerns.
It is notable that we did not ask participants to report on their state anxiety following the manipulation. This is because past research has shown that by asking participants to pause, in the course of the procedure, to reflect on their emotions can lessen the emotional experience and reduce the effect of the manipulation (Elliot and Devine, 1994; Lieberman et al., 2007). Nevertheless, previous studies using this type of public/private manipulation have shown that a public condition reliably produces strong social anxiety, relative to a private condition (Plant and Devine, 1998; Lambert et al., 2003; Plant et al., 2003; Amodio, 2009). Furthermore, by examining the interactive effect of a situational threat and trait social anxiety, we could test a very specific person × situation prediction, whereby the situational effect would only enhance outgroup face processing among highly socially anxious individuals. This specificity would bolster our ability to infer that the effect was related to social anxiety.
METHODS
Participants
Forty-three right-handed, White American, native English-speaking Introductory Psychology students participated individually for extra course credit.
Design and procedure
After providing informed consent, participants were prepared for EEG recording and received task instructions. Experimenters were White females. Participants were told that on each trial, they would see an image (White face, Black face or car) followed by a word, and that they should categorize the word as pleasant or unpleasant via button press. All participants were told that responses on certain trials could reveal an influence of racial bias (e.g. errors in Black–pleasant trials) and that they should be aware of the types of errors they made. This instruction was given so that participants in each condition would be personally motivated to respond without prejudice (Amodio et al., 2006).
Participants were randomly assigned to the private or public condition. Participants assigned to the private condition were told that their responses would remain confidential, such that they should not be concerned with external pressures to appear nonprejudiced. Participants assigned to the public condition were told that the experimenter would observe their responses for signs of racial prejudice. This procedure has been used as a strong manipulation of social concern in past behavioral and ERP research (Plant et al., 2003; Amodio et al., 2006).
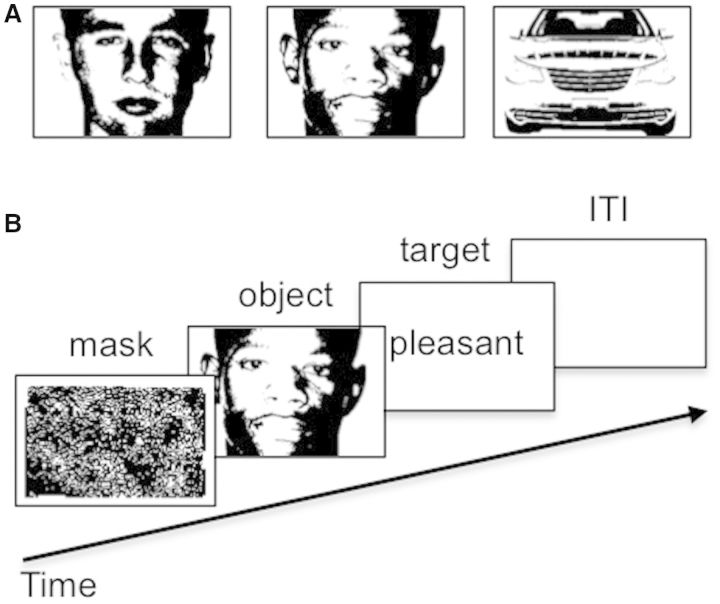
The task included 20 practice trials and 240 experimental trials. Trials representing the six possible types of prime-target combinations were randomly ordered and equally probable. Trials began with a pattern mask that served as a fixation point (600 ms), followed by a prime image (300 ms) and then a target word which remained onscreen until a response was made (Figure 1A). Participants were encouraged to respond within 600 ms, but responses were recorded until 1500 ms. A ‘Too slow!’ message followed responses that exceeded the deadline. This deadline was used to elicit a sufficient distribution of errors for analysis. Participants received accuracy feedback on practice trials but not experimental trials. Stimuli and recording triggers were presented using DMDX software (Forster and Forster, 2003).
Fig. 1.
Example illustrations of two-toned face and car images, which were equated on luminance and contrast (A) and a trial of the sequential evaluative priming task in which these visual stimuli were presented (B).
Prime stimuli included two-tone images of 10 Black male faces, 10 White male faces and 10 cars (front view; Figure 1B). All images were 250 × 165 pixels (1 × 0.66° visual angle), with equal proportions of black and white pixels, and thus equalized for luminance and contrast. Previous research using these stimuli indicated that the race of two-toned Black and White faces is highly discriminable (Ofan et al., 2011). Target words included 10 pleasant and 10 unpleasant words that were semantically unrelated to Black or White stereotypes or to cars (e.g. laughter, beauty, pain and disgusting). All stimuli were presented in the center of the computer screen. Importantly, by including clear images of faces (and cars) and presenting them alone and at fixation, this design held attention to the prime stimuli constant.
Questionnaires
Participants completed the Social Phobia Inventory (SPIN; Connor et al., 2000) in a mass testing session conducted at the beginning of the academic semester. The SPIN is a widely used 17-item self-report measure of social phobia. Each item is rated on a 0–4 scale. Total scores can range from 0 to 68; scores above 19 suggest the potential for social anxiety disorder, and scores above 50 reflect extreme social anxiety. Subjects rated their agreement with a list of statements describing common anxiety-related concerns, such as ‘Being criticized scares me a lot’ and ‘Fear of embarrassment causes me to avoid doing things or speaking to people’. The scale was reliable, α = 0.85, and prior research has established the scale’s internal consistency and discriminant validity (Connor et al., 2000; Anthony et al., 2006).
Behavioral data processing
Responses with latencies between 200 and 1000 ms were included in analyses, thereby excluding responses associated with action slips or inattention (0.08% of trials). Response latency scores for correct responses were submitted to natural log transformation and averaged as a function of trial type for analysis (but presented as untransformed values). Accuracy scores were computed as a function of trial type.
EEG recording and processing
EEG was recorded from 11 Ag–AgCl electrodes (5 midline electrodes: Fz, Fcz, Cz, Pz and Oz; 6 lateral–occipital electrodes: P7/8, PO7/8 and PO9/10), embedded in a stretch-lycra cap (Electrode Arrays, El Paso, TX, USA), positioned according to the 10–10 system, referenced online to the left earlobe (kΩ < 5), with a forehead ground. Vertical and horizontal eye movements were recorded to facilitate artifact scoring. Signals were amplified using a Neuroscan Synamps2 (El Paso, TX, USA) with AC coupling, .15–100 Hz bandpass-filtered and digitized at 1000 Hz. Off-line, EEG was re-referenced to average earlobes, scored for movement artifact, submitted to a regression-based eyeblink-correction procedure and then digitally filtered through a 1–15 Hz bandpass.
Analyses of the N170 component focused on amplitude scores derived from the PO10 recording site, where the N170 effect was maximal. To create the ERP waveforms, a 900 ms stimulus-locked epoch was selected for each artifact-free trial beginning 100 ms prior to the prime onset. Baseline correction procedures subtracted the average voltage during the 100 ms prestimulus period from each epoch, and then epochs were averaged within their respective trial type. For each subject, the N170 was scored as the peak negative amplitude between 150 and 210 ms following face onset.
RESULTS
Four participants were excluded from analysis: one for excessive EEG artifact and three for extreme SPIN scores (>49), yielding 39 participants for analysis (mean SPIN score = 18.26, s.d. = 9.60).
Task behavior
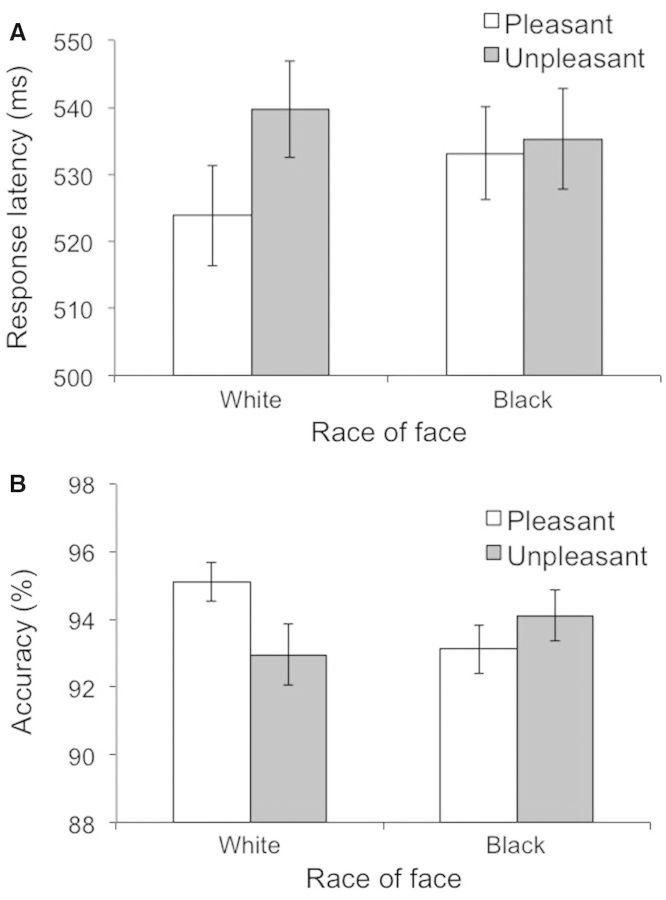
A preliminary analysis of participants’ task behavior revealed the typical pattern of implicit racial bias. A 2 (race: Black vs White face) × 2 (target word: positive vs negative) analysis of variance (ANOVA) conducted on response latencies produced a significant interaction, F(1,37) = 5.46, P = 0.02 (Figure 2A). Simple effect tests indicated that pleasant words were categorized more quickly following White faces (M = 523.86, s.d. = 46.68) than Black faces (M = 533.18, s.d. = 43.33), t(38) = 2.17, P = 0.04. Responses to negative words following White faces (M = 539.72, s.d. = 44.75) and Black faces (M = 535.28, s.d. = 47.12) did not differ significantly in latency, t(38) = 1.56, P = 0.13. An ANOVA testing the effects of race and target word on response accuracy also produced a significant interaction, F(1,37) = 7.08, P = 0.01 (Figure 2B). Simple effect tests indicated that pleasant words were categorized more accurately following White faces (M = 95.12%, s.d. = 3.62) than Black faces (M = 93.12%, s.d. = 4.54), t(38) = 2.51, P = 0.02. Accuracy in responses to negative words following White faces (M = 92.96%, s.d. = 5.62) and Black faces (M = 94.11%, s.d. = 4.75) did not differ significantly, t(38) = 1.39, P = 0.17.2 Together, these findings replicate the typical pattern of racial bias found using reaction-time tasks; here, as in several previous studies, the effect was driven primarily by stronger positive associations with White ingroup faces than with Black outgroup faces (Fazio et al., 1995; Dovidio et al., 1997; Conrey et al., 2005; Ofan et al., 2011). This pattern also indicated that participants had substantial reason to fear social evaluation in the public condition.
Fig. 2.
White faces were more strongly associated with positive words than negative words, in comparison with Black faces, as indicated by response latencies (A) and accuracy rates (B) as a function of trial type.
N170 effects
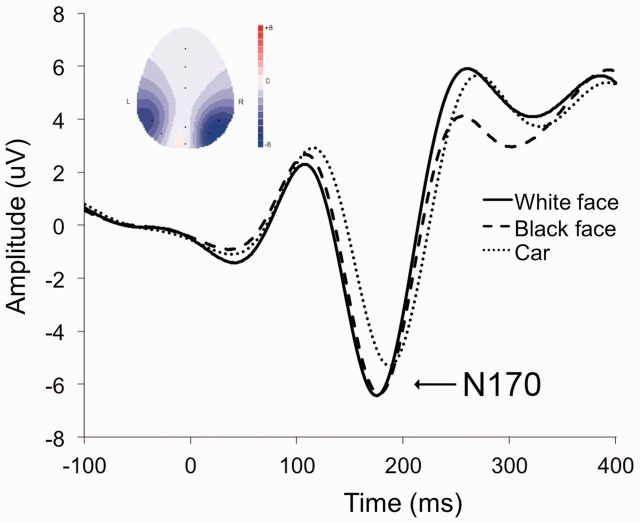
Preliminary analyses to establish the N170 as a face-specific response confirmed that the N170 was larger to faces (Black: M = −7.33, s.d. = 4.12; White: M = −7.36, s.d. = 4.55) than to cars (M = −6.21, s.d. = 4.3), P < 0.01 (Figure 3), and that the face-related N170 was more pronounced in the right hemisphere (M = −6.97, s.d. = 4.08) than the left hemisphere (M = −4.87, s.d. = 6.40), F(1, 37) = 3.93, P = 0.05. This pattern replicated typical findings in the N170 literature (e.g. Bentin et al., 1996; Carmel and Bentin, 2002). Hence, subsequent analyses focused on right-hemisphere N170 responses to faces.
Fig. 3.
Event-related potential waveforms recorded at the right temporo-occipital site, illustrating a larger N170 response to both White and Black faces than to car images. On the X-axis, zero represents the onset of the face or car image on the screen. The inset topographic voltage map for the peak N170 amplitude shows that is most pronounced over the right temporo-occipital brain region.
On average, across conditions, N170 responses to Black and White faces did not differ, P = 0.95, as in previous studies (Carmel and Bentin, 2002; Caldara et al., 2003, 2004; Ofan et al., 2011; but see also Ito and Urland, 2005; Walker et al., 2008). However, our main goal was to test whether the relative difference in N170 amplitude between Black and White faces varied as a function of condition and trait social anxiety. To this end, a difference score was computed by subtracting the N170 amplitude for White faces from that of Black faces, separately for each participant, as in Ofan et al. (2011). This ‘race N170’ score reflected the relative difference between the processing of Black and White faces, such that more negative scores reflect greater processing of Black faces than White faces. This allowed us to test our main hypothesis using multiple regression.
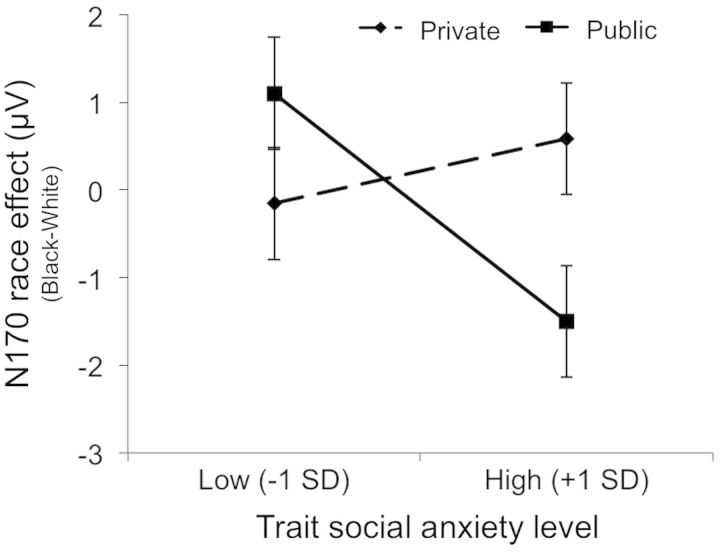
The interactive effect of response condition (public vs private, coded as 1 and −1, respectively) and the continuous measure of trait social anxiety on the race N170 score was tested using regression. This analysis produced only a significant interaction, β = −0.39, t = 2.54, P = 0.02 (predicted values are illustrated in Figure 4). Simple-slope analyses indicated that in the public condition, participants with higher dispositional social anxiety exhibited larger N170 race effects, β = −0.60, t = −2.69, P = 0.01, suggesting greater processing of Black faces. This pattern was not observed in the private condition, β = 0.17, t = 0.83, P = 0.41. That is, greater processing of Black than White faces was observed only in public and only among participants with high trait social anxiety. This pattern of results supported our main hypothesis.
Fig. 4.
Predicted values of the N170 race effect, illustrating the interaction of response condition and participants’ degree of trait anxiety, computed at 1 s.d. below and above each mean. Error bars represent 1 s.e.m.
Additional analyses that compared the N170 to car images, in relation with either Black faces, White faces or the average of White and Black faces, did not produce significant effects of trait anxiety, response condition or their interaction (P’s ranged from 0.13 to 0.79). These analyses further suggest that the interactive effect of social anxiety and response condition pertained to the relative difference in the N170 to Black compared with White faces.
Relationship between N170 and task behavior
Although not the primary focus of this experiment, we conducted supplementary analyses to examine the association between the N170 effects and task behavior. A large body of research has shown that anxiety is typically associated with behavioral freezing (Blanchard and Blanchard, 1969; Davis, 1992) and disrupted attentional focus (i.e. attention toward the source of threat and away from a secondary task; Easterbrook, 1959; Aston-Jones and Cohen, 2005). In the context of the priming task used here, greater processing of the face primes (a threat cue for some participants) could interfere with the categorization of the target words (see also Ofan et al., 2011). Thus, we expected that, in public, larger N170 responses to Black compared with White faces would be associated with worse overall accuracy on face trials (response slowing would be predicted, too, but given the short response deadline, the effect was expected to emerge in terms of accuracy rather than latency). We did not expect to find this relationship in the private condition.
Average response accuracy scores for face trials were submitted to a regression analysis, in which the condition, N170 race score, and their interaction were included as predictors. The only significant effect to emerge from this analysis was the Condition × N170 interaction, β = 0.35, t = 2.25, P = 0.03. Simple-slope analyses indicated that in the public condition, the tendency to exhibit a larger N170 to Black than White faces was associated with worse task performance, β = 0.41, t = 1.76, P = 0.087. That is, enhanced processing of the threat-related faces appeared to detract from the main task goal of classifying target stimuli (as in Ofan et al., 2011). In the private condition, the N170 score was not significantly associated with behavior, β = −0.30, t = −01.41, P = 0.17. Additional analyses indicated that this interaction pattern was evident for each trial type, although not all reached significance (β’s ranging from −0.18 to −0.45, P’s ranging from 0.01 to 0.31), and that these effects were not significantly different from each other. Overall, this pattern suggests that in a socio-evaluative situation, enhanced processing of anxiety-relevant faces may shift processing resources away from one’s main task goal overall (i.e. across trials types) and toward the source of the threat.2
Although the experimental task was optimized for an analysis of accuracy rates, we conducted a supplementary analysis testing the effects of condition, N170 race score and their interaction on the average response latency across trial types while adjusting for trait social anxiety. A significant effect emerged only for the N170 race score, β = −0.35, t = −2.20, P = 0.03, indicating that participants with larger N170 amplitudes to Black than White faces responded more slowly on the task. Additional analysis indicated that this main effect was evident for each trial type (β’s ranging from −0.25 to −0.37, P’s ranging from 0.02 to 0.13), and these effects were not significantly different from each other. Although the interaction was not significant, β = −0.12, t = −0.78, P = 0.44, the main effect of the N170 race score is consistent with the idea that the tendency to engage greater visual processing of Black than White face primes may reflect a degree of response freezing—the primary behavioral expression of anxiety—and hence with worse performance on the speeded categorization of target words.
DISCUSSION
Social anxiety emerges in social situations in which an individual fears the negative evaluation of others. Here, we demonstrated that situationally induced social anxiety enhances the neural processing of faces that represent the source of one’s socio-evaluative concern. When responding in public, under observation for signs of racial prejudice, high-anxiety participants evidenced greater visual encoding of Black faces than White faces, as indicated by the N170 index of neural activity. In this case, participants knew that responses to Black faces could reveal their racial prejudice to a disapproving observer, and thus Black faces were strongly relevant to their socio-evaluative concerns. But when responding in private, away from the scrutiny of the evaluative observer, the same faces no longer received preferential processing. These results suggest that social anxiety, as it arises from an evaluative social context, can affect the initial stages of face processing—in effect, enhancing the perception of faces that signal a social threat.
It is notable that we did not assess participants’ reported social anxiety directly in this experiment. We omitted this type of measure to avoid drawing participants’ attention to their subjective feelings, as past research has shown that subjective focus on one’s emotional response to a manipulation can reduce the effect of the manipulation on other psychological processes (Elliot and Devine, 1994). Instead, we used a person × situation approach to show that the situational manipulation of socio-evaluative threat only produced an effect among participants with a dispositional tendency for social anxiety. The selective effect afforded by this design strengthened our inference that our results were due to social anxiety. Furthermore, previous research using a similar type of public response manipulation has shown that it successfully induces anxiety (e.g. Lambert et al., 2003). Nevertheless, it would be useful for future research on social anxiety and face perception to include unobtrusive indicators of anxiety, such as physiological measures of autonomic activity, to further strengthen such inferences.
A major contribution of this research is that it highlights the critical role of the social situation in social anxiety processes. Much prior research has examined social anxiety effects on face processing in nonsocial situations, illuminating basic anxiety effects on social stimuli (e.g. Schupp et al., 2004). The present research provides a critical advance in this line of inquiry, however, by situating this process in a realistic social context that produces strong socio-evaluative concerns. In this way, our research captured the essence of the social anxiety experience and showed that it can enhance the processing of faces that are relevant to one’s socio-evaluative concern.
N170 modulation as a motivated response strategy
Although our findings focus on the effect of social anxiety on the N170 response to faces, we propose that the N170 effect represents a strategic shift in perceptual sensitivity that serves the goal of protecting oneself from social disapproval. This shift may be adaptive in some situations but not others. For example, Amodio (2010) found that greater early perceptual attention to Black than White faces, as indicated by the P2 ERP component, was associated with a behavioral pattern of greater control and less racial bias. In this case, participants knew that Black faces might prime them to respond with prejudice and, thus, in the absence of socio-evaluative threat, Black faces served as a cue for engaging a more controlled response strategy. Importantly, however, this adaptive pattern occurred among participants with egalitarian racial attitudes who completed the task in private, absent social anxiety concerns.
By comparison, in an investigation of face encoding processes by Ofan et al. (2011), larger N170 responses to Black than White faces were associated with worse behavioral control. In this study, participants completed the task in private, and half were alerted to the possibility that their performance could reveal racial bias. Here, greater early visual processing of faces interfered with effective behavioral control. That is, task performance depended on accurate responding to word targets, and so the enhanced processing of faces may have distracted participants’ focus away from the targets. In this context, the greater processing of Black faces interfered with successful task completion.
Indeed, the pattern of N170 effects on behavior in the present study matched this pattern observed by Ofan et al. (2011). That is, in public, but not in private, larger N170 differences to Black than White faces were associated with lower task accuracy. Social anxiety appeared to increase processing of the threat while impairing effective action on the task. In terms of task performance, our results suggest that social anxiety promotes a response strategy that may be maladaptive. However, it is likely that in another context, such as when a social threat must be dealt with directly, the enhanced processing of faces representing the source of threat would be adaptive. More broadly, these findings are consistent with the idea that motivated changes in perception constitute a self-regulatory function.
Studying social anxiety in the intergroup context
Although we presented our research as examining general mechanisms of social anxiety and visual processing, we were also interested in the implications of these findings for intergroup relations. Past research on prejudice and stereotyping has shown that intergroup anxiety can amplify implicit prejudice (Amodio and Hamilton, 2012), interfere with cognitive control (Lambert et al., 2003; Amodio, 2009) and harm cross-race friendships (West et al., 2009). However, it has not previously addressed whether intergroup anxiety might also affect how people ‘see’ members of the ingroup and outgroup. This question is important because subtle biases in visual perception may be difficult to detect, and thus resistant to control, yet they lay the foundation for subsequent judgments and impressions of a person. Our research offers new evidence that intergroup anxiety can indeed tune the visual perception of faces, beyond attentional effects of intergroup anxiety suggested by previous research (e.g. Monteith et al., 2002; Richeson and Trawalter, 2008; Amodio, 2010; Bean et al., 2012). Specifically, we found that anxiety about appearing prejudiced to an observer enhanced the encoding of outgroup faces, which were directly relevant to the source of anxiety, as compared with ingroup faces. This suggests that intergroup anxiety effects on cognition and behavior may arise from biases in early visual processing. To the extent this is true, it may be possible to develop new interventions to reduce bias in perception, in addition to interventions that target biases in downstream responses (e.g. Monteith et al., 2002; Mendoza et al., 2010).
The present research also highlights the relevance of social psychological research on intergroup bias for the understanding of basic mechanisms of anxiety and visual perception. The intergroup domain provides a rich context for examining the interplay of visual face processing, personal goals (e.g. to respond without prejudice), social concerns and behavior in an experimentally controlled manner. Indeed, there is a substantial literature on intergroup anxiety in the field of social psychology, though its contact with other literatures on social anxiety is limited. These social psychological studies have investigated how social anxiety arising from intergroup situations affects a range of psychological processes, including categorization (i.e. stereotyping), cognitive control, attitudes, physiological responses and behavior (e.g. Rankin and Campbell, 1955; Britt et al., 1996; Lambert et al., 2003; Plant and Devine, 2003; Littleford et al., 2005; Richeson and Trawalter, 2008; Trawalter et al., 2009; Amodio, 2009; Amodio and Hamilton, 2012). Although designed to address social psychological processes, findings from these studies can also inform basic psychological mechanisms of social anxiety. Thus, the intergroup anxiety literature has much to contribute, with its theoretical advances and powerful methods, to the broader field of research on social anxiety.
Evidence for the top–down modulation of the N170
Our findings are also relevant to the longstanding debate on whether early stages of visual face processing are subject to top–down influences. Traditional models posit that face perception is a hierarchical process influenced only by bottom–up informational streams (Bruce and Young, 1986). According to this view, the configural processing of a face is relatively veridical with respect to the visual percept, and thus this form of processing, indexed by the N170, should not be subject to top–down modulation. However, this view has been challenged in recent years, with an increasing number of investigations demonstrating top–down effects on the N170 and other neural indicators of early face processing (e.g. Golby et al., 2001; Van Bavel et al., 2008; Ofan et al., 2011). For example, knowledge of whether a face represents a member of one’s own arbitrary social group has been found to moderate the N170 amplitude toward faces that are otherwise visually indistinguishable (Ratner and Amodio, 2013).
The present work provides new evidence that situation-based social anxiety, and the motivational strategies it may invoke, produces top–down signaling to early visual mechanisms to strategically modulate the initial encoding of a face. By demonstrating that socio-affective responses modulate early face processing, this research provides new insight into how socio-evaluative concerns can shape one’s perceptual experience. Broadly, it advances the debate on whether the face-related N170, and other early components of neural face processing, may be influenced by the top–down effects of prior information, emotion and context.
CONCLUSION
Social anxiety is unique among other forms of anxiety in that it emerges in the context of social situations. By examining responses in a socio-evaluative context, the present findings suggest that social anxiety may influence the way we ‘see’ other people, informing basic theories of visual perception and suggesting new perspectives on how visual processes may relate to social behavior.
Footnotes
1 It is notable that some researchers reserve the term “intergroup anxiety” to describe anxiety about appearing prejudiced to an outgroup member only. However, the term is also used more broadly to refer to anxiety about appearing prejudiced to any peer or authority figure who might disapprove of a prejudiced response, as in the present research.
2 When accuracy rates for trials involving car primes were included in a 3 (Prime: White face vs. Black face vs. car) x 2 (Target word: positive vs. negative) ANOVA, the interaction remained significant, F(2, 76) = 4.78, p = .01, driven by the effects of race described in the main text. A paired t-test indicated that accuracy was not significantly different on car-positive (M = .94, SD = .06) and car-negative trials (M = .96, SD = .04), t(38) = 1.70, p = .10.
References
- Amodio DM. Intergroup anxiety effects on the control of racial stereotypes: a psychoneuroendocrine analysis. Journal of Experimental Social Psychology. 2009;45:60–7. [Google Scholar]
- Amodio DM. Coordinated roles of motivation and perception in the regulation of intergroup responses: frontal cortical asymmetry effects on the P2 event-related potential and behavior. Journal of Cognitive Neuroscience. 2010;22:2609–17. doi: 10.1162/jocn.2009.21395. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Hamilton HK. Intergroup anxiety effects on implicit racial evaluation and stereotyping. Emotion. 2012;12:1273–80. doi: 10.1037/a0029016. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Harmon-Jones E, Devine PG. Individual differences in the activation and control of affective race bias as assessed by startle eyeblink responses and self-report. Journal of Personality and Social Psychology. 2003;84:738–53. doi: 10.1037/0022-3514.84.4.738. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Kubota JT, Harmon-Jones E, Devine PG. Alternative mechanisms for regulating racial responses according to internal vs external cues. Social Cognitive and Affective Neuroscience. 2006;1:26–36. doi: 10.1093/scan/nsl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony MM, Coons MJ, McCabe RE, Ashbaugh A, Swinson RP. Psychometric properties of the social phobia inventory: further evaluation. Behaviour Research and Therapy. 2006;44:1177–85. doi: 10.1016/j.brat.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Tice DM. Anxiety and social exclusion. Journal of Social and Clinical Psychology. 1990;9:165–95. [Google Scholar]
- Bean MG, Slaten DG, Horton WS, Murphy MC, Todd AR, Richeson JA. Prejudice concerns and race-based attentional bias: new evidence from eyetracking. Social Psychological and Personality Science. 2012;3:722–9. [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–65. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of Comparative Physiological Psychology. 1969;67:370–5. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Britt TW, Boniecki KA, Vescio TK, Biernat M, Brown LM. Intergroup anxiety: a person x situation approach. Personality and Social Psychology Bulletin. 1996;22:1177–88. [Google Scholar]
- Bruce V, Young A. Understanding face recognition. British Journal of Psychology. 1986;77:305–27. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Caldara R, Rossion B, Bovet P, Hauert CA. Event-related potentials and time course of the ‘other-race’ face classification advantage. Neuroreport. 2004;15:905–10. doi: 10.1097/00001756-200404090-00034. [DOI] [PubMed] [Google Scholar]
- Caldara R, Thut G, Servoir P, Michel CM, Bovet P, Renault B. Face versus non-face object perception and the ‘other-race’ effect: a spatiotemporal event-related potential study. Clinical Neurophysiology. 2003;114:515–28. doi: 10.1016/s1388-2457(02)00407-8. [DOI] [PubMed] [Google Scholar]
- Carmel D, Bentin S. Domain specificity versus expertise: factors influencing distinct processing of faces. Cognition. 2002;83:1–29. doi: 10.1016/s0010-0277(01)00162-7. [DOI] [PubMed] [Google Scholar]
- Cauquil AS, Edmonds GE, Taylor MJ. Is the face-sensitive N170 the only ERP not affected by selective attention? Neuroreport. 2000;11:2167–71. doi: 10.1097/00001756-200007140-00021. [DOI] [PubMed] [Google Scholar]
- Connor KM, Davidson JRT, Chuchill LE, Sherwood A, Weisler RH, Foa E. Psychometric properties of the social phobia inventory (SPIN): new self-rating scale. The British Journal of Psychiatry. 2000;176:379–86. doi: 10.1192/bjp.176.4.379. [DOI] [PubMed] [Google Scholar]
- Conrey FR, Sherman JW, Gawronski B, Hugenberg K, Groom C. Separating multiple processes in implicit social cognition: The Quad-Model of implicit task performance. Journal of Personality and Social Psychology. 2005;89:469–87. doi: 10.1037/0022-3514.89.4.469. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dovidio JF, Kawakami K, Johnson C, Johnson B, Howard A. On the nature of prejudice: Automatic and controlled processes. Journal of Experimental Social Psychology. 1997;33:510–40. [Google Scholar]
- Easterbrook JA. The effects of emotion on cue utilization and the organization of behavior. Psychological Review. 1959;66:183–201. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Elliot AJ, Devine PG. On the motivational nature of cognitive dissonance: dissonance as psychological discomfort. Journal of Personality and Social Psychology. 1994;67:382–94. [Google Scholar]
- Fazio RH, Jackson JR, Dunton BC, Williams CJ. Variability in automatic activation as an unobtrusive measure of racial attitudes: a bona fide pipeline? Journal of Personality and Social Psychology. 1995;69:1013–27. doi: 10.1037//0022-3514.69.6.1013. [DOI] [PubMed] [Google Scholar]
- Forster KI, Forster JC. DMDX: a windows display program with millisecond accuracy. Behavior Research Methods, Instruments, & Computers. 2003;35:116–24. doi: 10.3758/bf03195503. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Gabrieli JDE, Chiao JY, Eberhardt JL. Differential fusiform responses to same- and other-race faces. Nature Neuroscience. 2001;4:845–50. doi: 10.1038/90565. [DOI] [PubMed] [Google Scholar]
- He Y, Johnson MK, Dovidio JF, McCarthy G. The relation between race-related implicit associations and scalp-recorded neural activity evoked by faces from different races. Social Neuroscience. 2009;4:426–42. doi: 10.1080/17470910902949184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito TA, Urland GR. The influence of processing objectives on the perception of faces: an ERP study of race and gender perception. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:21–36. doi: 10.3758/cabn.5.1.21. [DOI] [PubMed] [Google Scholar]
- Jacques C, Rossion B. Electrophysiological evidence for temporal dissociation between spatial attention and sensory competition during human face processing. Cerebral Cortex. 2007;17:1055–65. doi: 10.1093/cercor/bhl015. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Archives of General Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa IT, Kolassa S, Bergmann S, et al. Interpretive bias in social phobia: an ERP study with morphed emotional schematic faces. Cognition & Emotion. 2008;23:69–95. [Google Scholar]
- Kolassa IT, Kolassaa S, Musial F, Miltner WHR. Event-related potentials to schematic faces in social phobia. Cognition & Emotion. 2007;21:1721–44. [Google Scholar]
- Kolassa IT, Miltner WHR. Psychophysiological correlates of face processing in social phobia. Brain Research. 2006;1118:130–41. doi: 10.1016/j.brainres.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Lambert AJ, Payne BK, Jacoby LL, Shaffer LM, Chasteen AL, Khan SR. Stereotypes as dominant responses: on the “social facilitation” of prejudice in anticipated public contexts. Journal of Personality and Social Psychology. 2003;84:277–95. doi: 10.1037//0022-3514.84.2.277. [DOI] [PubMed] [Google Scholar]
- Leary MR, Kowalski RM. Social Anxiety. New York: Guilford Press; 1995. [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity to affective stimuli. Psychological Science. 2007;18:421–8. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Littleford LN, Wright MO, Sayoc-Parial M. White students’ intergroup anxiety during same-race and interracial interactions: a multimethod approach. Basic and Applied Social Psychology. 2005;27:85–94. [Google Scholar]
- Marcin MS, Nemeroff CB. The neurobiology of social anxiety disorder: the relevance of fear and anxiety. Acta Psychiatrica Scandinavica. 2003;108:51–64. doi: 10.1034/j.1600-0447.108.s417.4.x. [DOI] [PubMed] [Google Scholar]
- Mendoza SA, Gollwitzer PM, Amodio DM. Reducing the expression of implicit stereotypes: reflexive control through implementation intentions. Personality and Social Psychology Bulletin. 2010;36:512–23. doi: 10.1177/0146167210362789. [DOI] [PubMed] [Google Scholar]
- Monteith MJ, Ashburn-Nardo L, Voils CI, Czopp AM. Putting the brakes on prejudice: on the development and operation of cues for control. Journal of Personality and Social Psychology. 2002;83:1029–50. doi: 10.1037//0022-3514.83.5.1029. [DOI] [PubMed] [Google Scholar]
- Mueller EM, Hofmann SG, Santesso DL, Meuret AE, Bitran S, Pizzagalli DA. Electrophysiological evidence of attentional biases in social anxiety disorder. Psychological Medicine. 2008;15:1–12. doi: 10.1017/S0033291708004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlberger A, Wieser MJ, Herrmann MJ, Weyers P, Trger C, Pauli P. Early cortical processing of natural and artificial emotional faces differs between lower and higher socially anxious persons. Journal of Neural Transmission. 2009;116:735–46. doi: 10.1007/s00702-008-0108-6. [DOI] [PubMed] [Google Scholar]
- Ofan RH, Rubin N, Amodio DM. Seeing race: N170 responses to race and their relation to automatic racial attitudes and controlled processing. Journal of Cognitive Neuroscience. 2011;10:3153–61. doi: 10.1162/jocn_a_00014. [DOI] [PubMed] [Google Scholar]
- Ohman A. Face the beast and fear the face: animal and social fears as prototypes for evolutionary analyses of emotion. Psychophysiology. 1986;23:123–45. doi: 10.1111/j.1469-8986.1986.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Plant EA, Devine PG. Internal and external motivation to respond without prejudice. Journal of Personality and Social Psychology. 1998;75:811–32. doi: 10.1177/0146167205275304. [DOI] [PubMed] [Google Scholar]
- Plant EA, Devine PG. Antecedents and implications of interracial anxiety. Personality and Social Psychology Bulletin. 2003;29:790–801. doi: 10.1177/0146167203029006011. [DOI] [PubMed] [Google Scholar]
- Plant EA, Devine PG, Brazy PC. The bogus pipeline and motivations to respond without prejudice: revisiting the fading and faking of racial prejudice. Group Processes and Intergroup Relations. 2003;6:187–200. [Google Scholar]
- Rankin RE, Campbell DT. Galvanic skin response to Negro and White experimenters. Journal of Abnormal and Social Psychology. 1955;51:30–3. doi: 10.1037/h0041539. [DOI] [PubMed] [Google Scholar]
- Ratner KG, Amodio DM. Seeing “us vs. them”: minimal group effects on the neural encoding of faces. Journal of Experimental Social Psychology. 2013;49:298–301. [Google Scholar]
- Richeson JA, Trawalter S. The threat of appearing prejudiced and race-based attentional biases. Psychological Science. 2008;19:98–102. doi: 10.1111/j.1467-9280.2008.02052.x. [DOI] [PubMed] [Google Scholar]
- Ro T, Russell C, Lavie N. Changing faces: a detection advantage in the flicker paradigm. Psychological Science. 2001;12:94–9. doi: 10.1111/1467-9280.00317. [DOI] [PubMed] [Google Scholar]
- Schlenker BR, Leary MR. Social anxiety and self-presentation: a conceptualization model. Psychological Bulletin. 1982;92:641–69. doi: 10.1037/0033-2909.92.3.641. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Ohman A, Junghofer M, Weike AI, Stockburger J, Hamm AO. The facilitated processing of threatening faces: an ERP analysis. Emotion. 2004;4:189–200. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- Stephan WG, Stephan CW. Intergroup anxiety. Journal of Social Issues. 1985;41:157–75. [Google Scholar]
- Theeuwes J, Van der Stigchel S. Face captures attention: evidence from inhibition of return. Visual Cognition. 2006;13:657–65. [Google Scholar]
- Trawalter S, Richeson JA, Shelton JN. Predicting behavior during interracial interactions: a stress and coping approach. Personality and Social Psychology Review. 2009;13:243–68. doi: 10.1177/1088868309345850. [DOI] [PubMed] [Google Scholar]
- Walker PM, Silvert L, Hewstone M, Nobre AC. Social contact and other-race face processing in the human brain. Social Cognitive and Affective Neuroscience. 2008;3:16–25. doi: 10.1093/scan/nsm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West TV, Shelton JN, Trail TE. Relational anxiety in interracial interactions. Psychological Science. 2009;20:289–92. doi: 10.1111/j.1467-9280.2009.02289.x. [DOI] [PubMed] [Google Scholar]
- Wiese H, Stahl J, Schweinberger SR. Configural processing of other-race faces is delayed but not decreased. Biological Psychology. 2009;81:103–9. doi: 10.1016/j.biopsycho.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Williams KD. Ostracism. Annual Review of Psychology. 2007;58:425–52. doi: 10.1146/annurev.psych.58.110405.085641. [DOI] [PubMed] [Google Scholar]