Abstract
There have been several reports on the association between the Val158Met genetic polymorphism of the catechol-O-methyltransferase (COMT) gene, as well as the serotonin transporter-linked polymorphic region (5-HTTLPR) of the serotonin transporter gene (SLC6A4), and frontolimbic region volumes, which have been suggested to underlie individual differences in emotion processing or susceptibility to emotional disorders. However, findings have been somewhat inconsistent. This study used diffeomorphic anatomic registration through exponentiated Lie algebra (DARTEL) whole-brain voxel-based morphometry to study the genetic effects of COMT Val158Met and SLC6A4 5-HTTLPR, as well as their interaction, on the regional gray matter volumes of a sample of 91 healthy volunteers. An interaction of COMT Val158Met × SLC6A4 5-HTTLPR genotypes with gray matter volume was found in bilateral parahippocampal gyrus, amygdala, hippocampus, vermis of cerebellum and right putamen/insula. In particular, the gray matter volume in these regions was smaller in individuals who were both COMT-Met and 5-HTTLPR-S carriers, or both COMT-Val and 5-HTTLPR-L homozygotes, as compared with individuals with intermediate combinations of alleles. The interaction of COMT Val158Met and SLC6A4 5-HTTLPR adds to the understanding of individual differences in emotion processing.
Keywords: catechol-O-methyltransferase, genetic interaction, gray matter, serotonin transporter gene, serotonin transporter-linked polymorphic region, voxel-based morphometry
INTRODUCTION
A number of studies in the last 10 years have associated some genetic polymorphisms with increased vulnerability to depressive disorders. In particular, the short allele (S) of the serotonin transporter-linked polymorphic region (5-HTTLPR) of the serotonin transporter gene (SLC6A4), as well as the Val allele of the Met158Val polymorphism of the catechol-O-methyltransferase (COMT) gene, have been strongly associated with a vulnerability to major depressive disorder (MDD) (Caspi et al., 2003; Kendler et al., 2005; Mandelli et al., 2007; Conway et al., 2010; Aberg et al., 2011; Karg et al., 2011).
Structural neuroimaging genetic studies looking for intermediate phenotypes of depression have also demonstrated brain volume differences associated with the above genes. For example, several groups (Canli et al., 2005; Pezawas et al., 2005; Frodl et al., 2008; Selvaraj et al., 2010) reported that carriers of 5-HTTLPR-S compared with long allele (L) had smaller gray matter volumes in the amygdala, hippocampus, anterior cingulate cortex, superior, middle and inferior frontal gyri, dorsolateral prefrontal cortex and superior temporal gyrus. This evidence is in agreement with the findings of smaller volumes of hippocampal/temporolimbic structures in people predisposed to MDD, e.g. the offspring of depressed patients (Chen et al., 2010) or the monozygotic twins of patients (Baare et al., 2010). In contrast, studies of COMT Val158Met effect on brain structure found increased volume of hippocampus in COMT-Met compared with Val allele carriers (Taylor et al., 2007; Cerasa et al., 2008). However, it must be highlighted that other important studies have failed to detect any genetic effects of SLC6A4 5-HTTLPR or COMT Val158Met on gray matter volumes (Zinkstok et al., 2006; Dutt et al., 2009).
It is known that genetic factors predisposing toward complex syndromes such as MDD often demonstrate an interaction, even in the absence of any main effects (Grigorenko et al., 2003). We have recently reported an interaction of COMT Val158Met and SLC6A4 5-HTTLPR with the Granger effective connectivity within the emotion processing circuit in healthy individuals (Surguladze et al., 2012). This study tested the hypothesis that an interaction between COMT Val158Met and SLC6A4 5-HTTLPR genotypes would be associated with the size of gray matter in brain regions involved in emotion processing. To this end, the effects of COMT Val158Met, SLC6A4 5-HTTLPR and their interaction on brain gray matter volume were assessed using structural MRI data acquired on a large sample of healthy volunteers and applying advanced voxel-based morphometry algorithms, namely: the ‘new segmentation’ and ‘diffeomorphic anatomic registration through exponentiated Lie algebra’ (DARTEL) method, which provides enhanced accuracy (Ashburner, 2007).
METHODS
Participants
The participants were 91 right-handed healthy Caucasian individuals (Table 1) with no family history of psychiatric disorder. Exclusion criteria were current or past psychiatric diagnosis as established by the Structured Clinical Interview for DSM-IV (SCID I) (APA, 1994). The study was approved by the Ethics Committee of the Institute of Psychiatry, King’s College London. After a complete description of the study to the subjects, written informed consent was obtained before the experiments began.
Table 1.
Characteristics of the healthy individuals participating in this study.
| All participants | Val and L′ homozygotes | Val homozygotes but S′ carriers | Met carriers but L′ homozygotes | Both Met and S′ carriers | |
|---|---|---|---|---|---|
| Sample size | 91 | 7 | 13 | 19 | 52 |
| Sex (% males) | 51 | 71 | 62 | 32 | 52 |
| Age (years)a | 33 ± 9 | 36 ± 13 | 34 ± 8 | 33 ± 9 | 31 ± 9 |
| BDIa | 4.4 ± 5 | 5.8 ± 4 | 3.5 ± 4 | 5.6 ± 8 | 3.9 ± 4 |
| Harm avoidancea | 90 ± 19 | 92 ± 28 | 88 ± 19 | 85 ± 18 | 91 ± 18 |
| Novelty seekinga | 106 ± 13 | 104 ± 12 | 108 ± 14 | 106 ± 13 | 106 ± 13 |
| Persistencea | 123 ± 18 | 115 ± 18 | 120 ± 22 | 128 ± 17 | 123 ± 18 |
| Reward dependencea | 104 ± 16 | 92 ± 21 | 100 ± 13 | 107 ± 15 | 106 ± 16 |
aValues are expressed as mean ± SD.
Participants underwent an evaluation of depressive symptoms with the Beck Depression Inventory (BDI) II (Beck et al., 1996). The mean total BDI on the day of scanning was 3.1 ± 3.7 (below the cut-off level of ‘minimal depression’), though one participant scored 18 (i.e. within the range of ‘mild depression’). Participants also completed the ‘harm avoidance’, ‘novelty seeking’, ‘reward dependence’ and ‘persistence’ scales of Cloninger’s Temperament and Character Inventory (TCI; Cloninger et al., 1993), whose dimensions have been shown to be genetically modulated (Heath et al., 1994).
Genotyping
DNA was extracted from cheek swabs using standard procedures. The genotype of the COMT Val158Met (rs4680) single nucleotide polymorphism (SNP) was determined by allelic discrimination assay (C_25746809_50) based on fluorogenic 5′ nuclease activity: a TaqMan SNP genotyping assay was performed the ABI Prism 7900HT and analyzed with Sequence Detection System software according to the manufacturer’s instructions (Applied Biosystems, Warrington, UK). Twenty participants were found to be Val homozygotes and the remaining 71 found to be Met carriers.
To determine the genotypes of the SLC6A4 5-HTTLPR insertion/deletion and of the rs25531 G/A SNP, a modified version of the protocol described by Wendland et al. (2006) was used. The 5′-TCCTCCGCTTTGGCGCCTCTTCC-3′ forward and 5′-TGGGGGTTGCAGGGGAGATCCTG-3′ reverse primers were used (Operon, Huntsville, AT) that amplify a 469 (short or S allele) and 512 (long or L allele) product. In a total volume of 20 μl, 25 ng of genomic DNA were amplified in the presence of 1× polymerase chain reaction (PCR) Buffer (Qiagen) and oligonucleotide primers. A combination of Q solution (Qiagen), c7–dGTP Q (ROCHE) and AmpliTaq Gold (ABI) were used to facilitate successful genotyping. Q (5×) solution improves suboptimal PCR systems caused by templates that have a high degree of secondary structure or that GC rich. The incorporation of c7–dGTP in the dNTP mix (the concentration mix contained 0.2 mM of A, C, T and 0.1 mM of G and c7) can eliminate spurious GC–hydrogen bonding and relax secondary structures and AmpliTaq Gold (1.25 U), provides increased sensitivity, specificity and yield over conventional PCR techniques. MgCl2 (ABI) of 1.8 mM was added to the final mix. Thermal cycling consisted of 15 min of initial denaturation at 95°C followed by 42 cycles of 94°C (30 s) 68°C (90 s) and 72°C (60 s) each with a final extension step of 10 min at 72°C. PCR product were loaded onto 3% agarose gel run for 1 hour at 120 V in 1× TBE and visualized by ethidium bromide (Sigma-Aldrich, St Louis, MO). Ten mocroliters of the remaining PCR product was digested for 12 h at 37°C with MSP1 (5 U/μl) (New England Biolabs, Ipswich, MA) which cuts the 5′-C/CGG-3′ sequence. Digestion with MSP1 resulted in the following fragments: a 469 bp product (short uncut) representing the SA allele; a 402 bp + 67 bp product (short cut) representing the SG allele; a 512 bp product (long uncut) representing the LA allele and a 402 bp + 110 bp product (long cut) representing the LG allele. This is an improved protocol for the amplification of 5-HTTLPR which resulted in clear and easily distinguishable bands. As the Long allele behaves like the Short allele in the presence of the rs25531 A/G substitution, therefore SLC6A4 5-HTTLPR polymorphisms were recoded as L′ (Long A) and S′ (Short A, Short G or Long G) (Parsey et al., 2006). Twenty-six participants were found to be L′ homozygotes, and the remaining 65 were found to be S′ carriers.
Conditional frequencies of COMT-Met carriers and 5-HTTLPR-S′ carriers were found to be highly balanced in the sample (χ2 = 0.1939, df = 1, P = 0.660). The frequency of COMT-Met carriers in the subsample of 5-HTTLPR-L′ homozygotes (73%, 19 out of 26 individuals) was very similar to the frequency of COMT-Met carriers in the subsample of 5-HTTLPR-S′ carriers (80%, 52 out of 65 individuals). Similarly, the frequency of 5-HTTLPR-S′ carriers in the subsample of COMT-Val homozygotes (65%, 13 out of 20 individuals) was very similar to the frequency of 5-HTTLPR-S′ carriers in the subsample of COMT-Met carriers (73%, 52 out of 71 individuals).
Finally, cross-tabulation of the COMT Val158Met and SLC6A4 5-HTTLPR alleles yielded four genetic groups with similar age and sex distribution: (i) individuals who were both COMT-Val and 5-HTTLPR-L′ homozygotes; (ii) individuals who were COMT-Val homozygotes but 5-HTTLPR-S′ carriers; (iii) individuals who were COMT-Met carriers but 5-HTTLPR-L′ homozygotes; and (iv) individuals who were both COMT-Met and 5-HTTLPR-S′ carriers. As shown in Table 1, the sample size of some of the genetic groups was small, a condition which does not increase the likelihood of false positive findings (Friston, 2012) but may decrease the power to detect small differences between groups. However, it must be noted that the power of statistical comparisons depends on the combined sample size of the different groups, and a post hoc power analysis revealed that the power to detect interaction effects in this study was equivalent to the power of a standard t test with 28 individuals per group.
There were no significant differences of age or female/male distribution related to the genetic groups. Age: COMT Val158Met × SLC6A4 5-HTTLPR two-factorial analysis of variance (ANOVA) P values > 0.05; female/male distribution: COMT Val158Met × SLC6A4 5-HTTLPR interaction log-linear model P values > 0.05. As detailed later, the age and sex variables will be included as covariates in the statistical analysis to decrease the residual variance and thus increase the power to detect differences between groups. Similarly, there were no significant differences in the BDI level and the four TCI dimension scores between the genetic groups (COMT Val158Met × SLC6A4 5-HTTLPR two-factorial ANOVA P values > 0.05), with the exception of a trend toward higher ‘reward dependence’ TCI score in COMT-Met carriers (106 ± 16 vs. 97 ± 16 in Val homozygotes; t test uncorrected P value = 0.04, corrected P value > 0.05).
Magnetic resonance imaging
Scanning was performed on a General Electric Signa 3T scanner at the Institute of Psychiatry, acquiring 196 T1-weigthed fast spoiled gradient echo coronal slices (TR/TE/TI 7.1/2.8/450 ms, flip angle 20°, FOV 280 mm, 256 × 256 matrix, voxel size 1.1 × 1.1 × 1.1 mm3). Preprocessing steps with SPM8 (Welcome Trust Centre for Neuroimaging, London) were as follows: (i) manual realignment and centering at the anterior commissure; (ii) segmentation into gray matter, white matter, cerebrospinal fluid and other tissues; (iii) iterative creation of a study-specific template; (iv) normalization of gray matter images to MNI space through the final template of the series and the flow fields; and (v) smoothing by an isotropic Gaussian kernel. Following DARTEL defaults, we conducted the latter step with an 8 mm (rather than 12 mm) FWHM kernel. This narrow kernel is probably recommended because high-resolution normalization algorithms such as DARTEL are thought to require a lower degree of smoothing, and narrow kernels have been found to be more sensitive to detect abnormalities in small brain structures such as the amygdala (Uchida et al., 2008). Finally, individual volumes were voxel-wise scaled by the Jacobian determinant of their transformation to prevent volume differences due to spatial deformations.
Analysis
Effects of COMT Val158Met and SLC6A4 5-HTTLPR were assessed by t tests comparing whole brain regional gray matter volume, e.g. between COMT-Met carriers and COMT-Val homozygotes, with age and sex as covariates. The interaction between COMT Val158Met and SLC6A4 5-HTTLPR was assessed with a COMT Val158Met × SLC6A4 5-HTTLPR two-factorial analysis of covariance (ANCOVA), with age and sex as covariates. All analyses were proportionally scaled by global gray matter. Statistical significance was assessed at the cluster level and based on the Gaussian random fields’ theory (Worsley and Friston, 1995). Specifically, clusters of gray matter volume difference were first defined using a voxel threshold of P < 0.001 and a size threshold of >100 voxels, but only those clusters with a false discovery rate (FDR) < 0.05 were considered as statistically significant to correct for multiple comparisons. Peak coordinates were Lancaster-transformed to Talairach space with the SDM online utilities (www.sdmproject.com/utilities) (Lancaster et al., 2007; Radua and Mataix-Cols, 2009). The gray matter volume of each cluster in each individual native space was then estimated by summing the values of its voxels and scaled by global gray matter—note that the value of a voxel in a modulated image is informative of its volume in native space. Potential associations between these volumes and mood and personality characteristics (BDI and TCI) were assessed with Pearson correlations.
A region of interest (ROI) analysis was also conducted of the amygdala and hippocampus, based on consistent findings of volume reductions in these regions associated with MDD (see meta-analyses; Videbech and Ravnkilde, 2004; Cole et al., 2011; Kempton et al., 2011; Arnone et al., 2012; Bora et al., 2012) or with risk of developing MDD (Chen et al., 2010). ROIs were defined according to the Harvard–Oxford atlas (www.fmrib.ox.ac.uk/fsl/data/atlas-descriptions.html#ho), which showed a good overlap with the study-specific template. The gray matter volume of each ROI was estimated in each individual as the sum of the values of its voxels and scaled by global gray matter. ROI volumes were then compared across genetic groups using the t tests and ANCOVA described earlier, with Bonferroni–Holm correction for multiple comparisons. Holm’s modification of the Bonferroni method gives strong control of the family-wise error rate, whilst it has increased power and is valid under arbitrary assumptions (Holm, 1979).
RESULTS
Voxel-based morphometry
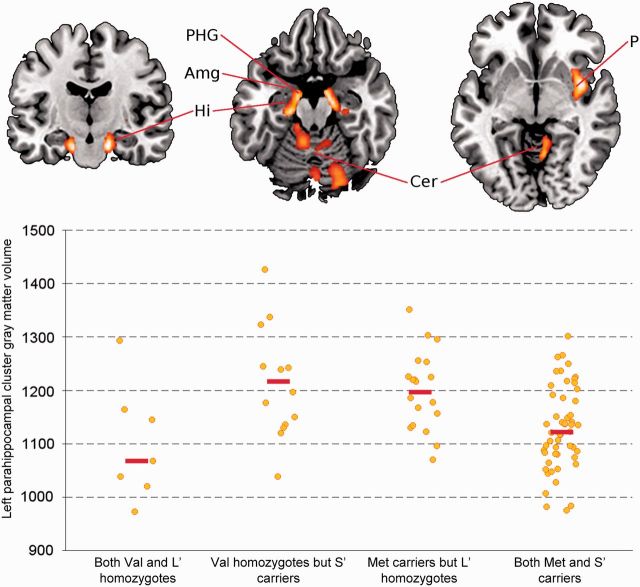
There was a significant interaction of COMT Val158Met × SLC6A4 5-HTTLPR that had effects on the gray matter volume of bilateral parahippocampal gyrus, cerebellar vermis and right putamen/insula (all corrected P ≤ 0.030). This interaction was accounted for by 8.33% smaller gray matter volume in the clusters of significant interaction in individuals who were both COMT-Met and 5-HTTLPR-S′ carriers, or both COMT-Val and 5-HTTLPR-L′ homozygotes, as compared with individuals with any other combinations of alleles (Table 2 and Figure 1).
Table 2.
Whole-brain voxel-based analysis of the COMT Val158Met × SLC6A4 5–HTTLPR interaction
|
COMT Val158Met × SLC6A4 5-HTTLPR interaction |
Volumes of the clustersa (mm3 in subjects’ native space) |
|||||
|---|---|---|---|---|---|---|
| Talairach coordinateb | Cluster P valuec | Val and L′ homozygotes | Val homozygotes but S′ carriers | Met carriers but L′ homozygotes | Both Met and S′ carriers | |
| Left parahippocampal gyrus (743 voxels)d | −14, −11, −19 | <0.001 | 1100 ± 109 | 1212 ± 105 | 1200 ± 74 | 1128 ± 77 |
| Right cerebellum (1766 voxels)d | 11, −43, −8 | —d | 3231 ± 309 | 3677 ± 305 | 3635 ± 257 | 3438 ± 258 |
| Right putamen/insula (1416 voxels) | 34, −2, −8 | 0.003 | 2404 ± 250 | 2657 ± 216 | 2648 ± 196 | 2470 ± 162 |
| Right parahippocampal gyrus (736 voxels) | 13, −11, −17 | 0.026 | 1043 ± 76 | 1138 ± 84 | 1129 ± 78 | 1058 ± 63 |
| Vermis cerebelli 6 (1217 voxels)e | 14, −72, −24 | 0.030 | 2438 ± 239 | 2787 ± 273 | 2777 ± 213 | 2646 ± 188 |
aThe ANCOVA was conducted using MNI-normalized images and returned a set of clusters. The gray matter volume of each cluster in each individual was then estimated by summing the values of its voxels. Note that the value of a voxel in a modulated image is informative of its volume in native space.
bLocation of the cluster peak t-value of the COMT Val158Met × SLC6A4 5-HTTLPR interaction term of the two factorial analysis of covariance (ANCOVA with age and sex as covariates).
cP value after FDR-correction for multiple comparisons.
dOne left parahippocampal/bilateral cerebellar cluster was split for display purposes in this table by slightly increasing the threshold.
eVolumes of two contiguous cerebellar clusters were combined in this table.
Fig. 1.
Interactive effects of COMT Val158Met and SLC6A4 5-HTTLPR on gray matter volume (whole brain analysis). Top: Regions where gray matter volume was smaller in individuals who were both COMT-Met and 5-HTTLPR-S′ carriers, or both COMT-Val and 5-HTTLPR-L′ homozygotes, as compared with those with other combinations of these alleles. Left side of the brain image corresponds to the left side of the brain. Bottom: Individual and genetic group median left parahippocampal cluster gray matter volumes. Amg, amygdala; Cer, cerebellum; Hi, hippocampus; P, putamen; PHG, parahippocampal gyrus. Clusters of gray matter volume difference were defined using a voxel threshold of P < 0.001 and a size threshold of >100 voxels, with statistical significance corrected for multiple comparisons (FDR < 0.05). Note that the part inferior of the bar plot has been truncated to 900 for display purposes.
As regards to the main effects, COMT Val158Met voxel-based ANCOVA demonstrated increased gray matter volume in a small part of right angular gyrus in COMT-Met carriers (peak Talairach coordinate: 52, −62, 34; cluster P value = 0.025), though this finding was not observed in the voxel-based COMT Val158Met × SLC6A4 5-HTTLPR two-factorial voxel-based ANCOVA and thus was not further considered. No statistically significant main effects of SLC6A4 5-HTTLPR were detected in the SLC6A4 5-HTTLPR voxel-based ANCOVA.
The COMT Val158Met × SLC6A4 5-HTTLPR interactions described earlier could also be observed when the volume of the clusters found in the earlier mentioned interaction analysis was separately analyzed for male and female individuals, and for younger and older individuals according to median age (<30 years vs. ≥30 years) with COMT Val158Met × SLC6A4 5-HTTLPR two-factorial ANOVAs. Specifically, the pattern of smaller gray matter volume in individuals who were both COMT-Met and 5-HTTLPR-S′ carriers, or both COMT-Val and 5-HTTLPR-L′ homozygotes, was observed in each of the brain regions in each of the four demographic groups, with the ANOVA interaction term achieving statistical significance in all cases—with the exception of right parahippocampal cluster in younger participants and left parahippocampal cluster in female participants, where only non-significant trends were detected (P = 0.080 and 0.103, respectively). There was no significant correlation between the cluster volumes detected in the interaction analysis and the BDI or TCI measures.
ROI analysis
The interaction effect of COMT Val158Met and SLC6A4 5-HTTLPR on hippocampus and amygdala ROIs was of a similar nature to that described earlier (Table 3). In particular, the hippocampal and amygdalar volumes were smaller in the individuals with both COMT-Met and 5-HTTLPR-S′ alleles, or those individuals who were both COMT-Val and 5-HTTLPR-L′ homozygotes, compared with the individuals with any other combinations of alleles.
Table 3.
ROI analysis of the COMT Val158Met × S6C4A5 5–HTTLPR interaction
|
COMT Val158Met and SLC6A4 5-HTTLPR interaction |
Volumes of the regions of interest (mm3 in subjects’ native space) |
|||||
|---|---|---|---|---|---|---|
| t valuea | P valueb | Val and L’ homozygotes | Val homozygotes but S′ carriers | Met carriers but L’ homozygotes | Both Met and S’ carriers | |
| Left amygdala | 2.31 | 0.023 | 2862 ± 350 | 3055 ± 267 | 2976 ± 203 | 2901 ± 214 |
| Right amygdala | 3.11 | 0.006 | 2745 ± 221 | 2928 ± 230 | 2944 ± 212 | 2813 ± 199 |
| Left hippocampus | 2.96 | 0.008 | 6173 ± 679 | 6613 ± 350 | 6554 ± 360 | 6336 ± 394 |
| Right hippocampus | 3.65 | 0.002 | 6045 ± 596 | 6623 ± 419 | 6568 ± 394 | 6324 ± 398 |
at value of the COMT Val158Met × SLC6A4 5-HTTLPR interaction term of the two factorial ANCOVA (with age and sex as covariates).
bP value after Bonferroni–Holm correction for multiple comparisons. Holm’s modification of the Bonferroni method gives strong control of the family-wise error rate whilst it has increased power and is valid under arbitrary assumptions (Holm, 1979).
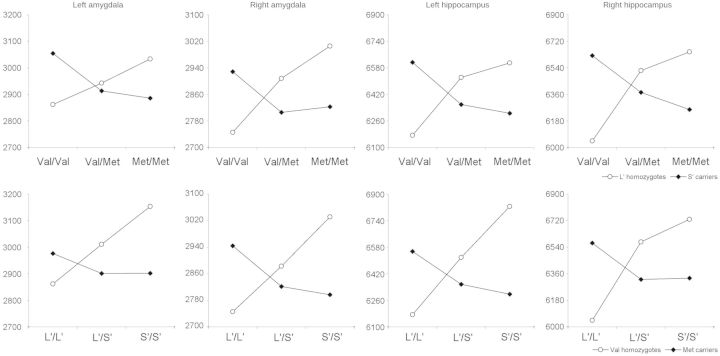
Finally, we repeated the interaction analyses separately assessing the three genotypes of COMT Val158Met and the three genotypes of SLC6A4 5-HTTLPR. As shown in Figure 2, amygdalar and hippocampal volumes were found to progressively vary depending on the number of COMT-Met alleles (Val/Val > Val/Met > Met/Met in 5-HTTLPR-S′ carriers; Met/Met > Val/Met > Val/Val in 5-HTTLPR-L′ homozygotes). Similarly, amygdalar and hippocampal volumes were found to also progressively vary depending on the number of 5-HTTLPR-S′ alleles (L′/L′ > L′/S′ > S′/S′ in COMT-Met carriers; S′/S′ > L′/S′ > L′/L′ in COMT-Val homozygotes). However, findings derived from this exploratory, descriptive analysis should be taken with caution given the small sample size of some of the genotype combinations.
Fig. 2.
Interactive effects of COMT Val158Met and SLC6A4 5-HTTLPR on gray matter volume (ROI analysis).
DISCUSSION
In this study, a COMT Val158Met × SLC6A4 5-HTTLPR interaction upon gray matter volume was observed, by which individuals who carried both COMT-Met and 5-HTTLPR-S′ alleles or none, had symmetrical decreases in gray matter volume in bilateral parahippocampal gyrus, cerebellum, right putamen/insula, amygdala and hippocampus, compared with individuals who were homozygous for either (but not simultaneously for both) Val or L′ allele.
These results may help to shed light on the potential reasons behind the diversity of previous findings. For example, in COMT-Met carriers, the effects of SLC6A4 5-HTTLPR would be such that 5-HTTLPR-S′ carriers would have smaller gray matter volumes than 5-HTTLPR-L′ homozygotes, which is in accordance with reports of lower amygdalar volumes in 5-HTTLPR-S′ carriers (Pezawas et al., 2005). On the other hand, in 5-HTTLPR-L′ homozygotes, the effects of COMT Val158Met would be such that COMT-Met carriers would have larger gray matter volumes than COMT-Val homozygotes. Again, this was the case in previous studies reporting larger hippocampal volumes in COMT-Met carriers (Taylor et al., 2007; Cerasa et al., 2008).
However, in samples where the frequencies of COMT-Met and 5-HTTLPR-S′ carriers are balanced, no main effects of one or the other gene should be detected, as it was the case in this study when the effects of COMT Val158Met and SLC6A4 5-HTTLPR were separately assessed. Indeed, studies composed of mainly COMT-Val homozygotes or 5-HTTLPR-S′ carriers could even report results opposite to those published. In the subsample of COMT-Val homozygotes, for instance, parahippocampal gray matter volumes were larger (rather than smaller) in 5-HTTLPR-S′ carriers. Similarly, in the subsample of 5-HTTLPR-S′ carriers these volumes were smaller (rather than larger) in COMT-Met carriers.
The underlying mechanisms of the above interaction need further investigation. The effects of COMT Val158Met and SLC6A4 5-HTTLPR genes on brain structure have been proposed to be related to the role monoamines play in synaptic plasticity during cortical maturation. In particular, dopaminergic innervation has been found to change during the course of cortical development (Rosenberg and Lewis, 1995), an observation which has been proposed to be related to COMT Val158Met-dependent variation of gray matter volumes (Taylor et al., 2007). There is also evidence that cortical (Gaspar et al., 2003), and hippocampal (Zhang et al., 2006; Choi et al., 2007) development is influenced by serotonin, which could explain the SLC6A4 5-HTTLPR-dependent variation of gray matter volumes (Pezawas et al., 2005).
Importantly, the regions identified in this study (hippocampus, amygdala, cerebellum, ventral striatum) have been proposed in a recent study (Glahn et al., 2012) as potential endophenotypes of recurrent MDD. This is in line with previous findings establishing the neuropathology of MDD. For example, meta-analyses (Koolschijn et al., 2009; Kempton et al., 2011; Arnone et al., 2012; Bora et al., 2012) have demonstrated smaller volumes of putamen and hippocampus, and a review (Beyer and Ranga Krishnan, 2002) has highlighted moderate cerebellar volume reductions in individuals with MDD. The size of the amygdala in MDD has not been found to consistently deviate from normal, however (Kempton et al., 2011).
Findings of this study indicate that both hypo- and hypermetabolism of dopamine and serotonin may be associated with reduced volumes of the brain regions involved in emotion processing. Thus, smaller gray matter volumes were shown in individuals with both COMT-Met and 5-HTTLPR-S′ low activity alleles, and also in individuals homozygous for both COMT-Val and 5-HTTLPR-L′ high activity alleles. It could be thus suggested that both extremes of neurotransmitter metabolism negatively impact on regional brain volume development, resembling the inverted U-shape relationship previously suggested between COMT-Val and COMT-Met alleles and dopaminergic function (Meyer-Lindenberg et al., 2005). Importantly, the interaction between these alleles on gray matter volumes was very similar to our previous finding regarding effective connectivity measured in the same sample of individuals (Surguladze et al., 2012). Here, effective connectivity in emotion processing neural circuitry was reduced in individuals who carried both COMT-Met and 5-HTTLPR-S′ alleles. Individuals who were both COMT-Val and 5-HTTLPR-L′ homozygotes showed normal connectivity values, though a slight trend could be observed towards reduced connectivity. It is therefore plausible that there may be developmental effects of the interaction of COMT Val158Met and SLC6A4 5-HTTLPR on both gray matter volume and effective connectivity.
It is important to highlight several limitations of this study. First, the sample size of some of the genetic groups was small, a condition which does not increase the likelihood of false positive findings (Friston, 2012) but may decrease the power to detect small differences between groups. However, it must be noted that the power of statistical comparisons depends on the sample size of the different groups and not only on the sample size of the smallest group. Indeed, a simulation work estimated that the power to detect interaction effects with the sample sizes of this study was equivalent to that achieved in a standard t test with 28 participants per group. Second, no associations between brain volume and the phenotypes related to emotion processing variance (i.e. TCI and BDI) could be detected. The presence of such an association would have provided external validity to the suggestion of a genetic interaction effect on the regions involved in emotional processes. Thus far, this proposal is supported by the existing literature indicating the role of amygdala, hippocampus, cerebellum and putamen in the earlier mentioned processes (Koolschijn et al., 2009; Kempton et al., 2011; Arnone et al., 2012; Bora et al., 2012). Further studies involving larger sample sizes are warranted to explore possible associations between individual measures of emotion processing, genetic interaction effects and regional brain volumes.
To sum up, the results of this study showed interaction effects of COMT Val158Met and SLC6A4 5-HTTLPR polymorphisms on the gray matter volumes of structures involved in emotion processing in healthy individuals. These effects may underlie the individual differences in emotion processing, and potentially inform about the mechanisms of susceptibility to MDD. These findings also help to disentangle some of the inconsistencies in the findings of previous studies that separately assessed the effects of COMT Val158Met and SLC6A4 5-HTTLPR polymorphisms upon brain structure.
Conflict of Interest
None declared.
Acknowledgments
The authors thank Dr. P. Brittain for his helpful comments on the draft. This study was supported by the Wellcome Trust grant to MLP. During the study period, W.E.H. was supported by the French Association of Biological Psychiatry and Neuropsychopharmacology (AFPBN) research grant.
REFERENCES
- Aberg E, Fandińo-Losada A, Sjöholm LK, Forsell Y, Lavebratt C. The functional Val158Met polymorphism in catechol-O-methyltransferase (COMT) is associated with depression and motivation in men from a Swedish population-based study. Journal of Affective Disorders. 2011;129(1–3):158–66. doi: 10.1016/j.jad.2010.08.009. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- Arnone D, Mcintosh AM, Ebmeier KP, Munafo MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: systematic review and meta-regression analyses. European Neuropsychopharmacology. 2012;22(1):1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffemorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Baare WF, Vinberg M, Knudsen GM, et al. Hippocampal volume changes in healthy subjects at risk of unipolar depression. Journal of Psychiatric Research. 2010;44(10):655–62. doi: 10.1016/j.jpsychires.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory—II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Beyer JL, Ranga Krishnan K. Volumetric brain imaging findings in mood disorders. Bipolar Disorders. 2002;4(2):89–104. doi: 10.1034/j.1399-5618.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. Journal of Affective Disorders. 2012;138(1-2):9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proceedings of National Academy of Science U S A. 2005;102(34):12224–9. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Gioia MC, Labate A, Liguori M, Lanza P, Quattrone A. Impact of catechol-O-methyltransferase Val(108/158) Met genotype on hippocampal and prefrontal gray matter volume. Neuroreport. 2008;19(4):405–8. doi: 10.1097/WNR.0b013e3282f5f784. [DOI] [PubMed] [Google Scholar]
- Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk of depression. Archives of General Psychiatry. 2010;67:270. doi: 10.1001/archgenpsychiatry.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IS, Cho JH, Kim JT, et al. Serotoninergic modulation of gabaergic synaptic transmission in developing rat ca3pyramidalneurons. Journal of Neurochemistry. 2007;103(6):2342–53. doi: 10.1111/j.1471-4159.2007.04945.x. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Archives of General Psychiatry. 1993;50(12):975–90. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Cole J, Costafreda SG, Mcguffin P, Fu CH. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. Journal of Affective Disorders. 2011;134(1–3):483–7. doi: 10.1016/j.jad.2011.05.057. [DOI] [PubMed] [Google Scholar]
- Conway CC, Hammen C, Brennan PA, Lind PA, Najman JM. Interaction of chronic stress with serotonin transporter and catechol-O-methyltransferase polymorphisms in predicting youth depression. Depression and Anxiety. 2010;27(8):737–45. doi: 10.1002/da.20715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt A, Mcdonald C, Dempster E, et al. The effect of COMT, BDNF, 5-HTT, NRG1 and DTNBP1 genes on hippocampal and lateral ventricular volume in psychosis. Psychological Medicine. 2009;39(11):1783–97. doi: 10.1017/S0033291709990316. [DOI] [PubMed] [Google Scholar]
- Friston K. Ten ironic rules for non-statistical reviewers. Neuroimage. 2012;61(4):1300–10. doi: 10.1016/j.neuroimage.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Frodl T, Koutsouleris N, Bottlender R, et al. Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Molecular Psychiatry. 2008;13(12):1093–101. doi: 10.1038/mp.2008.62. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nature Review Neuroscience. 2003;4:1002–12. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Curran JE, Winkler AM, et al. High dimensional endophenotype ranking in the search for major depression risk genes. Biological Psychiatry. 2012;71(1):6–14. doi: 10.1016/j.biopsych.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorenko EL, Plomin R, Defries JC, Craig IW, Mcguffin P. Behavioral Genetics in the Postgenomic Era. Washington, D.C.: American Psychological Association; 2003. Epistasis and the genetics of complex traits. [Google Scholar]
- Heath AC, Cloninger CR, Martin NG. Testing a model for the genetic structure of personality: a comparison of the personality systems of Cloninger and Eysenck. Journal of Personality and Social Psychology. 1994;66(4):762–75. doi: 10.1037//0022-3514.66.4.762. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Archives of General Psychiatry. 2011;68(5):444–54. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafo MR, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Archives of General Psychiatry. 2011;68(7):675–90. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Archives of General Psychiatry. 2005;62(5):529–35. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Koolschijn PC, Van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Human Brain Mapping. 2009;30(11):3719–35. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28(11):1194–205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli L, Serretti A, Marino E, Pirovano A, Calati R, Colombo C. Interaction between serotonin transporter gene, catechol-O-methyltransferase gene and stressful life events in mood disorders. International Journal of Neuropsychopharmacology. 2007;10(4):437–47. doi: 10.1017/S1461145706006882. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana B, et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nature Neuroscience. 2005;8(5):594–6. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, et al. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. American Journal of Psychiatry. 2006;163(1):48–51. doi: 10.1176/appi.ajp.163.1.48. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8(6):828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. British Journal of Psychiatry. 2009;195:391–400. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. Journal of Comparative Neurology. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Godlewska BR, Norbury R, et al. Decreased regional gray matter volume in S′ allele carriers of the 5-HTTLPR triallelic polymorphism. Molecular Psychiatry. 2010;16:471, 472–473. doi: 10.1038/mp.2010.112. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Radua J, El-Hage W, et al. Interaction of catechol O-methyltransferase and serotonin transporter genes modulates effective connectivity in a facial emotion processing circuitry. Translational Psychiatry. 2012;2:e70. doi: 10.1038/tp.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Züchner S, Payne ME, et al. The COMT Val158Met polymorphism and temporal lobe morphometry in healthy adults. Psychiatry Research. 2007;155(2):173–7. doi: 10.1016/j.pscychresns.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida RR, Del-Ben CM, Araujo D, et al. Correlation between voxel based morphometry and manual volumetry in magnetic resonance images of the human brain. Anais da Academia Brasileira de Ciencias. 2008;80(1):149–56. doi: 10.1590/s0001-37652008000100010. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. American Journal of Psychiatry. 2004;161(11):1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Molecular Psychiatry. 2006;11:224–6. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of FMRI time-series revisited-again. Neuroimage. 1995;2(3):173–81. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Zhang L, Guadarrama L, Corona-Morales AA, Vega-Gonzalez A, Rocha L, Escobar A. Rats subjected to extended l-tryptophan restriction during early postnatal stage exhibit anxious-depressive features and structural changes. Journal of Neuropathology Experimental Neurology. 2006;65(6):562–70. doi: 10.1097/00005072-200606000-00004. [DOI] [PubMed] [Google Scholar]
- Zinkstok J, Schmitz N, Van Amelsvoort T, et al. The COMT Val158Met polymorphism and brain morphometry in healthy young adults. Neuroscience Letters. 2006;405(1–2):34–9. doi: 10.1016/j.neulet.2006.06.034. [DOI] [PubMed] [Google Scholar]