Abstract
People feel bad for inflicting harms upon others; this emotional state is termed interpersonal guilt. In this study, the participant played multiple rounds of a dot-estimation task with anonymous partners while undergoing fMRI. The partner would receive pain stimulation if the partner or the participant or both responded incorrectly; the participant was then given the option to intervene and bear a proportion of pain for the partner. The level of pain voluntarily taken and the activations in anterior middle cingulate cortex (aMCC) and bilateral anterior insula (AI) were higher when the participant was solely responsible for the stimulation (Self_Incorrect) than when both committed an error (Both_Incorrect). Moreover, the gray matter volume in the aMCC predicted the individual’s compensation behavior, measured as the difference between the level of pain taken in the Self_Incorrect and Both_Incorrect conditions. Furthermore, a mediation pathway analysis revealed that activation in a midbrain region mediated the relationship between aMCC activation and the individual’s tendency to compensate. These results demonstrate that the aMCC and the midbrain nucleus not only play an important role in experiencing interpersonal guilt, but also contribute to compensation behavior.
Keywords: interpersonal guilt, anterior middle cingulate, fMRI, gray matter volume, mediation pathway analysis
INTRODUCTION
How would you feel if you lost the bicycle borrowed from your friend, which was the last present given to him by his grandmother before she died (de Hooge et al., 2011)? This is an example of interpersonal guilt. In philosophy, guilt is understood as ‘the moral feeling produced by conscience, itself the internalized voice of moral authority’ (Griswold, 2007). The societal significance of guilt is wide and extensive (Baumeister et al., 1994). It functions as a moral emotion, protecting and enhancing social relationships by punishing interpersonal wrongdoings and restoring equities (Baumeister et al., 1994; Haidt, 2003). Moreover, the prospect of guilt prevents people from committing wrongful deeds (Chang et al., 2011); the lack of guilt is a characteristic manifestation of psychopaths, who have normal moral knowledge but behave abnormally immoral (Blair, 2006; Kiehl, 2006).
Over the last decade, several neuroimaging studies have been carried out to investigate the neural mechanisms underlying the processing of guilt. These studies predominately used imagination or recall of a guilt-related situation as emotion-inducing stimuli (Shin et al., 2000; Takahashi et al., 2004; Berthoz et al., 2006; Zahn et al., 2009; Basile et al., 2011). Since the script-based imagination and recall may require psychological processes that are nonessential to the experience of guilt, the results of these studies are mixed. Nonetheless, several brain regions have been consistently implicated and the activation of these regions have also been observed for the experience of negative affect, physical pain, and ‘social pain’ (Shackman et al., 2011; Eisenberger, 2012). For instance, anticipating and imagining a guilt-evoking situation activate the anterior cingulate cortex (ACC), the anterior insula (AI) and the lateral orbitofrontal cortex (LOFC) (Shin et al., 2000; Basile et al., 2011; Chang et al., 2011; Wagner et al., 2011). These activations may reflect an ‘unpleasant arousal akin to anxiety’ (Tennen and Herzberger, 1987), such as the anxiety over being socially excluded (Baumeister et al., 1994). This anxiety may in turn promote compensation and prosocial behaviors, thereby restoring the impaired social relationship (Griswold, 2007).
While imagining or recalling particular situations may, in fact, be successful in eliciting guilt-like feelings, the every-day experience of guilt is interpersonal and complex and needs to be analyzed in these more realistic settings (Baumeister et al., 1994; Sanfey, 2007). Moreover, interpersonal guilt, as a type of moral emotion, or ‘the voice of conscience’, may stimulate moral behavior, such as compensation (Baumeister et al., 1994; Griswold, 2007), the neural basis of which has not been examined in previous studies. In this study, we address three previously unanswered questions (but see Koban et al., in press): (i) What are the brain responses to interpersonal guilt? (ii) What is the neural pathway through which the initial cortical response to interpersonal guilt is translated into compensation behaviors? (iii) What is the structural basis for guilt and compensation behavior?
We combined functional/structural MRI technique and an interpersonal game paradigm to investigate these three questions. While undergoing fMRI scanning, the participant was playing a dot-estimation task (Fliessbach et al., 2007) with an anonymous partner. In each round of the game the partner, a confederate, was randomly chosen from three possible candidates. The participant underwent two scanning sessions. In the first session (Figure 1), the participant was told that a painful stimulation could befall their partner if one or both of them estimated incorrectly. There were four possible outcomes: both estimated correctly (Both_Correct), only the partner estimated incorrectly (Partner_Incorrect), only the participant estimated incorrectly (Self_Incorrect), and both estimated incorrectly (Both_Incorrect) (Table 1). Before stimulation, the participant indicated the level of pain he or she would be willing to take for the partner, which was used as an online measure of the level of guilt and compensation (Batson et al., 1981; de Hooge et al., 2011). We hypothesized that in the Self_Incorrect condition, where the participant’s mistake is the direct cause of the partner’s suffering, the feeling of interpersonal guilt will be more intense than the Both_Incorrect and the Partner_Incorrect conditions. Therefore, the compensation behavior, i.e. taking painful stimulation for the partner, will be higher in the Self_Incorrect condition as compared with the other two conditions. In the second session, the participant was told that he/she would perform the same task with the same three partners. No pain stimulation was delivered to either side. This session was included to control for potential confounding factors such as social comparison (see below). After scanning, the participant was asked to rate separately, on a 9-point scale, their feelings of guilt, distress, anger and fear in the three pain-present conditions in the first session.
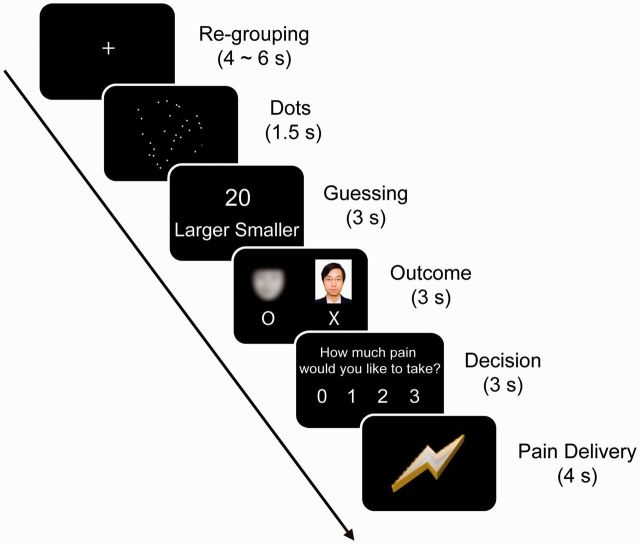
Fig. 1.
Task displays, timing and design. In the first scanning session, each trial began by informing the participant that the program has randomly chosen one out of the three confederates as his/her partner in the current trial, the identity of whom was unknown. The participant and his/her partner had to quickly estimate the number of dots on the screen. The participant was told to press a corresponding button, using the left or right thumb, to indicate whether his/her estimation was larger or smaller than the number (randomly chosen from 19, 20 and 21) appeared on the next screen. There were always 20 dots on the screen, the positions of which were randomly generated. The positions of ‘Smaller’ and ‘Larger’ were counterbalanced across participants. The correctness of their estimations was presented under the photo of the participant and under a blurred picture of face representing the partner. Before and after the outcome presentation, a fixation cross (not shown in the figure) was presented for a variable of interval ranging from 1 to 3 s. These are for the purpose of fMRI signal deconvolution. The participant indicated the level of pain he/she would be willing to take by pressing 1 of 4 corresponding buttons ranging from 0 (‘no pain’) to 3 (‘moderate pain’), using his/her left or right thumb. The participant was told that the more pain he/she took, the less his/her partner had to suffer. Finally, a pain stimulation of the participant’s choice was delivered to him/her.
Table 1.
Experimental design
| Self | Partner | Condition | Pain |
|---|---|---|---|
| O | O | Both_Correct | − |
| O | X | Partner_Incorrect | + |
| X | O | Self_Incorrect | + |
| X | X | Both_Incorrect | + |
Note. O: correct feedback, X: incorrect feedback; +: pain present, −: pain absent.
We first used a conventional general linear model-based analysis to identify guilt-related activations and brain correlates of individual’s sensitivity to guilt. Then to identify the neural pathways through which the brain responses to guilt are translated to behavioral responses, we utilized a recently developed procedure to test mediation relationship and to locate multiple brain mediators (Mediation Effect Parametric Mapping, MEPM; Wager et al., 2008, 2009a). With this procedure, Wager et al. (2009a) found that periaqueductal gray (PAG), a midbrain nucleus, mediated the brain processes of social threat and the physiological responses to the threat (see also Buhle et al., in press). This finding highlights the importance of the cortical–subcortical interaction in the translation of brain processes of social-affective stimuli into physiological and experiential responses. If interpersonal guilt can be conceptualized as anxiety and social threat, as argued above, then it is possible that the midbrain nucleus mediates the relation between the cortical affective processing of interpersonal guilt and the behavioral responses to the guilt.
MATERIALS AND METHODS
Participants
Twenty-seven healthy right-handed graduate and undergraduate students took part in the fMRI scanning. Because of excessive head movements (>3 mm), 3 were excluded from data analysis, leaving 24 participants (mean age 22.0 years; age range: 19–24 years; 11 female) for data analysis. None of the participants reported any history of psychiatric, neurological or cognitive disorders. Informed written consent was obtained from each participant before scanning. The study was carried out in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Department of Psychology, Peking University.
Procedure
Each participant came to the scanning room individually. Upon arrival he/she met three confederates and was told that they would later play an interactive game together through an intranet, but in separate rooms. At least one confederate had the same sex as the participant and at least one had the opposite sex. An intra-epidermal needle electrode was attached to the left wrist of the participant for cutaneous electrical stimulation (Inui et al., 2002). Participant-specific pain threshold was calibrated and three levels of pain stimulation were set as 1, 3 and 15 repeated pulses (with 0.5 ms duration of each pulse and a 10 ms interval between consecutive pulses) of epidermal electrical stimulation. The intensity of each pulse (in the unit of mA) was four times of the participant’s pain threshold. The participant was then asked to rate the intensity of three levels of pain stimulation on a scale of 0 (‘not painful’) to 10 (‘unbearable’). The mean intensity ratings were (mean ± SD) 2.5 ± 1.0, 5.1 ± 1.5 and 8.2 ± 1.5 for the low, medium and high intensity, respectively. All participants reported that the three levels of pain stimulation were clearly distinguishable. In the first scanning session, the participant was to perform the task described in Figure 1. There were four possible outcomes: both estimated correctly (Both_Correct), only the partner estimated incorrectly (Partner_Incorrect), only the participant estimated incorrectly (Self_Incorrect) and both estimated incorrectly (Both_Incorrect) (Table 1). The first fMRI scanning session consisted of 64 trials (16 for each experimental condition) and lasted for ∼25 min. Note that the estimation outcome (correct vs incorrect) was predetermined by a computer program such that each condition had an equal number of trials. This is for maximizing the fMRI statistical power and a postscan interview showed that no participant actually noticed this manipulation. The second scanning session was similar to the first except that no pain stimulation was delivered. The second scanning session consisted of 64 trials and lasted for about 17 min. After scanning, each participant rated, on a scale of 1 (‘not at all’) to 9 (‘very strong’), their feeling of guilt, anger and fear for the three conditions in the first scanning session.
Data acquisition
Images were acquired using a Siemens 3.0 Tesla Trio scanner with a standard head coil at the Key Laboratory of Cognition and Personality (Ministry of Education) of Southwest University, China. T2*-weighted functional images were acquired in 36 axial slices parallel to the AC–PC line with no interslice gap, affording full-brain coverage. Images were acquired using an EPI pulse sequence, with a TR of 2200 ms, a TE of 30 ms, a flip angle of 90°, an FOV of 220 mm × 220 mm and 3.4 mm × 3.4 mm × 3.5 mm voxels. A high-resolution, whole-brain structural scan (1 mm3 isotropic voxel MPRAGE) was acquired after functional imaging.
GLM-based image analysis
Image preprocessing and analysis used the Statistical Parametric Mapping software SPM8 (Wellcome Trust Department of Cognitive Neurology, London, UK). Images were slice-time corrected, motion corrected, re-sampled to 2 × 2 × 2 isotropic voxel, normalized to Montreal Neurological Institute (MNI) space and spatially smoothed using an 8-mm FWHM Gaussian filter, and temporally filtered using a high-pass filter with 1/128 Hz cutoff frequency. The first-level (within-participant) statistical analysis was conducted with SPM8. Briefly, separate regressors in the GLM were specified for fMRI responses to the recasting cue, random dot presentation, estimation responses, outcome feedback, costly helping responses and pain delivery. Values for the ‘Self_Incorrect > Both_Incorrect’ contrast at the outcome stage were subjected to second-level random effects analysis using the one-sample t test in SPM8. The same contrast in the second session was defined as an exclusive mask (P < 0.05 uncorrected) for the first session results.
A robust regression analysis (Wager et al., 2005) was carried out on the same contrast to locate, in an anatomically defined midbrain region of interest (ROI), regions in which activity correlated with the tendency to compensate (i.e. the Compensation Index; see ‘Results: Behavioral result’ section). In addition, we defined a parametric model to examine the neural correlate of intra-participant variation in the level of pain chosen. A new GLM was built in which the three error-containing conditions were combined in one regressor. This regressor was further modulated by a parametric regressor that contained trial-wise level of pain chosen by the participant.
Mediation pathway analysis
The MEPM analysis is based on a standard three-variable path model with a bootstrap test for the statistical significance of the product a*b (Wager et al., 2008, 2009a). This analysis was conducted on ‘Self_Incorrect vs Both_Incorrect’ contrast values. For the ROI analysis in aMCC, we extracted the parameter estimates from a 6-mm edge cube around the peak voxel of the aMCC and of the midbrain revealed by the above second-level contrasts and used them as mediator and predictor in a mediation model. The Compensation Index was the dependent variable in this model. For the whole-brain exploratory analysis, the chosen threshold of P < 0.017 controlled the false positive rate (FDR) < 0.05 corrected in the whole brain.
Voxel-based morphometry analysis
The voxel-based morphometry (VBM) was conducted using the VBM toolbox developed by Christian Gaser (University of Jena, Department of Psychiatry, freely available online http://dbm.neuro.uni-jena.de/vbm/). Images were first segmented into gray matter (GM) and white matter. Coregistration of GM images across participants was achieved using the DARTEL (Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra) algorithm. The resulting template image was transformed to MNI stereotactic space using affine and nonlinear spatial normalization. Then the images were smoothed with a Gaussian kernel (full-width at half-maximum, FWHM = 10 mm). In a multiple regression to the GM volume in each voxel in an anatomically defined ACC mask we used the Compensation Index as covariate of interest, and used participants’ age, gender and total brain volume as covariates of no interest were entered into a multiple regression to the GM volume in each voxel in the whole brain.
Multi-level kernel density analysis
Meta-analysis of neuroimaging studies is useful for testing the consistency of the recruitment of certain brain regions by certain psychological process and for evaluating the specificity of certain brain activation in indicating certain psychological computation (Wager et al., 2009b). The meta-analytic approach adopted here, and used in a couple of recent papers (Ekin et al., 2007; Wager et al., 2007; Kober et al., 2008; Wager et al., 2008), is Multi-Level Kernel Density Analysis (MKDA) developed by Tor Wager and is freely accessible on his website (http://wagerlab.colorado.edu/). In MKDA, the coordinates in a standard stereotaxic space (i.e. Montreal Neurological Institute space) are treated as a sparse representation of activated locations. The question asked is whether the distribution of reported peaks shows any pattern or randomly distributed across the entire brain. Here we obtained a database of activation peaks from 15 published studies on emotion regulation and mapped the location of emotion regulation sources and sites (see Supplementary Data).
RESULTS
Behavioral results
Table 2 summarizes the level of pain voluntarily taken by the participants in the first scanning session and their self-reported feelings in the postscan manipulation check. Participants took the highest level of pain in the Self_Incorrect condition and took less in the Both_Incorrect condition and still less in the Partner_Incorrect condition, F (2, 46) = 65.09, P < 0.001. To control for unwanted influences on the fMRI results, e.g. the influence from the participant’s evaluation of his/her own performance in the dot-estimation task, we only compared the Self_Incorrect and Both_Incorrect conditions, in both of which the participant estimated incorrectly. To normalize the difference in the level of pain taken, we computed for each participant an index of tendency to compensation (i.e. Compensation Index) by dividing the difference between the level of pain taken in the Self_Incorrect and that in the Both_Incorrect by the sum of the two (mean Compensation Index = 0.12, SD = 0.08).
Table 2.
Behavioral results
| Item | Partner incorrect | Both incorrect | Self incorrect | F (2, 46) |
|---|---|---|---|---|
| Pain taken | 2.0a (0.6) | 2.8b (0.6) | 3.1c (0.6) | 65.09*** |
| Responsibility | 3.0a (1.8) | 4.6b (1.6) | 7.1c (1.6) | 35.31*** |
| Guilt | 1.8a (0.9) | 3.4b (1.7) | 5.3c (2.3) | 33.43*** |
| Distress | 2.0a (1.5) | 2.8a (2.0) | 4.0b (2.4) | 10.51*** |
| Fear | 2.6 (1.8) | 3.3 (2.3) | 2.9 (1.9) | 1.47 |
| Anger | 2.0 (1.6) | 2.6 (2.3) | 2.0 (1.3) | 1.89 |
Note. Standard deviations are shown in parentheses. Significant differences (critical α < 0.05, Bonferroni corrected) in pair-wise comparison are denoted by different subscripts. ***P < 0.001.
It should be noted that task difficulty and the sense of responsibility are directly related to whether individuals would actually feel guilty (Hoffman, 1982). We included a postscan question that asked the participants to indicate the perceived difficulty of the dot-estimation task. We found that the participants thought the task to be mildly difficult (5.0 ± 2.4, on 1–9 scale). Moreover, the participants felt more responsible, guilty and distressful for the partner’s suffering in the Self_Incorrect than in the Both_Incorrect condition (Table 2). These findings confirmed the validity of our paradigm in guilt induction.
Guilt-related neural activation
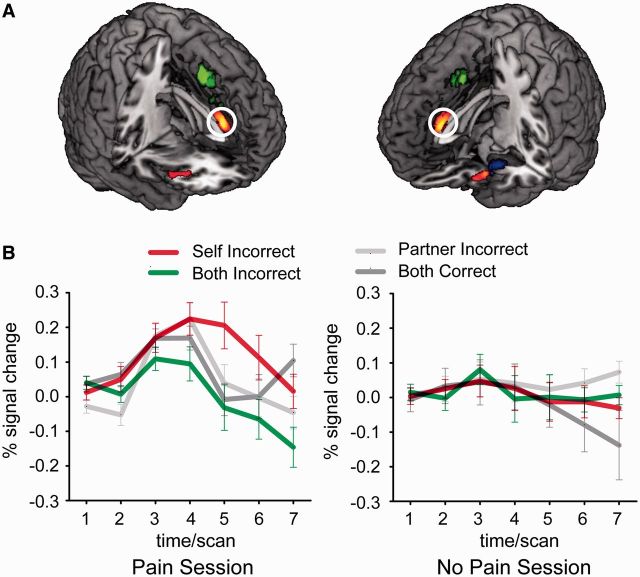
We first identified the voxels in which activation was higher in the Self_Incorrect condition than in the Both_Incorrect condition in the first session (voxel-level P < 0.001, cluster-level PFWE < 0.05). This contrast, after being exclusively masked with the same contrast in the second session (see ‘Materials and Methods’ section), revealed a single activation in the aMCC (peak voxel in MNI coordinates: 0, 34, 16; k = 270; Figure 2A). The application of the exclusive mask ensured that the findings reported here cannot be explained in terms of social comparison (Fliessbach et al., 2007), although the same pattern of brain activations was obtained without masking. To further ensure that the activation of aMCC was only present in the Pain but not in the No Pain (control) session, we extracted and plotted the regional signal change of aMCC in both sessions (Figure 2B). As can be seen, differential activations between the ‘Self_Incorrect’ and the ‘Both_Incorrect’ conditions were only present for the Pain session. Noted that we used the term ‘aMCC’ following the suggestion of Shackman et al. (2011) and Vogt (2009); where the cingulate cortex is parcellated on the basis of regional differences in microanatomy, connectivity, and physiology. In some previous publications, especially those related to ‘social pain’, this area is sometime included in a region called dorsal ACC (dACC; Eisenberger, 2012).
Fig. 2.
Results of the ‘Self_Incorrect > Both_Incorrect’ contrast and of the meta-analysis. (A) Results of the contrast ‘Self_Incorrect > Both_Incorrect’ in the first session is shown in yellow-to-red (P < 0.001 uncorrected, k > 50). Results of the meta-analysis of the emotion regulation literature are shown in green (regulation source) and blue (regulation site) (PFWE < 0.05). The activations observed in this study are clearly distinct from the source of emotion regulation. (B) Timecourse of percent fMRI signal change in the aMCC ROI in the first (Pain, left) and second (No Pain, right) session. The timecourse is locked to the onset of the estimation outcome. Error bars indicate standard error (s.e.m.).
The bilateral insula failed to reach the whole-brain cluster level threshold. However, since bilateral AI/LOFC is consistently implicated in imagining and recalling guilt-related situations (Chang et al., 2011; Wagner et al., 2011), and since these areas in the present analysis did show activation in the whole-brain analysis at a slightly liberal cluster-level threshold (P < 0.001 uncorrected, k > 50), we conducted a spatially restricted analysis using anatomically defined ROI masks based on the automatic anatomical labeling (AAL) system (Maldjian et al., 2003). The mask consisted of the insula and the inferior frontal gyrus (pars orbitalis). Activations were thresholding at PFWE < 0.05 both at the voxel- and the cluster-level. Significant activations were found both in the left (−30, 16, −18; k = 116) and in the right insula (36, 30, −8; k = 90).
The intra-participant parametric analysis of outcome-related brain activity and pain chosen revealed that activations in right (i.e. contralateral to the stimulated hand) putamen, mid-insula and superior parietal cortex were negatively correlated with the level of pain chosen (Supplementary Figure S1). These regions have been implicated in anticipation and experience of pain (Hui et al., 2000; Bingel et al., 2002). It is conceivable that the anticipation of physical pain had prevented the participant from choosing higher level of pain, rendering them to behave more selfishly. The reversed contrast did not reveal any significant activation.
It may be argued that the activation increase observed in the aMCC and bilateral insula arose from the received and/or anticipated pain rather than the feeling of guilt per se. This is plausible because the participants generally selected higher pain stimulation in the ‘Self_Incorrect’ relative to the ‘Both_Incorrect’ condition. However, we found that during the pain delivery stage, activations in bilateral dorsal-posterior insula, dorsal middle cingulate cortex and bilateral primary sensory area positively correlated with the level of pain stimulation. Importantly, these brain regions are clearly separated from those observed in the contrast of ‘Self_Incorrect > Both_Incorrect’ (Supplementary Figure S2), suggesting that activations of aMCC and bilateral insula were not caused by pain stimulation per se.
Although our analysis of brain activations focused on the ‘Self_Incorrect vs Both_Incorrect’ contrast, we did check the activations of the ‘Partner_Incorrect vs Both_Correct’ contrast. The ‘Partner_Incorrect > Both_Correct’ contrast revealed activations in dACC, posterior cingulate cortex and left AI, i.e. the empathy network (Lamm et al., 2011; Bernhardt and Singer, 2012). The reversed contrast revealed activations in the ventral striatum and bilateral amygdala, which are implicated in responding to salient positive outcome (Pessoa and Adolphs, 2000; Delgado et al., 2008). These results confirmed the validity of our experimental manipulation, suggesting that the participants believed in the setup and were emotionally involved in the task.
We have focused our neuroimaging analysis on the outcome-stage brain activity rather than the decision-stage activity for two reasons. First, the participant could know the consequence of his/her guess performance at the outcome stage. The emergence of guilt, as soon as the bad consequence for the partner was clear, should be automatic and immediate. Thus it is conceivable to expect the neural processing of guilt to be initiated at the outcome stage. Second, the neural signals associated with the decision stage might suffer more from confounding factors than those associated with the outcome phase, such as motor responses and pain anticipation. When we looked into the data associated with the decision stage, we did not find any significant activation for the contrast ‘Self_Incorrect > Both_Incorrect’. When we used a relatively liberal threshold (P < 0.005 uncorrected with 20 contiguous voxels), we found activations within the primary motor area, which probably reflect the motor component in the decision stage.
Functional specifity analysis
It could be argued that the aMCC activation observed in the above analysis arises from the suppression of the unpleasant feeling of guilt, rather than the experience of guilt per se. To test this hypothesis, we conducted a MKDA (Wager et al., 2009b) based on 15 published studies on emotion regulation, including cognitive reappraisal, suppression, emotional conflict control, etc (see ‘Materials and Methods’ section). Activations were dichotomized into source and site, with the source being regions that increase activation during regulation and the site being regions that decrease activation during regulation. Results showed that the source of emotion regulation was predominantly located in the supplementary motor area (SMA; Figure 2A, green) while the site mostly was located in the left amygdala (Figure 2A, blue) (PFWE < 0.05). This finding is consistent with a recent meta-analysis of emotion regulation that took into account a larger number of studies (Ochsner et al., 2012). As can be seen, the aMCC activation in this study is clearly distinct from the regulation source, suggesting that aMCC plays a role other than emotion regulation. Taken together, we are confident to conclude that the activations in the aMCC observed here reflect the experience of interpersonal guilt.
Structural correlate of the individual sensitivity to guilt
The aMCC activation reported above highlighted the physiological dynamics underlying the processing of interpersonal guilt. It is not clear whether this brain region is also structurally involved. We thus used voxel-based morphometry (VBM) (Ashburner and Friston, 2000) to test for a correlation between the GM volume of aMCC and the Compensation Index. We found that, over the 24 participants, the GM volume in an aMCC cluster (0, 34, 24; k = 98; PFWE < 0.05 both at the voxel- and the cluster-level) positively correlated with their sensitivity to guilt (Figure 3; see ‘Materials and Methods’ section). No significant results were obtained outside the mask, in a whole-brain exploratory analysis.
Fig. 3.
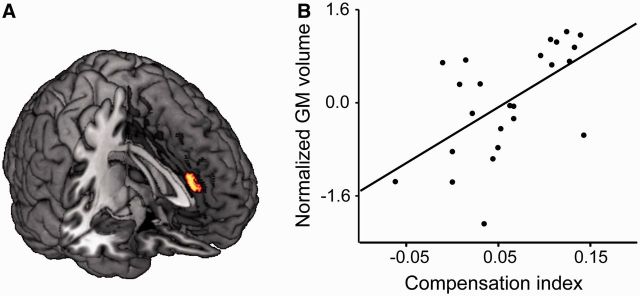
Results of the multiple regression analysis on the cingulate GM volume. The GM volume in an aMCC cluster positively correlated with the individual sensitivity to guilt (Compensation Index).
Mediation pathway analysis
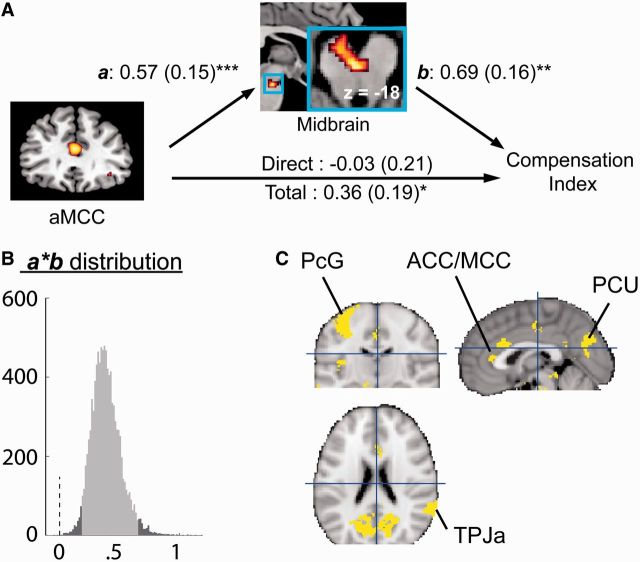
To investigate the neural pathway mediating the brain-to-behavioral translation of guilt-related responses, we need first to identify the brain correlates of the individuals’ tendency to compensate. We thus carried out a voxel-wise robust regression analysis (Wager et al., 2005) to correlate Compensation Index with guilt-related brain activation (‘Self_Incorrect > Both_Incorrect’). Robust regression is useful for examining questions about individual differences because it down-weights potential outliers that could exert undue leverage on results (Ochsner et al., 2009). A conventional statistical threshold for this analysis, false discovery rate (FDR) corrected qFDR < 0.05, was used. The regression was conducted only within the midbrain region defined by anatomical mask (the AAL system, see above), and a significant cluster was found within this region (−2, −20, −20; k = 7; Figure 4A). This anatomical region was chosen because it has been implicated in representing social threat and in translating the brain processes of social threat to physiological responses to the threat (Wager et al., 2009a).
Fig. 4.
Mediation analysis results. (A) Path diagram shows the relationships between regions in the path model. The predictor region (aMCC) is shown on the left, which predicts activations in a midbrain nucleus. This is the a path for the mediator region. The mediator region’s connection to individual sensitivity to guilt (Compensation Index) is the b path. It was calculated controlling for aMCC activity. The lines are labeled with path coefficients, and standard errors are shown in parentheses. The direct path was calculated controlling for the mediator. (B) The bootstrapped mediation effect (path a*b) for the aMCC. (C) Results of the whole-brain exploratory MEPM analysis for indirect cortical influence of Compensation Index, whose effect is mediated by the midbrain. Activation clusters are shown at qFDR < 0.05. Confirming the ROI-based analysis, the ACC/MCC is within the cortical network that influences Compensation Index indirectly via the midbrain. ***P < 0.001, **P < 0.01, *P < 0.05, two-tailed.
It should be noted that we do not claim this area to be the PAG, which was identified in a couple of previous neuroimaging studies on physical and social threat (for a review, see Buhle et al., in press). Due to the low spatial resolution of BOLD signal (relative to the size of the midbrain nucleus), we cannot say anything decisive about what this activation is. A speculation could be that this activation is centered in the caudal ventral tegmental area (VTA) and/or rostral medial tegmental nucleus, both of which play important roles in aversive processes (Laviolette et al., 2002; Laviolette and van der Kooy, 2003; Jhou et al., 2009). The whole-brain explorative analysis of robust regression did not yield significant results after cluster-level FDR correction. Nevertheless, when we used a relatively liberal criterion, i.e. a minimum of 20 contiguous voxels each significant at P < 0.001, we found that an empathy-related network, including the cuneus, precuneus, and superior parietal lobule (Supplementary Figure S3), whose activations were positively correlated with the Compensation Index.
If interpersonal guilt can be conceptualized as a type of anxiety and social threat, as argued above, then it is possible that the midbrain mediates the relation between the cortical affective processing of interpersonal guilt and the behavioral responses to the guilt. We thus carried out a recently developed procedure to test the hypothesis that the midbrain mediates the relation between the cortical affective processing of interpersonal guilt and the experiential-behavioral responses to the guilt (MEPM) (Wager et al., 2008, 2009a). First, we tested whether the aMCC exerts indirect influence, via the mediation of midbrain, on the sensitivity to guilt. Parameter estimates corresponding to the estimation outcome were extracted from a 6-mm edge cube around the peak voxel of the aMCC revealed by the contrast ‘Self_Incorrect > Both_Incorrect’ and around the midbrain revealed by the robust regression. With the aMCC activation as predictor, the midbrain activation as mediator, and the Compensation Index as outcome, the MEPM estimated the strength and significance of the mediation relationship. Confirming our prediction, the mediation effect of midbrain was significant (P = 0.004; nonparametric bootstrap test for whether the mediation strength is significantly different from zero) such that the relationship between aMCC activation and Compensation Index was fully mediated by midbrain activation (Figure 4A, B and Supplementary Figure S4). We then conducted a whole-brain exploratory search for indirect influence of Compensation Index, whose relationship was mediated by midbrain, with a qFDR < 0.05 threshold. Confirming the ROI-based analysis, the aMCC was found significantly activated in the whole-brain analysis (Figure 3C). In addition, an empathy-related network (Lamm et al., 2011; Bernhardt and Singer, 2012), including the right anterior temporoparietal junction (TPJa), the precentral gyrus (PcG) and the precuneus (PCU), was found to contribute to individual’s tendency to compensation via the mediation of midbrain (Table 3 and Figure 3C).
Table 3.
Results of whole-brain MEPM analysis
| Regions | MNI Coordinates |
Max T-value | Voxel size | ||
|---|---|---|---|---|---|
| x | y | z | |||
| MFG | −30 | 22 | 42 | 6.54 | 278 |
| aMCC | 2 | 24 | 22 | 6.03 | 199 |
| PcG | −36 | −18 | 56 | 6.76 | 902 |
| MTG | 52 | −38 | −4 | 6.60 | 221 |
| TPJa | 64 | −44 | 22 | 7.13 | 216 |
| Cerebellum | 26 | −48 | −22 | 7.50 | 406 |
Note. STS = superior temporal sulcus, MOG = middle occipital gyrus, IFG = inferior frontal gyrus
DISCUSSION
Past research has not adequately investigated the brain and behavioral responses of social emotions in life-like circumstances; our study on interpersonal guilt in social interactive context is an important extension to this past research. Due to its interpersonal nature (Baumeister et al., 1994), the feeling of and the responses to guilt is most natural in a social interactive context. Utilizing a game paradigm and functional/structural MRI, we showed the recruitment of the aMCC-AI network in response to an interpersonal guilt situation. This finding is in line with a recent neuroimaging study using a similar behavioral paradigm as the current one (Koban et al., in press). Moreover, the structural variability (GM volume) in the aMCC predicted individual difference in the sensitivity to guilt. Furthermore, we discovered that the cortical processing of guilt was translated by the midbrain to behavioral responses, suggesting that the human neural system processes the interpersonal guilt as social threat and anxiety (Wager et al., 2009a) and attempts to minimize it through compensation behaviors.
The cingulate cortex and the insula are known to participate in a multitude of sensory, affective, cognitive and motivational processes. Their coactivations are seen in experiencing physical and social pain, empathy for pain, disgust, taste, etc (Lamm et al., 2011; Bernhardt and Singer, 2012; Bernhardt et al., in press). Theories have been proposed concerning the functional interplay between AI and ACC/MCC in various cognitive and affective processing. Craig (2002, 2009) argued that insular cortex plays a major role in interoception, i.e. translating bodily states to conscious emotional feeling states; ACC/MCC, in turn, forms the motivational and action-related output. Similarly, Medford and Critchley (2010) suggested that while the AI forms an input region of a system that is based on self-awareness, the global emotional feeling states are ultimately re-represented in cingulate cortex to generate and regulate appropriate responses. Viewed in this context, activations of AI and aMCC here may arise from participants’ increased distress and anxiety associated with guilt and the motivation to take actions to reduce this distress (Baumeister et al., 1994; Griswold, 2007). In social interaction, distressing events, such as being isolated by others or betrayed by one’s romantic partner, cause substantial feeling of pain (MacDonald et al., 2005) and reliably elicit activations in cingulate cortex and insula (Eisenberger, 2012). This argument is also consistent with our postscan rating that the feeling of guilt and distress are significantly more intense in the ‘Self_Incorrect’ than in the ‘Both_Incorrect’ condition (Table 2). Therefore, we interpret the activations in aMCC and AI as reflecting the distressing emotional state arising from causing harm to the partner, i.e. interpersonal guilt.
We did not observe activations of dACC and SMA as one previous study did (Chang et al., 2011). Interestingly, however, the dACC and the SMA clusters observed in that study overlapped with the emotion regulation source obtained through the meta-analysis (Figure 2A), indicating that those brain regions may serve to suppress the selfish impulse of the trustee in a Trust Game context. This is in line with the authors’ finding that when the activation levels of dACC and SMA were higher, the participants tended to refrain from selfish behaviors. In our study, however, it is not necessary for the participant to suppress the feeling of guilt in order to act prosocially; instead, it was the feeling of guilt that drove the compensation behaviors. Thus it is conceivable that the emotion regulation-related brain regions did not show up in the present study. These results suggest that the current mathematical models of guilt be expanded to include situations other than monetary bargaining games (Fehr and Schmidt, 1999; Battigalli and Dufwenberg, 2007).
The network of AI and ACC/MCC does not work in isolation. Devinsky et al. argued that these regions work together with limbic and subcortical regions such as amygdala, midbrain and ventral striatum, forming a coherent network that assesses the motivational content of internal and external stimuli to generate and regulate goal-directed behaviors (Devinsky et al., 1995). Recent neuroimaging studies confirmed this hypothesis by demonstrating the prefrontal–subcortical pathway in generating and regulating social and physical threat (Wager et al., 2007, 2009a). Our finding that the midbrain nucleus mediated the relationship between the guilt-related activation in the aMCC and the guilt-induced compensation behaviors bridges two otherwise separate fields of knowledge: the daily experience and psychological evidence that people feel guilty and tend to compensate for the harms they inflict on others, on the one hand, and the neurobiological evidence that the midbrain nuclei mediate the cortical affective processing of and the behavioral responses to threatening stimuli, on the other hand. Although the participants were not confronted with explicit evaluation or required to interact with the partners after the experiment, the concern for reputation, which is ubiquitous in human society (Fehr and Gächter, 2002) and especially in East Asian cultures where the sense of shame and guilt is emphasized (Benedict, 1946), could still make them feel anxious or threatened when they inflicted harm on others.
An interesting question related to this finding is whether the participants’ motivation to compensate is altruistic, directing toward the end-state goal of reducing the other’s distress, or egoistic, directing toward the end-state goal of reducing their own distress (Batson et al., 1981; Nelissen and Zeelenberg, 2009; Nelissen, 2012). Although our experiment cannot provide a decisive answer to this question, the results of the mediation analysis suggested that the compensation behavior may be driven by both motivations: both the mentalizing network, including the right TPJ and precuneus, and the personal distress-related regions, such as the dACC and aMCC, contribute, via the mediation of midbrain, to the individual difference in compensation tendency.
We found that the GM volume within a focal region of aMCC predicted individual sensitivity to guilt (Figure 2C). The structural variability of the brain contributes to individual differences in cognitive and affective processing and behaviors (Kanai and Rees, 2011). Lesion of monkey ACC induces substantial changes in complex social behaviors, such as diminished shyness and fear of humans, increased emotional blunting and lowered patience (Devinsky et al., 1995). Clinical studies of human patients with cingulate lesion reported a deficit in understanding and expressing complex emotions in social interaction and using these emotions to constrain their social behaviors (Rudebeck et al., 2008; Krajbich et al., 2009). We extended the understanding of the functional significance of the cingulate cortex in social and affective processing by demonstrating, in a healthy population, that the variation in aMCC GM volume contributes to the individual sensitivity to guilt. This finding also has important implications for the understanding of neural basis of psychopathy, which shows dissociation between normal moral knowledge and abnormal moral behaviors (Blair, 2006; Kiehl, 2006). Taken together the previous finding that the anticipation of guilt prevents people from committing wrongful deeds (Chang et al., 2011), our result suggests that psychopaths may have impaired AI and/or ACC/MCC functions.
In conclusion, by employing functional and structural MRI during an interpersonal interactive game, we elicited and measured interpersonal guilt in the social interaction context. We showed that feeling of guilt induces the activation of the insula-cingulate network that has been consistently implicated in the processing of physical and social pain, threat and distress. Extending previous studies on social emotions, we demonstrated the neural pathway through which the experience of certain affective states is translated to behaviors. We highlighted the important role of midbrain nucleus in this function, which fits with the theoretical framework concerning the function of the midbrain nucleus in mediating brain–body interaction. Finally, utilizing structural imaging technique, we showed that the aMCC GM volume contributes to the individual difference related to compensation behavior. These findings may shed light on the understanding of the neural basis of psychopathy.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The authors thank Li Zhang, Chen Zhao, and Ping Xiao for technical support during fMRI data acquisition and thank Dr. Tor Wager for his help in fMRI data analysis. The authors are also grateful to the two anonymous reviewers for their constructive suggestions concerning the revision of the manuscript.
This study was supported by National Basic Research Program from the Ministry of Science and Technology of China (973 Program: 2010CB833904) and by grants from the National Natural Science Foundation of China (30110972, 91232708).
REFERENCES
- Ashburner J, Friston KJ. Voxel-based morphometry – the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Basile B, Mancini F, Macaluso E, Caltagirone C, Frackowiak RSJ, Bozzali M. Deontological and altruistic guilt: evidence for distinct neurobiological substrates. Human Brain Mapping. 2011;32:229–39. doi: 10.1002/hbm.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson CD, Duncan BD, Ackerman P, Buckley T, Birch K. Is empathic emotion a source of altruistic motivation? Journal of Personality and Social Psychology. 1981;40:290–302. [Google Scholar]
- Battigalli P, Dufwenberg M. Guilt in games. American Economic Review. 2007;97:170–6. [Google Scholar]
- Baumeister R, Stillwell AM, Heatherton TF. Guilt: an interpersonal approach. Psychological Bulletin. 1994;115:243–67. doi: 10.1037/0033-2909.115.2.243. [DOI] [PubMed] [Google Scholar]
- Benedict R. The Chrysanthemum and the Sword: Patterns of Japanese culture. New York, NY: Houghton Mifflin; 1946. [Google Scholar]
- Bernhardt BC, Singer T. The neural basis of empathy. Annual Review of Neuroscience. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Klimecki OM, Leiberg S, Singer T. Structural covariance networks of the dorsal anterior insula predict females’ individual differences in empathic responding. Cerebral Cortex. in press doi: 10.1093/cercor/bht072. Advance online publication: doi:10.1093/cercor/bht072. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Grezes J, Armony JL, Passingham RE, Dolan RJ. Affective response to one’s own moral violations. Neuroimage. 2006;31:945–50. doi: 10.1016/j.neuroimage.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Büchel C. Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain. 2002;99:313–21. doi: 10.1016/s0304-3959(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Blair J. The emergence of psychopathy: implications for the neuropsychological approach to developmental disorders. Cognition. 2006;101:414–42. doi: 10.1016/j.cognition.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Kober H, Ochsner KN, et al. Common representation of pain and negative emotion in the midbrain periaqueductal gray. Social Cognitive and Affective Neuroscience. in press;8:609–16. doi: 10.1093/scan/nss038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Smith A, Dufwenberg M, Sanfey AG. Triangulating the neural, psychological, and economic bases of guilt aversion. Neuron. 2011;70:560–72. doi: 10.1016/j.neuron.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel: now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- de Hooge IE, Nelissen RMA, Breugelmans SM, Zeelenberg M. What is moral about guilt? Acting “prosocially” at the disadvantage of others. Journal of Personality and Social Psychology. 2011;100:462–73. doi: 10.1037/a0021459. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84:3072–7. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nature Reviews Neuroscience. 2012;13:421–34. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An fMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Fehr E, Schmidt KM. A theory of fairness, competition, and cooperation. Quarterly Journal of Economics. 1999;114:817–68. [Google Scholar]
- Fehr E, Gächter S. Altruistic punishment in humans. Nature. 2002;415:137–40. doi: 10.1038/415137a. [DOI] [PubMed] [Google Scholar]
- Fliessbach K, Weber B, Trautner P, et al. Social comparison affects reward-related brain activity in the human ventral striatum. Science. 2007;318:1035–8. doi: 10.1126/science.1145876. [DOI] [PubMed] [Google Scholar]
- Griswold CL. Forgiveness: A Philosophical Exploration. New York, NY: Cambridge University Press; 2007. [Google Scholar]
- Haidt J. The moral emotions. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of Affective Sciences. New York, NY: Oxford University Press; 2003. pp. 852–70. [Google Scholar]
- Hoffman ML. Development of prosocial motivation: empathy and guilt. In: Eisenberg N, editor. The Development of Prosocial Behavior. San Diego, CA: Academic Press; 1982. pp. 281–313. [Google Scholar]
- Hui KKS, Liu J, Makris N, et al. Acupuncture modulates the limbic system and subcortical gray structures of the human brain: evidence from fMRI studies in normal subjects. Human Brain Mapping. 2000;9:13–25. doi: 10.1002/(SICI)1097-0193(2000)9:1<13::AID-HBM2>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui K, Tran TD, Hoshiyama M, Kakigi R. Preferential stimulation of Aδ fibers by intra-epidermal needle electrode in humans. Pain. 2002;96:247–52. doi: 10.1016/S0304-3959(01)00453-5. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nature Reviews Neuroscience. 2011;12:231–42. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: Evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142:107–28. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban L, Corradi-Dell’Acqua C, Vuilleumier P. Integration of error agency and representation of others’ pain in the anterior insula. Journal of Cognitive Neuroscience. in press doi: 10.1162/jocn_a_00324. Advanced online publication: doi:10.1162/jocn_a_00324. [DOI] [PubMed] [Google Scholar]
- Krajbich I, Adolphs R, Tranel D, Denburg NL, Camerer CF. Economic games quantify diminished sense of guilt in patients with damage to the prefrontal cortex. Journal of Neuroscience. 2009;29:2188–92. doi: 10.1523/JNEUROSCI.5086-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Alexson TO, van der Kooy D. Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. The Journal of Neuroscience. 2002;22:8653–60. doi: 10.1523/JNEUROSCI.22-19-08653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. Blockade of mesolimbic dopamine transmission dramatically increases sensitivity to the rewarding effects of nicotine in the ventral tegmental area. Molecular Psychiatry. 2003;8:50–9. doi: 10.1038/sj.mp.4001197. [DOI] [PubMed] [Google Scholar]
- MacDonald G, Kingbury R, Shaw S. Adding insult to injury: social pain theory and response to soical exclusion. In: Williams KD, Forgas JP, von Hippel W, editors. The Social Outcast: Ostracism, Social Exclusion, Rejection, and Bullying. New York, NY: Psychological Press; 2005. pp. 77–90. [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Structure & Function. 2010;214:535–49. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen RMA. Guilt-induced self-punishment as a sign of remorse. Social Psychological and Personality Science. 2012;3:139–44. [Google Scholar]
- Nelissen RMA, Zeelenberg M. When Guilt Evokes Self-Punishment: Evidence for the Existence of a Dobby Effect. Emotion. 2009;9:118–22. doi: 10.1037/a0014540. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Hughes B, Robertson ER, Cooper JC, Gabrieli JDE. Neural systems supporting the control of affective and cognitive conflicts. Journal of Cognitive Neuroscience. 2009;21:1842–55. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience. 2010;11:773–83. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Bannerman DM, Rushworth MFS. The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cognitive Affective & Behavioral Neuroscience. 2008;8:485–97. doi: 10.3758/CABN.8.4.485. [DOI] [PubMed] [Google Scholar]
- Sanfey AG. Social decision-making: Insights from game theory and neuroscience. Science. 2007;318:598–602. doi: 10.1126/science.1142996. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12:154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Dougherty DD, Orr SP, et al. Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biological Psychiatry. 2000;48:43–50. doi: 10.1016/s0006-3223(00)00251-1. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y. Brain activation associated with evaluative processes of guilt and embarrassment: an fMRI study. Neuroimage. 2004;23:967–74. doi: 10.1016/j.neuroimage.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Tangney JP, Dearing RL. Shame and Guilt. New York, NY: The Guilford Press; 2002. [Google Scholar]
- Tennen H, Herzberger S. Depression, self-esteem, and the absence of self-protective attributional biases. Journal of Personality and Social Psychology. 1987;52:72–80. doi: 10.1037//0022-3514.52.1.72. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage. 2005;26:99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta J-K. Placebo effects on human μ-opioidactivity during pain. Proceedings of the National Academy of Sciences. 2007;104:11056–61. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN. Brain mediators of cardiovascular responses to social threat, Part II: prefrontal-subcortical pathways and relationship with anxiety. Neuroimage. 2009a;47:836–51. doi: 10.1016/j.neuroimage.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Lindquist MA, Nichols TE, Kober H, van Snellenberg JX. Evaluating the consistency and specificity of neuroimaging data using meta-analysis. Neuroimage. 2009b;45:S210–21. doi: 10.1016/j.neuroimage.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, N’Diaye K, Ethofer T, Vuilleumier P. Guilt-specific processing in the prefrontal cortex. Cerebral Cortex. 2011;21:2461–70. doi: 10.1093/cercor/bhr016. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Paiva M, et al. The neural basis of human social values: evidence from functional MRI. Cerebral Cortex. 2009;19:276–83. doi: 10.1093/cercor/bhn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.