Abstract
Psychopathic traits affect social functioning and the ability to make adaptive decisions in social interactions. This study investigated how psychopathy affects the neural mechanisms that are recruited to make decisions in the ultimatum game. Thirty-five adult participants recruited from the community underwent functional magnetic resonance imaging scanning while they performed the ultimatum game under high and low cognitive load. Across load conditions, high psychopathy scorers rejected unfair offers in the same proportion as low scorers, but perceived them as less unfair. Among low scorers, the perceived fairness of offers predicted acceptance rates, whereas in high scorers no association was found. Imaging results revealed that responses in each group were associated with distinct patterns of brain activation, indicating divergent decision mechanisms. Acceptance of unfair offers was associated with dorsolateral prefrontal cortex activity in low scorers and ventromedial prefrontal cortex activity in high scorers. Overall, our findings point to distinct motivations for rejecting unfair offers in individuals who vary in psychopathic traits, with rejections in high psychopathy scorers being probably induced by frustration. Implications of these results for models of ventromedial prefrontal cortex dysfunction in psychopathy are discussed.
Keywords: psychopathy, functional magnetic resonance imaging, ultimatum game, ventromedial prefrontal cortex
INTRODUCTION
Psychopathy is a disorder characterized by affective, interpersonal and behavioral traits that predispose the individual to a variety of antisocial behaviors (Hare, 1991). Psychopathic traits include disregard for the rights of others, lack of empathy and remorse, impulsivity, and egocentricity (Hare, 1991; Blair, 2005; Blair et al., 2006). These traits may affect social functioning and compromise the ability to make adaptive decisions in social interaction settings. In experimental tasks, psychopathic individuals have previously shown atypical patterns of cooperation (Rilling et al., 2007; Mokros et al., 2008), and difficulties in social exchange and reasoning about social rules (Ermer and Kiehl, 2010). These socio-affective processing deficits are associated with abnormal patterns of brain functioning, which in turn may be linked to structural abnormalities (Birbaumer et al., 2005; de Oliveira-Souza et al., 2008; Veit et al., 2010; Yang et al., 2011; Fairchild et al., 2013). However, recent empirical reports indicate that atypical functioning in brain regions involved in social cognitive and moral tasks may occur in the absence of behavioral differences between subjects with high and low psychopathic tendencies (Gordon et al., 2004; Glenn et al., 2009; Sommer et al., 2010; Marsh et al., 2011; Pujol et al., 2012). This suggests that, in some circumstances, subjects with extreme psychopathy scores might recruit neurocognitive processes that are distinct from those used by low scorers to achieve comparable behavioral outcomes. However, although suggested in the interpretation of previous findings (e.g. Glenn et al., 2009), this hypothesis has never been formally tested.
In the present study, we used functional magnetic resonance imaging (fMRI) to examine the neural processes involved in making social decisions as a function of psychopathy. We investigated responses to unfairness in an economic bargaining paradigm, the ultimatum game (UG), in which two players must decide how to split an amount of money: the proposer suggests a division to the responder, who decides whether to accept the offer, knowing that if he or she accepts, the stake will be divided according to the offer, and if he or she rejects, both players get nothing. From an economic standpoint, the rational decision in the UG is to accept any offer. However, subjects typically decline offers of <40% of the stake (Camerer, 2003; Oosterbeek et al., 2004). This response, commonly referred to as altruistic punishment, is considered a prosocial behavior because the subject forfeits monetary gain to punish the other player for violating a fairness norm (Frith and Frith, 2008). Brain regions implicated in negative emotional reactions to uneven splits of money and consequent rejection of unfair offers include the anterior insula and the amygdala (Sanfey et al., 2003; Haruno and Frith, 2010; Gospic et al., 2011; Harle and Sanfey, 2012; Osumi et al., 2012). During the acceptance of unfair offers the dorsolateral prefrontal cortex (dlPFC) is recruited (Sanfey et al., 2003; Gospic et al., 2011; Harle and Sanfey, 2012), which may reflect the cognitive effort required to override automatic negative emotional responses to unfairness (Sanfey et al., 2003). Koenigs and Tranel (2007) also highlighted the role of the ventromedial prefrontal cortex (vmPFC) in the UG, demonstrating that vmPFC lesion patients made exaggerated irrational decisions by rejecting even more unfair offers than controls. This finding was interpreted as the result of a failure to down-regulate negative emotions to unfair offers (e.g. anger), providing further evidence of the involvement of the vmPFC in emotion-guided decisions (Bechara, 2004) and suggesting that non-altruistic motives like frustration or revenge can also lead to costly punishment in the UG.
How psychopathic traits affect decision making in the UG is unclear. Reports of the performance of psychopathic individuals in the UG are inconsistent, with high psychopathy scorers having alternately been reported to accept more unfair offers (Osumi and Ohira, 2010; Osumi et al., 2012) and to reject more unfair offers (Koenigs et al., 2010) than low psychopathy scorers. Increased acceptance of unfair offers by psychopathic individuals was interpreted as the result of diminished sensitivity to unfairness. This interpretation was further supported by reports of no electrodermal differentiation between unfair and fair offers (Osumi and Ohira, 2010) and decreased amygdala activity to unfair offers (Osumi et al., 2012) in high psychopathy scorers. These findings are consistent with observations that psychopathic individuals are generally less responsive to stimuli that elicit automatic emotional responses in healthy subjects. During emotionally charged moral judgments and other emotional processing tasks, individuals with psychopathic traits display reduced activation in the amygdala, medial prefrontal cortex and striatum, but increased activation in the dlPFC (Intrator et al., 1997; Kiehl et al., 2001; Gordon et al., 2004; Rilling et al., 2007; Glenn et al., 2009; Marsh and Cardinale, 2012). Involvement of the dlPFC suggests that psychopathic subjects may recruit effortful abstract reasoning processes during these tasks (Seidman et al., 1994; Crescentini et al., 2011) to produce perhaps more strategic and cognitively demanding responses (Steinbeis et al., 2012). Overall, these findings suggest that individuals with psychopathic traits are more rational decision makers in the UG, displaying reduced emotional reactions to unfairness and favoring economic utility, possibly at the expense of greater cognitive effort.
However, Koenigs et al. (2010) reported an opposite behavioral pattern. Their study demonstrated a similarity between the UG performance of vmPFC lesion patients and subjects with psychopathy, with both groups rejecting unfair offers more frequently than the comparison groups. This finding was interpreted as a result of deficient emotion regulation in high psychopathy scorers due to vmPFC dysfunction. Models of vmPFC dysfunction in psychopathy have also linked this region to abnormal processing of reinforcement information (Birbaumer et al., 2005; Finger et al., 2008), which is thought to compromise the individuals’ ability to make adaptive decisions, thus increasing their vulnerability to frustration by not receiving the desired outcomes (Blair, 2010). These findings suggest that, although highly psychopathic individuals may favor economic utility over fairness, this does not necessarily result in rational decisions in the UG, as abnormalities in emotion regulation and reward processing during the game could lead to the rejection of unfair offers as a result of revenge or frustration.
Consideration of the specific neural mechanisms underlying UG decisions in individuals who vary in psychopathic traits may help to explain this apparent inconsistency. In the present study, we incorporated two features designed to disambiguate how psychopathy affects economic decision making. First, to identify the role of compliance with a fairness norm as compared with other motivations (e.g. Koenigs and Tranel, 2007), we examined individual perceptions of the fairness of offers and analyzed their relation to behavioral responses. Then, we used a linear regression approach to investigate the brain regions in which activation predicted both perceptions of fairness and responses to unfair offers in high and low psychopathy scorers, as assessing the relationship between individual performance and brain activation has been suggested to be a more informative approach to analyzing fMRI data than performing simple contrasts (Christakou et al., 2009). Second, we manipulated cognitive load during the UG in order to disentangle automatic emotion-based from controlled strategy-based decisions, under the assumption that controlled responses are more sensitive to the amount of available cognitive resources than automatic responses (Bargh and Ferguson, 2000; Barrett et al., 2004; Evans, 2008).
In line with UG literature, we hypothesized that for low psychopathy scorers behavioral responses would be predicted by the perceived fairness of offers and would not be affected by the increase in cognitive load. At the neural level, we predicted that UG decisions would be mainly associated with amygdala and dlPFC activity. For high psychopathy scorers, two competing sets of predictions were formulated, based on the divergent evidence outlined above. If high psychopathy scorers are essentially rational decision makers who make strategic responses solely according to economic utility, we would expect their responses to be associated with the perceived fairness of offers and to be affected by the increase in cognitive load. At the neural level, we would expect behavioral responses to be linked with cognitive control-related activity, namely in the dlPFC. Alternatively, if psychopathic individuals are non-rational decision makers whose emotion regulation difficulties lead to anger-motivated responses, we would expect their responses not to be associated with the perceived fairness of offers and not to be affected by the increase in cognitive load. At the neural level, responses to unfair offers would be predicted by activity in the vmPFC.
To our knowledge, this is only the second study using fMRI to investigate responses to unfairness in individuals with psychopathic traits, and the first to directly examine the neural basis of decisions by high and low psychopathy scorers in the UG.
METHODS
Participants
Thirty-six participants (20 females) were recruited from the Georgetown University community through advertisements developed for psychopathy research, which have been shown to produce oversampling of high psychopathy scorers (Widom, 1977; Marsh and Cardinale, 2012). Participants were screened for neurological and psychiatric disorders, as well as brain injuries, which constituted exclusion criteria. All participants were right-handed and reported not taking any psychotropic medication at the time of screening. Average intelligence quotient (IQ) was assessed using the Kaufman Brief Intelligence Test (K-BIT; Kaufman, 1990) (Table 1).
Table 1.
Sample characterization: age, IQ and psychopathy scores for the whole sample and for each group
| Characteristics | Whole sample (n = 35) |
Low psychopathy (n = 17, 12 F) |
High psychopathy (n = 18, 8 F) |
|||
|---|---|---|---|---|---|---|
| Minimum–Maximun | Mean (s.d.) | Minimin–Maximum | Mean (s.d.) | Minimum–Maximum | Mean (s.d.) | |
| Age | 18–24 | 21.06 (1.80) | 18–24 | 21.24 (2.05) | 18–24 | 20.89 (1.58) |
| IQ | 94–132 | 112.43 (12.02) | 94–132 | 112.65 (11.65) | 95–131 | 112.22 (12.69) |
| TriPM | ||||||
| Total score | 23–120 | 71.31 (25.42) | 23–63 | 50.88 (11.96) | 66–120 | 90.61 (18.61) |
| Boldness | 20–55 | 39.83 (9.49) | 20–44 | 33.12 (6.85) | 26–55 | 46.17 (6.96) |
| Meanness | 1–51 | 15.71 (11.02) | 1–23 | 8.59 (5.57) | 10–51 | 22.44 (10.72) |
| Desinhibition | 0–37 | 15.77 (10.58) | 0–30 | 9.18 (6.92) | 9–37 | 22.00 (9.71) |
The study was approved by the Institutional Review Board at Georgetown University, and all participants provided informed written consent in accordance with the Declaration of Helsinki.
Psychopathy measures
Psychopathy was assessed using the Triarchic Psychopathy Measure (TriPM; Patrick, 2010), a 58-item self-report instrument conceptually based on the Triarchic Model of psychopathy (Patrick et al., 2009). For each item, subjects indicate how accurately the item applies to them using a 4-point scale (0 = true; 1 = somewhat true; 2 = somewhat false; 3 = false). The TriPM measures psychopathic traits in a dimensional manner, consistent with the idea that psychopathy can be more accurately assessed continuously than categorically (Skeem et al., 2011). The Triarchic Model describes psychopathy as a conjunction of three phenotypic components, boldness, meanness, and disinhibition, which are evaluated by three subscales of the TriPM. This is a relatively new self-report measure of psychopathy but has been reported to have good construct validity and to be able to successfully tap the core traits of psychopathy (Sellbom and Phillips, 2013; Stanley et al., 2013; Marion et al., 2012).
For group analyses, subjects were divided in two groups (high and low psychopathy) by median split of the total TriPM score. Following the split, mean TriPM scores in the high and low psychopathy groups were 90.61 (s.d. = 18.61) and 50.88 (s.d. = 11.96), respectively. Groups did not differ in age or IQ. Average psychopathy scores in males were higher than in females, but the distribution of men and women in the groups was not significantly different (χ2 = 2.44, P > 0.05) (Table 1).
fMRI scanning task
Participants played a series of one-shot UGs featuring two cognitive load conditions (No Load and Load) during fMRI scanning.
The No Load condition corresponded to the classical UG (Figure 1A). Pictures of other players were selected from the Radboud Faces Database (RaFD) (Langner et al., 2010). All selected pictures displayed closed mouth, eyes facing forward, neutral facial expressions performed by Caucasian actors and an equal number of male and female actors were used. After the picture of the proposer, the stake was displayed, followed by the offer. Participants were instructed to only make a response when the response slide was displayed, using two response buttons held in the right (‘Reject’) and left hand (‘Accept’), respectively. A feedback slide was displayed at the end of the run, showing what each player had won in that round.
Fig. 1.
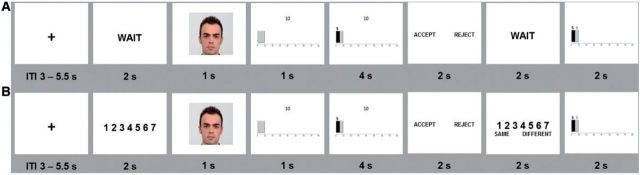
Task design and stimulus presentation for the No Load (A) and Load (B) condition.
In the Load condition, participants were required to play a memory task concurrent with the UG. The introduction of a secondary task was intended to affect the availability of cognitive resources for the main task. Cognitive load manipulations have been shown to be effective in selectively interfering with the production of controlled responses (Greene et al., 2008) and have been used previously in the context of economic decision paradigms (Schulz et al., 2012; Haruno and Frith, 2010). In this condition, a sequence of seven digits was shown before the other player’s picture. Participants were instructed to memorize the digits, disregarding their order in the sequence, because at the end of each round they would be asked to recognize them. Thus, before the feedback slide, another seven-digit sequence was displayed, and participants responded ‘Same’ or ‘Different’, via button press (Figure 1B).
Participants played 48 UG rounds in each condition, in which 24 featured unfair (offers were 20–33% of the stake) and 24 featured fair trials (offers were 40–50% of the stake). There were only two possible offered amounts, 5 and 15, and the stake sized varied, following previous paradigms (Crockett et al., 2008; Van der Veen and Sahibdin, 2011) (Table 2). Each offer was repeated four times within condition.
Table 2.
UG offers
| Amount offered | Stake size |
|
|---|---|---|
| Unfair | Fair | |
| 5 | 15 | 10 |
| 20 | 11 | |
| 25 | 12.5 | |
| 15 | 45 | 30 |
| 60 | 33 | |
| 75 | 37.5 | |
The task was programmed and delivered in Presentation 0.71 (2003, Neurobehavioral Systems, Inc.).
Procedures
Following previous studies (Crockett et al., 2008; Van der Veen and Sahibdin, 2011), participants were informed that the offers they would see during the task were made by people who participated in the study previously and they would have the chance to make offers themselves, after the scan. Subjects were presented with splits of tokens and not monetary units to control for subjective evaluations of the amounts. Participants’ compensation was calculated by converting the total amount of tokens earned during the task to US dollars, according to a predefined conversion rate.
Before entering the scanner, subjects were given the task instructions and played eight practice rounds (four of each condition) on a laptop. Inside the scanner, they completed two runs of the task that corresponded to each of the two conditions. The order of presentation of the conditions was counterbalanced across participants. After the MRI scan, participants were presented once with all the offers shown during the UG and asked to rate their fairness using a 7-point scale (1 = very unfair; 7 = very fair). This task was programmed and delivered in Presentation 0.71 (2003, Neurobehavioral Systems, Inc.) running on a laptop.
Finally, to assure that subjects believed the cover story, they were queried regarding: (i) their reasons to accept and reject offers and (ii) their beliefs about the real objectives of the study. Subjects were also given the opportunity to write down any additional comments about the study. Only then subjects were fully debriefed. One participant was dropped from the analysis because he reported not believing that the offers were really made by other players, resulting in a final sample composed of 35 subjects.
fMRI data acquisition and preprocessing
Participants were scanned on a 3.0 Tesla MRI system (Siemens Magnetom Trio, Erlangen, Germany), at Georgetown University’s Center for Functional and Molecular Imaging (CFMI), fitted with a circularly polarized 12-channel head coil. A mirror mounted on the coil allowed participants to view the task via projection. Head movements were minimized through padding.
A high-resolution T1-weighted structural scan (MPRAGE, magnetization-prepared rapid gradient echo) was acquired between two functional scans (TR = 1900 ms, TE = 2.52 ms, slices = 176, slice thickness = 1.0 mm, FOV = 256 × 256 mm). In the two functional runs of the task a T2*-weighted gradient echo-planar imaging (TR = 2700 ms, TE = 30 ms, slices = 51, slice thickness = 3 mm, FOV = 256 × 256 mm, acquisition matrix = 64 × 64, flip angle = 90°) was used. The first four TRs of each functional run were excluded from analysis due to magnet stabilization.
Imaging data were pre-processed and analyzed in Analysis of Functional Neuroimaging (AFNI) (Cox, 1996). For each subject, functional images from the two functional runs were concatenated, despiked, motion corrected, spatially smoothed using a 6.0-mm full-width half-maximum Gaussian filter, and then masked to exclude activation outside the brain. The time series were then normalized such that the resulting regression coefficients represent a percent signal change from the mean. Regressors were created that represented four task conditions (Fair No Load, Fair Load, Unfair No Load, Unfair Load) and two contrasts across conditions (Unfair No Load > Fair No Load, Unfair Load > Fair Load). A final regressor of no interest was created for offers to which participants did not provide a valid response and for all events in the task that were not a fixation or an offer. All regressors were created by convolving the train of stimulus events with a gamma-variate hemodynamic response function (Cohen, 1997). Linear regression modeling was performed using the full set of regressors to model baseline drift and residual motion artifact. The baseline was modeled by a first-order function and motion artifacts were modeled using the six estimated rigid-body motion parameters. This produced a beta coefficient and associated t-statistic for each voxel and regressor. Participants’ anatomical scans were individually registered to the Talairach and Tournoux Atlas (Talairach and Tournoux, 1988).
Statistical analysis
Behavioral data
Average acceptance rates (%) for fair and unfair offers were calculated for each participant in each condition. In addition, we computed difference scores indexing the difference between the percentages of fair and unfair offers accepted, such that the higher the difference score, the greater the number of rejected unfair offers.
To investigate the effects of fairness, cognitive load and psychopathy on acceptance rates, we performed a mixed factors analysis of variance (ANOVA), with fairness (Fair, Unfair) and cognitive load (No Load, Load) as within-subject factors and psychopathy group (High, Low psychopathy) as between-subjects factor. To confirm the results, we performed another mixed factors ANOVA on the difference scores, with load as within-subject factor and psychopathy group as between-subjects factor. Associations between acceptance rates and psychopathy (total score and subscales) were also explored using correlation analysis, as a dimensional approach is consistent with theoretical conceptions of psychopathy and often results in increased power to detect significant effects.
Average fairness ratings for fair and unfair offers were computed for each subject and represent a measure of perceived fairness. Higher values corresponded to subjects’ perceptions of greater fairness. Correlations between fairness ratings and psychopathy scores were performed. Finally, we examined the associations between fairness ratings and acceptance rates within each group.
The threshold for statistical significance was set at P < 0.05, two-tailed, for all analyses. Multiple comparisons were addressed through Sidak correction and the Greenhouse–Geisser procedure was used to correct departures from sphericity, when necessary.
fMRI data
We conducted whole-brain analyses setting the threshold at P < 0.001, uncorrected, with an extent threshold of 10 contiguous voxels, a procedure that has been suggested to successfully balance Type I and Type II errors in fMRI research (Lieberman and Cunningham, 2009; Marsh and Cardinale, 2012; White et al., 2012).
In order to explore underlying patterns of activation that guide responses to unfair offers among high and low psychopathy scorers, we performed whole-brain linear regression analysis in each group, using activation in the Unfair > Fair contrast in the No Load condition as dependent variable. Difference scores of acceptance rates in the same condition and fairness ratings of unfair offers were entered as predictors. This approach allowed us to explore in which areas activation was uniquely associated with acceptance rates and perceived fairness and, therefore, to isolate the neural mechanisms involved in UG decision from the mechanisms involved in subjective perceptions of fairness. Mean parameter estimates were extracted from functionally defined clusters identified in the regression analyses.
Additionally, as a task validation measure and to explore potential psychopathy × cognitive load interactions, we conducted two whole-brain 2 (Load, No Load) × 2 (High psychopathy, Low psychopathy) mixed factors ANOVA, using brain activation to Unfair and Fair offers independently as dependent variables. We were specifically interested in examining the clusters in which an effect of cognitive load was identified in order to assure that our secondary memory task was successful in increasing the task demands.
RESULTS
Behavioral results
Regarding acceptance rates, we obtained a main effect of Fairness [F(1,33) = 46.69, P < 0.001, ηp2 = 0.59], with fair offers being accepted more often (91.8%) than unfair offers (53.7%). We did not observe any main effects of cognitive load or psychopathy, or any significant interactions (Table 3). Similarly, difference scores were not affected by cognitive load or psychopathy.
Table 3.
Means and standard deviations for acceptance rates and fairness ratings in each group
| Group |
Low psychopathy | High psychopathy | ||
|---|---|---|---|---|
| Mean (s.d.) | Mean (s.d.) | |||
| Acceptance rates (%) | Unfair | No Load | 51.21 (39.94) | 55.16 (34.18) |
| Load | 55.14 (41.19) | 53.30 (38.17) | ||
| Fair | No Load | 89.23 (21.25) | 93.27 (14.39) | |
| Load | 88.73 (22.57) | 96.07 (10.74) | ||
| Fairness ratings | Unfair | 2.38 (0.59) | 2.67 (0.58) | |
| Fair | 5.38 (1.05) | 5.63 (0.68) | ||
Performance in the secondary memory task was analyzed, to assure the efficacy of the cognitive load manipulation. Average accuracy in the task was 78.2% and no differences between groups were found.
Correlation analysis revealed no significant associations between either raw acceptance rates or difference scores and psychopathy (for either total or subscales scores).
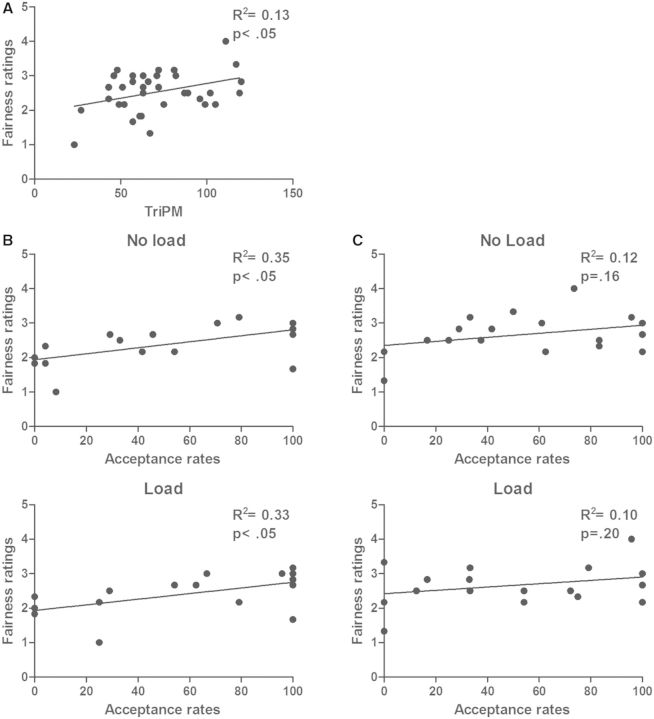
We obtained a significant positive association between fairness ratings for unfair offers and psychopathy total score (r = 0.37, P = 0.03) (Figure 2A). A t-test revealed a trend toward higher ratings of unfair offers in high vs low psychopathy scorers (P = 0.1). Finally, we examined the associations between acceptance rates and fairness ratings of unfair offers in each group. In the low psychopathy group, acceptance rates were positively associated with fairness ratings both in the No Load (r = 0.59, P = 0.012) and Load conditions (r = 0.58, P = 0.016) (Figure 2B). In the high psychopathy group, these associations were not significant (No Load: r = 0.34, P = 0.162; Load: r = 0.32, P = 0.201) (Figure 2C).
Fig. 2.
(A) Association between fairness ratings of unfair offers and psychopathy total score (P < 0.05). (B) Association between acceptance rates and fairness ratings of unfair offers for the No Load (upper) and Load (bottom) condition, in the low psychopathy group (P < 0.05). (C) No significant association between acceptance rates and fairness ratings was found in the high psychopathy group.
fMRI results
Regression analyses
Results for low psychopathy scorers showed (P < 0.001, uncorrected, 10 voxel threshold) that acceptance rates were significantly associated with activation in the left middle frontal gyrus [Montreal Neurological Institute (MNI) coordinates, x, y, z = −49, 16, 29], with increased activity in this cluster being associated with higher acceptance of unfair offers. In addition, both acceptance rates and fairness ratings were associated with activation in the left superior frontal gyrus (x, y, z = −4, 43, 60), with increased activity in this cluster being associated with higher acceptance of unfair offers and perceptions of greater unfairness (Table 4 and Figure 3).
Table 4.
Clusters identified by whole-brain linear regression analyses in the Unfair > Fair contrast (No Load), with acceptance rates and fairness ratings as predictors (MNI coordinates are reported; P < 0.001, uncorrected, 10 voxel threshold)
| Cluster | BA | x | y | z | Voxels |
|---|---|---|---|---|---|
| High psychopathy | |||||
| Acceptance rates | |||||
| R ACC | 24, 32 | 2 | 42 | 8 | 15 |
| Fairness ratings | |||||
| R medial frontal gyrus | 8 | 2 | 31 | 43 | 11 |
| Low psychopathy | |||||
| Acceptance rates | |||||
| R superior parietal lobule | 7, 5 | 35 | −51 | 65 | 10 |
| R parahippocampal gyrus | 30, 19 | 15 | −43 | −2 | 10 |
| L middle frontal gyrus | 9 | −49 | 16 | 29 | 10 |
| L superior frontal gyrus | 8 | −4 | 43 | 60 | 10 |
| Fairness ratings | |||||
| R inferior parietal lobule | 19, 40 | 44 | −71 | 44 | 13 |
| L superior frontal gyrus | 8 | −4 | 43 | 60 | 10 |
R = Right; L = left.
Fig. 3.
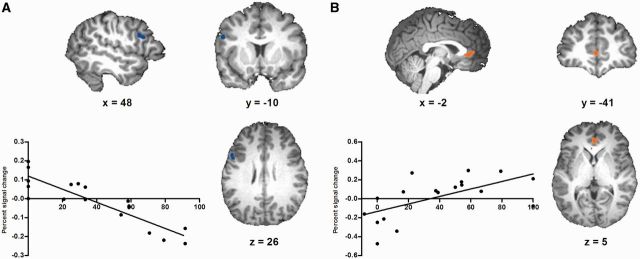
Clusters identified by whole-brain linear regression analyses for the low (A) and high (B) psychopathy group in the Unfair > Fair contrast. (A) In the low psychopathy group, difference scores of acceptance rates were negatively associated with activity in the dlPFC, such that higher activation in this region was associated with increased acceptance of unfair offers. The scatter plot depicts the association between mean percent signal changes extracted from that cluster and difference scores. (B) In the high psychopathy group, difference scores of acceptance rates were negatively associated with activity in the rostral ACC/vmPFC, such that higher activation in this region was associated with increased rejection of unfair offers. The scatter plot depicts the association between mean percent signal changes in that cluster and difference scores.
In contrast, results in high psychopathy scorers showed that acceptance rates were associated with activation in the right rostral anterior cingulate (x, y, z = 2, 42, 8), such that increased rejection of unfair offers was associated with higher activity in this cluster. In addition, fairness ratings were associated with activation in the right medial frontal gyrus (x, y, z = 2, 31, 43), with increased activity in this cluster being associated with perceptions of greater fairness (Table 4 and Figure 3). No overlapping areas were identified that corresponded to both fairness ratings and acceptance rates in high psychopathy scorers.
Additional analyses (ANOVA)
For Unfair offers, a significant effect of cognitive load was observed (P < 0.005, uncorrected, 10 voxel threshold) in a network of regions that included a cluster in the left superior extending to middle frontal gyrus (18 voxels; x, y, z = −22, 33, 59). Likewise, for Fair offers, we identified a cluster in the left superior/middle frontal gyrus (31 voxels; x, y, z = −25, 30, 59). According to previous results about the association between dlPFC activation and working memory load (Barch et al., 1997; Manoach et al., 1997), these results suggest that our manipulation was effective in increasing the task demands. No group differences were observed as a function of cognitive load.
DISCUSSION
In this study, we used fMRI to examine how psychopathic traits affect decisions in the UG. The UG provides a way of exploring how individuals weigh social and individual gain when making social decisions, thereby representing a valuable tool to investigate social decision making in psychopathy. In light of conflicting results from previous research, we formulated two sets of predictions concerning the processes recruited by higher scorers in psychopathy. To test our predictions, we incorporated two novel features into our design and analysis: (i) assessment of subjective perceptions of fairness and (ii) a cognitive load manipulation.
Our results showed that both high and low psychopathy scorers tend to reject about 50% of unfair offers. This response pattern has been consistently demonstrated in the UG literature and has been interpreted as the result of both negative emotional reactions to unfairness and motivation to actively punish the other player for his unfairness (Fehr and Gachter, 2002; Sanfey et al., 2003; de Quervain et al., 2004; Fowler et al., 2005). More importantly, we demonstrated that this response does not change when individuals are under more cognitively demanding conditions. This finding supports our second set of hypotheses, suggesting that the rejection of an unfair offer is mainly an automatic response in both groups.
Results also showed that, although high and low psychopathy scorers accepted unfair and fair offers in roughly the same proportion, subjects with higher psychopathic traits tended to perceive unfair offers as subjectively less unfair. Furthermore, as we hypothesized, in low psychopathy scorers the acceptance of unfair offers tracked closely with perceived fairness, whereas for high scorers no significant association was found. This suggests that, although both groups provided similar responses in the UG, the motivations to reject unfair offers across groups, as well as their underlying neural mechanisms, may be distinct.
In fact, as we predicted, in low psychopathy scorers the rejection of unfair offers was associated with dlPFC (BA 9) activity, with increased activation in this region being associated with higher acceptance of unfair offers. The dlPFC has been previously shown to be involved in inhibiting pre-potent responses (Suzuki et al., 2011) and making normative choices (Baumgartner et al., 2011; Steinbeis et al., 2012). This pattern of results corroborates the idea that accepting unfair offers requires cognitive control, probably to override an automatic negative reaction to the violation of a fairness norm. Moreover, regions associated with acceptance rates overlapped with regions associated with subjective fairness perceptions, further supporting the idea that in low psychopathy scorers the perceived fairness of offers guides the decision to accept or reject them.
In contrast, high psychopathy scorers recruited a cluster in the rostral anterior cingulate cortex (ACC)/vmPFC (BA 24 and 32), with higher activation in this area being associated with rejection of unfair offers. The vmPFC has been previously implicated in economic games (Rilling et al., 2007; Baumgartner et al., 2011; Suzuki et al., 2011) and is known to have an important role in emotion regulation, especially in automatic emotional regulation processes (Phillips et al., 2008). Damage in this region is associated with blunted affect and reduced tolerance to provocation or frustration (Barrash et al., 2000; Bechara, 2004; Anderson et al., 2006). Koenigs and Tranel (2007) demonstrated that vmPFC lesion patients with reported problems in emotion regulation made exaggerated irrational decisions in the UG, which was interpreted as the result of an angry reaction to the unfair treatment by another individual. A later study (Koenigs et al., 2010) showed that the performance of individuals with elevated primary psychopathy scores was similar to that of vmPFC lesion patients. These findings are in line with models of vmPFC dysfunction in psychopathy, which suggest that abnormal vmPFC function is responsible for problems in stimulus reinforcement learning (e.g. Birbaumer et al., 2005; Finger et al., 2011) and reversal learning processes (e.g. Budhani et al., 2006; Finger et al., 2008). These problems compromise the ability to adapt to changing reinforcement contingencies and to obtain the desired outcomes, thus increasing the vulnerability to frustration in psychopathic individuals and, consequently, the risk for reactive aggression (Blair, 2010). Taking these findings into account, together with reports of increased vmPFC activity when individuals expect higher donations from others (Cooper et al., 2010), we interpret the vmPFC activation observed in high psychopathy scorers as the result of the maintenance of high reward expectations throughout the game, with the conflict between such expectations and the unfairness of offers resulting in frustration and rejection. This interpretation is further supported by reports of reciprocal vmPFC and dlPFC activation in decision-making situations that vary in emotional saliency, with vmPFC being more active in ‘hot’ (emotionally salient) decisions and dlPFC in ‘cold’ (emotionally neutral) decisions (Goel and Dolan, 2003). In high psychopathy scorers it was also shown that, unlike in low scorers, no regions were involved in both acceptance rates and fairness ratings. The lack of overlap in the regions associated with UG responses and fairness perceptions further supports the hypothesis that in high psychopathy scorers UG decisions are not guided by fairness norm concerns.
Thus, in conjunction with the behavioral findings, these results lend support to the second set of hypotheses, suggesting that in high psychopathy scorers the response to unfair offers is probably not driven by a concern for fairness as normative preference for egalitarian divisions of resources, but instead may reflect an angry reaction to frustration for not obtaining the desired outcomes.
Although amygdala activity has been shown to be associated with a negative reaction to unequal divisions of resources (Haruno and Frith, 2010; Gospic et al., 2011; Osumi et al., 2012), our results did not reveal amygdala activation to be a significant predictor of rejection rates in the low psychopathy group. This is consistent with the findings of Haruno and Frith (2010), who demonstrated that in healthy participants inequity aversion is only associated with amygdala activity in highly prosocial individuals (and not in subjects with a more individualistic social orientation). Within our groups, individual differences in social value orientation were not considered and no attempt was made to recruit unusually prosocial low-psychopathy participants, which could potentially explain why no effects were obtained in brain regions previously associated with negative reactions to unfairness, such as the amygdala, in the low psychopathy group.
Our findings should be interpreted in light of the fact that the study was conducted in a community sample (which was larger than that used in either of the two previous studies addressing UG responses in psychopathy), under the assumption that psychopathic traits are continuously distributed in the population (Markon et al., 2011). Because we used a recently developed self-report measure of psychopathy, the replication of the present findings using alternative instruments would be important to confirm the reported effects. This is particularly important given the absence of group differences in acceptance rates across groups, although this finding is not surprising considering the inconsistency of previous UG results in psychopathy, and the frequency with which distinct neural socio-affective processes are observed in high and low psychopathy scorers in the absence of overt behavioral differences (e.g. Harenski et al., 2010; Sommer et al., 2010; Pujol et al., 2012). Replication will also be important in light of the fact that mean scores on the TriPM in community samples are not yet well established, such that we were unable to confirm how psychopathy scores in our sample compared with those of similar community samples.
In conclusion, this study was the first to adopt a linear regression approach in addition to the simple analysis of fMRI contrasts to directly investigate the neural mechanisms involved in economic decision making in individuals with varying psychopathic traits. Our findings may help to disambiguate previous findings by incorporating novel task features that clarify the relative influence of strategic and fairness considerations in the decisions made by each group. Our results also highlighted the role of the vmPFC in emotional regulation in social interactions, and implicate atypical functioning in this structure in the behavioral patterns of individuals with psychopathic traits. More importantly, they showed that similar behavioral responses can emerge from distinct underlying neural mechanisms, highlighting the importance of continuing to investigate the neural basis of adaptive and maladaptive social decision making in psychopathy.
FUNDING
This work was supported by a doctoral scholarship awarded to JBV (SFRH/BD/76254/2011) and a research grant (PTDC/PSI-PCO/114953/2009) from the Portuguese Foundation for Science and Technology (Fundação para a Ciência e Tecnologia).
Conflict of Interest
None declared.
Acknowledgments
This work was supported by a doctoral scholarship awarded to J.B.V. (SFRH/BD/76254/2011) and a research grant (PTDC/PSI-PCO/114953/2009) from the Portuguese Foundation for Science and Technology (Fundação para a Ciência e Tecnologia).
REFERENCES
- Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. Journal of the International Neuropsychological Society. 2006;12(2):224–35. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologia. 1997;35(10):1373–80. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Bargh JA, Ferguson MJ. Beyond behaviorism: on the automaticity of higher mental processes. Psychological Bulletin. 2000;126(6):925–45. doi: 10.1037/0033-2909.126.6.925. [DOI] [PubMed] [Google Scholar]
- Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Developmental Neuropsychology. 2000;18(3):355–81. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Tugade MM, Engle RW. Individual differences in working memory capacity and dual-process theories of the mind. Psychological Bulletin. 2004;130(4):553–73. doi: 10.1037/0033-2909.130.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Knoch D, Hotz P, Eisenegger C, Fehr E. Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nature Neuroscience. 2011;14(11):1468–74. doi: 10.1038/nn.2933. [DOI] [PubMed] [Google Scholar]
- Bechara A. Disturbances of emotion regulation after focal brain lesions. International Review of Neurobiology. 2004;62:159–93. doi: 10.1016/S0074-7742(04)62006-X. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Veit R, Lotze M, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Archives of General Psychiatry. 2005;62(7):799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Developmental Psychopathology. 2005;17(3):865–91. doi: 10.1017/S0954579405050418. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Psychopathy, frustration, and reactive aggression: the role of ventromedial prefrontal cortex. British Journal of Psychology. 2010;101(Pt 3):383–99. doi: 10.1348/000712609X418480. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Peschardt KS, Budhani S, Mitchell DG, Pine DS. The development of psychopathy. Journal of Child Psychology and Psychiatry. 2006;47(3–4):262–76. doi: 10.1111/j.1469-7610.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- Budhani S, Richell RA, Blair RJ. Impaired reversal but intact acquisition: probabilistic response reversal deficits in adult individuals with psychopathy. Journal of Abnormal Psychology. 2006;115(3):552–8. doi: 10.1037/0021-843X.115.3.552. [DOI] [PubMed] [Google Scholar]
- Camerer CF. Psychology and economics. Strategizing in the brain. Science. 2003;300(5626):1673–5. doi: 10.1126/science.1086215. [DOI] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Giampietro V, Rubia K. Right ventromedial and dorsolateral prefrontal cortices mediate adaptive decisions under ambiguity by integrating choice utility and outcome evaluation. Journal of Neuroscience. 2009;29(35):11020–8. doi: 10.1523/JNEUROSCI.1279-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cooper JC, Kreps TA, Wiebe T, Pirkl T, Knutson B. When giving is good: ventromedial prefrontal cortex activation for others' intentions. Neuron. 2010;67(3):511–21. doi: 10.1016/j.neuron.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crescentini C, Seyed-Allaei S, De Pisapia N, Jovicich J, Amati D, Shallice T. Mechanisms of rule acquisition and rule following in inductive reasoning. Journal of Neuroscience. 2011;31(21):7763–74. doi: 10.1523/JNEUROSCI.4579-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Tabibnia G, Lieberman MD, Robbins TW. Serotonin modulates behavioral reactions to unfairness. Science. 2008;320(5884):1739. doi: 10.1126/science.1155577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira-Souza R, Hare RD, Bramati IE, et al. Psychopathy as a disorder of the moral brain: fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage. 2008;40(3):1202–13. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Fischbacher U, Treyer V, et al. The neural basis of altruistic punishment. Science. 2004;305(5688):1254–8. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- Ermer E, Kiehl KA. Psychopaths are impaired in social exchange and precautionary reasoning. Psychological Science. 2010;21(10):1399–405. doi: 10.1177/0956797610384148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. Dual-processing accounts of reasoning, judgment and social cognition. Annual Review of Psychology. 2008;59:255–78. doi: 10.1146/annurev.psych.59.103006.093629. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ, Goodyer IM. Brain structure abnormalities in adolescent girls with conduct disorder. Journal of Child Psychology and Psychiatry. 2013;54(1):86–95. doi: 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr E, Gachter S. Altruistic punishment in humans. Nature. 2002;415(6868):137–40. doi: 10.1038/415137a. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Blair KS, et al. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. American Journal of Psychiatry. 2011;168(2):152–62. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Mitchell DG, et al. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Archives of General Psychiatry. 2008;65(5):586–94. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler JH, Johnson T, Smirnov O. Human behaviour: egalitarian motive and altruistic punishment. Nature. 2005;433(7021):1. doi: 10.1038/nature03256. p following 32; discussion following 32. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Implicit and explicit processes in social cognition. Neuron. 2008;60(3):503–10. doi: 10.1016/j.neuron.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Raine A, Schug RA. The neural correlates of moral decision-making in psychopathy. Molecular Psychiatry. 2009;14(1):5–6. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Reciprocal neural response within lateral and ventral medial prefrontal cortex during hot and cold reasoning. Neuroimage. 2003;20(4):2314–21. doi: 10.1016/j.neuroimage.2003.07.027. [DOI] [PubMed] [Google Scholar]
- Gordon HL, Baird AA, End A. Functional differences among those high and low on a trait measure of psychopathy. Biological Psychiatry. 2004;56(7):516–21. doi: 10.1016/j.biopsych.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Gospic K, Mohlin E, Fransson P, Petrovic P, Johannesson M, Ingvar M. Limbic justice—amygdala involvement in immediate rejection in the ultimatum game. PLoS Biology. 2011;9(5):e1001054. doi: 10.1371/journal.pbio.1001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JD, Morelli SA, Lowenberg K, Nystrom LE, Cohen JD. Cognitive load selectively interferes with utilitarian moral judgment. Cognition. 2008;107(3):1144–54. doi: 10.1016/j.cognition.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist-Revised. Toronto, Ontario: Multi-Health Systems; 1991. [Google Scholar]
- Harenski CL, Harenski KA, Shane MS, Kiehl KA. Aberrant neural processing of moral violations in criminal psychopaths. Journal of Abnormal Psychology. 2010;119(4):863–74. doi: 10.1037/a0020979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harle KM, Sanfey AG. Social economic decision-making across the lifespan: an fMRI investigation. Neuropsychologia. 2012;50(7):1416–24. doi: 10.1016/j.neuropsychologia.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno M, Frith CD. Activity in the amygdala elicited by unfair divisions predicts social value orientation. Nature Neuroscience. 2010;13(2):160–1. doi: 10.1038/nn.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intrator J, Hare R, Stritzke P, et al. A brain imaging (single photon emission computerized tomography) study of semantic and affective processing in psychopaths. Biological Psychiatry. 1997;42(2):96–103. doi: 10.1016/S0006-3223(96)00290-9. [DOI] [PubMed] [Google Scholar]
- Kaufman AS. K-BIT: Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, et al. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry. 2001;50(9):677–84. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Kruepke M, Newman JP. Economic decision-making in psychopathy: a comparison with ventromedial prefrontal lesion patients. Neuropsychologia. 2010;48(7):2198–204. doi: 10.1016/j.neuropsychologia.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the ultimatum game. Journal of Neuroscience. 2007;27(4):951–6. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner O, Dotsch R, Bijlstra G, Wigboldus DHJ, Hawk ST, van Knippenberg A. Presentation and validation of the Radboud Faces Database. Cognition and Emotion. 2010;24(8):1377–88. [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4(4):423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Schlaug G, Siewert B, et al. Prefrontal cortex fMRI signal changes are correlated with working memory load. Neuroreport. 1997;8(2):545–9. doi: 10.1097/00001756-199701200-00033. [DOI] [PubMed] [Google Scholar]
- Marion BE, Sellbom M, Salekin RT, Toomey JA, Kucharski LT, Duncan S. An examination of the association between psychopathy and dissimulation using the MMPI-2-RF validity scales. Law and Human Behavior. 2012 doi: 10.1037/lhb0000008. [DOI] [PubMed] [Google Scholar]
- Markon KE, Chmielewski M, Miller CJ. The reliability and validity of discrete and continuous measures of psychopathology: a quantitative review. Psychological Bulletin. 2011;137(5):856–79. doi: 10.1037/a0023678. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Cardinale EM. When psychopathy impairs moral judgments: neural responses during judgments about causing fear. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nss097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, et al. Reduced amygdala-orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Research. 2011;194(3):279–86. doi: 10.1016/j.pscychresns.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokros A, Menner B, Eisenbarth H, Alpers GW, Lange KW, Osterheider M. Diminished cooperativeness of psychopaths in a prisoner's dilemma game yields higher rewards. Journal of Abnormal Psychology. 2008;117(2):406–13. doi: 10.1037/0021-843X.117.2.406. [DOI] [PubMed] [Google Scholar]
- Oosterbeek H, Randolf S, Van De Kuilen G. Cultural differences in ultimatum game experiments: evidence from a meta-analysis. Experimental Economics. 2004;7:171–88. [Google Scholar]
- Osumi T, Nakao T, Kasuya Y, Shinoda J, Yamada J, Ohira H. Amygdala dysfunction attenuates frustration-induced aggression in psychopathic individuals in a non-criminal population. Journal of Affective Disorders. 2012;142(1–3):331–8. doi: 10.1016/j.jad.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Osumi T, Ohira H. The positive side of psychopathy: emotional detachment in psychopathy and rational decision-making in the ultimatum game. Personality and Individual Differences. 2010;49:451–6. [Google Scholar]
- Patrick CJ. Florida State University; 2010. Operationalizing the Triarchic Conceptualization of Psychopathy: Preliminary Description of Brief Scales for Assessment of Boldness, Meanness, and Disinhibition. [Google Scholar]
- Patrick CJ, Fowles DC, Krueger RF. Triarchic conceptualization of psychopathy: developmental origins of disinhibition, boldness, and meanness. Developmental Psychopathology. 2009;21(3):913–38. doi: 10.1017/S0954579409000492. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13(9):829, 833–57. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Batalla I, Contreras-Rodriguez O, et al. Breakdown in the brain network subserving moral judgment in criminal psychopathy. Social Cognitive and Affective Neuroscience. 2012;7(8):917–23. doi: 10.1093/scan/nsr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J, Glenn A, Jairam M, et al. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biological Psychiatry. 2007;61(11):1260–71. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the ultimatum game. Science. 2003;300(5626):1755–8. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Schulz JF, Fischbacher U, Thöni C, Utikal V. Affect and fairness: dictator games under cognitive load. Journal of Economic Psychology. 2012 doi:10.1016/j.joep.2012.08.007. [Google Scholar]
- Seidman LJ, Yurgelun-Todd D, Kremen WS, et al. Relationship of prefrontal and temporal lobe MRI measures to neuropsychological performance in chronic schizophrenia. Biological Psychiatry. 1994;35(4):235–46. doi: 10.1016/0006-3223(94)91254-8. [DOI] [PubMed] [Google Scholar]
- Sellbom M, Phillips TR. An examination of the triarchic conceptualization of psychopathy in incarcerated and nonincarcerated samples. Journal of Abnormal Psychology. 2013;122(1):208–14. doi: 10.1037/a0029306. [DOI] [PubMed] [Google Scholar]
- Skeem JL, Polaschek DLL, Patrick CJ, Lilienfeld SO. Psychopathic personality: bridging the gap between scientific evidence and public policy. Psychological Science in the Public Interest. 2011;12:95–162. doi: 10.1177/1529100611426706. [DOI] [PubMed] [Google Scholar]
- Sommer M, Sodian B, Dohnel K, Schwerdtner J, Meinhardt J, Hajak G. In psychopathic patients emotion attribution modulates activity in outcome-related brain areas. Psychiatry Research. 2010;182(2):88–95. doi: 10.1016/j.pscychresns.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Stanley JH, Wygant DB, Sellbom M. Elaborating on the construct validity of the triarchic psychopathy measure in a criminal offender sample. Journal of Personality Assessment. 2013;95(4):343–50. doi: 10.1080/00223891.2012.735302. [DOI] [PubMed] [Google Scholar]
- Steinbeis N, Bernhardt BC, Singer T. Impulse control and underlying functions of the left DLPFC mediate age-related and age-independent individual differences in strategic social behavior. Neuron. 2012;73(5):1040–51. doi: 10.1016/j.neuron.2011.12.027. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Niki K, Fujisaki S, Akiyama E. Neural basis of conditional cooperation. Social Cognitive and Affective Neuroscience. 2011;6(3):338–47. doi: 10.1093/scan/nsq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme; 1988. [Google Scholar]
- Van der Veen FM, Sahibdin PP. Dissociation between medial frontal negativity and cardiac responses in the ultimatum game: effects of offer size and fairness. Cognitive, Affective & Behavioral Neuroscience. 2011;11(4):516–25. doi: 10.3758/s13415-011-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit R, Lotze M, Sewing S, Missenhardt H, Gaber T, Birbaumer N. Aberrant social and cerebral responding in a competitive reaction time paradigm in criminal psychopaths. Neuroimage. 2010;49(4):3365–72. doi: 10.1016/j.neuroimage.2009.11.040. [DOI] [PubMed] [Google Scholar]
- White SF, Marsh AA, Fowler KA, et al. Reduced amygdala response in youths with disruptive behavior disorders and psychopathic traits: decreased emotional response versus increased top-down attention to nonemotional features. American Journal of Psychiatry. 2012;169(7):750–8. doi: 10.1176/appi.ajp.2012.11081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS. A methodology for studying noninstitutionalized psychopaths. Journal of Consulting and Clinical Psychology. 1977;45(4):674–83. [PubMed] [Google Scholar]
- Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Morphological alterations in the prefrontal cortex and the amygdala in unsuccessful psychopaths. Journal of Abnormal Psychology. 2011;119(3):546–54. doi: 10.1037/a0019611. [DOI] [PubMed] [Google Scholar]