Abstract
While abnormal processing of performance feedback has been associated with obsessive–compulsive disorder (OCD), neural responses to different kinds of feedback information, especially to ambiguous feedback are widely unknown. Using fMRI and a performance adaptive time-estimation task, we acquired blood oxygenation level-dependant responses and emotional ratings to positive, negative and ambiguous performance feedback in patients and healthy controls. Negative and ambiguous feedback led to increased levels of anxiety, guilt and shame in patients. Both negative and ambiguous feedback, as compared to positive feedback, induced increased activation of the insular cortex in patients. Furthermore, patients showed no differential activation to negative feedback in the putamen and to ambiguous feedback in the ventromedial prefrontal cortex (VMPFC). Finally, negative feedback induced increased activation in the midcingulate cortex in patients compared to controls. Findings indicate that both negative and ambiguous performance feedbacks are associated with abnormal negative emotions and altered brain activation, in particular increased insula activation, while activation in the putamen and VMPFC does not differentiate between feedback types in OCD patients. This suggests a parallel pattern of increased and decreased neural sensitivity to different kinds of feedback information and a general emotional hyperresponsivity to negative and ambiguous performance feedback in OCD.
Keywords: obsessive–compulsive disorder, neuroimaging, feedback processing, emotion, performance monitoring, ambiguity
INTRODUCTION
Obsessive–compulsive disorder (OCD) is a severe psychopathological disorder with an estimated lifetime prevalence of 2.3% (Ruscio et al., 2010) which is characterized by the presence of recurrent obsessions and/or compulsions. Previous work suggests that OCD is associated with hyperactive performance monitoring (Pitman, 1987) and symptoms of OCD seem to be maintained by patients’ dysfunctional appraisals of the outcomes of their own actions (Salkovskis et al., 1999; Coles et al., 2005). In particular, patients are characterized by inflated responsibility, intolerance of uncertainty and perfectionism, including excessive concerns over mistakes (Salkovskis et al., 1999). The associated feelings of incompleteness or imperfection represent a major source of distress in OCD (Coles et al., 2005). Thus, negative performance outcomes might be associated with enhanced negative emotions such as anxiety, guilt, shame and uncertainty, which are thought to contribute to OCD symptoms (Fergus et al., 2010), although a potential link between performance outcomes and emotional responses in OCD patients has not been experimentally investigated yet. Furthermore, pathological beliefs about the tolerability of doubt and uncertainty are thought to contribute to the development and maintenance of OCD (Steketee et al., 1997). Since uncertainty has been conceptualized as an emotional reaction elicited by ambiguous stimuli (Grenier et al., 2005), the delivery of ambiguous performance feedback should in particular represent an aversive situation for OCD patients, in whom a postulated ‘just not right’ experience might be amplified when ambiguous feedback is given (Coles et al., 2005). Even though emotional responsiveness to uncertain and negative feedback might be increased in OCD, this may not be necessarily associated with increased learning from negative feedback (Nielen et al., 2009) suggesting a dissociation between emotional responses to feedback and utilization of feedback signals for adaptive responses.
Efforts have been made to reveal the functional neuroanatomy underlying OCD based on different cognitive or symptom provocation paradigms (Menzies et al., 2008; Rotge et al., 2008). According to influential models (Graybiel and Rauch, 2000), findings provide some evidence for altered activations in fronto-striatal loops (Menzies et al., 2008). Despite strong arguments for a role of altered processing of negative and ambiguous feedback in the pathophysiology of OCD, no neuroimaging studies have—to the best of our knowledge—investigated the processing of ambiguous feedback in OCD. Furthermore, no imaging studies have explored the processing of feedback valence in OCD outside the context of monetary incentives (Remijnse et al., 2006; Figee et al., 2011; Jung et al., 2011) with the latter studies yielding only partially consistent results. Remijnse et al. (Remijnse et al., 2006) explored brain activation of OCD patients during a reversal task with monetary reward and loss feedback and showed attenuated activation of orbitofrontal cortex (OFC) and caudate nucleus in OCD patients to reward feedback. During loss feedback, however, no differential brain activation was observed. More recent studies reported diminished activation of the nucleus accumbens during reward anticipation and of medial frontal cortex during reward delivery in patients (Figee et al., 2011), and higher insular activation during loss anticipation relative to neutral anticipation (Jung et al., 2011). None of these studies reported activation of the cingulate cortices, which are typically involved in error and conflict processing in OCD (Saxena et al., 2009).
In the current study we investigated emotional and brain responses to different categories of performance feedback in OCD relative to healthy controls. We used an adaptive time-estimation task which incorporated balanced feedback on correct and incorrect estimation performance (Miltner et al., 1997), as well as a third category during which ambiguous performance feedback was presented. As dysfunctions in orbitofrontal, cingulate and striatal regions in OCD patients have been consistently reported (Menzies et al., 2008), we expected alterations in feedback processing to be primarily reflected in altered engagement of these structures in patients. Furthermore, we hypothesized that negative as well as ambiguous feedback should be associated with amplified emotional responses and increased brain activation in areas suggested to be involved in negative affect and performance monitoring.
MATERIALS AND METHODS
Participants
Data were analysed from 24 patients with a DSM-IV diagnosis of OCD (15 females) and 24 healthy control subjects (13 females), who were closely matched for age, gender, education and general intelligence (Table 1). The current diagnostic status of OCD patients was assessed by the structured clinical interview for DSM-IV (SCID; (Wittchen et al., 1996)), the mini international neuropsychiatric inventory (MINI; (Sheehan et al., 1998)), the Yale–Brown obsessive compulsive scale (Y-BOCS; (Goodman et al., 1989)) and the obsessive compulsive inventory-revised (OCI-R; German version: (Gonner et al., 2008)). Diagnostic interviews were conducted by an experienced clinical psychologist. All subjects were recruited from local clinical and community populations. Patients were excluded for current comorbid episodes of major depression and neurological disorders. Eleven patients had been diagnosed with one or more comorbid Axis I disorder in the past. These Axis I disorders included depressive episodes (n = 7), anorexia (n = 1) and panic disorder (n = 3). One Patient had an additional diagnosis of histrionic and borderline disorder. Seven patients had received pharmaceutical treatment—four subjects were medicated with SSRIs, two subjects were medicated with SNRIs and one subject was medicated with a tricyclic antidepressant. Six of these seven patients also received cognitive-behavioral therapy (CBT). Moreover, seven patients had received CBT but no pharmacological treatment. The study was approved by the Ethics Committee of the University of Jena.
Table 1.
Sample characteristics of OCD patients and control subjects
| OCD patients |
Control subjects |
Group differences | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 28.1 | 8.1 | 25.8 | 6.2 | t(43.19) = −1.08; P = 0.29 |
| Illness duration (years) | 8.52 | 6.3 | |||
| Estimated intelligencea | 109b | 9.4 | 114c | 13.5 | t(37.29) = −1.65; P = 0.11 |
| Y-BOCS | 19.0 | 6.8 | |||
| OCI-R total | 34.7 | 11.9 | 7.0 b | 4.7 | t(29.97) = −10.6; P < 0.01 |
| OCI-R washing | 4.1 | 3.0 | 0.5 | 1.1 | t(28.72) = −5.53; P < 0.01 |
| OCI-R checking | 7.2 | 3.4 | 0.9 | 1.7 | t(33.46) = −8.02; P < 0.01 |
| OCI-R obsessing | 7.5 | 3.0 | 1.3 | 1.5 | t(34.01) = −9.21; P < 0.01 |
| OCI-R hoarding | 4.2 | 3.7 | 2.5 | 1.7 | t(32.58) = −2.06; P < 0.01 |
| OCI-R neutralizing | 5.3 | 4.0 | 0.6 | 1.0 | t(25.91) = −5.54; P < 0.01 |
| OCI-R ordering | 6.4 | 3.9 | 1.2 | 1.4 | t(28.66) = −6.15; P < 0.01 |
| HAM-Dd | 5.9 b | 3.2 | |||
| N | % | N | % | ||
| Gender | |||||
| Females | 15 | 62.5 | 13 | 54.2 | |
| Males | 9 | 37.5 | 11 | 45.8 | |
| Educatione | 1:8:12:3 | 0:2:16:6 | χ2(1) = 0.34; P = 0.56 | ||
| Medication, Number | 7 | 29.2 | 0 | 0 | |
aMultiple Choice Vocabulary Test (MWT; Forms A and B).
bData from one subject are missing.
cData from two subjects are missing.
dHamilton Rating Scale for Depression.
e9 years : 10 years : 12 years : >12 years
Stimuli and task
The time-estimation task required participants to estimate an interval of 1 s as accurately as possible. An auditory cue of 50 ms duration marked the onset of each time-estimation trial. Participants were required to press a button with their right index finger just when they thought that 1 s had elapsed. During auditory cue presentation and for the following 4200–7600 ms (depending on a random SOA; see below), a central white fixation cross on a black background was presented. Performance feedback was administered visually after offset of fixation cross presentation and consisted of either of the letters A, B or C, which were presented for 1 s, respectively (Figure 1). Each of these letters was assigned to one of three feedback classes: it either indicated a correct response, a false response or did not convey any unambiguous information regarding estimation success. The specific assignment of letters to conditions was balanced across subjects in order to control for specific effects of visually presented feedback stimuli which were projected onto a screen inside the scanner bore.
Fig. 1.
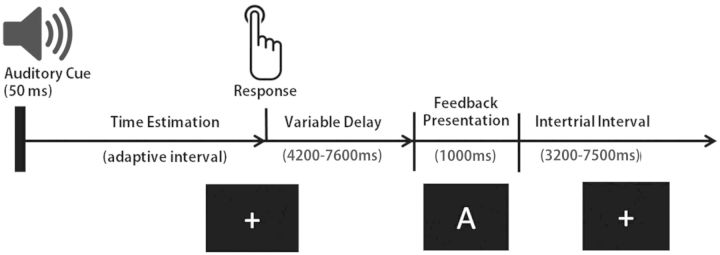
Schematic representation of the sequence of trial events.
In order to decorrelate response- and stimulus-related activation patterns, stimulus onsets were systematically jittered. Specifically, intervals between button presses and feedback presentation were offset within a range of 4200–7600 ms; intertrial intervals were offset within a range of 3200–7500 ms. Furthermore, a sliding response window algorithm was used to balance the relative presentation frequency of the three different feedback conditions. This is an important factor in the time-estimation task as it prevents subjects from building up strong expectations about the information conveyed by feedback. That is, because the local probability of a quickly learned behavioral response being sufficiently accurate is adapted trial-wise, depending on the individual’s accumulated performance level, habit formation is unlikely to take place quickly. Thus, the response situation remains underdetermined throughout the experiment and feedback presentation is needed in order to clarify the response outcome.
Prior to the experiment, subjects were trained on the task and successively completed 2 runs of 66 estimation trials, respectively. Post-experimentally, subjects rated their subjective experiences during feedback evaluation on several 9-point Likert scales (1 = ‘not at all’, 9 = ‘very much’) accounting for feelings of anxiety, guilt, shame and uncertainty; in one control and one OCD patient, these rating scores are missing due to a technical error. Further, subjects were asked to reproduce the correct assignment of letters to feedback conditions. Behavioral data were analysed by means of repeated measures ANOVAs and MANOVAs using SPSS software (version 19; SPSSInc., Chicago, IL) or by the non-parametric Mann–Whitney-U statistic when requirements of a parametric analysis were not fulfilled.
fMRI data acquisition, pre-processing and analysis
Functional magnetic resonance data were acquired at 3 T on a Siemens Magnetom Trio (Siemens, Medical Systems, Erlangen, Germany) using a standard 12-channel Siemens Head Matrix Coil. Two runs of 376 volumes, each consisting of 35 slices (slice thickness = 3 mm; interslice gap = 0.50 mm; in-plane resolution = 3 × 3 mm2) were recorded by means of a T2*-weighted gradient-echo, echoplanar sequence with a repetition time (TR) of 2300 ms, an echo time (TE) of 30 ms and a flip angle (FA) of 90°, yielding a data matrix of 64 × 64 voxels within a field of view (FOV) of 192 mm. Acquisition orientation was obliquely tilted ∼30° relative to the anterior commissure-posterior commissure line (Deichmann et al., 2003). Additionally, a T1-weighted MPRAGE structural volume was recorded for anatomical localization and a shimming field was applied prior to functional imaging.
Pre-processing and analysis of the functional data were performed using Brain Voyager QX software (BVQX 1.10 and 2.3; Brain Innovation B. V., Maastricht, The Netherlands). The first four volumes of each run were discarded as dummies in order to ensure steady state tissue magnetization. Realignment to the first volume of each run was performed via least squares estimation of six rigid body parameters in order to reduce effects of head movements on volume time course analysis. Further data pre-processing comprised a correction for slice time errors and spatial (8 mm full-width at half-maximum [FWHM] isotropic Gaussian kernel) as well as temporal (high pass filter: 8 cycles per run; low pass filter: 2.8 s FWHM; Linear Trend Removal) smoothing. Anatomical and functional images were co-registered and normalized to the Talairach space (Talairach and Tournoux, 1988).
The statistical analysis of fMRI data was based on the general linear model (GLM) with adjustment for autocorrelation following a global AR(1) model. The stimulation protocols comprised predictors for auditory cue presentations, positive, negative and ambiguous feedback, as well as missed trials. Based on these protocols, reference functions were derived. The expected blood oxygenation level dependent (BOLD) signal change for each predictor was modeled by convolving these reference functions with a 2-gamma hemodynamic response function to account for the delayed onset and typical shape of the BOLD signal time course. Beta-weights for the random-effects group GLM were based on z-standardized time course data and determined by a least squares estimation. The main focus of analysis was on a priori defined feedback-relevant Regions of Interest (ROIs) which included the insula, the midcingulate cortex (MCC), ventral and dorsal striatum and OFC and ventromedial prefrontal cortex (VMPFC) (Nieuwenhuis et al., 2005). Based on these regions’ Talairach coordinates as provided by Talairach Client software (Lancaster et al., 2000) search regions were defined. A cluster-size threshold estimation procedure was used (Goebel et al., 2006) to correct for multiple comparisons within these search regions. Significant clusters of contiguously activated voxels within these search regions were determined by a Monte Carlo simulation based on 1000 iterations. After setting the voxel-level false positive rate to P < 0.005 (uncorrected), and specifying the FWHM of the spatial filter, the simulation resulted in a minimum cluster size of contiguously activated voxels corresponding to a false positive rate of 5%.
RESULTS
Behavioral
On average, participants received 31.4% positive (SEM: +/−0.6%), 33.7% negative (SEM: +/−0.5%) and 32.2% ambiguous (SEM: +/−0.4%) feedback. There were no significant between-group differences as to the number of presented feedbacks in any condition (Roy’s Largest Root = 0.13; F[3,44] = 1.901; P = 0.143) nor did any significant within-subjects differences emerge for presentation frequencies of positive (F[1,46] = 2.172; P = 0.147), negative (F[1,46] = 1.596; P = 0.213), or ambiguous feedback (F[1,46] = 0.889; P = 0.351).
Across all subjects, the mean range of the time interval within which a response was regarded as correct was 338 ms (SEM: 17.8); even though there was a tendency for longer intervals in OCD patients (OCD: M = 367 ms, SEM = 30.7; Controls: M = 309 ms, SEM = 16.7), the group difference was not significant (t46 = 1.650, P = 0.106). More importantly, the average number of violated response deadlines (35.2 across all subjects) did not differ significantly between the groups (t46 = 1.570, P = 0.125). All of these findings strongly imply that the sliding time window algorithm effectively balanced the presentation frequency of the different feedback conditions by adjusting the window’s width.
Self-reports
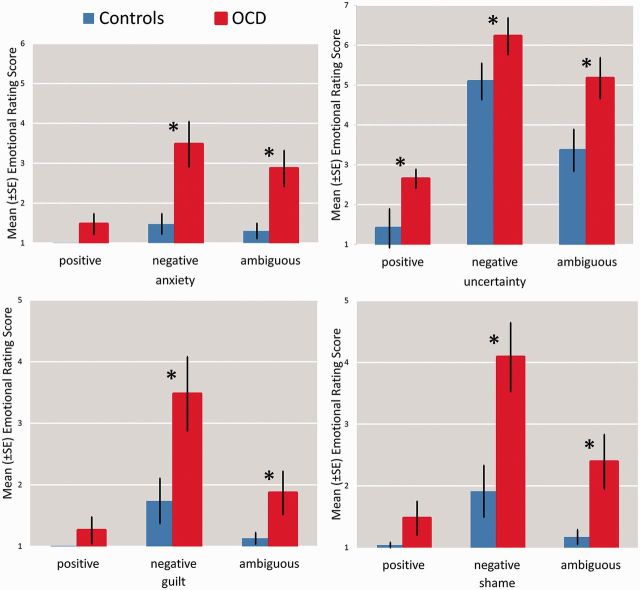
All subjects were able to reproduce the assignment of letters to feedback conditions correctly. Analyses of self-report scores by means of a repeated measures ANOVA revealed a main effect of group for uncertainty (F[1,43] = 9.40; P < 0.05), independent of feedback category in OCD patients (Figure 2). According to the Mann–Whitney-U test, anxiety (negative: Z = −3.44; P < 0.05; ambiguous: Z = −2.94; P < 0.05), guilt (negative: Z = −3.00; P < 0.05; ambiguous: Z = −2.16; P < 0.05) and shame (negative: Z = −3.09; P < 0.05; ambiguous: Z = −2.70; P < 0.05) ratings differed between groups for negative and ambiguous feedback, but not for positive feedback (Figure 2).
Fig. 2.
Post-experimental ratings of anxiety, uncertainty, shame and guilt in OCD patients and controls for positive, negative and ambiguous feedback.
Neuroimaging between-group differences
Negative vs positive
Analysis of brain activation revealed several significant clusters in OCD patients relative to controls for the contrast negative > positive feedback (Table 2). There were significant activations in the right posterior putamen and bilaterally in insular cortex as well as MCC. The effect in the putamen was due to the fact that positive and negative feedback did not induce different activations in patients (P > 0.05, corr.), while controls showed relatively stronger activation to positive as compared to negative feedback (P < 0.05, corr.) (Figure 3). Furthermore, the clusters within the right and left insula reflected amplified activation to negative feedback in OCD patients (Figure 3). In addition, a cluster within the MCC also resulted from patients’ elevated activation in response to negative feedback as compared to positive feedback, while controls showed the opposite trend (Figure 3). There was no increased activation in controls compared to patients for this contrast.
Table 2.
Significant between-groups differences in activation for the three contrasts of interest
| Talairach coordinates |
Talairach coordinates |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Lateralization | x | y | z | T scores | Cluster size (mm3) | x | y | z | T scores | Cluster size (mm3) |
| OCD > Controls | Controls > OCD | ||||||||||
| Negative feedback > positive feedback | |||||||||||
| Insula | R | 32 | −11 | 16 | 3.57 | 2538 | |||||
| L | −39 | −10 | 22 | 3.41 | 243 | ||||||
| Putamen/claustrum | R | 32 | −4 | 5 | 5.16 | 1431 | |||||
| Midcingulate cortex | R | 13 | 9 | 32 | 3.37 | 378 | |||||
| Ambiguous feedback > Positive feedback | |||||||||||
| Insula | R | 39 | −1 | 7 | 2.84 | 135 | |||||
| L | −41 | −7 | 20 | 3.01 | 216 | ||||||
| Ventromedial prefrontal cortex | L/R | 0 | 65 | −2 | 3.27 | 405 | |||||
| Ambiguous feedback > Negative feedback | |||||||||||
| Ventromedial prefrontal cortex | L | −3 | 62 | −2 | 3.28 | 2457 | |||||
| Putamen/claustrum | R | 24 | −10 | −3 | 4.28 | 189 | |||||
Fig. 3.
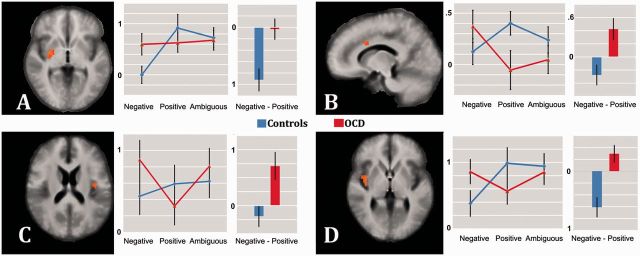
Between-groups differences in activation to negative relative to positive feedback. Significant activation differences between OCD and controls in putamen (A; z = 1), MCC (B; x = 10), left (C; z = 4) and right insula (D; z = 17) are shown. Images are in radiological convention. OCD (red) and control group (blue) parameter estimates are shown group-wise for each condition (left) and contrast with ordinates’ scales indicating parameter estimates.
Ambiguous vs positive
For the contrast ambiguous > positive feedback, the insular cortex showed significantly stronger bilateral activation in OCD patients relative to controls. Between-group activation differences in the left insular cortex were due to increased activation to ambiguous feedback, an effect which was similar to findings for negative vs positive feedback (Figure 4; Table 2).
Fig. 4.
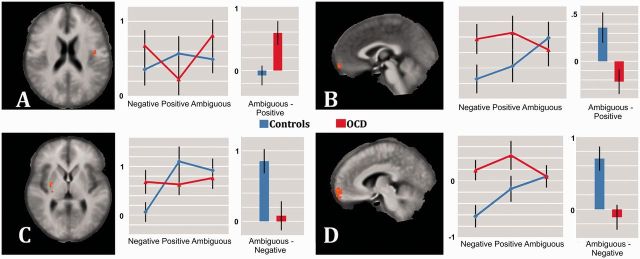
Between-groups differences in activation to ambiguous relative to negative feedback and ambiguous relative to positive feedback. Significant activation differences between OCD and controls in left insula (A; z = 20), vmPFC (B; x = −1; and D; x = −6) and putamen (C; z = 5) are shown. Images are in radiological convention. OCD (red) and control group (blue) parameter estimates are shown group-wise for each condition (left) and contrast with ordinates’ scales indicating parameter estimates.
Controls showed for this contrast increased activation in VMPFC as compared to patients. This was due to increased activation to ambiguous as compared to positive feedback in controls (P < 0.05, corr.) and lack of a significantly differential response in patients (P > 0.05, corr.).
Ambiguous vs negative
The contrast ambiguous feedback > negative feedback yielded no areas with significantly increased activation in patients as compared to controls. However, controls showed increased responses within VMPFC and in the right putamen. Both clusters reflected reduced differential activation to ambiguous vs negative feedback in patients, while controls showed generally amplified activation to ambiguous vs negative feedback (Figure 4; Table 2).
We found no differences between medicated and unmedicated patients for any of the peak effects described above, except for the putamen cluster identified by the contrast ambiguous > negative feedback. At this location, unmedicated patients showed increased activation compared to medicated patients (P < 0.05, corr.). Further, we used criterion cut-off scores presented by Gonner et al. (2009) for the six OCI-R subscales (see also Table 1) in order to divide OCD patients in subgroups according to their main symptom domains and tested the main contrasts as described above for differences between these subgroups within our ROIs. No differences between these subgroups could be obtained from the resulting volume maps fully corrected for the number of tests performed.
DISCUSSION
We investigated emotional responses and brain activation to positive and negative valid as well as ambiguous performance feedback in OCD patients and healthy control subjects. Negative and ambiguous feedback led to increased ratings of anxiety, guilt and shame in patients as compared to controls. Both negative and ambiguous feedback relative to positive feedback induced increased activation in the insular cortex in OCD. Furthermore, negative vs positive feedback was associated with increased brain activation in MCC. In contrast to healthy control subjects, patients showed no differential activation to negative vs positive feedback in the putamen and to ambiguous vs valid feedback in the VMPFC.
The emotional rating data suggest that OCD patients exhibit increased aversive emotional responses to negative as well as to ambiguous feedback. Moreover, this rating pattern was not only found for anxiety but also for shame and guilt ratings, emotions whose role in the etiology of OCD has repeatedly been emphasized. Specifically, feelings of guilt (Mancini and Gangemi, 2004) and shame (Fergus et al., 2010) have been strongly linked to a pathological inflation of responsibility obsessions. In OCD, dysfunctional beliefs about uncertainty have been hypothesized as one of several contributing factors in the pathogenesis of anxiety and indecisiveness (Salkovskis et al., 1999; Coles et al., 2005). Cognitions in OCD are at least in part characterized by patients’ heightened insecurity and excruciating doubt about the implications of self-appraised and usually egodystonic thought-contents. In consequence, those individuals are likely to develop cognitive styles within which ambiguity, ambivalence and unpredictability appear threatening and unbearable and call for immediate resolution by application of lucid rules.
Paralleling the patterns of emotional ratings, we detected increased insular cortex activation during negative and ambiguous feedback as compared to positive feedback in OCD patients. In recent years, the role of the insular cortex in homeostatic integration, interoception and emotional perception has been explored (Craig, 2009; Paulus and Stein, 2010). Activation of the insular cortex has been broadly reported in the context of negative and aversive emotional experiences and has been hypothesized to play a key role in linking belief systems to perceptions of internal states (Paulus and Stein, 2010). Consequently, functional alterations of the insula are assumed to contribute to manifestations of anxiety disorders, where valence and meaning of spontaneously fluctuating bodily signals are often misinterpreted to indicate threatening and anxiety-provoking deviations from a homeostatic equilibrium.
Furthermore, we also observed that controls showed increased activation to positive vs negative feedback in the putamen, while OCD patients showed similar activations to the different kinds of feedback. Remarkably, a coordinate-based meta-analysis of fMRI activation differences between OCD patients and controls has localized the most prominent cluster of between-group differences in BOLD-R within the putamen (Menzies et al., 2008), which seems to be in accordance with classical neural models of OCD. Next to its role in the selection, execution and evaluation of motor programs, the putamen features prominently in cognitive and motivational theories. It has been implied in the anticipation and evaluation of reward-related feedback, particularly in elicitation of a neural response to reward prediction errors (Haruno and Kawato, 2006). The putamen has been suggested to be important for integration of stimulus–action–reward associations (Haruno and Kawato, 2006) and might mediate action selection by computing and evaluating their values in terms of sensory contexts and rewards. Therefore, the lack of a differential response of the putamen in OCD patients to different kinds of feedback could indicate insensitivity to action outcomes in the context of future action selection. Accounts that activation of the putamen has been shown to track differences in the flexibility of motor learning (Schonberg et al., 2007) and appears to be involved in categorization learning (Daniel and Pollmann, 2010) lend further credence to this interpretation.
In the VMPFC we observed that healthy controls showed a clear differentiation between feedback types with highest activation to ambiguous and lowest activation to negative feedback. This differentiation was absent in OCD patients. Patients showed no difference between negative and ambiguous feedback. Next to its role in the default network (Buckner et al., 2008), the VMPFC has been implicated in a broad range of valuation paradigms (Grabenhorst and Rolls, 2011), most notably in the processing of subjective stimulus values (Hare et al., 2011), especially under ambiguity (Levy et al., 2010) or uncertainty about current expectations (Rushworth and Behrens, 2008). While the exact parcellation of the VMPFC and the respective functional roles are still being discussed (Kringelbach, 2005), studies generally report changes in specific aspects of emotional and behavioral regulation. Seminal neuropsychological findings in humans suggest that lesions of the VMPFC attenuate the contribution of internally generated goals to behavioral adaptation and make the organism more susceptible to information conveyed by external stimuli as indicated by a compulsive execution of the actions elicited by these stimuli (Bechara et al., 1994; Rudebeck et al., 2008). Located at the confluence of information coding context-specific reward properties, the VMPFC of healthy subjects would be in a key position to identify the emotional and behavioral values of stimuli which indicate an absence of action-relevant information.
A specifically increased response was also found in MCC during negative feedback relative to positive feedback in OCD. Brain imaging studies on error processing have strongly emphasized the role of the MCC in hyperactive performance monitoring in OCD (Saxena et al., 2009). Enhanced activation of this region during negative feedback presentation is in line with interpretations of increased monitoring of negative outcomes in OCD and can be assumed to be closely linked to OCD psychopathology (Schlosser et al., 2010). Thus, excessive error signals generated by MCC may explain OCD patients’ characteristic feeling that something is wrong and that a certain behavioral change is needed to correct the problem. As a consequence, exaggerated MCC activation in response to negative feedback or error commission may constitute the neural basis of a wide range of compulsive behaviors (e.g. repeated checking or exaggerated fear of committing an error).
In conclusion, we found significant alterations in the functional neuroanatomy of OCD patients during feedback processing. Findings indicate that both negative and ambiguous performance feedbacks are associated with abnormal negative emotions and changed brain activation, in particular increased insula activation, while activation in the putamen and VMPFC lacks the normal differential responses depending on feedback type in OCD patients.
Conflict of Interest
None declared.
Acknowledgments
The authors report no financial relationships with commercial interests. The study was supported by the German Federal Ministry of Education and Research (grant 01GW0740 to W.H.R.M., T.S. and R.S.).
REFERENCES
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1-3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Coles ME, Heimberg RG, Frost RO, Steketee G. Not just right experiences and obsessive-compulsive features: experimental and self-monitoring perspectives. Behaviour Research and Therapy. 2005;43(2):153–67. doi: 10.1016/j.brat.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Daniel R, Pollmann S. Comparing the neural basis of monetary reward and cognitive feedback during information-integration category learning. Journal of Neuroscience. 2010;30(1):47–55. doi: 10.1523/JNEUROSCI.2205-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19(2 Pt 1):430–41. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Fergus TA, Valentiner DP, McGrath PB, Jencius S. Shame- and guilt-proneness: relationships with anxiety disorder symptoms in a clinical sample. Journal of Anxiety Disorders. 2010;24(8):811–5. doi: 10.1016/j.janxdis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Figee M, Vink M, de Geus F, Vulink N, Veltman DJ, Westenberg H, Denys D. Dysfunctional reward circuitry in obsessive-compulsive disorder. Biological Psychiatry. 2011;69(9):867–74. doi: 10.1016/j.biopsych.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping. 2006;27(5):392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonner S, Hahn S, Leonhart R, Ecker W, Limbacher K. Identification of main symptoms in OCD patients by use of symptom scales. Criterion validity and diagnostic accuracy of the OCI-R. Verhaltenstherapie. 2009;19(4):251–8. [Google Scholar]
- Gonner S, Leonhart R, Ecker W. The Obsessive-Compulsive Inventory-Revised (OCI-R): validation of the German version in a sample of patients with OCD, anxiety disorders, and depressive disorders. Journal of Anxiety Disorders. 2008;22(4):734–49. doi: 10.1016/j.janxdis.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The yale-brown obsessive compulsive scale. I. Development, use, and reliability. Archives of General Psychiatry. 1989;46(11):1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. 2000;28(2):343–7. doi: 10.1016/s0896-6273(00)00113-6. [DOI] [PubMed] [Google Scholar]
- Grenier S, Barrette AM, Ladouceur R. Intolerance of uncertainty and intolerance of ambiguity: similarities and differences. Personality and Individual Differences. 2005;39(3):593–600. [Google Scholar]
- Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. Journal of Neuroscience. 2011;31(30):11077–87. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno M, Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. Journal of Neurophysiology. 2006;95(2):948–59. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- Jung WH, Kang DH, Han JY, Jang JH, Gu BM, Choi JS, Jung MH, Choi CH, Kwon JS. Aberrant ventral striatal responses during incentive processing in unmedicated patients with obsessive-compulsive disorder. Acta Psychiatrica Scandinavica. 2011;123(5):376–86. doi: 10.1111/j.1600-0447.2010.01659.x. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10(3):120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Snell J, Nelson AJ, Rustichini A, Glimcher PW. Neural representation of subjective value under risk and ambiguity. Journal of Neurophysiology. 2010;103(2):1036–47. doi: 10.1152/jn.00853.2009. [DOI] [PubMed] [Google Scholar]
- Mancini F, Gangemi A. Fear of guilt from behaving irresponsibly in obsessive–compulsive disorder. Journal of Behavior Therapy and Experimental Psychiatry. 2004;35(2):109–20. doi: 10.1016/j.jbtep.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neuroscience and Biobehavioral Reviews. 2008;32(3):525–49. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience. 1997;9(6):788–98. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Nielen MM, den Boer JA, Smid HG. Patients with obsessive-compulsive disorder are impaired in associative learning based on external feedback. Psychological Medicine. 2009;39(9):1519–26. doi: 10.1017/S0033291709005297. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Slagter HA, von Geusau NJ, Heslenfeld DJ, Holroyd CB. Knowing good from bad: differential activation of human cortical areas by positive and negative outcomes. European Journal of Neuroscience. 2005;21(11):3161–8. doi: 10.1111/j.1460-9568.2005.04152.x. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Structure & Function. 2010;214(5-6):451–63. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman RK. A cybernetic model of obsessive-compulsive psychopathology. Comprehensive Psychiatry. 1987;28(4):334–43. doi: 10.1016/0010-440x(87)90070-8. [DOI] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MM, van Balkom AJ, Cath DC, van Oppen P, Uylings HB, Veltman DJ. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Archives of General Psychiatry. 2006;63(11):1225–36. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- Rotge JY, Guehl D, Dilharreguy B, Cuny E, Tignol J, Bioulac B, Allard M, Burbaud P, Aouizerate B. Provocation of obsessive-compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. Journal of Psychiatry & Neuroscience. 2008;33(5):405–12. [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Bannerman DM, Rushworth MF. The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cognitive Affective & Behavioral Neuroscience. 2008;8(4):485–97. doi: 10.3758/CABN.8.4.485. [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Molecular Psychiatry. 2010;15(1):53–63. doi: 10.1038/mp.2008.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience. 2008;11(4):389–97. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Salkovskis P, Shafran R, Rachman S, Freeston MH. Multiple pathways to inflated responsibility beliefs in obsessional problems: possible origins and implications for therapy and research. Behaviour Research and Therapy. 1999;37(11):1055–72. doi: 10.1016/s0005-7967(99)00063-7. [DOI] [PubMed] [Google Scholar]
- Saxena S, O'Neill J, Rauch SL. The role of cingulate cortex dysfunction in obsessive-compulsive disorder. In: Vogt BA, editor. Cingulate Neurobiology and Disease. New York: Oxford University Press; 2009. pp. 587–617. [Google Scholar]
- Schlosser RG, Wagner G, Schachtzabel C, Peikert G, Koch K, Reichenbach JR, Sauer H. Fronto-cingulate effective connectivity in obsessive compulsive disorder: a study with fMRI and dynamic causal modeling. Human Brain Mapping. 2010;31(12):1834–50. doi: 10.1002/hbm.20980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberg T, Daw ND, Joel D, O'Doherty JP. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. Journal of Neuroscience. 2007;27(47):12860–7. doi: 10.1523/JNEUROSCI.2496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59 (Suppl. 20, 22–33); quiz 34–57. [PubMed] [Google Scholar]
- Steketee G, Frost RO, Amir N, Bouvard M, Carmin C, Clark AD, Jr, Cottroux J, Eisen J, Emmelkamp P. Cognitive assessment of obsessive-compulsive disorder. Behaviour Research and Therapy. 1997;35(7):667–81. doi: 10.1016/s0005-7967(97)00017-x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain : 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Stuttgart: Thieme; 1988. [Google Scholar]
- Wittchen, H.U., Wunderlich, U., Gruschwitz, S., Zaudig, M., editors. (1996). Strukturiertes Klinisches Interview für DSM-IV (SKID). Göttingen, Germany: Beltz-Test.