Abstract
Recent research on classical fear-conditioning in the anxiety disorders has identified overgeneralization of conditioned fear as an important conditioning correlate of anxiety pathology. Unfortunately, only one human neuroimaging study of classically conditioned fear generalization has been conducted, and the neural substrates of this clinically germane process remain largely unknown. The current generalization study employs a clinically validated generalization gradient paradigm, modified for the fMRI environment, to identify neural substrates of classically conditioned generalization that may function aberrantly in clinical anxiety. Stimuli include five rings of gradually increasing size with extreme sizes serving as cues of conditioned danger (CS+) and safety (CS−). The three intermediately sized rings serve as generalization stimuli (GSs) and create a continuum-of-size from CS+ to CS−. Results demonstrate ‘positive’ generalization gradients, reflected by declines in responding as the presented stimulus differentiates from CS+, in bilateral anterior insula, dorsomedial prefrontal cortex, and bilateral inferior parietal lobule. Conversely, ‘negative’ gradients, reflected by inclines in responding as the presented stimulus differentiates from CS+ were instantiated in bilateral ventral hippocampus, ventromedial prefrontal cortex and precuneus cortex. These results as well as those from connectivity analyses are discussed in relation to a working neurobiology of conditioned generalization centered on the hippocampus.
Keywords: anxiety, conditioned generalization, fear-conditioning, fMRI, neurobiology
INTRODUCTION
The past two decades have seen dramatic progress in the neuroscience of anxiety due, in no small part, to animal findings specifying the neurobiology of classically conditioned fear. Fortuitously, this neurally mapped process of fear learning is widely expressed in humans, and has been centrally implicated in the etiology of clinical anxiety (for a review, see Mineka and Zinbarg, 2006). Studying classical fear-conditioning correlates of anxiety pathology thus represents a unique opportunity to bring recent advances in animal neuroscience to bear on working, brain-based models of clinical anxiety. Meta-analytic results of lab-based conditioning studies in the anxiety disorders implicate overgeneralization of fear from a conditioned danger cue (CS+) to a perceptually similar conditioned safety cue (CS−) as one of the more robust conditioning abnormalities in anxiety patients ( Lissek et al. , 2005 ). Neurobiological explorations of conditioned generalization may thus yield important insights on the pathophysiology of clinical anxiety.
Unfortunately, there is surprisingly little psychobiological, or even behavioral studies of generalization of classically conditioned fear in humans using systematic methods developed in animals known as ‘generalization gradient’ techniques (for reviews, see Kalish, 1969 ; Mackintosh, 1974 ; Honig and Urcuioli, 1981 ). This method includes measurement of conditioned fear responses to both the CS+ and generalization stimuli (GSs) parametrically varying in similarity to the CS+ and yields generalization slopes—or gradients—with the strongest fear-responding to the CS+, and decreasing levels of fear to GSs of decreasing similarity to the CS+. The strength of generalization is captured by the steepness of the gradient, with less steep decreases in responding reflecting stronger generalization. Because of the paucity of such work in humans, we designed a fear-conditioning paradigm capable of generating continuous generalization gradients in humans ( Lissek et al. , 2008 ). Clinical applications of this paradigm have demonstrated overgeneralization in panic disorder ( Lissek et al. , 2010 ), generalized anxiety disorder ( Lissek, 2012 ), and post-traumatic stress disorder ( Lissek and Grillon, 2012 ); all characterized by less steep declines in conditioned fear as the presented stimulus differentiates from CS+. The current effort applies this behaviorally and clinically validated generalization gradient paradigm in the functional magnetic resonance imaging (fMRI) environment to: (i) neurally characterize conditioned fear-generalization in humans based on a model derived from animal work and (ii) identify potential neural processes responsible for overgeneralization in clinical anxiety.
To date, only one neuroimaging study of classically conditioned fear generalization has been conducted ( Dunsmoor et al. , 2011 , 2012 ). This pioneering experiment elicits ‘intensity-based’ fear generalization. That is, generalization driven simultaneously by: (i) the unconditioned emotional intensity of GSs (i.e. facial expressions displaying low, medium or high levels of fear) and (ii) the perceptual resemblance of GSs to the CS+. Dunsmoor and colleagues identified fMRI activations related to intensity-based fear generalization in the insula, striatum, thalamus and subgenual cingulate. Such effects reflect the joint effects of the unconditioned emotional intensity of the GS and the degree to which the GS resembles the CS+. The current study employs CSs and GSs of neutral emotional valence, and thus represents the first effort to assess fMRI correlates of classically conditioned fear generalization uninfluenced by effects of unconditioned emotional intensity. Of note, two fMRI studies have very recently been conducted ( Greenberg et al., 2013a , b ) using an elegant instructed threat generalization paradigm. In these experiments, prior to the start of the study, participants are explicitly instructed that the shock US will be paired with CS+, but will never be paired with any of the GSs. Results link instructed generalization to the anterior insula, anterior cingulate cortex, caudate nucleus and ventromedial prefrontal cortex (vmPFC). Though these results contribute importantly to our understanding of fear generalization, they have limited comparability to the rich animal literature on classical conditioning, as instructed threat is a highly cognitive learning process beyond the capacity of lower mammals. This fMRI study, employing classically conditioned generalization, may thus contribute importantly toward bridging animal and human findings in this area of work.
A working neurobiology of conditioned fear generalization
Figure 1 depicts a recently proposed neural model of classically conditioned fear generalization in humans ( Lissek, 2012 ). This model attributes generalization of conditioned fear to a network of brain areas centering on the hippocampus and extending to sensory cortex, brain areas associated with fear excitation (e.g. amygdala and insula) and fear inhibition (e.g. vmPFC). Evidence for this model derives from classical conditioning research in both animals and humans.
Fig. 1.
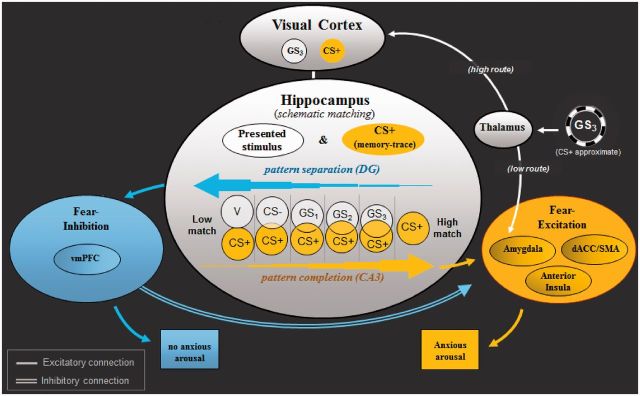
Neural model of conditioned fear generalization. Following acquisition of fear to CS+, when exposed to a stimulus resembling CS+ (i.e. GS 3 ), the thalamus is thought to relay sensory information about GS 3 to amygdala-based fear circuits via a ‘quick and dirty’ route resulting in a fast initial fear response to GS 3 . The thalamus simultaneously sends sensory GS 3 information to visual cortices for higher level sensory processing—a slower route through which neural representations of GS 3 are activated in visual cortex. Next, through hippocampally based ‘schematic matching’, the overlap between patterns of brain activity representing GS 3 and the previously encoded CS+ is assessed. Given sufficient overlap, CA3 neurons in hippocampus are thought to initiate ‘pattern completion’ (e.g. Treves and Rolls, 1994 ), whereby a subset of cues from a previous experience (i.e. CS+) activates the stored pattern representing that experience. Pattern completion by the hippocampus is then proposed to result in activation of brain structures associated with fear excitation (denoted in yellow: anterior insula, dACC, amygdala), culminating in the autonomic, neuroendocrine and behavioral constituents of the fear response. In the event of insufficient overlap between neural representations of GS 3 and the CS+, dentate gyrus neurons in the hippocampus are thought to initiate ‘pattern separation’ (e.g. McHugh et al. , 2007 ), resulting in the spread of activation to structures associated with fear inhibition (denoted in blue: vmPFC). Such activations are then proposed to attenuate ongoing activity in amygdala-based fear circuits initiated earlier by the ‘quick and dirty’ route and serve to stem anxious arousal. GS 1 , GS 2 , GS 3 = ring-shaped generalization stimuli; CS+ = ring-shaped danger-cue; CS− = ring shaped safety cue; vCS− = V-shaped safety cue; DG = dentate gyrus; CA3 = cornu ammonis region 3; vmPFC = ventromedial prefrontal cortex; dACC = dorsal anterior cingulate cortex; SMA = supplementary motor cortex area.
Animal evidence
Lesions of either hippocampus ( Wild and Blampied, 1972 ; Solomon and Moore, 1975 ) or cortical inputs to the hippocampus (i.e. postrhinal and perirhinal cortex: Bucci et al. , 2002 ) increase generalization of fear from CS+ to CS− in animals. These findings suggest that hippocampal activations are necessary for successful discrimination of CS+ from CS−, potentially attributable to the ‘pattern separation’ function of the hippocampus (e.g. O'Reilly and Rudy, 2001 ), through which brain representations of resembling, yet distinct, sensory experiences are discriminated. As such, human responses to stimuli most distinguishable from CS+ are predicted to undergo the most hippocampally mediated discrimination, with decreasing levels as the presented stimulus becomes more similar to the CS+.
Additional animal findings demonstrate overgeneralization of conditioned fear to auditory CSs following lesions of the auditory cortex ( Jarrell et al. , 1987 ; Teich et al. , 1988 ; but also see Armony et al. , 1997 ), and the medial geniculate nucleus of the thalamus ( Antunes and Moita, 2010 ). Such results support a contribution to generalization from sensory areas of the brain where stimulus features of CS+ and GSs are represented and putatively discriminated by the hippocampus.
A further area implicated in generalization of classical conditioning in animals is the ventromedial orbitofrontal cortex (OPFC; Zelinski et al., 2010 ). Specifically, rats with OPFC lesions generalize freezing behavior from a context paired with shock to an unpaired context, whereas intact animals display freezing only in the paired context. Such results suggest that OPFC activations are needed to inhibit fear to stimulus events resembling the CS+, and support predictions of inverse relations between OPFC activations and generalized conditioned-fear responses to GSs.
Human evidence
GSs have long been known to elicit the same response evoked by CS+, with gradual declines in responding as the GS differentiates from CS+ (e.g. Mackintosh, 1974 ). Thus, brain activations to the CS+ vs CS− found by multiple human neuroimaging studies of classical fear-conditioning, in the amygdala, anterior insula, dorsal anterior cingulate cortex (dACC) and dorsomedial prefrontal cortex (dmPFC; for reviews see Sehlmeyer et al., 2009 ; Etkin et al., 2011 ) are predicted to decrease as the presented GS diverges from CS+. Conversely, activations in human brain areas associated with inhibition of fear to previously dangerous, but currently safe conditioned stimuli, such as the vmPFC (e.g. Phelps et al. , 2004 ; Milad et al. , 2007 ; Schiller et al., 2008 ), are predicted to gradually increase as the GS becomes less similar to CS+. Results from recent instructed threat studies of generalization are consistent with these predictions, and find gradually ‘decreasing’ activations in anterior insula, dACC and dmPFC; and gradually ‘increasing’ activations in vmPFC, as the presented GS differentiates from CS+ ( Greenberg et al. , 2013a , b ).
Together, these animal and human findings support a neural model of classically conditioned fear generalization ( Lissek, 2012 ) in which CS+ and GS representations in sensory cortex undergo ‘schematic matching’, or same–different assessment by the hippocampus ( Otto and Eichenbaum , 1992 ; Sander et al. , 2005 ). With decreasing representational overlap between CS+ and GS, the hippocampus increasingly pattern-separates GS from CS+, and correspondingly activates fear inhibition areas of the brain (vmPFC). With greater representational overlap, the hippocampus increasingly responds to the GS by completing the pattern of brain activity representing the CS+ (see Nakazawa et al. , 2004 ), culminating in a corresponding level of activation of fear excitation areas of the brain (amygdala, anterior insula, dACC and dmPFC). A central aim of the current effort is to identify the degree to which fMRI activations and their connectivity support this predicted neural model.
METHODS
Participants
In total, 20 healthy participants (11 females) with a mean age of 24.00 years (s.d. = 4.70), and average State and Trait Anxiety Inventory scores ( Spielberger et al. , 1983 ) of 27.64 (s.d. = 6.04) and 30.93 (s.d. = 9.60), were recruited from the community and reimbursed for their time. Prior to testing, participants gave written informed consent that had been approved by the NIMH-IRB. Exclusion criteria included the typical magnetic resonance exclusions (e.g. metal in the body) as well as: (i) past or current Axis-I psychiatric disorder as per Structured Clinical Interview for DSM-IV, (SCID-I/NP; First et al. , 2001 ), (ii) major medical condition that interfered with the objectives of the study, (iii) current use of medications altering central nervous system function, (iv) current use of illicit drugs as per urine test and (v) pregnancy.
Conditioned, unconditioned and generalization stimuli
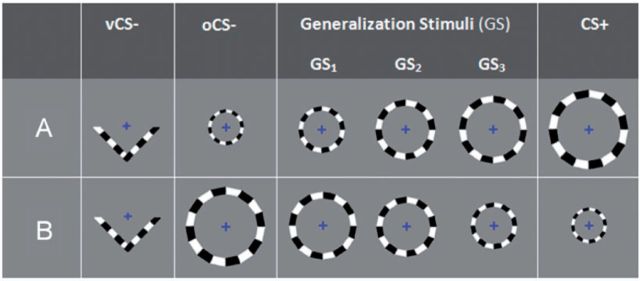
Five checkerboard-textured counterphase-flickering (10 Hz) rings of parametrically increasing size and one ‘V-shaped’ stimulus of the same counterphase-flickering type ( Figure 2 ) served as conditioned stimuli (CS+ and CS−) and generalization stimuli (GSs). Such stimuli were designed to activate the calcarine sulcus along a continuum of visual eccentricity (e.g. Murray et al., 2006 ) as part of a longer range goal to use this generalization paradigm to retinotopically map representations of CSs and GSs in sensory cortex. Important for the purposes of this article is the size and shape of these stimuli rather than their retinotopic-mapping characteristics, as retinotopy was unsuccessful in this study. The dimensions and size increments for employed rings are described in Figure 2 .
Fig. 2.
Conditioning and generalization stimuli for counterbalancing groups A and B. Half of the participants were assigned to counterbalancing group A and half to B. For both counterbalancing groups A and B , GS 3 consisted of the ring closest in size to CS+, with GS 2 and GS 1 further decreasing in similarity to CS+. Ring diameters in centimetres (and visual angles) from smallest to largest were: 6.63 (0.93°), 8.02 (1.12°), 9.38 (1.31°), 10.98 (1.54°) and 12.46 (1.75°). vCS− = v-shaped conditioned safety cue; oCS− = ring-shaped conditioned safety cue; GS 1 , GS 2 and GS 3 = three classes of generalization stimuli; CS+ = conditioned danger cue.
The current paradigm included one CS+ and the following two CS−: (i) either the largest or smallest ring—referred to as the oCS− and (ii) a ‘V–shaped’ stimulus—referred to as the vCS−. Though all subjects were conditioned with the same vCS−, the oCS− was the largest ring for 50% of subjects and the smallest ring for the remaining half. Subjects for whom the oCS− was the largest ring were conditioned with the smallest ring as CS+ and vice versa. The three intermediately sized rings served as GSs (i.e. GS 1 , GS 2 and GS 3 ) and formed a continuum-of-size between the CS+ and oCS− with GS 3 , GS 2 and GS 1 demarcating the GS with most to least similarity to the CS+ regardless of CS+ size. The vCS− was included to test the degree to which conditioned generalization accrues to all ‘ringed’ stimuli following reinforcement of the ring-shaped CS+. That is, heightened activations in fear-related brain areas to all sized rings, relative to the V-shaped vCS− would be identified as neural correlates of this broader form of generalization to all circular stimuli. Furthermore, the inclusion of the vCS− allows for an assessment of brain responses to the CS+ ( vs vCS−) that are independent of putative generalization effects to all ringed stimuli. Such an assessment is important because brain activations to the CS+ will be used as functional regions of interest in which to test gradients of fear generalization, and should thus be orthogonal to the generalization process. The CS+ vs vCS− contrast provides such an index of conditioning that is independent of generalization effects.
All CSs and GSs were presented for 4 s on a rear-projection viewing screen mounted at the foot of the scanner with a viewing distance of 6.71 feet (204.47 cm). Inter-trial-intervals for CSs and GSs were either 2.4 or 4.8 s, during which time participants focused their gaze on crosshairs in the center of the screen. The unconditioned stimulus (US) was a 100 ms electric shock (3–5mA) delivered to the right ankle. Prior to the start of the experiment, a sample shock procedure was performed during which participants received between one and three sample shocks and a level of shock rated by participants as being ‘highly uncomfortable but not painful’ was established. Shock intensity varied by subject and had an average intensity of 4.6 mA (s.d. = 0.80).
Behavioral ratings
Throughout testing, a behavioral task developed to maintain visual gaze at the center of the visual field ( Schwartz et al. , 2005 ) was applied. This task consists of a string of colored crosshairs (blue, yellow, red, green and purple) presented serially for a duration of 800 ms each in a quasi-random order in the center of the viewing screen during 4 s presentation of CSs/GSs (five crosshairs per stimulus) as well as inter-trial interval (ITI) periods lasting 2.4 s (three crosshairs) or 4.8 s (four crosshairs). Participants were instructed to continuously monitor the stream of colored crosshairs and rate their perceived level of risk for shock as quickly as possible following each red cross using a three button, fiber optic response pad (Lumina LP-404 by Cedrus), where 0 = ‘no risk’, 1 = ‘moderate risk’ and 2 = ‘high risk’. Risk ratings were recorded with Presentation software (Neurobehavioral Systems). For half of CS/GS trials, one of five crosshairs was red and the remaining trials included no red crosshairs. Additionally, on reinforced CS+ trials, the red crosshair never appeared in the fourth or fifth position to avoid interference from shock on behavioral responses. Finally, self-reported anxiety to CS+, oCS−, and vCS− were retrospectively assessed following pre-acquisition, acquisition and generalization sequences using 10-point Likert scale.
Design
The generalization paradigm included three phases: (i) pre-acquisition—consisting of 20 trials of each stimulus type (CS+, GS 1 , GS 2 , GS 3 , oCS− and vCS−) all presented in the absence of any shock US; (ii) acquisition —including 15 CS+, 15 oCS−, and 15 vCS−, with 12 of 15 CS+ co-terminating with shock (80% reinforcement schedule) and (iii) ‘generalization test’—including 20 trials of each stimulus type (unreinforced CS+, GS 1 , GS 2 , GS 3 , oCS−, vCS−) and an additional 10 CS+ co-terminating with shock (33% reinforcement schedule) to prevent extinction of the conditioned response during the generalization sequence, while leaving 20 unreinforced CS+ to index responses uninfluenced by the shock US. Trials for all three phases of the study were arranged in quasi-random order such that no more than two stimuli of the same class occurred consecutively. An additional constraint for the generalization sequence was the arrangement of trials into six blocks of 13 trials (two unreinforced CS+, one reinforced CS+, two oCS×, two vCS×, two GS 1 , two GS 2 , two GS 3 ) to ensure an even distribution of trial types throughout runs.
fMRI data acquisition
A 3T General Electric Signa system (GE Medical Systems, Waukesha, WI, USA) equipped with an eight-channel receive-only head coil was used to acquire functional T2*-weighted echo-planar images (EPIs) depicting the blood-oxygen-level-dependent (BOLD) contrast (Repetition time: 2300 ms, echo time: 23 ms, flip angle: 90°). Whole-brain acquisitions consisted of 37 sagittally oriented slices of 3.5 mm thickness and 1.7 × 1.7 mm 2 in-plane resolution (matrix: 128 × 128, field of view: 22 cm). A total of 1162 functional volumes were collected across five EPI runs with 235 volumes acquired for runs 1 and 2 (pre-acquisition), 170 for run 3 (acquisition) and 261 for runs 4 and 5 (generalization test). The first four EPI volumes in each run were discarded to avoid T1 equilibrium effects. High-resolution T1-weighted magnetization prepared rapid acquisition gradient-echo sequence were obtained to serve as anatomical reference. Foam pads securing participants in the headcoil where used to limit head movement during data acquisition.
Procedure
Participants were not instructed of the CS/US contingency but were told they might learn to predict the shock if they attend to the presented stimuli. Shock electrodes were then attached and a shock workup procedure was completed. Participants next practiced using the button box to respond to red crosshairs appearing both at the center of CSs and GSs and during ITI periods. Participants were then placed in the magnet. Structural scans were acquired followed by preacquisition, acquisition, and generalization test. Participants rated their anxiety responses to CS+, oCS− and vCS− after pre-acquisiton, acquisition and generalization scans.
fMRI data analysis
Analysis of Functional Neural Images (AFNI) software ( Cox, 1996 ) was used for image analysis. Echo-planar time series data were time corrected to adjust for non-simultaneous slice acquisition within each volume, registered to the seventh volume of the first functional imaging scan, spatially smoothed to minimize effects of anatomical variability (FWHM = 4 mm), normalized to percent signal change relative to voxel means for the entire task and concatenated. Additionally, subjects with more than 3.0 mm of head motion in any dimension from one EPI brain volume to the next were removed (only one such subject was dropped). During individual-level analyses, functional activation maps were computed by regressing each voxel’s fMRI response time course onto an ideal response function consisting of a Gamma-variate function convolved with the time-series of each of six stimulus types (i.e. vCS−, oCS−, GS 1 , GS 2 , GS 3 and unreinforced CS+) at pre-acquisition and generalization test separately. Modeled as covariates of no interest were baseline drift, participant-specific movement parameters and the time course of motor button presses. Additionally, at generalization, the time course of CS+ paired with shock (10 trials) was also entered as a covariate of no interest. The fMRI data at acquisition were not analyzed, because the majority of responses to CS+ were contaminated by US administrations and because such data were not crucial for testing central hypotheses of interest.
Group-level analyses of generalization test data were completed in two stages. First, brain areas sensitive to the conditioning manipulation were identified as functional regions of interest (fROIs). Specifically, whole-brain analyses of the contrast between responses to the 20 unreinforced CS+ vs the 20 vCS− were conducted using a voxelwise probability of P ≤ 0.001 and a cluster probability of P ≤ 0.05. The probability of obtaining clusters of a particular size was estimated with the AFNI program AlphaSim. The vCS− rather than oCS− was contrasted against unreinforced CS+ because the CS+ vs vCS− contrast, but not CS+ vs oCS−, yields a measure of conditioning independent of fear generalization that may occur to all circular stimuli. In the second stage, beta weights averaged across voxels within these functional ROIs were plotted across conditioned and generalization stimuli and analyzed for effects of generalization. Such analyses began with one-way repeated measures ANOVAs with six levels (vCS, oCS−, GS 1 , GS 2 , GS 3 and unreinforced CS+) and were followed, when appropriate, by tests of linear and quadratic components. Criterion alpha for ANOVAs and follow-up statistics was set at P = 0.05.
Functional connectivity analysis
Inter-relations between brain areas associated with the generalization process were tested using psychophysiological interaction (PPI; Friston et al. , 1997 ) with functionally defined seed regions in the hippocampus—the central node of the theorized network of brain areas subserving generalization. PPI identifies neural couplings affected by psychological context. We used PPI to test two primary predictions: (i) increased neural coupling between the hippocampus and brain areas associated with fear excitation in the context of stimuli with more vs less resemblance to CS+ and 2) increased neural coupling between the hippocampus and brain areas associated with fear inhibition in the context of stimuli with less vs more resemblance to CS+. Following previous work on functional connectivity with PPI, we set criterion alpha at P ≤ 0.001 ( Passamonti et al. , 2009 ).
Behavioral data analysis
At pre-acquisition, acquisition and generalization test, levels of conditioning were assessed with paired sample t -tests comparing risk ratings to CS+ vs oCS− and CS+ vs vCS−. Additionally, risk ratings at pre-acquisition and generalization test were analyzed with one-way, repeated measures ANOVAs with six levels (vCS, oCS−, GS 1 , GS 2 , GS 3 and unreinforced CS+), and were followed, when appropriate, by tests of linear and quadratic components. Criterion alpha for these behavioral analyses was set at P = 0.05.
RESULTS
Subjective ratings
Pre-acquisition
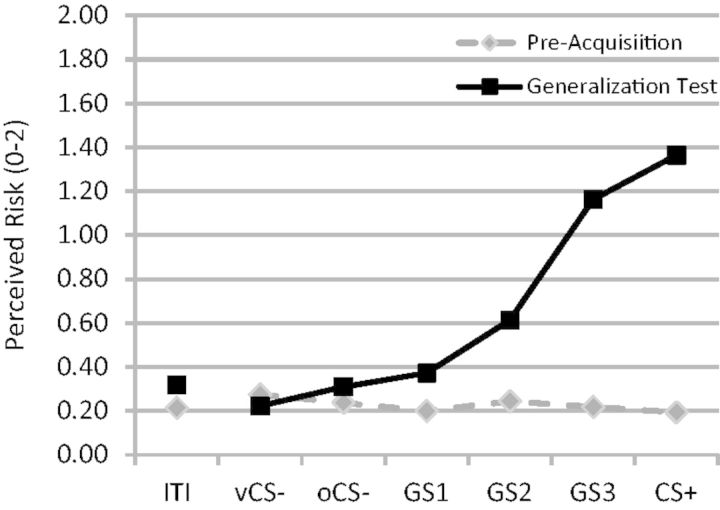
Prior to conditioning, there were no differences between CS+ and oCS− for retrospective ratings of anxiety ( P = 0.17) or online behavioral ratings of perceived threat ( P = 0.39). Additionally, CS+ and vCS− did not differ on retrospective ratings of anxiety ( P = 0.42), though a trend for larger online risk ratings for vCS− vs CS+ was found ( P = 0.08). Finally, online ratings did not differ across conditioned and generalization stimuli whether including or excluding the vCS− (both P values > 0.30; Figure 3 ).
Fig. 3.
Online ratings of shock risk (0 = no risk, 1 = some risk, 2 = high risk) both before acquisition training (pre-acquisition) and after (generalization test). ITI = inter-trial interval; vCS− = v-shaped conditioned safety cue; oCS− = ring-shaped conditioned safety cue; GS 1 , GS 2 and GS 3 = three classes of generalization stimuli; CS+ = conditioned danger cue.
Acquisition
Successful acquisition of conditioned fear was evidenced by increases in online ratings of threat to CS+ (M = 1.40, s.d. = 0.43) relative to both oCS− (M = 0.66, s.d. = 0.60; t (19) = 5.09, P < 0.0001) and vCS− (M = 0.62, s.d. = 0.58; t (19) = 5.06, P < 0.0001). Additionally, greater retrospective ratings of anxiety were found for CS+ ( = 7.44, s.d. = 2.19) compared to both oCS− (M = 3.60, s.d. = 2.67; t (19) = 4.71, P < 0.0001) and vCS− (M = 2.86, s.d. = 2.48; t (19) = 5.69, P < 0.0001). Finally, differences between oCS− and vCS− were not found for risk or anxiety ratings ( P values > 0.19).
Generalization
Conditioned fear persisted through the generalization sequence as evidenced by greater online risk ratings to CS+ vs both oCS−, t (19) = 10.82, P < 0.0001 and vCS−, t (19) = 12.62, P < 0.0001 as well as greater retrospective anxiety to CS+ vs both oCS−, t (19) = 5.66, P < 0.0001 and vCS−, t (19) = 10.73, P < 0.0001. Generalization of conditioned fear was evidenced by increasing levels of reported risk from vCS− to oCS− to GS 1, to GS 2, to GS 3 to CS+, F (5,15) = 43.34, P < 0.0001 ( Figure 3 ). This generalization gradient consisted of both linear [ F (1,19) = 206.18, P < 0.0001] and quadratic [ F (1,19) = 14.35, P = 0.001] components.
fMRI activations
fROIs
Brain regions activating differentially to CS+ vs vCS− that survived a voxelwise P value of 0.001 and a cluster P value of 0.05 are listed in Table 1 . Areas responding more to CS+ than vCS− included: (i) left anterior insula; (ii) right anterior insula; (iii) dmPFC [Brodmann area (BA) 6]; (iv) left inferior parietal lobule (IPL; BA 40); (v) right IPL (BA 40) and (vi) right middle frontal gyrus (MFG; BA 10). The reverse contrast (vCS−>CS+) yielded significant activations in areas such as: (i) vmPFC (BA 10); (ii) left ventral hippocampus (VH); (iii) right VH and (iv) precuneus. These 10 brain areas served as fROIs within which effects of generalization gradients were tested both before and after acquisition training.
Table 1.
Brain areas responding differentially to CS+ vs vCS− that served as fROIs within which to plot gradients of generalization across vCS−, oCS−, GS 1 , GS 2 , GS 3 and CS+
| Brain region | Direction | Volume (μl) |
Peak coordinates
a |
t -value | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Left anterior insula | CS+ > CS− | 1123 | −28.9 | 14.8 | 10.0 | 6.54 |
| Right anterior insula | CS+ > CS− | 3520 | 28.9 | 18.1 | 3.0 | 6.72 |
| dmPFC/SMA (BA 6) | CS+ > CS− | 632 | 1.7 | 11.3 | 45.0 | 5.47 |
| Left IPL (BA 40) | CS+ > CS− | 819 | −61.2 | −38.0 | 31.0 | 5.57 |
| Right IPL (BA 40) | CS+ > CS− | 2863 | 49.3 | −48.2 | 34.5 | 6.75 |
| Right MFG | CS+> CS− | 516 | 42.5 | 26.6 | 27.5 | 5.39 |
| Right SFG (BA10) | CS+ > CS− | 2883 | 25.5 | 50.4 | 17.0 | 6.34 |
| Right STG (BA 22) | CS+ > CS− | 314 | 44.2 | −21.0 | −4.0 | 5.78 |
| vmPFC | CS− > CS+ | 3085 | 5.1 | 45.3 | −7.5 | 7.03 |
| Left ventral hippo | CS− > CS+ | 334 | −23.8 | −19.3 | 11.0 | 6.91 |
| Right ventral hippo | CS− > CS+ | 253 | 28.9 | −19.3 | 11.0 | 5.21 |
| PCu | CS− > CS+ | 5472 | 1.7 | −56.7 | 27.5 | 8.37 |
a LPI.
L = left; R = right; BA = Brodmann Area; CS+ = conditioned danger cue; CS− = v-shaped conditioned safety cue; t -value = clusterwise; dmPFC = dorsomedial prefrontal cortex; SMA = supplementary motor area; IPL = inferior parietal lobule; MFG = middle frontal gyrus; SFG = superior frontal gyrus; STG = superior temporal gyrus; vmPFC = ventromedial prefrontal cortex; hippo = hippocampus; Pcu = precuneus.
Pre-acquisition
Prior to acquisition training, none of the 10 fROI’s produced linear or quadratic increases in BOLD signal as the presented stimulus increased in similarity to the CS+.
Generalization test
Following acquisition, BOLD activations in several fROI’s fell along ‘positive’ generalization gradients with strongest responding to CS+ and gradual decreases to GS 3 , GS 2 , GS 1 , oCS−, and vCS− ( Figure 4 ). Consistent with predictions, these ‘positive’ generalization gradients were found in right anterior insula [linear: F (1,20) = 25.72, P < 0.0001, quadtratic: F (1,20) = 10.06, P = 0.005], left anterior insula [linear: F (1,20) = 26.92, P < 0.0001, quadratic: F (1,20) = 15.32, P = 0.001] and dmPFC [linear: F (1,20) = 28.39, P < 0.0001, quadratic: F (1,20) = 5.53, P = 0.03]. Additional positive gradients were found in left IPL [linear: F (1,20) = 17.88, P < 0.0001, quadratic: F (1,20) = 6.99, P = 0.016], right IPL [linear: F (1,20) = 28.97, P < 0.0001, quadratic: F (1,20) = 8.59, P = 0.008] and right MFG [linear: F (1,20) = 7.06, P = 0.015, quadratic: ns].
Fig. 4.
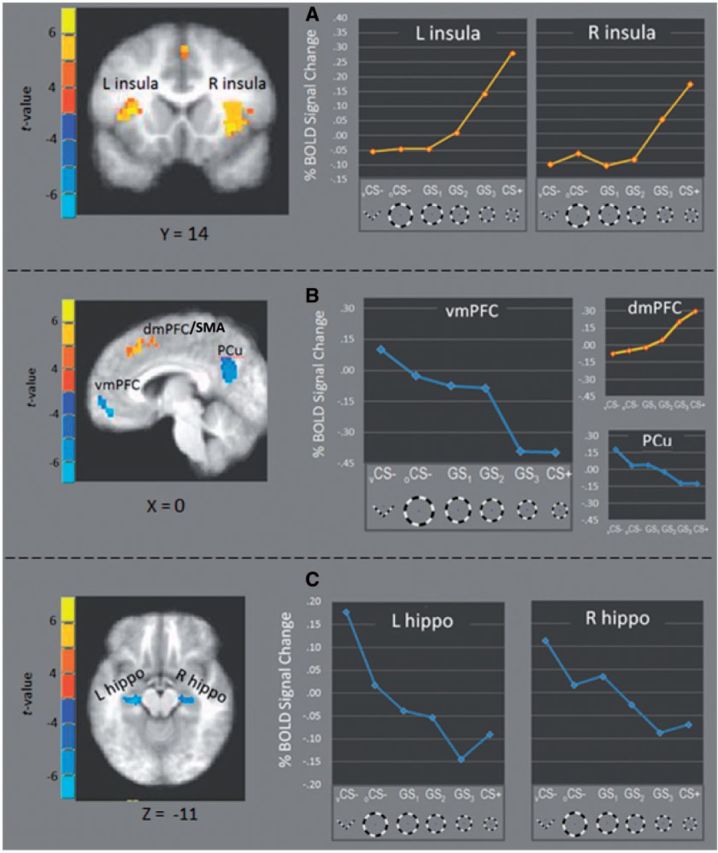
fROIs responding more strongly to CS+ vs CS− (yellow) and CS− versus CS+ (blue) fell along positive and negative generalization gradients, respectively. Specifically, activations in left and right anterior insula ( A ), as well as dmPFC ( B ), fell along positive gradients with increasing signal change as presented stimuli became more similar to CS+. Conversely, activations in vmPFC, and PCC (B) as well as left and right anterior hippocampus ( C ) formed negative gradients with decreasing levels as presented stimuli became more similar to CS+. L = left; R = right; vmPFC = ventromedial prefrontal cortex; dmPFC = dorsomedial prefrontal cortex; SMA = supplementary motor cortex; Pcu = precuneus; hippo = hippocampus.
Also found were fROIs responding strongest to vCS− with degraded reactivity to oCS−, GS 1 , GS 2 , GS 3 and CS+. As shown in Figure 4 B and C, these ‘negative’ generalization gradients were instantiated in vmPFC [linear: F (1,20) = 59.65, P < 0.0001, quadratic: ns], left VH [linear: F (1,20) = 65.57, P < 0.0001, quadratic: P = 0.11], right VH [linear: F (1,20) = 30.55, P < 0.0001, quadratic: ns] and precuneus [linear: F (1,20) = 57.37, P < 0.0001, quadratic: ns].
Brain–behavior correlations
Conditioned fear
Average activations in the 10 fROIs were correlated with online ratings of perceived shock risk and retrospective anxiety ratings. Risk ratings to neither CS+ vs vCS− nor CS+ vs oCS− correlated with responses to these contrasts in any of the fROIs (all P values > 0.15). Retrospective anxiety to CS+ vs vCS− was negatively correlated with activations to this contrast in left VH ( r = −0.49, P = 0.02). Additionally, retrospective anxiety to CS+ vs oCS− was correlated with activations in right anterior insula ( r = 0.47, P = 0.03); right MFG ( r = 0.50, P = 0.02); and right IPL ( r = 0.48, P = 0.03).
Generalization
Two types of contrasts were used to test brain–behavior relations for conditioned generalization. The first assessed responses to GS 3 , the closest approximation of the CS+, vs vCS−. This contrast was chosen because the strongest effect of generalization of conditioned fear should occur to GS 3 as it most closely resembles CS+. No significant correlations between behavior and activations in any of the 10 fROIs were found using this contrast (all P values > 0.15). The second contrast indexing levels of generalization assessed a broader generalization to all ring-shaped stimuli. It was computed as the average response to all circular stimuli (oCS−, GS 1 , GS 2 , GS 3 and CS+) minus response to the vCS−. This contrast yielded a positive correlation between perceived risk of shock and activation in right IPL lobule ( r = 0.42, P = 0.05), and a negative correlation between risk ratings and left IPL ( r = −0.54, P = 0.01).
Connectivity results
Results from PPI analyses are listed in Table 2 . Because of the centrality of the hippocampus to our neural model of generalization, fROIs identified by first level analyses in the left and right VH served as seeds for PPI analyses. Generalization-related modifications in connectivity between the seed and target regions were assessed across GS 3vs vCS− and all ringed stimuli vs vCS−. With left VH as seed (maxima at Talairach xyz = −24, −19, −11), functional coupling with left amygdala and right anterior insula were stronger during GS 3vs vCS− (both P values < 0.001, uncorrected). Additionally, all ringed stimuli (oCS−, GS 1 , GS 2 , GS 3 and CS+) vs vCS−, a measure of broad generalization to all things circular, produced greater left VH coupling with amygdala and right anterior insula (both P values < 0.001, uncorrected). With right VH as seed (maxima at Talairach xyz = 29, −19, −11), PPI results revealed increased functional coupling during GS 3vs vCS− in right amygdala and right anterior insula (both P values < 0.001, uncorrected) and decreased neural coupling during vCS− vs GS 3 in precuneus cortex and vmPFC (both P values < 0.001, uncorrected). Finally, all rings vs vCS− increased coupling between right VH and right anterior insula and vCS− vs all rings elicited greater coupling between right VH and vmPFC (both P values < 0.001, uncorrected). Such findings are consistent with hippocampal activations of the amygdala and insula during stimuli resembling the CS+ and hippocampal activation of vmPFC and, to some degree, the precuneus during stimuli with least resemblance to CS+.
Table 2.
Results from psychophysiological interaction analyses with left and right VH as seed regions
| Seed region | Target region | Effect |
Peak coordinates
a |
t -value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| L VH | Left amygdala | GS 3 > vCS− | −23 | −3 | −12 | 4.74 |
| Right anterior insula | GS 3 > vCS− | 43 | 10 | −7 | 4.96 | |
| vmPFC | vCS−> GS 3 | 1 | 23 | −12 | 4.45 | |
| Right amygdala | All rings > vCS− | 24 | −1 | −13 | 7.13 | |
| Right anterior insula | All rings > vCS− | 36 | 7 | −1 | 6.05 | |
| R VH | Right amygdala | GS 3 > vCS− | 22 | −2 | −9 | 4.66 |
| Right anterior insula | GS 3 > vCS− | 45 | 6 | −7 | 4.11 | |
| Precuneus | vCS−> GS 3 | 6 | −59 | 15 | 5.22 | |
| vmPFC | vCS−> GS 3 | 0 | 23 | −13 | 4.12 | |
| Right anterior insula | All rings > vCS− | 44 | 11 | −8 | 5.15 | |
| vmPFC | vCS−> all rings | −5 | 45 | −7 | 3.91 | |
a LPI.
L = left; R = right; VH = ventral hippocampus; vmPFC = ventromedial prefrontal cortex; GS 3 = generalization stimulus most similar to conditioned danger cue; vCS− = ‘v-shaped’ conditioned safety cue; all rings = average response across all ringed stimuli (i.e. oCS−, GS 1 , GS 2 , GS 3 and CS+). All effects were significant at P ≤ 0.001, uncorrected.
DISCUSSION
Results point to a number of brain regions sensitive to classically conditioned fear generalization. Activations in such areas fell along either ‘positive’ generalization gradients—with highest reactivity to the conditioned danger cue (CS+) and decreased responses as the target stimulus differentiates from CS+, or negative gradients—with highest reactivity to conditioned safety cues (vCS− and oCS−) and decreased responses as the target stimulus becomes more similar to CS+.
Negative generalization gradients were instantiated in left and right VH. Such findings are consistent with the predicted hippocampal role in discrimination of CS+, from a resembling GS, via pattern separation ( Lissek, 2012 ). When faced with a new stimulus event (i.e. GS) that resembles a past event stored in memory (i.e. CS+), the hippocampus is thought to perform a ‘schematic match’, or same–different determination, between the cortical representations of the current and past events ( Otto and Eichenbaum, 1992 ; Sander et al. , 2005 ). With decreasing representational overlap, the hippocampus increasingly differentiates the current and past event through pattern separation ( O'Reilly and Rudy, 2001 ), and forms a new memory trace for the current event that is distinct from the old. In this study, hippocampal activations were strongest during presentations of stimuli with the least schematic match to CS+, or in other words, stimuli most likely to elicit pattern separation. Furthermore, hippocampal activations gradually weakened as the schematic match increased and pattern separation became less appropriate. Such findings implicate the VH in the pattern separation necessary for successful discriminative conditioning.
According to the neural model of generalization ( Lissek, 2012 ), hippocampal instantiations of pattern separation should determine the level of activation in brain areas associated with fear inhibition such as the vmPFC. Consistent with this model, greater functional connectivity between the hippocampus and vmPFC was found during processing of stimuli most likely to elicit pattern separation (i.e. vCS−), and the negative generalization gradients across stimuli in the hippocampus was mirrored in the vmPFC. Such a result may indicate that increasing hippocampal activations to GSs with less CS+ similarity and, in turn, greater safety value, resulted in corresponding increases in vmPFC-mediated fear inhibition. This greater vmPFC reactivity to stimuli with increasing safety value, is also consistent with much human work linking vmPFC to inhibition of fear to previously dangerous, but currently safe, conditioned stimuli (e.g. Phelps et al. , 2004 ; Milad et al. , 2007 ; Schiller et al. , 2008 ).
In addition to hippocampal activation of fear inhibition brain areas commensurate with the ‘dissimilarity’ of a given GS from CS+, our model proposes separate areas of the hippocampus (i.e. CA3 neurons) activating brain areas associated with fear excitation, commensurate with the degree of ‘similarity’ between a given GS and the CS+ ( Lissek, 2012 ). This latter prediction derived from the notion that a greater schematic match between a presented GS and the previously encountered CS+, would increase the likelihood of hippocampally mediated pattern completion ( Nakazawa et al. , 2004 ) resulting in excitation of the total pattern of brain activity subserving the CS+ including fear excitation brain areas, culminating in a generalized fear response to the GS.
Though no found hippocampal activation showed positive gradients reflective of pattern completion, GSs with stronger schematic matches to CS+ resulted in stronger activation of such brain areas associated with fear excitation as the anterior insula (e.g. Büchel et al. , 1998 ; Nitschke et al. , 2006 , Klumpp et al. , 2012 ), dmPFC (for a review, see Etkin et al. , 2011 ) and IPL ( Radua et al. , 2010 ). Additionally, PPI analyses revealed greater functional connectivity between hippocampus and both amygdala and insula to all stimuli sharing a ring shape with the CS+, lending further evidence for proposed increases in hippocampal activation of fear-related brain areas to stimuli with better schematic matches to CS+.
Of note, several past human studies have identified enhanced hippocampal activations to CS+ vs CS−, a pattern of results seemingly inconsistent with present findings of hippocampal increases to CS− vs CS+. These past findings, however, arise largely from studies assessing hippocampal contributions to either trace conditioning (e.g. Knight et al. , 2004 ) or contextual conditioning ( Alvarez et al. , 2008 ), two conditioning processes not assessed by this study. Such studies implicate the hippocampus in either declarative memory necessary for successful conditioning when CS+ and US are temporally non-overlapping (trace conditioning), or spatial mapping of environments necessary to acquire fear-conditioning to contexts in which a noxious US is delivered (contextual conditioning). The absence of hippocampal activations to CS+ vs CS− in this study may thus be due to our use of delay conditioning (i.e. the CS and US temporally overlap) rather than trace conditioning and discrete cue conditioning (CS+ consists of a single element) rather than contextual conditioning.
Clinical hypotheses
Though completed on healthy participants, this study affords predictions regarding neural substrates for classically conditioned generalization abnormalities of the kind generated by this same paradigm in anxiety patients. In this study, positive neural gradients of conditioned generalization in bilateral anterior insula, SMA and bilateral IPL all evidence quadratic declines in BOLD responses, with steep decreases from CS+ to the closest two approximations of the CS+ (GS 3 , GS 2 ) and approximately equal levels of low responding to stimuli least similar to the CS+ (GS 1 , oCS−, and vCS−). This precipitous, quadratic decline closely mirrors the shape of behavioral measures of classically conditioned fear-generalization in both intact animals (e.g. Armony et al., 1997 ) and healthy humans ( Lissek et al. , 2008 ). In contrast, behavioral gradients in anxiety patients have been shown to deviate from this ‘normative’ quadratic shape by forming more gradual, linear declines from CS+ to CS− ( Lissek et al. , 2010 ; Lissek, 2012 ; Lissek and Grillon, 2012 ). Such findings support the prediction that abnormally shallow, linear declines in BOLD signal from CS+ to CS− in anterior insula, dmPFC and IPL subserve the behavioral overgeneralization seen clinically and experimentally in anxiety patients.
Conflict of Interest
None declared.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Mental Health and by the Extramural Research Program of the National Institute of Mental Health (grant no. K99 MH080130 to S.L.).
REFERENCES
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. Journal of Neuroscience. 2008;28:6211–9. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes R, Moita MA. Discriminative auditory fear learning requires both tuned and nontuned auditory pathways to the amygdala. Journal of Neuroscience. 2010;30:9782–7. doi: 10.1523/JNEUROSCI.1037-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE. Stimulus generalization of fear responses: effects of auditory cortex lesions in a computational model and in rats. Cerebral Cortex. 1997;7:157–65. doi: 10.1093/cercor/7.2.157. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Saddoris MP, Burwell RD. Contextual fear discrimination is impaired by damage to the postrhinal or perirhinal cortex. Behavioral Neuroscience. 2002;116:479–88. [PubMed] [Google Scholar]
- Büchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–57. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, LaBar KS. Brain activity associated with omission of an aversive event reveals the effects of fear learning and generalization. Neurobiology of Learning and Memory. 2012;97:301–12. doi: 10.1016/j.nlm.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Prince SE, Murty VP, Kragel PA, LaBar KS. Neurobehavioral mechanisms of human fear generalization. NeuroImage. 2011;55:1878–88. doi: 10.1016/j.neuroimage.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) Research version. New York: New York State Psychiatric Institute; 2001. [Google Scholar]
- Friston KJ, Büchel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica-Parodi LR. Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depression and Anxiety. 2013a;30:242–50. doi: 10.1002/da.22016. [DOI] [PubMed] [Google Scholar]
- Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica-Parodi LR. Neural reactivity tracks fear generalization gradients. Biological Psychology. 2013b;92:2–8. doi: 10.1016/j.biopsycho.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Honig WK, Urcuioli PJ. The legacy of Guttman and Kalish (1956): twenty-five years of research on stimulus generalization. Journal of the Experimental Analysis of Behavior. 1981;36:405–45. doi: 10.1901/jeab.1981.36-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell T, Gentile C, Romanski L, McCabe P, Schneiderman N. Involvement of cortical and thalamic auditory regions in retention of differential bradycardiac conditioning to acoustic conditioned stimuli in rabbits. Brain Research. 1987;412:285–94. doi: 10.1016/0006-8993(87)91135-8. [DOI] [PubMed] [Google Scholar]
- Kalish HI. Stimulus generalization. In: Marx MH, editor. Learning: Processes. Oxford, England: Macmillan; 1969. [Google Scholar]
- Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biological Psychology. 2012;89:273–6. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. Journal of Neuroscience. 2004;24:218–28. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: the case for conditioned overgeneralization. Depression and Anxiety. 2012;29:257–63. doi: 10.1002/da.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin S, et al. Generalization of conditioned fear-potentiated startle in humans: experimental validation and clinical relevance. Behaviour Research and Therapy. 2008;46:678–87. doi: 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Grillon C. Learning models of PTSD. In: Beck JG, Sloan DM, editors. The Oxford Handbook of Traumatic Stress Disorders. New York, NY: Oxford University Press; 2012. [Google Scholar]
- Lissek S, Powers AS, McClure EB, et al. Classical fear-conditioning in the anxiety disorders: a meta-analysis. Behaviour Research and Therapy. 2005;43:1391–424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin SJ, Heller RE, et al. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. American Journal of Psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh NJ. The Psychology of Animal Learning. New York, NY: Academic Press; 1974. [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–9. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. Conditioning and ethological models of anxiety disorders: stress-in-dynamic-context anxiety models. In: Hope DA, editor. Perspective on Anxiety, Panic, & Fear. Vol. 43. Lincoln and London: University of Nebraska Press; 1996. pp. 135–210. [PubMed] [Google Scholar]
- Murray SO, Boyaci H, Kersten D. The representation of perceived angular size in human primary visual cortex. Nature Neuroscience. 2006;9:429–34. doi: 10.1038/nn1641. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nature Reviews Neuroscience. 2004;5:361–72. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewica KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–16. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychological Review. 2001;108:311–45. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Neuronal activity in the hippocampus during delayed non-match to sample performance in rats: evidence for hippocampal processing in recognition memory. Hippocampus. 1992;2:323–34. doi: 10.1002/hipo.450020310. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Rowe JB, Schwarzbauer C, Ewbank MP, von dem Hagen E, Calder AJ. Personality predicts the brain's response to viewing appetizing foods: the neural basis of a risk factor for overeating. Journal of Neurosciences. 2009;29:43–51. doi: 10.1523/JNEUROSCI.4966-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, Ledoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Radua J, Phillips ML, Russell T, et al. Neural response to specific components of fearful faces in healthy and schizophrenic adults. Neuroimage. 2010;49:939–46. doi: 10.1016/j.neuroimage.2009.08.030. [DOI] [PubMed] [Google Scholar]
- Sander D, Grandjean D, Scherer KR. A systems approach to appraisal mechanisms in emotion. Neural Networks. 2005;18:317–52. doi: 10.1016/j.neunet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. Journal of Neurosciences. 2008;28:11517–25. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Vuilleumier P, Hutton C, Maravita A, Dolan RJ, Driver J. Attentional load and sensory competition in human vision: modulation of fMRI responses by load at fixation during task-irrelevant stimulation in the peripheral visual field. Cerebral Cortex. 2005;15:770–86. doi: 10.1093/cercor/bhh178. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schöning S, Zwitserlood P, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PR, Moore JW. Latent inhibition and stimulus generalization of the classically conditioned nictitating membrane response in rabbits (Orcytolagus cuniculus) following dorsal hippocampal ablation. Journal of Comparative and Physiological Psychology. 1975;89:1192–203. doi: 10.1037/h0077183. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg P, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1983. [Google Scholar]
- Teich AH, McCabe PM, Gentile CG, et al. Role of auditory cortex in the acquisition of differential heart rate conditioning. Physiology and Behavior. 1988;44:405–12. doi: 10.1016/0031-9384(88)90044-3. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls E. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–91. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Wild JM, Blampied NM. Hippocampal lesions and stimulus generalization in rats. Physiology of Behavior. 1972;9:505–11. doi: 10.1016/0031-9384(72)90005-4. [DOI] [PubMed] [Google Scholar]
- Zelinski EL, Hong NS, Tyndall AV, Halsall B, McDonald RJ. Prefrontal cortical contributions during discriminative fear conditioning, extinction, and spontaneous recovery in rats. Experimental Brain Research. 2010;203:285–97. doi: 10.1007/s00221-010-2228-0. [DOI] [PubMed] [Google Scholar]