Abstract
There is increasing evidence for a role of the amygdala in processing gaze direction and emotional relevance of faces. In this event-related functional magnetic resonance study we investigated amygdala responses while we orthogonally manipulated head direction, gaze direction and facial expression (angry, happy and neutral). This allowed us to investigate effects of stimulus ambiguity, low-level factors and non-emotional factors on amygdala activation. Averted vs direct gaze induced increased activation in the right dorsal amygdala regardless of facial expression and head orientation. Furthermore, valence effects were found in the ventral amygdala and strongly dependent on head orientation. We observed enhanced activation to angry and neutral vs happy faces for observer-directed faces in the left ventral amygdala while the averted head condition reversed this pattern resulting in increased activation to happy as compared to angry and neutral faces. These results suggest that gaze direction drives specifically dorsal amygdala activation regardless of facial expression, low-level perceptual factors or stimulus ambiguity. The role of the amygdala is thus not restricted to the detection of potential threat, but has a more general role in attention processes. Furthermore, valence effects are associated with activation of the ventral amygdala and strongly influenced by non-emotional factors.
Keywords: amygdala, attention, emotion, gaze
INTRODUCTION
Processing of gaze direction is of fundamental importance in social cognition. Gaze direction provides information about the attentional focus of another person. Recently it has been suggested that the amygdala is involved in processing gaze direction of faces (Kawashima et al., 1999; Hoffman et al., 2007; Gamer and Büchel, 2009; Straube et al., 2010b; Boll et al., 2011), even though the functional role of the amygdala is not clear yet. While some studies observed increased amygdala activation to direct gaze (Kawashima et al., 1999), others reported enhanced amygdala activation to averted gaze (Hadjikhani et al., 2008; Straube et al., 2010b). Several factors have been proposed to modulate amygdala activation to gaze information, such as ambiguity (Adams et al., 2003), duration of picture presentation (Adams et al., 2012) or size of the eyewhite area (EWA; Hardee et al., 2008). Furthermore, it has been suggested that the amygdala becomes predominantly activated when gaze indicates potential threat, as expressed in angry and fearful faces (Adams et al., 2003; Hadjikhani et al., 2008; N'Diaye et al., 2009).
Interestingly, recent findings showed increased activation of the amygdala to averted gaze also for happy expressions that are known to provide safety signals in both monkeys and humans (Hoffman et al., 2007; Straube et al., 2010b). This suggests that the role of the amygdala is not restricted to the detection of and the response to threat but that the amygdala is more generally involved in gaze processing and attention. Direct support for this assumption also comes from lesion studies that demonstrated that patients with amygdala lesions do not show an attentional shift to the direction of averted gaze, and are not able to glean information about social signals expressed by faces and the eyes in particular (Adolphs et al., 2005; Spezio et al., 2007; Okada et al., 2008).
The relevant factors leading to the increased amygdala activation to averted gaze are still unknown. Straube et al. (2010b) used faces presented in frontal-view which showed either direct or averted gaze. While they could rule out that threat or threat-related ambiguity is the relevant factor to explain the amygdala activation to averted gaze, further confounding factors could not be excluded. The effects observed in this study might be related to a general ambiguity in averted gaze stimuli, as the pictures displayed faces directed to the observer, but with averted gaze. An observer-directed face with an averted gaze might confuse the observer as the target of the facial expression is less clear. Furthermore, there is a perceptual difference between conditions such that the EWA is increased in an observer-directed face that displays an averted gaze (Hardee et al., 2008). General stimulus ambiguity and low-level perceptual factors such as size of the EWA have been proposed to activate the amygdala (Whalen, 1998; Whalen et al., 2004; Straube et al., 2010a). Thus, studies are needed which include a combination of emotional expression, head orientation and gaze direction to assess the relevance of stimulus ambiguity and low-level perceptual factors for the role of the amygdala in processing gaze direction. Furthermore, head direction might be associated with additional effects concerning amygdala activation, for example increased activation to averted head direction. Perrett et al. (1992) suggested that processing of gaze and head direction is hierarchical, with gaze direction being processed prior to head direction when it is accessible. This would lead to the hypothesis that gaze direction affects activation in the amygdala independently of head direction.
In addition, recent studies (Gamer et al., 2010; Straube et al., 2010b) showed differential amygdala activation patterns depending on the valence of the facial expression and the direction of gaze. Remarkably, a recent study in monkeys found a spatial dissociation of the activation in the amygdala for emotion and gaze processing (Hoffman et al., 2007). Averted gaze led to activation in the dorsal and extended amygdala, whereas the effect of emotion was evident in the basolateral amygdala. Thus, gaze effects seem to be associated with attentional functions of the central nucleus within the dorsal amygdala, whereas responses to stimulus valence seem to mainly activate the ventral amygdala that covers the basolateral parts of the amygdala, which have demonstrated stronger involvement in stimulus processing than in the organization of behavioral responses (Kim et al., 2004; Hoffman et al., 2007; Boll et al., 2011). However, it is unknown whether effects of valence of facial expression on amygdalar responses are completely independent of attentional cues such as gaze or head direction.
Taken together, several research questions remain to be explored. It remains unclear whether previously reported effects of gaze direction on amygdala activation depended on ambiguity of attentional conditions or on low-level factors in stimulus display. This can only be tested by including different head directions in the paradigm to control for these confounding factors. Furthermore, there might also be an additional effect of head direction, with increased activation to the averted head direction, as the averted head direction also serves as an attentional cue. In addition, effects of facial expression and gaze should be spatially dissociated within different parts of the amygdala; however, it is not known whether amygdalar responses to facial expressions of different valence are influenced by gaze or head direction.
This study aimed at investigating the effects of head orientation, gaze direction and valence of facial expression on amygdala activation. To test whether averted gaze per se drives amygdala activation, we presented pictures of faces in frontal- and profile-view, displaying happy, neutral and angry facial expressions with either direct or averted gaze. By modulating the head direction, we controlled for increased EWA and general stimulus ambiguity. The following hypotheses were investigated: (i) if the amygdala responds to averted gaze as an indicator of a person’s attentional direction, this should be evident in a main effect of averted gaze direction on amygdala activation regardless of facial expression and head direction; (ii) effects of attention (to gaze) and emotion (to angry/neutral vs happy faces) should be dissociable within the amygdala with attention effects taking place in the dorsal and emotion effects taking place in the ventral amygdala; and (iii) if effects of facial expressions are independent of contextual factors, there should be no effect of head direction or gaze direction on differential activations that are induced by facial expression.
MATERIALS AND METHODS
Participants
Sixteen right-handed healthy subjects with normal or corrected-to-normal vision participated in the study. Participants gave written informed consent for the study. The study was approved by the ethics committee of the University of Jena. Three subjects had to be excluded from data analyses due to abnormal brain activation or to equipment malfunction, leaving a final sample of 13 subjects (mean age = 23.2 ± 2.9 years; 11 females).
Stimuli
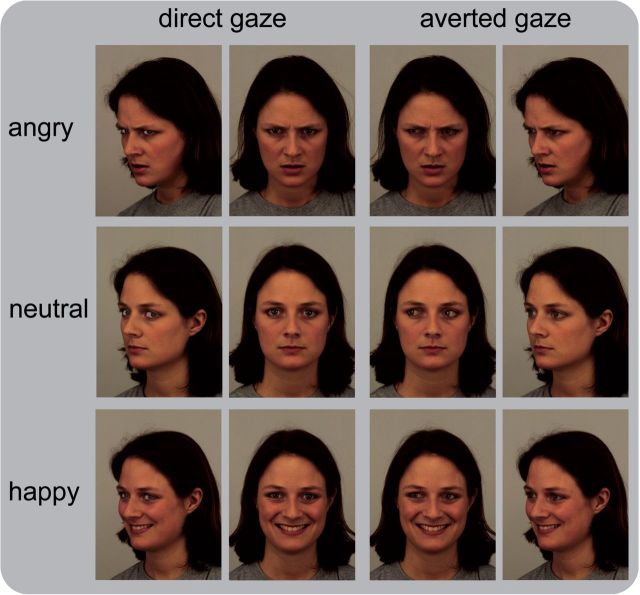
Images were taken from the Karolinska Directed Emotional Faces set (Lundqvist et al., 1998). Images were selected according to two criteria: clear visibility of the eyes and vividness of facial expression. The final picture set contained 168 different pictures of 14 amateur actors (seven females, seven males) displaying happy, neutral and angry facial expressions with either direct or averted gaze in frontal-view as well as profile-view (Figure 1). Gaze direction was manipulated using Adobe Photoshop CS 4 software. The position of the iris of both eyes was shifted to the left in frontal-view pictures (averted gaze) and to the right in profile-view pictures (direct gaze).
Fig. 1.
Example of stimuli. Pictures comprised faces of seven females and seven males. The combination of expression, head direction and gaze direction is shown for one female face.
Experimental design
Pictures were presented via a back-projection screen onto an overhead mirror that was placed in the MR scanner bore, positioned above and slightly in front of the subject’s head. Each picture appeared only once in a run and was presented for 1000 ms. The inter-stimulus interval between two subsequent pictures was 3500 ms. Additionally, 28 null-events depicting a fixation cross were randomly intermixed into the sequence of face stimuli (Josephs and Henson, 1999). During presentation of face pictures, participants performed a gender decision task to ensure that participants paid attention to the stimuli. Responses were measured via one of two buttons of an optic fiber response box with either the first or the middle finger of the right hand. The randomization-order of the pictures and the assignment of buttons to the answer alternatives were counterbalanced across subjects. Presentation of stimuli as well as recording of responses was accomplished with Presentation software (version 14.2; Neurobehavioral Systems, Inc., Albany, CA). After scanning, participants were asked to evaluate the valence and arousal of each picture that they had seen in the scanner by using a nine-point Likert scale (valence: 1 = very unpleasant, 5 = neutral, 9 = very pleasant; arousal: 1 = not arousing, 9 = very arousing).
Data acquisition and analysis
Scanning was performed in a 1.5 T magnetic resonance scanner (‘Magnetom Vision Plus’; Siemens Medical Systems, Erlangen, Germany). After acquisition of a T1-weighted anatomical scan (192 slices, TE = 6 ms, matrix = 256 × 256 mm, resolution = 1 × 1 × 1 mm), two runs of T2*-weighted echo planar images consisting of 300 volumes each were acquired (TE = 50 ms, flip angle = 90°, matrix = 64 × 64, field of view = 192 mm, TR = 2980 ms). Each volume comprised 30 axial slices (thickness = 3 mm, gap = 1 mm, in-plane resolution = 3 × 3 mm). The slices were acquired parallel to the line between anterior and posterior commissure and then rotated through 30° (Deichmann et al., 2003). Before imaging, a shimming procedure was performed to improve field homogeneity. The first four volumes of each run were discarded from the analysis to ensure that steady-state tissue magnetization was reached.
Preprocessing and analysis of the functional data were performed with Brain Voyager QX software (version 2.2; Brain Innovation, Maastricht, The Netherlands). First, all functional volumes of one run were realigned to the first volume in order to minimize artifacts due to head movements, and a slice time correction was conducted. Further data preprocessing comprised spatial (5 mm full-width half-maximum isotropic Gaussian kernel) as well as temporal smoothing (high pass filter: five cycles per run, low pass filter: 2.8 s, linear trend removal). The anatomical and functional images were co-registered and normalized to the Talairach space (Talairach and Tournoux, 1988).
Statistical analysis was performed by multiple linear regression of the signal time course at each voxel. The expected blood oxygen level dependent signal change for each event type (=predictor) was modeled by a hemodynamic response function. Within-group statistical comparisons were conducted using a mixed-effect analysis that considers inter-subject variance and permits population-level inferences. In the first step, predictor estimates were computed for each individual. In the second step, predictor estimates were analyzed across subjects with a repeated measures analysis of variance (ANOVA) followed by planned t-tests. Analysis was performed in the amygdala as region of interest (ROI), which was defined with help of Talairach daemon software (Lancaster et al., 1997, 2000) and the atlas of the human brain (Mai et al., 2004). Peak activations were labeled to specific subregions of the amygdala using the Mai et al. (2004) atlas. According to previous studies that reported functional dissociations within the amygdala in primates (Hoffman et al., 2007; Gamer et al., 2010; Boll et al., 2011), we refer to dorsal and ventral parts of the amygdala. The assignment of subregions of the amygdala to dorsal and ventral parts was done by using high-resolution anatomical maps from a brain atlas (Mai et al., 2004). The dorsal part of the amygdala comprises the corticomedial group with the central nucleus (see also Boll et al., 2011). The basolateral nucleus and lateral nucleus of the amygdala were assigned to the ventral part of the amygdala. Statistical parametric maps resulting from the voxelwise analysis were considered significant for statistical values that survived a cluster-based correction for multiple comparisons as implemented in Brain Voyager (Goebel et al., 2006) which is based on a 3D extension of the randomization procedure described by Forman et al. (1995). Initially, voxel-level threshold was set to P < 0.005 (uncorrected). Thresholded maps were then submitted to an ROI specific correction criterion, which was based on the estimate of the map’s spatial smoothness and on an iterative procedure (Monte Carlo simulation) for estimating cluster-level false-positive rates. After 1000 iterations, the minimum cluster size threshold that yielded a cluster-level false-positive rate of 5% was applied to the statistical maps. Behavioral data were analyzed by means of repeated measures ANOVA using SPSS (version 19; SPSS Inc., Chicago, IL, USA). A probability level of P < 0.05 was considered as statistically significant. Greenhouse–Geisser corrections were used when appropriate to correct violations of sphericity. All data are expressed by mean ± s.e.m. (standard error of mean).
RESULTS
Rating data
Analysis of post-scanning ratings of valence and arousal showed a significant main effect of emotion (valence: F[1.34,16.07] = 84.35, P < 0.001; arousal: F[2,24] = 19.41, P < 0.001). Post hoc t-tests revealed that angry faces were evaluated significantly more unpleasant (t[12] =−8.02, P < 0.001) and significantly more arousing (t[12] = 5.16, P < 0.001) than neutral faces and also as significantly more unpleasant (t[12] = −10.04, P < 0.001) and significantly more arousing (t[12] = 3.92, P < 0.01) than happy faces. Furthermore, happy faces were rated as significantly more pleasant (t[12] = 7.92, P < 0.001) and significantly more arousing (t[12] = 3.03, P < 0.01) than neutral faces.
For valence, there was also a significant main effect of gaze direction (F[1,12] = 6.67, P < 0.05). Subsequent t-tests revealed that faces with direct gaze were evaluated significantly more unpleasant than faces with averted gaze (t[12] =−2.58, P < 0.05). Furthermore, there was a significant interaction of facial expression and head orientation (F[2,24] = 16.57, P < 0.001), with more positive ratings for happy faces when the head was observer-directed (F[1,12] = 19.77, P < 0.001). There was also a significant interaction of valence and gaze direction (F[1.37,16.39] = 4.33, P < 0.05), due to significantly higher positive ratings for happy faces with direct gaze (F[1,12] = 7.31, P < 0.05), and a significant interaction of head orientation and gaze direction (F[1,12] = 14.93, P < 0.01) due to significantly more positive ratings for the congruent conditions. We also observed a significant three-way interaction of facial expression, head direction and gaze direction (F[2,24] = 3.55, P < 0.05). Direct gaze of angry faces was rated as significantly more unpleasant than the averted gaze when the observer-directed head direction was compared to the averted head direction (F[1,12] = 6.21, P < 0.05).
For arousal, there were significant main effects of gaze direction (F[1,12] = 12.86, P < 0.005) and head direction (F[1,12] = 6.22, P < 0.05). Subsequent t-tests indicated that faces with an observer-directed gaze or head were rated as significantly more arousing than faces displaying an averted gaze (t[12] = 3.59, P < 0.005) or averted faces (t[12] = 2.50, P < 0.05). For arousal, we also found a significant interaction between facial expression and head orientation (F[2,24] = 8.24, P < 0.005) with significantly higher ratings for the observer-directed head direction for angry and happy faces compared to neutral faces (angry: F[1,12] = 12.17, P < 0.005; happy: F[1,12] = 8.87, P < 0.05). Furthermore there was a significant interaction of head orientation and gaze direction (F[1,12] = 10.61, P < 0.01) due to significantly higher ratings for the congruent direct conditions. Finally, a significant interaction between facial expression, head orientation and gaze direction (F[2,24] = 4.45, P < 0.05) expressed significantly increased arousal ratings for observer-directed happy faces with direct gaze compared to neutral faces (F[1,12] = 6.58, P < 0.05) (Table 1).
Table 1.
Rating data
| Angry |
Neutral |
Happy |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Head averted |
Head straight |
Head averted |
Head straight |
Head averted |
Head straight |
|||||||
| Gaze averted | Gaze straight | Gaze averted | Gaze straight | Gaze averted | Gaze straight | Gaze averted | Gaze straight | Gaze averted | Gaze straight | Gaze averted | Gaze straight | |
| Valence | 3.15 | 2.72 | 2.87 | 2.72 | 5.17 | 4.57 | 4.68 | 5.00 | 6.16 | 5.92 | 6.28 | 6.66 |
| (0.72) | (0.77) | (0.69) | (0.84) | (0.39) | (0.48) | (0.51) | (0.46) | (0.61) | (0.82) | (0.77) | (0.63) | |
| Arousal | 4.43 | 5.13 | 4.90 | 5.35 | 2.46 | 3.29 | 2.76 | 2.68 | 3.42 | 3.68 | 3.64 | 3.89 |
| (1.97) | (2.04) | (2.07) | (2.10) | (1.44) | (1.76) | (1.59) | (1.64) | (1.45) | (1.54) | (1.53) | (1.57) | |
Values are represented as mean (s.d.).
Functional magnetic resonance imaging data
Main effects
There was a significant effect of gaze in the right dorsal amygdala (coordinates of peak voxel [x,y,z] = 21,−13,−9; size = 108 mm3; F[1,12] = 17.28, P < 0.05, corr.; Figure 2). As indicated in Figure 2, the significant main effect of gaze in the amygdala was based on increased activation to averted vs direct gaze across facial expression and head directions. According to the Mai et al. (2004) atlas, the peak activation was observed in the central nucleus of the amygdala. There were no significant main effects of head direction or emotional expression.
Fig. 2.
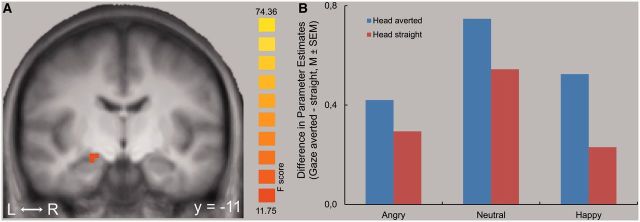
Main effect of gaze direction. (A) Increased activation to averted gaze compared to direct gaze in the right amygdala. Statistical parametric maps of the ROI analysis are overlaid on a T1 scan (P < 0.005, corrected; radiological convention: left = right; y = −11). (B) The plot shows the difference of mean parameter estimates (averted gaze – direct gaze) for the maximally activated voxel for each of the three facial expressions.
Interactions
In the right and left ventral parts of the amygdala, we found a significant interaction between facial expression and head direction (right: coordinates of peak voxel [x,y,z] = 17,−3,−20; size = 162 mm3; F[2,24] = 19.28, P < 0.05, corr.; left: coordinates of peak voxel [x,y,z] =−21,−5,−13; size = 540 mm3; F[2,24] = 11.97, P < 0.05, corr.; Figure 3). According to the atlas of Mai et al. (2004) both coordinates refer to the lateral amygdala.
Fig. 3.
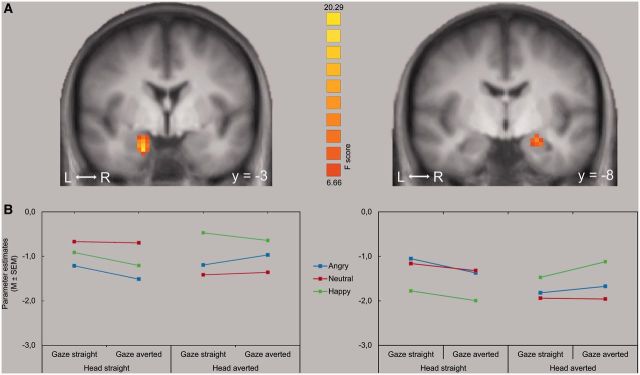
Interaction between head direction and emotion. (A) Increased activation in the right and left amygdala to the interaction of head direction and valence of facial expression. Statistical parametric maps of the ROI analysis are overlaid on a T1 scan for the right and left amygdala (P < 0.001, corrected; radiological convention: left = right). (B) The plots show the mean parameter estimates for the maximally activated voxel for each of the three facial expressions in the respective amygdala.
For observer-directed faces, t-tests revealed significantly increased activation in the left amygdala to angry and neutral faces compared to happy faces (t[12] = 5.30, P < 0.005, and t[12] = 3.23, P < 0.005). However, for the profile-view faces, the pattern was reversed. Here, the left amygdala activation was significantly increased for happy as compared to angry (t[12] = 3.63, P < 0.005) and to neutral (t[12] = 3.69, P < 0.005) faces.
In the right amygdala, happy faces elicited also significantly more activation than angry (t[12] = 3.88, P < 0.005) and neutral faces (t[12] = 4.27, P < 0.005) for the averted head direction. However, for the observer-directed head direction, neutral faces showed significantly increased activation as compared to happy faces (t[12] = 4.57, P < 0.005) and to angry faces (t[12] = 3.30, P < 0.005).
There was no significant interaction between facial expression and gaze or head direction and gaze in the amygdala. There was also no significant three-way interaction between these factors.
DISCUSSION
In this event-related functional magnetic resonance imaging study, we found increased amygdala activation for faces with averted gaze compared to faces with direct gaze, regardless of facial expression (angry, happy and neutral) or head orientation. This finding indicates that the amygdala is involved in attention processes and that averted gaze functions as an indicator of socially relevant information that drives amygdala activation. Furthermore, there were different activation patterns in the amygdala to facial expressions depending on head orientation, suggesting strong effects of non-emotional factors on amygdala activation.
Amygdala activation to gaze direction
The majority of previous findings on the role of gaze direction on amygdala activation has been interpreted in terms of threat relevance of faces or ambiguity of the source of threat (Adams et al., 2003; Hadjikhani et al., 2008; N'Diaye et al., 2009). In contrast to the conclusions drawn by these studies, recent findings suggest that the amygdala has a more general role in attention processes and is not restricted to the detection of threat (Hoffman et al., 2007; Straube et al., 2010b). For instance, Straube et al. (2010b) found enhanced amygdala activation to averted gaze also for happy faces, indicating that threat or threat-related ambiguity is not the relevant factor for this effect. However, it was still unclear whether averted gaze per se drives amygdala activation or whether other factors such as general stimulus ambiguity or low-level perceptual factors play a role (Whalen et al., 2004; Hardee et al., 2008; Straube et al., 2010a).
The present finding of enhanced amygdala responses to averted gaze regardless of facial expression and head direction supports the hypothesis that the amygdala is strongly involved in attentional functions associated with gaze processing. Because we also varied head direction we can rule out that general stimulus ambiguity or low-level perceptual factors such as increased EWA led to the activation of the amygdala. The finding of the present study is in line with several studies suggesting a critical role for the amygdala in attention processes (Adolphs et al., 2005; Gamer and Büchel, 2009; Adolphs, 2010). In particular, Gamer and Büchel (2009) showed that amygdala activation correlates with eye movements to the eye region in fearful faces and emphasized the role of the amygdala in reflexive gaze initiations to salient stimuli. Further evidence comes from lesion studies. Attention to eye information and attentional cuing effects by averted gaze are disturbed in patients with amygdala lesions (Adolphs et al., 2005; Adolphs and Spezio, 2006; Akiyama et al., 2007; Spezio et al., 2007). The finding of a main effect of gaze direction but not head direction in the amygdala is also consistent with previous studies which proposed a hierarchical model for the processing of these cues (Perrett et al., 1992). However, there might be cell populations within the amygdala that are responding specifically to full profile faces and averted profile faces (Tazumi et al., 2010), which might be detected with increased spatial resolution of responses in the amygdala in future studies.
Furthermore, the arousal ratings of the present study also indicate that arousal alone cannot explain amygdala activation to averted gaze. In humans, direct gaze is typically more arousing than averted gaze. Several studies proposed that this strong arousal might lead to stronger amygdala activation to direct gaze [for a review, see Senju and Johnson (2009)]. However, although the rating data of the present study also showed that direct gaze was regarded as more arousing, this was not associated with increased amygdala activation.
In the present study we localized the largest effect of gaze in the dorsal amygdala, which is in accordance with previous studies in humans (Gamer et al., 2010; Straube et al., 2010b) and monkeys (Hoffman et al., 2007). The dorsal amygdala comprises the central amygdaloid nucleus as part of the corticomedial nuclei group. These structures have been proposed to be mainly involved in attentional and executive functions (Davis and Whalen, 2001). A role in vigilance and attention to behaviorally relevant faces has for example been shown in a recent study by Boll et al. (2011).
Effects of valence of facial expression on amygdala activation
This study tested the hypothesis that effects of facial expression (valence effects) would be found in the ventral amygdala. And indeed, in contrast to gaze direction which significantly modulated the activity of the dorsal amygdala, effects of facial expression mainly influenced activation in ventral parts of the amygdala. The ventral amygdala comprises the basolateral nuclei, which form the main visual input region of the amygdala (Amaral et al., 1992; Sah et al., 2003) and which have been proposed to be involved in basic facial feature encoding (Boll et al., 2011) and processing of emotional facial expressions (Hoffman et al., 2007; Gamer et al., 2010). This dissociation of dorsal and ventral parts within the amygdala is also in line with a recent meta-analysis study by Mende-Siedlecki et al. (2013), in which the authors found differences in the response of neuronal populations in ventral and dorsal parts of the amygdala for valence (ventral amygdala) and salience (dorsal amygdala) of a facial expression.
Effects in the ventral amygdala in the present study might not necessarily reflect effects of valence of these facial expressions. For example, it was shown that the response to facial expressions is influenced by the ambiguity and intensity of the expressions and the attractiveness of faces (Rotshtein et al., 2001; Adams et al., 2003; Winston et al., 2003, 2007; Straube et al., 2010b), including differential activation to different negative expressions. The amygdala response also seems to be determined by the general arousal that is induced by emotional stimuli (i.e. Anderson et al., 2003; Costa et al., 2010; Hamann and Mao, 2002; Sabatinelli et al., 2005; Small et al., 2003; Straube et al., 2005, 2007, 2011). Thus, even when comparing happy and angry facial expressions it is difficult to distinguish between valence and arousal, since threat-related faces are more arousing than happy faces (see also Straube et al., 2005). Furthermore, it has been proposed that distinct subpopulations of neurons respond to positive and negative stimuli (Paton et al., 2006).
In contrast, we suggest that arousal cannot explain the pattern of results since both high (angry faces) and low arousing faces (neutral faces) show a differential activation as compared to happy faces. In this study, we observed enhanced amygdala activation to angry and neutral faces as compared to happy faces in the left amygdala for the direct head orientation. This replicates previous findings (Hoffman et al., 2007; Straube et al., 2010b). Remarkably, the presentation of faces in profile-view completely reversed the pattern. Now happy faces induced increased activation as compared to angry and neutral faces, in both the left and the right amygdala. This strongly suggests that head orientation affects amygdala responses to facial expressions. It also shows that amygdala activation is not simply dependent on facial expression or valence of facial expression, but that contextual factors such as head orientation affect these responses; depending on head orientation opposite outcomes can be observed. One explanation for this unexpected finding might be that the social relevance of an averted happy face is less clear and therefore more processing resources are engaged when observing happy faces in a profile-view. This would lead to a gain of affective information processing, associated with increased basolateral amygdala activation.
CONCLUSION
Taken together, our findings reveal that the dorsal amygdala is sensitive to averted gaze regardless of facial expression and head direction and thus regardless of stimulus ambiguity, low-level perceptual factors or threat relevance. This indicates that the amygdala has a more general role in gaze processing and attentional functions. Furthermore, there was a spatial dissociation of main effects of gaze in the dorsal amygdala and effects of facial expression in the ventral amygdala. Finally, valence effects were strongly dependent on head orientation, suggesting strong influences of non-emotional factors on amygdala responses to facial expressions.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) [project STR 987/3-1].
REFERENCES
- Adams RB, Jr, Gordon HL, Baird AA, Ambady N, Kleck RE. Effects of gaze on amygdala sensitivity to anger and fear faces. Science. 2003;300:1536. doi: 10.1126/science.1082244. [DOI] [PubMed] [Google Scholar]
- Adams RB, Jr, Franklin RG, Kveraga K, et al. Amygdala responses to averted vs direct gaze fear vary as a function of presentation speed. Social Cognitive and Affective Neuroscience. 2012;7:568–77. doi: 10.1093/scan/nsr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Spezio M. Role of the amygdala in processing visual social stimuli. Progress in Brain Research. 2006;156:363–78. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Kato M, Muramatsu T, Umeda S, Saito F, Kashima H. Unilateral amygdala lesions hamper attentional orienting triggered by gaze direction. Cerebral Cortex. 2007;17:2593–600. doi: 10.1093/cercor/bhl166. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggelton JP, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- Anderson AK, Christoff K, Stappen I, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Boll S, Gamer M, Kalisch R, Büchel C. Processing of facial expressions and their significance for the observer in subregions of the human amygdala. Neuroimage. 2011;56:299–306. doi: 10.1016/j.neuroimage.2011.02.021. [DOI] [PubMed] [Google Scholar]
- Costa VD, Lang PJ, Sabatinelli D, Versace F, Bradley MM. Emotional imagery: assessing pleasure and arousal in the brain's reward circuitry. Human Brain Mapping. 2010;31:1446–57. doi: 10.1002/hbm.20948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–41. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic-resonance-imaging (fMRI)—use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gamer M, Büchel C. Amygdala activation predicts gaze toward fearful eyes. Journal of Neuroscience. 2009;29:9123–26. doi: 10.1523/JNEUROSCI.1883-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Büchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9400–5. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with BrainVoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Hoge R, Snyder J, de Gelder B. Pointing with the eyes: the role of gaze in communicating danger. Brain and Cognition. 2008;68:1–8. doi: 10.1016/j.bandc.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann SB, Mao H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport. 2002;13:15–19. doi: 10.1097/00001756-200201210-00008. [DOI] [PubMed] [Google Scholar]
- Hardee JE, Thompson JC, Puce A. The left amygdala knows fear: laterality in the amygdala response to fearful eyes. Social Cognitive and Affective Neuroscience. 2008;3:47–54. doi: 10.1093/scan/nsn001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facial-expression and gaze-selective responses in the monkey amygdala. Current Biology. 2007;17:766–72. doi: 10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Josephs O, Henson RNA. Event-related functional magnetic resonance imaging: modelling, inference and optimization. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1999;354:1215–28. doi: 10.1098/rstb.1999.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima R, Sugiura M, Kato T, et al. The human amygdala plays an important role in gaze monitoring. A PET study. Brain. 1999;122:779–83. doi: 10.1093/brain/122.4.779. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, et al. Contextual modulation of amygdala responsivity to surprised faces. Journal of Cognitive Neuroscience. 2004;16:1730–45. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, et al. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Human Brain Mapping. 1997;5:238–42. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. 1998. The Karolinska Directed Emotional Faces—KDEF, CD ROM from Department of Clinical Neuroscience, Psychology Section, Karolinska Institutet, ISBN 91-630-7164-9. [Google Scholar]
- Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. San Diego, CA: Academic Press; 2004. [Google Scholar]
- Mende-Siedlecki P, Said CP, Todorov A. The social evaluation of faces: a meta-analysis of functional neuroimaging studies. Social Cognitive and Affective Neuroscience. 2013;8:285–99. doi: 10.1093/scan/nsr090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N'Diaye K, Sander D, Vuilleumier P. Self-relevance processing in the human amygdala: gaze direction, facial expression, and emotion intensity. Emotion. 2009;9:798–806. doi: 10.1037/a0017845. [DOI] [PubMed] [Google Scholar]
- Okada T, Sato W, Kubota Y, et al. Involvement of medial temporal structures in reflexive attentional shift by gaze. Social Cognitive and Affective Neuroscience. 2008;3:80–8. doi: 10.1093/scan/nsm027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–70. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett DI, Hietanen JK, Oram MW, Benson PJ. Organization and functions of cells responsive to faces in the temporal cortex. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 1992;335:23–30. doi: 10.1098/rstb.1992.0003. [DOI] [PubMed] [Google Scholar]
- Rotshtein P, Malach R, Hadar U, Graif M, Hendler T. Feeling or features: different sensitivity to emotion in high-order visual cortex and amygdala. Neuron. 2001;32:747–57. doi: 10.1016/s0896-6273(01)00513-x. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–70. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL, De Armentia ML, Power J. The amygdaloid complex: anatomy and physiology. Physiological Reviews. 2003;83:803–34. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Senju A, Johnson MH. The eye contact effect: mechanisms and development. Trends in Cognitive Sciences. 2009;13:127–34. doi: 10.1016/j.tics.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–11. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Huang PYS, Castelli F, Adolphs R. Amygdala damage impairs eye contact during conversations with real people. Journal of Neuroscience. 2007;27:3994–7. doi: 10.1523/JNEUROSCI.3789-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Dietrich C, Mothes-Lasch M, Mentzel HJ, Miltner WHR. The volatility of the amygdala response to masked fearful eyes. Human Brain Mapping. 2010a;31:1601–8. doi: 10.1002/hbm.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Langohr B, Schmidt S, Mentzel HJ, Miltner WHR. Increased amygdala activation to averted versus direct gaze in humans is independent of valence of facial expression. Neuroimage. 2010b;49:2680–6. doi: 10.1016/j.neuroimage.2009.10.074. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52:163–8. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- Straube T, Sauer A, Miltner WH. Brain activation during direct and indirect processing of positive and negative words. Behavioral Brain Research. 2011;222:66–72. doi: 10.1016/j.bbr.2011.03.037. [DOI] [PubMed] [Google Scholar]
- Straube T, Weiss T, Mentzel HJ, Miltner WH. Time course of amygdala activation during aversive conditioning depends on attention. Neuroimage. 2007;34:462–9. doi: 10.1016/j.neuroimage.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Stuttgart: Thieme; 1988. [Google Scholar]
- Tazumi T, Hori E, Maior RS, Ono T, Nishijo H. Neural correlates to seen gaze-direction and head orientation in the macaque monkey amygdala. Neuroscience. 2010;169:287–301. doi: 10.1016/j.neuroscience.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7:177–88. [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- Winston JS, O'Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. Neuroimage. 2003;20:84–97. doi: 10.1016/s1053-8119(03)00303-3. [DOI] [PubMed] [Google Scholar]
- Winston JS, O'Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]