Abstract
Lack of empathy is a hallmark of social impairments in individuals with autism spectrum disorder (ASD). However, the concept empathy encompasses several socio-emotional and behavioral components underpinned by interacting brain circuits. This study examined empathic arousal and social understanding in individuals with ASD and matched controls by combining pressure pain thresholds (PPT) with functional magnetic resonance imaging (study 1) and electroencephalography/event-related potentials and eye-tracking responses (study 2) to empathy-eliciting stimuli depicting physical bodily injuries. Results indicate that participants with ASD had lower PPT than controls. When viewing body parts being accidentally injured, increased hemodynamic responses in the somatosensory cortex (SI/SII) but decreased responses in the anterior mid-cingulate and anterior insula as well as heightened N2 but preserved late-positive potentials (LPP) were detected in ASD participants. When viewing a person intentionally hurting another, decreased hemodynamic responses in the medial prefrontal cortex and reduced LPP were observed in the ASD group. PPT was a mediator for the SI/SII response in predicting subjective unpleasantness ratings to others’ pain. Both ASD and control groups had comparable mu suppression, indicative of typical sensorimotor resonance. The findings demonstrate that, in addition to reduced pain thresholds, individuals with ASD exhibit heightened empathic arousal but impaired social understanding when perceiving others’ distress.
Keywords: autism spectrum disorder (ASD), empathy, pressure pain thresholds (PPT), functional MRI (fMRI), electroencephalography/event-related potentials (EEG/ERP)
INTRODUCTION
Autism spectrum disorder (ASD) is a complex neurodevelopmental disorder associated with problems with social interaction and communication. Lack of empathy is one of social impairments in ASD (Gillberg, 1992; Frith and Happé, 2005; Baron-Cohen et al., 2009). ASD individuals’ failure to perceive and/or respond to others’ affective expressions was postulated to result in difficulties in social orienting (Hobson, 1993; Dawson et al., 1998). The exact nature of empathic deficits in ASD, however, is not well understood. Given the complexity of the phenomenological experience of empathy, investigating the neurobiological underpinnings requires breaking down the construct into component processes that empathy encompasses (Batson, 2009).
Empathy is a multidimensional construct composed of dissociable neurocognitive components that interact and operate in parallel fashion (Blair, 2005; Shamay-Tsoory, 2009; Baird et al., 2011; Decety, 2011; Zaki and Ochsner, 2012), including cognitive, emotional and sensorimotor resonance components. Cognitive empathy is similar to some extent to the construct of theory of mind (ToM), i.e. the ability to explain, predict and interpret behavior by attributing mental states such as desire, beliefs, intentions and emotions to oneself and to other people (Decety and Svetlova, 2012). Emotional empathy involves the capacity to either share or become affectively aroused by others’ emotions, commonly referred to emotional contagion or empathic arousal. While there is no clear agreement on which component of empathy is impaired in ASD, a general consensus seems to support that impaired cognitive empathy in ASD is associated with mentalizing dysfunction (Baron-Cohen et al., 1985; Ozonoff et al., 1991; Baron-Cohen and Swettenham, 1997) and reduced hemodynamic responses in the brain regions involved in ToM, i.e. the medial prefrontal cortex (mPFC) and posterior superior temporal sulcus/temporoparietal junction (pSTS/TPJ) (Baron-Cohen et al., 1999; Frith, 2001; Pelphrey et al., 2011). However, the extent to which emotional empathy is impaired in ASD is not well known. Some studies reported that ASD individuals fail to activate amygdala and fusiform gyrus when perceiving facial expressions (Pelphrey and Carter, 2008), whereas other studies found that gaze fixation influenced their activation (Dalton et al., 2005) and that amygdala hyperarousal was a result of reduced neural habituation (Kleinhans et al., 2009). Recently, empathic deficits in ASD have been proposed to result from disrupted bottom-up engagement of sensorimotor resonance (Winkielman et al., 2009; Keysers et al., 2011), implemented by the mirror neuron system (MNS) (Cattaneo and Rizzolatti, 2009). Consistent with this hypothesis, one study found reduced hemodynamic responses in regions involved in the MNS to emotional faces (Dapretto et al., 2006), but subsequent studies were unable to replicate these findings (Dinstein et al., 2010; Fan et al., 2010; Schulte-Ruther et al., 2011). Another hypothesis proposed an empathy imbalance in ASD, associated with a deficit of cognitive empathy but a surfeit of emotional empathy (Smith, 2009).
Pain is a special psychological state with great evolutionary significance. The perception of pain in others acts as an empathic signal, alerting individuals that a conspecific is at risk, attracting their attention and motivating social behaviors (Craig, 2009). The neural response to the distress of others has been used as a proxy to provide insight into the neurobiological underpinnings of empathy (e.g. Decety, 2007; Decety and Michalska, 2010). A number of neuroimaging studies have reliably documented that the neural network involved in perceiving others in pain and direct experience of pain includes the anterior mid-cingulate cortex (aMCC), anterior insula cortex (AIC), supplementary motor area, periaqueductal gray and somatosensory cortex (SI/SII) (for a meta-analysis, see Lamm et al., 2011). Moreover, when perceiving an individual hurt by another intentionally, the mPFC and pSTS/TPJ, regions consistently found to be engaged in social understanding (Decety et al., 2008; Akitsuki and Decety, 2009; Decety and Michalska, 2010), are additionally recruited. Several studies have used electroencephalographic event-related potentials (EEG/ERP) to investigate the temporal dynamics of neural responses to perceiving others’ pain (Fan and Han, 2008; Han et al., 2008; Li and Han, 2010; Chen et al., 2012; Cheng et al., 2012), documented frontocentral N2, late positive potentials (LPP) and mu suppression. N2 was associated with affective arousal and attention novelty (Cuthbert et al., 2000; Olofsson and Polich, 2007; Ibanez et al., 2012). It has been suggested that LPP could be a neurophysiological indicator for emotion regulation (Olofsson et al., 2008; Dennis and Hajcak, 2009). Mu suppression, supposedly generated in the sensorimotor cortex, is sensitive to the perception of stimuli depicting painful situations (Cheng et al., 2008; Perry et al., 2010). Examining the neuroimaging and neurophysiological response in ASD while viewing someone being harmed could provide insight into the neural processes underlying empathic deficits.
To provide a better understanding of the nature of empathic deficits in ASD, we used a well-validated functional magnetic resonance imaging (fMRI) and EEG/ERP paradigm (Decety et al., 2008; Akitsuki and Decety, 2009; Chen et al., 2012; Cheng et al., 2012) to investigate the component processes involved in perceiving others in pain. It was hypothesized that if the sensorimotor resonance component of empathy is impaired in ASD, then these individuals with ASD would exhibit reduced hemodynamic responses in the SI/SII and failure to suppress the mu rhythm induced by the perception of others in pain. If emotional component of empathy is altered, participants with ASD would change the responses in the neural network involved in perceiving others’ pain, including the aMCC, AIC and N2. If the cognitive component of empathy is impaired, we anticipate reduced activation in the neural network subserving social understanding and mentalizing, which comprises the mPFC and pSTS/TPJ, along with reduced LPP when the pain is intentionally inflicted by another individual. Furthermore, considering the potential influence of autistic traits and atypical sensory processing on the hemodynamic and electrophysiological responses to empathy-eliciting stimuli (Hilton et al., 2010), we conducted a mediation analysis to test whether the pressure pain threshold (PPT) is a significant mediator for the relationship between the responses in the SI/SII, aMCC and AIC and ratings of unpleasantness to perceiving others’ pain as well as multiple regression analyses to test to the extent to which LPP co-varies with PPT and Autism-Spectrum Quotient (AQ).
MATERIALS AND METHODS
Participants
The study sample consisted of 24 participants with ASD and 21 typically developing controls (TDC) in the fMRI experiment and 20 ASD and 20 TDC in the EEG/ERP experiment (Table 1). Fourteen of the subjects (7 ASD) participated in both experiments. Additional 20 individuals who were tested in the fMRI experiment (n = 7; 3 TDC) and in the EEG/ERP experiment (n = 13; 5 TDC) were not included in the final sample because of motion artifacts or technical failures. All participants were Chinese males aged 16–29 years with IQ > 90 as estimated by the Wechsler Intelligence Scale. Participants with a comorbid psychiatric or medical condition, history of head injury, or genetic disorder associated with autism were excluded. The ASD group consisted of non-medicated individuals with ASD, recruited from a community autism program. The diagnosis of ASD was confirmed using the DSM-IV diagnostic criteria as well as the Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994). The TDC group was recruited from local schools and screened for major psychiatric and neurological illnesses by a physician. All participants and their parents gave written informed consent for the study, which was approved by the local Ethics Committee (Yang-Ming University Hospital) and conducted in accordance with the Declaration of Helsinki.
Table 1.
Demographic and clinical variables of the study participants
| fMRI |
EEG/ERP |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TDC (N = 21 males) |
ASD (N = 24 males) |
TDC (N = 20, 2 females) |
ASD (N = 20, 2 females) |
|||||||
| Mean | SD | Mean | SD | P | Mean | SD | Mean | SD | P | |
| Age | 19.3 | 3.4 | 18.4 | 2.8 | 0.33 | 21.0 | 3.3 | 20.4 | 4.3 | 0.63 |
| IQ | 111.5 | 10.3 | 107.0 | 11.2 | 0.18 | 109.7 | 14.3 | 105.7 | 6.9 | 0.45 |
| TAS-20-C | 47.3 | 2.8 | 61.7 | 3.8 | <0.001 | 48.7 | 6.1 | 62.2 | 4.4 | <0.001 |
| ADI-R | ||||||||||
| Social | — | — | 19.5 | 7.2 | — | — | — | 19.8 | 6.4 | — |
| Comm. | — | — | 15.6 | 6.3 | — | — | — | 16.2 | 5.8 | — |
| Repeti. | — | — | 4.9 | 2.2 | — | — | — | 5.3 | 2.7 | — |
| UNPLE | 3.3 | 0.7 | 1.8 | 0.8 | <0.001 | 2.7 | 0.6 | 2.4 | 1.2 | 0.66 |
| PPT | 2.9 | 0.1 | 2.3 | 0.3 | <0.001 | 3.2 | 0.6 | 2.7 | 0.6 | 0.008 |
TDC, typically developing control; ASD, autism spectrum disorder; IQ, intelligence quotient; TAS-20-C, Chinese translation of the 20-item Toronto Alexithymia Scale; ADI-R, Autism Diagnostic Interview-Revised; Social, social interaction (cuff-off = 10); Comm., communication (cut-off = 8); Repeti., repetitive behavior (cut-off = 3); UNPLE, unpleasantness ratings; PPT, pressure pain threshold (kg/cm2).
General procedures
Before fMRI scanning and EEG/ERP recording, each participant underwent assessments of the AQ (Baron-Cohen et al., 2001), 20-item Toronto Alexithymia Scale (TAS-20-C) (Zhu et al., 2007) and PPT examination (Supplementary Materials). After scanning and recording, participants were asked to rate the unpleasantness of the stimuli, using a 6-point scale on the Facial Pain Scale-Revised (FPS-R) (Bieri et al., 1990). Eye tracking was monitored during these post-scanning ratings.
Visual stimuli
A total of 48 stimuli consisting of successive presentation of images depicting body parts (hands and feet) being injured or not were used. The injuries were either accidental or intentionally inflicted by another individual (see Decety et al., 2008; Akitsuki and Decety, 2009; Decety and Michalska, 2010 for validation). Each animation consisted of three digital color pictures, which were edited to the same size (205 × 154 pixels). The durations of the first, second and third pictures were 1000, 200 and 1000 ms, respectively. A total of four categories were included:
One person is in a painful situation accidentally caused (Solo Pain, SP).
One person is involved in a non-painful situation (Solo No-pain, SN).
One person is in a painful situation, intentionally caused by another individual (Dyad Pain, DP).
Two individuals are present and moving, but there is no pain in the interaction (Dyad No-pain, DN).
fMRI experiment
MRI data acquisition, image processing and statistical analysis
Structural and functional MRI data were acquired on a 3 T MRI scanner (Siemens Magnetom Tim Trio, Erlanger, German) equipped with a high-resolution 12-channel head array coil. Image processing and analysis was carried out using SPM5 (Wellcome Department of Imaging Neuroscience, London, UK). Please refer to Supplementary Materials for details. Participants went through four sessions of functional and one session of high-resolution structural scanning. Functional scanning consisted of four blocks during which stimuli from one of the four categories were shown.
ROI definition and analysis
Three regions of interest (ROIs), including aMCC, AIC and SI/SII, were selected individually for each subject, using a combination of anatomical and functional criteria (Supplementary Table S1). To assess the relationship between the hemodynamic responses to empathy-eliciting stimuli, subjective unpleasantness ratings to others’ pain, sensitivity to first-hand pain, alexithymic traits and autistic traits, multiple regression analyses were performed to determine which ROIs were significantly associated with unpleasantness ratings, PPT values, TAS-20-C and AQ.
Mediation analysis
Based on a standard three-variable path model with a bootstrap test for the statistical significance of the product a*b, a single-level version of the mediation path model was used to get further insight of linkage between the behavioral output and brain hemodynamic response (Supplementary Figure S1). Matlab coding implementing mediation analyses, developed by Dr. Wager and his colleagues, is freely available at http://www.columbia.edu/cu/psychology/tor/ (Wager et al., 2008).
EEG/ERP experiment
Recordings and statistical analysis
Each participant was asked to watch the visual stimuli while oculomotor activity was measured with a Tobii eye tracker (Supplementary Figure S2). Following each stimulus, participants were instructed to choose one of the response buttons and judge whether the scenarios depicted pain or no-pain. A total of three Solo and three Dyad sessions were included. The order of the sessions was randomized and counterbalanced across participants.
Time windows for the ERP components of interest (N2 and LPP) were chosen according to visual inspection of the grand-averaged data as well as previous studies (Chen et al., 2012; Cheng et al., 2012). Statistical analyses were conducted, separately for each context (Solo vs Dyad), using a mixed analysis of variance (ANOVA) with two within-subject factors: (i) stimulus (Pain vs No-pain) (ii) region (Fz, Cz, Pz, Oz) and one between-subject factor: group (ASD vs TDC) was computed. The dependent variable was the mean amplitudes of N2 and LPP at the selected electrode sites. Tukey’s test was conducted only when preceded by significant effects.
Mu rhythm
The ∼10 Hz power spectrum densities were analyzed from the averaged epochs for the first picture and the third picture, respectively (Supplementary Materials). Statistical analysis used a mixed ANOVA. Tukey’s test was conducted only when preceded by significant effects.
RESULTS
Behavioral performance
The participants’ demographics, subjective ratings and pain thresholds are listed in Table 1 and Supplementary Table S2. The data on the pressure algometry indicated that participants with ASD compared with TDC were more sensitive to first-hand pain [t (81) = 28.82, P < 0.001, effect size d = 0.91]. During EEG recordings, most of the participants could correctly make pain judgments (mean accuracy = 92.2%). ASD did not differ from TDC.
Oculomotor performance
For the Solo scenarios, the mixed ANOVA model showed that the stimulus (P = 0.93), the group (P = 0.72), and their interaction (P = 0.49) did not reach significance. For the Dyad, neither the stimulus (P = 0.21) nor the group (P = 0.80) had an effect, but their interaction was significant (P = 0.031, d = 0.73). Post hoc analysis indicated that TDC had longer gaze duration on Dyad Pain relative to Dyad No-pain (P = 0.024, d = 0.21), whereas no such differences were observed in ASD (P = 0.51). This result is suggestive of atypical attention processing for social understanding in ASD.
Functional MRI results
Whole brain analyses for perceiving stimuli depicting painful relative to non-painful situations [(SP + DP) − (SN + DN)] showed that TDC participants exhibited significant signal increase in regions implicated in both the affective/motivational and sensory discriminative components of physical pain processing, including aMCC, AIC, left precentral and SI/SII. ASD showed activation in the SI/SII bilaterally (Figure 1A). When viewing stimuli depicting injuries caused by another individual [(DP + DN) − (SP + SN)], TDC showed activation in the superior temporal gyrus, pSTS/TPJ, temporal pole, mPFC, posterior cingulate and superior frontal gyrus. Participants with ASD showed activation in the left precentral gyrus, right inferior temporal gyrus and left lingual gyrus (Figure 1B).
Fig. 1.
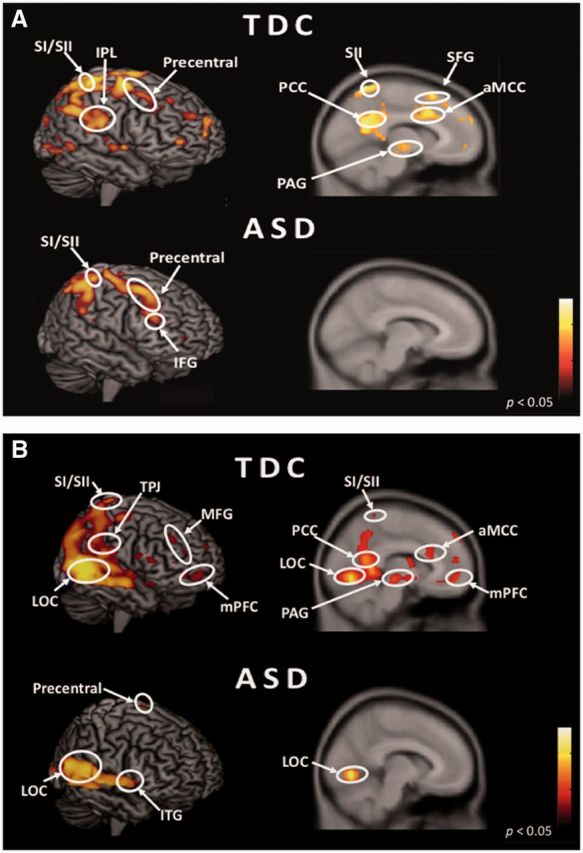
Hemodynamic responses to perceiving the injury accidentally caused and intentionally inflicted by another individual. (A) Pain effect [Pain vs No-pain: (SP + DP) − (SN + DN)] in participants with typically developing control (TDC) and autism spectrum disorder (ASD). (B) Social effect [Dyad vs Solo: (DP + DN) − (SP + SN)] in TDC and ASD. SI/SII, somatosensory cortex; anterior mid-cingulate cortex (aMCC); TPJ, temporoparietal junction; LOC, lateral occipital cortex; PCC, posterior cingulate cortex; PAG, periaqueductal gray; SFG, superior frontal gyrus; MFG, middle frontal gyrus; IFG, inferior frontal gyrus; mPFC, medial prefrontal cortex; ITG, inferior temporal gyrus.
A direct voxel-by-voxel comparison between two groups showed greater signal change in the aMCC and AIC in TDC compared with ASD, whereas ASD relative to TDC was associated with greater hemodynamic signal in the SI/SII and inferior frontal gyrus in response to perceiving others’ pain (Supplementary Table S3). The interaction of group by social understanding indicates that TDC relative to ASD showed stronger activation in the right mPFC, bilateral posterior cingulate and pSTS/TPJ, whereas ASD compared to TDC had no significant activation (Supplementary Table S4). The interaction of pain and social understanding by group demonstrated that TDC relative to ASD showed more activation in the caudate nucleus and right AIC (Supplementary Table S5).
Correlation with subjective ratings, pain thresholds and alexithymic traits
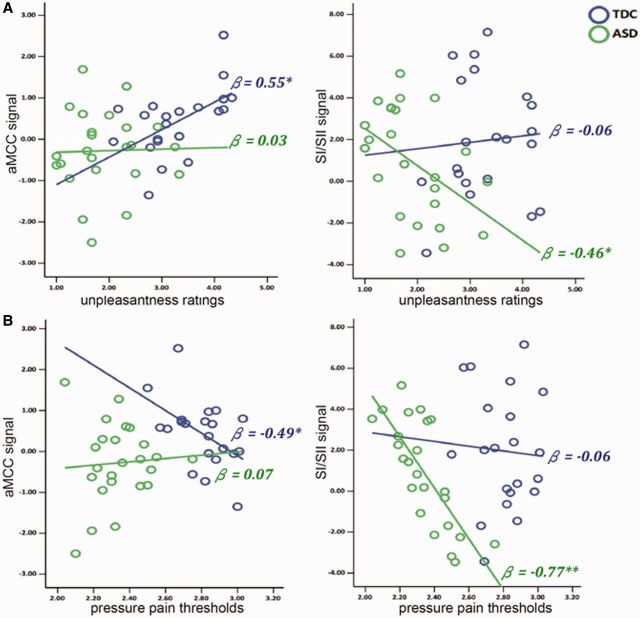
A multiple regression model predicting unpleasantness ratings from beta-values at all three ROIs (stepwise) indicated an at least partly independent contribution of the aMCC in TDC individuals [standardized β = 0.55, t (20) = 2.82, P = 0.011], while the inclusion of AIC and SI/SII did not improve the fit of the model. Increased aMCC activation in response to perceiving others’ pain was related to more unpleasantness ratings. In contrast, increased SI/SII activation was related to decreased unpleasantness ratings in ASD [standardized β = −0.46, t (23) = −2.44, P = 0.023], while the inclusion of AIC and aMCC did not improve the fit of the model (Figure 2A).
Fig. 2.
Correlations between hemodynamic responses, unpleasantness ratings and pain thresholds. (A) Unpleasantness ratings are correlated with hemodynamic responses in anterior mid-cingulate cortex (aMCC) in TDC (β = 0.55) but with somatosensory cortex (SI/SII) in ASD (β = −0.46). (B) Pressure pain thresholds (PPT) are associated with aMCC in TDC (β = −0.49) but with SI/SII in ASD (β = −0.77).
A multiple regression model predicting PPT from beta-values at all three ROIs indicated an at least partly independent contribution of aMCC in TDC [standardized β = −0.49, t (21) = −2.51, P = 0.022], while the inclusion of AIC and SI/SII did not improve the fit of the model. Increased sensitivity to first-hand pain was associated with stronger aMCC activation to perceiving others’ pain. In contrast, there was a tight coupling between PPT values and SI/SII activations in ASD [standardized β = −0.77, t (23) = −5.69, P < 0.001] (Figure 2B). The linkage of hemodynamic responses with PPT and unpleasantness suggests that the sensitivity to first-hand pain might play a pivotal role for empathy deficits in ASD. In addition, multiple regression analyses did not find any correlation between TAS-20-C scores and hemodynamic responses in TDC as well as ASD.
Mediation analysis results
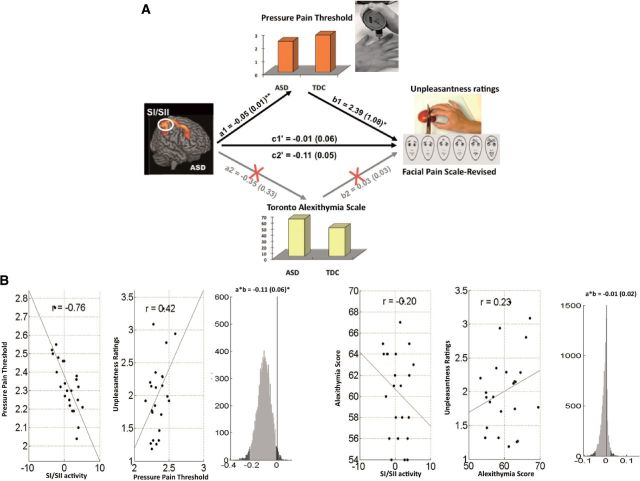
In ASD, PPT was a significant mediator of the SI/SII–unpleasantness ratings relation (Figure 3). Lower PPT was associated with stronger SI/SII activation, but reduced unpleasantness ratings (a = −0.05, SE = 0.01, P < 0.01; b = 2.39, SE = 1.08, P < 0.05; a*b = −0.11, Z = −1.98, P = 0.04). Pain sensitivity in ASD was a mediator for SI/SII in processing the feeling about others’ pain. However, alexithymia was not a significant mediator in predicting the SI/SII–unpleasantness relation (a = −0.35, SE = 0.33, P = 0.28; b = 0.03, SE = 0.03, P = 0.24; a*b = −0.01, Z = −0.22, P = 0.83).
Fig. 3.
Mediation analysis results in individuals with ASD. (A) Path diagram showing the relationships between cortical responses, pressure pain thresholds (PPT), alexithymia scores and unpleasantness ratings in the path model. The predictor region in the SI/SII is shown at left, which predicts the PPT at top and alexithymia (bottom). These are a paths for each mediator. The lines are labeled with path coefficients, and standard errors are shown in parentheses. The mediator factors (PPT and alexithymia) connections to the outcome (unpleasantness) are the b paths. They are calculated controlling for the SI/SII activity and for the mediator factor, as is standard in mediation models. **P < 0.01, *P < 0.05, two-tailed. (B) Partial regression scatterplots for the SI/SII−PPT relation (left panel) and for the PPT−unpleasantness relation (center panel). The right panel shows an example of a bootstrapped mediation effect (path ab) for the PPT. (C) Partial regression scatterplots for the SI/SII−alexithymia relation (left panel) and for the alexithymia−unpleasantnesss relation (center panel). The right panel shows an example of a bootstrapped mediation effect (path a*b) for the alexithymia.
In TDC, PPT was a significant mediator for the aMCC−unpleasantness relation (Supplementary Figure S3). Lower PPT was associated with stronger aMCC activation and increased unpleasantness ratings (a = −0.08, SE = 0.03, P = 0.034; b = −1.90, SE = 0.86, P = 0.034; a*b = 0.16, Z = 2.06, P = 0.039), suggesting that PPT in TDC was a mediator for the aMCC response to pain empathy. However, alexithymia scores were not a significant mediator of the aMCC−unpleasantness relation (a = −0.40, SE = 0.77, P = 0.52; a*b = 0.05, Z = 0.72, P = 0.47) in spite of a negative correlation with unpleasantness ratings (b = −0.1, SE = 0.04, P = 0.022).
Neither PPT nor alexithymia scores significantly mediated the relationship between AIC signal changes and unpleasantness ratings (PPT: a = −0.02, SE = 0.04, P = 0.65; b = −2.46, SE = 0.52, P = 0.022; a*b = 0.05, Z = 0.41, P = 0.68; alexithymia: a = −0.05, SE = 0.09, P = 0.88; b = 0.07, SE = 0.04, P = 0.12; a*b = −0.01, Z = −0.03, P = 0.97).
ERP/EEG results
N2 and LPP to SP vs SN
The ANOVA analyses of N2 amplitudes indicated a stimulus effect (Fz: P = 0.020; Cz: P = 0.010) and a stimulus × group interaction (Cz: P = 0.039, d = 0.69). Post hoc analysis at Cz indicated that Solo Pain was differentiated from Solo No-pain in ASD (P = 0.004, d = 0.29), but not in TDC (P = 0.67).
LPP had a stimulus effect (Fz: P = 0.004, d = 1.01; Cz: P < 0.001, d = 1.24; Pz: P = 0.001, d = 1.19). There was neither a group effect nor a stimulus × group interaction (Figure 4).
Fig. 4.
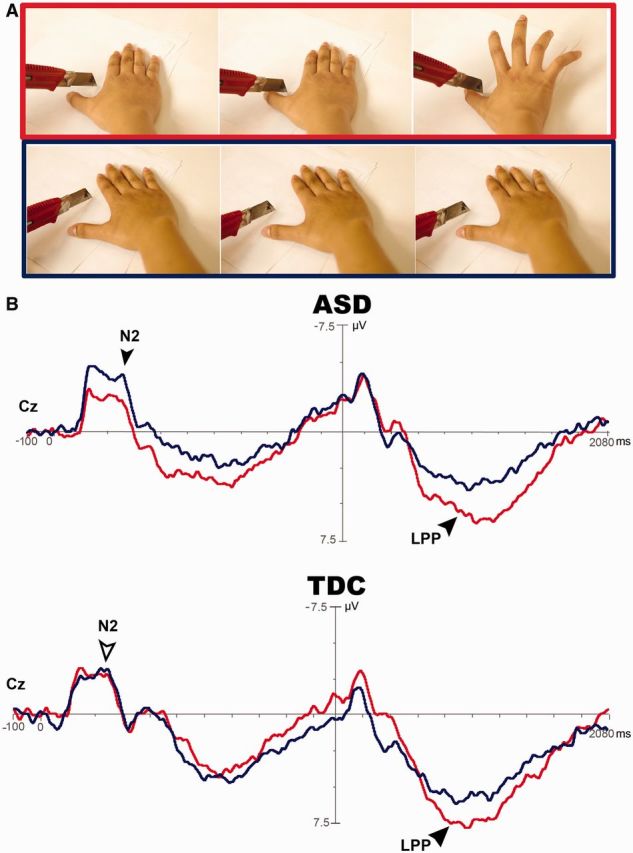
Cortical responses to perceiving someone accidentally in pain. Solo Pain (red line) vs Solo No-pain (blue line) elicits central N2 in individuals with ASD (P = 0.017, black arrow) rather than in TDC (P > 0.05, white arrow). The late-positive potential (LPP) is differentiated in ASD as well as in TDC (P < 0.001, black arrow).
N2 and LPP to DP vs DN
The ANOVA analyses of N2 amplitudes showed a stimulus × group interaction (Fz: P = 0.026, d = 0.77), but no effect of the stimulus or the group (Figure 5). Post hoc analysis indicated that N2 had a more negative deflection in response to painful relative to non-painful stimuli in ASD (P = 0.017, d = 0.31), but no such a response was detected in TDC (P = 0.41).
Fig. 5.
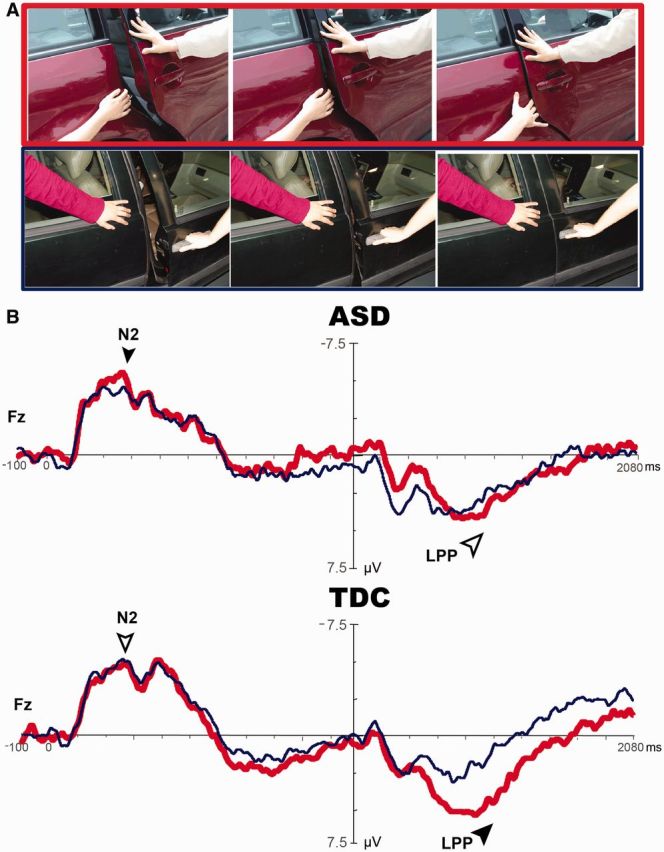
Cortical responses to perceiving an individual hurt by another intentionally. Dyad Pain (red line) vs Dyad No-pain (blue line) elicits frontal N2 in ASD (P = 0.017, black arrow) rather than in TDC (P > 0.05, white arrow). Importantly, LPP is differentiated in TDC (P = 0.002, black arrow), but not in ASD (P > 0.05, white arrow).
LPP had a stimulus effect (Fz: P = 0.004; Cz: P = 0.036) in addition to a stimulus × group interaction (Fz: P = 0.032, d = 0.76). Post hoc analysis indicated that LPP in TDC had a more positive deflection in response to painful relative to non-painful stimuli (P = 0.002, d = 0.88), but none was detected in ASD (P = 0.57) (Figure 5).
Correlation of LPP with autistic traits and pain thresholds
Frontal LPP to Dyad Pain in ASD were negatively correlated with AQ-total scores [r (17) = −0.52, P = 0.034] and the imagination subscale [r (19) = −0.50, P = 0.031]. However, in TDC, no such association was detected. Additionally, frontal LPP to Dyad Pain was positively correlated with PPT values in ASD [r (19) = 0.46, P = 0.046], but not in TDC.
Mu suppression indicative of sensorimotor resonance
For SP vs SN, during the first picture, the ANOVA analyses did not find any significant effect of the stimulus, group and their interaction. During the third picture, only the stimulus (P < 0.001, d = 1.37), not the group or their interaction, was significant (P > 0.05). Both in TDC (P < 0.001, d = 0.22) and ASD (P = 0.045, d = 0.18), SP induced stronger mu suppression than SN (Figure 6A).
Fig. 6.
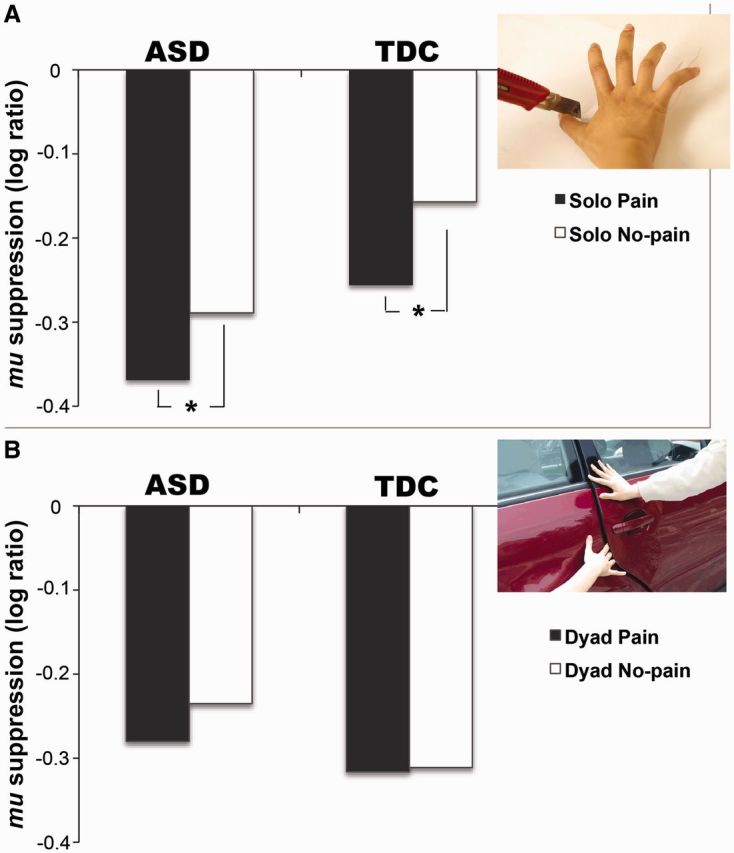
Sensorimotor resonance of empathy for pain. (A) For mu suppression in response to the third picture of Solo Pain vs Solo No-pain, only the stimulus has an effect (P < 0.001). Neither the group nor their interaction reaches significance (P > 0.05). In both TDC and ASD, Solo Pain induces stronger mu suppression than Solo No-pain (*P < 0.05). (B) For mu suppression to the third picture of Dyad Pain vs Dyad No-pain, there is no effect in the stimulus, the group and their interaction (P > 0.05).
For DP vs DN, the stimulus and the group had no effect during the first picture, but their interaction was marginally significant (P = 0.049, d = 0.67). DP induced stronger mu suppression than DN (P = 0.021, d = 0.20) in ASD, whereas there was comparable suppression in TDC. During the third picture, the stimulus, group and their interaction were not significant (Figure 6B). Further, gaze duration was not associated with mu suppression irrespective of Solo or Dyad scenarios.
DISCUSSION
Although it has been suggested that ASD is characterized by empathic deficits, the available empirical evidence is, at best, contradictory. Examining the different component processes of empathy can contribute to a better understanding of interpersonal deficits in ASD.
Using a well-validated pain empathy fMRI and EEG/ERP paradigm, we demonstrated that ASD participants preserved SI/SII activation and mu suppression, indicating typical sensorimotor resonance. The AIC hemodynamic activation in response to passively viewing others’ pain was decreased but the N2 response to actively making pain judgment was heightened. Perceiving intentional harm was not associated with increased activation in the mPFC and pSTS/TPJ, nor elicited the LPP in ASD as compared with TDC. Furthermore, the mediation analysis confirmed that the pain thresholds in ASD were a significant mediator for the relationship between SI/SII signal changes and unpleasantness ratings of others’ pain. Lower pain thresholds were coupled with more autistic traits and reduced LPP. These results suggest that atypical sensory processing in ASD influences empathic processing.
Hypersensitivity to first-hand pain in individuals with ASD, as indicated by reduced PPT values, was associated with stronger SI/SII activation in response to perceiving others’ pain. This finding could complement the embodiment account of empathy deficits in ASD. In this account, sensorimotor engagement is central for emotion processing (Winkielman et al., 2009; Keysers et al., 2011), but evidence for this is at best ambiguous and inconsistent. Automatic mimicry to emotional faces was impaired in one electromyographic study (McIntosh et al., 2006) but the opposite findings were reported in another (Magnée et al., 2007). One transcranial magnetic stimulation study reported that individuals with ASD, in contrast to neurotypical controls, when watching others in pain, did not show any amplitude reduction of motor-evoked potentials recorded from the muscle vicariously affected by pain (Minio-Paluello et al., 2009). One EEG study demonstrated impaired mu suppression (Oberman et al., 2005), while two subsequent studies failed to replicate the findings (Raymaekers et al., 2009; Fan et al., 2010). Observing emotional faces evoked less hemodynamic responses in the inferior frontal gyrus, a core area of the MNS (Dapretto et al., 2006), whereas the response of that region to perceiving actions showed no difference between ASD and controls (Williams et al., 2006; Grèzes et al., 2009). Recently, one study using an fMRI repetition suppression design confirmed that ASD individuals exhibit typical MNS activity (Dinstein et al., 2010). Our results, which control for attention to the stimuli using eye tracking, support the latter finding and demonstrate that SI/SII activation and mu suppression to the sight of others’ pain is preserved in ASD. Furthermore, the mediation analysis confirmed that ASD individuals’ sensitivity to first-hand pain mediates the SI/SII response in rating their unpleasantness about others’ pain. With regard to the impact of aberrant sensory processing on sensorimotor resonance of empathy, these results indicate that previous findings of atypical MNS activity in response to viewing biological actions in ASD should not be immediately attributed to a dysfunctional MNS.
Atypical attention to social stimuli in ASD, a well-documented finding (Klin et al., 2002; Dalton et al., 2005), is likely to contribute to the explanation of preserved MNS activity, as least as measured via mu suppression. It has been demonstrated that ASD individuals have an abnormal visual fixation pattern when facing social situations (Klin et al., 2002). Here, in line with the previous studies (Fan et al., 2010; Chen et al., 2012), ASD participants exhibited typical mu suppression and oculomotor performance in SP vs SN. However, for Dyad (DP vs DN), they failed to differentiate gaze duration and showed abnormally inverted mirroring (mu suppression: DN > DP). Moreover, heightened hemodynamic response in the amygdala has been associated with longer gaze fixation in autism (Dalton et al., 2005). Here, the data demonstrated reduced hemodynamic activation in the AIC and lower unpleasantness ratings to the pain of others when ASD participants viewed the stimuli, and heightened N2 and comparable unpleasantness ratings when careful attention was required for actively making pain judgment. These findings indicate that ASD may tend to use avoidant patterns of attention to restrict affective hyperarousal, particularly in socioemotional processing.
Social context can impact how people perceive the intentionality and make judgments about morality when viewing others in pain. In line with previous studies (Akitsuki and Decety, 2009; Decety et al., 2009), in which the affective and cognitive components of empathy were dissociated, the aMCC, AIC and SI/SII activation to the accidentally inflicted pain whereas the mPFC, pSTS/TPJ, temporal pole and posterior cingulate to the intentional harm were detected in TDC. Importantly, when passively viewing others’ pain during fMRI and actively making pain judgment during EEG/ERP, ASD participants showed dissociation between emotional and cognitive empathy, as demonstrated by heightened frontal N2 for affective arousal along with reduced mPFC and LPP response specific for social understanding. The N2 in the context of pain empathy has been interpreted to reflect early affective arousal, whereas LPP represents late cognitive evaluation (Chen et al., 2012; Mella et al., 2012). The current results concur with the empathy imbalance hypothesis of autism (Smith, 2009). According to this account, people with ASD tend to have low cognitive empathy but high affective arousal. This would make these individuals vulnerable to empathic overarousal and personal distress without necessarily being conscious of the origin of such feelings. Supporting this, the hemodynamic activation in the AIC was reduced to passive observation of others’ pain. The AIC is polysensory cortex involved in mapping internal states of bodily and subjective feeling (Craig, 2003) and its activation has been demonstrated to correlate with arousal level to affective signals (Critchley et al., 2000). The AIC has extensive reciprocal connections with limbic forebrain areas (Nieuwenhuys, 2012), and has been the most consistently and robustly activated region across all studies of pain empathy (Gu et al., 2010; Lamm et al., 2011; Gu et al., 2012). In this context, the AIC was proposed to integrate emotional and cognitive valuation with a full representation of the sentient self (Kurth et al., 2010). Another key region involved in empathy for pain is the aMCC. This region implements a domain-general process and integrates negative affect, pain and cognitive control (Shackman et al., 2011). Recently though, it has been proposed that AIC and aMCC form the core of a salience network integrating external stimuli with internal states to guild behaviors (Menon and Uddin, 2010). Previous studies that measured cardiovascular, neuroendocrine and neurochemical indices to novel and stressful stimuli are also consistent with the hypothesis of abnormal arousal in ASD (Tordjman et al., 1997; Hirstein et al., 2001). In self-report questionnaires designed to assess empathy, individuals with Asperger syndrome relative to controls scored significantly higher on emotional empathy (Rogers et al., 2007; Dziobek et al., 2008).
The finding of lower pain thresholds in ASD contradicts the prevailing belief of pain insensitivity described by previous literature (Gillberg and Wahlstrom, 1985). There are some methodological issues with these early studies that could explain this difference. The assessment of pain sensitivity was mostly based on retrospective parental report, not on validated objective approaches (Nader et al., 2004). Some studies have reported lower vibrotactile thresholds (Blakemore et al., 2006) and another study found increased sensitivity to thermal pain in ASD (Cascio et al., 2008). Results from mediation analysis indicated that reduced pain thresholds specific to ASD aberrantly mediate the sensory discrimination to the sight of others in pain. In healthy controls, however, pain thresholds mediate the affective-motivation aspect of empathy. Altered pain thresholds may be related to abnormal serotonin levels in individuals with autism (Militerni et al., 2000), which, in turn, interacts with trait empathy in modulating prosocial behavior (Crockett et al., 2010). Along with previous reports about the relationship between sensory processing and social competence (Hilton et al., 2010), these findings may illuminate how atypical low-level sensory hypersensitivity in ASD affects higher-level empathy (Mottron and Burack, 2001; Baron-Cohen et al., 2009).
One fMRI study reported that the empathic brain response to pain observed in autism results from the large comorbidity between alexithymia and autism (Bird et al., 2010). However, we did not find that alexithymia was a significant mediator in processing pain empathy in spite of the presence of a negative correlation between alexithymic levels and unpleasantness ratings. This finding indicates that alexithymic traits might be comorbid to empathic deficits in ASD, but not the causal sequence between specific cortical activities and subjective unpleasantness to others’ pain. The methodologies between the two studies are quite different and may account for this discrepancy. The study by Bird et al. (2010) asked participants to imagine that their partners were receiving electrical shocks, whereas our study presented the visual stimuli depicting body parts in daily painful situations.
Callous traits, like ASD, index lack of empathy (Lockwood et al., 2013; Marsh et al., 2013). Interestingly, a very different pattern of fMRI and ERP responses has been found in youths with callous traits when they were exposed to the same stimuli (Decety et al., 2009; Cheng et al., 2012). Participants with callous traits demonstrated higher pain thresholds compared with controls. To SP vs SN, they showed atypical activation in amygdala and ventral striatum but reduced N2 and LPP. To DP vs DN, they retained LPP but exhibited the AIC activation. Both individuals with ASD and callous traits had typical sensorimotor resonance. As characteristic of empathy deficits, psychopathy and autism can be considered as diametrically opposed disorders with respect to the empathy imbalance hypothesis.
CONCLUSION
This study breaks down the construct into component processes to examine the neurobiological underpinnings of empathy in ASD. The lower pain thresholds but intact mu suppression in response to perceiving others’ pain in ASD compared with controls suggests that the sensorimotor contribution to interpersonal sensitivity is unlikely to be damaged. Stronger N2 along with reduced LPP and mPFC in response to the stimuli depicting intentional harm shows dissociation between affective arousal and social understanding. Such empathy imbalance in ASD may be a source of emotional distress without the individuals necessarily being conscious of the origin of such feeling, and this is likely to contribute to difficulties in engaging emotionally with others.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN Online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The authors thank Dr. Yu-Yu Wu for helpful support on clinical assessments. The study was funded by National Science Council (NSC 99-2314-B-010-037-MY3; NSC 100-2628-H-010-001-MY3), National Yang-Ming University Hospital (RD 2011-005), the Health Department of Taipei City Government (10201-62-064) and a grant from the Ministry of Education (Aim for the Top University Plan). Dr. Jean Decety was supported by an NSF grant (BCS-0718480).
REFERENCES
- Akitsuki Y, Decety J. Social context and perceived agency affects empathy for pain: an event-related fMRI investigation. Neuroimage. 2009;47:722–34. doi: 10.1016/j.neuroimage.2009.04.091. [DOI] [PubMed] [Google Scholar]
- Baird AD, Scheffer IE, Wilson SJ. Mirror neuron system involvement in empathy: a critical look at the evidence. Social Neuroscience. 2011;6:327–35. doi: 10.1080/17470919.2010.547085. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ashwin E, Ashwin C, Tavassoli T, Chakrabarti B. Talent in autism: hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:1377–83. doi: 10.1098/rstb.2008.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, et al. Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience. 1999;11:1891–8. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Swettenham J. Theory of mind in autism: its relationship to executive function and central coherence. In: Cohen DJ, Volkmar F, editors. Handbook of Autism and Pervasive Developmental Disorders. New York, NY: John Wiley and Sons; 1997. [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Batson CD. These things called empathy: eight related but distinct phenomena. In: Decety J, Ickes W, editors. The Social Neuroscience of Empathy. Cambridge, MA: MIT press; 2009. [Google Scholar]
- Bieri D, Reeve RA, Champion GD, Addicoat L, Ziegler JB. The Faces Pain Scale for the self-assessment of the severity of pain experienced by children: development, initial validation, and preliminary investigation for ratio scale properties. Pain. 1990;41:139–50. doi: 10.1016/0304-3959(90)90018-9. [DOI] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain. 2010;133:1515–25. doi: 10.1093/brain/awq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Consciousness and Cognition. 2005;14:698–718. doi: 10.1016/j.concog.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Tavassoli T, Calo S, et al. Tactile sensitivity in Asperger syndrome. Brain and Cognition. 2006;61:5–13. doi: 10.1016/j.bandc.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Cascio C, McGlone F, Folger S, et al. Tactile perception in adults with autism: a multidimensional psychophysical study. Journal of Autism and Developmental Disorders. 2008;38:127–37. doi: 10.1007/s10803-007-0370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo L, Rizzolatti G. The mirror neuron system. Archives of Neurology. 2009;66:557–60. doi: 10.1001/archneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- Chen C, Yang CY, Cheng Y. Sensorimotor resonance is an outcome but not a platform to anticipating harm to others. Social Neuroscience. 2012;7:578–90. doi: 10.1080/17470919.2012.686924. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Hung AY, Decety J. Dissociation between affective sharing and emotion understanding in juvenile psychopaths. Developmental Psychopathology. 2012;24:623–36. doi: 10.1017/S095457941200020X. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Yang CY, Lin CP, Lee PL, Decety J. The perception of pain in others suppresses somatosensory oscillations: a magnetoencephalography study. Neuroimage. 2008;40:1833–40. doi: 10.1016/j.neuroimage.2008.01.064. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig KD. The social communication model of pain. Canadian Psychology. 2009;50:22–32. [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. Journal of Neuroscience. 2000;20:3033–40. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Hauser MD, Robbins TW. Serotonin selectively influences moral judgment and behavior through effects on harm aversion. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17433–8. doi: 10.1073/pnas.1009396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8:519–26. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nature Neuroscience. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28:479–85. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Decety J. A social cognitive neuroscience model of human empathy. In: Harmon-Jones E, Winkielman P, editors. Social Neuroscience: Integrating Biological and Psychological Explanations of Social Behavior. New York: Guilford Publications; 2007. [Google Scholar]
- Decety J. Dissecting the neural mechanisms mediating empathy. Emotion Review. 2011;3:92–108. [Google Scholar]
- Decety J, Michalska KJ. Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Developmental Science. 2010;13:886–99. doi: 10.1111/j.1467-7687.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y. Who caused the pain? An fMRI investigation of empathy and intentionality in children. Neuropsychologia. 2008;46:2607–14. doi: 10.1016/j.neuropsychologia.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y, Lahey BB. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biological Psychology. 2009;80:203–11. doi: 10.1016/j.biopsycho.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Svetlova M. Putting together phylogenetic and ontogenetic perspectives on empathy. Developmental Cognitive Neuroscience. 2012;2:1–24. doi: 10.1016/j.dcn.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, Hajcak G. The late positive potential: a neurophysiological marker for emotion regulation in children. Journal of Child Psychology and Psychiatry. 2009;50:1373–83. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Thomas C, Humphreys K, Minshew N, Behrmann M, Heeger DJ. Normal movement selectivity in autism. Neuron. 2010;66:461–9. doi: 10.1016/j.neuron.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziobek I, Rogers K, Fleck S, et al. Dissociation of cognitive and emotional empathy in adults with Asperger Syndrome using the Multifaceted Empathy Test (MET) Journal of Autism and Developmental Disorders. 2008;38:464–73. doi: 10.1007/s10803-007-0486-x. [DOI] [PubMed] [Google Scholar]
- Fan Y, Han S. Temporal dynamic of neural mechanisms involved in empathy for pain: an event-related brain potential study. Neuropsychologia. 2008;46:160–73. doi: 10.1016/j.neuropsychologia.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Fan YT, Decety J, Yang CY, Liu JL, Cheng Y. Unbroken mirror neurons in autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2010;51:981–8. doi: 10.1111/j.1469-7610.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- Frith U. Mind blindness and the brain in autism. Neuron. 2001;32:969–79. doi: 10.1016/s0896-6273(01)00552-9. [DOI] [PubMed] [Google Scholar]
- Frith U, Happé F. Autism spectrum disorder. Current Biology. 2005;15:R786–90. doi: 10.1016/j.cub.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Gillberg C. Autism and autistic-like conditions. Journal of Child Psychology and Psychiatry. 1992;33:813–42. doi: 10.1111/j.1469-7610.1992.tb01959.x. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Wahlstrom J. Chromosome abnormalities in infantile autism and other childhood psychoses: a population study of 66 cases. Developmental Medicine & Child Neurology. 1985;27:293–304. doi: 10.1111/j.1469-8749.1985.tb04539.x. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Wicker B, Berthoz S, de Gelder B. A failure to grasp the affective meaning of actions in autism spectrum disorder subjects. Neuropsychologia. 2009;47:1816–25. doi: 10.1016/j.neuropsychologia.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Gu X, Gao Z, Wang X, et al. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135:2726–35. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Liu X, Guise KG, Naidich TP, Hof PR, Fan J. Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. Journal of Neuroscience. 2010;30:3739–44. doi: 10.1523/JNEUROSCI.4844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Fan Y, Mao L. Gender difference in empathy for pain: an electrophysiological investigation. Brain Research. 2008;1196:85–93. doi: 10.1016/j.brainres.2007.12.062. [DOI] [PubMed] [Google Scholar]
- Hilton CL, Harper JD, Kueker RH, et al. Sensory responsiveness as a predictor of social severity in children with high functioning autism spectrum disorders. Journal of Autism and Developmental Disorders. 2010;40:937–45. doi: 10.1007/s10803-010-0944-8. [DOI] [PubMed] [Google Scholar]
- Hirstein W, Iversen P, Ramachandran VS. Autonomic responses of autistic children to people and objects. Proceedings of the Royal Society of London, Series B: Biological Sciences. 2001;268:1883–8. doi: 10.1098/rspb.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson RP. Autism and the Development of Mind. East Sussex, UK: Lawrence Eribaum Associates; 1993. [Google Scholar]
- Ibanez A, Melloni M, Huepe D, et al. What event-related potentials (ERPs) bring to social neuroscience? Social Neuroscience. 2012;7:632–49. doi: 10.1080/17470919.2012.691078. [DOI] [PubMed] [Google Scholar]
- Keysers C, Thioux M, Gazzola V. Mirror neuron system and social cognition. In: Decety J, Cacioppo JT, editors. The Oxford Handbook of Social Neuroscience. New York, NY: Oxford University Press; 2011. [Google Scholar]
- Kleinhans NM, Johnson LC, Richards T, et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. American Journal of Psychiatry. 2009;166:467–75. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59:809–16. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function. 2010;214:519–34. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Li W, Han S. Perspective taking modulates event-related potentials to perceived pain. Neuroscience Letter. 2010;469:328–32. doi: 10.1016/j.neulet.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Lockwood PL, Sebastian CL, McCrory EJ, et al. Association of callous traits with reduced neural response to others’ pain in children with conduct problems. Current Biology. 2013;23:1–5. doi: 10.1016/j.cub.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Magnée MJ, De Gelder B, Van Engeland H, Kemner C. Facial electromyographic responses to emotional information from faces and voices in individuals with pervasive developmental disorder. Journal of Child Psychology and Psychiatry. 2007;48:1122–30. doi: 10.1111/j.1469-7610.2007.01779.x. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Einger EC, Fowler KA, et al. Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. Journal of Child Psychology and Psychiatry. 2013;54:900–10. doi: 10.1111/jcpp.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh DN, Reichmann-Decker A, Winkielman P, Wilbarger JL. When the social mirror breaks: deficits in automatic, but not voluntary, mimicry of emotional facial expressions in autism. Developmenal Science. 2006;9:295–302. doi: 10.1111/j.1467-7687.2006.00492.x. [DOI] [PubMed] [Google Scholar]
- Mella N, Studer J, Gilet AL, Labouvie-Vief G. Empathy for pain from adolescence through adulthood: an event-related brain potential study. Frontiers in Psychology. 2012;3:501. doi: 10.3389/fpsyg.2012.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Militerni R, Bravaccio C, Falco C, Puglisi-Allegra S, Pascucci T, Fico C. Pain reactivity in children with autistic disorder. The Journal of Headache and Pain. 2000;1:53–6. [Google Scholar]
- Minio-Paluello I, Baron-Cohen S, Avenanti A, Walsh V, Aglioti SM. Absence of embodied empathy during pain observation in Asperger syndrome. Biological Psychiatry. 2009;65:55–62. doi: 10.1016/j.biopsych.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Mottron L, Burack JA. Enhanced perceptual functioning in the development of autism. In: Burack JA, Charman T, Yirmiya N, Zelazo PR, editors. The Developemnt of Autism: Perspectives from Theory and Research. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2001. [Google Scholar]
- Nader R, Oberlander TF, Chambers CT, Craig KD. Expression of pain in children with autism. The Clinical Journal of Pain. 2004;20:88–97. doi: 10.1097/00002508-200403000-00005. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R. The insula cortex: a review. Progress in Brain Reseach. 2012;195:123–63. doi: 10.1016/B978-0-444-53860-4.00007-6. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cognitive Brain Research. 2005;24:190–8. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: an integrative review of ERP findings. Biological Psychology. 2008;77:247–65. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Polich J. Affective visual event-related potentials: arousal, repetition, and time-on-task. Biological Psychology. 2007;75:101–8. doi: 10.1016/j.biopsycho.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ. Executive function deficits in high-functioning autistic individuals: relationship to theory of mind. Journal of Child Psychology and Psychiatry. 1991;32:1081–105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ. Brain mechanisms for social perception: lessons from autism and typical development. Annals of the New York Academy of Sciences. 2008;1145:283–99. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Vander Wyk BC. Research review: Constraining heterogeneity: the social brain and its development in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2011;52:631–44. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A, Bentin S, Bartal IB, Lamm C, Decety J. “Feeling” the pain of those who are different from us: modulation of EEG in the mu/alpha range. Cognitive, Affective & Behavioral Neuroscience. 2010;10:493–504. doi: 10.3758/CABN.10.4.493. [DOI] [PubMed] [Google Scholar]
- Raymaekers R, Wiersema JR, Roeyers H. EEG study of the mirror neuron system in children with high functioning autism. Brain Research. 2009;1304:113–21. doi: 10.1016/j.brainres.2009.09.068. [DOI] [PubMed] [Google Scholar]
- Rogers K, Dziobek I, Hassenstab J, Wolf O, Convit A. Who cares? Revisiting empathy in Asperger syndrome. Journal of Autism and Developmental Disorders. 2007;37:709–15. doi: 10.1007/s10803-006-0197-8. [DOI] [PubMed] [Google Scholar]
- Schulte-Ruther M, Greimel E, Markowitsch HJ, et al. Dysfunctions in brain networks supporting empathy: an fMRI study in adults with autism spectrum disorders. Social Neuroscience. 2011;6:1–21. doi: 10.1080/17470911003708032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Review Neuroscience. 2011;12:154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory S. Empathic processing: its cognitive and affective dimensions and neuroanatomical basis. In: Decety J, Ickes W, editors. The Social Neuroscience of Empathy. Cambridge, MA: MIT press; 2009. [Google Scholar]
- Smith A. The empathy imbalance hypothesis of autism: a theoretical approach to cognitive and emotional empathy in autistic development. The Psychological Record. 2009;59:489–510. [Google Scholar]
- Tordjman S, Anderson GM, McBride PA, et al. Plasma beta-endorphin, adrenocorticotropin hormone, and cortisol in autism. Journal of Child Psychology and Psychiatry. 1997;38:705–15. doi: 10.1111/j.1469-7610.1997.tb01697.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JH, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and ‘mirror neuron' functioning in autistic spectrum disorder. Neuropsychologia. 2006;44:610–21. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Winkielman P, Mclntosh DN, Oberman LM. Embodied and disembodied emotion processing: learning from and about typical and autistic individuals. Emotion Review. 2009;1:178–90. [Google Scholar]
- Zaki J, Ochsner K. The cognitive neuroscience of sharing and understanding others' emotions. In: Decety J, editor. Empathy—From Bench to Bedside. Cambridge: MIT press; 2012. [Google Scholar]
- Zhu X, Yi J, Yao S, Ryder AG, Taylor GJ, Bagby RM. Cross-cultural validation of a Chinese translation of the 20-item Toronto Alexithymia Scale. Comprehensive Psychiatry. 2007;48:489–9. doi: 10.1016/j.comppsych.2007.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.