Abstract
Atypical visual processing of biological motion contributes to social impairments in autism spectrum disorders (ASD). However, the exact temporal sequence of deficits of cortical biological motion processing in ASD has not been studied to date. We used 64-channel electroencephalography to study event-related potentials associated with human motion perception in 17 children and adolescents with ASD and 21 typical controls. A spatio-temporal source analysis was performed to assess the brain structures involved in these processes. We expected altered activity already during early stimulus processing and reduced activity during subsequent biological motion specific processes in ASD. In response to both, random and biological motion, the P100 amplitude was decreased suggesting unspecific deficits in visual processing, and the occipito-temporal N200 showed atypical lateralization in ASD suggesting altered hemispheric specialization. A slow positive deflection after 400 ms, reflecting top-down processes, and human motion-specific dipole activation differed slightly between groups, with reduced and more diffuse activation in the ASD-group. The latter could be an indicator of a disrupted neuronal network for biological motion processing in ADS. Furthermore, early visual processing (P100) seems to be correlated to biological motion-specific activation. This emphasizes the relevance of early sensory processing for higher order processing deficits in ASD.
Keywords: event-related-potentials, motion perception, P100, N200, hemisphere-asymmetry
INTRODUCTION
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impairments in social interaction, communication, and repetitive and stereotyped behaviours and interests (American Psychiatric Association, 2000). Impaired visual processing of biological motion has been the focus of recent neuropsychological and brain imaging studies in ASD (Kaiser and Pelphrey, 2012). Recognition of biological motion, including eye movements, facial expressions and body gestures, is crucial for the development of adequate social interaction and adaptive responses, and usually emerges early in development (Grossmann and Johnson, 2007; Simion et al., 2008). Furthermore, biological motion perception deficits might be a marker of disorders characterized by difficulties in social interaction and social cognition (Pavlova, 2012).
Classical human motion stimuli are the so-called point-light displays introduced by Johansson (1973). Children with autism showed impaired recognition of human motion from these point-light displays (Blake et al., 2003), failed to orient to such motions patterns (Klin et al., 2009; Annaz et al., 2012), and showed an abnormal development of human motion recognition abilities during development (Annaz et al., 2010). Similarly, in adolescents with ASD higher detection thresholds for human motion (Koldewyn et al., 2010) and a reduced sensitivity towards irregularities in human motion were reported (Price et al., 2012). In other studies, especially with adult samples, impairments were less prominent (Murphy et al., 2009; Saygin et al., 2010; Jones et al., 2011; Rutherford and Troje, 2012; Nackaerts et al., 2012). Still, adults with ASD were less sensitive to human motion when presented by masked displays (Kaiser et al., 2010a).
The neural mechanisms underlying biological motion perception also differ between ASD and typically developing controls (TCs). Neuroimaging studies showed reduced or atypical activation of temporal regions, including the superior temporal sulcus (STS) and gyrus, and the fusiform gyrus in ASD (Herrington et al., 2007; Freitag et al., 2008; Kaiser et al., 2010b; Koldewyn et al., 2011). Other studies questioned the specificity of biological motion perception difficulties. Atypical neural activation also to arbitrarily moving point-light displays (Freitag et al., 2008) and reduced activity in the middle temporal area (MT+/V5) to motion stimuli (Herrington et al., 2007) were also reported in ASD. Another study found abnormal brain activation already in the primary visual cortex (Brieber et al., 2010).
In contrast to functional magnetic resonance imaging, electroencephalography (EEG) has a higher temporal resolution. Thus, EEG represents an excellent tool to assess the temporal sequence of different neuronal sub-processes. EEG studies on biological motion perception in healthy populations have described several event-related potentials (ERPs). Two studies found increased activation peaks 100 ms after stimulus-onset especially in right occipital regions to human compared to non-biological motion (Hirai et al., 2009; Krakowski et al., 2011). This P100 can be interpreted as a marker of elementary stimulus feature processing (Allison et al., 1999) and early motion detection (Krakowski et al., 2011). After this first component, a stronger negative deflection to human compared to non-biological motion stimuli peaking around 200 ms is frequently described (Hirai et al., 2003, 2009; Jokisch et al., 2005; Hirai and Kakigi, 2008). It seems to reflect more specific motion processing (Hirai and Kakigi, 2008) including detection of motion direction (Niedeggen and Wist, 1999) in MT+/V5. Using high-density electrical mapping and source analysis on difference waves (biological–random motion), Krakowski et al. described a dipolar activity starting after approximately 300 ms. Its source was located in posterior middle temporal regions bilateral near the posterior STS (Krakowski et al., 2011), a neuronal region important for social information processing (Hein and Knight, 2008). Finally, a positive deflection at centro-parietal electrodes starting approximately after 400 ms was described (P400+), with greater amplitudes during human motion processing when the human motion aspect of the stimuli was actively attended (Krakowski et al., 2011). This component was interpreted as a marker of top-down cognitive processes involved in active decoding of stimulus content and might be a sub-component of the so-called late positive complex (LPC). A notably later and more broadly distributed LPC around 500–600 ms has also been observed, for example when object parts were difficult to recover (Schendan and Kutas, 2007).
To date, no study has been performed assessing different stages of biological motion compared to non-biological motion processing in ASD using ERPs. Because of the contradictory findings of specifically impaired biological motion processing vs generally impaired motion processing in children and adolescents with ASD, we first studied ERPs reflecting biological as well as random motion processing. We expected reduced P100 amplitudes in ASD as an indicator of a broader perception deficit and reduced N200 amplitudes as an indicator of disrupted motion processing, especially in the biological motion condition in ASD.
Second, we assessed the biological motion-specific ERPs by dipole analysis. Dipole analysis allows studying the activity of a source located in deeper structures of the neo-cortex. Here, we expected to find reduced biological motion-specific activity in ASD.
Finally, we explored if early processing stages (P100) have an impact on higher order processing stages. Strong correlations may imply that extraction of specific social features such as biological motion detection is tuned by basic visual motion perception deficits in ASD.
METHODS
Participants and psychological assessment
We included 21 TCs and 17 children with ASD. Only boys aged between 6 and 15 years were studied, because ASD disproportionately affects males. Both groups did not differ with regard to age (t = –0.34, P = 0.73; see Table 1 for details). All subjects were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971) and had normal or corrected-to-normal vision.
Table 1.
Descriptive data and behavioural data (mean ± s.d.)
| Mean ± s.d. | ASD-patients (N = 17) | TCs (N = 21) | Statistics |
|---|---|---|---|
| Age | 11.9 ± 2.2 | 11.63 ± 2.4 | t = −0.34, P = 0.73 |
| IQ (Raven) percent rank | 77.6 ± 30.2 | 59.1 ± 38.2 | Z = −1.5, P = 0.14 |
| Correct responses to ‘walker’ (%) | 85.7 ± 4.8 (95.2%) | 84.8 ± 6.8 (94.2%) | Z = −0.4, P = 0.73 |
| Correct responses to ‘scramble’ (%) | 85.2 ± 4.2 (94.7%) | 83.8 ± 9.5 (93.1%) | Z = −0.5, P = 0.62 |
| Reaction time to ‘walker’ (s) | 1.41 ± 0.27 | 1.35 ± 0.29 | t = −0.63, P = 0.53 |
| Reaction time to ‘scramble’ (s) | 1.45 ± 0.28 | 1.43 ± 0.28 | t = −0.26, P = 0.80 |
ASD-patients were diagnosed according to ICD-10 (WHO, 1992) at the Department of Child and Adolescent Psychiatry of the Goethe-University of Frankfurt (Germany) by experienced clinicians, based on the German version of the Autism Diagnostic Observation Schedule (ADOS Modul 2–4; Rühl et al., 2004) and the Autism Diagnostic Interview–Revised (ADI-R; Bölte et al., 2006). Individuals with high-functioning autism (N = 13) met autism criteria in the three domains of the ADI-R and in the two domains of the ADOS; individuals with Asperger Syndrome (N = 4) met autism criteria in the social interaction and stereotyped behaviour domain of the ADI-R and autism spectrum or autism criteria on the two domains of the ADOS.
Exclusion criteria were as follows: intellectual disability according to a standardized IQ assessment (percentile rank < 2 corresponding to IQ < 70; for details see below), any neurological disorder (including epilepsy), preterm birth (<2000 g), comorbid psychiatric disorders (e.g. attention-deficit/hyperactivity disorder, anxiety disorder), dyslexia and psycho-pharmacotherapy. Comorbid psychiatric disorders were controlled by a semi-structured interview according to ICD-10 criteria (Kinder-DIPS; Schneider, 2009).
TCs were recruited from local schools and screened with the German version of the Child Behavior Checklist (Arbeitsgruppe Kinder-, Jugend- und Familiendiagnostik, 1998) for any clinically relevant symptoms. Participants with T-scores >60 on the second order scales or >70 on any first order subscales, respectively, were excluded.
IQ was assessed using the standard (above 11.5 years) or coloured (below 11.5 years) progressive matrices (Bulheller and Häcker, 2002, 2003). The matrices test is a non-verbal, multiple-choice IQ test which measures deductive reasoning. Because the manual only supplies percentile ranks, a non-parametrical test for a group comparison was conducted (Mann–Witney U-test). No group difference was observed (Z = −1.5; P = 0.14; see Table 1 for details).
Subjects received a small fee for their participation. All participants and parents signed informed consent. The study was approved by the Ethics Committee of the Goethe-University Frankfurt (Germany) in accordance with the Declaration of Helsinki.
Experimental design, procedure and stimuli presentation
A 30 × 37.5 cm flat-screen was placed 80 cm in front of the participant in a dark room. The biological motion stimuli (‘walker’ condition) consisted of moving point-light displays without contours. Fifteen female and 15 male walkers were marked by 15 white dots at the joints against a black background, tracking movements at the joints of the limps. It was created using Labview version 6 (http//www.ni.com/labview). The walkers were shown in a frontal view walking with a speed of approximately two steps per second. Stimuli were based on motion capture data as previously described (Troje, 2002). The ‘scrambled’ (i.e. control-) condition was derived from these walkers. The spatial position of the 15 dots was permutated but leaving the shape of each trajectory for each individual dot intact. This manipulation retains the acceleration individually for each dot, but masks the acceleration profile indicative for biological motion. In sum, 30 different walkers and 30 scrambled motion stimuli were shown. Due to different starting points, dots were not strictly symmetrically organized. For the scrambled condition, dots were placed similar to the walker condition to cover the same visual field. Each individual stimulus was shown for 1 s, in a randomized order once per block. This block was repeated three times, thus each stimulus-class was presented 90 times. All stimuli were presented centrally on the screen. Between the stimuli, a white fixation-cross was presented for 2 s. In order to separate biological motion recognition and motor response, participants were instructed to react when the fixation-cross appeared. They were instructed to press the left mouse button, if they had seen a walking person before, and the right mouse button, if they recognized just a motion pattern (scramble condition). Correctness was emphasized over speed (Figure 1).
Fig. 1.
Example of experimental stimuli and stimuli presentation.
Children were allowed two breaks. Break duration was regulated by the participant. The stimulus sequence was controlled by Presentation™ software (http://www.neurobs.com/).
Electrophysiological assessment
Continuous direct current EEG (DC-EEG) from 64 channels at a sampling rate of 500 Hz was recorded against a reference at Fpz using BrainVision MR-Plus amplifiers and Brain Vision Recorder software (Brain Products GmbH, Munich, Germany). An anti-aliasing low-pass filter with 250 Hz high cut-off was applied online. Sixty-four sintered Ag–AgCl electrodes (impedances < 10 kΩ) were fixed by equidistant electrode caps (Easycap GmbH, Herrsching, Germany). Vertical and horizontal electro-oculograms were recorded from electrodes placed 1 cm above and below the left eye and lateral to the outer canthi.
Signal pre-processing
EEG data were analysed using the Brain Vision-Analyzer 2 (Brain Products, Munich, Germany). Offline, raw EEG was low-pass filtered at 40 Hz (high cut-off) and recordings were transformed to an average reference. Continuous recordings were segmented into epochs of 3.5 s (starting 500 ms before stimulus-onset until 3 s after onset). The first 500 ms of this epoch served as baseline. Only trials with correct responses were included into the analysis. The recordings were corrected automatically for eye movements and blinks by the algorithm of Gratton and Coles (Brain Vision Analyzer). Because no slow DC potentials were observable, no linear regressions to eliminate such trends were required. Artifacts were rejected automatically if the signal amplitude exceeded 150 μV (individual channel mode). This step was controlled by visual inspection, and remaining artifacts were removed by an experienced EEG technician who was blind to the study hypotheses. Electrodes with too high impedance or too many artefacts (in more than one-third of all segments) were interpolated by nearest neighbours.
ERP analysis
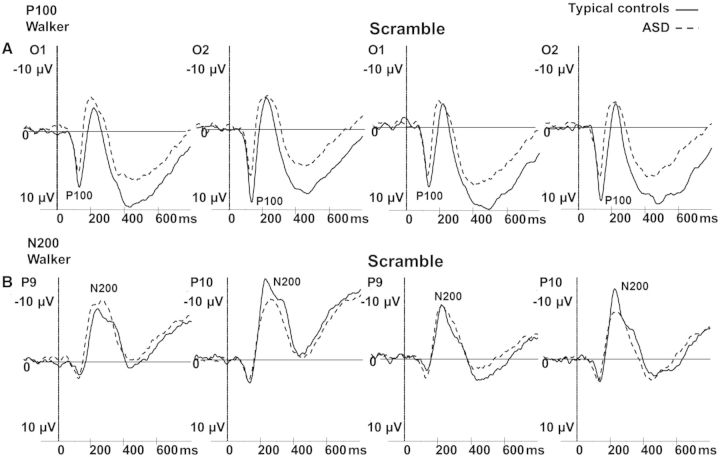
Scalp regions and time windows of interest were defined according to previous studies and visual inspection of group grand averages of our data (Jokisch et al., 2005; Hirai et al., 2009; Krakowski et al., 2011): we determined the first positive peak (P100) between 100 and 200 ms at O1 (left hemisphere) and O2 (right hemisphere) and the first negative peak (N200) between 170 and 280 ms at P9 (left hemisphere) and P10 (right hemisphere) in each data set (for topographic scalp maps see figure 2). Latencies (ms) and amplitudes (μV) were exported for further analysis.
Fig. 2.
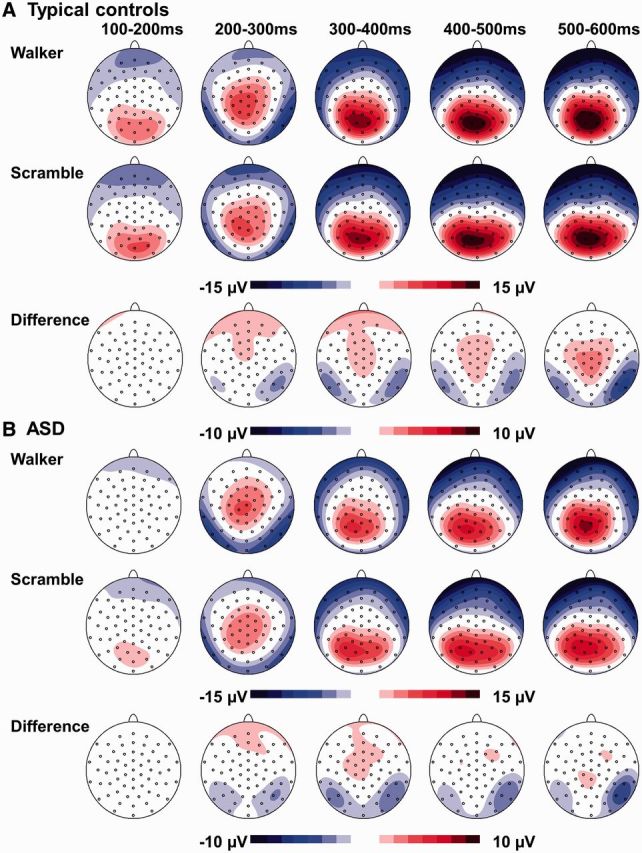
Topographic scalp maps in (A) typical controls and (B) autistic children (ASD) in both experimental conditions and the difference maps (walker–scramble).
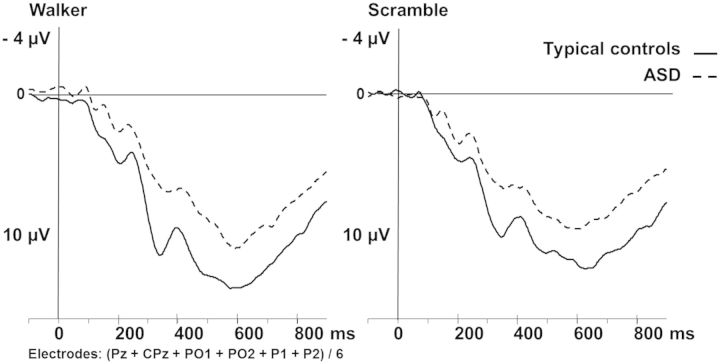
In some studies, a second negative component was observed (Hirai et al., 2003, 2009; Hirai and Kakigi, 2008; Jokisch et al., 2005), but in our data this second component was not observable on the scalp surface. For the P400+ starting after 400 ms, mean activity at pooled centro-parietal leads (Pz, CPz, PO1, PO2, P1, P2) was assessed between 400 and 800 ms. Wider and later time windows for peak detection and mean activity calculations were chosen because the components became prominent later in our data. This might be due to brain immaturity in children and adolescents (Taylor and Smith, 1995; Taylor et al., 1999).
Analysis of difference waves
In order to further elucidate the cortical areas implicated in biological motion processing, we performed spatio-temporal dipole source analysis using Brain Electrical Source Analysis (BESA GmbH, version 5.1.8, Munich, Germany) according to Krakowski et al. (2011). We calculated the difference waveforms between the human motion and the scrambled stimuli in order to eliminate basic motion from biological motion processing.
In TCs, the difference waveforms indicated a stable topography with an occipito-temporal negativity and a central/centroparietal positivity (Figure 2) in good agreement with the topography described in adults (Krakowski et al., 2011). In contrast to Krakowski et al., the activation on our data peaked approximately 100 ms later. Therefore, we fitted two bilaterally symmetrical dipoles in the time interval of 450–550 ms to the grand average of TCs (because of the better signal-to-noise ratio in grand averages compared to individual subject averages). Later time windows were not chosen, to minimize an overlap between the specific human motion activation and the P400+. Residual variance and dipole energy were minimized. Because the introduction of further dipoles did not improve the model (Supplementary Figure 1), the two-dipole solution was kept.
Subjects with ASD showed a more diffuse surface topography than TCs and presented lower amplitudes (see Results). Because of these low amplitudes, no reliable explanation of the surface topography was possible in the ASD-group, which was also due to very high residual variances. Thus, no different source model was used for the ASD-group, but the source model fitted on TCs was applied to all subjects. The dipole activations during the time window of 450–550 ms after stimulus-onset were exported for further statistical analysis in order to quantify the difference in strength of the dipole activation between ASD and TCs. In an additional complementary approach, to describe the more diffuse brain activity in ASD, we also compared the amount of explained variance of the surface potential by the dipole model between groups. To that aim, dipole orientations were refit on each single subject average and the explained variance (100% − residual variance) was exported for statistical analysis.
Statistical data analyses
Statistical tests and correlation coefficients were chosen according to the prior distribution of the dependent variables of interests. To test for group differences in correct responses, Mann–Witney U-tests and t-tests to test for differences in reaction times were performed. Differences in ERP amplitudes and latencies were analysed by repeated-measures ANCOVAS with the within-subjects factors MOTION TYPE (‘walker’/‘scramble’) and HEMISPHERE (right, left), the between-subjects factor GROUP (ASD/TCs) and AGE as a covariate. The centro-parietal P400+ was analysed by a repeated-measures ANCOVA with the within-factor MOTION TYPE, between-subjects factor GROUP and AGE as covariate. Dipole moments obtained by source analysis were also analysed by a repeated-measures ANCOVA with the within-factor HEMISPHERE and factor GROUP, with AGE as covariate. Explained variance of the surface potential by the source model was compared between groups by an ANCOVA with the between-subject factor GROUP and AGE as a covariate. Significance levels were set to P = 0.05. Post hoc Scheffé tests were applied in case of significant interaction effects.
We found a right hemisphere lateralization of the N200, which was less prominent in the ASD-group (see Results). To quantify this lateralization, we subtracted the amplitude (averaged for ‘walker’ and ‘scramble’) at P9 (left hemisphere) from the amplitude at P10 (right hemisphere). Smaller (negative) values therefore indicate stronger right hemispheric lateralization. The correlation between P100 (averaged for both conditions and hemispheres) and the following processing stages including the N200 lateralization, activity in dipoles (averaged for both hemispheres) and the P400+ (averaged for both conditions) was calculated using the Pearson-correlation coefficient (see Results) for each group separately. Because these correlation analyses were performed in an exploratory way, no alpha correction was applied. Statistical differences between the correlation coefficients of ASD and TCs were tested with Fisher r-to-z transformations.
The statistical analysis was performed using Statistica (StatSoft, Tulsa, USA) software packages.
RESULTS
Behavioural data
The number of correct responses did not differ between both groups, neither for scrambled stimuli (Z = −0.5; P = 0.62) nor for ‘walker’ stimuli (Z = −0.4; P = 0.73). There were also no differences regarding reaction times (after stimulus-onset) for the walker (P = 0.53) and scrambled condition (P = 0.80; see Table 1 for further details).
ERP analysis
TCs showed higher P100 amplitudes than children with ASD in both conditions (main effect GROUP (F1,35 = 4.9; P = 0.03). P100 amplitude decreased with increasing age within both groups (main effect AGE (F1,35 = 9.8; P = 0.004; r = −0.46). Regarding P100 latency, TCs showed longer latencies than autistic children in both conditions (main effect GROUP F1,35 = 6.7; P = 0.014).
An interaction of GROUP*HEMISPHERE was found for the N200 amplitude (F1,35 = 6.1; P = 0.018). Post hoc tests showed a right hemisphere dominance (in the processing of both types of motion stimuli) in the control group (P = 0.02), but not in the ASD-group (P = 0.99). No latency differences of the N200 were found (Figure 3).
Fig. 3.
ERPs to both experimental conditions and in typical controls (solid line) and autistic children (ASD; dashed line) for (A) the P100 at electrodes O1 (left hemisphere) and O2 (right hemisphere), and (B) the N200 at electrodes P9 (left hemisphere) and P10 (right hemisphere).
Regarding the P400+ we found a trend for the factor GROUP (F1,35 = 3.5; P = 0.07). TCs showed slightly higher amplitudes in both conditions compared to ASD-children (Supplementary Table 1) (Figure 4).
Fig. 4.
P400+ at electrodes CPz, Pz, PO1, PO2, P1 and P2 (averaged) walker condition and scramble condition for typical controls (dashed line) and ASD.
Dipole source analysis—difference wave biological minus random motion
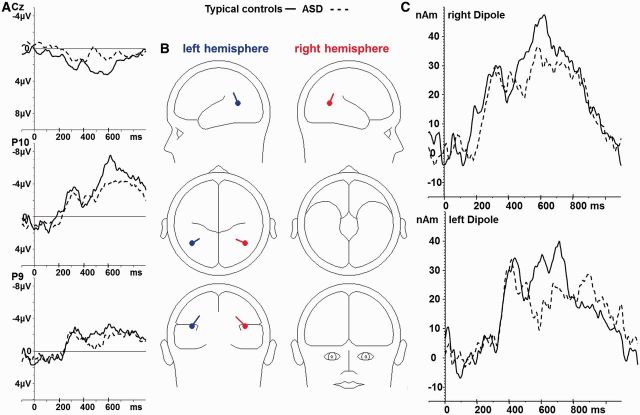
The equivalent dipoles were located at the occipito-temporal junction, between the posterior STS and MT+/V5. Both dipoles were located at a certain depth in the brain, indicating that they reflected the centre of gravity of larger active brain areas. The occipito-temporal negativity and midcentral/midcentroparietal positivity were explained by the same sources by volume conduction. The time-course of their activity is shown in Figure 5.
Fig. 5.
(A) Difference waves at the surface electrodes Cz, P10 (right hemisphere) and P9 (left hemisphere) in typical controls (solid line) and ASD (dashed line). (B) Dipole localization. (C) Dipole waves, displaying activity in the left and right occipito-temporal junction.
A trend for a main effect of GROUP (F1,35 = 3.16; P = 0.08) on dipole moments was found. TCs showed slightly stronger activity in both dipoles than that of children with ASD. The two-dipole model explained 88.6% of the variance of the difference waves elicited by human vs scrambled motion in TCs and 60.3% in ASD. This dipole model also explained significantly less variance in the ASD-group compared to TCs when dipole orientations were refit on single subject averages (F1,35 = 4.80; P = 0.035; Supplementary Table 1).
Correlation trends between the P100 and later visual ERP components
No significant correlation between P100 amplitude and the N200 lateralization was observed in TCs (r = −0.39; P = 0.09) or in ASD (r = −0.10; P = 0.71). Correlations did not differ between groups (z = −0.87; P = 0.38). The averaged P100 and LPC showed a correlation in TCs (r = 0.53; P = 0.01) and in ASD (r = 0.53, P = 0.03), which also did not differ between groups (z = 0; P = 1). We furthermore observed a correlation in both samples between P100 and dipole activity (TCs: r = 0.49; P = 0.02; ASD: r = 0.58; P = 0.02). Again, there was no statistical difference between both correlations (z = −0.35; P = 0.72) (Figure 6).
Fig. 6.
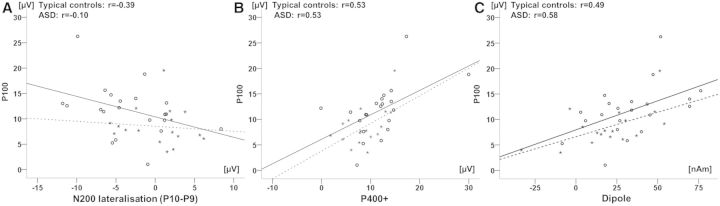
Correlation between P100 amplitude (averaged for both conditions and hemispheres) separately for each group and (A) N200 lateralisation (P10–P9, averaged for both conditions), (B) P400+ amplitude (averaged for both conditions), (C) dipole activity (averaged for both hemispheres). Typical controls are indicated by circles and subjects with ASD by stars.
DISCUSSION
The aim of our study was to clarify which components in the temporal sequence of neural processes are disturbed during biological and scrambled motion perception in children and adolescents with ASD. We addressed this issue using multi-channel EEG and source analysis. We found especially reduced amplitudes in P100 in biological and scrambled motion processing. The correlation analysis indicated that P100 amplitudes may partly explain reduced subsequent activation, e.g. in the biological motion specific dipole activity. Because these correlation analyses were performed without alpha correction, they should be interpreted with caution. In the following paragraphs, we will discuss the implications of the different neuronal processing stages in detail.
First, we observed a stimulus-unspecific positive activation after 100 ms within the visual cortex. This component is thought to reflect stimulus feature extraction, e.g. contrast, luminance, motion detection (Allison et al., 1999; Niedeggen and Wist, 1999; Krakowski et al., 2011), or pattern processing (e.g. Kubova et al., 1995; Hoffmann et al., 1999), and is supposed to be generated within the visual areas V1 and V2 (Prieto et al., 2007). Compared to TCs, the activity during this first processing stage was generally reduced in children with ASD as marked by lower amplitudes and decreased latencies. Thus, ASD seems to be associated with deficits in early visual motion processing which in turn may have an impact on higher order visual processing stages. The reduced P100 in our ASD-sample could also be related to a deficit in processing of complex stimuli. Stimuli used in our experiment showed some complexity as they contained different information, e.g. luminance, motion and form. Visual processing impairments in ASD especially of complex stimuli have been discussed previously (Bertone et al., 2003, 2005; Pellicano et al., 2005). It has been hypothesized that reduced lateral and horizontal connectivity within the visual cortex in individuals with ASD may underlie these difficulties (Bertone et al., 2005; Vandenbroucke et al., 2008; Keita et al., 2011). According to this theory, perception of simple visual stimuli is not affected in ASD, because only single processing unit areas are involved in the processing of simple stimuli, whereas processing of more complex stimuli (e.g. complex motion patterns) will be disrupted because activity of several processing units needs to be synchronized. Alternatively reduced P100 amplitudes could also be caused by abnormal visual attention allocation to the motion patterns. Because we did not use eye tracking it cannot be ruled out, that ASD-patients might have scanned the stimuli in a different way than TCs. Indeed in a recent study (Nackaerts et al., 2012), ASD-subjects produced more saccades and shorter fixation-durations in a biological motion recognition task. This pattern was interpreted as an indicator of a local processing bias in accordance with the ‘weak central coherence theory’ of autism (Frith and Happé, 1994). Thus, early inappropriate top-down modulation might be responsible for a reduced activity in early processing stages (Happe and Frith, 2006). However, a recent study found normal visual scanning patterns but no reduced modulation of face-sensitive ERPs in ASD (Wagner et al., 2013). Thus, future studies should assess visual scanning of biological motion stimuli and neuronal activation simultaneously.
In a second processing stage, both stimuli evoked negative amplitudes after 200 ms. The amplitude peak was found at electrodes P10 and P9 probably located over MT+/V5. While the P100 is supposed to be involved in motion detection, the N200 seems to be involved in the processing of motion direction (Niedeggen and Wist, 1999). It has also been suggested that N200 reflects the result of the integration of various consecutive visual scenes when changing locations of visual objects are interpreted as motion in a specific direction (Jokisch et al., 2005). The N200 showed a lateralization to the right hemisphere in TCs. A dominance of the right hemisphere in the processing of motion direction has been described before (Niedeggen and Wist, 1999; Prieto et al., 2007). The ASD-group did not show this hemispheric asymmetry.
It may be discussed if the lateralization to the right might be affected by motor reaction to the stimulus (walker or scrambled) which was always performed with the right hand. As lateralization was observed in the posterior temporal region, and, preparatory neural activity to a motor movement as a rule is observable in premotor and supplementary motor regions of the brain (Cunnington et al., 2003; Baker et al., 2011), this is highly unlikely. In addition, preparatory potentials are marked by slowly increasing neural activity (Baker et al., 2012) and not by a fast peaking potential, as the N200. In addition, as both groups performed equally well on the behavioural level, and all individuals were right handed, the group differences in N200 lateralization cannot be explained by right-hand motor responses.
Similar to our study, lack or abnormal structural and functional hemispheric asymmetry in ASD has been described in studies on language perception in the auditory (Boddaert et al., 2004; Kleinhans et al., 2008; Gage et al., 2009; Eyler et al., 2012) and frontal language cortex (de Fosse et al., 2004). Furthermore, reduced hemispheric asymmetry in the visual system was observed in adults (McPartland et al., 2004) and children (Webb et al., 2006) diagnosed with ASD and in infants at high-risk for ASD (McCleery et al., 2009). Hemispheric asymmetries appear very early in typical developing populations (Glasel et al., 2011; Kasprian et al., 2011). Because transcortical processing needs time and capacity, hemispheric specialization is important for effective and fast stimulus processing, e.g. during speech production [for a discussion, see Vallortigara and Rogers (2005)]. If such asymmetries fail to develop in ASD, this may negatively influence the development of specialized networks, and therefore may explain impaired language development or abnormal development of visual processing in this population (Eyler et al., 2012).
Interestingly, the lateralization of the N200 was not influenced by the P100 in our study. Therefore, abnormal lateralization of the N200 seems to be independent of early visual processing difficulties in ASD. Although P100 appears earlier than N200, and visual stimuli are likely to be processed in sequential stages, there is some evidence that V5 also receives input directly from subcortical structures (Standage and Benevento, 1983; Niedeggen and Wist, 1999) and is stimulated not only via V1/V2. Reports of prenatal influences on hemispheric asymmetries (Kasprian et al., 2011) support the notion of early developmental differences in hemispheric specializations, which may be independent from V1/V2 processing impairments.
After 400 ms we found a stimulus unspecific positive deflection at centro-parietal areas in both groups. While earlier components reflect automatic processes, this P400+ is supposed to be a marker of a top-down, active cognitive process involved in decoding the stimulus content (Schendan and Kutas, 2007; Krakowski et al., 2011). ASD-children showed a trend towards reduced activity in this top-down processing stage. A reduced P400+, probably a sub-component of the LPC, could be a marker of less efficient processing when a stimulus cannot be identified (Schendan and Kutas, 2007). Larger sample sizes or more demanding stimuli are probably needed to find significant differences between typical controls and ASD. In contrast to Krakowski et al., we did not observe a difference between activation to biological and random motion for the P400+ in both groups. This might be due to less automated and efficient processes in children compared to adults in human and biological motion perception.
As a second process after 400 ms post stimulus-onset, we analysed the difference waves between both conditions (‘walker’–‘scrambled’) by dipole source analysis. By comparing this contrast, effects of non-biological motion processing are expected to be removed with the aim to investigate brain systems specifically involved in the detection of biological motion (Jokisch et al., 2005). In this kind of analysis, dipoles have to be interpreted as equivalent dipoles, i.e. not indicating an exact anatomical localization of brain activation, but a centre of gravity. Its strength is the temporal resolution, which allows differentiating unspecific early processing and later specific biological motion perception stages. We found dipole activity approximately in the occipital–temporal junction bilaterally starting after 450 ms in TCs. Such specific activations to biological motion were previously described in the posterior temporal cortex, including regions important for social perception (Hein and Knight, 2008; Pavlova, 2012). The dipole activity in the control group might therefore be centred in this region. Activation of the intraparietal sulcus may also be reflected by the dipole, as it is involved in the detection of spatial relations (Grefkes and Fink, 2005; Wandell et al., 2007) which is also important for biological motion detection. The ASD-group showed a more diffuse and slightly reduced activity after 450 ms post stimulus-onset in this dipole. Furthermore, the amount of cortex activation explained by both dipoles was lower in the ASD-group. This result points towards a disrupted network responsible for the processing of biological motion patterns (Herrington et al., 2007; Freitag et al., 2008; Kaiser et al., 2010b; Koldewyn et al., 2011). In contrast to the distinctive EEG activation pattern, we found no differences on the behavioural level between ASD and TCs. This might indicate that in ASD different and compensatory processing pathways are used to process biological motion patterns. The deviant and diffuse brain activation during biological motion specific processing may indicate these compensatory mechanisms, which seem to involve less specific and more distributed temporal and parietal brain areas in ASD compared to TCs. This is in line with one recent study, which applied connectivity analysis (McKay et al., 2012). The activation of two distinct neuronal networks for motion and form information was found in ASD whereas in TCs only one network was active, passing information from temporal to parietal regions. Several other studies support the notion of impaired functional connectivity ASD (Vissers et al., 2012) but further studies are needed to elucidate the exact nature of such deficits.
Similar to our results regarding the P400+, the dipole activation also correlated to P100 amplitude. Biological motion-specific processing abnormalities in ASD might therefore be caused by early processing deficits. If deficits in primary unspecific visual perception emerge early in development, they may have an effect on the development, organization and connectivity of not only neural networks within the visual system but also other higher ordered networks. Such early abnormalities in the processing of basal visual information (luminance, contrast) were indeed found in infants at risk for ASD (McCleery et al., 2007). Thus the disrupted visual perception abilities might be causal to a disturbed perception of biological motion, which might further be related to imitation impairments in ASD (Freitag et al., 2008). Disrupted imitation abilities, appearing early in ASD (Williams et al., 2004), are sometimes hypothesized to be causal to the deviant development of language and communication abilities (Stone and Yoder, 2001; Heimann et al., 2006; Toth et al., 2006). But such developmental processes should specifically be studied by longitudinal studies.
CONCLUSION
We observed processing deficits in the visual cortex as early as 100 ms after stimulus presentation during perception of biological and random motion stimuli in ASD. Because perception deficits were not limited to biological motion, individuals with ASD seem to be affected by a more generally disturbed processing of random motion. In addition, the reduced hemispheric lateralization and diffuse brain activation during biological motion-specific processing point towards less specialized and thus possibly less effective neuronal processing in ASD. Longitudinal studies need to assess, if this reduced specialization is caused by early visual perception deficits in primary visual areas in ASD. Furthermore, these results should be replicated with more naturalistic stimuli, e.g. moving hands vs moving objects (Cook et al., 2009). Finally, future studies should address biological motion processing in other ASD populations not meeting our inclusion and exclusion criteria.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
CONFLICT OF INTEREST
None declared.
Supplementary Material
Acknowledgments
First, we thank all families, who supported our work by participating in our study. We also thank Jennifer Böhm and Katharina Hof for assistance with the data collection, Benjamin Teufert for data calculations and our clinical colleagues for helping us with recruiting participants. This work was supported by the foundation of Marie Christine Held and Erika Hecker to A.K., the German Research foundation ‘Deutsche Forschungsgemeinschaft’ to C.M.F. (FR2069/2-1), and by the LOEWE-Program ‘Neuronal Coordination Research Focus Frankfurt’ (NeFF) to C.M.F. and S.B.
REFERENCES
- Allison T, Puce A, Spencer DD, McCarthy G. Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non-face stimuli. Cerebral Cortex. 1999;9:415–30. doi: 10.1093/cercor/9.5.415. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edn. Arlington: American Psychiatric Association; 2000. [Google Scholar]
- Annaz D, Campbell R, Coleman M, Milne E, Swettenham J. Young children with autism spectrum disorder do not preferentially attend to biological motion. Journal of Autism and Developmental Disorders. 2012;42:401–8. doi: 10.1007/s10803-011-1256-3. [DOI] [PubMed] [Google Scholar]
- Annaz D, Remington A, Milne E, et al. Development of motion processing in children with autism. Developmental Science. 2010;13:826–38. doi: 10.1111/j.1467-7687.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- Arbeitsgruppe Kinder, Jugendlichen- und Familiendiagnostik. CBCL/4-18 Elternfragebogen über das Verhalten von Kindern und Jugendlichen. Göttingen: Hogrefe; 1998. [Google Scholar]
- Baker KS, Mattingley JB, Chambers CD, Cunnington R. Attention and the readiness for action. Neuropsychologia. 2011;49:3303–13. doi: 10.1016/j.neuropsychologia.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Baker KS, Piriyapunyaporn T, Cunnington R. Neural activity in readiness for incidental and explicitly timed actions. Neuropsychologia. 2012;50:715–22. doi: 10.1016/j.neuropsychologia.2011.12.026. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Motion perception in autism: a ‘complex’ issue. Journal of Cognitive Neuroscience. 2003;15:218–25. doi: 10.1162/089892903321208150. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain. 2005;128:2430–41. doi: 10.1093/brain/awh561. [DOI] [PubMed] [Google Scholar]
- Blake R, Turner LM, Smoski MJ, Pozdol SL, Stone WL. Visual recognition of biological motion is impaired in children with autism. Psychological Science. 2003;14:151–7. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Belin P, et al. Perception of complex sounds in autism: abnormal auditory cortical processing in children. American Journal of Psychiatry. 2004;161:2117–20. doi: 10.1176/appi.ajp.161.11.2117. [DOI] [PubMed] [Google Scholar]
- Bölte S, Rühl D, Schmötzer G, Poustka F. Diagnostisches Interview für Autismus—revidiert. ADI-R; deutsche Fassung des Autism Diagnostic Interview—revised (ADI-R) von Michael Rutter, Ann Le Couteur und Catherine Lord. Manual. Göttingen: Huber/Hogrefe; 2006. [Google Scholar]
- Brieber S, Herpertz-Dahlmann B, Fink GR, Kamp-Becker I, Remschmidt H, Konrad K. Coherent motion processing in autism spectrum disorder (ASD): an fMRI study. Neuropsychologia. 2010;48:1644–51. doi: 10.1016/j.neuropsychologia.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Bulheller S, Häcker HO. Coloured Progressive Matrices. Frankfurt: Swets Test Services; 2002. Deutsche Bearbeitung und Normierung nach J.C. Raven. [Google Scholar]
- Bulheller S, Häcker HO. Ravens’s Progressive Matrices and Vocabulary Scales. Frankfurt: Swets Test Services; 2003. Deutsche Bearbeitung und Normierung nach J.C. Raven. [Google Scholar]
- Cook J, Saygin A, Swain R, Blakemore S. Reduced sensitivity to minimum-jerk biological motion in autism spectrum conditions. Neuropsychologia. 2009;47:3275–8. doi: 10.1016/j.neuropsychologia.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and readiness for voluntary movement: a high-field event-related fMRI study of the Bereitschafts-BOLD response. NeuroImage. 2003;20:404–12. doi: 10.1016/s1053-8119(03)00291-x. [DOI] [PubMed] [Google Scholar]
- Eyler LT, Pierce K, Courchesne E. A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain. 2012;135:949–60. doi: 10.1093/brain/awr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fosse L, Hodge SM, Makris N, et al. Language-association cortex asymmetry in autism and specific language impairment. Annals of Neurology. 2004;56:757–66. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- Freitag CM, Konrad C, Haeberlen M, et al. Perception of biological motion in autism spectrum disorders. Neuropsychologia. 2008;46:1480–94. doi: 10.1016/j.neuropsychologia.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Frith U, Happé F. Autism: beyond ‘theory of mind’. Cognition. 1994;50:115–32. doi: 10.1016/0010-0277(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Gage NM, Juranek J, Filipek PA, et al. Rightward hemispheric asymmetries in auditory language cortex in children with autistic disorder: an MRI investigation. Journal of Neurodevelopmental Disorders. 2009;1:205–14. doi: 10.1007/s11689-009-9010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasel H, Leroy F, Dubois J, Hertz-Pannier L, Mangin J, Dehaene-Lambertz G. A robust cerebral asymmetry in the infant brain: the rightward superior temporal sulcus. NeuroImage. 2011;58:716–23. doi: 10.1016/j.neuroimage.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. Journal of Anatomy. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH. The development of the social brain in human infancy. European Journal of Neuroscience. 2007;25:909–19. doi: 10.1111/j.1460-9568.2007.05379.x. [DOI] [PubMed] [Google Scholar]
- Happe F, Frith U. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36:5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Heimann M, Strid K, Smith L, Tjus T, Erik Ulvund S, Meltzoff AN. Exploring the relation between memory, gestural communication, and the emergence of language in infancy: a longitudinal study. Infant and Child Development. 2006;15:233–49. doi: 10.1002/icd.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G, Knight RT. Superior temporal sulcus—it‘s my area: or is it? Journal of Cognitive Neuroscience. 2008;20:2125–36. doi: 10.1162/jocn.2008.20148. [DOI] [PubMed] [Google Scholar]
- Herrington JD, Baron-Cohen S, Wheelwright SJ, et al. The role of MT+/V5 during biological motion perception in Asperger Syndrome: an fMRI study. Research in Autism Spectrum Disorders. 2007;1:14–27. [Google Scholar]
- Hirai M, Fikushima H, Hiraki K. An event-related potentials study of biological motion perception in humans. Neuroscience Letters. 2003;344:41–44. doi: 10.1016/s0304-3940(03)00413-0. [DOI] [PubMed] [Google Scholar]
- Hirai M, Kakigi R. Differential cortical processing of local and global motion information in biological motion: an event-related potential study. Journal of Vision. 2008;8 doi: 10.1167/8.16.2. Article No. 2. [DOI] [PubMed] [Google Scholar]
- Hirai M, Watanabe S, Honda Y, Kakigi R. Developmental changes in point-light walker processing during childhood and adolescence: an event-related potential study. Neuroscience. 2009;161:311–25. doi: 10.1016/j.neuroscience.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Dorn TJ, Bach M. Time course of motion adaptation: motion-onset visual evoked potentials and subjective estimates. Vision Research. 1999;39:437–44. doi: 10.1016/s0042-6989(98)00186-2. [DOI] [PubMed] [Google Scholar]
- Johansson G. Visiual-perception of biological motion and a model for its analysis. Perception & Psychophysics. 1973;14:201–11. [Google Scholar]
- Jokisch D, Daum I, Suchan B, Troje NF. Structural encoding and recognition of biological motion: evidence from event-related potentials and source analysis. Behavioral Brain Research. 2005;157:195–204. doi: 10.1016/j.bbr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Jones CRG, Swettenham J, Charman T, et al. No evidence for a fundamental visual motion processing deficit in adolescents with autism spectrum disorders. Autism Reserach. 2011;4:347–57. doi: 10.1002/aur.209. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Delmolino L, Tanaka JW, Shiffrar M. Comparison of visual sensitivity to human and object motion in autism spectrum disorder. Autism Research. 2010a;3:191–5. doi: 10.1002/aur.137. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proceedings of the National Academy of Sciences of the United States of America. 2010b;107:21223–8. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser MD, Pelphrey KA. Disrupted action perception in autism: behavioral evidence, neuroendophenotypes, and diagnostic utility. Developmental Cognitive Neuroscience. 2012;2:25–35. doi: 10.1016/j.dcn.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprian G, Langs G, Brugger PC, et al. The prenatal origin of hemispheric asymmetry: an in utero neuroimaging study. Cerebral Cortex. 2011;21:1076–83. doi: 10.1093/cercor/bhq179. [DOI] [PubMed] [Google Scholar]
- Keita L, Mottron L, Dawson M, Bertone A. Atypical lateral connectivity: a neural basis for altered visuospatial processing in autism. Biological Psychiatry. 2011;70:806–11. doi: 10.1016/j.biopsych.2011.07.031. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Mueller R-A, Cohen DN, Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Research. 2008;1221:115–25. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–61. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K, Whitney D, Rivera SM. The psychophysics of visual motion and global form processing in autism. Brain. 2010;133:599–610. doi: 10.1093/brain/awp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K, Whitney D, Rivera SM. Neural correlates of coherent and biological motion perception in autism. Developmental Science. 2011;14:1075–88. doi: 10.1111/j.1467-7687.2011.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowski AI, Ross LA, Snyder AC, Sehatpour P, Kelly SP, Foxe JJ. The neurophysiology of human biological motion processing: a high-density electrical mapping study. NeuroImage. 2011;56:373–83. doi: 10.1016/j.neuroimage.2011.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubova Z, Kuba M, Spekreijse H, Blakemore C. Contrast dependence of motion-onset and apttern-reversal evoked-potentials. Vision Research. 1995;35:197–205. doi: 10.1016/0042-6989(94)00138-c. [DOI] [PubMed] [Google Scholar]
- McCleery JP, Akshoomoff N, Dobkins KR, Carver LJ. Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biological Psychiatry. 2009;66:950–7. doi: 10.1016/j.biopsych.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery JP, Allman E, Carver LJ, Dobkins KR. Abnormal magnocellular in infants at risk for autism. Biological Psychiatry. 2007;62:1007–14. doi: 10.1016/j.biopsych.2007.02.009. [DOI] [PubMed] [Google Scholar]
- McKay LS, Simmons DR, McAleer P, Marjoram D, Piggot J, Pollick FE. Do distinct atypical cortical networks process biological motion information in adults with autism spectrum disorders? NeuroImage. 2012;59:1524–33. doi: 10.1016/j.neuroimage.2011.08.033. [DOI] [PubMed] [Google Scholar]
- McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2004;45:1235–45. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- Murphy P, Brady N, Fitzgerald M, Troje NF. No evidence for impaired perception of biological motion in adults with autistic spectrum disorders. Neuropsychologia. 2009;47:3225–35. doi: 10.1016/j.neuropsychologia.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Nackaerts E, Wagemans J, Helsen W, et al. Recognizing biological motion and emotions from point-light displays in autism spectrum disorders. Plos One. 2012;7:e44473. doi: 10.1371/journal.pone.0044473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedeggen M, Wist ER. Characteristics of visual evoked potentials generated by motion coherence onset. Cognitive Brain Research. 1999;8:95–105. doi: 10.1016/s0926-6410(99)00009-9. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. Assessment and analysis of handedness—Edingburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pavlova MA. Biological Motion processing as a hallmark of social cognition. Cerebral Cortex. 2012;22:981–95. doi: 10.1093/cercor/bhr156. [DOI] [PubMed] [Google Scholar]
- Pellicano E, Gibson L, Maybery M, Durkin K, Badcock DR. Abnormal global processing along the dorsal visual pathway in autism: a possible mechanism for weak visuospatial coherence? Neuropsychologia. 2005;43:1044–53. doi: 10.1016/j.neuropsychologia.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Price KJ, Shiffrar M, Kerns KA. Movement perception and movement production in Asperger's Syndrome. Research in Autism Spectrum Disorders. 2012;6:391–8. [Google Scholar]
- Prieto EA, Barnikol UB, Soler EP, et al. Timing of V1/V2 and V5+ activations during coherent motion of dots: an MEG study. NeuroImage. 2007;37:1384–95. doi: 10.1016/j.neuroimage.2007.03.080. [DOI] [PubMed] [Google Scholar]
- Rühl D, Bölte S, Feineis-Matthews S, Poustka F. German Version of the Autism Diagnostic Observation Schedule. Bern: Huber; 2004. [Google Scholar]
- Rutherford MD, Troje NF. IQ predicts biological motion perception in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42:557–65. doi: 10.1007/s10803-011-1267-0. [DOI] [PubMed] [Google Scholar]
- Saygin AP, Cook J, Blakemore S-J. Unaffected perceptual thresholds for biological and non-biological form-from-motion perception in autism spectrum conditions. Plos One. 2010;5:e13491. doi: 10.1371/journal.pone.0013491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendan HE, Kutas M. Neurophysiological evidence for the time course of activation of global shape, part, and local contour representations during visual object categorization and memory. Journal of Cognitive Neuroscience. 2007;19:734–49. doi: 10.1162/jocn.2007.19.5.734. [DOI] [PubMed] [Google Scholar]
- Schneider S, Unnewehr S, Margraf J. Diagnostisches Interview bei psychischen Störungen im Kindes- und Jugendalter. Kinder-DIPS. Heidelberg: Springer; 2009. [DOI] [PubMed] [Google Scholar]
- Simion F, Regolin L, Bulf H. A predisposition for biological motion in the newborn baby. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:809–13. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standage GP, Benevento LA. The organization of connections between the pulvinar and visual area MT in the macaque monkey. Brain Research. 1983;262:288–94. doi: 10.1016/0006-8993(83)91020-x. [DOI] [PubMed] [Google Scholar]
- Stone WL, Yoder PJ. Predicting spoken language level in children with autism spectrum disorders. Autism. 2001;5:341–61. doi: 10.1177/1362361301005004002. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, McCarthy G, Saliba E, Degiovanni E. ERP evidence of developmental changes in processing of faces. Clinical Neurophysiology. 1999;110:910–5. doi: 10.1016/s1388-2457(99)00006-1. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Smith ML. Age-related ERP changes in verbal and nonverbal memory tasks. Journal of Psychophysiology. 1995;9:283–97. [Google Scholar]
- Toth K, Munson J, Meltzoff AN, Dawson G. Early predictors of communication development in young children with autism spectrum disorder: joint attention, imitation, and toy play. Journal of Autism and Developmental Disorders. 2006;36:993–1005. doi: 10.1007/s10803-006-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troje NF. Decomposing biological motion: a framework for analysis and synthesis of human gait patterns. Journal of Vision. 2002;2:371–87. doi: 10.1167/2.5.2. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behavioral and Brain Sciences. 2005;28:575–89. doi: 10.1017/S0140525X05000105. [DOI] [PubMed] [Google Scholar]
- Vandenbroucke MWG, Scholte HS, van Engeland H, Lamme VAF, Kemner C. A neural substrate for atypical low-level visual processing in autism spectrum disorder. Brain. 2008;131:1013–24. doi: 10.1093/brain/awm321. [DOI] [PubMed] [Google Scholar]
- Vissers ME, Cohen MX, Geurts HM. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neuroscience and Biobehavioral Reviews. 2012;36:604–25. doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Wagner JB, Hirsch SB, Vogel-Farley VK, Redcay E, Nelson CA. Eye-tracking, autonomic, and electrophysiological correlates of emotional face processing in adolescents with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2013;43:188–99. doi: 10.1007/s10803-012-1565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron. 2007;56:366–83. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Dawson G, Bernier R, Panagiotides H. ERP evidence of atypical face processing in young children with autism. Journal of Autism and Developmental Disorders. 2006;36:881–90. doi: 10.1007/s10803-006-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JHG, Whiten A, Singh T. A systematic review of action imitation in autistic spectrum disorder. Journal of Autism and Developmental Disorders. 2004;34:285–99. doi: 10.1023/b:jadd.0000029551.56735.3a. [DOI] [PubMed] [Google Scholar]
- World Health Organization, WHO. Geneva: WHO; 1992. The ICD-10 classification of mental and behavioural disorders. Clinical descriptions and diagnostic guidelines. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.