Abstract
Individuals with antisocial behavior place a great physical and economic burden on society. Deficits in emotional processing have been recognized as a fundamental cause of antisocial behavior. Emerging evidence also highlights a significant contribution of attention allocation deficits to such behavior. A comprehensive literature search identified 12 studies that were eligible for inclusion in the meta-analysis, which compared 291 individuals with antisocial problems and 247 controls. Signed Differential Mapping revealed that compared with controls, gray matter volume (GMV) in subjects with antisocial behavior was reduced in the right lentiform nucleus (P < 0.0001), left insula (P = 0.0002) and left frontopolar cortex (FPC) (P = 0.0006), and was increased in the right fusiform gyrus (P < 0.0001), right inferior parietal lobule (P = 0.0003), right superior parietal lobule (P = 0.0004), right cingulate gyrus (P = 0.0004) and the right postcentral gyrus (P = 0.0004). Given the well-known contributions of limbic and paralimbic areas to emotional processing, the observed reductions in GMV in these regions might represent neural correlates of disturbance in emotional processing underlying antisocial behavior. Previous studies have suggested an FPC role in attention allocation during emotional processing. Therefore, GMV deviations in this area may constitute a neural basis of deficits in attention allocation linked with antisocial behavior.
Keywords: antisocial personality disorder, Brodmann area (BA) 9, conduct disorder, frontal pole, psychopathy
INTRODUCTION
Individuals with antisocial behavior, such as conduct disorder (CD), antisocial personality disorder (ASPD) and callous-unemotional traits or psychopathy, place a great physical and economic burden on society (Moffitt, 1993; Kratzer and Hodgins, 1997; Loeber and Stouthamer-Loeber, 1998). People with such disorders have symptoms of emotional detachment and a propensity for disinhibited, impulsive behavior combined with a general callousness and lack of insight for the impact that such behavior has on others (Cleckley, 1941; Anderson and Kiehl, 2012). Though not all children with CD have life-course persistent symptoms, genetic studies have suggested that continuous antisocial behavior is heritable (Moffitt, 2005) and children with CD or callous-unemotional traits frequently develop an ASPD or psychopathy in adulthood (Frick and Viding, 2009). Neurodevelopmental theories also suggest that brain abnormalities in early life are associated with lifelong antisocial behavior (Frick and Viding, 2009; Gao et al., 2009), indicating that individuals with CD, callous-unemotional traits, ASPD and psychopathy share a common neural basis.
Whether antisocial behavior is characterized by fundamental deficits in attention or emotion is a long-standing debate (Sadeh and Verona, 2012). Although numerous studies have confirmed that deficits in emotional processing are involved in the pathophysiology of antisocial behavior (Blair, 2007; Sadeh and Verona, 2012), individuals with antisocial behavior also perform abnormally on emotionally neutral tasks (Jutai and Hare, 1983; Blair et al., 2006; Vitale et al., 2007; Zeier et al., 2009; Sadeh et al., 2013). Thus, the pathophysiology model of abnormal emotional processing cannot fully account for such deficits in emotionally neutral information processing. Based on psychological experiments in which appropriate emotional responses were reported when attention was focused on emotional stimuli (Glass and Newman, 2006; Newman et al., 2010; Baskin-Sommers et al., 2011), it is hypothesized that it is not only emotional processing deficits but also attention deficits, especially attention allocation and maintenance during emotional processing, that contributes to the pathophysiology of antisocial behavior (Sadeh and Verona, 2012).
Neuroimaging studies have investigated the neural bases of the pathophysiology of antisocial behavior. The amygdala, one of the centers of emotional processing (Phelps and LeDoux, 2005), is the region most commonly implicated in functional and structural abnormalities of the brain in individuals who display antisocial behavior (Blair et al., 2006; Yang et al., 2009). In addition to the amygdala, the paralimbic system is also recognized as a center of emotional processing and has often been investigated in antisocial behavior research (Blair et al., 2006; Blair, 2007; Raine et al., 2010; Finger et al., 2012; Ly et al., 2012). Among the paralimbic regions, the orbitofrontal cortex (OFC) and ventromedial prefrontal cortex (vmPFC) are those most commonly studied in the field of antisocial behavior, and a number of structural magnetic resonance imaging (MRI) studies have reported abnormalities in these regions in people with antisocial behavior (Boccardi et al., 2011; Hyatt et al., 2012). Functional MRI (fMRI) studies have consistently revealed abnormal activity in the OFC/vmPFC in individuals with antisocial problems during tasks related to emotional processing in value-oriented or social situation, such as making judgments about legal actions (Marsh et al., 2011), social cooperation tasks (Rilling et al., 2002, 2007) and gambling tasks (Mitchell et al., 2002; Blair, 2003). Thus, dysfunction of the amygdala and paralimbic regions has been proposed as fundamental to the pathophysiology of abnormal emotional processing in people with antisocial behavior (Koenigs, 2012).
Neuroimaging studies with healthy volunteers indicate that the FPC is associated with allocating and maintaining attention on emotional stimuli (Koechlin et al., 1999; Burgess et al., 2007; Tsujimoto et al., 2011). Given that the FPC is a potential neural correlate of attention allocation deficits during emotional processing in individuals with antisocial behavior, we hypothesized that structural abnormalities would be observed in the FPC of such individuals.
A number of whole-brain voxel-based morphometry (VBM) studies of people with antisocial problems have reported various regional gray matter volume (GMV) abnormalities. Some have reported abnormalities in the FPC but results are inconsistent (Tiihonen et al., 2008; Gregory et al., 2012). A contributing factor to the inconsistency of results may be each study’s insufficient sample size. Therefore, integration of these results with a statistically conservative threshold would address our hypothesis. To clarify whether VBM studies demonstrate abnormality in the areas related to attention allocation deficit, we conducted a systematic review and meta-analysis of unbiased VBM studies.
METHOD
Study selection
A comprehensive literature search of VBM studies published in peer-reviewed journals between 2001 (the date of the first VBM study in subjects with antisocial behavior) and April 2013 that compared individuals with ASPD, CD, callous-unemotional trait, disruptive behavior disorder and psychopathy with healthy subjects was conducted using the MEDLINE, Embase and Web of Knowledge databases. The search keywords were ‘antisocial’, ‘conduct’, ‘disruptive’, ‘oppositional defiant’, ‘callous-unemotional’, ‘psychopathy’ or ‘psychopath’, plus ‘morphometry’, ‘voxel-based’, ‘VBM’ or ‘voxel-wise’. The titles and abstracts of the studies were examined to determine whether or not they should be included. The reference lists of the included articles were also examined to search for additional relevant studies to be included. We defined the individuals with ASPD, CD, disruptive behavior disorder, callous-unemotional trait and psychopathy as individuals with antisocial behavior.
Selection of studies
Studies were included in our database if (i) they reported a voxel-wise comparison between patients with antisocial behavior and controls for GMV; and similar to previous studies (Radua and Mataix-Cols, 2009; Radua et al., 2010), (ii) they reported whole-brain results in stereotactic coordinates and used thresholds for significance corrected for multiple comparisons, or uncorrected with spatial extent thresholds. The literature search was performed without language restriction. If the study did not provide sufficient data, we emailed the corresponding author to obtain more data. In cases where the author did not respond, we excluded the study. Two of the authors (Y.A. and R.I.) independently screened the studies.
Comparison of regional GMVs
For coordinate-based meta-analysis, we used Signed Differential Mapping (SDM) software (www.sdmproject.com/software/) (Radua and Mataix-Cols, 2009; Radua et al., 2010, 2011; Bora et al., 2011; Nakao et al., 2011) to analyze GM abnormalities in patients with antisocial behavior. Briefly, a map of GMV differences, comprising the reported stereotactic coordinates for each significant group difference, was generated for each study. In SDM, unlike in other coordinate-based, meta-analytic methods, both positive and negative differences are reconstructed in the same map, which prevents a particular voxel from appearing significant in opposite directions. Importantly, when using SDM, the effects of negative studies are also included in the meta-analysis. Meta-analytic statistical maps were subsequently obtained by calculating the corresponding statistics from the study maps, weighted by the square root of the sample size of each study, to enable studies with large sample sizes to contribute proportionally more. A random effect model is applied to integrate the effect sizes of the studies (Radua et al., 2012). The statistical significance of each voxel was determined using randomization tests (P < 0.001) as in previous studies (Radua and Mataix-Cols, 2009; Radua et al., 2010; Bora et al., 2011).
Data extraction
We extracted the number of participants of both groups, and the coordinates and effect sizes of peak voxels. When different types of statistical values were reported, such as z-values, they were converted to t-values, accounting for the number of participants in both groups and the number of covariates. In addition, we extracted the mean age of participants.
In one study, which reported peak coordinates and threshold without statistical values of the coordinates, we determined the threshold value as the effect size of the coordinates (Fahim et al., 2011). The uncorrected statistical thresholds were set at P < 0.001 based on previous literature (Radua and Mataix-Cols, 2009; Radua et al., 2010; Bora et al., 2011).
RESULTS
Study selection for database
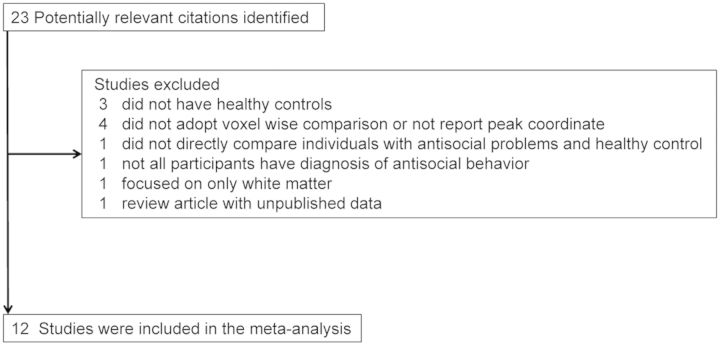
The literature search produced 23 potential candidates for the meta-analysis. Three studies were excluded because they did not involve healthy control comparisons (Ermer et al., 2012, 2013; Cope et al., 2012). Four studies were discarded because they did not adopt voxel-wise comparison or did not report peak coordinate (Yang et al., 2005; McAlonan et al., 2007; Schiffer et al., 2011; Sato et al., 2011). One study was discarded because it did not ensure any diagnosis of antisocial behavior we defined above in all the participants (Dalwani et al., 2011). One study was excluded because it did not directly compare individuals with antisocial behavior and healthy individuals (Sasayama et al., 2010). One study was discarded because it focused on only white matter (Wu et al., 2011). One study was not included because it was a review article with unpublished data (Vloet et al., 2008) (Figure 1).
Fig. 1.
Process of study selection.
Demographic characteristics
A comprehensive literature search identified 12 independent studies which were eligible for inclusion in the meta-analysis (Sterzer et al., 2007; de Oliveira-Souza et al., 2008; Huebner et al., 2008; Müller et al., 2008; Tiihonen et al., 2008; De Brito et al., 2009; Fahim et al., 2011; Fairchild et al., 2011, 2013; Gregory et al., 2012; Stevens and Haney-Caron, 2012; Bertsch et al., in press) (Table 1). In total, these 12 studies compared 291 individuals with antisocial problems and 247 control subjects. Nine studies included only male subjects (Sterzer et al., 2007; Huebner et al., 2008; Müller et al., 2008; Tiihonen et al., 2008; De Brito et al., 2009; Fahim et al., 2011; Fairchild et al., 2011; Gregory et al., 2012; Bertsch et al., in press), while one study recruited only female subjects (Fairchild et al., 2013). Seven reports involved children with antisocial problems; among these seven, five studied children with CD (Sterzer et al., 2007; Huebner et al., 2008; Fairchild et al., 2011, 2013; Stevens and Haney-Caron, 2012), one studied conduct problems (De Brito et al., 2009) and one studied disruptive behavioral disorder (Fahim et al., 2011). Five studies recruited adults, two included individuals with ASPD with psychopathy (Tiihonen et al., 2008; Gregory et al., 2012), one involved psychopathy (de Oliveira-Souza et al., 2008) and two recruited individuals with ASPD (Müller et al., 2008; Bertsch et al., in press). All the studies in the meta-analysis had excluded individuals with mental retardation.
Table 1.
Summary of studies included in the meta-analysis
| Study | Diagnosis | No. of ASB (male) | Age of ASB | FIQ | No. of CTRL (male) | Age of CTRL | FIQ | Handedness | Substance abuse | Comorbidity | Strength of magnetic field | FWHM (mm) | Significance | Covariates |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bertsch et al., in press | ASPD | 13 (13) | 28.9 | 99.6 | 14 (14) | 26.1 | 104 | NA | 54% | BPD | 1.5 | 8 | P < 0.005, uncorrected | Total intracranial volume |
| 12 (12) | 27.3 | 97.6 | 58% | No | ||||||||||
| De Brito et al. (2009) | Callous–unemotional traits | 25 (25) | 11.75 | 107 | 23 (23) | 11.6 | 95.4 | NA | No | NA | 3 | 8 | P < 0.001, uncorrected | GMV, FIQ, hyperactivity– inattention symptoms |
| de Oliveira-Souza et al. (2008) | Psychopathy | 15 (8) | 32 | NA | 15 (8) | 32 | NA | NA | NA | No | 1.5 | 8 | P < 0.005, uncorrected | ICV |
| Fahim et al. (2011) | Disruptive behavior | 22 (22) | 8.39 | NA | 25 (25) | 8.36 | NA | All right | NA | No | 1.5 | 10 | P < 0.05, corrected, FDR | NA |
| Fairchild et al., 2013 | CD | 22 (0) | 17.23 | 99.8 | 20 (0) | 17.6 | 106 | One left handed | NA | ADHD, MDD | 3 | 8 | P < 0.001, uncorrected | GMV |
| Fairchild et al. (2011) | CD | 63 (63) | 17.78 | 99.1 | 27 (27) | 18.5 | 101 | NA | 9% | Substance abuse | 3 | 8 | P < 0.001, uncorrected | GMV |
| Gregory et al. (2012) | ASPD with psychopathy | 17 (17) | 38.9 | 89.9 | 22 (22) | 32.4 | 99.4 | NA | yes | No | 1.5 | 8 | P < 0.05, corrected FDR | Age, IQ |
| Huebner et al. (2008) | CD | 23 (23) | 14.5 | 96.7 | 23 (23) | 14.2 | 98.9 | NA | No | No | 1.5 | 10 | P < 0.05 corrected for cluster level P < 0.001 uncorrected for voxel level | ICV |
| Muller et al. (2008) | ASPD | 17 (17) | 33 | NA | 17 (17) | 30.6 | NA | NA | 53% | Substance dependence | 1.5 | 10 | P < 0.05, corrected | Time of drug intake |
| Tiihonen et al. (2008) | ASPD and psychopathy | 26 (26) | 32.5 | NA | 25 (25) | 34.6 | NA | NA | 77% | Substance abuse | 1 | 8 | P < 0.05, corrected | Age, ICV |
| Sterzer et al. (2007) | CD | 12 (12) | 12.75 | 101 | 12 (12) | 12.5 | 107 | NA | NA | ADHD | 1.5 | 8 | P < 0.05, corrected | Global grey matter sign, Age, IQ |
| Stevens and Haney-Caron (2012) | CD | 24 (16) | 16 | NA | 24 (16) | 16 | NA | NA | 4% | Substance abuse | 3 | 8 | P < 0.05, uncorrected | No |
ASB, antisocial behavior; FIQ, full-scale intelligence quotient; FWHM, full-width at half-maximum; FDR, false discovery rate; CD, conduct disorder; ASPD, antisocial personality disorder; ADHD, attention deficit hyperactivity disorder; MDD, major depressive disorder; NA, not available; GMV, gray matter volume; ICV, intracranial volume.
Regional differences in GMV
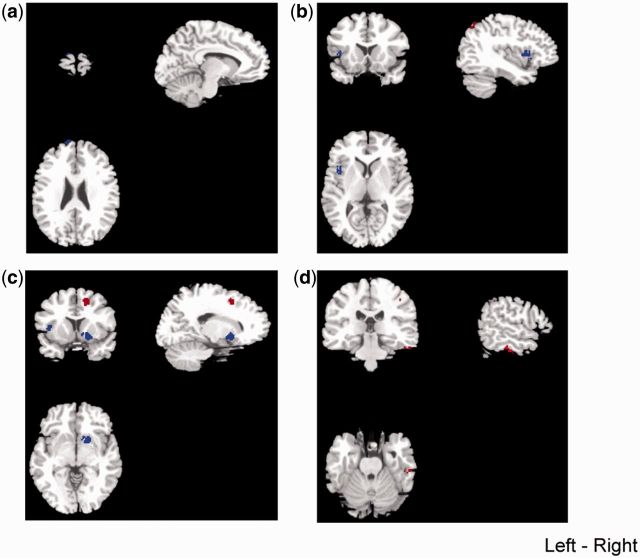
A meta-analysis revealed that individuals with antisocial behavior had significantly smaller-than-normal GMV in the left superior frontal gyrus in its frontopolar portion (Talairach coordinates: x = −10, y = 62, z = 26; SDM value = −2.261, P = 0.0006; 16 voxels) (Table 2 and Figure 2a), in the left anterior insula (Talairach coordinates: x = −40, y = 8, z = 10; SDM value = −2.389, P = 0.0002; 53 voxels) (Table 2 and Figure 2b) and in the right lentiform nucleus (Talairach coordinates: x = 18, y = 6, z = −4; SDM value = −2.541, P < 0.0001; 110 voxels) (Table 2 and Figure 2c). Although the meta-analysis demonstrated a tendency of smaller-than-normal GMV in the amygdala in individuals with antisocial behavior, it did not reach statistical significance (Talairach coordinates: x = −28, y = 2, z = −16; SDM value = −1.957, P = 0.0049).
Table 2.
Results of meta-analysis of VBM studies comparing individuals with antisocial behavior and controls
| Anatomical location | Maximum |
Cluster |
||||
|---|---|---|---|---|---|---|
| Talairach coordinate | SDM value | P-value (uncorrected) | Total cluster size | Breakdown | Sub-cluster size | |
| Smaller gray matter volume (individuals with antisocial behavior < controls) | ||||||
| Right lentiform nucleus | 18, 6, −4 | −2.541 | <0.0001 | 110 | rt. putamen | 69 |
| rt. lateral globus pallidus | 21 | |||||
| rt. caudate head | 18 | |||||
| Other sub-lobar region | 2 | |||||
| Left insula | −40, 8, 10 | −2.389 | 0.0002 | 53 | lt. insula | 46 |
| lt. precentral gyrus | 7 | |||||
| Left superior frontal gyrus | −10, 62, 26 | −2.261 | 0.0006 | 16 | lt. superior frontal gyrus | 16 |
| Larger gray matter volume (individuals with antisocial behavior > controls) | ||||||
| Right fusiform gyrus | 46, −22, −24 | 1.385 | <0.0001 | 60 | rt. fusiform gyrus | 33 |
| rt. inferior temporal gyrus | 27 | |||||
| Right inferior parietal lobule | 38, −30, 42 | 1.040 | 0.0003 | 39 | rt. inferior parietal lobule | 37 |
| rt. postcentral gyrus | 2 | |||||
| Left superior parietal lobule | −36, −68, 44 | 1.002 | 0.0004 | 41 | lt. superior parietal lobule | 35 |
| lt. inferior parietal lobule | 5 | |||||
| lt. precuneus | 1 | |||||
| Right cingulate gyrus | 12, 8, 44 | 1.001 | 0.0004 | 63 | rt. cingulate gyrus | 15 |
| rt. medial frontal gyrus | 40 | |||||
| rt. superior frontal gyrus | 8 | |||||
| Right postcentral gyrus | 58, −20, 30 | 1.001 | 0.0004 | 43 | rt. postcentral gyrus | 38 |
| rt. inferior parietal lobule | 5 | |||||
SDM, signed differential mapping.
Fig. 2.
Regions of decreases (blue) or increases (red) in regional gray matter volume in individuals with antisocial behavior, compared with controls voxel threshold P <0.001. (a) Left frontopolar cortex; (b) left insula; (c) lentiform nucleus; (d) right fusiform.
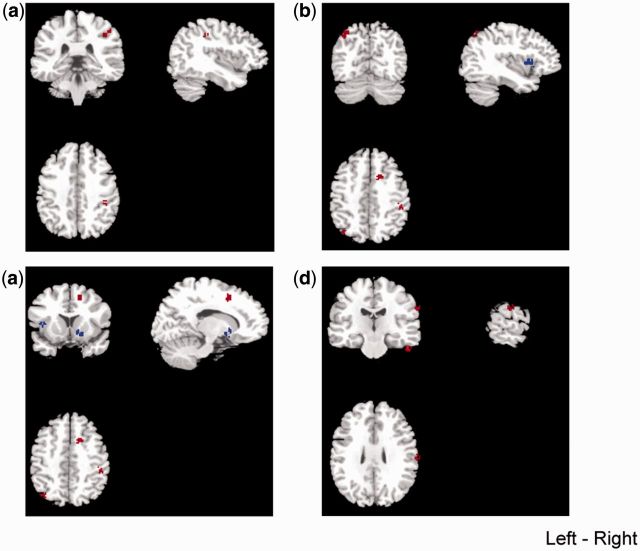
Furthermore, the analysis also showed a significant increase in GMV in the right fusiform gyrus (Talairach coordinates: x = 46, y = −22, z = −24; SDM value = 1.385, P < 0.0001; 60 voxels) (Table 2 and Figure 2d), in the right inferior parietal lobule (Talairach coordinates: x = 38, y = −30, z = 42; SDM value = 1.040, P = 0.0003; 39 voxels) (Table 2 and Figure 3a), in the left superior parietal lobule (Talairach coordinates: x = −36, y = −68, z = 44; SDM value = 1.002, P = 0.0004; 41 voxels) (Table 2 and Figure 3b), in the right cingulate gyrus (Talairach coordinates: x = 12, y = 8, z = 44; SDM value = 1.001, P = 0.0004; 41 voxels) (Table 2 and Figure 3c) and right postcentral gyrus (Talairach coordinates: x = 58, y = −20, z = 30; SDM value = 1.001, P = 0.0004; 43 voxels) (Table 2 and Figure 3d) in subjects with antisocial behavior, compared with control subjects. The statistical conclusions for differences in regional brain volumes were preserved after controlling for the effect of age.
Fig. 3.
Regions of decreases (blue) or increases (red) in regional gray matter volume in individuals with antisocial behavior, compared with controls voxel threshold P <0.001. (a) Right inferior parietal lobule; (b) left superior parietal lobule; (c) right cingulate gyurs; (d) right postcentral gyrus.
DISCUSSION
To the best of our knowledge, this is the first meta-analysis of studies integrating VBM in individuals with antisocial behavior. The analysis identified a significant regional GMV reduction in the left FPC as well as in the paralimbic region, such as the anterior insula, in individuals with antisocial behaviors compared with healthy controls, supporting our hypothesis. The current analysis also found significantly increased GMV in the right fusiform gyrus and the right inferior parietal lobule.
Although the function of the FPC is yet to be elucidated (Tsujimoto et al., 2011), it is thought to be responsible for holding in mind a goal while exploring and processing secondary goals, a process generally required in planning and reasoning, which integrates working memory and attentional resource allocation (Koechlin et al., 1999; Burgess et al., 2007). The FPC is also recognized to be responsible for cognitive branching—the maintenance of pending information related to a previous behavioral episode during an ongoing behavioral episode for future use (Koechlin and Hyafil, 2007; Charron and Koechlin, 2010). Recently, Boorman et al. (2009, 2011) showed that the FPC not only represents pending information or intentions for future use but also encodes the reward-based evidence favoring the best counterfactual option for future decisions. These results indicate that the FPC is not simply involved in attention allocation but also plays an important role in complex social decision based on its fundamental role. This notion is supported by results of a number of fMRI studies that have reported that the FPC is responsible for guiding complex social decisions such as moral judgment (Greene et al., 2001; Moll et al., 2002) or charitable donation (Moll et al., 2006). In addition, one study with transcranial direct current stimulation (tDCS) showed that inhibiting the excitability of the FPC with cathodal tDCS did not lead to impairment, but rather to a significant within-subject improvement of deceptive behavior (Karim et al., 2007). These previous studies have strongly indicated the possibility that abnormality in the FPC results in antisocial behavior.
Previous studies in individuals with antisocial behavior have identified an association between the impulsive trait and working memory deficit (Carlson et al., 2009; Venables et al., 2011). It is also thought that the FPC is associated with keeping information in working memory (Koechlin et al., 1999; Charron and Koechlin 2010). Therefore, a reduction in GMV in the FPC may relate to impulsivity. Interestingly, one study describing two case reports of individuals who sustained injury to the FPC reported that damage in this region resulted in impulsive antisocial behavior (Anderson et al., 1999).
The current analysis showed GMV reduction in the anterior insula that consists of the paralimbic regions. The anterior insula has a strong connection with the amygdala (Naqvi and Bechara, 2009; Meyer-Lindenberg and Tost, 2012; Sescousse et al., 2013) and is involved in emotional processing and empathy (Vogt, 2005; de Vignemont and Singer, 2006; Fan et al., 2011; Morita et al., in press; Ponz et al., in press). The previous studies demonstrated abnormal activation of the anterior insula during empathy or emotional processing tasks in individuals with antisocial behavior (Herpertz et al., 2008; Sadeh et al., 2013). In addition, the previous study reported a thinner-than-normal cortex in the anterior insula in individuals with psychopathy (Ly et al., 2012). Although we have predicted abnormality in the amygdala as a potential neural correlate of abnormal emotional processing in individuals with antisocial behavior, the current meta-analysis suggested that abnormality in the anterior insula may also be responsible for abnormal emotional processing among them.
The analysis identified significantly smaller-than-normal GMV in the lentiform nucleus, mainly the putamen. Neuroimaging studies have repeatedly reported that the putamen is involved in reward-based learning (O’Doherty et al., 2004, 2006; Samejima et al., 2005; Liu et al., 2011, Wunderlich et al., 2012). Furthermore, it was also demonstrated that the putamen, along with the insula, is involved in judgment of distribution of justice (Hsu et al., 2008). As reward-based learning is disturbed in individuals with antisocial behavior (Finger et al., 2011), abnormality in these structures is suggested to be a potential pathophysiology of antisocial behavior (Glenn and Yang, 2012). However, the current finding should be interpreted with caution, because of comorbid attention deficit personality disorder (ADHD). ADHD is a frequent comorbidity in individuals with antisocial behavior clinically and sub-clinically, and some of the included studies in the current meta-analysis recruited individuals with comorbid diagnosis of ADHD (Sterzer et al., 2007; Fairchild et al., 2013). As it has been shown that there is a smaller-than-normal GMV in the right lentiform nucleus in individuals with ADHD (Nakao et al., 2011), comorbid ADHD may have influenced the results.
The meta-analysis further identified a GMV increase in the fusiform gyrus as a potential neural basis of antisocial behavior. Although we could not statistically test relationship between symptoms of antisocial behavior and abnormality of GMV in the fusiform gyrus, previous fMRI studies suggest how abnormal GMV in the fusiform gyrus attributes to antisocial behavior. The fusiform gyrus is thought to be directly involved in the process of social categorization via top-down modulation of social and face perception (Sabatinelli et al., 2011; Schwarz et al., 2013; Shkurko, in press) and emotions of guilt and shame (Takahashi et al., 2004; Michl et al., in press). It is also thought that the right fusiform gyrus is a center of rapid learning regarding the moral status of others (Singer et al., 2004). In addition, individuals with Klinefelter syndrome, a chromosomal condition (XXY) whose phenotype is high risk for antisocial behavior, displayed less activation of the fusiform gyrus during judgment of faces with regard to trustworthiness (van Rijn et al., 2012). These evidence suggest that abnormal GMV in the fusiform gyrus is related to deviated face recognition (Dolan and Fullam, 2006) and sense of guilt and shame (Tangney et al., 2011) in individuals with antisocial behavior.
The analysis also identified an increase in GMV in the inferior parietal lobule as a potential neural correlate of antisocial behavior. This area has been suggested to contain mirror neurons (Molenberghs et al., 2012), indicating that disturbance in this region results in various social dysfunctions. For example, the inferior parietal lobule is involved in gaze processing (Pelphrey et al., 2003, 2004), action perception in understanding intentions (Gallese et al., 2004), comprehending impressions of others (Mende-Siedlecki et al., in press), predicting the actions of from their gaze (Ramsey et al., 2012) and risk-taking action (Tamura et al., 2012). Based on previous fMRI studies, increased GMV in the inferior parietal lobule may reflect inappropriate eye gazing of individuals with antisocial behavior (Dadds et al., 2008). The analysis also demonstrated larger-than-normal GMV in the left superior parietal lobule. The superior parietal lobule, which is often activated together with the inferior parietal lobule (Culham et al., 1998), is involved in spatial attention (Molenberghs et al., 2007) and is reported to be abnormally activated for fearful congruent in individuals with antisocial behavior (White et al., 2012). The current analysis also demonstrated larger-than-normal GMV in the postcentral gyrus of subjects with antisocial behavior. Recent studies suggested that the right postcentral gyrus was associated with emotional processing and empathy (Bernhardt and Singer, 2012; Morelli et al., in press; Sarkheil et al., in press). Thus, this abnormality may relate to a disturbance of emotional processing and empathy in individuals with antisocial behavior. The cingulate gyrus is also demonstrated to be larger-than-normal in individuals with antisocial behavior. As a number of previous fMRI studies reported abnormal activation of the BOLD signal during moral- or shame-related tasks (Raine and Yang, 2006; Christensen et al., in press; Michl et al., in press), the structural abnormality may contribute to these dysfunctions.
As a number of functional neuroimaging studies of individuals with antisocial behavior have repeatedly shown functional abnormality in the amygdala and OFC/vmPFC (Phelps and LeDoux, 2005; Blair, 2007; Yang et al., 2009; Hyatt et al., 2012), we have predicted GMV reduction in these regions. But contrary to the prediction, the analysis did not show significant GMV reduction in the amygdala and OFC/vmPFC. This dissociation between functional and structural alteration is surprising. A possible explanation for this negative result is the heterogeneity of the participants. We integrated people with several different disorders into the analysis, because all were at higher risk of antisocial behavior. Further, it is thought that people with these disorders share a common neural basis of antisocial behavior. However, some participants with CD and ASPD had a dual diagnosis of psychopathy. The diagnosis of CD and ASPD has also been criticized for over-emphasizing behavioral outcomes (such as criminality) and neglecting core psychological features (Blair, 2007). With this in mind, it is possible that we have integrated individuals with similar behavioral phenotypes but with partially different neural correlates. Another explanation is that functional abnormality derives from abnormality in the white matter instead of the gray matter. As abnormal connectivity between the amygdala and the OFC/vmPFC has been reported in individuals with antisocial behavior (Passamonti et al., 2012), white matter abnormality without GMV reduction may contribute to their well-established functional abnormality.
Limitations
There are some methodological considerations in the reported meta-analysis. First, although we have integrated only whole-brain VBM studies in individuals with antisocial behavior, there is considerable heterogeneity between studies, in terms of participants and methodologies. For example, as we discussed above, we may have included studies with individuals with similar phenotypes but different neural or psychological bases for their symptoms. In addition, there is significant diversity in the methodology of imaging between the studies we included, such as smoothing function used and strength of magnetic field. Further, the studies adopted different statistical analyses. Thus, although we used a conservative threshold in our analysis to minimize study heterogeneity, the results should nevertheless be treated with caution. Second, the majority of participants within the integrated studies had psychiatric comorbidity, such as substance abuse or subclinical features of other psychiatric disorders, including depression, anxiety disorder, autism, and attention deficit hyperactivity disorder. It is known that these comorbid conditions have an impact on structure of the frontotemporal cortex (Yamasue et al., 2003, 2004; Aoki et al, 2012a,b,c; Lucantonio et al., 2012). Therefore, it is possible that the abnormal GMV was an artifact of the comorbid psychiatric disorders. In addition, differences in the subjects’ behavioral and emotional traits may also affect GMV (Takeuchi et al., 2011; Morishima et al., 2012; Takeuchi et al., in press). We could not conduct sensitivity analysis or meta-regression due to an insufficient number of studies, although our conservative meta-analysis of unbiased studies demonstrated significant abnormalities in GMV. Thus, although we robustly found GMV abnormalities, the functions of which may relate to a psychological trait of individuals with antisocial behavior, we could not directly address the relationship between abnormalities in brain structure and behavior. Third, as non-significant data have a higher possibility of not being published, there exists strong publication bias. In addition, although SDM reconstructs both positive and negative differences in the same map (signed map) (Radua and Mataix-Cols, 2009; Radua et al., 2010), peak-based meta-analyses are based on highly significant data (i.e. P < 0.001 uncorrected) rather than raw statistical brain maps, and this approach may result in less accurate results.
CONCLUSION
In conclusion, the meta-analysis of unbiased whole-brain VBM studies of individuals with antisocial behavior demonstrated significantly abnormal GMV reductions in the FPC and parahippocampal gyrus, including the amygdala, and GMV increases in the right fusiform gyrus and the right inferior parietal lobule. These abnormalities may correspond to deficits in keeping information in the working memory during allocation of attention, emotional processing and inappropriate face information processing in social context. The current analysis emphasized that attention deficit is also an important factor in the pathophysiology of individuals with antisocial behavior.
Conflict of Interest
None declared.
Acknowledgments
Part of this study was a funded by the Grants-in-Aid for Scientific Research (22689034 to H.Y.).
REFERENCES
- Anderson NE, Kiehl KA. The psychopath magnetized: insights from brain imaging. Trends in Cognitive Sciences. 2012;16:52–60. doi: 10.1016/j.tics.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–7. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Abe O, Yahata N, et al. Absence of age-related prefrontal NAA change in adults with autism spectrum disorders. Translational Psychiatry. 2012a;2:e178. doi: 10.1038/tp.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Aoki A, Suwa H. Reduction of N-acetylaspartate in the medial prefrontal cortex correlated with symptom severity in obsessive-compulsive disorder: meta-analyses of 1H-MRS studies. Translational Psychiatry. 2012b;2:e153. doi: 10.1038/tp.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Kasai K, Yamasue H. Age-related change in brain metabolite abnormalities in autism: a meta-analysis of proton magnetic resonance spectroscopy studies. Translational Psychiatry. 2012c;2:e69. doi: 10.1038/tp.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin-Sommers AR, Curtin JJ, Newman JP. Specifying the attentional selection that moderates the fearlessness of psychopathic offenders. Psychological Science. 2011;22:226–34. doi: 10.1177/0956797610396227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Singer T. The neural basis of empathy. Annual Reviews Neuroscience. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Grothe M, Prehn K, et al. Brain volumes differ between diagnostic groups of violent criminal offenders. European Archives of Psychiatry Clinical Neuroscience. in press doi: 10.1007/s00406-013-0391-6. [DOI] [PubMed] [Google Scholar]
- Blair KS, Newman C, Mitchell DGV, et al. Differentiating among prefrontal substrates in psychopathy: neuropsychological test findings. Neuropsychology. 2006;20:153–65. doi: 10.1037/0894-4105.20.2.153. [DOI] [PubMed] [Google Scholar]
- Blair RJR. Neurobiological basis of psychopathy. The British Journal of Psychiatry. 2003;182:5–7. doi: 10.1192/bjp.182.1.5. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11:387–92. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Peschardt KS, Budhani S, Mitchell DGV, Pine DS. The development of psychopathy. Journal of Child Psychology and Psychiatry. 2006;47:262–76. doi: 10.1111/j.1469-7610.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- Boccardi M, Frisoni GB, Hare RD, et al. Cortex and amygdala morphology in psychopathy. Psychiatry Research. 2011;193:85–92. doi: 10.1016/j.pscychresns.2010.12.013. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Yücel M, Pantelis C. The effects of gender on grey matter abnormalities in major psychoses: a comparative voxelwise meta-analysis of schizophrenia and bipolar disorder. Psychological Medicine. 2011:1–13. doi: 10.1017/S0033291711001450. [DOI] [PubMed] [Google Scholar]
- Boorman ED, Behrens TE, Woolrich MW, Rushworth MF. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62:733–43. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Boorman ED, Behrens TE, Rushworth MF. Counterfactual choice and learning in a neural network centered on human lateral frontopolar cortex. PLoS Biology. 2011;9:e1001093. doi: 10.1371/journal.pbio.1001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Dumontheil I, Gilbert SJ. The gateway hypothesis of rostral prefrontal cortex (area 10) function. Trends in Cognitive Sciences. 2007;11:290–8. doi: 10.1016/j.tics.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Thái S, McLarnon ME. Visual P3 amplitude and self-reported psychopathic personality traits: frontal reduction is associated with self-centered impulsivity. Psychophysiology. 2009;46:100–13. doi: 10.1111/j.1469-8986.2008.00756.x. [DOI] [PubMed] [Google Scholar]
- Charron S, Koechlin E. Divided representation of concurrent goals in the human frontal lobes. Science. 2010;328:360–3. doi: 10.1126/science.1183614. [DOI] [PubMed] [Google Scholar]
- Christensen JF, Flexas A, de Miguel P, Cela-Conde CJ, Munar E. Roman Catholic beliefs produce characteristic neural responses to moral dilemmas. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nss121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleckley HM. The Mask of Sanity, An Attempt to Reinterpret the So-called Psychopathic Personality. St Louis, MO: Mosby; 1941. [Google Scholar]
- Cope LM, Shane MS, Segall JM, et al. Examining the effect of psychopathic traits on gray matter volume in a community substance abuse sample. Psychiatry Research. 2012;204:91–100. doi: 10.1016/j.pscychresns.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, Tootell RB. Cortical fMRI activation produced by attentive tracking of moving targets. Journal of Neurophysiology. 1998;80:2657–70. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Masry ElY, Wimalaweera S, Guastella AJ. Reduced eye gaze explains “fear blindness” in childhood psychopathic traits. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:455–63. doi: 10.1097/CHI.0b013e31816407f1. [DOI] [PubMed] [Google Scholar]
- Dalwani M, Sakai JT, Mikulich-Gilbertson SK, et al. Reduced cortical gray matter volume in male adolescents with substance and conduct problems. Drug and Alcohol Dependence. 2011;118:295–305. doi: 10.1016/j.drugalcdep.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, et al. Size matters: increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132:843–52. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- de Oliveira-Souza R, Hare RD, Bramati IE, et al. Psychopathy as a disorder of the moral brain: fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage. 2008;40:1202–13. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- de Vignemont F, Singer T. The empathic brain: how, when and why? Trends in Cognitive Science. 2006;10:435–41. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Dolan M, Fullam R. Face affect recognition deficits in personality-disordered offenders: association with psychopathy. Psychological Medicine. 2006;36:1563–9. doi: 10.1017/S0033291706008634. [DOI] [PubMed] [Google Scholar]
- Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in criminal psychopathy. Journal of Abnormal Psychology. 2012;121:649–58. doi: 10.1037/a0026371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in incarcerated male adolescents with psychopathic traits. Journal of American Academy of Child and Adolescent Psychiatry. 2013;52:94–103. doi: 10.1016/j.jaac.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim C, He Y, Yoon U, Chen J, Evans A, Pérusse D. Neuroanatomy of childhood disruptive behavior disorders. Aggressive Behavior. 2011;37:326–37. doi: 10.1002/ab.20396. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ, Goodyer IM. Brain structure abnormalities in adolescent girls with conduct disorder. Journal of Child Psychology and Psychiatry. 2013;54:86–95. doi: 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G, Passamonti L, Hurford G, et al. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. The American Journal of Psychiatry. 2011;168:624–33. doi: 10.1176/appi.ajp.2010.10081184. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35:903–11. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh A, Blair KS, Majestic C, Evangelou I, Gupta K, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Research. 2012;202:239–44. doi: 10.1016/j.pscychresns.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Blair KS, et al. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. American Journal of Psychiatry. 2011;168:152–62. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ, Viding E. Antisocial behavior from a developmental psychopathology perspective. Development and Psychopathology. 2009;21:1111–31. doi: 10.1017/S0954579409990071. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends in Cognitive Sciences. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Gao Y, Glenn AL, Schug RA, Yang Y, Raine A. The neurobiology of psychopathy: a neurodevelopmental perspective. Canadian Journal of Psychiatry. 2009;54:813–23. doi: 10.1177/070674370905401204. [DOI] [PubMed] [Google Scholar]
- Glass SJ, Newman JP. Recognition of facial affect in psychopathic offenders. Journal of Abnormal Psychology. 2006;115:815–20. doi: 10.1037/0021-843X.115.4.815. [DOI] [PubMed] [Google Scholar]
- Glenn AL, Yang Y. The potential role of the striatum in antisocial behavior and psychopathy. Biological Psychiatry. 2012;72:817–22. doi: 10.1016/j.biopsych.2012.04.027. [DOI] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:2105–8. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Gregory S, Ffytche D, Simmons A, et al. The antisocial brain: psychopathy matters: a structural MRI investigation of antisocial male violent offenders. Archives of General Psychiatry. 2012;69:962–72. doi: 10.1001/archgenpsychiatry.2012.222. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Huebner T, Marx I, et al. Emotional processing in male acolescents with childhood-onset conduct disorder. Journal of Child Psychology and Psychiatry. 2008;49:781–91. doi: 10.1111/j.1469-7610.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- Hsu M, Anen C, Quartz SR. The right and the good: distributive justice and neural encoding of equity and efficiency. Science. 2008;320:1092–5. doi: 10.1126/science.1153651. [DOI] [PubMed] [Google Scholar]
- Huebner T, Vloet TD, Marx I, et al. Morphometric brain abnormalities in boys with conduct disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:540–7. doi: 10.1097/CHI.0b013e3181676545. [DOI] [PubMed] [Google Scholar]
- Hyatt CJ, Haney-Caron E, Stevens MC. Cortical thickness and folding deficits in conduct-disordered adolescents. Biological Psychiatry. 2012;72:207–14. doi: 10.1016/j.biopsych.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutai JW, Hare RD. Psychopathy and selective attention during performance of a complex perceptual-motor task. Psychophysiology. 1983;20:146–51. doi: 10.1111/j.1469-8986.1983.tb03280.x. [DOI] [PubMed] [Google Scholar]
- Karim AA, Schneider M, Lotze M, Veit R, Sauseng P, Braun C, Birbaumer N. The truth about lying: inhibition of the anterior prefrontal cortex improves deceptive behavior. Cereb Cortex. 2010;20:205–13. doi: 10.1093/cercor/bhp090. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–51. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–8. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Koenigs M. The role of prefrontal cortex in psychopathy. Reviews in the Neurosciences. 2012;23:253–62. doi: 10.1515/revneuro-2012-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzer L, Hodgins S. Adult outcomes of child conduct problems: a cohort study. Journal of Abnormal Child Psychology. 1997;25:65–81. doi: 10.1023/a:1025711525255. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2011;35:1219–36. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R, Stouthamer-Loeber M. Development of juvenile aggression and violence. Some common misconceptions and controversies. American Psychologist. 1998;53:242–59. doi: 10.1037//0003-066x.53.2.242. [DOI] [PubMed] [Google Scholar]
- Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G. The impact of orbitofrontal dysfunction on cocaine addiction. Nature Neuroscience. 2012;15:358–66. doi: 10.1038/nn.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly M, Motzkin JC, Philippi CL, et al. Cortical thinning in psychopathy. The American Journal of Psychiatry. 2012;169:743–9. doi: 10.1176/appi.ajp.2012.11111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, et al. Reduced amygdala-orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Research. 2011;194:279–86. doi: 10.1016/j.pscychresns.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, et al. Mapping brain structure in attention deficit-hyperactivity disorder: a voxel-based MRI study of regional grery and white matter volume. Psychiatry Research Neuroimaging. 2007;154:171–80. doi: 10.1016/j.pscychresns.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Mende-Siedlecki P, Cai Y, Todorov A. The neural dynamics of updating person impressions. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nss040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Tost H. Neural mechanisms of social risk for psychiatric disorders. Nature Neuroscience. 2012;15:663–8. doi: 10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- Michl P, Meindl T, Meister F, et al. Neurobiological underpinnings of shame and guilt: a pilot fMRI study. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nss114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DGV, Colledge E, Leonard A, Blair RJR. Risky decisions and response reversal: is there evidence of orbitofrontal cortex dysfunction in psychopathic individuals? Neuropsychologia. 2002;40:2013–22. doi: 10.1016/s0028-3932(02)00056-8. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychological Reviews. 1993;100:674–701. [PubMed] [Google Scholar]
- Moffitt TE. Genetic and environmental influences on antisocial behaviors: evidence from behavioral-genetic research. Advances in Genetics. 2005;55:41–104. doi: 10.1016/S0065-2660(05)55003-X. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neuroscience and Biobehavioral Reviews. 2012;36:341–9. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Mesulam MM, Peeters R, Vandenberghe RR. Remapping attentional priorities: differential contribution of superior parietal lobule and intraparietal sulcus. Cerebral Cortex. 2007;17:2703–12. doi: 10.1093/cercor/bhl179. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Eslinger PJ, et al. The neural correlates of moral sensitivity: a functional magnetic resonance imaging investigation of basic and moral emotions. Journal of Neuroscience. 2002;22:2730–6. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human fronto-mesolimbic networks guide decisions about charitable donation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15623–8. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli SA, Rameson LT, Lieberman MD. The neural components of empathy: predicting daily prosocial behavior. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nss088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima Y, Schunk D, Bruhin A, Ruff CC, Fehr E. Linking brain structure and activation in temporoparietal junction to explain the neurobiology of human altruism. Neuron. 2012;75:73–9. doi: 10.1016/j.neuron.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Morita T, Tanabe HC, Sasaki AT, Shimada K, Kakigi R, Sadato N. The anterior insular and anterior cingulate cortices in emotional processing for self-face recognition. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nst011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller JL, Gänßbauer S, Sommer M, et al. Gray matter changes in right superior temporal gyrus in criminal psychopaths. Evidence from voxel-based morphometry. Psychiatry Research. 2008;163:213–22. doi: 10.1016/j.pscychresns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. The American Journal of Psychiatry. 2011;168:1154–63. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends in Neuroscience. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JP, Curtin JJ, Bertsch JD, Baskin-Sommers AR. Attention moderates the fearlessness of psychopathic offenders. Biological Psychiatry. 2010;67:66–70. doi: 10.1016/j.biopsych.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49:157–66. doi: 10.1016/j.neuron.2005.11.014. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–4. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Fairchild G, Fornito A, et al. Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PLoS One. 2012;7:e48789. doi: 10.1371/journal.pone.0048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JD, Allison T, McCarthy G. Brain activation evoked by perception of gaze shifts: the influence of context. Neuropsychologia. 2003;41:156–70. doi: 10.1016/s0028-3932(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Viola RJ, McCarthy G. When strangers pass: processing of mutual and averted social gaze in the superior temporal sulcus. Psychological Science. 2004;15:598–603. doi: 10.1111/j.0956-7976.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Ponz A, Montant M, Liegeois-Chauvel C, et al. Emotion processing in words: a test of the neural re-use hypothesis using surface and intracranial EEG. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nst034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. The British Journal of Psychiatry. 2009;195:393–402. doi: 10.1192/bjp.bp.108.055046. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D, Philips ML, et al. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. European Psychiatry. 2012;27:605–11. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Radua J, van den Heuvel OA, Surguladze S, Mataix-Cols D. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Archives of General Psychiatry. 2010;67:701–11. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]
- Radua J, Via E, Catani M, Mataix-Cols D. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychological Medicine. 2011;41:1539–50. doi: 10.1017/S0033291710002187. [DOI] [PubMed] [Google Scholar]
- Raine A, Lee L, Yang Y, Colletti P. Neurodevelopmental marker for limbic maldevelopment in antisocial personality disorder and psychopathy. The British Journal of Psychiatry. 2010;197:186–92. doi: 10.1192/bjp.bp.110.078485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A, Yang Y. Neural foundations to moral reasoning and antisocial behavior. Social Cognitive and Affective Neuroscience. 2006;1:203–13. doi: 10.1093/scan/nsl033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey R, Cross ES, de C. Hamilton AF. Predicting others' actions via grasp and gaze: evidence for distinct brain networks. Psychological Research. 2012;76:494–502. doi: 10.1007/s00426-011-0393-9. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biological Psychiatry. 2007;61:1260–71. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35:395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, et al. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. 2011;54:2524–33. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Sadeh N, Spielberg JM, Heller W, et al. Emotion disrupts neural activity during selective attention in psychopathy. Social Cognitive and Affective Neuroscience. 2013;8:235–46. doi: 10.1093/scan/nsr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N, Verona E. Visual complexity attenuates emotional processing in psychopathy: implications for fear-potentiated startle deficits. Cognitive Affective and Behavioral Neuroscience. 2012;12:346–60. doi: 10.3758/s13415-011-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima K, Ueda Y, Doy K, Kimura M. Representation of action-specific reward values in the striatum. Science. 2005;310:1337–40. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- Sarkheil P, Goebel R, Schneider F, Mathiak K. Emotion unfolded by motion: a role for parietal lobe in decoding dynamic facial expressions. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nss092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasayama D, Hayashida A, Yamasue H, et al. Neuroanatomical correlates of attention-deficit-hyperactivity disorder accounting for comorbid oppositional defiant disorder and conduct disorder. Psychiatry and Clinical Neuroscience. 2010;64:394–402. doi: 10.1111/j.1440-1819.2010.02102.x. [DOI] [PubMed] [Google Scholar]
- Sato JR, de Oliveira-Souza R, Thomaz CE, et al. Identification of psychopathic individuals using pattern classification of MRI images. Social Neuroscience. 2011;6:627–39. doi: 10.1080/17470919.2011.562687. [DOI] [PubMed] [Google Scholar]
- Schiffer B, Müller BW, Scherbaum N, et al. Disentangling structural brain alterations associated with violent behavior from those associated with substance use disorders. Archives of General Psychiatry. 2011;68:1039–1049. doi: 10.1001/archgenpsychiatry.2011.61. [DOI] [PubMed] [Google Scholar]
- Schwarz KA, Wieser MJ, Gerdes ABM, Mühlberger A, Pauli P. Why are you looking like that? How the context influences evaluation and processing of human faces. Social Cognitive and Affective Neuroscience. 2013;8:438–45. doi: 10.1093/scan/nss013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Caldu X, Segura B, Dreher JC. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2013;37:681–96. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Shkurko AV. Is social categorization based on relational ingroup/outgroup opposition? A meta-analysis. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nss085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Kiebel SJ, Winston JS, Dolan RJ, Frith CD. Brain responses to the acquired moral status of faces. Neuron. 2004;41:653–62. doi: 10.1016/s0896-6273(04)00014-5. [DOI] [PubMed] [Google Scholar]
- Starcke K, Brand M. Decision making under stress: a selective review. Neuroscience and Biobehavioral Reviews. 2012;36:1228–48. doi: 10.1016/j.neubiorev.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Christina Stadler C, Poustka F, Kleinschmidta A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37:335–42. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Stevens S, Haney-Caron E. Comparison of brain volume abnormalities between ADHD and conduct disorder in adolescence. Journal of Psychiatry Neuroscience. 2012;37:389–98. doi: 10.1503/jpn.110148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y. Brain activation associated with evaluative processes of guilt and embarrassment: an fMRI study. Neuroimage. 2004;23:967–74. doi: 10.1016/j.neuroimage.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, et al. Regional gray matter density is associated with achievement motivation: evidence from voxel-based morphometry. Brain Structure and Function. in press doi: 10.1007/s00429-012-0485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, et al. Regional gray matter density associated with emotional intelligence: evidence from voxel-based morphometry. Human Brain Mapping. 2011;32:1497–510. doi: 10.1002/hbm.21122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M, Moriguchi Y, Higuchi S, et al. Neural network development in late adolescents during observation of risk-taking action. PLoS One. 2012;7:e39527. doi: 10.1371/journal.pone.0039527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney JP, Stuewig J, Hafez L. Shame, guilt and remorse: implications for offender populations. Journal of Forensic Psychiatry and Psychology. 2011;22:706–23. doi: 10.1080/14789949.2011.617541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J, Rossi R, Laakso MP, et al. Brain anatomy of persistent violent offenders: more rather than less. Psychiatry Research. 2008;163:201–12. doi: 10.1016/j.pscychresns.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Genovesio A, Wise SP. Frontal pole cortex: encoding ends at the end of the endbrain. Trends in Cognitive Sciences. 2011;15:169–76. doi: 10.1016/j.tics.2011.02.001. [DOI] [PubMed] [Google Scholar]
- van Rijn S, Swaab H, Baas D, de Haan E, Kahn RS, Aleman A. Neural systems for social cognition in Klinefelter syndrome (47,XXY): evidence from fMRI. Social Cognitive and Affective Neuroscience. 2012;7:689–97. doi: 10.1093/scan/nsr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Patrick CJ, Hall JR, Bernat EM. Clarifying relations between dispositional aggression and brain potential response: overlapping and distinct contributions of impulsivity and stress reactivity. Biological Psychology. 2011;86:279–88. doi: 10.1016/j.biopsycho.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale JE, Brinkley CA, Hiatt KD, Newman JP. Abnormal selective attention in psychopathic female offenders. Neuropsychology. 2007;21:301–12. doi: 10.1037/0894-4105.21.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vloet TD, Konrad K, Huebner T, Herpertz S, Herpertz-Dahlmann B. Structural and functional MRI- findings in children and adolescents with antisocial behavior. Behav Sci Law. 2008;26:99–111. doi: 10.1002/bsl.794. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience. 2005;6:533–44. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Williams WC, Brislin SJ, et al. Reduced activity within the dorsal endogenous orienting of attention network to fearful expressions in youth with disruptive behavior disorders and psychopathic traits. Development and Psychopathology. 2012;24:1105–16. doi: 10.1017/S0954579412000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Zhao Y, Liao J, Yin H, Wang W. White matter abnormalities in young males with antisocial personality disorder—evidence from voxel-based morphometry-diffeomorphic anatomical registration using exponentiated lie algebra analysis. Neural Regeneration Research. 2011;6:1965–70. [Google Scholar]
- Wunderlich L, Dayan P, Dolan RJ. Mapping value based planning and extensive trained choice in the human brain. Nature Neuroscience. 2012;15:786–93. doi: 10.1038/nn.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasue H, Iwanami A, Hirayasu Y, et al. Localized volume reduction in prefrontal, temporolimbic, and paralimbic regions in schizophrenia: an MRI parcellation study. Psychiatry Research. 2004;131:195–207. doi: 10.1016/j.pscychresns.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9039–43. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr KL, Colletti P, Toga AW. Localization of deformations within the amygdala in individuals with psychopathy. Archives of General Psychiatry. 2009;66:986–94. doi: 10.1001/archgenpsychiatry.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Volume reduction in prefrontal gray matter in unsuccessful criminal psychopaths. Biological Psychiatry. 2005;57:1103–8. doi: 10.1016/j.biopsych.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Zeier JD, Maxwell JS, Newman JP. Attention moderates the processing of inhibitory information in primary psychopathy. Journal of Abnormal Psychology. 2009;118:554–63. doi: 10.1037/a0016480. [DOI] [PMC free article] [PubMed] [Google Scholar]