Abstract
As a social species, humans evolved to detect information from the social behavior of others. Yet, the mechanisms used to evaluate social interactions, the brain networks implicated in such recognition, and whether individual differences in own social behavior determine response to similar behavior in others remain unknown. Here we examined social synchrony as a potentially important mechanism in the evaluation of social behavior and utilized the parenting context, an evolutionarily salient setting of significant consequences for infant survival, to test this issue. The brain response of healthy postpartum mothers to three mother–infant interaction vignettes was assessed. Videos included a typical synchronous interaction and two pathological interactions of mothers diagnosed with postpartum depression and anxiety that showed marked deviations from social synchrony. Mothers’ own interactions with their 4- to 6-month-old infants were videotaped and micro-coded for synchrony. Results indicated that the recognition of social synchrony involved activations in the dorsal anterior cingulate cortex (dACC), fusiform, cuneus, inferior parietal lobule, supplementary motor area and NAcc. Mother’s own synchrony with her infant correlated with her dACC response to synchrony in others. Findings are consistent with models suggesting that social action underpins social recognition and highlight social synchrony and the mother–infant bond as one prototypical context for studying the brain basis of social understanding.
Keywords: social synchrony, mothering, social brain, maternal depression, maternal anxiety
INTRODUCTION
As a social species, humans' brains evolved to collect meaningful information from observing the social behavior of others (Dunbar, 1998). Noticing and interpreting social signals in our surrounding occurs frequently and, to some extent, automatically, and humans can recognize immediately whether social interactions are adaptive or pathological, benevolent or ill (Spunt and Lieberman, 2013). However, the behavioral signals humans use to make social judgments and the brain mechanisms involved in such detection are largely unknown. In this study, we examine whether social synchrony—the coordination of nonverbal behaviors between social partners during interpersonal exchange (Feldman, 2007a)—may be one mechanism through which humans decode social information. We suggest that social synchrony, an experience learned within the parent–infant bond, provides a unique exemplar of patterned behavior that is deeply rooted in mammalian biology and is anchored within specific brain networks that underlie the human capacity to show empathic concern, understand others' mind, and become collaborative members of the social world (Feldman, 2012a, b). Our overall hypothesis is that synchrony, as an early-learned experience, may serve as a reference point for evaluating social behavior. We further tested whether neural circuits involved in the expression of behavioral synchrony may mediate the recognition of synchrony in others.
Social synchrony underlies the development of affiliative bonds and, thus, its detection in social contexts may be important for bond formation and, consequently, for adequate social functioning. Immediately after birth, mammalian mothers express a unique set of species-typical behaviors that enables adequate maternal care, supports infant survival, and is coordinated with the infant’s physiological state and social signals (Feldman, 2007a, b, c; Niedenthal, 2007; Barrier et al., 2012). Human pair bonding similarly involves synchronous coordination between the behaviors of romantic partners (Schneiderman et al., 2012), and close friendships are expressed in social synchrony between friends (Feldman et al., 2013). Interestingly, the three forms of attachment in mammals—parental, pair and filial—are supported by the oxytocin system and share a brain network that is activated when individuals are exposed to cues of own child or partner or the distress of a close friend (Bartels and Zeki, 2004; Swain et al., 2005; Atzil et al., 2011; Farrow et al., 2011). Nelson and Pankspepp (1998) suggest that patterns of maternal behavior form a brain network of social signaling that differentiates adaptive from maladaptive social behavior. Similarly, human studies indicate that mother–infant synchrony provides the foundation for the development of social understanding and empathy across childhood and adolescence (Feldman, 2007b, c, 2012a, b). These findings support the conclusion that early experiences within the mother–infant bond may be used by humans and other mammals to differentiate adaptive from maladaptive social cues. The findings may also suggest that human adults may draw upon early synchronous experiences for understanding the social behavior of others (Silk, 1999).
Conditions associated with disruptions to maternal–infant bonding, in particular postpartum depression, are accompanied by a significant reduction in the maternal repertoire and the elimination of social synchrony (Field, 1994). In an elegant animal model, Meaney and colleagues differentiated rat dams characterized by high vs low maternal behavior (licking and grooming) and found differences in brain circuits and neuroendocrine systems that support social affiliation and stress regulation in mother and child (Meaney, 2010). Similar to infants of low licking-and-grooming dams, infants of depressed mothers are at a high developmental risk, showing greater propensity to psychopathology (Goodman and Gotlib, 1999), deviant social behavior, dysfunctions in stress management (Feldman et al., 2009) and higher amygdala volume (Lupien et al., 2011). Since the depressed maternal style represents a marked deviation from the typical synchronous style and poses a significant risk for infant development, it may be detected as maladaptive by both postpartum mothers and human adults.
The recognition of others' mental states, including affective states, involves activation of regions that support self-reference processing and mentalization, such as the medial prefrontal cortex (mPFC) and posterior cingulate gyrus (Whitfield-Gabrieli et al., 2011). This network complements the mirror neuron system that includes sensorimotor areas, inferior parietal lobule (IPL), insula, temporal-parietal junction (TPJ), cuneus and inferior frontal gyrus (IFG), which is implicated in the automatic processing of others’ motor goals (Iacoboni and Dapretto, 2006). Since mother–infant synchrony requires both automatic and mental processing, mothers' response to social synchrony may involve the activation of both networks. The processing of social behaviors activates circuits implicated in the simulation of motor action as well as higher cognitive functions that require inferences about mental states (Spunt and Lieberman, 2012). An emotional experience is often perceived in others and expressed by the self via similar neural networks (Lombardo et al., 2010). Since mothering is a salient example of embodied experience, as mothers need to coordinate with the motor patterns of their infants and infer their mental states from observed behavior, it is likely that brain regions supporting mother–infant synchrony will also be involved when mothers react to the expression of synchrony in others.
Synchronous maternal behavior has been associated with both evolutionary-ancient motivational systems, such as reward systems, and higher-level socio-cognitive circuits (Atzil et al., 2011, 2012). Specifically, mothers exhibiting more behavioral synchrony showed greater responses to their own infants in the nucleus accumbens (NAcc) and anterior cingulate cortex (ACC) (Atzil et al., 2011). Moreover, among synchronous mothers, NAcc response correlated with maternal plasma oxytocin and was functionally connected with emotion modulation, theory of mind and empathy brain networks. These results support the link between mother–infant behavioral synchrony and brain circuits implicated in reward and social understanding when mothers observe their own infants. However, this study did not examine the mother’s brain response to social interactions but to her own infant video and did not test whether the mother’s own behavioral synchrony modulates her brain response to synchrony in others. Such question is of theoretical and clinical importance and touches upon the way in which early social interactions and their key parameters may shape the way individuals evaluate adaptive vs non-adaptive social behavior.
In sum, the current study examined the hypothesis that the neural mechanisms implicated in the mother’s own synchrony will also be involved in her differential response to social synchrony in others. We tested the brain response of healthy postpartum mothers to typical and pathological mother–infant interactions that vary in the degree of social synchrony. Mothers of 6-month-old infants were scanned while observing three unfamiliar mother–infant interaction vignettes depicting interactions between: (i) healthy synchronous mother, (ii) mother diagnosed with major depressive disorder (MDD) and (iii) mother diagnosed with generalized anxiety disorder (GAD) and their infants. The depressed mother–infant interaction provided a unique context where social synchrony is eliminated and the exchange poses danger to infant well-being. The anxious interaction was included to tease apart the effects of the ‘amount’ of maternal behavior from the effects of social synchrony. Anxious mothers express adequate amounts of social behavior; however, their behaviors are not coordinated with the infant’s signals (Feldman et al., 2009). Thus, both the depressed and anxious interactions represent marked deviations from the typical pattern of social synchrony, but only the depressed interaction involves elimination of social cues. Mothers' own synchrony with her infant was micro-coded from observations of mother–infant interaction at home. Two hypotheses were formed. First, we predicted that brain regions associated with social salience (fusiform, dACC, parietal cortex [Litt et al., 2011]), simulation, reward (NAcc [Atzil et al., 2011]), social mentalization, and mirroring (Iacoboni and Dapretto, 2006; Whitfield-Gabrieli et al., 2011) will show differential response to synchronous vs nonsynchronous interactions. Second, we expected that individual differences in the mother’s own synchrony would mediate the degree of her brain response to social synchrony in others.
METHODS
Procedure and analyses
Participants
Twenty-seven mothers of 4- to 6-month-old infants participated. Mothers' age averaged 29.0 years (SD = 3.45), education averaged 15.76 years (SD = 1.85), and all mothers were healthy, with no history of mental illnesses, married, gave birth to healthy singleton infants, and were of middle-class backgrounds. Subjects were recruited through advertisements in the community. The study was approved by the institutional review board and all participants signed informed consents.
Procedure
The study included two sessions. In the first, families were visited at home and mother–infant interactions were videotaped. Films were coded offline for mother–infant synchrony. Following this, mothers underwent brain scanning at the Tel-Aviv Sourasky Medical Center.
Stimuli preparation
Three mothers were recorded interacting with their infants: (i) a typical, healthy mother interacting with her infant, (ii) a mother diagnosed with postpartum depression interacting with her infant, and (iii) a mother diagnosed with postpartum anxiety interacting with her infant. The depressed and anxious interactions were of mothers recruited at birth who reported elevated depression (BDI > 11) or anxiety (STAI > 43) symptoms (but not co-morbidity) on the second day post-birth, at 6 months, and at 9 months, and were diagnosed at 9 months by a psychiatrist for the existence of MDD or GAD, when interactions were filmed.
As our goal was to examine maternal brain response to natural, ecologically valid social cues, all videotaping was conducted in the homes of the families. To minimize differences related to filming conditions, mothers were asked to sit close to their infants on comfortable non-confined space (such as sofas or carpets), place their infants within arms' reach, and play with them as they typically did. No other instructions were given in order to capture the natural style of mother’s. Videotaping was conducted from a distance of 1.20 m from mother and child, to minimize differences related to distance, and focused on the face and upper body of mother and child. No toys were provided to minimize differences between films. In order to compare the mothers’ own synchrony with their infants to their brain responses to the unfamiliar films, all mother–infant interactions of participating mothers followed the same procedure in their homes.
fMRI paradigm
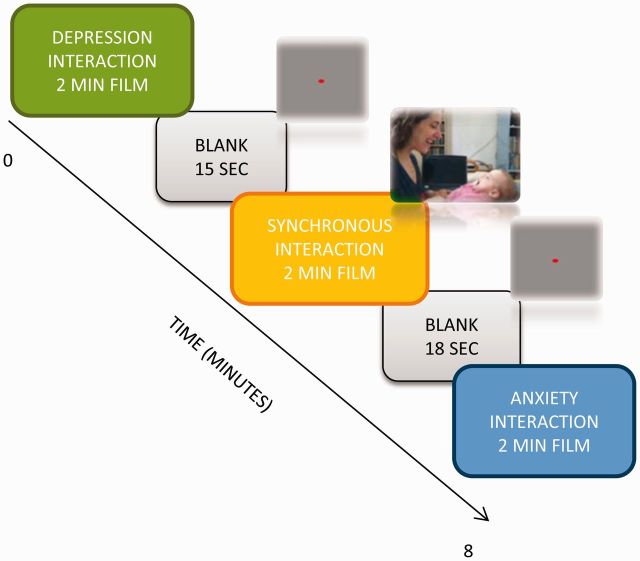
Subjects were presented with a series of parenting-related video vignettes while lying in the scanner. Stimuli included three 2-min movies of (i) a typical, healthy mother interacting with her infant, (ii) a mother diagnosed with postpartum depression interacting with her infant and (iii) a mother diagnosed with postpartum anxiety interacting with her infant (Figure 1). Each film lasted 2 min, which were presented once with 15–18 s of fixation between films, and analyzed as one 2-min block. Stimuli were counterbalanced and were randomly presented in five different orders.
Fig. 1.
Experimental paradigm. Subjects were presented with three films each containing a 2-min mother–infant interaction as follows. (i) Interaction between a mother diagnosed with postpartum depression and her infant containing minimal maternal behavior and no social synchrony. (ii) Interaction between a healthy mother and her infant containing adequate amount of maternal behavior and social synchrony. (iii) Interaction between a mother diagnosed with generalized anxiety disorder and her infant containing minimal social synchrony. Clips were previewed by rest with fixation period of 1 min. A rest with fixation periods of 15–18 s was presented between films.
Behavioral coding of mother–infant synchrony
Coding of mother–infant synchrony was conducted for both the fMRI stimuli (unfamiliar healthy and pathological interactions) and mother–own-infant interactions. Interactions were micro-coded by trained coders on a computerized system (Noldus, Wageningen, the Netherlands) in 0.01 s frames, consistent with our previous research on parent–infant synchrony (Feldman and Eidelman, 2004). Four non-verbal categories of parenting behaviors were coded, each including a set of mutually exclusive codes. Parent gaze—to infant, to object or environment, gaze aversion; parent affect—positive, neutral, negative; parent vocalizations—motherese (high-pitched, sing-song vocalization), adult speech to infant, adult speech to other adult, none; and parent touch—affectionate touch, functional touch, proprioceptive touch, stimulatory touch, none. Infant behavior was coded in a separate viewing for similar categories, including infant gaze, affect, and vocalizations. Inter-rater reliability, conducted for 10% of the interactions, averaged 98% (k = 0.84). Social synchrony was indexed by a conditional probability indexing the mothers coordinating their social gaze and affectionate touch with episodes of infant social gaze, positive affect or vocalizations and was defined as mean durations of synchronous episodes, consistent with previous research (Feldman et al., 2011).
Postpartum mothers’ assessment of synchronous vs pathological cues
In addition to micro-coding the exact amount of synchrony in the three movies presented to the mothers, five mothers who did not participate in the current study blindly observed the three films and were asked to rate on a three-point scale whether the interaction was ‘good and enjoyable’ and whether mothers were coordinated with their infants. All mothers rated the synchronous film as ‘good and enjoyable’ and ‘coordinated’, the depressed interaction as neither enjoyable nor coordinated, and the anxious interaction as somewhat enjoyable and not coordinated. These findings demonstrate that postpartum mothers respond to the dimension of synchrony and can easily differentiate interactions that are coordinated and growth promoting from those lacking in social synchrony.
fMRI acquisition
Imaging was performed on a GE 3T Sigma Horizon echo speed scanner with a resonant gradient echoplanar imaging system. Functional images were acquired using a single-shot echo-planar T2*-weighted sequence. The following parameters were used: 128 × 128 matrix; field of view of 2020 cm; 39 slices with 3 mm thickness and no gap; TR/TE 3000/35; and flip angle 90°, acquisition orientation was of the fourth ventricle plane. In addition, each functional scan was accompanied by a three-dimensional anatomical scan using T1-SPGR sequence (1 × 1 × 1 mm3).
fMRI analysis
Each of the three films were presented once and analyzed as 2-min blocks. Preprocessing and statistical analyses were conducted using the general linear model (GLM) framework implemented in Brain Voyager QX version 2.1.
Preprocessing
3D motion correction was conducted, using trilinear interpolation, linear trend removal and high pass filtering. A 4 mm full width at half maximum Gaussian smoothing was used to overcome differences in inter-subject localization. Functional 2D data were manually aligned and coregistered with 3D anatomical data which were normalized into Talairach space. To account for a hemodynamic response, predictors were convolved with 6 s hemodynamic response filter for all participants.
Whole brain analysis
The first six functional volumes, before signal stabilization, were excluded from analysis. Statistical maps were prepared for each subject using a GLM, in which the various activation blocks were defined as district predictors. Following, subjects' statistical maps were entered into random-effects group analyses, with statistical maps threshold of P < 0.005, with an extent threshold of 25 voxels, which was determined using a Monte Carlo simulation method, which was calculated using NeuroElf’s (http://neuroelf.net/) instantiation of AlphaSim (Forman et al., 1995). This cluster-based method of thresholding is often more sensitive to activation when one can reasonably expect multiple contiguous activated voxels, and is widely used in fMRI research (Kross et al., 2011). Whole brain maps were then used to localize relevant voxels and to visually demonstrate the signal change in the dACC, fusiform and NAcc, which were of theoretical a priori interest in the current study.
Correlations between own mother–infant synchrony and brain response to synchronous vs nonsynchronous interactions
In addition to assessing the brain regions that differentially responded to the synchronous vs nonsynchronous interactions, we were interested in locating brain regions that can be predicted by the mothers' behavioral synchrony scores. For that end, we used a whole brain ANCOVA analysis with a mother’s synchrony score as a covariate in the contrast synchrony interaction vs depression Interaction (P = 0.005, k = 25).
RESULTS
Behavioral synchrony
As a first step, we examined whether the degree of social synchrony in the three unfamiliar films showed significant differences. In all behavioral categories—gaze, affect, vocalizations and touch—the depressed mother showed substantially lower amounts of maternal behavior as compared to the healthy mother, while the mother diagnosed with GAD scored at mid-point. Mean proportions of time a mother gazed at the infant’s face—the core behavior that enables social interaction—were lowest in the depressed interaction (8.78%), and highest in the healthy (77.24%) and anxious (80%) interactions. Mother–infant synchrony occurred 30% of the time in the healthy interaction, but was observed for merely a split-second (0.0078%) in the depressed interaction and 18% in the anxious interaction. These results showed that both pathological interactions are characterized by marked deviations from social synchrony, with the depressed interaction demonstrating substantial decrease in maternal behavior in addition to no social synchrony (Figure 2).
Fig. 2.
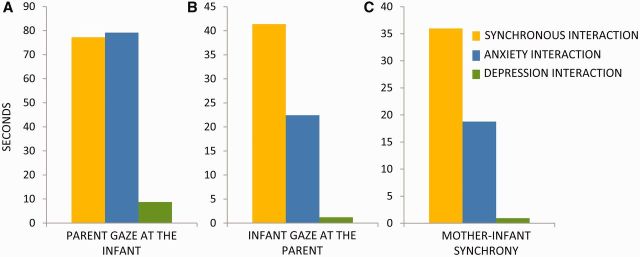
Behavioral analysis of the three films presented to mothers as fMRI stimuli. (A) Parent gaze. The total proportion of time mother gazed at the infant’s face. (B) Infant’s social engagement. The total proportion of time infant gazed at mother’s face. (C) Synchrony. The total proportion of time mothers coordinated social gaze, positive vocalizations and affective touch with the infant’s social gaze and positive affect.
Whole-brain GLM
Two whole-brain contrasts were calculated to explore the brain responses to the healthy vs the pathological interactions. The first contrast compared the synchronous and the depressed interaction and the second compared the synchronous and the anxious interaction.
Synchronous vs depression interaction
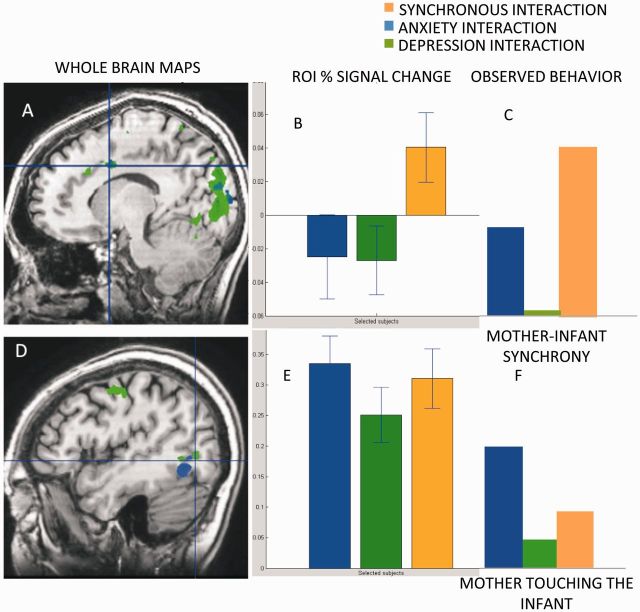
This whole-brain analysis revealed several brain regions that were more active when mothers observed the synchronous as compared to the depression interaction (P < 0.005, k = 25, random effect, N = 27; Figure 3 and Table 1). These include the posterior part of the middle and superior temporal gyri, cuneus, fusiform gyrus, inferior occipital cortex, cerebellum, superior and inferior parietal lobule, dACC and supplementary motor area (SMA)/motor cortex. When lowering the threshold (P = 0.02, k = 5, Table A1), other regions such as the NAcc and insula also showed greater response to the synchronous interaction. Notably, no brain region showed greater activations to the depression interaction. For visualization purposes, t-tests were calculated for the Fusiform: t = 5.08, P = 0.000 (Figure 3A), dACC: t = 5.26, P = 0.000 (Figure 3B) and NAcc: t = 2.95, P = 0.007 (Figure 3C).
Fig. 3.
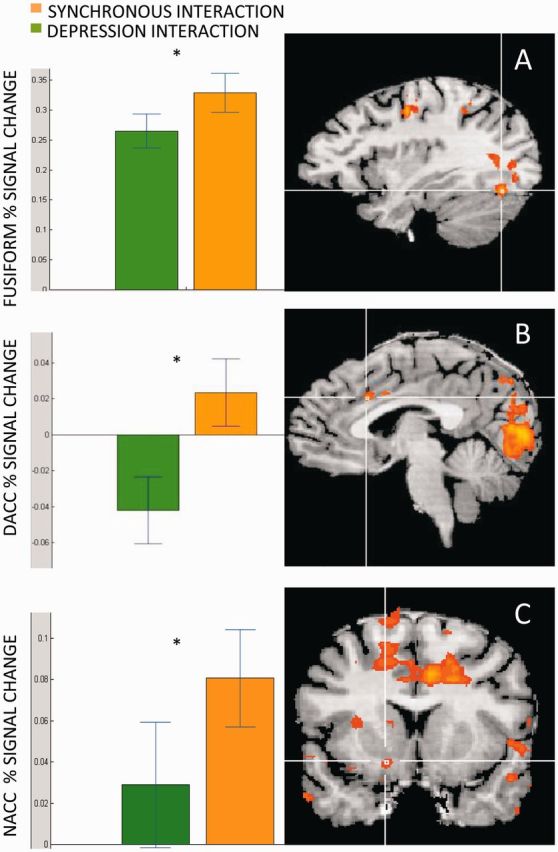
Brain areas showing greater response to the synchronous compared to the depressed interaction. Fusiform (A), dACC (B) and NACC (C) showed greater activations when mothers observed the synchronous interaction (orange) compared to the depression interaction (green).
Table 1.
Brain activations in the contrast synchronous vs depression interaction
| Synchrony film> depression film | Peak X | Peak Y | Peak Z | t | p | k |
|---|---|---|---|---|---|---|
| Right middle temporal gyrus | 36 | −55 | 7 | 5.823734 | 0.000004 | 155 |
| Right inferior temporal gyrus | 42 | −67 | 1 | 4.609706 | 0.000094 | 99 |
| Right middle temporal gyrus | 48 | −58 | 1 | 3.81377 | 0.000758 | 44 |
| Left cuneus | −6 | −85 | 7 | 5.724349 | 0.000005 | 1258 |
| Left cuneus | −12 | −85 | 31 | 5.486945 | 0.000009 | 179 |
| Left fusiform gyrus | −33 | −73 | −14 | 5.391277 | 0.000012 | 82 |
| Left inferior occipital gyrus | −42 | −73 | 1 | 5.277966 | 0.000016 | 179 |
| Left cuneus | 0 | −79 | 28 | 5.027919 | 0.000031 | 31 |
| Right cuneus | 9 | −79 | 22 | 5.001453 | 0.000033 | 29 |
| Left culmen | −18 | −61 | −8 | 4.844227 | 0.000051 | 28 |
| Left superior temporal gyrus | −57 | −43 | 16 | 4.69743 | 0.000075 | 57 |
| Left precuneus | 0 | −61 | 52 | 5.326772 | 0.000014 | 219 |
| Left superior parietal lobule | −24 | −52 | 43 | 4.599651 | 0.000097 | 83 |
| Right superior temporal gyrus | 63 | −16 | 1 | 5.230619 | 0.000018 | 34 |
| Right superior temporal gyrus | 60 | −40 | 10 | 5.149761 | 0.000023 | 48 |
| Left precentral gyrus | −21 | −16 | 61 | 5.113294 | 0.000025 | 511 |
| Left precentral gyrus | −33 | −10 | 43 | 5.037417 | 0.00003 | 40 |
| Right superior frontal gyrus | 12 | 2 | 67 | 4.825377 | 0.000053 | 135 |
| Right precentral gyrus | 54 | −4 | 46 | 4.496356 | 0.000127 | 72 |
| Right Precentral Gyrus | 39 | −10 | 46 | 4.255092 | 0.00024 | 63 |
| Left cingulate gyrus | −15 | 2 | 34 | 4.914289 | 0.000042 | 137 |
| Left medial frontal gyrus | −21 | 26 | 31 | 4.282782 | 0.000223 | 52 |
| Left superior temporal gyrus | −51 | −13 | 4 | 4.762651 | 0.000063 | 62 |
| Right middle frontal gyrus | 30 | 41 | 34 | 4.696496 | 0.000075 | 37 |
| Left tuber | −51 | −46 | −20 | 4.233436 | 0.000254 | 34 |
| Left medial frontal gyrus | −24 | 41 | 7 | 3.616691 | 0.00126 | 26 |
X, Y, Z represent Talairach coordinates. Cluster size > 25, P < 0.005, minimum activation threshold = 2.78. N = 27. Threshold = 2.78. P = 0.005. Cluster = 25.
Synchronous vs anxiety interaction
This whole-brain analysis showed several brain regions that were more active when mothers observed the synchronous vs the anxiety interaction (P < 0.005, k = 25, random effect, N = 27, see Table 2). These include the cuneus, anterior cingulate, and dACC. Brain regions that showed higher activations to the anxious interaction included the primary auditory cortex and the posterior part of the middle temporal gyrus/fusiform.
Table 2.
Brain activations in the contrast synchronous vs anxiety interaction
| Synchrony film> anxiety film | Peak X | Peak Y | Peak Z | t | p | k |
|---|---|---|---|---|---|---|
| Left cuneus | −9 | −94 | 10 | 4.577863 | 0.000102 | 103 |
| Right anterior_cingulate | 18 | 41 | 7 | 4.235737 | 0.000252 | 97 |
| Left dorsal anterior cingulate gyrus | −12 | 2 | 34 | 3.815599 | 0.000755 | 35 |
| Deactivations | ||||||
| Right superior temporal gyrus | 57 | −31 | 16 | −4.784 | 0.0001 | 87 |
| Right middle temporal gyrus | 51 | −58 | −8 | −5.96 | 0.0001 | 69 |
X, Y, Z represent Talairach coordinates. Cluster size > 25, p < 0.005, minimum activation threshold = 2.78. N = 27. Threshold = 2.78. P = 0.005. Cluster = 25.
Overlay of depression and anxiety contrasts
To describe brain regions that responded differently to the synchronous vs the nonsynchronous interactions, we overlaid the two maps (healthy > depressed, healthy > anxious). Results of the overlay revealed brain regions that were more active when mothers viewed the synchronous interactions compared to the pathological interactions. These include the cuneus, fusiform and dACC. However, whereas the dACC showed greater response to the synchronous compared to the nonsynchronous interactions (Figure 4B and C), the fusiform (Figure 4D) showed greater response to the anxious film (response was highest in the anxious film, lower to the synchronous film and lowest to the depressed film), regardless of the degree if synchrony (Figure 4E and F).
Fig. 4.
Brain response in the overlap between the two contrasts of synchrony > depression and synchrony > anxiety. (A) dACC in the overlay of maps synchrony > depression and synchrony > anxiety. (B) Dorsal cingulate percentage signal change respond more during the synchrony interaction (in orange, levels of synchrony are demonstrated in (C). (D) Fusiform activation in the overlay of maps synchrony > depression and synchrony > anxiety. (E) Fusiform percentage signal change. The more maternal behavior is presented in the film (F), the stronger the percentage signal change, regardless of synchrony.
Links between own synchrony and brain response to social synchrony
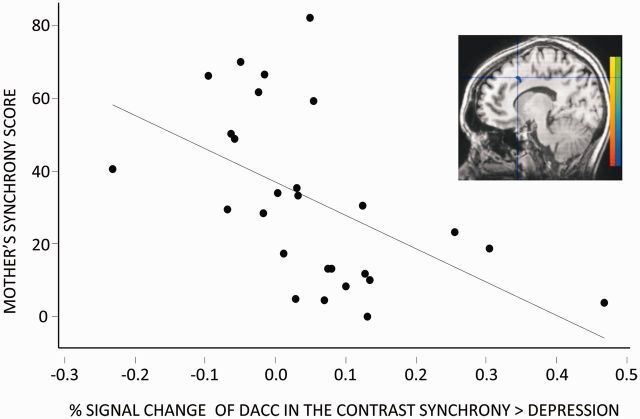
Mother–infant behavioral synchrony scores ranged from 0% to 81.86% (mean = 31.91, SD = 23.65, median = 29.38). To evaluate whether there are brain regions observed in the contrast synchronous vs depression interaction that can be predicted by the mother’s own behavioral synchrony score, we computed a covariant analysis, where we evaluated which voxel’s signal is correlated to the mothers' synchrony scores. Results show that the dACC (R = −0.608, P = 0.001) and precentral gyri (R = −0.591, P-value = 0.001) were each predicted by the mothers' own behavioral synchrony scores (Table 3, Figure 5).
Table 3.
Brain activations in the contrast synchronous vs depression interaction with mothers’ own synchrony scores as covariate
| Synchrony film> depression film × synchrony scores | Peak X | Peak Y | Peak Z | R | p | k |
|---|---|---|---|---|---|---|
| Precentral gyrus | 42 | −31 | 55 | −0.591 | 0.001 | 34 |
| Cingulate gyrus | 12 | 14 | 40 | −0.608 | 0.001 | 30 |
X, Y, Z represent Talairach coordinates. Cluster size > 25, P < 0.005, minimum activation threshold = 2.78. N = 27. Threshold = 2.78. P = 0.005. Cluster = 25.
Fig. 5.
dACC activation in the contrast synchronous interaction vs depression interaction is predicted by mothers’ behavioral synchrony scores. Pearson correlation between percentage signal change of the dACC in the contrast synchrony > depression interaction and the mothers’ synchrony scores (R(Pearson) = −0.608, P = 0.001).
DISCUSSION
\Results of the current study demonstrate that the human brain responds to variations in social synchrony. Specifically, the findings show that postpartum mothers differentiate synchronous interactions from those characterized by marked reduction in social synchrony. Brain areas that showed greater response to the synchronous interactions as compared to interactions that contained minimal social synchrony included reward, simulation and mentalization areas, such as the dACC, NAcc, SMA, cuneus, IPL, fusiform and STS. Furthermore, activation of the dACC was not only higher during the synchronous interaction but could also be predicted by the mother’s own synchrony with her infant. These findings are consistent with models on the embodied nature of the human social brain (Lombardo et al., 2010) and show that mothers detect synchrony in others using the same brain regions related to the expression of synchrony with their own infants. Findings also suggest that the dACC may play a special role in the link between participating in a synchronous exchange and detecting synchrony in others, possibly through mechanisms of embodiment.
To our knowledge, this is the first study to test ecologically valid social interactions as fMRI stimuli and examine the links between active expression of social behavior and the evaluation of similar behavior in others. Authors have recently called to shift the focus in neuroscience research to ecologically valid social contexts, dynamic rather than static presentations and real-life rather than computer-generated stimuli, particularly in the domains of social and affective neuroscience (Schippers et al., 2010; Hasson and Honey, 2012). Exploring the brain as it responds to real-life social interactions is thought to provide new opportunities for theoretical and empirical advancement. Although it is clear that there is always a tradeoff between experimental control and ecological validity, most social interactions occur within naturalistic contexts and the brain must make quick predictions with regards to their key parameters. From an evolutionary viewpoint, it is critical that the human brain acquires mechanisms for assessing the quality of early care, particularly whether certain caregiving practices pose risk for infant well-being. This is important not only for individual survival but also for the consolidation of human societies. Among social species, the mother–infant bond provides the central context for infant entry into the social world and becoming a functional member of the social group. Mechanisms that enable a quick detection of functional vs dysfunctional early relationships are therefore critical not only at the level of the individual, but also for the continuity of the social species.
Social interaction and the processing of affective information are suggested to build on mechanisms of simulation and embodiment (Niedenthal and Brauer, 2012). To understand emotions in others, individuals use their own body and neural body representations to simulate themselves making the same gestures in similar contexts (Niedenthal and Brauer, 2012), thus synchronizing own behavior with that of the social partner. During synchronous mother–infant interactions, both mother and child embody each other’s affective and physiological states to achieve biological and behavioral synchrony (Feldman et al., 2011). Furthermore, when a postpartum mother is observing another mother interacting with her infant, she can rely on her immediate experiences to embody and grasp the partners’ social signals and, as our findings show, mothers who are better able to synchronize with their own child also show more robust activations to synchrony in simulation-related areas. Authors have interpreted the simulation of perceived emotional and motor gestures and states in terms of mirror neurons (Keysers and Gazzola, 2007; Niedenthal and Brauer, 2012). In addition, the anterior insula and ACC have been implicated in simulation and embodiment (Niedenthal and Brauer, 2012), and some researchers consider these regions as key players in processing the affective value of somato-sensation (Keysers et al., 2010). Thus, it is not surprising that both maternal synchrony with her infant and her ‘reading’ synchrony in others rely on the ACC and possibly on embodiment. Further support of the hypothesis that behavioral synchrony relies on neural mechanisms of embodiment come from our previous study which demonstrated the connection between maternal synchrony and the mirror system, anterior insula and ACC (Atzil et al., 2011).
Our findings are consistent with previous reports that emphasized the role of the ACC in regulating reward, social understanding (Bush et al., 2002; Williams et al., 2004; Sheth et al., 2012) and the overlap between the experience of physical pain and social pain (Eisenberger et al., 2003). The dACC comprises an overlapping hub between two large-scale intrinsic networks: the dorsal anterior-insula network, which is linked with cognitive performance, and the ventral anterior insula network that shapes performance in affective tasks (Touroutoglou et al., 2012). In addition, the dACC is involved in social cognition (Payer et al., 2008), which requires the integration of affective and cognitive processing. Finally, the dACC synchronizes with the hippocampus during complex multi-domain processes (Guitart-Masip et al., 2013). It is thus possible that one role of the dACC is to integrate parallel processes of cognition, affect and sociability and to regulate action in light of this integration by sending efferent to the motor system. Previous studies from our laboratory showed that behavioral synchrony is associated with the integration of reward, affect and social-cognitive brain networks (Atzil et al., 2011, 2012). The current study similarly highlights the role of reward in social synchrony, as seen by the greater activation of the NAcc and dACC to the synchronous interaction. Possibly, the detection of social synchrony involves the dACC in the integration of the affective and social-cognitive processes that are required for decoding the reward value of social information and the findings seem to suggest that this link is specific to the dACC. For instance, the anxiety interaction, although containing minimal synchrony, included substantially more maternal touch of the infant as compared to the other two interactions and the fusiform, which is sensitive to touch, showed higher activation to this interaction. On the other hand, the dACC appears to be specifically sensitive to social synchrony and showed higher activation in the synchronous interactions than during the two nonsynchronous interactions. It is possible that the reward-related information processed by the dACC is critical in order to make accurate judgments on the desirability of social events and assists in the planning of adequate motor response.
In addition to its role in social reward and the integration of affective and social-cognitive processes, a recent meta-analysis of imaging studies on the neural basis of empathy described a dACC–SMA axis that was observed across all studies of human empathy, whether to the pain, social exclusion, anxiety or happiness of familiar and unfamiliar others (Fan et al., 2011). The authors highlight the dACC as a neurochemically unique structure with strong connectivity to limbic areas that is implicated in the understanding of others' social goals, intentions and affect. Our results showing that the dACC moderated the link between maternal synchrony to her own infant and the detection of synchrony in others may suggest that while synchrony requires mothers to share a range of infant states, from physical to emotional to cognitive-exploratory, it also requires higher cognitive inferences about mental state from observed behavior and is related to activations in reward areas, which are particularly salient among synchronous mothers (Atzil et al., 2011). Our findings, therefore, highlight the mother–child bond and the experience of social synchrony as a potentially useful window to study socio-cognitive processes that are based on observed behavior and occur within real relationships and concrete repeatedly experienced social contexts.
The depressed interaction showed minimal maternal behavior and no social synchrony, exhibiting marked deviation from the typical pattern of maternal care. This deviant social interaction was detected both consciously by the reporting mothers and automatically by the brain response of the scanned mothers. While much research has documented the brain processes that are impaired in depressed individuals, our study is the first to examine the brain response of healthy individuals to the social behavior of the depressed. Interestingly, a recent study has shown that postnatally depressed mothers exhibit lower response in the NAcc, fusiform and dACC to their infant cries as compared to nondepressed mothers (Laurent and Ablow, 2012). The current findings show that these same areas are deactivated when mothers view the interaction between a depressed mother and her infant. It has been argued that depressed individuals induce aversive response from social partners as those who are faced with their deviant social behavior attempt to reduce the discomfort by avoiding social contact (Coyne, 1976; Gurtman, 1986). Possibly, since depressed individuals provide less response-contingent reinforcement during social exchanges, social partners prefer interactions that are more rewarding and contain more social synchrony. Such explanation is consistent with our findings, which show that the depressed interaction induced lower brain response in reward-related area. However, much further research is required to examine the effects of depression on the deactivation of reward circuits not only among depressed individuals but also among their social partners.
Mothers' response to the synchronous interactions in both contrasts shared a common network of brain activations, including midline structures in the prefrontal cortex and the cuneus, dACC and temporal cortices. These brain regions have been consistently implicated in ToM, mentalization, declarative memory and episodic memory (Buckner and Vincent, 2007). Our findings may suggest that mothers show greater activations in mentalization areas in response to the synchronous social cues. Importantly, deactivation of these areas during the observation of nonsynchronous interactions was substantially more robust in the case of depression and included greater deactivations in midline and lateral parietal regions, NAcc and fusiform as compared to anxiety. Furthermore, the fusiform showed greater activation to the anxiety interaction, possibly as anxious mothers showed greater amounts of touch and this structure is sensitive to patterns of touch. The activation of brain regions associated with metallization during the processing of social cues is consistent with the hypothesis is that the ‘mentalizing’ network recombines stored information in real time to create not only cognitions but also emotions and perceptions of people and events in the world (Barret and Satpute, 2013).
Several study limitations should be remembered in the interpretation of the findings. Perhaps the most notable limitation is that the three stimuli presented to the mothers were each filmed in a different home ecology and we could not fully control for context-related factors that may have influenced the findings. Although we tried to minimize these caveats by using the same filming protocol and micro-coding and controlling for a range of behavioral and postural factors, studies that use ecologically valid stimuli must compromise on exact similarity among conditions. Yet, we believe that information received from ecological observations is important and research should complement laboratory-based, computer-generated stimuli with those collected in ‘messy’ real-life situations for a fuller understanding of the brain basis of social behavior. Another limitation is that we did not follow the experiment by asking mothers to rate the degree of empathy or identification they felt toward each of the films and such information may have been valuable in the interpretation of the data.
Further research is required to assess whether the current findings on social synchrony extend to situations that require social cohesion outside the parenting context, such as athletes, performing artists or medical crews that call for a tight coordination between the behaviors of multiple social partners. It would be of interest to explore whether participation in such social groups increases the individual’s capacity to detect synchrony accurately. Similarly, it would be of empirical and clinical value to study whether conditions associated with severe social pathology, such as autism, depression or schizophrenia, are associated not only with the inability to engage in well-coordinated social behavior but also in difficulty detecting social synchrony in others.
Acknowledgments
Supported by the German-Israeli Science Foundation-GIF (grant no. 1114-101.4/2010) to RF and the I-CORE program of the planning and budgeting committee and the Israeli Science Foundation (Grant No. 51/11) to TH and RF. Research at Prof. Feldman's lab during the study period was supported by the Israeli Science Foundation (#08-1308), the US-Israel Bi-National Science Foundation (#2011-349), the NARSAD Foundation, and the Irving B. Harris Foundation.
Appendix
Table A1.
Brain activations in the contrast synchronous vs depression interaction
| Synchrony film> depression film | Peak X | Peak Y | Peak Z | t | p | k |
|---|---|---|---|---|---|---|
| Right middle temporal gyrus | 36 | −55 | 7 | 5.823734 | 0.000004 | 6197 |
| Left cuneus | −6 | −85 | 7 | 5.724349 | 0.000005 | 1186 |
| Left cuneus | −12 | −85 | 31 | 5.486945 | 0.000009 | 223 |
| Left fusiform gyrus | −33 | −73 | −14 | 5.391277 | 0.000012 | 121 |
| Left precuneus | 0 | −61 | 52 | 5.326772 | 0.000014 | 169 |
| Left middle occipital gyrus | −42 | −73 | 1 | 5.277966 | 0.000016 | 245 |
| Left precuneus | −21 | −73 | 22 | 5.272156 | 0.000016 | 19 |
| Left cuneus | −21 | −85 | 28 | 5.2171 | 0.000019 | 7 |
| Right superior temporal gyrus | 60 | −40 | 10 | 5.149761 | 0.000023 | 87 |
| Left precentral gyrus | −21 | −16 | 61 | 5.113294 | 0.000025 | 429 |
| Left precentral gyrus | −33 | −10 | 43 | 5.037417 | 0.00003 | 43 |
| Left cuneus | 0 | −79 | 28 | 5.027919 | 0.000031 | 44 |
| Right cuneus | 9 | −79 | 22 | 5.001453 | 0.000033 | 53 |
| Left cuneus | −21 | −85 | 19 | 4.973629 | 0.000036 | 5 |
| Left cingulate gyrus | −15 | 2 | 34 | 4.914289 | 0.000042 | 271 |
| Left lingual gyrus | −18 | −61 | −8 | 4.844227 | 0.000051 | 46 |
| Right medial frontal gyrus | 12 | 2 | 67 | 4.825377 | 0.000053 | 419 |
| Left transverse temporal gyrus | −51 | −13 | 4 | 4.762651 | 0.000063 | 94 |
| Left superior temporal gyrus | −60 | −25 | 7 | 4.707959 | 0.000073 | 80 |
| Left declive | −18 | −67 | −17 | 4.707017 | 0.000073 | 17 |
| Left superior temporal gyrus | −57 | −43 | 16 | 4.69743 | 0.000075 | 160 |
| Right middle frontal gyrus | 30 | 41 | 34 | 4.696496 | 0.000075 | 133 |
| Left precentral gyrus | −54 | 2 | 1 | 4.677734 | 0.000079 | 39 |
| Left middle temporal gyrus | −45 | −55 | 10 | 4.66789 | 0.000081 | 26 |
| Left lingual gyrus | −9 | −67 | −5 | 4.641159 | 0.000087 | 22 |
| Right middle occipital gyrus | 42 | −67 | 1 | 4.609706 | 0.000094 | 44 |
| Right cuneus | 12 | −82 | 31 | 4.604163 | 0.000096 | 7 |
| Left superior parietal lobule | −24 | −52 | 43 | 4.599651 | 0.000097 | 284 |
| Left cingulate gyrus | −6 | 8 | 37 | 4.599423 | 0.000097 | 6 |
| Right medial frontal gyrus | 3 | −10 | 55 | 4.531183 | 0.000116 | 10 |
| Right postcentral gyrus | 54 | −4 | 46 | 4.496356 | 0.000127 | 166 |
| Right sub-gyral | 18 | −7 | 58 | 4.483133 | 0.000132 | 18 |
| Left inferior temporal gyrus | −48 | −73 | −8 | 4.391094 | 0.000168 | 5 |
| Left precuneus | −15 | −55 | 61 | 4.38834 | 0.000169 | 46 |
| Left cingulate gyrus | −3 | 17 | 31 | 4.34108 | 0.000191 | 6 |
| Right precuneus | 12 | −58 | 58 | 4.32761 | 0.000198 | 47 |
| Left middle occipital gyrus | −21 | −85 | 4 | 4.283283 | 0.000223 | 9 |
| Left middle frontal gyrus | −21 | 26 | 31 | 4.282782 | 0.000223 | 93 |
| Right precentral gyrus | 39 | −10 | 46 | 4.255092 | 0.00024 | 68 |
| Left cuneus | −12 | −82 | 40 | 4.15835 | 0.000309 | 8 |
| Left cingulate gyrus | −12 | 2 | 49 | 4.123909 | 0.000338 | 6 |
| Right precentral gyrus | 36 | −7 | 55 | 4.060318 | 0.000399 | 5 |
| Right cuneus | 15 | −67 | 4 | 3.995401 | 0.000473 | 14 |
| Left middle occipital gyrus | −30 | −82 | 7 | 3.9464 | 0.000538 | 5 |
| Right superior frontal gyrus | 15 | 41 | 43 | 3.924084 | 0.00057 | 36 |
| Left superior temporal gyrus | −45 | −37 | 10 | 3.900079 | 0.000606 | 7 |
| Right medial frontal gyrus | 6 | 41 | 25 | 3.856824 | 0.000678 | 53 |
| Right cingulate gyrus | 15 | −28 | 40 | 3.849413 | 0.000692 | 57 |
| Left cuneus | −3 | −79 | 43 | 3.840784 | 0.000707 | 11 |
| Right middle temporal gyrus | 48 | −58 | 1 | 3.81377 | 0.000758 | 22 |
| Left insula | −45 | −28 | 25 | 3.753224 | 0.000887 | 51 |
| Right declive | 33 | −58 | −17 | 3.716979 | 0.000974 | 30 |
| Left superior parietal lobule | −27 | −64 | 58 | 3.700445 | 0.001016 | 7 |
| Right cuneus | 21 | −82 | 31 | 3.695329 | 0.001029 | 9 |
| Right middle temporal gyrus | 60 | −37 | 1 | 3.642017 | 0.00118 | 12 |
| Left middle frontal gyrus | −24 | 41 | 7 | 3.616691 | 0.00126 | 91 |
| Right cingulate gyrus | 9 | −1 | 46 | 3.615192 | 0.001264 | 11 |
| Left precuneus | −18 | −64 | 40 | 3.614171 | 0.001268 | 16 |
| Right middle frontal gyrus | 30 | −7 | 40 | 3.603248 | 0.001304 | 8 |
| Left inferior parietal lobule | −36 | −34 | 31 | 3.587424 | 0.001358 | 20 |
| Right fusiform gyrus | 45 | −55 | −20 | 3.545646 | 0.00151 | 18 |
| Left anterior cingulate | −3 | 38 | 22 | 3.529206 | 0.001575 | 14 |
| Right caudate | 39 | −25 | −8 | 3.521751 | 0.001605 | 21 |
| Right superior temporal gyrus | 48 | −46 | 16 | 3.504964 | 0.001675 | 26 |
| Left middle frontal gyrus | −15 | 32 | 40 | 3.479513 | 0.001787 | 20 |
| Left postcentral gyrus | −48 | −16 | 46 | 3.467338 | 0.001843 | 40 |
| Left inferior parietal lobule | −33 | −46 | 43 | 3.465581 | 0.001851 | 15 |
| Left transverse temporal gyrus | −36 | −34 | 7 | 3.458814 | 0.001883 | 11 |
| Right middle frontal gyrus | 21 | 50 | 7 | 3.437995 | 0.001985 | 53 |
| Right sub-gyral | 42 | −46 | −11 | 3.434504 | 0.002002 | 22 |
| Left anterior cingulate | −6 | 17 | 19 | 3.41259 | 0.002116 | 11 |
| Right cingulate gyrus | 24 | −46 | 25 | 3.391633 | 0.002231 | 16 |
| Left precentral gyrus | −48 | −10 | 25 | 3.369376 | 0.00236 | 16 |
| Right precuneus | 15 | −40 | 52 | 3.352963 | 0.002459 | 11 |
| Left medial frontal gyrus | −9 | −10 | 58 | 3.335973 | 0.002567 | 7 |
| Left cingulate gyrus | −12 | −13 | 40 | 3.335645 | 0.002569 | 16 |
| Left declive | −36 | −58 | −20 | 3.28297 | 0.002931 | 13 |
| Right middle frontal gyrus | 24 | 32 | 28 | 3.27687 | 0.002976 | 8 |
| Right precuneus | 9 | −61 | 46 | 3.262665 | 0.003083 | 19 |
| Right culmen | 36 | −49 | −26 | 3.243642 | 0.003233 | 10 |
| Right inferior frontal gyrus | 30 | 32 | −2 | 3.239307 | 0.003268 | 6 |
| Left inferior parietal lobule | −54 | −25 | 34 | 3.230817 | 0.003337 | 9 |
| Left postcentral gyrus | −33 | −28 | 46 | 3.211684 | 0.0035 | 10 |
| Right superior frontal gyrus | 21 | 47 | 19 | 3.198853 | 0.003613 | 11 |
| Left precentral gyrus | −36 | −16 | 31 | 3.15995 | 0.003978 | 12 |
| Right caudate | 15 | 26 | −2 | 3.122265 | 0.004365 | 36 |
| Left middle temporal gyrus | −66 | −37 | 1 | 3.086197 | 0.004769 | 8 |
| Left paracentral lobule | −9 | −37 | 52 | 3.05565 | 0.00514 | 11 |
| Right anterior cingulate | 18 | 38 | 7 | 3.054423 | 0.005155 | 18 |
| Left superior frontal gyrus | −21 | 56 | 25 | 3.021274 | 0.005589 | 17 |
| Left insula | −42 | −19 | −8 | 2.998683 | 0.005904 | 12 |
| Right insula | 30 | −40 | 16 | 2.992995 | 0.005986 | 13 |
| Right cingulate gyrus | 15 | 26 | 34 | 2.981074 | 0.006162 | 5 |
| Right cingulate gyrus | 6 | 11 | 34 | 2.958525 | 0.006507 | 7 |
| Right cuneus | 21 | −67 | 16 | 2.9157 | 0.007215 | 13 |
| Right pyramis | 27 | −61 | −35 | 2.878651 | 0.007885 | 14 |
| Left superior frontal gyrus | −12 | 53 | 34 | 2.852895 | 0.008386 | 9 |
| Right cingulate gyrus | 18 | 8 | 43 | 2.826535 | 0.008929 | 24 |
| Right superior temporal gyrus | 63 | −16 | 1 | 5.230619 | 0.000018 | 89 |
| Right superior temporal gyrus | 63 | −16 | 1 | 5.230619 | 0.000018 | 72 |
| Right superior temporal gyrus | 57 | 2 | −5 | 3.526175 | 0.001587 | 11 |
| Right superior temporal gyrus | 54 | −16 | −2 | 3.497421 | 0.001707 | 6 |
| Right declive | 12 | −64 | −23 | 4.431082 | 0.000151 | 22 |
| Right middle occipital gyrus | 48 | −76 | −8 | 4.263717 | 0.000234 | 28 |
| Right inferior occipital gyrus | 39 | −79 | −11 | 4.103423 | 0.000357 | 5 |
| Left fusiform gyrus | −51 | −46 | −20 | 4.233436 | 0.000254 | 82 |
| Left fusiform gyrus | −45 | −37 | −17 | 3.651731 | 0.001151 | 16 |
| Left tuber | −42 | −64 | −38 | 4.059591 | 0.0004 | 44 |
| Left pyramis | −18 | −67 | −38 | 3.452895 | 0.001911 | 20 |
| Left tuber | −33 | −67 | −35 | 3.322744 | 0.002653 | 6 |
| Right fusiform gyrus | 45 | −4 | −35 | 3.535775 | 0.001549 | 28 |
| Left lentiform nucleus | −21 | −16 | −5 | 3.48067 | 0.001782 | 28 |
| Left thalamus | −18 | −13 | 13 | 3.248124 | 0.003197 | 10 |
| Right sub-gyral | 27 | −43 | 61 | 3.277154 | 0.002974 | 28 |
| Left superior frontal gyrus | −9 | 17 | 58 | 3.27685 | 0.002976 | 12 |
| Right sub-gyral | 24 | −34 | 64 | 3.273734 | 0.002999 | 14 |
| Right declive | 27 | −79 | −23 | 3.270238 | 0.003025 | 10 |
| Right insula | 30 | −28 | 13 | 3.24306 | 0.003237 | 19 |
| Left inferior frontal gyrus | −48 | 29 | 10 | 3.153066 | 0.004046 | 18 |
| Right superior parietal lobule | 27 | −55 | 55 | 3.149966 | 0.004077 | 9 |
| Left inferior frontal gyrus | −51 | 20 | 16 | 3.08715 | 0.004758 | 5 |
| Left inferior frontal gyrus | −33 | 35 | 1 | 3.053882 | 0.005162 | 7 |
| Left superior temporal gyrus | −45 | 8 | −17 | 3.053442 | 0.005167 | 14 |
| Left thalamus | −12 | −31 | −2 | 3.013889 | 0.00569 | 5 |
| Right precentral gyrus | 36 | 20 | 37 | 2.976198 | 0.006235 | 26 |
| Right inferior frontal gyrus | 39 | 11 | 25 | 2.885397 | 0.007759 | 5 |
| Left posterior cingulate | −6 | −34 | 19 | 2.965508 | 0.006398 | 11 |
| Right lentiform nucleus | 18 | −4 | 7 | 2.904048 | 0.00742 | 21 |
| Right middle temporal gyrus | 60 | −10 | −20 | 2.893358 | 0.007612 | 5 |
| Right middle temporal gyrus | 57 | −34 | −17 | 2.879601 | 0.007867 | 10 |
| Left inferior frontal gyrus | −33 | 32 | −8 | 2.861849 | 0.008208 | 13 |
| Left Inferior Frontal Gyrus | −24 | 35 | −11 | 2.795519 | 0.00961 | 5 |
| Left posterior cingulate | −18 | −55 | 19 | 2.833784 | 0.008776 | 20 |
| Left medial frontal gyrus | −15 | 50 | 4 | 2.796397 | 0.009591 | 5 |
| Right subcallosal gyrus | 18 | 11 | −20 | 2.75601 | 0.01055 | 9 |
| Right nucleus accumbens | 15 | 8 | −8 | 2.740939 | 0.010931 | 5 |
| Right lentiform nucleus | 15 | −4 | −2 | 2.686311 | 0.012421 | 6 |
| Right thalamus | 9 | −28 | 16 | 2.598199 | 0.015232 | 5 |
| Left precentral gyrus | −51 | −1 | 34 | 2.594307 | 0.015369 | 11 |
| Right claustrum | 30 | 8 | 13 | 2.539992 | 0.017403 | 6 |
| Right caudate | 12 | 11 | 10 | 2.424758 | 0.02257 | 8 |
X, Y, Z represent Talairach coordinates. Cluster size > 5, P < 0.02, mean activation threshold = 2.16. Threshold: 2.16. P = 0.02. k = 5.
Conflict of Interest
None declared.
REFERENCES
- Atzil S, Hendler T, Feldman R. Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology. 2011;36(13):2603–15. doi: 10.1038/npp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Zagoory-Sharon O, Winetraub Y, Feldman R. Synchrony and specificity in the maternal and the paternal brain: relations to oxytocin and vasopressin. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51(8):798–811. doi: 10.1016/j.jaac.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Satpute AB. Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr Opin Neurobiol. 2013;23(3):361–72. doi: 10.1016/j.conb.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrier AC, Ruelle E, Haskell MJ, Dwyer CM. Effect of a difficult calving on the vigour of the calf, the onset of maternal behaviour, and some behavioural indicators of pain in the dam. Preventive Veterinary Medicine. 2012;103(4):248–56. doi: 10.1016/j.prevetmed.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21(3):1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37(4):1091–6. doi: 10.1016/j.neuroimage.2007.01.010. discussion 1097–9. [DOI] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):523–8. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JC. Depression and the response of others. The Journal of Abnormal Psychology. 1976;85(2):186–93. doi: 10.1037//0021-843x.85.2.186. [DOI] [PubMed] [Google Scholar]
- Dunbar RIM. The social brain hypothesis. Evolutionary Anthropology: Issues, News, and Reviews. 1998;6(5):178–90. [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience & Biobehavioral Reviews. 2011;35(3):903–11. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Farrow C, Haycraft E, Meyer C. Similarities between eating attitudes among friendship groups in childhood: the moderating role of child anxiety. Journal of Pediatric Psychology. 2011;36(10):1144–52. doi: 10.1093/jpepsy/jsp105. [DOI] [PubMed] [Google Scholar]
- Feldman R. Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry. 2007a;48(3–4):329–54. doi: 10.1111/j.1469-7610.2006.01701.x. [DOI] [PubMed] [Google Scholar]
- Feldman R. On the origins of background emotions: from affect synchrony to symbolic expression. Emotion. 2007b;7(3):601–11. doi: 10.1037/1528-3542.7.3.601. [DOI] [PubMed] [Google Scholar]
- Feldman R. Mother-infant synchrony and the development of moral orientation in childhood and adolescence: direct and indirect mechanisms of developmental continuity. American Journal of Orthopsychiatry. 2007c;77(4):582–97. doi: 10.1037/0002-9432.77.4.582. [DOI] [PubMed] [Google Scholar]
- Feldman R. Oxytocin and social affiliation in humans. Hormones and Behavior. 2012a;61(3):380–91. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Feldman R. Parent-infant synchrony: A bio-behavioral model of mutual influences in the formation of affiliative bonds. Monographs of the Society for Research in Child Development. 2012b;77(2):42–51. [Google Scholar]
- Feldman R, Eidelman AI. Parent-infant synchrony and the social-emotional development of triplets. Developmental Psychology. 2004;40(6):1133–47. doi: 10.1037/0012-1649.40.6.1133. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Influs M, Gutbir T, Ebstein RP. Parental oxytocin and early caregiving jointly shape childrens oxytocin response and social reciprocity. Neuropsychopharmacology. 2013;38:1154–62. doi: 10.1038/npp.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Developmental Science. 2011;14(4):752–61. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(9):919–27. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Feldman R, Magori-Cohen R, Galili G, Singer M, Louzoun Y. Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behavior and Development. 2011;34:569–77. doi: 10.1016/j.infbeh.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Field T. The effects of mothers physical and emotional unavailability on emotion regulation. Monographs of the Society for Research in Child Development. 1994;59(2–3):208–27. [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106(3):458–90. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M, Barnes GR, Horner A, Bauer M, Dolan RJ, Duzel E. Synchronization of medial temporal lobe and prefrontal rhythms in human decision making. Journal of Neuroscience. 2013;33(2):442–51. doi: 10.1523/JNEUROSCI.2573-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtman MB. Depression and the response of others: reevaluating the reevaluation. Journal of Abnormal Psychology. 1986;95(1):99–101. doi: 10.1037//0021-843x.95.1.99. [DOI] [PubMed] [Google Scholar]
- Hasson U, Honey CJ. Future trends in Neuroimaging: Neural processes as expressed within real-life contexts. Neuroimage. 2012;62(2):1272–8. doi: 10.1016/j.neuroimage.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nature Reviews Neuroscience. 2006;7(12):942–51. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends in Cognitive Sciences. 2007;11(5):194–6. doi: 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nature Reviews Neuroscience. 2010;11(6):417–28. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(15):6270–5. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC. A cry in the dark: depressed mothers show reduced neural activation to their own infants cry. Social Cognitive and Affective Neuroscience. 2012;7(2):125–34. doi: 10.1093/scan/nsq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt A, Plassmann H, Shiv B, Rangel A. Dissociating valuation and saliency signals during decision-making. Cerebral Cortex. 2011;21(1):95–102. doi: 10.1093/cercor/bhq065. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, et al. Shared neural circuits for mentalizing about the self and others. Journal of Cognitive Neuroscience. 2010;22(7):1623–35. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Parent S, Evans AC, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(34):14324–9. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene x environment interactions. Child Development. 2010;81(1):41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22(3):437–52. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Niedenthal PM. Embodying emotion. Science. 2007;316(5827):1002–5. doi: 10.1126/science.1136930. [DOI] [PubMed] [Google Scholar]
- Niedenthal PM, Brauer M. Social functionality of human emotion. Annual Review of Psychology. 2012;63(1):259–85. doi: 10.1146/annurev.psych.121208.131605. [DOI] [PubMed] [Google Scholar]
- Payer DE, Lieberman MD, Monterosso JR, Xu J, Fong TW, London ED. Differences in cortical activity between methamphetamine-dependent and healthy individuals performing a facial affect matching task. Drug and Alcohol Dependence. 2008;93(1–2):93–102. doi: 10.1016/j.drugalcdep.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippers MB, Roebroeck A, Renken R, Nanetti L, Keysers C. Mapping the information flow from one brain to another during gestural communication. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(20):9388–93. doi: 10.1073/pnas.1001791107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin during the initial stages of romantic attachment: relations to couples interactive reciprocity. Psychoneuroendocrinology. 2012;37(8):1277–85. doi: 10.1016/j.psyneuen.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, et al. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 2012;488(7410):218–21. doi: 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB. Why are infants so attractive to others? The form and function of infant handling in bonnet macaques. Animal Behaviour. 1999;57(5):1021–32. doi: 10.1006/anbe.1998.1065. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Lieberman MD. Dissociating modality-specific and supramodal neural systems for action understanding. Journal of Neuroscience. 2012;32(10):3575–83. doi: 10.1523/JNEUROSCI.5715-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt RP, Lieberman MD. The busy social brain: evidence for automaticity and control in the neural systems supporting social cognition and action understanding. Psychological Science. 2013;24(1):80–86. doi: 10.1177/0956797612450884. [DOI] [PubMed] [Google Scholar]
- Swain JE, Leckman JF, Volkmar FR. The wolf boy: reactive attachment disorder in an adolescent boy. Psychiatry (Edgmont) 2005;2(11):55–61. [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A, Hollenbeck M, Dickerson BC, Feldman Barrett L. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage. 2012;60(4):1947–58. doi: 10.1016/j.neuroimage.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Moran JM, Nieto-Castanon A, Triantafyllou C, Saxe R, Gabrieli JD. Associations and dissociations between default and self-reference networks in the human brain. Neuroimage. 2011;55(1):225–32. doi: 10.1016/j.neuroimage.2010.11.048. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nature Neuroscience. 2004;7(12):1370–5. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]