Abstract
3,4-Methylenedioxymethamphetamine (MDMA, ‘ecstasy’) is used recreationally to improve mood and sociability, and has generated clinical interest as a possible adjunct to psychotherapy. One way that MDMA may produce positive ‘prosocial’ effects is by changing responses to emotional stimuli, especially stimuli with social content. Here, we examined for the first time how MDMA affects subjective responses to positive, negative and neutral emotional pictures with and without social content. We hypothesized that MDMA would dose-dependently increase reactivity to positive emotional stimuli and dampen reactivity to negative stimuli, and that these effects would be most pronounced for pictures with people in them. The data were obtained from two studies using similar designs with healthy occasional MDMA users (total N = 101). During each session, participants received MDMA (0, 0.75 and 1.5 mg/kg oral), and then rated their positive and negative responses to standardized positive, negative and neutral pictures with and without social content. MDMA increased positive ratings of positive social pictures, but reduced positive ratings of non-social positive pictures. We speculate this ‘socially selective’ effect contributes to the prosocial effects of MDMA by increasing the comparative value of social contact and closeness with others. This effect may also contribute to its attractiveness to recreational users.
Keywords: MDMA, social cognition, ecstasy, emotion
INTRODUCTION
The amphetamine analog 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) is often used recreationally in social settings, reportedly because it enhances mood, and uniquely increases feelings of sociability and connectedness with others (Bravo, 2001; Ter Bogt and Engels, 2005; Sumnall et al., 2006). MDMA is a potent releaser of the monoamine neurotransmitters norepinephrine, serotonin and dopamine, which are involved in physiological arousal, mood regulation and drug reinforcement. There is also evidence that MDMA releases oxytocin, a neuropeptide involved in affiliative behaviors (Dumont et al., 2009; Hysek et al., 2012a, in press). It has been proposed that this increase in oxytocin mediates the effects of MDMA on prosocial behavior in rats and subjective feelings of sociability in humans (Thompson et al., 2007; Dumont et al., 2009). Although the ‘prosocial’ effects of MDMA appear to contribute to both its recreational use and abuse potential (Ter Bogt and Engels, 2005; McGregor et al., 2008), comparatively little is known about which basic emotional processes the drug alters to produce these effects. MDMA may produce ‘prosocial’ effects in several ways: by directly producing positive and prosocial subjective states, by altering responses to stimuli encountered under the influence of the drug (e.g. enhancing responses to positive stimuli and dampening responses to negative stimuli) or by affecting responses to social stimuli in particular. A better understanding of these effects could help researchers understand why MDMA is used, and how it alters behavior. Here, we examined the effects of MDMA or placebo on a measure of emotional reactivity to social compared to non-social stimuli, to examine whether the effects of MDMA are specific to social stimuli.
Controlled, double-blind studies show that MDMA alters subjective mood states as well as emotional and social processing. The drug dose-dependently increases euphoria, positive mood states and feelings of sociability (Tancer and Johanson, 2001; Harris et al., 2002; Bedi et al., 2010; Hysek et al., 2012a, 2013; Kirkpatrick et al., 2012). MDMA improves recognition of positive mental states, such as friendliness in others (Hysek et al., 2012a), and increases the degree of arousal reported in response to pictures of people in positive emotional situations (Hysek et al., 2013). Conversely, MDMA impairs recognition of negative states such as expressions of anger or fear (Bedi et al., 2010; Hysek et al., 2012a). Brain imaging reveals similar modifications in neural responses to emotional expressions, with MDMA (1.5 mg/kg) increasing ventral striatum response to happy facial expressions and decreasing amygdala response to angry facial expressions (Bedi et al., 2009). However, these previous studies do not provide evidence to determine whether MDMA changes responses to positive and negative emotional stimuli in general, or whether its effects are specific to social stimuli. This is the question addressed here.
We investigated the effects of oral MDMA (0, 0.75 and 1.5 mg/kg) on reactivity to emotionally positive, negative and neutral pictures with or without social content, in occasional MDMA users (N = 101). We hypothesized that the drug would dose-dependently increase reactivity to positive emotional stimuli and dampen reactivity to negative stimuli, and that this effect would be greater for social pictures compared with non-social pictures.
MATERIALS AND METHODS
Study design
We pooled data from two studies using similar within-subjects, double-blind designs with only minor methodological differences. Occasional MDMA users attended three (Study 1) or four outpatient sessions (Study 2), separated by at least 5 days. In Study 1, they received placebo, 0.75 and 1.5 mg/kg MDMA, and in Study 2, they received placebo, 0.75 and 1.5 mg/kg MDMA and one of two doses of oxytocin (20 or 40 IU; not reported here). Drug doses were administered at one session each, with no drugs co-administered. In both studies, drug doses were counterbalanced relative to session order, and drug sequences were assigned randomly to participants. At each session, we collected measures of subjective effects, cardiovascular effects and responses to emotional pictures. The measures reported here were the only measures shared between the two studies; thus, additional results from these studies are published separately elsewhere (Kirkpatrick et al., in press; M. C. Wardle and H. de Wit, submitted for publication). In both studies, the pictures were presented as part of a block of tests given during expected peak effect, along with additional measures testing responses to social stimuli only (e.g. identification of emotional expressions). The picture task was the only measure to directly compare social to non-social stimuli. Task order was counterbalanced in both studies to minimize any order effects.
Participants
Healthy participants (58 male, 43 female), ages 18–35 were recruited through flyers and online advertisements. Participants completed a 2 h in-person psychiatric and medical evaluation, including physical examination, electrocardiogram, modified structured clinical interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; First et al., 1996) and self-reported drug and health history. Inclusion criteria were 4–40 times self-reported ecstasy use with no adverse responses; high-school education; English fluency; body mass index >19 and <30; no regular medication (except birth control); no medical conditions contraindicating MDMA; no past year DSM-IV Axis I diagnosis, excluding non-treatment-seeking substance abuse; no history of stimulant dependence; no women who were pregnant or planning a pregnancy. Smokers smoking more than 25 cigarettes per week were also excluded, to avoid nicotine withdrawal during study procedures. Participants were primarily Caucasian (n = 85, 84%), in their 20 s (mean = 24.1 years, s.d. = 4.2), with some college education (mean = 14.8 years, s.d. = 1.4) and moderate recreational drug use (Table 1).
Table 1.
Demographics (N = 101; female = 43, male = 58)
| Mean (s.d.) | |
|---|---|
| BMI | 23.3 (2.8) |
| MDMA use (lifetime) | 13.3 (10.5) |
| Current drug use | |
| Alcohol (drinks/week: N = 92) | 10.6 (8.5) |
| Caffeine (cups/day: N = 77) | 1.9 (1.5) |
| Marijuana (days/month: N = 78) | 10.2 (11.4) |
| Tobacco (cigarettes/day: N = 47) | 5.0 (4.6) |
Participants were instructed to consume normal amounts of caffeine and nicotine, and to fast for 2 h before the session. Participants were instructed to refrain from alcohol and over-the-counter drugs for 24 h before and 12 h after the session. Participants were also instructed to refrain from marijuana for 7 days before and 24 h after the session, and from all other recreational drugs for 48 h before and 24 h after the session. Compliance was verified using breath (Alcosensor III, Intoximeters Inc., St. Louis, MO, USA) and urine tests (ToxCup, Branan Medical Corporation, Irvine, CA, USA). Although these were the minimum requirements for compliance, typical abstention times for recreational drugs were longer. Seventy three percent of participants reported no illicit drug use in the last month, and among those who did report last month use, mean time since last use was 12 days. Women not using hormonal contraceptives were scheduled during the follicular phase (White et al., 2002). Female participants were tested for pregnancy prior to each session.
During the consent procedure, participants were told that the purpose of the study was to investigate individual differences in drug responses, and that they might receive a stimulant (e.g. amphetamine or ecstasy), a sedative (e.g. valium), a hallucinogen (e.g. LSD), a cannabinoid (e.g. marijuana) or a placebo. In Study 2 only, participants were also told they might receive a hormone (e.g. oxytocin). Participants agreed to receive any drug from the list, and all sessions were conducted double blind, with neither the experimenter nor the participant informed about the contents of the capsule in advance. This blind was maintained until the debriefing, at which point participants were told which drugs and doses they had received. All participants provided written informed consent, and all procedures were carried out in accordance with the Declaration of Helsinki and approved by the University of Chicago Institutional Review Board.
Procedure
Sessions were conducted from 9:00 am to 2:00 pm in a comfortable ‘living room’ style laboratory. At arrival, participants provided breath and urine samples for drug and pregnancy testing, and at 9:15 am, they completed baseline measures of subjective and cardiovascular effects. At 9:30 am, participants ingested a capsule containing MDMA powder (0.75 and 1.5 mg/kg, maximum dose of 125 mg, with lactose filler) or placebo (lactose only), encapsulated in 00 opaque capsules by the University of Chicago Hospitals Investigational Pharmacy. When no measures were scheduled, participants relaxed, watched a movie from a selection available or read. At 10:00 am and every 30–60 min thereafter, subjective and cardiovascular effects were assessed. From 10:40 am to 11:30 am, participants completed computerized tasks including picture ratings. At 2:00 pm, participants completed an end of session questionnaire, which asked the participant to identify the drug that they had received that day. Participants were then discharged provided their subjective and cardiovascular measures had returned to baseline.
Subjective mood
To measure subjective mood, we used a Visual Analog Scale (VAS) comprised of 13 adjectives rated on a 1–100 (not at all–extremely) line. This included two ‘entactogenic’ effects, ‘playful’ and ‘loving’ which Bedi et al. (2010) found to be sensitive to the unique effects of MDMA on social emotions, and two typical stimulant-like effects ‘elated’ and ‘stimulated’.
Cardiovascular measures
Blood pressure and heart rate were measured using portable monitors (Life Source, A&D Company, Tokyo, Japan). Heart rate results were similar in dose dependence and time course to blood pressure, so we use mean arterial pressure (MAP; [Systolic BP + 2 × Diastolic BP]/3) as our measure of cardiovascular effects of the drug.
Responses to emotional stimuli
We used pictures from the International Affective Picture System (IAPS; Lang et al., 1999) as emotional stimuli. IAPS pictures are normatively rated on valence (positivity vs negativity) and arousal. Although IAPS pictures are not normatively rated for social relevance, based on previous research (Cacioppo et al., 2009; Gros et al., 2009) we defined ‘social’ pictures as those depicting at least two people or parts of people (e.g. two people talking, a hand pointing a gun at another person), and ‘non-social’ pictures as those depicting no people or parts of people (e.g. a slice of pizza, a car accident with no bodies visible). Thus, there were six subtypes: social/negative, non-social/negative, social/neutral, non-social/neutral, social/ positive and non-social/positive. To avoid adaptation, at each session the participant saw a different set of pictures. We constructed 3 sets of 54 pictures for Study 1, with 9 pictures per subtype per set, and 4 sets of 36 pictures for Study 2, with 6 pictures per subtype per set1. We attempted to match valence and arousal across sets and social vs non-social pictures, using the normative ratings provided with the IAPS pictures (Lang et al., 1999). We counterbalanced picture set with drug dose, such that each picture set was paired approximately the same number of times with each drug dose. Pictures were presented in fixed random order, with no more than two of the same valence in a row. Picture trials consisted of a 3 s pre-picture fixation, a 6 s picture period, then subjective ratings. Participants rated pictures using the evaluative space grid (Larsen et al., 2009), which allows independent 0 (not at all) to 4 (extreme) ratings of positivity and negativity, and a 0 (not at all) to 9 (extreme) rating of arousal.
Drug identifications
At the end of each session, we asked participants to identify the class of drug that they thought they had received that day as ‘1. a stimulant (e.g. amphetamine or ecstasy), 2. A hallucinogen (e.g. LSD), 3. A sedative (e.g. Valium), 4. A cannabinoid (e.g. marijuana), or 5. A placebo’.
Statistical analyses
We used linear mixed effect models (LMEMs) in the lme4 package (v 0.999999-0; Bates et al., 2011) of the R statistical computing environment (v. 2.15.2; R Development Core Team, 2011) as our primary statistical approach.
For subjective and cardiovascular measures, which were taken repeatedly across sessions, we first summarized each session by calculating area under the curve (AUC) relative to the participant’s baseline score for that session. We then used the AUC scores in LMEMs (one each for VAS playful, VAS loving, VAS elated, VAS stimulated and MAP) with dose as an independent (fixed) factor, and participant as a random effect.
For response to emotional stimuli we constructed mean ratings of arousal, positivity and negativity for each picture subtype within each session. We then used these means in LMEMs (one each for arousal, positivity and negativity) using dose, valence of picture and social content of picture as independent (fixed) factors and participant and dose within participant as random effects.
In all analyses we examined any dose effects using orthogonal polynomial contrasts, which constituted our primary analyses of interest. We tested for both linear effects of drug (which would suggest a dose-dependent relationship between dose and outcome), and quadratic effects (which would suggest a U shaped relationship between dose and outcome). If a significant effect of drug was identified, we then used paired t-tests comparing each dose to placebo to further describe the effect and identify the effective doses. We also included participant sex and study (Study 1 vs Study 2) as potential moderators. We additionally examined number of self-reported previous occasions of ecstasy use as a potential continuous moderator, but it did not affect any of the outcomes in this study, and is omitted from the final models for simplicity. Finally, we included a fixed session effect, to account for any order effects. Effect sizes are reported as unstandardized coefficients (B) with standard errors (s.e.). We calculated P-values using the t distribution with n − 1 degrees of freedom (see Wardle and de Wit, 2012 for rationale).
RESULTS
Subjective and cardiovascular drug effects
MDMA (0.75 and 1.5 mg/kg) significantly and dose-dependently increased self-reports of playfulness, lovingness, elated and stimulated, linear drug effect on playfulness B = 2696.9, s.e. = 682.1, t(98) = 3.95, P < 0.001, linear drug effect on loving B = 3311.89, s.e. = 572.75, t(98) = 5.78, P < 0.001, linear drug effect on elated B = 5125.84, s.e. = 301.00, t = 8.22, P < 0.001, linear drug effect on stimulated B = 7088.3, s.e. = 575.9, t = 12.31, P < 0.001. Participants in Study 2 had overall higher loving and elated scores [B = 1000.31, s.e. = 492.5, t(98) = 2.03, P = 0.05, and B = 1196.5, s.e. = 604.9, t(98) = 1.98, P = 0.05, respectively], but effects of MDMA did not differ across studies in the AUC analysis (which accounts for baseline levels of loving and elated). Sex did not moderate the subjective effects of MDMA. MDMA (0.75 and 1.5 mg/kg) also significantly and dose-dependently increased MAP, B = 3240.0, s.e. = 230.3, t(98) = 14.07, P < 0.001. MDMA increased MAP to a greater extent in Study 2 vs Study 1, linear drug effect × study interaction B = −1226.98, s.e. = 459.4, t(98) = 2.67, P = 0.008. Sex did not moderate the effects of MDMA on blood pressure.
Responses to pictures
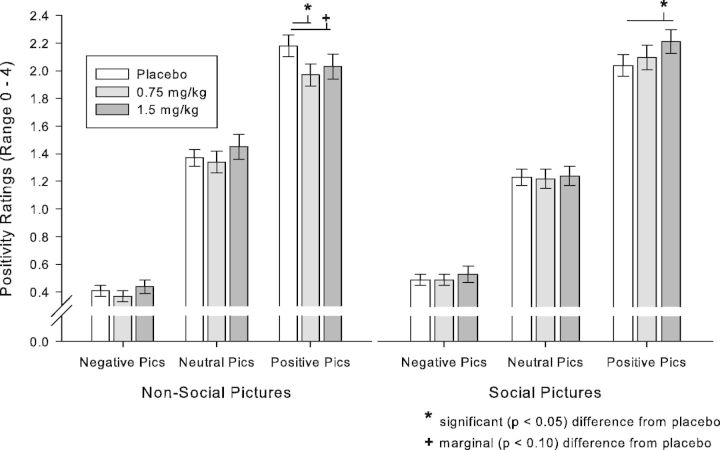
MDMA differentially affected positivity ratings of the pictures, depending on picture sociability and valence, linear drug × linear valence × social content interaction B = 0.35, s.e. = 0.15, t(98) = 2.37, P = 0.02. Follow-up t-tests showed that 1.5 mg/kg MDMA significantly increased the positivity of positive social pictures [t(98) = −2.46, P = 0.02], while 0.75 mg/kg MDMA significantly [t(98) = 2.66, P = 0.009], and 1.5 mg/kg MDMA marginally [t(98) = 1.66, P = 0.10] decreased the positivity of positive non-social pictures. This effect of MDMA on positivity ratings is shown in Figure 1. MDMA did not significantly affect arousal or negativity for any type of picture. There were no differences between studies in arousal, negativity or positivity, or in the effect of drug on those scores, and there were no sex differences.
Fig. 1.
MDMA (1.5 mg/kg) significantly increased ratings of positivity for positive pictures with social content, while 0.75 mg/kg MDMA significantly, and 1.5 mg/kg MDMA marginally decreased ratings of positivity for positive pictures without social content. *P < 0.05, significant, +P = 0.10, marginal.
Drug identifications
A majority of participants correctly identified MDMA as a stimulant. At the placebo dose, 51% identified it as a placebo, 7% identified it as a stimulant and 42% identified it as one of the other drugs listed. At the 0.75 mg/kg dose, 8% identified it as a placebo, 62% identified it as a stimulant and 30% identified it as one of the other drugs listed. At the 1.5 mg/kg dose, 6% identified it as a placebo, 75% identified it as a stimulant and 19% identified it as one of the other drugs listed.
DISCUSSION
MDMA increased positive responses to pleasant pictures with social content, while decreasing positive responses to pleasant pictures without social content. This suggests a ‘socially selective’ effect whereby the drug enhances social rewards while devaluing non-social ones. The MDMA doses used also produced typical changes in both subjective and cardiovascular measures, including increased positive and prosocial feelings, and increased blood pressure, indicates our doses were effective in producing the typically reported subjective effects of MDMA. Unsurprisingly, given the strong and relatively identifiable subjective effects of MDMA, most participants correctly identified it, especially at the high dose, as a stimulant drug.
These findings of increased positive responses to pleasant pictures with social content are consistent with the idea that MDMA increases positive responses to social stimuli. In rats, MDMA increases social behavior, particularly passive physical contact or ‘adjacent lying’ (Morley and McGregor, 2000; Morley et al., 2005; Thompson et al., 2007, 2009; Ramos et al., 2013). The drug also appears to enhance the incentive value of social experiences. MDMA treated rats in social conditions show increased activation in reward-related brain areas compared to either placebo treated rats in social conditions or MDMA treated rats in isolated conditions (Thompson et al., 2009). These findings in rats are consistent with the increased subjective pleasure in positive social stimuli seen in this study. The present findings are also consistent with previous human imaging findings, in which MDMA increased activity in the ventral striatal area when participants viewed happy facial expressions (Bedi et al., 2009). Although subjective ratings were not obtained in the imaging study, the increased activity in a reward-related brain area is consistent with our present findings. Finally, they are somewhat consistent with previous results indicating that MDMA increased reported arousal in response to pictures of individuals in positive social situations (Hysek et al., 2013), although here we saw a change in positivity ratings rather than arousal.
In contrast, there are few precedents for the observed decrease in positive responses to non-social stimuli. Although this is the first study explicitly comparing the effects of MDMA on social and non-social stimuli, studies in laboratory animals suggest that MDMA may enhance the value of rewards regardless of their social nature. For example, MDMA lowers the threshold for the rewarding effects of direct brain stimulation in rats (Hubner et al., 1988; Lin et al., 1997). It is difficult to speculate on the reason for this difference in the absence of more studies comparing the effects of MDMA on social vs non-social rewards in both humans and rats. However, this could represent a species differences, given the greater importance of social contacts to humans, and the concurrent differences in brain organization, and particularly in distribution of oxytocin receptors, between species with different types of social organization (Insel and Shapiro, 1992). It would be particularly interesting for future studies in laboratory animals to examine the effects of MDMA on the incentive value of non-social vs social rewards.
Importantly, although it has been proposed that MDMA may contribute to psychotherapy by decreasing emotional responses to negative material (Johansen and Krebs, 2009), we did not see any evidence here for ‘dampening’ of negative responses, despite having a well-powered within-subject design. MDMA consistently reduces the ability to identify negative emotional expressions in others (Bedi et al., 2010; Hysek et al., 2012a, 2013), but identifying an expression is somewhat different than having an emotional response to that expression. In a previous study, MDMA reduced neural responses to threatening faces in healthy volunteers (Bedi et al., 2009), but subjective responses to the faces were not assessed. MDMA also did not alter arousal in response to pictures of negative social situations (Hysek et al., 2013) in a previous study in healthy volunteers. Thus, the effects of MDMA on emotional responses to negative stimuli are less clear.
The neuropharmacological mechanisms underlying the observed effects of MDMA on responses to social stimuli are not known. However, it may be informative to compare the effects of related psychoactive drugs, specifically similar stimulants, other serotonergic drugs and oxytocin. First, MDMA acts as a stimulant, but we did not see a ‘socially selective’ pattern using a similar design with the classic stimulant d-amphetamine. Instead, we found that amphetamine enhanced positive responses to all types of pictures, regardless of whether they contained social content (Wardle and de Wit, 2012). One difference between these drugs is the comparatively greater serotonergic effect of MDMA compared with a stronger dopaminergic profile for amphetamine (Kankaanpää et al., 1998). Serotonin is also thought to be critical to the social effects of MDMA (Thompson et al., 2007; Hysek et al., 2012b). However, acute serotonin reuptake inhibitor (SSRI) administration, which also enhances serotonin, albeit by a different mechanism (Harmer, 2008), does not produce similar effects. Rather than enhancing responses to positive stimuli only, SSRIs increase emotional responses to both positive and negative stimuli (Harmer, 2008). It is unknown whether SSRIs act more strongly on social stimuli vs non-social stimuli. Finally, it has often been proposed that the oxytocin release triggered by MDMA is responsible for its prosocial effects (McGregor et al., 2008). Interestingly, intranasal oxytocin is the only related drug that has produced a ‘socially selective’ pattern of results in previous studies (Norman et al., 2011). However, rather than affecting positive social stimuli, 20 IU of oxytocin in healthy adults reduced arousal responses to negative social pictures without altering responses to non-social pictures (Norman et al., 2011). In each of these comparisons, it should be noted that there are potential dose equivalency issues. For example, it is unknown whether acute SSRI administration produces an equivalent serotonin increase to MDMA. Nevertheless, MDMA appears to have a unique profile of effects on responses to emotional stimuli when compared to related drugs at typically used doses. Future studies employing precursor depletion or antagonist methods may help determine the exact neuropharmacological basis of these effects, as would studies directly comparing the effects of MDMA with those of other related drugs in the same participants.
This study does have several limitations. First, the relatively homogeneous sample, which excluded DSM-IV disorders and heavier drug users, may limit our ability to detect reinforcing effects of the drug, which may be more evident in ‘at risk’ populations. A second limitation is the artificial nature of our stimuli. Pictures of social interaction may not elicit the same responses as actual interaction opportunities. For example, they do not include aspects such as physical touch, which are thought to be important to the effects of MDMA on social behavior in laboratory animals (Thompson et al., 2009). Further, despite our efforts to match social and non-social stimuli on valence and arousal, social stimuli were rated slightly more extremely (particularly the negative ones) by our sample than non-social ones, perhaps reflecting the inherently more engaging nature of social objects to humans. Finally, our participants, all of whom had some previous MDMA experience, were relatively likely to be able to identify the drug as a stimulant. Thus, it should be considered that the effects observed likely represent a combination of pharmacological and expectancy effects. Use of marginal doses with less pronounced subjective effects may help disentangle these components of MDMA response. However, it is important to note that effects of MDMA in typical recreational use will likely also reflect the combination of pharmacological and expectancy effects.
In conclusion, we observed for the first time that MDMA has a ‘socially selective’ effect in humans, whereby it increases the reward value of positive social stimuli, while decreasing the value of non-social positive stimuli. This phenomenon appears likely to contribute to the unique ‘entactogenic’ effects of MDMA by increasing the comparative value of social contact and closeness with others. These effects may also contribute to the abuse of this unusual stimulant drug, given that MDMA users report that such prosocial effects motivate MDMA use.
Acknowledgments
The authors would like to thank Celina Joos, Charles Frye, Jon Solamillo and Aoibhin Curran for help with data collection, and the University of Chicago Investigational Pharmacy service for preparing the drug capsules. This work was supported by two grants from the National Institutes of Health National Institute on Drug Abuse [grant numbers R01 DA002812, R21 DA026570] to H.d.W., and M.C.W. and M.G.K. were partially supported by a National Institute on Drug Abuse Training Grant [T32 DA007255].
Footnotes
1 Picture sets for Study 1 were the same as in Wardle and de Wit (2012), and can be found in the footnote on p. 143 of that article. IAPS numbers for each subset of Study 2, followed by IAPS normative mean valence (V, 1 = extremely unpleasant − 9 = extremely pleasant) and mean arousal (A, 1 = extremely unarousing − 9 = extremely arousing): Set 1 non-social/negative = 7380, 9911, 9180, 9373, 1280, 7360, V = 3.06, A = 5.29; Set 1 social/negative = 9425, 9903, 6561, 9584, 2694, 9926, V = 3.15, A = 5.24; Set 1 non-social/neutral = 7830, 7190, 7285, 7207, 1935, 7055, V = 5.23, A = 3.77; Set 1 social/neutral = 7620, 2580, 9700, 2595, 2397, 2597, V = 5.28, A = 3.58; Set 1 non-social/positive = 1640, 7352, 5450, 7480, 5700, 5260, V = 6.90, A = 5.26; Set 1 social/positive = 4606, 8467, 3291, 8116, 8420, 2216, V = 7.03, A = 5.37; Set 2 non-social/negative = 9560, 9301, 1274, 1111, 1220, 9008, V = 2.96, A = 5.23; Set 2 social/negative = 9420, 2053, 3216, 6562, 6836, 2718, V = 3.06, A = 5.22; Set 2 non-social/neutral = 7500, 1616, 5661, 9472, 7546, 7054, V = 5.02, A = 3.89; Set 2 social/neutral = 8010, 4605, 2485, 2393, 2606, 2593, V = 5.38, A = 3.81; Set 2 non-social/positive = 1660, 7289, 8500, 7508, 5480, 8502, V = 6.97, A = 5.28; Set 2 social/positive = 8600, 4598, 4601, 4599, 7502, 8496, V = 7.00, A = 5.38; Set 3 non-social/negative = 9300, 9620, 9290, 9471, 9110, 9480, V = 3.05, A = 5.09; Set 3 social/negative = 6212, 6022, 2700, 2455, 9045, 9594, V = 3.00, A = 5.07; Set 3 non-social/neutral = 7100, 5120, 7590, 7183, 7037, 7242, V = 5.01, A = 3.51; Set 3 social/neutral = 2850, 9582, 2695, 9913, 2579, 2396, V = 4.71, A = 4.06; Set 3 non-social/positive = 7284, 7481, 8162, 5849, 8170, 5660, V = 6.87, A = 5.01; Set 3 social/positive = 2358, 2605, 2344, 4624, 2352, 8499, V = 6.88, A = 4.95; Set 4 non-social/negative = 9140, 9320, 9470, 9621, 9010, 9390, V = 3.12, A = 4.90; Set 4 social/negative = 9421, 6838, 2691, 4621, 4635, 2312, V = 3.07, A = 4.97; Set 4 non-social/neutral = 5510, 7233, 7283, 7182, 5395, 1947, V = 5.35, A = 3.67; Set 4 social/neutral = 4000, 7496, 2272, 2435, 2704, 7506, V = 5.21, A = 4.34; Set 4 non-social/positive = 7450, 5991, 7410, 8531, 5600, 7270, V = 7.00, A = 5.00; Set 4 social/positive = 4625, 2594, 4650, 2373, 2345, 4626, V = 6.90, A = 5.04
REFERENCES
- Bates DM, Meachler M, Bolker B. lme4: Linear Mixed-effects Model Using S4 Classes. 2011. [Google Scholar]
- Bedi G, Hyman D, de Wit H. Is ecstasy an “empathogen”? Effects of ±3,4-methylenedioxymethamphetamine on prosocial feelings and identification of emotional states in others. Biological Psychiatry. 2010;68(12):1134–40. doi: 10.1016/j.biopsych.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Phan KL, Angstadt M, de Wit H. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology. 2009;207(1):73–83. doi: 10.1007/s00213-009-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo GL. What Does MDMA Feel Like? Ecstasy: The Complete Guide: A Comprehensive Look at the Risks and Benefits of MDMA. Rochester, VT: Park Street Press; 2001. [Google Scholar]
- Cacioppo JT, Norris CJ, Decety J, Monteleone G, Nusbaum H. In the eye of the beholder: individual differences in perceived social isolation predict regional brain activation to social stimuli. Journal of Cognitive Neuroscience. 2009;21(1):83–92. doi: 10.1162/jocn.2009.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont GJH, Sweep FCGJ, van der Steen R, et al. Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Social Neuroscience. 2009;4(4):359–66. doi: 10.1080/17470910802649470. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Strutured Clinical Interview for DSM-IV Axis I Disorders. New York: Biometrics Research Department; 1996. [Google Scholar]
- Gros DF, Hawk LW, Jr, Moscovitch DA. The psychophysiology of social anxiety: Emotional modulation of the startle reflex during socially-relevant and-irrelevant pictures. International Journal of Psychophysiology. 2009;73:207–11. doi: 10.1016/j.ijpsycho.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Harmer CJ. Serotonin and emotional processing: does it help explain antidepressant drug action? Neuropharmacology. 2008;55(6):1023–8. doi: 10.1016/j.neuropharm.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Harris D, Baggott M, Mendelson J, Mendelson J, Jones R. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2002;162(4):396–405. doi: 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- Hubner C, Bird M, Rassnick S, Kornetsky C. The threshold lowering effects of MDMA (ecstasy) on brain-stimulation reward. Psychopharmacology. 1988;95(1):49–51. doi: 10.1007/BF00212765. [DOI] [PubMed] [Google Scholar]
- Hysek C, Domes G, Liechti M. MDMA enhances “mind reading” of positive emotions and impairs “mind reading” of negative emotions. Psychopharmacology. 2012a;222:293–302. doi: 10.1007/s00213-012-2645-9. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Simmler LD, et al. MDMA enhances emotional empathy and prosocial behavior. Social Cognitive and Affective Neuroscience. in press doi: 10.1093/scan/nst161. doi:10.1093/scan/nst161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Nicola VG, et al. Duloxetine inhibits effects of MDMA (“ecstasy”) in vitro and in humans in a randomized placebo-controlled laboratory study. PLoS One. 2012b;7(5):e36476. doi: 10.1371/journal.pone.0036476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(13):5981–5. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen P, Krebs T. How could MDMA (ecstasy) help anxiety disorders? A neurobiological rationale. Journal of Psychopharmacology. 2009;23(4):389–91. doi: 10.1177/0269881109102787. [DOI] [PubMed] [Google Scholar]
- Kankaanpää A, Meririnne E, Lillsunde P, Seppälä T. The acute effects of amphetamine derivatives on extracellular serotonin and dopamine levels in rat nucleus accumbens. Pharmacology Biochemistry and Behavior. 1998;59(4):1003–9. doi: 10.1016/s0091-3057(97)00527-3. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Gunderson E, Perez A, Haney M, Foltin R, Hart C. A direct comparison of the behavioral and physiological effects of methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2012;219(1):109–22. doi: 10.1007/s00213-011-2383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick MG, Lee R, Wardle MC, Jacob S, de Wit H. Effects of MDMA and intranasal oxytocin on social and emotional processing. Neuropsychopharmacology. in press doi: 10.1038/npp.2014.12. doi:10.1038/npp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: NIMH Center for the study of emotion and attention, University of Florida; 1999. [Google Scholar]
- Larsen JT, Norris CJ, McGraw AP, Hawkley LC, Cacioppo JT. The evaluative space grid: a single-item measure of positivity and negativity. Cognition and Emotion. 2009;23(3):453–80. [Google Scholar]
- Lin H, Jackson D, Atrens D, Christie M, McGregor I. Serotonergic modulation of 3, 4-methylenedioxymethamphetamine (MDMA)-elicited reduction of response rate but not rewarding threshold in accumbal self-stimulation. Brain Research. 1997;744(2):351–7. doi: 10.1016/S0006-8993(96)01210-3. [DOI] [PubMed] [Google Scholar]
- McGregor I, Callaghan P, Hunt G. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? British Journal of Pharmacology. 2008;154(2):358–68. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley K, McGregor I. (±)-3, 4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”) increases social interaction in rats. European Journal of Pharmacology. 2000;408(1):41–9. doi: 10.1016/s0014-2999(00)00749-4. [DOI] [PubMed] [Google Scholar]
- Morley KC, Arnold JC, McGregor IS. Serotonin (1A) receptor involvement in acute 3, 4-methylenedioxymethamphetamine (MDMA) facilitation of social interaction in the rat. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29(5):648–57. doi: 10.1016/j.pnpbp.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, et al. Selective influences of oxytocin on the evaluative processing of social stimuli. Journal of Psychopharmacology. 2011;25(10):1313–9. doi: 10.1177/0269881110367452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Ramos L, Hicks C, Kevin R, et al. Acute prosocial effects of oxytocin and vasopressin when given alone or in combination with 3,4-methylenedioxymethamphetamine in rats: involvement of the V1A receptor. Neuropsychopharmacology. 2013;38:2249–59. doi: 10.1038/npp.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumnall HR, Cole JC, Jerome L. The varieties of ecstatic experience: an exploration of the subjective experiences of ecstasy. Journal of Psychopharmacology. 2006;20(5):670–82. doi: 10.1177/0269881106060764. [DOI] [PubMed] [Google Scholar]
- Tancer ME, Johanson C-E. The subjective effects of MDMA and mCPP in moderate MDMA users. Drug and Alcohol Dependence. 2001;65(1):97–101. doi: 10.1016/s0376-8716(01)00146-6. [DOI] [PubMed] [Google Scholar]
- Ter Bogt TFM, Engels RCME. “Partying” hard: party style, motives for and effects of MDMA use at rave parties. Substance Use and Misuse. 2005;40(9–10):1479–502. doi: 10.1081/JA-200066822. [DOI] [PubMed] [Google Scholar]
- Thompson MR, Callaghan P, Hunt GE, Cornish J, McGregor IS. A role for oxytocin and 5-HT1A receptors in the prosocial effects of 3, 4 methylenedioxymethamphetamine (“ecstasy”) Neuroscience. 2007;146(2):509–14. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Thompson MR, Hunt GE, McGregor IS. Neural correlates of MDMA (“Ecstasy”)-induced social interaction in rats. Social Neuroscience. 2009;4(1):60–72. doi: 10.1080/17470910802045042. [DOI] [PubMed] [Google Scholar]
- Wardle M, de Wit H. Effects of amphetamine on reactivity to emotional stimuli. Psychopharmacology. 2012;220(1):143–53. doi: 10.1007/s00213-011-2498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TL, Justice AJH, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacology, Biochemistry and Behavior. 2002;73(4):729–41. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]