Abstract
1,25-dihydroxyvitamin D3 [1,25(OH)2D3] is the biologically active ligand for the vitamin D receptor (VDR). VDR−/− mice have a hair follicle cycling defect resulting in alopecia. However, mice lacking 25-hydroxyvitamin D3 1α-hydroxylase (CYP27B1−/−) and that have no circulating 1,25(OH)2D3, have normal follicle function. These mouse models indicate that VDR functions independently of 1,25(OH)2D3 in regulating hair follicle cycling. Here, we show that VDR−/− mice rapidly develop chemically-induced skin tumors whereas CYP27B1−/− and wildtype mice do not, indicating that VDR, not the 1,25(OH)2D3 ligand, is essential for protection against skin tumorigenesis. Because the majority of human skin cancer results from exposure to ultraviolet light (UV), the susceptibility of VDR−/− mice to this carcinogen was also evaluated. VDR−/− mice developed UV-induced tumors more rapidly and with greater penetrance than VDR+/+ mice. P53 protein levels were upregulated at similar rates in UV-treated keratinocytes of VDR−/− and VDR+/+ mice. However, rates of thymine dimer repair and UV-induced apoptosis were significantly lower in VDR−/− epidermis compared to wildtype. UV-induced epidermal thickening was also attenuated in the VDR−/− skin, indicating that VDR plays a critical role in the repair and removal of severely damaged keratinocytes and adaptation of the skin to chronic UV exposure.
Introduction
Approximately one million people are diagnosed with non-melanoma skin cancer each year, which accounts for half of all diagnosed tumors. The incidence of non-melanoma skin cancer is increasing and, while rarely fatal, poses significant health care challenges (Albert and Weinstock, 2003). The vitamin D endocrine system protects against multiple forms of carcinogenesis, but its significance in skin tumorigenesis is only beginning to be appreciated (Bikle, 2004; Zinser et al., 2002). In addition, the molecular mechanisms which govern the activity of the vitamin D endocrine system in the skin remain unclear.
The biologically active form of vitamin D is 1,25-dihydroxyvitamin D3 [1,25(OH)2D3]. This hormone binds the vitamin D receptor (VDR) and promotes association with its heterodimeric partner, the retinoid X receptor (RXR) (Cheskis and Freedman, 1994; Kliewer et al., 1992). The VDR-RXR heterodimer regulates transcription by binding to specific response elements in the promoter regions of target genes (Kerner et al., 1989). Synthesis of 1,25(OH)2D3 is highly regulated and involves several tissues. Vitamin D prohormone is produced primarily in the skin, where 7–dehydrocholesterol is converted to vitamin D by ultraviolet light. Bioactivation of vitamin D occurs via sequential hydroxylation at C-25 in the liver and at C-1 in the kidney (Holick, 2007). The renal 25-hydroxyvitamin D 1α-hydroxylase (1αOHase) enzyme is required to generate circulating levels of 1,25(OH)2D3 in humans and other mammals (Dardenne et al., 2001; Gray et al., 1972; Panda et al., 2001; St-Arnaud et al., 1997).
Knockout mouse models have provided important physiological insights into the vitamin D endocrine system. Four independent strains of mice have been generated in which the VDR has been inactivated (i.e., receptor knockout mice) (Erben et al., 2002; Li et al., 1997; Van Cromphaut et al., 2001; Yoshizawa et al., 1997). Two additional strains of mice were engineered to target the CYP27B1 gene, which encodes the renal 1αOHase enzyme (i.e., ligand knockout mice) (Dardenne et al., 2001; Panda et al., 2001). CYP27B1−/− mice are devoid of circulating 1,25(OH)2D3. As expected, the receptor and ligand knockout mice have similar phenotypes relating to mineral homeostasis, manifesting as hypocalcemia, hyperparathyroidism, and skeletal defects such as rickets. Interestingly, this phenotype is corrected by feeding the mice a diet high in calcium, phosphorus, and lactose, suggesting that the significance of the vitamin D endocrine system in the skeletal system is secondary to its role in maintaining adequate absorption of dietary calcium by the intestine (Dardenne et al., 2003; Li et al., 1998).
Importantly, the phenotypes of vitamin D receptor and ligand knockout mice (VDR−/− and CYP27B1−/−, respectively) differ greatly in the skin. VDR−/− mice develop an initial postnatal coat of hair, but subsequent hair follicle cycling fails leading to alopecia (Li et al., 1998). Hair follicles degenerate to form dermal cysts and utriculi (Li et al., 1998), and epidermal keratinocytes proliferate at approximately twice the normal rate (Zinser et al., 2002). This phenotype is not corrected by a high calcium diet, indicating that it is a direct consequence of VDR inactivation (Li et al., 1998). In contrast, CYP27B1−/− mice have normal hair follicle cycling, a normal keratinocyte proliferative index, and they display only minor differences in the expression of skin differentiation marker proteins (Bikle et al., 2004). The lack of phenotype in CYP27B1−/− skin compared with the VDR−/− skin strongly suggests that VDR regulates transcription in keratinocytes independently of its 1,25(OH)2D3 ligand. Indeed, recent data demonstrating a 1,25(OH)2D3-independent transcriptional activity of the VDR selectively in human and mouse keratinocytes provides key support for this concept (Ellison et al., 2007).
Genetic inactivation of VDR also increases the susceptibility of mice to chemically-induced skin tumorigenesis. VDR−/− mice rapidly develop skin tumors following oral administration of dimethylbenzathracene (DMBA), whereas wildtype mice are resistant to tumor development (Zinser et al., 2002). Pharmacological doses of 1,25(OH)2D3 have been shown to increase latency and reduce multiplicity of chemically-induced skin tumor formation in mice (Chida et al., 1985). However, it is presently unknown whether CYP27B1−/− mice show altered susceptibility to DMBA-induced skin carcinogenesis. While chemical carcinogenesis protocols provide a convenient way to study tumorigenesis in rodents over a relatively short time period, the major environmental risk factor in human skin carcinogenesis is exposure to ultraviolet light (Albert and Weinstock, 2003). Ultraviolet light, particularly UVB with wavelengths between 290 and 320 nm, causes DNA damage including cyclobutane pyrimidine dimers (Setlow and Setlow, 1962) and (6–4) photoproducts (Johns et al., 1964). Failure to adequately repair or remove a damaged cell may result in a mutated cell with the potential ability to clonally expand and become tumorigenic. Therefore, it is critical to evaluate whether VDR also plays a protective role against UV-induced tumorigenesis, and if so, to evaluate the mechanisms underlying this protective activity of VDR.
In this report, we provide evidence that VDR, but not its 1,25(OH)2D3 ligand, is required for in vivo resistance to chemically-induced skin tumorigenesis in mice. Furthermore, we show that VDR is essential for protection against UV-induced skin carcinogenesis. This striking difference in UV-induced tumor susceptibility may be due, in part, to a significant reduction in thymine dimer repair and keratinocyte apoptosis in VDR−/− skin following acute exposure to UV light. In addition, VDR−/− skin shows reduced epidermal thickening in response to repeated UV exposure, indicating that VDR−/− skin does not mount an appropriate protective response to UV, leaving basal keratinocytes more exposed to penetrating UV rays.
Results
Protection against chemically-induced skin tumorigenesis is VDR-dependent and 1αOHase-independent in mice
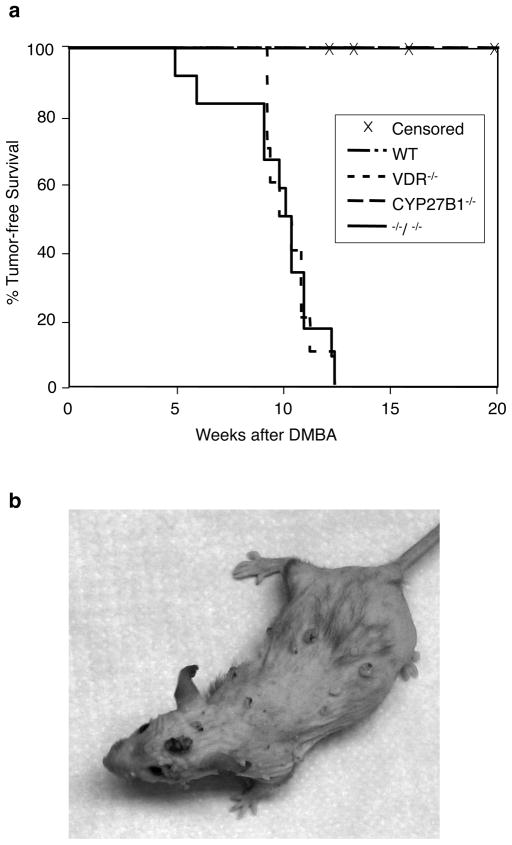
VDR−/− mice are more susceptible to chemically-induced skin carcinogenesis compared to wildtype mice (Zinser et al., 2002), suggesting a genoprotective role for VDR in skin. However, the significance of the 1αOHase enzyme and thus, the 1,25(OH)2D3 ligand, in preventing skin tumor development is not known. Therefore, we compared skin tumor development in wildtype, VDR+/−, VDR−/−, CYP27B1+/−, and CYP27B1−/− mice that were administered two oral doses of the chemical carcinogen DMBA using a dosing regimen described previously for VDR−/− mice (Zinser et al., 2002). Mice were monitored for skin tumor development weekly. All VDR−/− mice developed skin tumors within 10–12 weeks after receiving DMBA (Figure 1a). A representative VDR−/− mouse bearing multiple tumors is shown in Figure 1b. Skin tumors were classified as sebaceous hyperplasias (15%), adenomas (70%), or carcinomas (5%) depending on the ratio of well-differentiated sebocytes to epithelial keratinocytes. These tumors, like many of the tumors previously reported in VDR−/− skin (Zinser et al., 2002), likely originated from the folliculosebaceous unit. In addition, 10% of tumors were classified as warts because of signs of viral change, such as koilocytic change, church-spire parakeratin, and dilated blood vessels in the stroma. In striking contrast to the VDR−/− mice, no skin tumors formed in wildtype or CYP27B1−/− mice during the eight months of the study. In addition, no tumors were observed in the mice heterozygous for the VDR or CYP27B1 genes. Since CYP27B1−/− mice lack detectable levels of circulating 1,25(OH)2D3 (Dardenne et al., 2001; Panda et al., 2001), these data indicate that VDR, but not its 1,25(OH)2D3 ligand is required for protection from DMBA-induced tumorigenesis. To test whether loss of 1,25(OH)2D3 can further sensitize VDR−/− mice to tumorigenesis, we generated mice deficient in both VDR and CYP27B1 (double knockout mice) and administered DMBA as described above. Average time to tumor development was virtually identical in VDR−/− and VDR−/− CYP27B1−/− mice [10.6 (±1.1) and 10.7 (±1.2) weeks after DMBA exposure, respectively]. These data indicate that loss of 1,25(OH)2D3 through genetic deletion of the 1αOHase enzyme does not increase susceptibility of mice to tumorigenesis whether or not they express the VDR.
Figure 1. Protection against chemically-induced skin tumorigenesis is VDR-dependent and 1αOHase independent in mice.
VDR+/+ (n=11), VDR+/− (n=6), and VDR−/− mice (n=10), CYP27B1+/+ (n=10), CYP27B1 +/− (n=11), and CYP27B1−/− mice (n=10), and VDR−/− CYP27B1−/− mice (n=13) were given two doses of DMBA by oral gavage at 5 and 6 weeks of age. Mice were monitored weekly by palpation and visual inspection for tumor development. a) Probability of tumor development was analyzed according to Kaplan-Meier. VDR+/+ and CYP27B1+/+ wildtype mice were grouped for statistical analysis. Mice were censored if they died or if they required euthanasia due to extreme sickness before developing a skin tumor. b) A representative image of a VDR−/− mouse bearing several DMBA-induced skin tumors.
VDR protects mice from UV-induced skin tumorigenesis
Ultraviolet light causes the majority of human skin cancer, and because it both initiates and promotes tumorigenesis it is considered to be a complete carcinogen. To determine whether VDR protects the skin against UV-induced skin cancer, VDR−/− and wildtype mice were chronically exposed to UVB three times per week for 33 weeks, and monitored weekly for tumor development for 45 weeks. CYP27B1−/− mice were not included in this study because they lack an overt skin or hair phenotype and because they are completely resistant to the aggressive, rapid-onset, chemically-induced carcinogenesis paradigm.
VDR+/+ and VDR−/− mice were shaved and depilated as needed to maintain bare skin during the course of thrice-weekly UV treatments. VDR−/− mice began to develop tumors after approximately 18 weeks of UV exposure, and half of the VDR−/− mice had developed at least one tumor by 30 weeks into the study (Figure 2a). All of the VDR−/− mice that completed the 45-week study developed at least one tumor. In contrast, only three wildtype mice developed tumors by this time, and these tumors appeared much later than tumors in the VDR−/− mice (36–44 weeks after the start of UV). All but one of the VDR−/− tumors were classified as squamous in origin (Table 1). Sixty percent of VDR−/− tumors were squamous papillomas with various degrees of atypia and dysplasia. Benign papillomas (Figure 2d) typically had a larger amount of keratinized material than more atypical papillomas, indicating a greater degree of cellular differentiation. The moderate papillomas contained parakeratin, indicative of a defect in the differentiation process (Figure 2f). Moderate papillomas also contained more PCNA positive cells than benign papillomas (Figure 2g and 2e, respectively), which indicates a higher proliferative index. Nearly 20% of VDR−/− tumors were malignant squamous cell carcinomas in situ, showing a high degree of atypia, many mitotic figures, and disordered skin structure. An additional 20% of VDR−/− tumors were invasive squamous cell carcinomas containing a large percentage of proliferating cells (Figure 2i), significant atypia and disordered structure, and invasion of the surrounding tissue (Figure 2h). One invasive SCC occurred in a wildtype mouse, but the remainder of the VDR+/+ tumors were a dermal hyperproliferation with characteristics consistent with atypical fibroxanthoma. These tumors had a high proliferative index, as shown by the large number of PCNA expressing cells (Figure 2k), a disordered growth pattern, and in some cases contained black pigmentation (Figure 2j). One VDR−/− mouse developed this type of tumor, suggesting that AFX is not unique to the wildtype mice.
Figure 2. VDR protects mice from UV-induced skin tumorigenesis.
a) VDR+/+ (n=23) and VDR−/− (n=22) were shaved and treated with a depilatory lotion, then exposed to UV thrice weekly for 33 weeks as described in Materials and Methods. Mice were monitored weekly by palpation and visual inspection for tumor development. Probability of tumor development was analyzed according to Kaplan-Meier. Mice were censored if they died or if they required euthanasia due to extreme sickness before developing a skin tumor. b) Representative image of thickened but non-tumor bearing skin from a wildtype mouse after 26 weeks of regular UV exposure. Scale bar = 20 μm. c) Representative image of thickened but non-tumor bearing skin from a VDR−/− mouse after 29 weeks of regular UV exposure. Scale bar = 20 μm. Histological appearance of a representative benign papilloma (d and e), moderate papilloma (f and g), an invasive squamous cell carcinoma (SCC; h and i), and an atypical fibroxanthoma (AFX; j and k). Images were taken of hematoxylin and eosin stained paraffin-embedded sections (d, f, h, and j), and of sections immunohistochemically stained with an antibody against proliferating cell nuclear antigen (PCNA) to indicate proliferation (e, g, i, and k). PCNA positive cells appear brown. Scale bar (d–k) = 20 μm.
Table 1.
Histologic classification of tumors induced by UV irradiation in wildtype and VDR−/− mice
| Papilloma | ||||||||
|---|---|---|---|---|---|---|---|---|
| Benign | Mild | Moderate | Severe | SCC in situ | Inv SCC | AFX | Total | |
| WT | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 4 |
| KO | 4 | 5 | 6 | 1 | 5 | 5 | 1 | 27 |
UV induces p53 protein levels equally in wildtype and VDR−/− keratinocytes
Tumorigenesis occurs after normal cells have sustained multiple insults and have been subject to growth promotion (Matsumura and Ananthaswamy, 2002). Therefore, long-term susceptibility to tumorigenesis may be determined by the ability of the skin to deal with acute UV-induced damage. DNA damage causes stabilization and upregulation of the p53 tumor suppressor protein (Maltzman and Czyzyk, 1984). Phosphorylation increases p53 protein stability and transcriptional activity (Siliciano et al., 1997). The active p53 either halts the cell cycle so that cellular damage can be repaired, or initiates apoptosis. To determine if VDR−/− mice have a defect in the p53 response, primary mouse keratinocytes were isolated from wildtype and VDR−/− mice. Cells were exposed to UVB and protein was harvested at various time points. Western blot analysis indicated that p53 was upregulated as early as 4 h after UV exposure, protein levels peaked at 8 h, and subsequently declined at 24 and 48 h (Figure 3). Notably, p53 levels were similar in VDR+/+ and VDR−/− keratinocytes. We also observed UV-induced increases in the levels of phosphorylated serine15 p53 that were comparable in wildtype and VDR−/− keratinocytes at each time point after UV exposure (Figure 3), suggesting that this important, early response to UV in keratinocytes is not dramatically altered in VDR−/− keratinocytes.
Figure 3. UV induces p53 protein levels and p53 phosphorylation equally in wildtype and VDR−/− keratinocytes.
Keratinocytes were isolated from newborn wildtype and VDR−/− mice, allowed to grow for 24h, and irradiated with 50 mJ/cm2 of UVB through a thin film of PBS. Media were replaced and protein was harvested at the indicated time points. Protein expression was analyzed by western blot.
Incomplete repair of thymine dimers in VDR−/− epidermis compared to wildtype
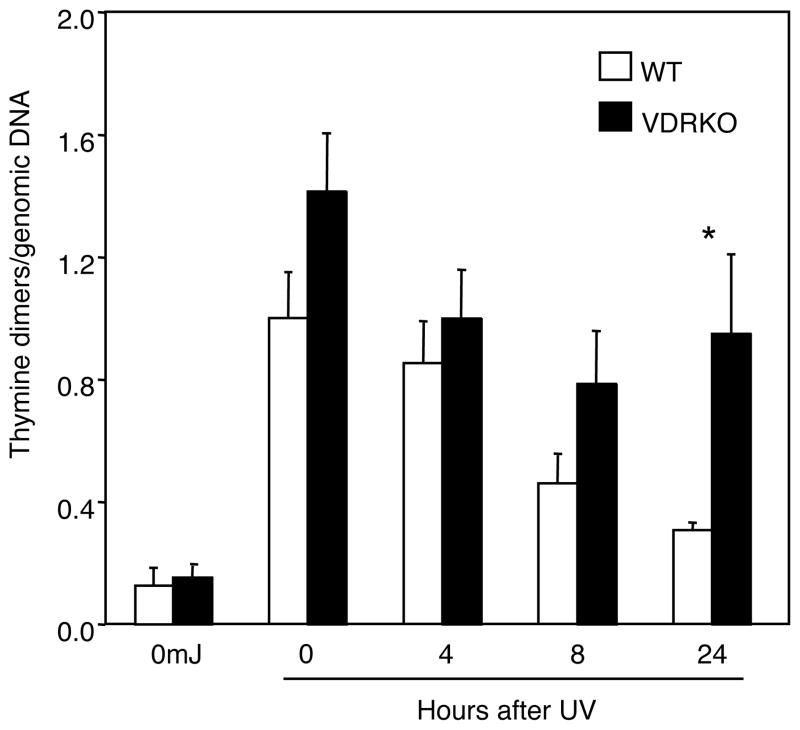
p53 upregulation institutes cell cycle arrest which allows time for DNA repair. If UV-induced thymine dimers are not repaired before a cell replicates this damage transforms into permanent mutations in the genetic code. To address an in vivo role of VDR in thymine dimer repair, wildtype and VDR−/− newborn mice were irradiated with UV and skin was harvested at the indicated time points. Genomic DNA was isolated from the epidermis and thymine dimers were quantified by southwestern slot blotting using an antibody recognizing this form of DNA damage. As expected, untreated epidermis lacked detectable thymine dimers (Figure 4). In wildtype epidermal samples, thymine dimer levels were highest immediately following irradiation and gradually decreased over time, nearly returning to baseline levels at 24 h after irradiation. In contrast, thymine dimer levels were higher in VDR−/− epidermis compared to wildtype at each time point, and the amount of thymine dimers remained significantly higher in VDR−/− epidermis than wildtype at 24 h after UV exposure (Figure 4), indicating that keratinocyte DNA repair pathways are compromised by VDR ablation.
Figure 4. Incomplete repair of thymine dimers in VDR−/− epidermis compared to wildtype.
Newborn mice were exposed to 120mJ/cm2 of UV and skin was collected at the indicated time points. Epidermal genomic DNA was isolated and thymine dimers were measured by southwestern slot blot. Thymine dimers were normalized to total genomic DNA with a radiolabeled mouse genomic DNA probe. Thymine dimers were measured in the epidermis of 2–3 individual mice for each time point, except for the unirradiated control. The data shown represents the mean from three independent measurements of thymine dimers relative to total genomic DNA from the epidermal samples. Error bars = SEM. *P<0.04
UVB-induced growth arrest is compromised in VDR−/− keratinocytes in vitro
Growth arrest is a critical, immediate response of keratinocytes to UV exposure so that effective repair of the damage may occur prior to DNA replication (Petrocelli et al., 1996). Failure to provide this important window for repair leads to propagation of the damage and cancer. Therefore, we tested whether VDR−/− keratinocytes displayed altered proliferative responses to UV exposure in vitro. Wildtype and VDR−/− keratinocytes were irradiated once with various doses of UVB and proliferation was monitored for three days using the MTT assay. Confirming previous reports (Sakai and Demay, 2000), non-irradiated wildtype and VDR−/− keratinocytes grew at similar rates in vitro (Figure 5a and 5b). Exposure of VDR+/+ keratinocytes to a 25 mJ/cm2 dose of UVB attenuated their growth rate at 24 and 48 h following a single UV exposure. In contrast, VDR−/− keratinocytes were virtually unaffected by this dose of UVB. A higher level of UVB exposure (50 mJ/cm2) effectively blocked wildtype keratinocyte growth at 24 and 48 h (Figure 5a). The VDR−/− keratinocytes continued to grow at this higher UV dose, albeit more slowly (Figure 5b). These data indicate that VDR−/− keratinocytes are defective in UV-induced growth arrest compared to their wildtype counterparts.
Figure 5. UVB-induced growth arrest is compromised in VDR−/− keratinocytes in vitro.
Primary keratinocytes were isolated from newborn wildtype and VDR−/− mice, allowed to grow for 24h, and irradiated with the indicated amount of UVB through a thin film of PBS. Media were replaced and cell proliferation was measured at the indicated time points using the MTT assay as described in Materials and Methods. Cell growth of a) wildtype and b) VDR−/− keratinocytes is plotted over time.
In vivo apoptotic defects in VDR−/− keratinocytes following a single dose of UV
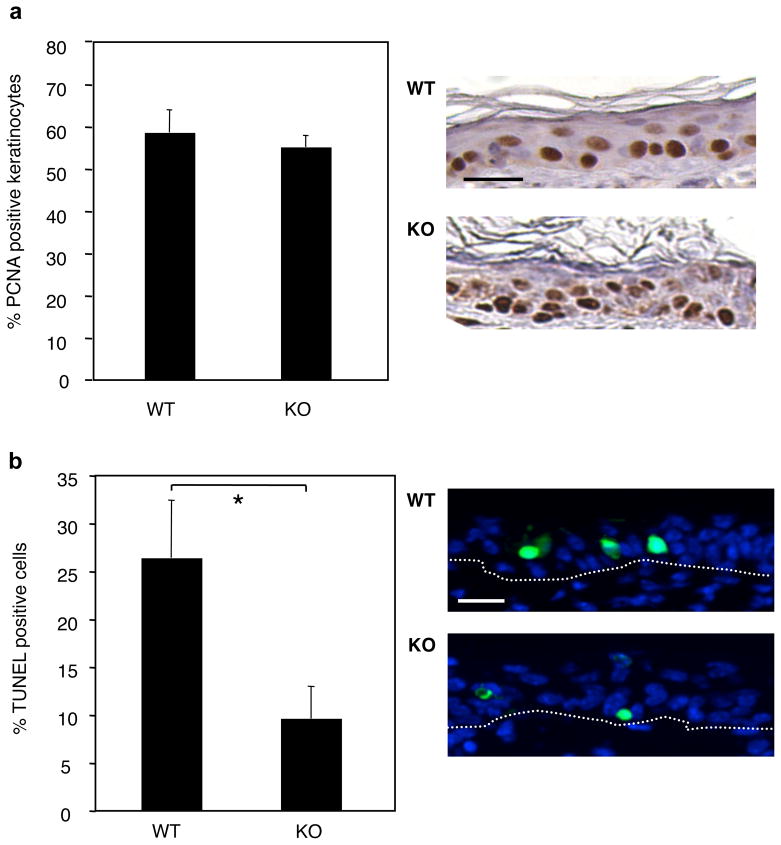
In addition to growth arrest and repair, keratinocytes undergo apoptosis if cellular damage is too severe to be repaired. Figure 6 examines proliferative and apoptotic responses of keratinocytes in vivo following exposure of VDR+/+ and VDR−/− mice to a single dose of UVB. Shaved and depilated dorsal skin of wildtype and VDR−/− mice were exposed to one 120 mJ/cm2 dose of UV and skin was harvested 24 h later. Proliferative keratinocytes were visualized using PCNA staining. Apoptotic cells were fluorescently labeled by the TUNEL method and nuclei were counterstained with DAPI. Differences in proliferation were not apparent in vivo, perhaps due to the nature of this single, high dose of UVB (Fig. 6a). However, VDR−/− skin contained less than half the number of apoptotic keratinocytes compared to wildtype skin (Figure 6b). This finding suggests that tumor susceptibility in VDR−/− mice may also derive from a decreased ability to eliminate damaged cells from the skin due to defective keratinocyte apoptosis.
Figure 6. In vivo apoptotic defects in VDR−/− keratinocytes following a single dose of UV.
Wildtype (WT, n=4) and VDR−/− (KO, n=4) mice were exposed to 120mJ/cm2 of UV and a biopsy was collected 24 h later. a) Skin samples were stained for PCNA expression and counterstained with hematoxylin (positive cells are brown). PCNA positive keratinocytes in WT and KO skin were counted as a percentage of total number of keratinocytes. Error bars=SEM. Representative sections are shown on the right. Scale bar = 20 μm. b) Apoptotic cells were labeled by the TUNEL method and nuclei were counterstained with DAPI. Apoptosis rates were calculated as number of TUNEL positive cells as a percentage of total epidermal keratinocytes from multiple fields of TUNEL labeled paraffin-embedded skin sections, viewed at 100× magnification. Error bars=SD. Differences were analyzed by the two-tailed Student’s t test, * p = 0.005. Representative fields of TUNEL-labeled sections are on the right, showing TUNEL positive cells in green and nuclei in blue. Scale bar = 20 μm. Dotted lines indicate the epidermal/dermal junction.
Defective epidermal thickening of VDR−/− skin in response to chronic UV exposure
Following UV-induced cell cycle arrest and repair, keratinocytes enter a hyperproliferative stage in order to replace cell loss due to apoptosis (i.e., sunburn cells) and to increase the number of epidermal cell layers to protect basal cells against additional UV exposure (de Winter et al., 2001). Differences in the ability to adapt to chronic UVB exposure may explain variation in long-term susceptibility to UV-induced tumorigenesis. Wildtype and VDR−/− mice (5–7 weeks old) were exposed to UVB six times over a period of two weeks. Skin samples were obtained 24 h after the final dose, stained for PCNA expression to indicate proliferating cells, and epidermal thickness was measured. Representative sections of VDR+/+ and VDR−/− skin after the final dose of UV are shown in Figure 7b. Virtually all keratinocytes in the basal layer were proliferating in both the VDR+/+ and VDR−/− skin, but the wildtype epidermis contained an abundance of keratinocytes proliferating above the basal layer. This effect required repeated doses since, as previously shown, PCNA positive keratinocytes in the epidermal layers were similar in wildtype and VDR−/− epidermis following a single, acute exposure to UVB (Figure 6a). Following more chronic exposure to six doses of UV, the PCNA-positive VDR+/+ keratinocytes were at a level of 72%, while the PCNA-positive VDR−/− keratinocytes were at a significantly lower level of 56% (p< 0.025, in Figure 7a). These differences in the proliferative index correlated to differences in epidermal thickness between wildtype and VDR−/− skin. Average epidermal thickness was similar in wildtype and VDR−/− mice that were not exposed to UV (14.7 ± 3.6μm and 17.4 ± 5.4 μm, respectively) (Figure 7c). Differences in epidermal thickness of 24 h VDR+/+ and VDR−/− skin were not apparent following a single, acute exposure 24 h post UV. However, following six doses of UV the average epidermal thickness of wildtype mice increased 8–fold compared to unexposed mice, whereas average thickness in VDR−/− mice increased less than 3-fold. This indicates a significant difference in UV-induced epidermal thickening in wildtype mice compared to VDR−/− mice. The reduced levels of keratinocyte proliferation and apoptosis in VDR−/− skin in response to UV exposure suggests that these mice do not mount a sufficient defense against the damage caused by UV radiation, which compounded over a chronic series of UV exposures leaves them more susceptible to UV-induced skin tumorigenesis.
Figure 7. Defective epidermal thickening of VDR−/− skin in response to chronic UV exposure.
Wildtype (WT, n=4) and VDR−/− (KO, n=4) mice were exposed to six doses of UV (120mJ/cm2) during a 2-week period. Skin samples were collected and fixed 24 h after the final dose. a) Skin samples were stained for PCNA expression and counterstained with hematoxylin. PCNA positive keratinocytes in WT and KO skin were counted as a percentage of total number of keratinocytes. Wildtype epidermis was significantly more proliferative than VDR−/− epidermis following 6 exposures of UV, * p < 0.025. Error bars=SEM. Differences were analyzed by the two-tailed Student’s t test. b) Representative sections of wildtype and VDR−/− skin, stained for PCNA expression. Brackets indicate epidermal thickness. Scale bar = 40 μm.
c) Epidermal thickness was calculated from 20–25 measurements from multiple fields of paraffin-embedded skin sections, viewed at 100× magnification. Error bars=SD. Differences in thickness were analyzed by the two-tailed Student’s t test. Wildtype epidermis was significantly thicker than VDR−/− epidermis following 6 exposures of UV, * p < 0.015.
Discussion
Arguably, one of the most remarkable observations derived from the vitamin D endocrine system knockout mouse models centers on the lack of a profound skin and hair phenotype in the CYP27B1−/− mice compared to the defects observed in VDR−/− skin. These mouse models are consistent with rare genetic diseases resulting from inactivation of VDR and CYP27B1 in humans. That is, many patients with hereditary vitamin D resistant rickets (resulting from inactivating mutations of VDR) present with alopecia, while patients with pseudo vitamin D deficiency rickets (resulting from inactivation of the 1αOHase enzyme) do not (Hughes et al., 1988; St-Arnaud et al., 1997). Indeed, classic dietary deficiency studies over the past century support these more modern genetic approaches, in that vitamin D deficiency does not significantly impact skin or hair. These and other observations led to the hypothesis that VDR may act independently of 1,25(OH)2D3 in keratinocytes. One of the most significant aspects of the present study is that the ligand-independence of VDR function in the skin can now be extended to a second global process, namely, protection against chemically-induced skin tumorigenesis. The CYP27B1−/− mice are remarkably resistant to chemically-induced skin tumor development using a paradigm in which all of the VDR−/− mice developed multiple tumors per animal. Clearly, lack of systemic 1,25(OH)2D3 has no impact on the sensitivity of mouse skin to chemically-induced tumorigenesis, while lack of VDR does. Mechanisms involved in this difference in cancer susceptibility remain to be defined.
Several possibilities exist to explain the apparent uncoupling of VDR and 1,25(OH)2D3 in both hair follicle cycling and in protection against DMBA-induced skin tumorigenesis. While systemic 1,25(OH)2D3 is undetectable in CYP27B1−/− mice (Dardenne et al., 2001; Panda et al., 2001), it is possible that local production of 1,25(OH)2D3 occurs in the CYP27B1−/− keratinocytes. Keratinocytes synthesize 1,25(OH)2D3 (Bikle et al., 1986) where it may act in an autocrine/paracrine manner to control keratinocyte differentiation and function. However, keratinocyte production of 1,25(OH)2D3 is thought to be mediated via the CYP27B1 gene product (Fu et al., 1997). Alternatively, a CYP27B1-independent pathway may catalyze local production of 1,25(OH)2D3. However, in vivo and in vitro experiments argue against CYP27B1-independent synthesis of 1,25(OH)2D3 in the skin. For example, VDR (L233S), a mutated form of VDR which is incapable of binding 1,25(OH)2D3, rescues the VDR−/− defect in hair follicle cycling when expressed in basal keratinocytes of the VDR−/− mouse (Skorija et al., 2005). The activity of VDR L233S supports the possibility that VDR activity in the skin is regulated by a previously unreported skin-selective ligand, or potentially no ligand at all. We recently showed in vitro evidence for a keratinocyte-selective, 1,25(OH)2D3-independent transactivation by VDR that supports this idea (Ellison et al., 2007). VDR activity is regulated by post-translational modifications such as phosphorylation. For example, okadaic acid, a protein phosphatase inhibitor, increases both 1,25(OH)2D3-dependent and independent activation of VDR by promoting interaction between VDR and the VDR-interacting protein 205 coactivators (Barletta et al., 2002). Thus, it is possible that keratinocyte growth factor pathways may impinge on VDR to activate this nuclear receptor in keratinocytes via a 1,25(OH)2D3-independent mechanism. Additional studies in this vein may produce novel strategies of targeting VDR pharmacologically in the skin.
Another key finding of these studies is that VDR protects against skin tumorigenesis induced by UV irradiation, the cause of skin cancer most relevant to human disease. VDR−/− mice exposed chronically to UV light developed tumors with a mean latency much shorter than wildtype controls (Figure 2a). The requirement for the 1,25(OH)2D3 ligand in protecting against UV-induced tumorigenesis remains an open question. Since DMBA and UV radiation induce tumorigenesis by different mechanisms, it is possible that 1,25(OH)2D3 may play a protective role here. Indeed, recent studies suggest that pharmacological application of vitamin D compounds may reduce cell death and enhance DNA repair in mouse skin following UV exposure (Dixon et al., 2007). In that study, analogs that are selective for a nongenomic, VDR-independent signaling pathway were effective and this raised the intriguing possibility that the 1,25(OH)2D3 ligand could protect against UV-induced skin tumorigenesis in a receptor-independent manner. This putative nongenomic pathway represents another paradigm in which the actions of VDR and its 1,25(OH)2D3 ligand may be uncoupled in skin physiology.
Early responses of keratinocytes to UV exposure and damage occur through p53-dependent and p53-independent pathways (Lee et al., 1997; Maltzman and Czyzyk, 1984; Melino et al., 2002). Data presented here indicate that VDR expression is not critical for UV-induced upregulation of p53 protein levels and activity in keratinocytes. Both total and phosphorylated p53 were upregulated to the same extent in VDR−/− and wildtype keratinocyte cultures (Figure 3). The intact p53 response pathway in VDR–/– keratinocytes predicts that DNA repair would be equal in wildtype and VDR−/− epidermis. However, thymine dimer repair was markedly attenuated in newborn VDR−/− epidermis as evidenced by the significantly higher levels of thymine dimers that remained in VDR−/− epidermis compared to wildtype controls 24 h following a single UV dose. While it will be important to extend these studies to older animals, to our knowledge it has previously been unreported that the VDR−/− mouse has defective DNA repair pathways. Cumulatively, these data support a role for VDR in regulating one or more p53-independent mechanisms involved in UV-induced DNA damage repair.
UV causes a multi-step cascade of events in exposed cells (Matsumura and Ananthaswamy, 2002). Initially, cell growth is arrested so that DNA damage may be repaired. Those keratinocytes that harbor extensive cellular damage beyond that which can be repaired undergo apoptosis. After an initial period of cell cycle arrest, proliferation ensues in order to replace cells lost to apoptosis. As a result of hyperproliferation, the epidermis becomes thickened so that upper layers of the epidermis protect the basal layer of keratinocytes from future exposure to UV. Our data indicate that VDR may play important roles in each of these cellular responses to UV. For example, acute UV exposure reduced the growth of wildtype keratinocytes, whereas VDR−/− keratinocytes remained more proliferative (Figure 5a and 5b). These data indicate that VDR is important for growth arrest immediately following UV exposure. Fewer apoptotic cells were detected in vivo in VDR−/− skin compared to VDR+/+ skin following a single dose of UVB. This suggests that VDR also plays a role in detecting the need for apoptosis or is involved in executing the cell death process itself (Figure 6b). In either case, it is likely that damaged keratinocytes remain in VDR−/− skin following UV exposure. Following repeated UV exposures over a two-week period, wildtype keratinocytes in the epidermis were more proliferative and epidermal thickness increased to a greater extent in wildtype skin compared to VDR−/− skin (Figure 7). These data suggest that VDR−/− mice are more sensitive to skin tumorigenesis induced by chronic UV treatment because they are unable to mount an effective thickening of their epidermal layer compared to the wildtype mice. Indeed, previous studies have illustrated an important role for the thickening of the epidermis in protecting basal keratinocytes from subsequent DNA damage caused by UV (de Winter et al., 2001). Finally, the enhanced basal proliferation of VDR−/− keratinocytes previously observed in vivo (Zinser et al., 2002), and the reduced proliferation and epidermal thickening of VDR−/− keratinocytes in response to chronic UV observed in these studies indicate that VDR exerts opposing actions on these two distinct proliferative pathways. The identification of VDR-regulated genes involved in these pathways will be the next important goal. To this end, the VDR−/− keratinocyte cell system will prove extremely valuable.
Material and Methods
Animal maintenance
All studies were approved by the Institutional Animal Care and Use Committee at Case Western Reserve University. Mice lacking the CYP27B1 gene (CYP27B1−/−) (Dardenne et al., 2001) and VDR gene (VDR−/−) (Li et al., 1997) were generously provided by R. St-Arnaud (Shriners Hospital for Children, Montreal, Quebec) and M. Demay (Massachusetts General Hospital and Harvard Medical School, Boston, MA), respectively. Both lines of mice are in an homogeneous C57BL/6 genetic background having been back-crossed greater than 10 times into this strain. VDR−/− CYP27B1−/− double knockout mice were generated by mating mice heterozygous for both genes. Mouse genotyping was performed on tail DNA as previously described (Ellison et al., 2007). Mice were fed a diet containing 2% calcium, 1.25% phosphorus, and 20% lactose supplemented with 2.2 IU vitamin D3/g (TD96348, Teklad, Madison, WI), which has been shown to normalize serum mineral homeostasis, skeletal growth, bone density, and body weight in CYP27B1−/− (Dardenne et al., 2003) and VDR−/− mice (Li et al., 1998).
Chemical carcinogen treatment and tumor monitoring
VDR+/+, VDR+/−, and VDR−/− mice (n=7), CYP27B1+/+, CYP27B1+/−, and CYP27B1−/− mice (n=11), and VDR−/− CYP27B1−/− double knockout mice (n=12) were treated with DMBA (100 mg/kg body weight) in corn oil by oral gavage at 5 and 6 weeks of age as described previously (Zinser et al., 2002). Mice were examined weekly for tumor development by visual inspection and palpation. Mice bearing a skin growth 1 mm or larger, persisting for more than 7 days, were scored positive.
UVB irradiation and tumor monitoring
Dorsal skin was shaved with electric clippers and depilated with Nair lotion 24 h before UVB exposure. Mice (5–7 weeks old at the onset of the study) were irradiated three times per week, with two to three days between treatments, and redepilated as needed. Dorsal skin was exposed to UV irradiation from a band of six FS-40 fluorescent lamps from which UVB and UVC wavelengths not normally present in natural solar radiation were filtered out using Kodacel cellulose film. After filtration with a Kodacel film, the UVB wavelengths ranged from approximately 290–320 nm. UVB emission was monitored with an IL-443 phototherapy radiometer (International Light, Newburyport, MA) equipped with an IL SED 240 detector fitted with a W side angle quartz diffuser and a SC5 280 filter. The dose of UVB varied to maximize UV exposure with minimal ulceration of the skin. Mice received 120 mJ/cm2 for two weeks. The dose was then increased 25% per week for 5 weeks, reaching the maximal dose of 400 mJ/cm2. This dose was administered for 9 weeks. With advancing age, the skin of several mice became increasingly ulcerated so the dose was reduced to 200 mJ/cm2 for the rest of the study. Mice received UVB treatments for 33 weeks, and were examined weekly for tumor development by visual inspection and palpation. Mice bearing a skin growth 1 mm or larger, persisting for more than 7 days, were scored positive.
Another cohort of mice (5–7 weeks old) were depilated as described above and exposed to 120 mJ/cm2. 24 h later mice were anesthetized with Avertin, and a biopsy was taken from the dorsal skin and fixed in neutral buffered formalin. Mice were exposed to 5 additional doses of UV and another sample of skin was collected. TUNEL staining was performed on the skin samples following the first dose of UV, and PCNA staining and epidermal thickness measurement was performed on all skin samples, as described below.
Tumor classification
Tumors were collected at death or by excision biopsy, fixed in neutral buffered formalin, and embedded in paraffin. 5μm sections were stained with hematoxylin and eosin, and evaluated in a blinded manner. DMBA tumors were classified as a sebaceous hyperplasia if more than 90% of the cells were well-differentiated mature sebocytes, or sebaceous adenoma if approximately 50% of the cells were well-differentiated mature sebocytes. UV-induced tumors were classified as a papilloma, either benign or with mild, moderate, or severe dysplasia, as a squamous cell carcinoma, either in situ or invasive, or as an atypical fibroxanthoma. Benign papillomas had few to no mitotic figures above the basal epidermal layer and no cytological atypia. Mild, moderate, and severe atypia papillomas had parakeratin, increasing numbers of mitotic figures above the basal layer and increasing likelihood of cytological atypia. Squamous cell carcinoma (SCC) in situ was characterized by parakeratosis, and high likelihood of hyperkeratosis, dyskeratosis, and acanthosis. There were many atypical keratinocytes with hyperchromatism, pleomorphism, atypical mitoses, and loss of orderly maturation (sometimes creating a “windblown” appearance). In some cases the epidermis showed evidence of a loss of polarity. Invasive squamous cell carcinoma was characterized by the descriptors mentioned above for SCC in situ, with the addition of invasion of the dermis by atypical keratinocytes and the lack of a defined basal layer. Some mice developed rapidly growing tumors consisting of dermal proliferation of atypical spindle cells, epithelioid cells, or multinucleated giant cells, and sometimes foamy cells often extending up against the epidermis. Tumors contained severe pleomorphism, hyperchromatism, and many very atypical mitoses, consistent with atypical fibroxanthoma (AFX).
Immunoblotting
Primary mouse keratinocytes were isolated as previously described (Ellison et al., 2007) and cultured in Keratinocyte-SFM (Invitrogen, Carlsbad, CA) on collagen-I plates (Corning, Lowell, MA). Cells were exposed to UVB through a thin film of PBS and media were replaced. Protein was harvested in sample buffer [62.5mM Tris (pH 6.8), 10% glycerol, and 2% sodium dodecyl sulfate] and protein concentration was determined by BCA assay (Pierce, Rockford, IL). β-mercaptoethanol (1%) and bromphenol blue (0.01%) were added and equal amounts of protein were separated on polyacrylamide gels. Protein was transferred to PVDF membrane (Immobilon-P, Millipore) and probed for p53 (sc-6243, 1:400, Santa Cruz Biotechnology, Santa Cruz, CA), phospho-serine15 p53 (1:1000, Cell Signaling Technology, Danvers, MA), or actin (1:20000, Sigma-Aldrich, St. Louis, MO). Membranes were washed and probed with the species appropriate secondary antibody at a 1:10000 dilution, then the signal was visualized by chemiluminescence.
Slot blot analysis of thymine dimers
Newborn VDR+/+ and VDR−/− mice were treated with 120mJ/cm2 of UV and skin was harvested at the indicated time points. Unirradiated skin was also collected as a control. Skin was floated in dispase (BD Biosciences, Bedford, MA) for 20 min at 37 C, then epidermis was removed with forceps. Epidermis was digested overnight in lysis solution with proteinase K (supplied with kit) and genomic DNA was purified by DNeasy columns (Qiagen Inc, Valencia, CA). Prior to DNA purification, the solution was treated for 2 min at room temperature with RNaseA (2 μg/μl, Sigma-Aldrich). After elution, DNA was further purified by standard phenol/chloroform extraction and ethanol precipitation. DNA concentration was determined using a spectrophotometer.
DNA was denatured by boiling in 0.4M NaOH/10mM EDTA for 10 min. Ice cold ammonium acetate (2M) was added to a final concentration of 1M. 50 ng per well of DNA was spotted onto a wet nitrocellulose membrane (Biotrace NT, VWR International, West Chester, PA) using a Bio-Dot SF apparatus (Bio-Rad Laboratories, Hercules, CA). The membrane was washed with 0.4M NaOH/10mM EDTA, removed from the apparatus, and baked under vacuum for 30 min at 80 C. The membrane was incubated with a primary antibody recognizing thymine dimers (1:2500, Clone KTM53, Kamiya Biomedical Company, Seattle, WA), followed by washing and incubation with a goat anti-mouse secondary antibody (1:10000, KPL, Gaitherburg, MD). Signal was visualized by chemiluminescence. Thymine dimer signal was normalized to total genomic DNA by probing with radiolabeled mouse genomic DNA. Genomic DNA was 32P-labeled using the Prime-a-Gene kit (Promega, Madison, WI) according to the manufacturer’s instructions.
MTT assay
Primary mouse keratinocytes were isolated and cultured as described above. 24 h after plating, cells were exposed to UV through a thin film of PBS and media were replaced. Proliferation rate was measured at the indicated time periods by incubating cells with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), 0.5 mg/mL final concentration, for 1 h. Cells were washed twice with PBS and cells were lysed with isopropanol with 0.04N HCl. Absorbance at 560 nm was measured using a plate spectrophotometer.
Histological analysis of skin
Epidermal thickness was calculated from 20–25 measurements from multiple fields of paraffin-embedded skin sections immunohistochemically stained for PCNA expression, viewed at 100× magnification. Measurements were made using Axiovision software (Carl Zeiss, Thornwood, NY). Differences in thickness were analyzed by the two-tailed Student’s t test in Microsoft Excel.
Immunohistochemistry was performed on paraffin-embedded sections using Vectastain ABC and peroxidase DAB kits (Vector Laboratories, Burlingame, CA). Briefly, skin sections were deparaffinized with xylenes and rehydrated in ethanol at decreasing concentrations. Sections were boiled for 20 min in 10mM citrate buffer, pH 6.0 for antigen retrieval, and endogenous peroxidase was blocked using 3% H2O2. For PCNA staining, sections were blocked in PBS with 1.5% horse serum, followed by an overnight incubation with the primary antibody (PCNA sc-56, Santa Cruz Biotechnology, Santa Cruz, CA), followed by a biotinylated horse anti-mouse secondary antibody (1:200, Vector Laboratories) and conjugation with the ABC complex. Sections were counterstained with Gill’s hematoxylin #2 (Fisher Scientific, Pittsburgh, PA), dehydrated, cleared, and mounted in Permount. A control (buffer without primary antibody) was performed for each tissue stained.
Apoptotic cells were detected in paraffin-embedded skin sections using the DeadEnd Fluorometric terminal deoxynucleotidyl transferase-mediated nick-end labeling (TUNEL) system (Promega, Madison, WI) according to the manufacturer’s instructions, with the following exceptions. Tissue sections were not fixed again following rehydration, and tissue was not treated with proteinase K. Instead, tissue was microwaved for 1 min at high power in 100mM citrate buffer, pH 6.0, then incubated for 30 min in Tris-HCl, pH 7.5, containing 3% bovine serum albumin and 20% normal bovine serum. Slides were washed twice in PBS before continuing with the manufacturer’s labeling protocol. Coverslips were mounted on slides with Vectashield mounting medium containing DAPI (Vector Laboratories).
Acknowledgments
The authors thank Tom McCormick and members of the laboratories of Drs. Ruth Keri and Richard Eckert for providing guidance for the design and execution of the experiments presented here. This work was supported by National Institutes of Health Grant R01DK53980 and by a Pilot and Feasibility seed grant from the Skin Disease Research Center at Case Western Reserve University (AR639750, to P.N.M.). T.I.E. was supported by NIGMS, National Institutes of Health, Institutional National Research Service Award T32GM 08803.
Abbreviations
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
- VDR
vitamin D receptor
- 1αOHase
25-hydroxyvitamin D3-1α-hydroxylase
- UV
ultraviolet
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- Albert MR, Weinstock MA. Keratinocyte carcinoma. CA Cancer J Clin. 2003;53:292–302. doi: 10.3322/canjclin.53.5.292. [DOI] [PubMed] [Google Scholar]
- Barletta F, Freedman LP, Christakos S. Enhancement of VDR-mediated transcription by phosphorylation: correlation with increased interaction between the VDR and DRIP205, a subunit of the VDR-interacting protein coactivator complex. Mol Endocrinol. 2002;16:301–314. doi: 10.1210/mend.16.2.0764. [DOI] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D and skin cancer. J Nutr. 2004;134:3472S–3478S. doi: 10.1093/jn/134.12.3472S. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, et al. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol. 2004;122:984–992. doi: 10.1111/j.0022-202X.2004.22424.x. [DOI] [PubMed] [Google Scholar]
- Bikle DD, Nemanic MK, Whitney JO, Elias PW. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry. 1986;25:1545–1548. doi: 10.1021/bi00355a013. [DOI] [PubMed] [Google Scholar]
- Cheskis B, Freedman LP. Ligand modulates the conversion of DNA-bound vitamin D3 receptor (VDR) homodimers into VDR-retinoid X receptor heterodimers. Mol Cell Biol. 1994;14:3329–3338. doi: 10.1128/mcb.14.5.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida K, Hashiba H, Fukushima M, Suda T, Kuroki T. Inhibition of tumor promotion in mouse skin by 1 alpha,25-dihydroxyvitamin D3. Cancer Res. 1985;45:5426–5430. [PubMed] [Google Scholar]
- Dardenne O, Prud’homme J, Arabian A, Glorieux FH, St-Arnaud R. Targeted inactivation of the 25-hydroxyvitamin D(3)-1(alpha)-hydroxylase gene (CYP27B1) creates an animal model of pseudovitamin D-deficiency rickets. Endocrinology. 2001;142:3135–3141. doi: 10.1210/endo.142.7.8281. [DOI] [PubMed] [Google Scholar]
- Dardenne O, Prud’homme J, Hacking SA, Glorieux FH, St-Arnaud R. Correction of the abnormal mineral ion homeostasis with a high-calcium, high-phosphorus, high-lactose diet rescues the PDDR phenotype of mice deficient for the 25-hydroxyvitamin D-1alpha-hydroxylase (CYP27B1) Bone. 2003;32:332–340. doi: 10.1016/s8756-3282(03)00023-1. [DOI] [PubMed] [Google Scholar]
- de Winter S, Vink AA, Roza L, Pavel S. Solar-simulated skin adaptation and its effect on subsequent UV-induced epidermal DNA damage. J Invest Dermatol. 2001;117:678–682. doi: 10.1046/j.0022-202x.2001.01478.x. [DOI] [PubMed] [Google Scholar]
- Dixon KM, Deo SS, Norman AW, Bishop JE, Halliday GM, Reeve VE, et al. In vivo relevance for photoprotection by the vitamin D rapid response pathway. J Steroid Biochem Mol Biol. 2007;103:451–456. doi: 10.1016/j.jsbmb.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Ellison TI, Eckert RL, MacDonald PN. Evidence for 1,25-dihydroxyvitamin D3-independent transactivation by the vitamin D receptor: uncoupling the receptor and ligand in keratinocytes. J Biol Chem. 2007;282:10953–10962. doi: 10.1074/jbc.M609717200. Epub 12007 Feb 10919. [DOI] [PubMed] [Google Scholar]
- Erben RG, Soegiarto DW, Weber K, Zeitz U, Lieberherr M, Gniadecki R, et al. Deletion of deoxyribonucleic acid binding domain of the vitamin D receptor abrogates genomic and nongenomic functions of vitamin D. Mol Endocrinol. 2002;16:1524–1537. doi: 10.1210/mend.16.7.0866. [DOI] [PubMed] [Google Scholar]
- Fu GK, Lin D, Zhang MY, Bikle DD, Shackleton CH, Miller WL, et al. Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol Endocrinol. 1997;11:1961–1970. doi: 10.1210/mend.11.13.0035. [DOI] [PubMed] [Google Scholar]
- Gray RW, Omdahl JL, Ghazarian JG, DeLuca HF. 25-Hydroxycholecalciferol-1-hydroxylase. Subcellular location and properties. J Biol Chem. 1972;247:7528–7532. [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Hughes MR, Malloy PJ, Kieback DG, Kesterson RA, Pike JW, Feldman D, et al. Point mutations in the human vitamin D receptor gene associated with hypocalcemic rickets. Science. 1988;242:1702–1705. doi: 10.1126/science.2849209. [DOI] [PubMed] [Google Scholar]
- Johns HE, Pearson ML, Leblanc JC, Helleiner CW. The Ultraviolet Photochemistry Of Thymidylyl-(3′-5′)-Thymidine. J Mol Biol. 1964;9:503–524. doi: 10.1016/s0022-2836(64)80223-0. [DOI] [PubMed] [Google Scholar]
- Kerner SA, Scott RA, Pike JW. Sequence elements in the human osteocalcin gene confer basal activation and inducible response to hormonal vitamin D3. Proc Natl Acad Sci U S A. 1989;86:4455–4459. doi: 10.1073/pnas.86.12.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992;355:446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Larner JM, Hamlin JL. A p53-independent damage-sensing mechanism that functions as a checkpoint at the G1/S transition in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1997;94:526–531. doi: 10.1073/pnas.94.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, et al. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998;139:4391–4396. doi: 10.1210/endo.139.10.6262. [DOI] [PubMed] [Google Scholar]
- Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, et al. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltzman W, Czyzyk L. UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol Cell Biol. 1984;4:1689–1694. doi: 10.1128/mcb.4.9.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura Y, Ananthaswamy HN. Short-term and long-term cellular and molecular events following UV irradiation of skin: implications for molecular medicine. Expert Rev Mol Med. 2002;2002:1–22. doi: 10.1017/S146239940200532X. [DOI] [PubMed] [Google Scholar]
- Melino G, De Laurenzi V, Vousden KH. p73: Friend or foe in tumorigenesis. Nat Rev Cancer. 2002;2:605–615. doi: 10.1038/nrc861. [DOI] [PubMed] [Google Scholar]
- Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, et al. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;98:7498–7503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocelli T, Poon R, Drucker DJ, Slingerland JM, Rosen CF. UVB radiation induces p21Cip1/WAF1 and mediates G1 and S phase checkpoints. Oncogene. 1996;12:1387–1396. [PubMed] [Google Scholar]
- Sakai Y, Demay MB. Evaluation of keratinocyte proliferation and differentiation in vitamin D receptor knockout mice. Endocrinology. 2000;141:2043–2049. doi: 10.1210/endo.141.6.7515. [DOI] [PubMed] [Google Scholar]
- Setlow RB, Setlow JK. Evidence that ultraviolet-induced thymine dimers in DNA cause biological damage. Proc Natl Acad Sci U S A. 1962;48:1250–1257. doi: 10.1073/pnas.48.7.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano JD, Canman CE, Taya Y, Sakaguchi K, Appella E, Kastan MB. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 1997;11:3471–3481. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorija K, Cox M, Sisk JM, Dowd DR, MacDonald PN, Thompson CC, et al. Ligand-independent actions of the vitamin D receptor maintain hair follicle homeostasis. Mol Endocrinol. 2005;19:855–862. doi: 10.1210/me.2004-0415. Epub 2004 Dec 2009. [DOI] [PubMed] [Google Scholar]
- St-Arnaud R, Messerlian S, Moir JM, Omdahl JL, Glorieux FH. The 25-hydroxyvitamin D 1-alpha-hydroxylase gene maps to the pseudovitamin D-deficiency rickets (PDDR) disease locus. J Bone Miner Res. 1997;12:1552–1559. doi: 10.1359/jbmr.1997.12.10.1552. [DOI] [PubMed] [Google Scholar]
- Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci U S A. 2001;98:13324–13329. doi: 10.1073/pnas.231474698. Epub 12001 Oct 13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- Zinser GM, Sundberg JP, Welsh J. Vitamin D3 receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis. 2002;23:2103–2109. doi: 10.1093/carcin/23.12.2103. [DOI] [PubMed] [Google Scholar]