Abstract
Extracellular nucleotides play important trophic roles in development and CNS injury but functions of distinct purinergic receptors and related signaling pathways have not been fully elucidated. Here we have identified opposing effects of P2X and P2Y receptors on the ability of FGF2 to induce proliferation in primary cultures of rat cortical astrocytes. Low concentrations of ATP enhanced DNA synthesis induced by FGF2 whereas high concentrations inhibited FGF2-induced proliferation. Comparison of concentration-response experiments with ATP and 2’,3’-O-(4-benzoyl)-benzoyl-ATP (BzATP) indicated that the inhibitory effect was mediated by P2X7 receptors. Interestingly, activation of P2X7 receptors led to a state of reversible growth arrest, rather than cell death. Selectivity studies showed that proliferation evoked by epidermal growth factor and platelet-derived growth factor was also inhibited by P2X7 receptors, but P2X1 or P2X3 receptors did not inhibit proliferation induced by FGF2. A marker of mitosis, phosphohistone-3, was reduced by BzATP and increased by UTP, suggesting the enhancing effect of ATP on FGF2-induced proliferation was mediated by P2 purine/pyrimidine receptors. Phosphorylation of growth arrest-related protein kinases p38/MAPK and SAPK/JNK was strongly increased by BzATP but only weakly affected by UTP. We conclude that P2Y purine/pyrimidine receptors enhance proliferation induced by FGF2 in astrocytes whereas stimulation of P2X7 receptors inhibits proliferation by shifting cells to a state of reversible growth arrest which may be mediated by protein kinase signaling. These trophic actions of P2X7 and P2Y purine/pyrimidine receptors may contribute to the regulation of CNS development, adult neurogenesis and the response of astrocytes to injury.
Keywords: Purinergic receptor, extracellular nucleotide, protein kinase, growth arrest, gliosis
INTRODUCTION
Extracellular ATP can exert long-term, trophic effects on many cell types, including astrocytes (Burnstock 1990, Neary et al 1996, Neary & Abbracchio 2001, Fields & Burnstock 2006, Burnstock 2007). Such long term effects include proliferation, differentiation, migration, growth arrest, and apoptosis. In the CNS, extracellular ATP plays key roles in development and during injury and repair. For instance, P2 purinergic receptors are expressed temporally and spatially during development, and extracellular nucleotides may regulate proliferation and differentiation of neural progenitor cells [for recent review, see (Zimmermann 2006), and references therein]. In addition, ATP is released upon injury such as trauma (Ahmed et al 2000, Neary et al 2005b), and addition of ATP to astrocyte cultures (Neary et al 1996, and references therein) or injection of ATP analogs into rat brain (Franke et al 1999, Franke et al 2001) elicits the hallmark features of astrogliosis, namely, increases in the number and size of astrocytic processes, GFAP expression and proliferation. Extracellular ATP can also act as a co-mitogen with polypeptide growth factors such as FGF2, another agent important in development and increased after CNS injury. Previously we demonstrated that extracellular ATP synergistically enhances DNA synthesis induced by FGF2 in astrocytes (Neary et al 1994). The synergistic effect was also observed with UTP, indicating that it is mediated by P2Y purine/pyrimidine receptors (Neary et al 2005a). Interestingly, stimulation of P2X receptors with BzATP led to an opposite response, i.e., inhibition of FGF2-induced proliferation.
An emerging feature of P2 receptor function is that the presence of P2Y and P2X receptors in the same cells can lead to opposing long-term effects such as proliferation and apoptosis, depending on the type of receptor activated (Harada et al 2000; Coutinho-Silva et al 2005). The aim of the current studies was to characterize the inhibitory actions of P2X receptors on the ability of FGF2 to induce DNA synthesis and mitosis in rat cortical astrocyte cultures and to compare these actions with stimulation of P2Y purine/pyrimidine-preferring receptors. Key questions addressed by these studies include (a) whether the inhibitory effect of P2X receptors on FGF2-induced proliferation is reversible, (b) whether the inhibitory effect is a general property of P2X receptors or is selective for a specific subtype, (c) whether the inhibitory effect is observed with other polypeptide growth factors such as epidermal growth factor (EGF) and platelet derived growth factor (PDGF) or is selective for FGF2, and (d) what signaling pathway(s) mediate the inhibitory effect. Responses are also compared to those stimulated by P2Y purine/pyrimidine-preferring receptors which elicit opposite effects in that they enhance rather than inhibit proliferation induced by FGF2. We report here that activation of P2X7 receptors in astrocytes leads to a state of reversible growth arrest which may be mediated at least in part by the stress-activated protein kinases SAPK/JNK and p38/MAPK.
MATERIALS AND METHODS
Cell culture
Primary cultures of astrocytes were obtained from neonatal rat (Fischer) cerebral cortices as previously described (Neary et al 1994); the experimental procedure was approved and monitored by the Animal Studies Subcommittee at the Miami VA Medical Center and the Animal Care and Use Committee, University of Miami Miller School of Medicine. Cells were seeded at densities of 0.2 and 0.5 million cells per well for 24 well plates and 35 mm dishes, respectively; cells were not replated before use. At least 95% of the cell population was astrocytes, as determined by staining with cell-specific markers (Neary et al 1994). Experiments were conducted with confluent 4–5 week old cultures (Neary et al 2005a). Prior to treatment, cells which had been maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% horse serum were shifted to the quiescent phase by incubation in DMEM containing 0.5% horse serum for 48–72 hr. Stock solutions of ATP and other nucleotides (Sigma Chemical Co., St. Louis, MO) as well as FGF2 (recombinant human FGF2; R&D Systems, Minneapolis, MN) and other polypeptide growth factors (EGF, Sigma Chemical Co.; PDGF, R&D Systems) were divided into single-use aliquots and stored at −80°C.
DNA synthesis
Quiescent cultures of rat cortical astrocytes were treated with FGF2 (25 ng/ml), other polypeptide growth factors and nucleotides at the concentrations indicated in figure legends in triplicate or quadruplicate wells of 24 well plates. Polypeptide growth factors and nucleotides were added to confluent, quiescent cultures at time zero, and media was not changed for the duration of the experiments. After 18 hr, 3H-thymidine (1 uCi, MP Biomedicals, Solon, OH) was added for an additional 4 hr, at which point 3H-thymidine incorporation was measured as previously described (Neary et al 1994). Data were expressed as cpm/mg protein. Protein concentrations were determined by the modified Lowry procedure with bovine serum albumin as standard (Peterson 1983).
Immunoblotting
Cells were lysed in Laemmli sample buffer and protein concentrations determined by the modified Lowry procedure (Peterson 1983). Cell lysates, containing 30–50 µg of protein were subjected to SDS-PAGE (11% acrylamide gels) and transferred to nitrocellulose filters with a Genie electrophoretic blotter (Idea Scientific Inc., Minneapolis, MN) for 1h at 12V in a transfer buffer containing 25 mM Tris, 192 mM glycine and 20% (v/v) methanol. Membranes were blocked and probed according to the manufacturer’s instructions for analysis with an Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NB). Immunoblotting was performed using antibodies specific for phospho-histone H3 (Upstate Cell Signaling Solutions, Lake Placid, NY), at a 1/500 dilution. Protein loading was checked with specific antibodies against β-actin (Sigma, St. Louis, MO) at a 1/200,000 dilution. Immunoblotting was also conducted to assess phosphorylation of p38 (Thr180/Tyr182) and JNK/SAPK (Thr183/Tyr185) using phospho-specific antibodies (both at 1/1000 dilution) obtained from Cell Signaling Technology, Beverly, MA; protein loading was checked using specific antibodies against total p38 and SAPK (both at 1/1000 dilution) also obtained from Cell Signaling Technology. Bands were visualized with an Odyssey Imaging System (Li-Cor Biosciences, Lincoln, NB) according to the manufacturer’s instructions using IRDye 800 labeled anti-mouse IgG and/or IRDye 700DX anti-rabbit IgG (Rockland Immunochemicals, Gilbertsville, PA) secondary antibodies at 1/5000 dilution. For quantification, ratios of phospho-histone 3 to β-actin, phospho-p38 to total p38, and phospho-SAPK to total SAPK were calculated, and data were expressed as fold stimulation compared to controls.
Cell Viability
An assay based on the reduction of MTS tetrazolium by viable cells to a colored formazan product was utilized, as previously described (Kucher & Neary 2005), according to the manufacturer’s instructions (Promega, Madison , WI). In this assay, absorbance readings are directly proportional to the number of living cells in each culture well.
Statistical Analysis
The number of experimental replications is given in the figure legends; experiments were conducted with cultures from different seedings. Data were analyzed by Student’s t test for two groups or repeated measures ANOVA for multiple groups followed by post hoc comparisons (Bonferroni test) using an Instat software package (GraphPad Software, San Diego, CA, USA). EC50 values were calculated with Prism 4 (GraphPad Software).
RESULTS
P2X7 receptors mediate inhibition of proliferation induced by FGF2 in astrocytes
Previous studies demonstrated that BzATP inhibited the ability of FGF2 to induce DNA synthesis in astrocytes, thereby suggesting a role for P2X7 receptors in attenuating FGF2-induced proliferation (Neary et al 2005a). To examine further the potential involvement of P2X7 receptors, several approaches were used. First, because P2X7 receptors are activated by high concentrations of ATP, we conducted concentration-response experiments in which astrocytes were treated with ATP ranging from 30 to 1000 uM in the presence of FGF2 (25 ng/ml). At 300 and 1000 uM ATP, DNA synthesis stimulated by FGF2 was significantly reduced (Fig. 1). By contrast, at 30 and 100 uM ATP, FGF2-induced DNA synthesis was enhanced (Fig. 1). As another approach, astrocytes were treated with BzATP ranging from 1 to 100 uM in the presence of FGF2 (25 ng/nl). Significant reductions in FGF2-induced DNA synthesis were observed at 30 and 100 uM BzATP (Figure 2). Thus, the concentration-response data in Figures 1 and 2 demonstrate that 30 and 100 uM ATP enhanced FGF2-induced DNA synthesis, whereas these concentrations of BzATP inhibited FGF2-induced DNA synthesis. The EC50 values for the inhibitory effects of BzATP and ATP on FGF2-induced DNA synthesis are 26 uM and 261 uM, respectively, values which are consistent with previous reports that BzATP is 10 to 30 times more potent than ATP at P2X7 receptors (North 2002) whereas for other P2X receptors, ATP is more potent than BzATP (Khakh et al 2001; Table 2). Because BzATP is a more potent agonist of P2X7 receptors than ATP and because P2X7 receptors are activated by high concentrations of ATP, these findings support the notion that P2X7 receptors attenuate FGF2-induced mitogenesis.
Figure 1.
Opposing effects of different concentrations of extracellular ATP on DNA synthesis induced by FGF2. Quiescent cultures of rat cortical astrocytes were treated with FGF2 (25 ng/ml) or in combination with different concentrations of ATP (30–1000 uM), and DNA synthesis was measured as described in Materials and Methods. H3-Thymidine incorporation in control cultures was 16,609 ± 995 cpm/mg protein (Mean ± SEM, n = 4). FGF2-induced DNA synthesis was significantly enhanced by 30 uM and 100 uM ATP whereas this effect was significantly inhibited by 300 uM and 1000 uM ATP (* p<0.05; ** p<0.01; *** p<0.001).
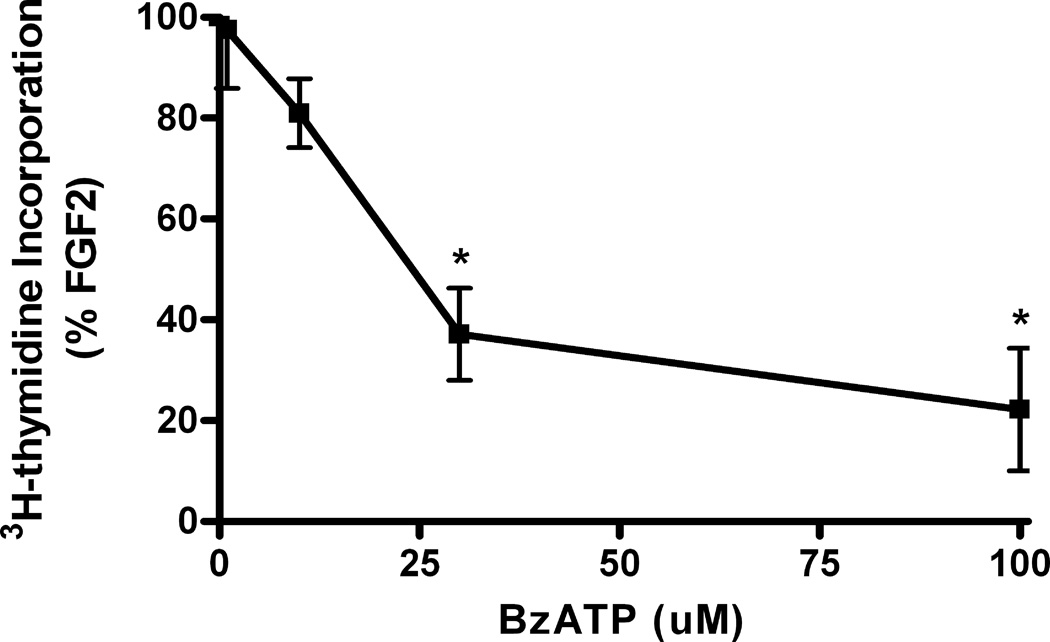
Figure 2.
Concentration-response of BzATP on DNA synthesis induced by FGF2. Quiescent cultures of rat cortical astrocytes were treated with FGF2 (25 ng/ml) or in combination with concentrations of BzATP ranging from 1 to 100 uM, and DNA synthesis was measured as described in Materials and Methods. Thymidine incorporation in FGF2-treated cultures was increased 606 ± 109 % (Mean ± SEM, n = 3) compared to control cultures. Data from cultures treated with FGF2 and BzATP are expressed as %FGF2. DNA synthesis induced by FGF2 was significantly decreased by 30 uM and 100 uM BzATP (* p<0.01).
Stimulation of P2X7 receptors leads to reversible growth arrest rather than cell death
Because P2X7 receptors have been linked to cell death and cytotoxicity in some cell types (Zanovello et al 1990, Ferrari et al 1997), we examined the possibility that the decrease in DNA synthesis could be due to a loss of cell viability upon activation of P2X7 receptors. However, no significant loss of cell viability was observed when astrocytes were treated with BzATP for 22 hr (Table I). As a positive control to test the effectiveness of the assay, cultures were treated independently with DMSO (10%) which brought about a significant reduction in cell viability (Table 1).
Table I.
BzATP does not affect cell viability.
| Treatment | Cell Viability (OD ± SEM) |
P value (n = 3) |
|---|---|---|
| Control | 0.754 ± 0.054 | |
| FGF2 | 0.815 ± 0.025 | P>0.05 |
| BzATP | 0.770 ± 0.017 | P>0.05 |
| BzATP + FGF2 | 0.748 ± 0.055 | P>0.05 |
| DMSO | 0.430 ± 0.036 | P<0.01 |
Astrocytes were treated with FGF2 (25 ng/ml), BzATP (100 uM) or dimethyl sulfoxide (DMSO; 10%) as indicated in the table. After 22 hr, cell viability was determined by means of a tetrazolium-based method according to the manufacture’s instructions (Promega, Madison , Wis., USA) as previously described (Kucher & Neary, 2005). Treatment of astrocytes with BzATP alone or in combination with FGF2 did not significantly affect cell viability, although cell viability was significantly reduced by DMSO.
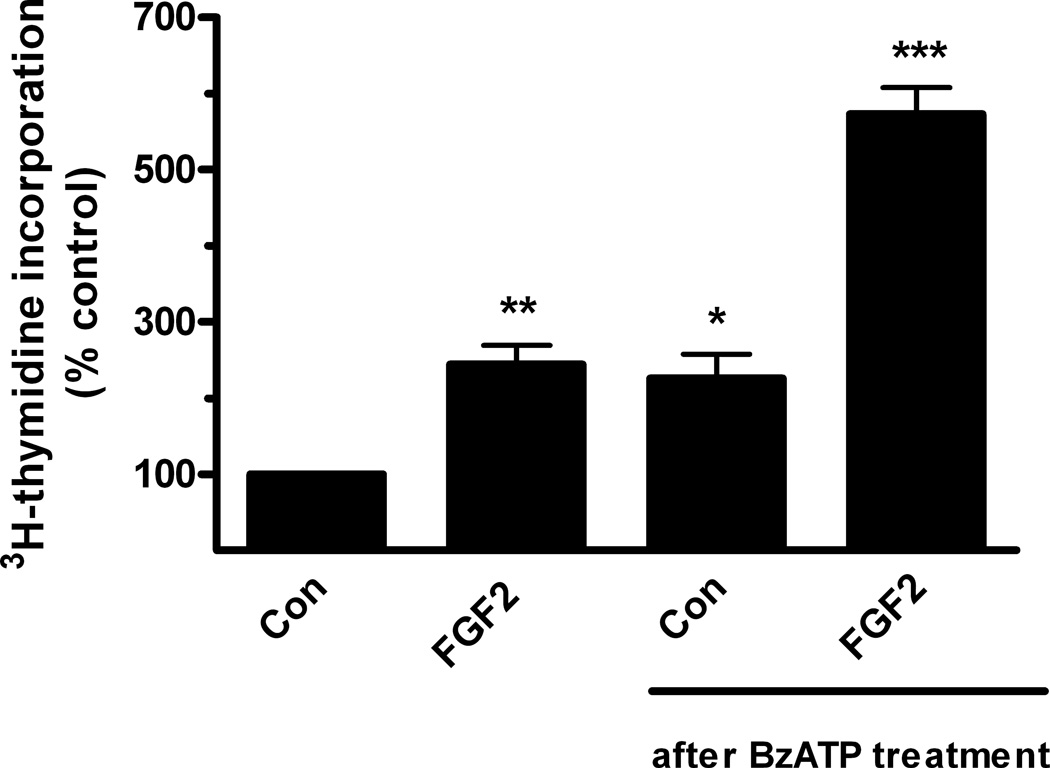
Because astrocytes remained viable in the presence of BzATP, this raised the question of whether astrocytes previously exposed to prolonged BzATP treatment can return to the proliferative state. To address this issue, astrocytes were treated with BzATP for 22 hr, then returned to normal culture medium and challenged with FGF2 (25 ng/ml). Under these conditions, FGF2 increased DNA synthesis about 2.5 fold, an increase which is similar to that observed in astrocytes not previously exposed to BzATP (Fig. 3). Although BzATP did not significantly reduce DNA synthesis in cells not challenged with FGF2 (85.2 ± 8.8% of control); p > 0.05, n = 4), basal DNA synthesis was increased following removal of BzATP (Fig. 3), thereby suggesting that P2X7 receptors may have a trophic effect on astrocytes after long term agonist stimulation and removal. Collectively, these experiments indicate that stimulation of P2X7 receptors on astrocytes does not lead to cell death; rather, it appears that activation of P2X7 receptors leads to induction of a non-proliferative, growth-arrested state which can be reversed by exposure to FGF2.
Figure 3.
The inhibitory effect of BzATP on FGF2-induced DNA synthesis is reversible. Quiescent cultures of rat cortical astrocytes were treated with BzATP (100 uM) for 22 hr. BzATP was removed by rinsing and cultures were returned to normal conditions (DMEM containing 0.5% horse serum). One day later, FGF2 (25 ng/ml) was added to these cultures or to quiescent cells not treated with BzATP, and DNA synthesis was measured as described in Materials and Methods. H3-Thymidine incorporation in untreated control cultures was 9076 ± 1828 cpm/mg protein (Mean ± SEM, n = 4). FGF2 stimulated significant increases in DNA synthesis in previously untreated cultures (**, p < 0.01) as well as those previously treated with BzATP (*** p < 0.001). DNA synthesis was also increased in cultures previously treated with BzATP when compared with untreated controls (*, p < 0.05).
Characterization of the selectivity of the inhibitory effect of P2X7 receptors on FGF2-induced proliferation
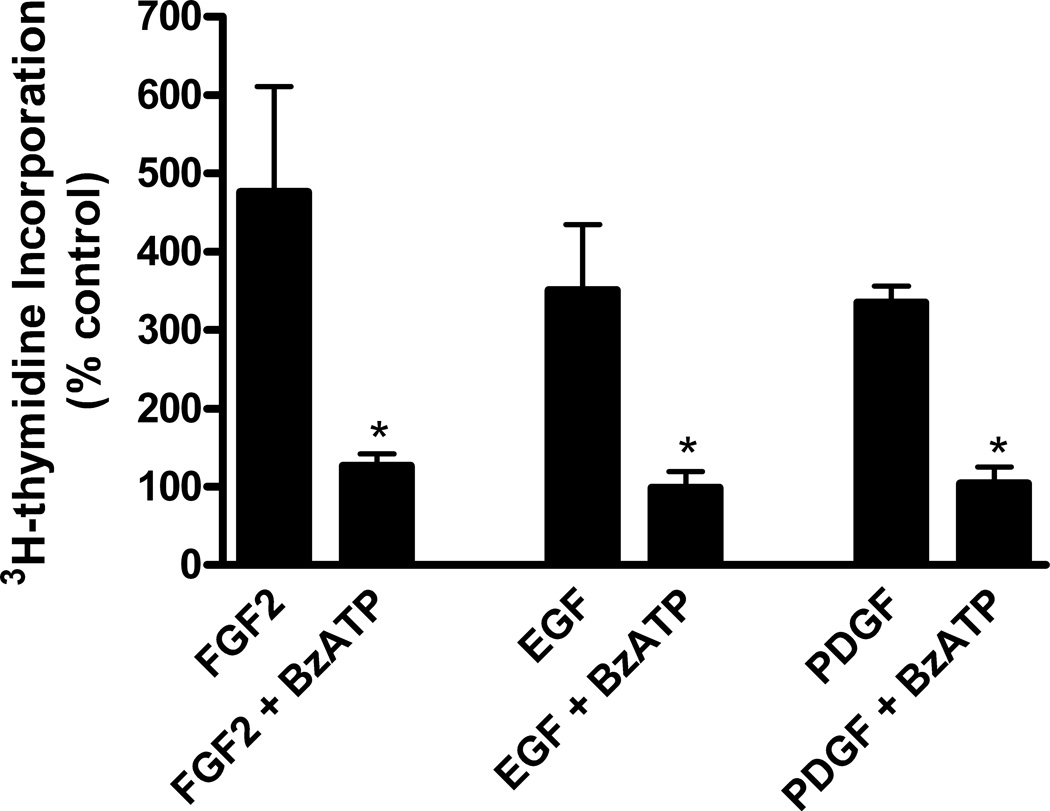
To examine whether stimulation of P2X7 receptors also inhibits proliferation of astrocytes induced by polypeptide growth factors other than FGF2, astrocytes were treated with EGF or PDGF in the presence or absence of BzATP. We found that BzATP significantly inhibited DNA synthesis stimulated by these polypeptide growth factors as well as FGF2 (Figure 4). However, it should be noted that, by contrast, the potentiating effect of P2Y receptors on DNA synthesis found with FGF2 was not observed with EGF and PDGF (Neary et al 1994).
Figure 4.
Activation of P2X7 receptors inhibits DNA synthesis induced by EGF and PDGF in addition to FGF2. Quiescent cultures of rat cortical astrocytes were treated with FGF2 (25 ng/ml), EGF (10 ng/ml), or PDGF (2.5 ng/ml), alone or in combination with BzATP (100 uM), and DNA synthesis was measured as described in Materials and Methods. H3-Thymidine incorporation in control cultures was 30,376 ± 4257 cpm/mg protein (Mean ± SEM, n = 3). Activation of P2X7 receptors significantly inhibited DNA synthesis induced by EGF, PDGF and FGF2 (* p < 0.05).
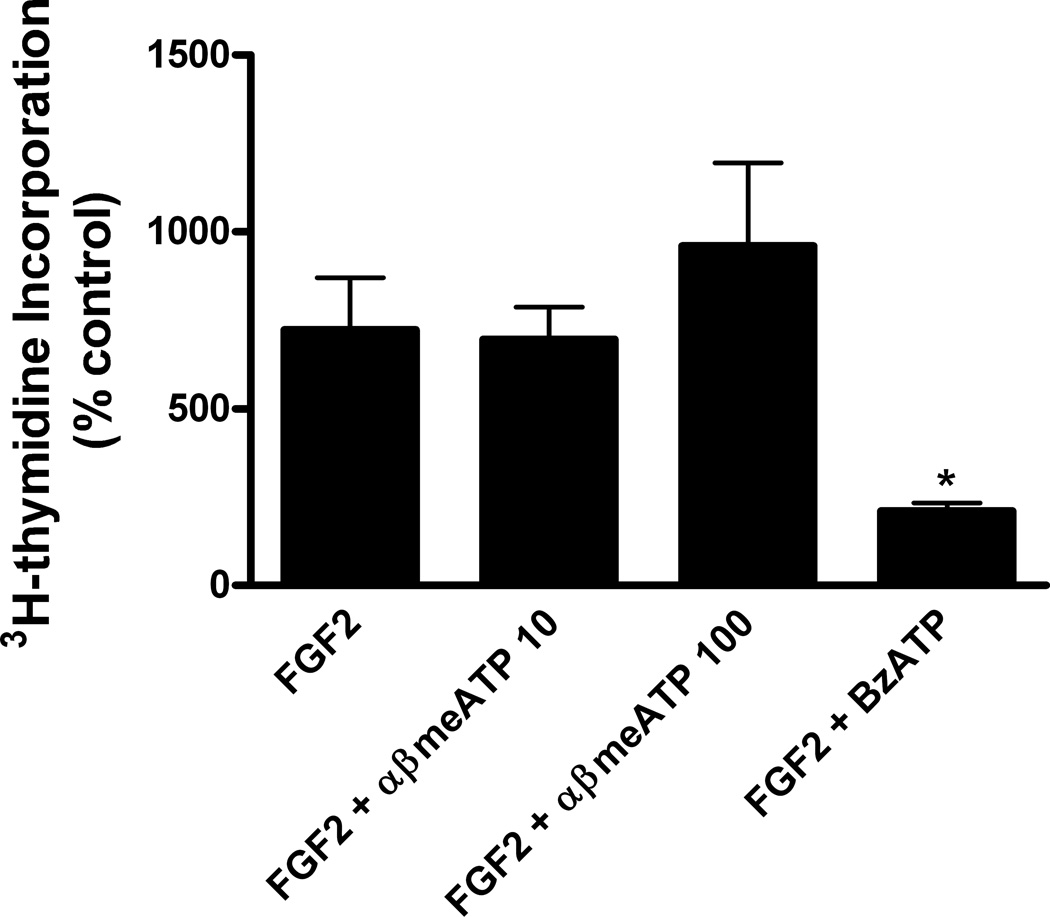
To investigate whether the inhibitory effect on DNA synthesis was a selective property of P2X7 receptors or could be evoked by activation of other P2X receptors, we utilized α,βmethylene ATP, an agonist of P2X1 and P2X3 receptors (Ralevic & Burnstock 1998). In order to conduct these experiments, it was necessary to prepare culture medium free of phenol red because it has been shown that this commonly used medium component is an antagonist of P2X1 and P2X3 receptors (King et al 2005). Under these conditions, we found that DNA synthesis induced by FGF2 was significantly inhibited by BzATP but not by either 10 or 100 uM α,βmethylene ATP (Figure 5). As a test of the effectiveness of α,βmethylene ATP, we found that it did stimulate phosphorylation of ERK1,2 (data not shown), indicating that P2X1 and P2X3 receptors are linked to protein kinase signaling when not antagonized by phenol red. Collectively, these selectivity experiments indicate that (1) the inhibitory effect of P2X7 receptors on proliferation is not a general effect common to all subtypes of P2X receptors and (2) stimulation of P2X7 receptors can also inhibit proliferation of astrocytes induced by other polypeptide growth factors.
Figure 5.
Activation of P2X1 and P2X3 receptors does not inhibit DNA synthesis induced by FGF2. Primary cultures of rat cortical astrocytes were shifted to the quiescent state by incubation in phenol red-free DMEM containing 0.5% horse serum for 48–72 hr. These cultures were then treated with FGF2 (25 ng/ml) alone or in the presence of αβ-methylene ATP (10 or 100 uM), an agonist of P2X1 and P2X3 receptors, or BzATP (100 uM), an agonist of P2X7 receptors, and DNA synthesis was measures as described in Materials and Methods. H3-Thymidine incorporation in control cultures was 13,018 ± 2392 cpm/mg protein (Mean ± SEM, n = 3). αβ-methylene ATP did not significantly affect FGF2-induced DNA synthesis whereas BzATP did (* p < 0.05), thereby indicating that the inhibitory effect of P2X7 receptors is not a general effect common to all subtypes of P2X receptors.
Comparison of effects of P2X7 and P2Y purine/pyrimidine receptors on mitosis
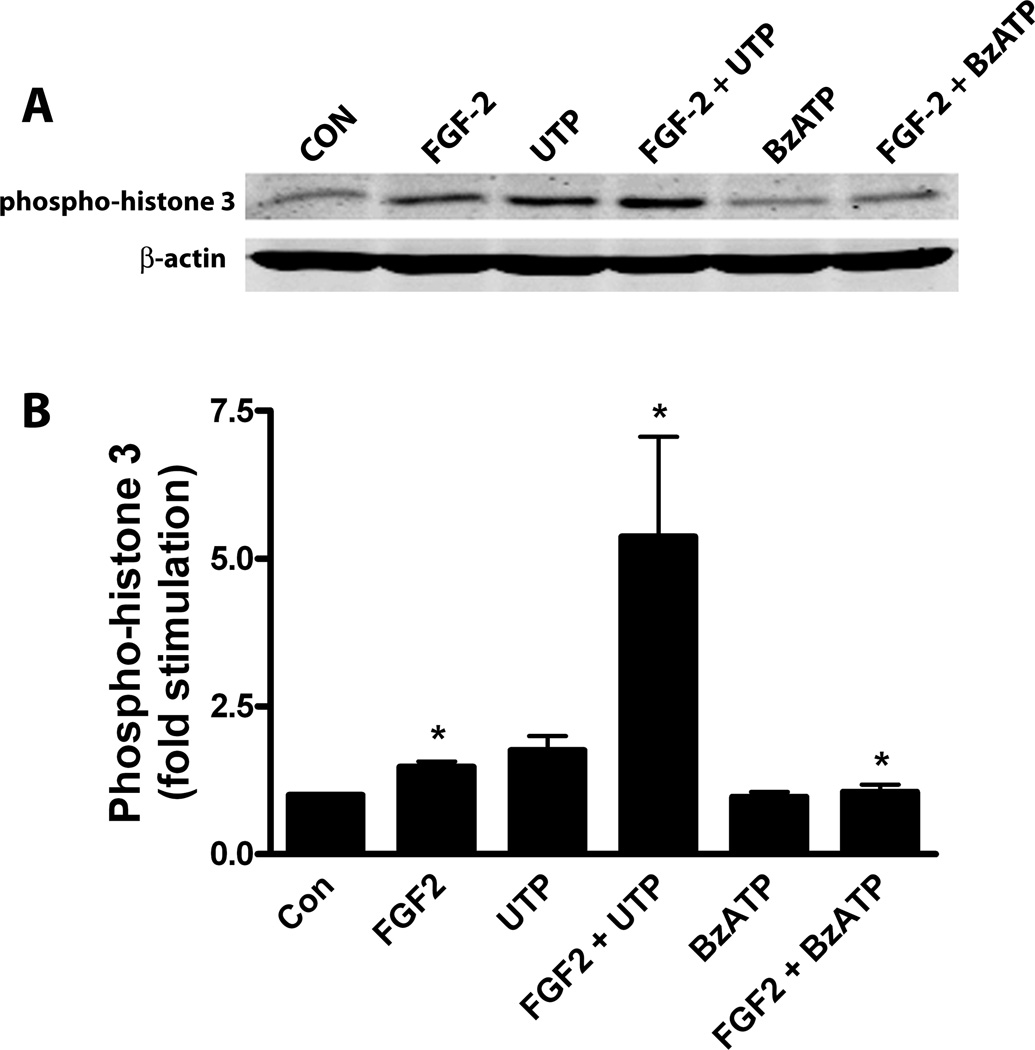
Phospho-histone 3 is a specific marker of cells undergoing mitosis (Colman et al 2006). To investigate the effects of P2X7 and P2Y purine/pyrimidine receptors on mitosis induced by FGF2, we conducted immunoblot experiments using an antibody specific for phospho-histone 3. Previous studies demonstrated that stimulation of P2Y purine/pyrimidine preferring receptors potentiated cell cycle entry and progression induced by FGF2 (Neary et al 2005a), and thus UTP was utilized in these and subsequent experiments. Astrocyte cultures were treated with FGF2 (25 ng/ml), alone or in the presence of UTP or BzATP. We found that phospho-histone 3 was elevated by FGF2 and that this effect was markedly increased when cultures were treated with both UTP and FGF2 (Figure 6A). By contrast, phospho-histone 3 was reduced when cultures were treated with BzATP and FGF2. Analysis of group data obtained by quantitative densitometry (Figure 6B) confirmed these observations. These findings indicate that P2Y purine/pyrimidine receptors potentiate mitosis induced by FGF2 whereas the stimulatory effect of FGF2 on mitosis is inhibited by P2X7 receptors.
Figure 6.
Effects of UTP and BzATP on the stimulation of the mitosis marker, phospho-histone 3, by FGF2. Quiescent cultures of rat cortical astrocytes were treated with FGF2 (25 ng/ml) alone or in combination with UTP (100 uM) or BzATP (100 uM) for 22 hr. Western blots were prepared and probed for phospho-histone 3 and for β-actin (loading control), as described in Materials and Methods. (A) Western blot representative of 6 experiments. (B) Ratios of phospho-histone 3/ β-actin were calculated, normalized to controls and group data were expressed as mean ± SEM (n= 6). Phospho-histone 3 levels in FGF2-treated cultures were increased compared to controls (*, p < 0.05). Compared to FGF2 alone, cultures treated with the combination of UTP and FGF2 had increased levels of phospho-histone 3 (*, p < 0.05) whereas those treated with FGF2 and BzATP had decreased levels of phospho-histone 3 (*, p < 0.05).
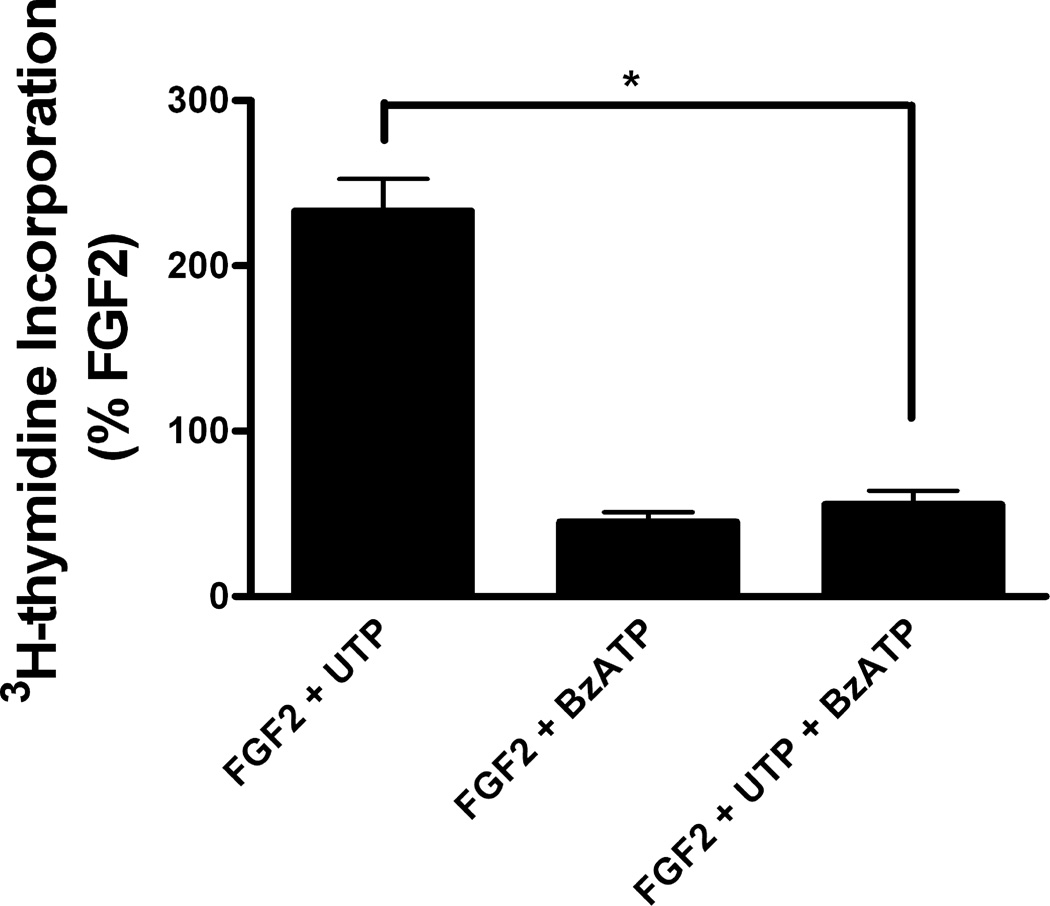
Another issue concerning the opposing effects of P2X7 and P2Y purine/pyrimidine receptors on proliferation induced by FGF2 regards the outcome of stimulation of both types of purinergic receptors. The difference in the effects of low and high concentration of ATP on DNA synthesis induced by FGF2 (Figure 1) suggested that activation of P2X7 receptors would overcome the potentiating effect of P2Y purine/pyrimidine receptors. To test how the inhibitory effect of P2X7 receptors would affect the potentiating effect of P2Y purine/pyrimidine receptors on FGF2-induced DNA synthesis, we conducted co-stimulatory experiments with FGF2, UTP and BzATP. When astrocytes treated with FGF2 (25 ng/ml) and UTP were also stimulated with BzATP, the potentiating effect of UTP was negated (Figure 7). It should be noted that BzATP is an antagonist of P2Y4 receptors with an IC50 value of 159 uM (Wildman et al 2003), and thus it is possible that part of the inhibitory effect of BzATP could be related to antagonism of P2Y4 receptors. Although we cannot exclude a role for other P2 receptors, a shift from a stimulatory effect to an inhibitory effect on FGF2-induced proliferation was observed at high concentrations of ATP (Fig. 1) which is consistent with a role for P2X7 receptors. In addition, these experiments confirmed previous results (Neary et al 2005a), in that UTP enhanced the proliferative effect of FGF2 on astrocytes whereas this was inhibited by BzATP (Figure 7).
Figure 7.
Activation of P2X7 receptors overcomes the potentiating effect of P2Y purine/pyrimidine receptors on FGF2-induced DNA synthesis. Quiescent cultures of rat cortical astrocytes were treated with FGF2 (25 ng/ml), in combination with UTP (100 uM) or BzATP (100 uM), or with all three agents, and DNA synthesis was measured as described in Materials and Methods. Thymidine incorporation in FGF2-treated cultures was increased 411 ± 54 % (Mean ± SEM, n = 7) compared to control cultures. Data from cultures treated with FGF2 and UTP and/or BzATP are expressed as %FGF2. DNA synthesis induced by FGF2 and UTP was significantly decreased by the addition of BzATP (* p<0.001).
P2X7 receptors signal to stress-activated protein kinase signaling pathways that mediate growth arrest
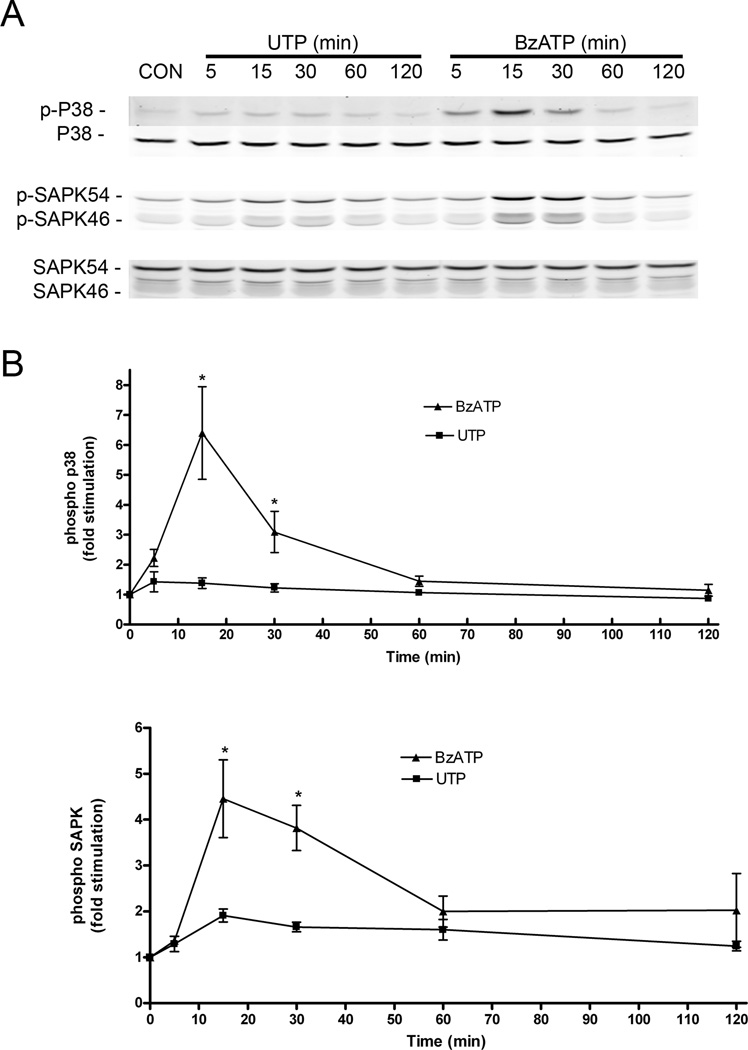
Previous studies suggested a role for ERK in the stimulatory and inhibitory effects of P2 receptors on FGF2-induced proliferation, based on differences in the intensity and duration of ERK activation (Neary et al 2005a) and subsequent STAT3 phosphorylation (Washburn & Neary 2006). To investigate additional signaling mechanism(s) that may underlie the inhibitory effect of P2X7 receptors on DNA synthesis induced by FGF2, we conducted experiments designed to measure the ability of BzATP to activate two members of the MAPK family that have been linked to growth arrest, namely, p38/MAPK and stress activated protein kinase (SAPK), also known as c-Jun-NH2-terminal kinase (JNK). Stimulation of p38/MAPK was determined by phosphorylation of Thr180 and Tyr182 while stimulation of SAPK was monitored by measuring phosphorylation of Thr183 and Tyr185. Time course studies revealed that both p38/MAPK and SAPK are strongly and transiently activated by BzATP (Figure 8). By contrast, these protein kinases were only weakly activated by UTP. The activation state of p38 and SAPK was unchanged compared to untreated controls after treatment with BzATP or UTP for 6, 18 or 22 hr (data not shown). These results suggest that the coupling of P2X7 receptors to SAPK and p38 may regulate down-stream, cell cycle regulatory events that lead to growth arrest.
Figure 8.
P2X7 receptors, but not P2Y purine/pyrimidine receptors, are strongly coupled to protein kinases associated with growth arrest. Quiescent cultures of rat cortical astrocytes were treated with UTP (100 uM) or BzATP (100 uM) for the times indicated, Cells were lysed and stimulation of phosphorylation of p38/MAPK and SAPK were determined by immunoblotting as described in Materials and Methods. A representative blot is shown in A. In B, group data of ratios of phosphorylated to total p38/MAPK and SAPK are shown (n = 4). BzATP stimulated phosphoryation of both p38/MAPK and SAPK more strongly than UTP at 15 and 30 min (*, p< 0.05).
DISCUSSION
The findings presented here demonstrate that in astrocytes, P2X7 receptors inhibit DNA synthesis and mitosis induced by FGF2 and other polypeptide growth factors such as EGF and PDGF without affecting cell viability. The inhibitory effect was not observed when P2X1 and P2X3 receptors were activated. Signaling from P2X7 receptors to the growth arrest related protein kinases, p38 and SAPK, may regulate the inhibitory effects on FGF2-induced proliferation. In contrast to P2X7 receptors, P2Y purine/pyrimidine preferring receptors enhance DNA synthesis and mitosis in astrocytes. Advances in understanding the opposing effects of signaling by P2X7 and P2Y purine/pyrimidine receptors may provide the opportunity to regulate long term, trophic actions of extracellular nucleotides associated with CNS development as well as injury and repair.
In some cell types, activation of P2X7 receptors has been reported to lead to cell death while in other cells proliferation has been observed. For instance, cytoxicity was demonstrated in lymphocytes (Zanovello et al 1990), microglia (Ferrari et al 1997), glomerular mesangial cells (Schulze-Lohoff et al 1998, Harada et al 2000) and thymocytes (Auger et al 2005) whereas in Jurkat cells (Budagian et al 2003) and a subpopulation of lymphoid cells (Baricordi et al 1999), proliferation was observed. Growth-promoting activity of the P2X7 receptor has been suggested to depend on the level of P2X7 activation, as in the case of microglial cells (Bianco et al 2006), or on P2X7-induced release of substance P, as in the case of nucleotide-activated human neuroblastoma cells (Raffaghello et al 2006). However, neither cell death nor growth was detected in astrocytes upon activation of P2X7 receptors. Instead, we found that astrocytes remained viable but were in a non-proliferatative, growth-arrested state. When P2X7 receptors were no longer stimulated, astrocytes could then return to the proliferative state upon stimulation with FGF2. The lack of an effect of BzATP on astrocyte death or growth observed here is in agreement with recent in vivo studies which demonstrated that stimulation of P2X7 receptors with BzATP did not lead to changes in either the number of GFAP-positive cells or GFAP/BrdU proliferating cells in rat brain (Franke et al 2007).
To investigate mechanisms that underlie the inhibitory effect of P2X7 signaling on FGF2-induced proliferation, we turned to p38/MAPK and SAPKs, protein kinases that have a key role in induction of growth arrest (Engelberg 2004). Avruch, Kyriakis, and colleagues identified p54 and p46 serine/threonine protein kinases that were activated by a large number of stress signals and that phosphorylated the transcription factor c-Jun; they termed these enzymes stress-activated protein kinases (SAPKs) (Kyriakis et al 1995). Other investigators studying the same kinases termed them c-Jun-NH2-terminal kinases (JNKs) based on their ability to phosphorylate c-Jun. Other protein kinases, p38/MAPKs, are also activated by stress signals. Thus, the general term SAPK is often used to refer to both p38 and JNKs. It should be noted that SAPKs are members of the mitogen-activated protein kinase (MAPK) family, which also includes extracellular signal-regulated protein kinase (ERK). These kinases are part of cascades consisting of upstream activating protein kinases termed MAPKK and MAPKKK. Data has been presented showing that each of the MAPKs (ERK, p38, JNK) can stimulate as well as inhibit proliferation (for review, see (Engelberg 2004). In addition, the same signal can activate all three cascades with each cascade responding at a specific level. Important factors in determining the biological outcome of MAPK activation are signal strength and duration (Marshall 1995). Other explanations for opposing effects of the same MAPK could be differences in functions of specific isoenzymes, cell-specific differences, or integration of several activities since a single stimulus can activate multiple cascades. Additional factors in the opposing roles of the same MAPKs may be differences between responses in transformed versus non-transformed cells and tissues.
Although further studies are needed to evaluate these possibilities in detail, we can eliminate cell-specific differences since the opposing effects on proliferation were observed in the same cell type. Our results with the SAPKs p38 and JNK are consistent with a role for signal strength and duration. For instance, it has been proposed that in normal cells, transient activation of SAPKs leads initially to inhibition of proliferation and to growth arrest (Engelberg 2004). Following a period of arrest and repair, SAPKs could support the resumption of proliferation. However, in situations of damage, SAPK activation would be more persistent and apoptosis would occur. In primary cultures of astrocytes used here, stimulation to P2X7 receptors led to a transient, strong activation of SAPKs, particularly p38. Consistent with the model of Engelberg, inhibition of FGF2-induced proliferation was observed under these conditions. When the P2X7 agonist was removed, astrocytes were able to resume proliferation in response to FGF2. However, stimulation of P2Y purine, pyrimidine-preferring receptors led to much weaker activation of SAPKs. Interestingly, under these conditions, proliferation was enhanced. Combined with our previous results with ERK (Neary et al 2005a), these findings suggest a role for the integration of ERK, p38 and SAPK signals in the mediating the trophic actions of extracellular nucleotides. Stimulation of P2Y purine/pyrimidine-preferring receptors leads to an initially intense but transient activation of ERK and weak p38 and SAPK signals which is consistent with proliferation. However, stimulation of P2X7 receptors leads to an initially less intense but more sustained ERK activation as well as strong but transient p38 and SAPK signals which is consistent with inhibition of proliferation and induction of growth arrest.
Several subtypes of P2Y, G protein-coupled receptors can regulate astrocyte proliferation. Early studies with synthetic nucleotides in cultured astrocytes suggested a role for P2Y1 and perhaps other types of P2Y receptors in proliferation (Rathbone et al 1992, Abbracchio et al 1994, Neary et al 1994). In vivo, infusion of ADPβS into rat brain stimulated astrocyte proliferation, suggesting a role for ADP-responding receptors such as P2Y1, P2Y12, or P2Y13 (Franke et al 2001, Franke et al 2004). Interactions of P2Y receptors with PDGF and EGF signaling stimulated proliferation of Muller glial cells (Milenkovic et al 2003), and agonists of P2Y1 and P2Y2/4/6 receptors, in combination with FGF2 and EGF, stimulated proliferation of neural precursor cells (Mishra et al 2006, Milosevic et al 2006). In the studies presented here, the enhancing effects of UTP on FGF2-induced DNA synthesis and mitosis suggest a role for P2Y purine/pyrimidine preferring receptors such as P2Y2, P2Y4 or P2Y6 in astrocyte proliferation. This is consistent with stuies on cyclin expression which showed that UTP potentiated cell cycle entry and progression in cultured astrocytes (Neary et al 2005a). Further studies are needed to distinguish the subtype(s) of P2Y purine/pyrimidine preferring receptors involved in astrocyte proliferation.
An emerging theme of P2 receptors is that the expression and activation of P2Y and P2X receptors in the same cells can lead to opposing long-term effects. For instance, previously it was reported that P2Y receptors were linked to proliferation whereas P2X receptors were related to apoptotic cell death in rat glomerular mesangial cells (Harada et al 2000) and human intestinal epithelial carcinoma cells (Coutinho-Silva et al 2005). In both cases, evidence indicated that P2Y purine/pyrimidine preferring receptors induced proliferation since this effect was stimulated by UTP. P2X7 receptors were implicated in apoptosis since this effect was brought about by BzATP or high concentrations of ATP. Similar subtypes of P2 receptors were involved in the opposing effects reported here in astrocytes, although in these cells, P2X7 receptors induced growth arrest rather cell death. The biological outcome could depend on the local concentration of extracellular ATP in which P2Y receptors would be activated by lower concentrations of ATP whereas P2X7 receptors would be activated by higher concentrations. The regulation of opposing long-term trophic effects could be relevant to tissue injury and repair. As previously proposed for neuroinflammatory responses (Kucher & Neary 2005), activation of P2Y purine/pyrimidine and P2X7 receptors may be a mechanism by which astrocytes can sense the severity of damage in a specific region of the CNS and modulate the trophic response accordingly. For example, in mild traumatic brain injury, the release of low levels of ATP would favor astrocyte proliferation. In the case of more severe injury, high levels of ATP could shift astrocytes into a growth arrested state. Subsequent exposure to polypeptide growth factors could then return astrocytes to a proliferative state during a later phase of the repair process.
In addition to injury, the long-term, trophic effects regulated by P2 receptors may also be involved in the development of the nervous system and adult neurogenesis, as reviewed recently by Zimmermann (2006). Recent evidence indicates that ATP is constitutively released from progenitor cells (Lin et al 2007). Various subtypes of P2X and P2Y receptors are expressed in stem cells, neural progenitor cells, and developing embryonic and early postnatal CNS tissues (Meyer et al 1999, Cheung & Burnstock 2002, Cheung et al 2003, Scemes et al 2003, Weissman et al 2004, Cheung et al 2005, Xiang & Burnstock 2005, Heo & Han 2006, Lin et al 2007). Stimulation of P2 receptors leads to increases in inward and spontaneous synaptic currents, membrane depolarization, and calcium responses, as well as activation of protein kinase signaling pathways (Fu & Poo 1991, Sugioka et al 1996, Pearson et al 2002, Ryu et al 2003, Scemes et al 2003, Weissman et al 2004, Tran et al 2004, Pearson et al 2005, Safiulina et al 2005, Mishra et al 2006, Lin et al 2007). In addition, P2 receptor-mediated proliferation and differentiation have been observed in neural progenitor cells and developing CNS tissues (Sugioka et al 1999, Pearson et al 2002, Sanches et al 2002, Ryu et al 2003, Scemes et al 2003, Weissman et al 2004, Pearson et al 2005, Heine et al 2006, Lin et al 2007). Interestingly, P2 receptors have also been implicated in adult neurogenesis (Braun et al 2003, Hogg et al 2004, Shukla et al 2005, Mishra et al 2006). Ecto-nucleotideases have been observed in developing CNS tissue and adult proliferating zones, indicating regulatory mechanisms for nucleotide and nucleoside signaling are present for embryonic and adult neurogenesis (Braun et al 2003, Shukla et al 2005, Mishra et al 2006, Lin et al 2007). In addition, recent reports have shown that extracellular nucleotides can act in combination with polypeptide growth factors such as FGF2, EGF, and PDGF, to stimulate proliferation of adult mouse neural stem cells (Mishra et al 2006) and human mesencephalic neural progenitor cells (Milosevic et al 2006). In view of these findings, we suggest that the enhancement of proliferation by P2Y purine/pyrimidine-prefering receptors and the induction of growth arrest by P2X7 receptors could be important factors in regulating embryonic CNS development and adult neurogenesis.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (NS46651) (J.T.N.) and the Department of Veterans Affairs (J.T.N.). The authors are also grateful to Dr. Brian King, Royal Free Hospital, University College London, UK, for discussions on antagonism of P2X receptors by phenol red used in culture media, and to Dr. Terry Crow, University of Texas Medical School, Houston, TX, for discussions on statistical analyses.
REFERENCES
- Abbracchio MP, Saffrey MJ, Hopker V, Burnstock G. Modulation of astroglial cell proliferation by analogues of adenosine and ATP in primary cultures of rat striatum. Neurosci. 1994;59:67–76. doi: 10.1016/0306-4522(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Ahmed SM, Rzigalinski BA, Willoughby KA, Sitterding HA, Ellis EF. Stretch-induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. J. Neurochem. 2000;74:1951–1960. [PubMed] [Google Scholar]
- Auger R, Motta I, Benihoud K, Ojcius DM, Kanellopoulos JM. A role for mitogen-activated protein kinase(Erk1/2) activation and non-selective pore formation in P2X7 receptor-mediated thymocyte death. J. Biol. Chem. 2005;280(30):28142–28151. doi: 10.1074/jbc.M501290200. [DOI] [PubMed] [Google Scholar]
- Baricordi OR, Melchiorri L, Adinolfi E, Falzoni S, Chiozzi P, Buell G, Di Virgilio F. Increased proliferation rate of lymphoid cells transfected with the P2X7 ATP receptor. J. Biol. Chem. 1999;274:33206–33208. doi: 10.1074/jbc.274.47.33206. [DOI] [PubMed] [Google Scholar]
- Bianco F, Ceruti S, Colombo A, Fumagalli M, Ferrari D, Pizzirani C, Matteoli M, Di Virgilio F, Abbracchio MP, Verderio C. A role for P2X7 in microglial proliferation. J. Neurochem. 2006;99(3):745–758. doi: 10.1111/j.1471-4159.2006.04101.x. [DOI] [PubMed] [Google Scholar]
- Braun N, Sevigny J, Mishra SK, Robson SC, Barth SW, Gerstberger R, Hammer K, Zimmermann H. Expression of the ecto-ATPase NTPDase2 in the germinal zones of the developing and adult rat brain. Eur. J. Neurosci. 2003;17(7):1355–1364. doi: 10.1046/j.1460-9568.2003.02567.x. [DOI] [PubMed] [Google Scholar]
- Budagian V, Bulanova E, Brovko L, Orinska Z, Fayad R, Paus R, Bulfone-Paus S. Signaling through P2X7 receptor in human T cells involves p56lck, MAP kinases, and transcription factors AP-1 and NF-kappa B. J. Biol. Chem. 2003;278(3):1549–1560. doi: 10.1074/jbc.M206383200. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Overview: Purinergic mechanisms. Ann. N. Y. Acad. Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Cheung KK, Burnstock G. Localization of P2X3 receptors and coexpression with P2X2 receptors during rat embryonic neurogenesis. J. Comp Neurol. 2002;443(4):368–382. doi: 10.1002/cne.10123. [DOI] [PubMed] [Google Scholar]
- Cheung KK, Chan WY, Burnstock G. Expression of P2X purinoceptors during rat brain development and their inhibitory role on motor axon outgrowth in neural tube explant cultures. Neuroscience. 2005;133(4):937–945. doi: 10.1016/j.neuroscience.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Cheung KK, Ryten M, Burnstock G. Abundant and dynamic expression of G protein-coupled P2Y receptors in mammalian development. Dev. Dyn. 2003;228(2):254–266. doi: 10.1002/dvdy.10378. [DOI] [PubMed] [Google Scholar]
- Colman H, Giannini C, Huang L, Gonzalez J, Hess K, Bruner J, Fuller G, Langford L, Pelloski C, Aaron J, Burger P, Aldape K. Assessment and prognostic significance of mitotic index using the mitosis marker phospho-histone H3 in low and intermediate-grade infiltrating astrocytomas. Am. J. Surg. Pathol. 2006;30(5):657–664. doi: 10.1097/01.pas.0000202048.28203.25. [DOI] [PubMed] [Google Scholar]
- Coutinho-Silva R, Stahl L, Cheung KK, de Campos NE, de Oliveira SC, Ojcius DM, Burnstock G. P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: effects of extracellular nucleotides on apoptosis and cell proliferation. Am. J. Physiol Gastrointest. Liver Physiol. 2005;288(5):G1024–G1035. doi: 10.1152/ajpgi.00211.2004. [DOI] [PubMed] [Google Scholar]
- Engelberg D. Stress-activated protein kinases-tumor suppressors or tumor initiators? Semin. Cancer Biol. 2004;14(4):271–282. doi: 10.1016/j.semcancer.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Collo G, Buell G, Di Virgilio F. ATP-mediated cytotoxicity in microglial cells. Neuropharmacol. 1997;36:1295–1301. doi: 10.1016/s0028-3908(97)00137-8. [DOI] [PubMed] [Google Scholar]
- Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat. Rev. Neurosci. 2006;7(6):423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H, Krügel U, Illes P. P2 receptor-mediated proliferative effects on astrocytes in vivo. Glia. 1999;28:190–200. [PubMed] [Google Scholar]
- Franke H, Krugel U, Schmidt R, Grosche J, Reichenbach A, Illes P. P2 receptor-types involved in astrogliosis in vivo. Br. J. Pharmacol. 2001;134(6):1180–1189. doi: 10.1038/sj.bjp.0704353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H, Krugel U, Grosche J, Heine C, Hartig W, Allagier C, Illes P. P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neurosci. 2004;127:431–441. doi: 10.1016/j.neuroscience.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Franke H, Schepper C, Illes P, Krugel U. Involvement of P2X and P2Y receptors in microglial activation in vivo. Purinergic Signaling. 2007;3:435–445. doi: 10.1007/s11302-007-9082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu WM, Poo MM. ATP potentiates spontaneous transmitter release at developing neuromuscular synapses. Neuron. 1991;6(5):837–843. doi: 10.1016/0896-6273(91)90179-4. [DOI] [PubMed] [Google Scholar]
- Harada H, Chan CM, Loesch A, Unwin R, Burnstock G. Induction of proliferation and apoptotic cell death via P2Y and P2X receptors, respectively, in rat glomerular mesangial cells. Kidney Int. 2000;57(3):949–958. doi: 10.1046/j.1523-1755.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- Heine C, Heimrich B, Vogt J, Wegner A, Illes P, Franke H. P2 receptor-stimulation influences axonal outgrowth in the developing hippocampus in vitro. Neurosci. 2006;138(1):303–311. doi: 10.1016/j.neuroscience.2005.11.056. [DOI] [PubMed] [Google Scholar]
- Heo JS, Han HJ. ATP stimulates mouse embryonic stem cell proliferation via protein kinase C, phosphatidylinositol 3-kinase/Akt, and mitogen-activated protein kinase signaling pathways. Stem Cells. 2006;24(12):2637–2648. doi: 10.1634/stemcells.2005-0588. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Chipperfield H, Whyte KA, Stafford MR, Hansen MA, Cool SM, Nurcombe V, Adams DJ. Functional maturation of isolated neural progenitor cells from the adult rat hippocampus. Eur. J. Neurosci. 2004;19(9):2410–2420. doi: 10.1111/j.0953-816X.2004.03346.x. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmcol. Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- King BF, Liu M, Townsend-Nicholson A, Pfister J, Padilla F, Ford AP, Gever JR, Oglesby IB, Schorge S, Burnstock G. Antagonism of ATP responses at P2X receptor subtypes by the pH indicator dye, Phenol red. Br. J. Pharmacol. 2005;145:313–322. doi: 10.1038/sj.bjp.0706187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucher BM, Neary JT. Bi-functional effects of ATP/P2 receptor activation on tumor necrosis factor-alpha release in lipopolysaccharide-stimulated astrocytes. J. Neurochem. 2005;92(3):525–535. doi: 10.1111/j.1471-4159.2004.02885.x. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Woodgett JR, Avruch J. The stress-activated protein kinases. A novel ERK subfamily responsive to cellular stress and inflammatory cytokines. Ann. N. Y. Acad. Sci. 1995;766:303–319. doi: 10.1111/j.1749-6632.1995.tb26683.x. [DOI] [PubMed] [Google Scholar]
- Lin JH, Takano T, Arcuino G, Wang X, Hu F, Darzynkiewicz Z, Nunes M, Goldman SA, Nedergaard M. Purinergic signaling regulates neural progenitor cell expansion and neurogenesis. Dev. Biol. 2007;302(1):356–366. doi: 10.1016/j.ydbio.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Meyer MP, Clarke JD, Townsend-Nicholson A, Burnstock G. Selective expression of purinoceptor cP2Y1 suggests a role for nucleotide signalling in development of the chick embryo. Dev. Dyn. 1999;214:152–158. doi: 10.1002/(SICI)1097-0177(199902)214:2<152::AID-AJA5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Milenkovic I, Weick M, Wiedemann P, Reichenbach A, Bringmann A. P2Y receptor-mediated stimulation of Muller glial cell DNA synthesis: dependence on EGF and PDGF receptor transactivation. Invest. Ophthalmol. Vis. Sci. 2003;44:1211–1220. doi: 10.1167/iovs.02-0260. [DOI] [PubMed] [Google Scholar]
- Milosevic J, Brandt A, Roemuss U, Arnold A, Wegner F, Schwarz SC, Storch A, Zimmermann H, Schwarz J. Uracil nucleotides stimulate human neural precursor cell proliferation and dopaminergic differentiation: involvement of MEK/ERK signalling. J. Neurochem. 2006;99(3):913–923. doi: 10.1111/j.1471-4159.2006.04132.x. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Braun N, Shukla V, Fullgrabe M, Schomerus C, Korf HW, Gachet C, Ikehara Y, Sevigny J, Robson SC, Zimmermann H. Extracellular nucleotide signaling in adult neural stem cells: synergism with growth factor-mediated cellular proliferation. Development. 2006;133(4):675–684. doi: 10.1242/dev.02233. [DOI] [PubMed] [Google Scholar]
- Neary JT, Abbracchio MP. Trophic Roles of Purines and Pyrimidines. In: Williams M, Abbracchio MP, editors. Handbook of Experimental Pharmacology: Purinergic and Pyrimidergic Signalling. Vol. 11. New York: Springer-Verlag; 2001. pp. 305–338. 305–338 pp. [Google Scholar]
- Neary JT, Kang Y, Shi Y-F. Cell cycle regulation of astrocytes by extracellular nucleotides and fibroblast growth factor-2. Purinergic Signalling. 2005a;1:329–336. doi: 10.1007/s11302-005-8075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, Kang Y, Tran MD, Feld J. Traumatic Injury Activates Protein Kinase B/Akt in Cultured Astrocytes: Role of Extracellular ATP and P2 Purinergic Receptors. J. Neurotrauma. 2005b;22:491–500. doi: 10.1089/neu.2005.22.491. [DOI] [PubMed] [Google Scholar]
- Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- Neary JT, Whittemore SR, Zhu Q, Norenberg MD. Synergistic activation of DNA synthesis in astrocytes by fibroblast growth factor and extracellular ATP. J. Neurochem. 1994;63:490–494. doi: 10.1046/j.1471-4159.1994.63020490.x. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Pearson R, Catsicas M, Becker D, Mobbs P. Purinergic and muscarinic modulation of the cell cycle and calcium signaling in the chick retinal ventricular zone. J. Neurosci. 2002;22(17):7569–7579. doi: 10.1523/JNEUROSCI.22-17-07569.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46(5):731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Peterson GL. Determination of total protein. Meth. Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Raffaghello L, Chiozzi P, Falzoni S, Di Virgilio F, Pistoia V. The P2X7 receptor sustains the growth of human neuroblastoma cells through a substance P-dependent mechanism. Cancer Res. 2006;66(2):907–914. doi: 10.1158/0008-5472.CAN-05-3185. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rathbone MP, Middlemiss PJ, Gysbers JW, DeForge S, Costello P, Del Maestro RF. Purine nucleosides and nucleotides stimulate proliferation of a wide range of cell types. In Vitro Cell Dev. Biol. 1992;28A:529–536. doi: 10.1007/BF02634137. [DOI] [PubMed] [Google Scholar]
- Ryu JK, Choi HB, Hatori K, Heisel RL, Pelech SL, McLarnon JG, Kim SU. Adenosine triphosphate induces proliferation of human neural stem cells: Role of calcium and p70 ribosomal protein S6 kinase. J. Neurosci. Res. 2003;72(3):352–362. doi: 10.1002/jnr.10507. [DOI] [PubMed] [Google Scholar]
- Safiulina VF, Kasyanov AM, Sokolova E, Cherubini E, Giniatullin R. ATP contributes to the generation of network-driven giant depolarizing potentials in the neonatal rat hippocampus. J. Physiol. 2005;565(Pt 3):981–992. doi: 10.1113/jphysiol.2005.085621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches G, de Alencar LS, Ventura AL. ATP induces proliferation of retinal cells in culture via activation of PKC and extracellular signal-regulated kinase cascade. Int. J. Dev. Neurosci. 2002;20(1):21–27. doi: 10.1016/s0736-5748(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Scemes E, Duval N, Meda P. Reduced expression of P2Y1 receptors in connexin43-null mice alters calcium signaling and migration of neural progenitor cells. J. Neurosci. 2003;23(36):11444–11452. doi: 10.1523/JNEUROSCI.23-36-11444.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Lohoff E, Hugo C, Rost S, Arnold S, Gruber A, Brune B, Sterzel RB. Extracellular ATP causes apoptosis and necrosis of cultured mesangial cells via P2Z/P2X7 receptors. Am. J. Physiol. 1998;275(6 Pt 2):F962–F971. doi: 10.1152/ajprenal.1998.275.6.F962. [DOI] [PubMed] [Google Scholar]
- Shukla V, Zimmermann H, Wang L, Kettenmann H, Raab S, Hammer K, Sevigny J, Robson SC, Braun N. Functional expression of the ecto-ATPase NTPDase2 and of nucleotide receptors by neuronal progenitor cells in the adult murine hippocampus. J. Neurosci. Res. 2005;80(5):600–610. doi: 10.1002/jnr.20508. [DOI] [PubMed] [Google Scholar]
- Sugioka M, Fukuda Y, Yamashita M. Ca2+ responses to ATP via purinoceptors in the early embryonic chick retina. J. Physiol. 1996;493.3:855–863. doi: 10.1113/jphysiol.1996.sp021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugioka M, Zhou WL, Hofmann HD, Yamashita M. Involvement of P2 purinoceptors in the regulation of DNA synthesis in the neural retina of chick embryo. Int. J. Dev. Neurosci. 1999;17(2):135–144. doi: 10.1016/s0736-5748(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J. Neurosci. Res. 2004;76(1):20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- Washburn KB, Neary JT. P2 purinergic receptors signal to STAT3 in astrocytes: Difference in STAT3 responses to P2Y and P2X receptor activation. Neuroscience. 2006;142(2):411–423. doi: 10.1016/j.neuroscience.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43(5):647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Wildman SS, Unwin RJ, King BF. Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br. J. Pharmacol. 2003;140:1177–1186. doi: 10.1038/sj.bjp.0705544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Burnstock G. Changes in expression of P2X purinoceptors in rat cerebellum during postnatal development. Brain Res. Dev. Brain Res. 2005;156(2):147–157. doi: 10.1016/j.devbrainres.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Zanovello P, Bronte V, Rosato A, Pizzo P, Di Virgilio F. Responses of mouse lymphocytes to extracellular ATP. II. Extracellular ATP causes cell type-dependent lysis and DNA fragmentation. J. Immunol. 1990;145:1545–1550. [PubMed] [Google Scholar]
- Zimmermann H. Nucleotide signaling in nervous system development. Pflugers Arch. 2006;452(5):573–588. doi: 10.1007/s00424-006-0067-4. [DOI] [PubMed] [Google Scholar]