Abstract
Objective
To describe the relationship between implantation-associated trauma and postoperative speech perception scores among adult and pediatric patients undergoing cochlear implantation using conventional length electrodes and minimally traumatic surgical techniques.
Study Design
Retrospective chart review (2002–2010).
Setting
Tertiary academic referral center.
Patients
All subjects with significant preoperative low-frequency hearing (≤70 dB HL at 250 Hz) who underwent cochlear implantation with a newer generation implant electrode (Nucleus Contour Advance, Advanced Bionics HR90K [1J and Helix], and Med El Sonata standard H array) were reviewed.
Intervention(s)
Preimplant and postimplant audiometric thresholds and speech recognition scores were recorded using the electronic medical record.
Main Outcome Measure(s)
Postimplantation pure tone threshold shifts were used as a surrogate measure for extent of intracochlear injury and correlated with postoperative speech perception scores.
Results
Between 2002 and 2010, 703 cochlear implant (CI) operations were performed. Data from 126 implants were included in the analysis. The mean preoperative low-frequency pure-tone average was 55.4 dB HL. Hearing preservation was observed in 55% of patients. Patients with hearing preservation were found to have significantly higher postoperative speech perception performance in the cochlear implantation-only condition than those who lost all residual hearing.
Conclusion
Conservation of acoustic hearing after conventional length cochlear implantation is unpredictable but remains a realistic goal. The combination of improved technology and refined surgical technique may allow for conservation of some residual hearing in more than 50% of patients. Germane to the conventional length CI recipient with substantial hearing loss, minimizing trauma allows for improved speech perception in the electric condition. These findings support the use of minimally traumatic techniques in all CI recipients, even those destined for electric-only stimulation.
Keywords: Cochlear implant(s), Hearing preservation, Hearing conservation, Auditory stimulation
In 1984, the first single-channel cochlear implant (CI) was approved by the Food and Drug Administration for implantation in adult patients with profound postlingual deafness. Since this time, the evolution of cochlear implantation has been marked by rapid technological advancement and surgical refinement resulting in unprecedented success. Innovations, including the selective scala tympani insertion, the addition of the multi-channel electrode, and increasingly sophisticated speech processing strategies, have revolutionized the early CI industry (1).
With early CI models, it was held that electrode insertion resulted in extensive intracochlear injury, thereby irreversibly destroying any residual acoustic potential. Within the last 20 years, however, there have been a series of publications documenting ever improving rates of hearing preservation (2) and corresponding histologic studies confirming attenuated intracochlear injury with newer generation implants (1,3). Within the last decade, there has been a paradigm shift toward the development of least-traumatic electrode designs and soft surgical techniques to improve CI outcomes (4). Today, even patients with substantial residual acoustic hearing are potential CI candidates (2,5).
Minimizing intracochlear trauma during implantation may offer several noteworthy advantages. First, for patients with “usable” preimplant low-frequency hearing, limiting trauma can allow for the preservation of native hearing, thereby accommodating concurrent electric-acoustic stimulation (EAS) (6). Second, lessening intracochlear damage may limit the amount of fibrosis and neo-ossification, making revision surgery for device failure or upgrade less problematic (7); this is becoming increasingly important as more patients are undergoing implantation during infancy and early childhood, thereby increasing the likelihood that reimplantation will be required during their lifetime. Third, limiting injury potentially allows for the application of future technologies, such as cellular regeneration or other novel cochlear nerve stimulation technologies.
Although not conclusively proven, it has been theorized that minimizing trauma during electrode insertion may result in improved outcomes with electrical stimulation (8). Insertional trauma may directly or indirectly cause injury to neurosensory elements and result in suboptimal electrode positioning away from the modiolus (4,9); it would therefore stand to reason that limiting such injuries would be paramount to optimal CI performance.
Previous studies evaluating hearing preservation with either short or conventional length electrodes have primarily focused on the incidence and sustainability of postoperative preserved hearing and the benefit of EAS. However, in the present study, we test the hypothesis that patients sustaining less intracochlear trauma during implantation with a conventional length electrode will demonstrate improved speech understanding in the electric-only condition. If substantiated, the results of this study would support the argument for minimizing insertion trauma in all conventional CI recipients realizing that reducing trauma optimizes electrical stimulation.
Recently, Balkany et al. (2) proposed the use of the postimplantation low-frequency pure tone threshold shift as a surrogate marker for the degree of acquired intracochlear trauma. Adopting this strategy, we evaluated audiometric data from 118 patients (126 implants) who demonstrated substantial preimplant low-frequency hearing. Those with hearing preservation after implantation were then compared with those with complete acoustic hearing loss to determine whether hearing preservation (a surrogate for attenuated intracochlear injury) conferred improved postimplantation speech perception performance in the CI–only condition.
METHODS
After institutional review board approval (IRB 10-003741), a retrospective chart review (2002–2010) was performed using the electronic medical record. Patients who underwent implantation with a newer generation conventional length electrode (Nucleus Contour Advance, Advanced Bionics HR90K (1J and Helix), and the Med El Sonata with a standard “H” electrode) who were capable of completing our adult CI evaluation battery were included. No patients underwent implantation with an EAS design electrode. To decrease bias associated with upper output limitations posed by commercial audiometers (2), only patients with substantial levels of preoperative acoustic hearing were evaluated; specifically, audiometric thresholds of 70 dB HL or better at 250 Hz were required for study inclusion. This criterion was chosen to include patients within test-retest variability of the 250-Hz criterion for the North American EAS/Hybrid clinical trials.
All subjects received comprehensive preimplant and postimplant audiologic evaluation including pure tone audiometry and speech perception testing. Standard pure tone audiometric procedures were used with a calibrated audiometer. A low-frequency pure-tone average (LF PTA) using the average threshold value, in dB HL, for 125, 250, and 500 Hertz was then calculated for each testing session. All patients were instructed to carefully differentiate auditory perception and vibrotactile sensation when they responded to pure tone testing. Per our CI program protocol, speech perception testing included preimplant and postimplant administration of Bamford-Kowal-Bench speech in noise test (BKB-SIN; Etymotic Research, 2005), Consonant Nucleus Consonant (10) (CNC) monosyllabic words, and Arizona Biomedical Sentences (AzBio). Speech perception performance was assessed using recorded stimuli at a calibrated presentation level of 60 dBA. A single loudspeaker placed at the zero-degree azimuth at a distance of 1 m from the patient was used for presentation of speech stimuli. Preoperative audiometric testing was obtained approximately 1 month before surgery for all patients, and data from the most recent postoperative testing session were used for comparison.
All devices were implanted using postauricular access through a limited mastoidectomy and facial recess approach. The basic elements of atraumatic surgery were followed. Intraoperative intravenous steroids were given in most cases (largely to help with postoperative nausea). Attempts were made to limit bone dust and blood from entering the cochlea; there was minimal or no suctioning of perilymph. The surgical procedure has evolved over the time frame of this study, and there was varying adherence to the standard soft-surgery technique by the individual surgeons. In general, after the mastoidectomy and facial recess were complete, the device was seated in a subperiosteal pocket. The cochleostomy was routinely made just anteroinferior to the round window, typically beginning with a 1.5-mm diamond burr to expose the endosteum. In most cases, the last remaining thin bone and endosteum were opened with a 1.0-mm diamond burr at low speed. The size of the cochleostomy was based on the specific device being implanted. The electrode array was inserted according to the manufacturer recommendations, and a full insertion was achieved in all cases. Soft tissue was placed around the electrode to seal the cochleostomy. When the round window membrane was in a favorable orientation and readily visible without significant drilling, a subset of patients underwent round window electrode insertion after gently incising the membrane with a hypodermic needle.
Patients were divided into 2 groups: those with some degree of acoustic hearing preservation as determined by detectable thresholds during pure tone audiometry and those with complete loss of detectable hearing. Comparisons of continuous variables between groups were evaluated using 2-sample t tests for variables that were approximately normally distributed and Wilcoxon rank sum tests otherwise. Comparisons of categorical variables between groups were evaluated using χ2 and Fisher’s exact tests. Correlations between continuous variables were evaluated using Pearson correlation coefficients. Differences in postoperative speech perception performance between groups after adjusting for preoperative audiometric thresholds and age differences were evaluated using multiple linear regression models. All tests were 2 sided, and p italic> 0.05 was considered statistically significant.
RESULTS
Between 2002 and 2010, 703 CI operations were performed. A total of 126 implants in 118 postlingually deafened patients (48 female and 70 male patients; mean age, 60.6 yr; standard deviation [SD], 19.6 yr; range, 7–89 yr) met inclusion criteria and were included in the present study. The mean implant experience at time of testing was 35.7 months (SD, 23.2 mo; range, 1.1–98.1 mo). The Nucleus Contour Advance (CI24RCA, CI24RE, and CI512) was the most frequently implanted electrode (82%), followed by the Advanced Bionics HR90K (1J and Helix) (15%) and the Med El Sonata (standard “H” array) (3%).
The mean overall preimplant LF PTA for the ear to be implanted was 55.4 dB HL (SD, 14.3 dB HL; n = 126). Mean preimplant CNC word recognition in the best-aided condition was 18.8% (SD, 14.0; n = 89). Sixty-nine patients (55%) had measurable postoperative acoustic hearing, whereas the remaining 57 (45%) lost all residual hearing after implantation. The mean age for the patients in the hearing preservation group was 56.4 years (SD, 21.4 yr) compared with 65.7 years (SD, 16.0 yr) for the patients in the nonhearing preservation group (p = 0.045) (Table 1). The mean months of experience for the hearing preservation group was 36.7 (median, 33.1; range, 2.4–98.1) compared with 28.8 (median, 25.1; range, 1.1–72.7) for the nonhearing preservation group (p = 0.07). Nine (13%) of the 69 patients in the hearing preservation group had less than 6 months of experience compared with 5 (9%) of the 57 patients in the nonhearing preservation group (p = 0.45). There was no statistical performance advantage seen in patients with more than 6 months of experience compared with those with less on CNC (64.8% versus 71.8%, p = 0.17), AzBio (80.3% versus 81.3%, p = 0.38), or BKB-SIN (8.8 versus 9.1, p = 0.99) testing.
TABLE 1.
Frequency and degree of postoperative hearing preserved after cochlear implantation by age group
| Hearing preservation group n (%) | Nonhearing preservation group n (%) | Postoperative low-frequency pure-tone average shift (dB) | |
|---|---|---|---|
| 0–19 yr | 9 (90) | 1 (10) | 35.9 |
| 20–49 yr | 7 (50) | 7 (50) | 43.0 |
| 50–59 yr | 9 (47) | 10 (53) | 43.9 |
| 60–69 yr | 26 (65) | 14 (35) | 40.8 |
| 70–79 yr | 13 (57) | 10 (43) | 46.6 |
| 80+ yr | 5 (25) | 15 (75) | 44.3 |
For the 69 patients with hearing preservation, mean pre-implant LF PTA and best-aided CNC word recognition was 51.5 dB HL (SD, 15.4 dB HL; n = 69) and 21.9%, (SD, 12.3%; n = 46), respectively. For the 57 patients experiencing loss of residual hearing, mean preimplant LF PTA and best-aided CNC word recognition was 60.1 dB HL (SD, 11.4 dB HL; n = 57) and 15.5% (SD, 14.9%; n = 43), respectively. Statistical analysis completed on preimplant LF PTA and best-aided CNC revealed that patients with hearing preservation had significantly better preoperative low-frequency hearing (p bold> 0.001) and CNC word recognition (p = 0.005) (Table 2).
TABLE 2.
Preoperative age and audiometric differences between the hearing and nonhearing preservation groups
| Hearing preservation group (55%) | Nonhearing preservation group (45%) | p | |
|---|---|---|---|
| Age at implantation (yr) | 56.4 | 65.7 | 0.045 |
| Preoperative low-frequency pure-tone average (dB HL) | 51.5 | 60.1 | <0.001 |
| Preoperative Consonant Nucleus Consonant (%) | 21.9 | 15.5 | 0.005 |
Mean postoperative implant-only CNC word recognition scores for the hearing preservation and nonhearing preservation groups were 70.6% (SD, 19.0%; n = 69) and 59.6% (SD, 20.6; n = 57) (p = 0.003), respectively. The mean postoperative implant-only AzBio sentence recognition in quiet scores for the hearing preservation and nonhearing preservation groups were 83.8% (SD, 15.0%; n = 52) and 76.6% (SD, 17.4; n = 47) (p = 0.029), respectively. Thus, on average, those patients with hearing preservation scored 11-percentage points higher on CNC word testing and 7.2-percentage points higher on AzBio sentence recognition in quiet compared with those who lost all hearing. Mean implant-only BKB-SIN sentence recognition in noise scores (in dB SNR-50) for the hearing preservation and nonhearing preservation groups were 8.2 (SD, 3.7; n = 53) and 9.6 dB SNR (SD, 3.7; n = 42), respectively—a lower score denoting of better performance. Sentence recognition in noise as measured on the BKB-SIN test was not found to be significantly different across the 2 subject groups (p = 0.065) (Table 3; Fig. 1).
TABLE 3.
Postimplantation speech perception differences between groups with univariate and multivariate statistical analysis (adjusting for age and preoperative audiometric group discrepancies)
| Hearing preservation group (55%) | Nonhearing preservation group (45%) | Univariate analysis | Multivariate analysis | |
|---|---|---|---|---|
| Postoperative Consonant Nucleus Consonant (%) | 70.6 | 59.6 | p = 0.003 | p = 0.034 |
| Postoperative Bamford-Kowal-Bench speech in noise test (%) | 83.8 | 76.6 | p = 0.029 | p = 0.300 |
| Postoperative Arizona Biomedical Sentences (db SNR) | 8.2 | 9.6 | p = 0.065 | p = 0.508 |
FIG. 1.
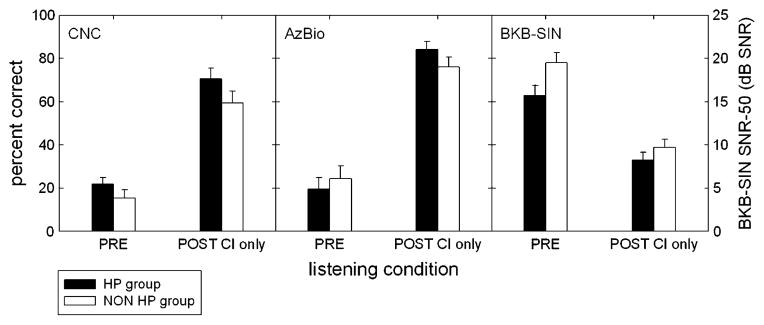
Mean preoperative and postoperative speech perception scores (CNC, AzBio, and BKB-SIN) for the hearing preservation and nonhearing preservation groups. The filled and unfilled bars represent scores for the hearing preservation and nonhearing preservation subjects, respectively.
Among patients experiencing hearing preservation, the mean postoperative LF PTA was 93.6 dB HL (n = 69). Mean degree of hearing loss (i.e., audiometric threshold shift) was 38.2 dB at 125 Hz, 44.0 dB at 250 Hz, 45.1 dB at 500 Hz, and 27.2 dB at 1,000 Hz (Fig. 2). Pearson correlation analyses were performed to evaluate the relationship between age, preoperative CNC word score performance, preoperative LF PTA, and postoperative speech perception performance. Age was significantly correlated with 2 of the 3 measures of postoperative speech perception: CNC words (r = −0.26; p = 0.004; n = 126), AzBio sentences (r = −0.26, p = 0.010; n = 99), and BKB-SIN (r = 0.19, p = 0.071; n = 95). There was no correlation between preoperative CNC word recognition and postoperative speech perception performance for CNC words (r = 0.16, p = 0.14; n = 89), AzBio sentences (r = 0.03, p = 0.77; n = 75), or BKB-SIN (r = −0.13, p = 0.27; n = 73). Preoperative audiometric LF PTA and postoperative performance was found to be significantly correlated with 2 of the 3 measures of postoperative speech perception: CNC words (r = −0.14, p = 0.12; n = 126), AzBio sentences (r = −0.20, p = 0.048; n = 99), and BKB-SIN (r = 0.22, p = 0.034; n = 95).
FIG. 2.

Mean low-frequency postoperative threshold shift among patients within the hearing preservation group.
Given preoperative age and audiometric discrepancies between groups, multiple linear regression analyses were performed to determine if hearing preservation, younger age, or lower LF PTA scores independently conferred improved postimplant speech perception performance. When adjusting for preoperative differences, patients with hearing preservation demonstrated improved CNC word scores (p = 0.034) independent of age and preoperative LF PTA differences; however, no statistical associations were seen with postoperative AzBio (p = 0.300) or BKB-SIN (p = 0.508) sentence scores (Table 3). Younger age was statistically associated with better postoperative implant-only CNC word scores (p = 0.018) and AzBio sentence scores (p = 0.012) but not BKB-SIN scores (p = 0.080). Finally, preoperative LF PTA as an independent variable was not statistically associated with postoperative CNC (p = 0.400), AzBio (p = 0.079), or BKB-SIN (p = 0.065) scores.
Other variables including round window versus cochleostomy electrode insertion, the use of a single intraoperative intravenous dose of dexamethasone (10 mg), surgeon experience, and the model type implanted were evaluated to determine if any additional factors carried a statistical association with regard to postoperative hearing preservation or improved speech perception scores. The mean postoperative AzBio score was 8.1-percentage points higher for patients receiving perioperative steroids (84.9% versus 76.8%) compared with those without (p = 0.011), whereas CNC and BKB-SIN scores were not statistically different with steroid use (p = 0.084 and p = 0.086, respectively). The device-specific hearing preservation rate for the HR90k device (Advanced Bionics Corp.) was 31.6% (6/19), (0% [0/1] for the 1J electrode and 33.3% [6/18] for the Helix electrode), 50% (2/4) for the Sonata (Med El GmbH) equipped with the “H” standard electrode, and 59.2% (61/103) for Cochlear Corp devices using the Contour Advance electrode; intermodel differences were not statistical (p = 0.081). Among patients within the hearing preservation group, the LF PTA shift was 49.3, 46.7, and 41.3 dB for the Advanced Bionics (Helix and 1J), Med EL Sonata with the standard electrode, and Nucleus Contour Advance Electrode equipped devices, respectively (p = 0.25). A total of 7 patients underwent a round window insertion 5 within the hearing preservation group and 2 within the nonhearing preservation group; the Nucleus Contour Advance electrode was used in all instances. There were no statistically significant associations between type of insertion and postoperative CNC (p = 0.071), AzBio (p = 0.099), or BK-SIN (p = 0.277) scores. Three otologic surgeons collectively implanted all 126 subjects; there were no statistical differences in ability to preserve hearing between the 3 surgeons (p = 0.160).
DISCUSSION
The present study demonstrates that hearing preservation with a conventional length electrode is possible. However, perhaps more novel, we found that minimizing insertional trauma confers improved speech perception in the CI–only condition. This finding is important because it supports the use of minimally traumatic techniques in all CI recipients, even those destined for electric only stimulation.
In 2006, Balkany et al. (2) described the potential use of postimplantation pure tone threshold shifts as a surrogate marker for extent of sustained insertional trauma. We chose to adopt this method and were able to correlate findings with postoperative speech recognition scores. Specifically, patients with postimplant hearing preservation demonstrated improved CNC word scores in the implant-only condition independent of age and preoperative LF PTA differences. The theory behind this approach is based on 2 suppositions.
First, the extent of acoustic hearing loss after implantation is heavily associated with the degree of injury. The impetus for the development of a shorter EAS electrode design is based on the notion that an abbreviated electrode will result in less inner ear injury during implantation and, therefore, may preserve higher degrees of acoustic hearing. Because direct or indirect electrode-associated injury is a primary cause of postimplantation hearing loss, one could indirectly estimate the extent of intracochlear trauma based on pure tone threshold shifts after implantation.
Second, many of the mechanisms that are responsible for loss of acoustic hearing also would affect CI electrical stimulation. Because electrical stimulation requires the relay of coded electrical data to spiral ganglion cells, diminished performance in this setting is likely related to either damage of such neurosensory elements or preclusion of efficacious/efficient electrode-to-neuron transmission. With this in mind, there are a number of published mechanisms of inner ear injury after implantation that deserve discussion (Table 4).
TABLE 4.
Mechanisms of implant-related cochlear injury
| Acute direct mechanical trauma from electrode or hydraulic forces |
| Fracturing of osseous spiral lamina containing dendrite processes |
| Injury to the modiolus (containing spiral ganglion cells) located along the medial scala tympani wall |
| Compression or tearing of superficial arterial supply or draining venous systems |
| Damage to the lateral wall (containing spiral ligament, organ of Corti, and stria vascularis) |
| Misdirection of electrode into the scala media or scala vestibuli |
| Acute non-mechanical injuries |
| Acoustic trauma from drilling |
| Disruption labyrinthine fluid homeostasis through excessive suctioning of perilymphatic fluid, introduction of blood into the scala tympani, and mixing of endolymphatic and perilymphatic fluids |
| Subacute or delayed deleterious events |
| Bacterial labyrinthitis from spread of middle ear flora into the cochlea |
| Foreign body reaction to electrode |
| Reactive fibrosis and/or ossification (resulting from mechanical trauma, bacterial infection, foreign body reaction, and introduction of bone dust into cochleostomy) |
| Molecular activation of proapoptosis and necrosis pathways resulting in delayed neural injury |
The primary focus of trauma associated performance loss among conventional CI recipients has centered on both direct and indirect causes of spiral ganglion cell degeneration. Histologic studies using human cadaveric temporal bone specimens have shown that direct injury to the modiolus or fracturing of the osseous spiral lamina is common even with newer generation electrode designs (11). Animal models have further demonstrated that even minor injury can lead to significant reduction of spiral ganglion cell populations in the region of injury (12).
Injury to the organ of Corti (13) or activation of molecular proapoptotic pathways (14) may indirectly result in spiral ganglion neural degeneration. Animal models have confirmed that loss of stimulating inner hair cells can lead to spiral ganglion cell degeneration (15,16). Injury to the organ of Corti and its constituent cells may result from direct electrode trauma or hydraulic injury (14), acoustic insult from drilling, or disruption of electrolyte homeostasis (4). Additionally, Eshraghi and Van de Water (14) were able to identify proapoptotic molecular pathway activation after cochlear implantation trauma using a guinea pig model; they have proposed that this mechanism may be at least partly responsible for delayed neurosensory cell degeneration after cochlear implantation.
The true significance of such injuries and the number of residual spiral ganglion cells required for adequate electrode stimulation in humans remains unknown. We would predict that changes in the number and distribution of spiral ganglion cells would affect CI performance; however, there are examples of patients who had excellent CI performance despite having lesser numbers of spiral ganglion cells on postmortem histologic analysis (17–19). Previous authors have discussed that, after a critical mass of spiral ganglion cell numbers has been reached, additional cell numbers do not necessarily confer improved speech recognition performance (20). In patients with already limited numbers of spiral ganglion cells, it is possible that even small losses may breach minimal number threshold requirements and impair CI performance. Although electrical stimulation from CI may confer a protective effect (12), any such advantage may be negated by substantial trauma.
In addition to injuring functional structures, traumatic insertions may be associated with suboptimal electrode placement (9,11,21). Perimodiolar positioning may carry several distinct advantages including lower threshold of stimulation, lower power consumption, more discrete spiral ganglion neural population stimulation, decreased likelihood of aberrant stimulation, and less risk of outer wall injury (1,22). Studies have shown that conventional length electrodes often acquire a lateral wall orientation in the distal basal turn of the cochlea and beyond (1). When moving from the basal cochlea to the apex, the turning radius of the cochlea shortens, and the cross-sectional area of the scala tympani lessens (23); the combination of these 2 factors results in increased outer wall impingement and may cause fracturing of the interscalar partition and excursion of the electrode into the scala media or scala vestibuli far from the intended site of stimulation (4,9,22). Studies using computed tomography of implanted patients have shown that those with electrode positions within the scala vestibuli have diminished speech performance scores (11,21,24).
Correlating the degree and type of intracochlear injury with speech perception performance remains critical as we move forward with new electrode designs and surgical technique. Unfortunately, there is no perfect method for correlating histologic injury to postoperative speech performance gain. The plurality of studies have used histologic analysis after electrode insertion in human cadaveric temporal bones to determine common patterns of trauma (1,4,9,25). With this strategy, only immediate mechanical trauma can be studied, and the implications of these injuries are theoretical without paired audiometric performance data. Animal models have been helpful for studying both acute and delayed effects of implantation (14,26). However, patterns of spiral ganglion cell degeneration from electrode trauma may be different in animal models compared with humans, thereby limiting clinical applicability (27,28). A third strategy has been the use of high-resolution computed tomography in implanted patients to correlate electrode location with speech performance (11,21,24). Beyond insertion depth and scalar location, the resolution limits posed by current imaging techniques prohibit the fine detail required to comment on injury to small radiolucent intracochlear structures. Finally, a handful of investigations have studied pledged temporal bone specimens from patients who were implanted during their lifetime whom have performance data available for review (17,18,29). Data from these studies may provide us with the most direct method of correlating speech performance data with type of intracochlear injury; however, the number of study specimens is very limited and commonly include early generation single-channel electrode designs.
The current study design carries several advantages over previous strategies. First, and most important, it allows for the comparison of speech recognition performance to the degree of sustained trauma in humans subjects. Second, it enables us to include a large number of patients with the most recent technologies because we do not have to rely on postmortem analysis to determine the extent of trauma. One limitation is the lack of information about the actual types of injuries incurred; the amount of hearing loss is used as a surrogate measure to estimate the extent of injury, and actual histologic analysis was not performed. Additionally, it is possible that progression of the underlying disease process may be at least partially responsible for some of the hearing loss seen after surgery. Finally, although we did include a significant number of patients with substantial degrees of preoperative low-frequency hearing, the current study design is not immune to the ceiling effect limitation posed by the upper output limit of commercial audiometers (2).
Although not the primary focus of our study, the relatively large number of patients with substantial degrees of preoperative low-frequency native hearing fortuitously allows us to compare the prevalence and degree of hearing preservation of conventional length electrodes with early published data from EAS clinical trials in patients with similar preoperative pure tone thresholds. Results from the Hybrid-10 (S8) (6) and Hybrid-L24 (30) demonstrate some degree of hearing preservation in more than 90% of all patients. Our study agrees with previous reports; shorter EAS designs are able to preserve residual hearing more consistently and to a higher degree than current conventional CI designs. Other investigations evaluating the prevalence of hearing preservation with conventional electrodes have reported success rates ranging from approximately 50% (31) to 90% (2). Inconsistent audiometric reporting and the use of varying surgical techniques make interstudy comparisons difficult.
CONCLUSION
In summary, minimizing insertional trauma remains critical to improving CI outcomes. Early results from EAS clinical trials have demonstrated that when combining soft surgical techniques with a thinner and shorter electrode design, minimizing trauma can allow for the preservation of usable acoustic hearing (6). Perhaps more relevant to the conventional length electrode recipient with more substantial hearing loss, minimally traumatic electrode insertion confers enhanced speech perception scores in the CI–only condition. The results of our study support the use of soft surgical technique and minimally traumatic electrode design in all patients undergoing implantation with a conventional length electrode to optimize CI performance.
Despite a standardized surgical approach used in all patients, the degree of sustained intracochlear injury was not predictable. Future studies evaluating alternate surgical techniques, modified electrode designs, or novel pharmacologic therapies will be beneficial for determining which strategies afford a more consistent capacity for atraumatic insertions.
Acknowledgments
No funding or other support was required for this study.
References
- 1.Roland JT., Jr A model for cochlear implant electrode insertion and force evaluation: results with a new electrode design and insertion technique. Laryngoscope. 2005;115:1325–39. doi: 10.1097/01.mlg.0000167993.05007.35. [DOI] [PubMed] [Google Scholar]
- 2.Balkany TJ, Connell SS, Hodges AV, et al. Conservation of residual acoustic hearing after cochlear implantation. Otol Neurotol. 2006;27:1083–8. doi: 10.1097/01.mao.0000244355.34577.85. [DOI] [PubMed] [Google Scholar]
- 3.Wright CG, Roland PS, Kuzma J. Advanced bionics thin lateral and Helix II electrodes: a temporal bone study. Laryngoscope. 2005;115:2041–5. doi: 10.1097/01.MLG.0000181461.63392.49. [DOI] [PubMed] [Google Scholar]
- 4.Roland PS, Wright CG. Surgical aspects of cochlear implantation: mechanisms of insertional trauma. Adv Otorhinolaryngol. 2006;64:11–30. doi: 10.1159/000094642. [DOI] [PubMed] [Google Scholar]
- 5.Gifford RH, Dorman MF, Shallop JK, et al. Evidence for the expansion of adult cochlear implant candidacy. Ear Hear. 2010;31:186–94. doi: 10.1097/AUD.0b013e3181c6b831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gantz BJ, Hansen MR, Turner CW, et al. Hybrid 10 clinical trial: preliminary results. Audiol Neurootol. 2009;14:32–8. doi: 10.1159/000206493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somdas MA, Li PM, Whiten DM, et al. Quantitative evaluation of new bone and fibrous tissue in the cochlea following cochlear implantation in the human. Audiol Neurootol. 2007;12:277–84. doi: 10.1159/000103208. [DOI] [PubMed] [Google Scholar]
- 8.Kiefer J, Gstoettner W, Baumgartner W, et al. Conservation of low-frequency hearing in cochlear implantation. Acta Otolaryngol. 2004;124:272–80. doi: 10.1080/00016480310000755a. [DOI] [PubMed] [Google Scholar]
- 9.Wardrop P, Whinney D, Rebscher SJ, et al. A temporal bone study of insertion trauma and intracochlear position of cochlear implant electrodes. I: Comparison of Nucleus banded and Nucleus Contour electrodes. Hear Res. 2005;203:54–67. doi: 10.1016/j.heares.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord. 1962;27:62–70. doi: 10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- 11.Aschendorff A, Kromeier J, Klenzner T, et al. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear. 2007;28:75S–9S. doi: 10.1097/AUD.0b013e318031542e. [DOI] [PubMed] [Google Scholar]
- 12.Leake PA, Hradek GT, Snyder RL. Chronic electrical stimulation by a cochlear implant promotes survival of spiral ganglion neurons after neonatal deafness. J Comp Neurol. 1999;412:543–62. doi: 10.1002/(sici)1096-9861(19991004)412:4<543::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann CE, Burgess BJ, Nadol JB., Jr Patterns of degeneration in the human cochlear nerve. Hear Res. 1995;90:192–201. doi: 10.1016/0378-5955(95)00165-1. [DOI] [PubMed] [Google Scholar]
- 14.Eshraghi AA, Van de Water TR. Cochlear implantation trauma and noise-induced hearing loss: apoptosis and therapeutic strategies. Anat Rec. 2006;288:473–81. doi: 10.1002/ar.a.20305. [DOI] [PubMed] [Google Scholar]
- 15.Spoendlin H. Factors inducing retrograde degeneration of the cochlear nerve. Ann Otol Rhinol Laryngol. 1984;112:76–82. doi: 10.1177/00034894840930s415. [DOI] [PubMed] [Google Scholar]
- 16.Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J Assoc Res Otolaryngol. 2005;6:136–47. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nadol JB, Jr, Shiao JY, Burgess BJ, et al. Histopathology of cochlear implants in humans. The Ann Otol Rhinol Laryngol. 2001;110:883–91. doi: 10.1177/000348940111000914. [DOI] [PubMed] [Google Scholar]
- 18.Fayad JN, Linthicum FH., Jr Multichannel cochlear implants: relation of histopathology to performance. Laryngoscope. 2006;116:1310–20. doi: 10.1097/01.mlg.0000227176.09500.28. [DOI] [PubMed] [Google Scholar]
- 19.Gassner HG, Shallop JK, Driscoll CL. Long-term clinical course and temporal bone histology after cochlear implantation. Cochlear Implants Int. 2005;6:67–76. doi: 10.1179/cim.2005.6.2.67. [DOI] [PubMed] [Google Scholar]
- 20.Blamey P. Are spiral ganglion cell numbers important for speech perception with a cochlear implant? Am J Otol. 1997;18:S11–2. [PubMed] [Google Scholar]
- 21.Finley CC, Holden TA, Holden LK, et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol. 2008;29:920–8. doi: 10.1097/MAO.0b013e318184f492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briggs RJ, Tykocinski M, Saunders E, et al. Surgical implications of perimodiolar cochlear implant electrode design: avoiding intracochlear damage and scala vestibuli insertion. Cochlear Implants Int. 2001;2:135–49. doi: 10.1179/cim.2001.2.2.135. [DOI] [PubMed] [Google Scholar]
- 23.Erixon E, Hogstorp H, Wadin K, et al. Variational anatomy of the human cochlea: implications for cochlear implantation. Otol Neurotol. 2009;30:14–22. doi: 10.1097/MAO.0b013e31818a08e8. [DOI] [PubMed] [Google Scholar]
- 24.Skinner MW, Holden TA, Whiting BR, et al. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol. 2007;197:2–24. [PubMed] [Google Scholar]
- 25.Roland JT, Jr, Zeitler DM, Jethanamest D, et al. Evaluation of the short hybrid electrode in human temporal bones. Otol Neurotol. 2008;29:482–8. doi: 10.1097/MAO.0b013e31816845eb. [DOI] [PubMed] [Google Scholar]
- 26.Eshraghi AA, Polak M, He J, et al. Pattern of hearing loss in a rat model of cochlear implantation trauma. Otol Neurotol. 2005;26:442–7. doi: 10.1097/01.mao.0000169791.53201.e1. discussion 47. [DOI] [PubMed] [Google Scholar]
- 27.Linthicum FH, Jr, Fayad JN. Spiral ganglion cell loss is unrelated to segmental cochlear sensory system degeneration in humans. Otol Neurotol. 2009;30:418–22. doi: 10.1097/mao.0b013e31819a8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teufert KB, Linthicum FH, Jr, Connell SS. The effect of organ of Corti loss on ganglion cell survival in humans. Otol Neurotol. 2006;27:1146–51. doi: 10.1097/01.mao.0000232006.16363.44. [DOI] [PubMed] [Google Scholar]
- 29.Fayad J, Linthicum FH, Jr, Otto SR, et al. Cochlear implants: histopathologic findings related to performance in 16 human temporal bones. Ann Otol Rhinol Laryngol. 1991;100:807–11. doi: 10.1177/000348949110001004. [DOI] [PubMed] [Google Scholar]
- 30.Lenarz T, Stover T, Buechner A, et al. Hearing conservation surgery using the Hybrid-L electrode. Results from the first clinical trial at the Medical University of Hannover. Audiol Neurootol. 2009;14:22–31. doi: 10.1159/000206492. [DOI] [PubMed] [Google Scholar]
- 31.Hodges AV, Schloffman J, Balkany T. Conservation of residual hearing with cochlear implantation. Am J Otol. 1997;18:179–83. [PubMed] [Google Scholar]


