Abstract
Hypothesis
Revision surgery using a newer-generation conventional length cochlear implant electrode will provide improved speech perception in patients that initially underwent hybrid electrode implantation and experienced post-operative loss of residual hearing and performance deterioration.
Clinical presentation
We present four patients who experienced delayed post-operative hearing loss following implantation with the Nucleus Hybrid S8 device and underwent reimplantation with the Nucleus Freedom or Nucleus 5 device using the Contour Advance array. Pure-tone thresholds and speech perception data were retrospectively reviewed.
Intervention
Four subjects underwent reimplantation with the Nucleus Freedom or Nucleus 5 device after experiencing deteriorating performance related to delayed acoustic hearing loss. Comparison of pre-revision performance to the most recent post-revision performance demonstrated improved speech perception performance in all subjects following reimplantation.
Conclusions
A small percent of patients will experience a significant loss of residual low-frequency hearing following hybrid implantation thereby becoming completely reliant on a shorter electrode for electrical stimulation. In the current series, reimplantation with a conventional length electrode provided improved speech perception performance in such patients. Revision surgery with a conventional length electrode should be considered in ‘short electrode’ recipients who experience performance deterioration following loss of residual hearing.
Keywords: Cochlear implant, Electroacoustic hearing
Introduction
Dual advancements in cochlear implant technology and surgical technique have improved speech perception performance and broadened selection criteria for patients who desire cochlear implantation (Rubinstein et al., 1999; Gifford et al.). One significant innovation in this regard has been the introduction of electroacoustic stimulation (EAS) for patients who have sufficient low-frequency hearing to disqualify them from traditional cochlear implantation yet not enough hearing to benefit from conventional hearing amplification (Gantz and Turner, 2003, 2004). This approach seeks to preserve residual low-frequency hearing by using a shortened, more slender electrode. A shallower electrode insertion combined with minimally traumatic surgical technique aims at providing electrical stimulation of absent basally located high frequencies and at the same time minimizes injury to apical structures responsible for low-frequency hearing.
The conventional length cochlear implant electrode provides excellent levels of speech understanding in quiet backgrounds for many patients. However, it may be limited in its ability to provide sufficient frequency resolution that appears to be requisite for speech recognition in background noise (Nelson et al., 2003) and even more so in the presence of competing talkers (Qin and Oxenham, 2003), both of which are frequent occurrences in everyday life. Combining acoustic and electrical stimulation has demonstrated improved speech understanding both in quiet (Gantz et al., 2005) and in the presence of background competing noise (Turner et al., 2004). Additionally, improved pitch perception experienced by electroacoustic hybrid implant recipients has been shown to aid in music appreciation (Gfeller et al., 2006).
Despite efforts to preserve residual hearing after cochlear implantation with a hybrid device, up to 10% of patients may ultimately lose their residual hearing over time and are left fully dependent on the electrical stimulation provided by their implant (Gstoettner et al., 2004). Given the recent advent of hybrid technologies the best management strategy for such patients remains unclear. To date there has been only one other report (two patients) in the international literature discussing reimplantation with a conventional length electrode following initial short hybrid electrode placement (Fitzgerald et al., 2008). With the increasing number of patients undergoing hybrid implantation, establishing an optimal management strategy will become ever more important. Herein, we report the post-operative results of four patients who initially underwent implantation with the Nucleus Hybrid S8 device were subsequently reimplanted with a standard Nucleus Freedom or Nucleus 5 device (Cochlear Corporation, Lane Cove, Australia) using the Contour Advance electrode.
Materials and methods
Subjects
All patients implanted at our institution with the Hybrid S8 device that experienced post-operative loss of residual hearing and subsequently underwent reimplantation with the Nucleus Freedom or Nucleus 5 device were retrospectively reviewed. Requisites for hybrid implant candidacy were as follows: severe-to-profound (threshold average of 2000, 3000, and 4000 Hz > 75 dB HL) sensorineural hearing loss for frequencies >1500 Hz; low-frequency thresholds up to and including 500 Hz should be no poorer than 60 dB HL; pre-operative aided speech perception scores between 10 and 60% on consonant-nucleus-consonant (CNC) words in the ear to be implanted; speech perception in the contralateral ear must be equal to or better than the ear to be implanted but not exceeding 80%. All patients were enrolled as a part of the manufacturer-sponsored Food and Drug Administration (FDA) clinical trial. Reimplantation with the Nucleus Freedom or Nucleus 5 device was performed only after patients experienced near-total or total low-frequency hearing loss and diminished speech recognition performance.
Audiologic testing
All patients received a comprehensive pre-operative audiologic evaluation including pure-tone audiometry, aided and unaided monosyllabic word recognition, aided sentence recognition testing both in quiet and noise, as well as tympanometry. In addition to the pre-operative assessment, all patients were evaluated post-operatively at 6-, 12-, and 24-month post-activation. Preservation or loss of residual hearing in the implanted ear was assessed by pure-tone audiometry. A low-frequency pure-tone average (LFPTA) was calculated using values between 125 and 750 Hz and was used as a measure of low-frequency hearing preservation. Post-implantation speech perception performance was measured in the following conditions: aided ipsilateral acoustic alone (A), binaural aided acoustic alone (binaural A), ipsilateral electric alone (E; non-implant ear occluded), electric plus ipsilateral aided acoustic (hybrid; contralateral ear occluded), bilateral electric alone (bilateral E, bilateral ears occluded), electric plus contralateral aided acoustic (bimodal; ipsilateral ear occluded), and electric plus binaural aided acoustic (combined). Monosyllabic word recognition performance was assessed with two, 50-item CNC word lists presented via a single loudspeaker at a calibrated level of 70 dB SPL per Hybrid S8 protocol. The loudspeaker was placed at 0° azimuth at a distance of 1 m from the subject. The three standard practice words (duck, bomb, and June) were presented with each list for all test conditions. Sentence recognition in noise was assessed using two paired lists of the Bamford–Kowal–Bench sentence in noise (BKB-SIN, Etymotic Research, 2005) test. The BKB-SIN test presents ten sentences each with a decreasing, or less favorable signal-to-noise ratio (SNR) in 3 dB steps. The level of the sentence stimuli was fixed at 70 dB SPL and the level of the multi-talker babble was increased starting at +21 dB SNR for the first sentence and ending at −6 dB SNR for the last sentence. The BKB-SIN test expresses performance in terms of a speech reception threshold (SRT), which provides the SNR required for the individual to achieve approximately 50% correct. Thus, a lower score is associated with better speech perception in noise. Every 1 dB improvement in the SNR can translate up to an 8–15% improvement in speech recognition performance. Individual improvement in CNC word score testing was analyzed using the binomial distribution statistic described by Thornton and Raffin (1978) for two, 50-item word lists; individual improvement in BKB-SIN scores using two list pairs requires at least a 3.1 dB difference to be statistically significant at the 95% confidence interval BKB-SIN User Manual. Group mean comparisons were performed using the paired t-test.
Surgical technique
In an effort to minimize trauma to the scala media, the Nucleus Hybrid S8 cochlear implant uses a reduced diameter electrode (0.2 × 0.4 mm) to limit intracochlear insertion depth to 10 mm (180–205°) (Fig. 1). All devices were placed according to the FDA study protocol as described by Gantz et al. (2005).
Figure 1.
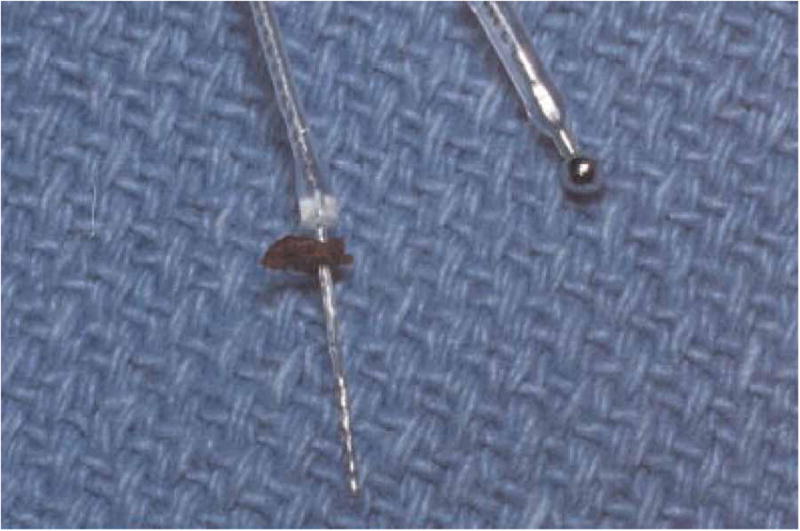
Nucleus Hybrid S8 electrode with a small piece of fascia abutting the Dacron collar (left); ground electrode (right).
Reimplantation was performed by using the same post-auricular incision to expose the implant. The electrode lead wire was carefully amputated approximately 1 cm lateral to the facial recess and the receiver stimulator package was removed. The subperiosteal pocket was then irrigated and the replacement device was seated into the existing pocket. After adequate exposure of the facial recess and removal of bony regrowth, adhesions around the cochleostomy were divided. The hybrid electrode was then slowly removed and the cochleostomy widened with a 1 mm drill. The Nucleus Freedom or Nucleus 5 device using the Contour Advance electrode was introduced using the Advance Off-Stylet technique per manufacturer protocols and a full insertion was achieved in all cases without appreciable resistance. Intraoperative impedance values and neural telemetry were obtained in all cases.
Results
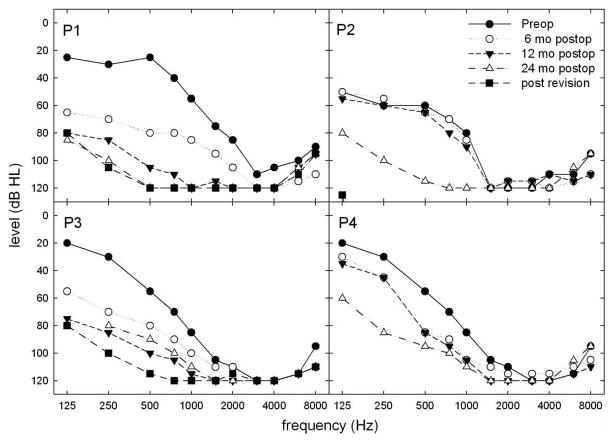
Four patients (P1–4) lost varying degrees of residual low-frequency hearing (Fig. 2) in the implanted ear over a period of 12–30 months and underwent reimplantation with the Nucleus Freedom or Nucleus 5 device. In the implanted ear, the degree of low-frequency post-operative threshold elevation at 6 months ranged from 0 to 44 dB (mean loss of 19.4 dB). There was little (0–6.25 dB) change in LFPTA thresholds for P2, P3, and P4 in the non-implanted ear. However, P1 experienced a 16.25 dB LFPTA elevation at 6 months and a 22.5 dB threshold elevation at 12 months in the non-implanted ear. There was no further significant change in the non-implanted ears beyond 12 months.
Figure 2.
Pre- and post-hybrid implantation pure tone values for P1–P4.
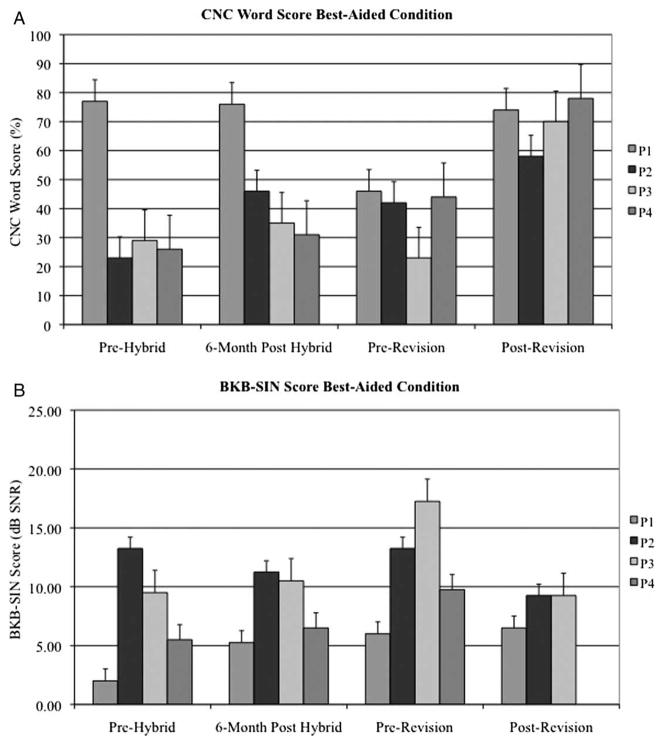
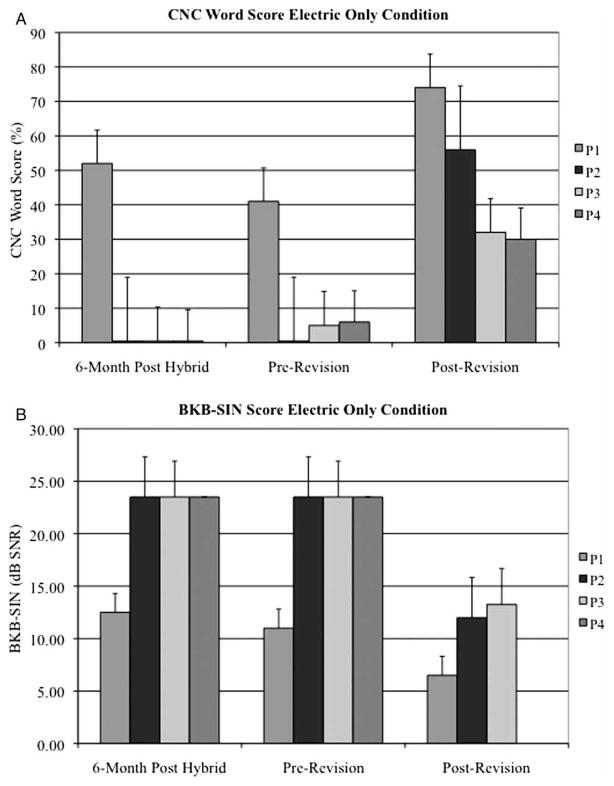
Mean CNC word scores significantly improved when comparing the immediate pre-revision performance to the most recent post-revision performance for both the best-aided (38.8 vs. 70.0%, P = 0.017) and electric only, condition (13.0 vs. 48.0%, P = 0.017). However, the mean speech perception in noise (BKB-SIN) score was not significantly different in either of the best-aided (12.2 dB SNR vs. 8.3 dB SNR, P = 0.26) or electric only condition (19.3 dB SNR vs. 10.6 dB SNR, P = 0.055) (Figs. 3A, 3B, 4A, 4B). A summary of speech perception testing is provided for all four subjects in Tables 1–7.
Figure 3.
Preoperative, 6 month post-hybrid implantation, immediate pre-revision, and post-revision (A) CNC word scores and (B) BKB-SIN sentence scores for P1–4 in the best-aided condition.
Figure 4.
Preoperative, 6 month post-hybrid implantation, immediate pre-revision, and post-revision (A) CNC word scores and (B) BKB-SIN sentence scores for P1–4 in the electric only condition.
Table 1.
Preimplant speech perception performance (CNC %, BKB-SIN dB SNR)
| CNC unaided scores
|
CNC aided scores
|
BKB-SIN aided scores
|
||||||
|---|---|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Binaural A | Right | Left | Binaural A | |
| P1 | 23 | 64 | 41 | 80 | 77 | 8.25 | 2.50 | 2.00 |
| P2 | 20 | 15 | 18 | 19 | 23 | 18.25 | 18.75 | 13.25 |
| P3 | 40 | 24 | 29 | 34 | 29 | 13.00 | 14.25 | 9.50 |
| P4 | 13 | 15 | 11 | 29 | 26 | 17.50 | 8.75 | 5.50 |
Table 7.
Post-revision BKB-SIN scores (dB SNR)
| E | Bimodal | Bilateral E | |
|---|---|---|---|
| P1 | 6.5 | NA | 6.5 |
| P2 | 12 | 9.25 | NA |
| P3 | 13.25 | 9.25 | NA |
| P4 | NT | NT | NA |
NT, not tested; NA, not applicable.
Case studies
P1, a 51-year-old male with familial hearing loss, suffered loss of residual low-frequency hearing (76 dB LFPTA loss in the implanted ear) approximately 24 months following implantation with the Hybrid S8 device. His CNC word scores 24 months after initial implantation were 41% (E) and 46% (bimodal); his BKB-SIN scores were 11 dB SNR (E) and 6 dB SNR (bimodal). He subsequently underwent explantation of the Hybrid S8 device and was simultaneously reimplanted with Nucleus Freedom devices bilaterally. His CNC scores 18 months post-revision were 74% (E) and 84% (bilateral E) for a post-revision benefit of 33% (E) and 28% (best-aided (bimodal) vs. post-revision (E)). This represents a significant improvement using the binomial distribution statistic (Thornton and Raffin, 1978). His post-revision BKB-SIN score was 6.5 (E and bilateral E) for a post-revision improvement in the SNR of −0.5 dB SNR (hybrid) and 4.5 dB SNR (E), the latter difference being statistically significant BKB-SIN User Manual. As previously noted, P1 experienced a 16.25 dB threshold elevation at 6 months and a 22.5 dB LFPTA threshold elevation at 12 months in the non-implanted ear.
P2, a 79-year-old male with familial hearing loss, experienced further deterioration of his low-frequency hearing (44 dB LFPTA loss in his implanted ear) approximately 18 months following implantation with the Hybrid S8 device. His pre-revision CNC word scores were 0% (E only), 28% (bimodal), and 42% (combined). His pre-revision BKB-SIN scores were 23.5 dB SNR (E), the lowest possible score on this test, 19 dB SNR (bimodal), and 13.25 dB SNR (combined). He subsequently underwent explantation of the Hybrid S8 device with reimplantation with the Nucleus Freedom device. Post-revision, the patient’s CNC scores were 56% (E) and 58% (bimodal), an improvement of 56% (E) and 30% (bimodal) as compared to his immediate pre-revision testing. As with P1, this represents a significant improvement using the binomial distribution statistic (Thornton and Raffin, 1978). His post-operative BKB-SIN scores were 12 dB SNR (E) and 9.25 dB SNR (bimodal), demonstrating a statistically significant improvement in the SNR of 11.5 dB (E) and 9.75 dB (bimodal) as compared to his immediate pre-revision testing BKB-SIN User Manual. There were no detectable post-implantation low-frequency audiometric threshold shifts in the contralateral non-implanted ear.
P3, a 69-year-old male with familial hearing loss, underwent explantation of his Hybrid S8 device and was implanted with a Nucleus Freedom device approximately 24 months following his initial Hybrid S8 implantation after losing residual low-frequency hearing (32.5 dB LFPTA loss in his implanted ear). His CNC word scores assessed immediately prior to revision surgery were 5% (E) and 21% (bimodal). His post-revision CNC scores were 32% (E) and 70% (bimodal) reflecting a 27% (E) and 49% (bimodal) benefit from his immediate pre-revision performance. His BKB-SIN scores assessed immediately prior to his revision surgery were 23.5 dB SNR (E) and 17.5 dB SNR (bimodal). His post-revision BKB-SIN scores were 13.25 dB SNR (E) and 9.25 dB SNR (bimodal), demonstrating a 10.25 dB SNR (E) and 8.25 dB SNR (bimodal) benefit following revision surgery with the Contour Advance electrode. The CNC word score (Thornton and Raffin, 1978) and BKB-SIN score BKB-SIN User Manual improvements were both statistically significant. There was only a 3.75 dB post-implant low-frequency audiometric thresholds shift in the contralateral non-implanted ear.
P4, a 62-year-old female with idiopathic progressive hearing loss experienced performance deterioration over a span of approximately 30 months (33.75 dB LFPTA loss in the implanted ear immediately prior to revision surgery) and was subsequently reimplanted with the Nucleus 5 device 4.5 years after initial hybrid implantation. Her CNC word scores immediately prior to surgery were 6% (E) and 44% (combined); the most recent post-revision score was 30% (E) and 78% (bimodal) demonstrating a 24% (E) and a 34% (bimodal) gain in the best-aided condition. This also represents a statistical improvement using the binomial distribution model (Thornton and Raffin, 1978). Pre-revision BKB-SIN scores were 23.5 dB SNR (E) and 9.75 dB SNR (combined); post-revision scores were not available at time of data collection. There was only a 6.25 dB LFPTA threshold shift seen just prior to reimplantation in the contralateral non-implanted ear.
Discussion
Preservation of at least some low-frequency residual hearing following implantation with the Nucleus Hybrid S8 device can be accomplished in over 90% of patients; however, substantial delayed hearing loss may result in progressive speech perception deterioration in a fraction of recipients (Gantz et al., 2009). Given the relative recent debut of shorter electroacoustic electrodes, there are less data in the literature to guide clinicians in the management of such patients. Many questions warrant further investigation:
1. After loss of residual hearing, hybrid implant recipients become solely reliant on a short electrode with a shallow insertion depth for electrical stimulation. If hybrid electrode implantees perform poorly in the cochlear implant only condition, will reimplantation with a conventional electrode provide substantial benefit?
Much effort has been devoted to determining prognostic indicators that might predict cochlear implant performance. Multivariate regression analysis has shown that pre-operative speech perception scores and duration of deafness predict over 80% of the variance in poor performing patients for both hybrid (Gantz et al., 2009) and conventional cochlear implant (Rubinstein et al., 1999) recipients. This would suggest that auditory physiologic mechanisms responsible for poor hybrid electrode performance may also render subsequent reimplantation (even with a longer electrode) unrewarding.
2. Will the mechanisms that caused post-implantation residual low-frequency acoustic hearing loss also hinder effective electrical stimulation?
Acoustic hearing loss after cochlear implantation can only be caused by one of two mechanisms: progression of the underlying disease process (i.e. familial hearing loss); or immediate and delayed deleterious events associated with cochlear implantation. Prior to initial implantation, all patients experienced slowly progressive symmetric hearing loss and only P1 experienced a limited amount of acoustic hearing loss in the contralateral ear following surgery; otherwise no additional significant contralateral threshold shifts following implantation were observed making disease progression a less likely cause.
Spiral ganglion cells of Rosenthal’s canal are required for cochlear implant electrical stimulation. Human cadaveric temporal bone studies have shown that injury to the delicate medial modiolar wall, the interscalar partition (osseous spiral lamina and basilar membrane), and lateral wall of the scala tympani is common even with least traumatic electrode designs (Roland and Wright, 2006). Acute mechanical insertional trauma to the organ of Corti and spiral ganglion cells may cause early post-operative hearing deterioration; postponed events such as apoptosis of inner hair cells (Eshraghi and Van de Water, 2006), fibrosis and neo-ossification may potentially result in delayed hearing loss. Animal studies have demonstrated that even minimal damage to the organ of Corti may result in delayed afferent neural degeneration of spiral ganglion cells (Sugawara et al., 2005; Spoendlin, 1984). While patterns of spiral ganglion cell degeneration may differ in humans, it remains plausible that injury to such structures may interfere with optimal electrical stimulation.
3. Will initial implantation using a shorter and thinner hybrid array preclude full insertion of a longer, thicker conventional cochlear implant electrode years later?
Histological and radiographic studies have shown that fibrosis and osteoneogenesis commonly occur around and beyond an implanted electrode (Somdas et al., 2007; Cervera-Paz and Linthicum, 2005). New tissue formation occurs as a result of injury to the endostium of the scala tympani or through the introduction of bone dust while drilling the cochleostomy. Such growth may interfere with electrode replacement Somdas et al. (2007), can cause elevated electrode impedance values (Clark et al., 1995), and may also negatively affect CI dynamic range performance (Kawano et al., 1998).
The Hybrid S8 implant uses a 10 mm electrode (180–205° angular insertion depth) and delivers electric stimulation via a six-channel system spaced over the distal 6 -mm of the array. The Contour Advance array, on the other hand, is inserted to a depth of approximately 18 mm (360–450° angular insertion depth) and delivers electric stimulation via 22 electrodes spaced over the distal 14.4 mm of the array. All four patients in our series exhibited improved word scores following reimplantation with a longer electrode. Furthermore, when evaluating individual subject benefit, the majority of implantees achieved improved speech perception in noise (BKB-SIN) when comparing immediate pre-revision scores with most recent post-revision values. Improved speech perception performance is likely the result of deeper electrode insertion. Reports from the EAS (Gantz and Turner, 2003; Gantz et al., 2005) and conventional cochlear implant literature support the notion that a deeper electrode insertion may provide improved speech perception up to a point; insertion depths beyond 400° do not seem to provide additional benefit and may carry an increased risk of intracochlear injury and possibly poorer speech recognition performance (Adunka et al., 2005; James et al., 2005; Gani et al., 2007).
Despite theoretical concerns, the four reported patients in our series demonstrated significantly improved performance following reimplantation with the conventional length electrode and full electrode insertions were obtained in all subjects. Our results corroborate an earlier publication by Fitzgerald et al. (2008). In their report, two patients were initially implanted with the 10 mm Nucleus Hybrid array and experienced profound hearing loss over 4–6 months. Reimplantation with the Nucleus Freedom Contour Advance electrode resulted in improvement in the cochlear implant only and best-aided condition.
Conclusion
Preliminary results from the EAS clinical trials have established hybrid implantation as a promising rehabilitation strategy for patients with serviceable low-frequency hearing and far advanced mid-to-high-frequency hearing loss; some degree of residual hearing can be preserved in over 90% of patients (Gantz et al., 2009). Unfortunately, for reasons not completely understood, a small group of implantees will experience delayed residual hearing. These patients become reliant on the electrical stimulation from the shorter electrode and often experience suboptimal speech perception performance. In the four reviewed cases, we found that reimplantation with a conventional length electrode is possible and provides improved performance.
This report then raises one of the key issue guiding future hybrid designs. There is likely a balance that could be met between minimally traumatic surgical techniques, a shortened electrode array (whether that is 10, 16, or 20 mm), and maximal electrical stimulation. Moving forward, the ideal hybrid electrode design must go beyond providing optimal EAS in patients with preserved residual hearing; it should be capable of providing sufficient electrical stimulation to compensate for delayed acoustic loss whether occurring months later from post-implantation effects or decades later from underlying disease progression.
Table 2.
6-month post-hybrid implant CNC word scores (%)
| A | Binaural A | E | Hybrid | Bimodal | Combined | |
|---|---|---|---|---|---|---|
| P1 | 7 | 37 | 52 | 64 | 76 | 69 |
| P2 | 17 | 22 | 0 | 38 | 18 | 46 |
| P3 | 11 | 27 | 0 | 27 | 26 | 35 |
| P4 | 15 | 30 | 0 | 12 | 23 | 31 |
Table 3.
6-month post-hybrid implant BKB-SIN scores (dB SNR)
| A | Binaural A | E | Hybrid | Bimodal | Combined | |
|---|---|---|---|---|---|---|
| P1 | 16.50 | 9.50 | 12.50 | 5.50 | 6.75 | 5.25 |
| P2 | 17.00 | 18.25 | 23.50 | 16.25 | 16.25 | 11.25 |
| P3 | 18.25 | 13.5 | 23.5 | 15.25 | 16.25 | 10.5 |
| P4 | 18.75 | 13.25 | 23.50 | 16.00 | 13.75 | 6.50 |
Table 4.
Pre-revision CNC word scores (%)
| A | Binaural A | E | Hybrid | Bimodal | Combined | |
|---|---|---|---|---|---|---|
| P1 | NT | NT | 41 | NT | 46 | NT |
| P2 | 8 | 27 | 0 | 36 | 28 | 42 |
| P3 | 3 | 13 | 5 | 15 | 21 | 23 |
| P4 | NT | NT | 6 | 28 | NT | 44 |
NT, not tested.
Table 5.
Pre-revision BKB-SIN scores (dB SNR)
| A | Binaural A | E | Hybrid | Bimodal | Combined | |
|---|---|---|---|---|---|---|
| P1 | NT | NT | 11 | NT | 6 | NT |
| P2 | 18.25 | 14.75 | 23.5 | 15.5 | 19 | 13.25 |
| P3 | 23.5 | 16.75 | 23.5 | 23.25 | 17.5 | 17.25 |
| P4 | NT | NT | 23.5 | 14.75 | NT | 9.75 |
NT, not tested.
Table 6.
Post-revision CNC word scores (%)
| E | Bimodal | Bilateral E | |
|---|---|---|---|
| P1 | 74 | NA | 84 |
| P2 | 56 | 58 | NA |
| P3 | 32 | 70 | NA |
| P4 | 30 | 78 | NA |
NA, not applicable.
Acknowledgments
Financial & material support
No funding orother support was required for this study.
Footnotes
Disclosures
Colin L.W. Driscoll, M.D. is a consultant for Cochlear Americas.
References
- Adunka O, Kiefer J, Unkelbach MH, Radeloff A, Gstoettner W. Evaluating cochlear implant trauma to the scala vestibuli. Clinical Otolaryngology. 2005;30:121–127. doi: 10.1111/j.1365-2273.2004.00935.x. [DOI] [PubMed] [Google Scholar]
- BKB-SIN User Manual. [accessed 2010 Dec 16];Etymotic. Available from: http://www.etymotic.com/pdf/bkbsin-user-manual.pdf.
- Cervera-Paz FJ, Linthicum FH., Jr Cochlear wall erosion after cochlear implantation. The Annals of Otology, Rhinology, and Laryngology. 2005;114:543–546. doi: 10.1177/000348940511400709. [DOI] [PubMed] [Google Scholar]
- Clark GM, Shute SA, Shepherd RK, Carter TD. Cochlear implantation: osteoneogenesis, electrode-tissue impedance, and residual hearing. The Annals of Otology, Rhinology and Laryngology. 1995;166:40–42. [PubMed] [Google Scholar]
- Eshraghi AA, Van de Water TR. Cochlear implantation trauma and noise-induced hearing loss: apoptosis and therapeutic strategies. The Anatomical Record. 2006;288:473–481. doi: 10.1002/ar.a.20305. [DOI] [PubMed] [Google Scholar]
- Fitzgerald MB, Sagi E, Jackson M, Shapiro WH, Roland JT, Jr, Waltzman SB, Svirsky MA. Reimplantation of hybrid cochlear implant users with a full-length electrode after loss of residual hearing. Otology and Neurotology. 2008;29:168–173. doi: 10.1097/mao.0b013e31815c4875. [DOI] [PubMed] [Google Scholar]
- Gani M, Valentini G, Sigrist A, Kos MI, Boex C. Implications of deep electrode insertion on cochlear implant fitting. Journal of the Association for Research in Otolaryngology. 2007;8:69–83. doi: 10.1007/s10162-006-0065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz BJ, Hansen MR, Turner CW, Oleson JJ, Reiss LA, Parkinson AJ. Hybrid 10 clinical trial: preliminary results. Audiology and Neuro-otology. 2009;14(Suppl 1):32–38. doi: 10.1159/000206493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz BJ, Turner CW. Combining acoustic and electrical hearing. The Laryngoscope. 2003;113:1726–1730. doi: 10.1097/00005537-200310000-00012. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner C. Combining acoustic and electrical speech processing: Iowa/Nucleus hybrid implant. Acta Oto-laryngologica. 2004;124:344–347. doi: 10.1080/00016480410016423. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. The Laryngoscope. 2005;115:796–802. doi: 10.1097/01.MLG.0000157695.07536.D2. [DOI] [PubMed] [Google Scholar]
- Gfeller KE, Olszewski C, Turner C, Gantz B, Oleson J. Music perception with cochlear implants and residual hearing. Audiology and Neuro-otology. 2006;11(Suppl 1):12–15. doi: 10.1159/000095608. [DOI] [PubMed] [Google Scholar]
- Gifford RH, Dorman MF, Shallop JK, Sydlowski SA. Evidence for the expansion of adult cochlear implant candidacy. Ear and Hearing. 2010;31:186–194. doi: 10.1097/AUD.0b013e3181c6b831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gstoettner W, Kiefer J, Baumgartner WD, Pok S, Peters S, Adunka O. Hearing preservation in cochlear implantation for electric acoustic stimulation. Acta Oto-laryngologica. 2004;124:348–352. doi: 10.1080/00016480410016432. [DOI] [PubMed] [Google Scholar]
- James C, Albegger K, Battmer R, Burdo S, Deggouj N, Deguine O, Dillier N, Gersdorff M, Lasig R, Lenarz T, Rodriguez MM, Mondain M, Offeciers E, Macias AR, Ramsden R, Sterkers O, Von Wallenberg E, Weber B, Fraysse B. Preservation of residual hearing with cochlear implantation: how and why. Acta Oto-laryngologica. 2005;125:481–491. doi: 10.1080/00016480510026197. [DOI] [PubMed] [Google Scholar]
- Kawano A, Seldon HL, Clark GM, Ramsden RT, Raine CH. Intracochlear factors contributing to psychophysical percepts following cochlear implantation. Acta Oto-laryngologica. 1998;118:313–326. doi: 10.1080/00016489850183386. [DOI] [PubMed] [Google Scholar]
- Nelson PB, Jin SH, Carney AE, Nelson DA. Understanding speech in modulated interference: cochlear implant users and normal-hearing listeners. The Journal of the Acoustical Society of America. 2003;113:961–968. doi: 10.1121/1.1531983. [DOI] [PubMed] [Google Scholar]
- Qin MK, Oxenham AJ. Effects of simulated cochlear-implant processing on speech reception in fluctuating maskers. The Journal of the Acoustical Society of America. 2003;114:446–454. doi: 10.1121/1.1579009. [DOI] [PubMed] [Google Scholar]
- Roland PS, Wright CG. Surgical aspects of cochlear implantation: mechanisms of insertional trauma. Advances in Oto-rhino-laryngology. 2006;64:11–30. doi: 10.1159/000094642. [DOI] [PubMed] [Google Scholar]
- Rubinstein JT, Parkinson WS, Tyler RS, Gantz B. Residual speech recognition and cochlear implant performance: effects of implantation criteria. The American Journal of Otology. 1999;20:445–52. [PubMed] [Google Scholar]
- Somdas MA, Li PM, Whiten DM, Eddington DK, Nadol JB., Jr Quantitative evaluation of new bone and fibrous tissue in the cochlea following cochlear implantation in the human. Audiology & Neuro-otology. 2007;12:277–284. doi: 10.1159/000103208. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Factors inducing retrograde degeneration of the cochlear nerve. The Annals of Otology, Rhinology and Laryngology. 1984;112:76–82. doi: 10.1177/00034894840930s415. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. Journal of the Association for Research in Otolaryngology. 2005;6:136–147. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton AR, Raffin MJ. Speech-discrimination scores modeled as a binomial variable. Journal of Speech and Hearing Research. 1978;21:507–518. doi: 10.1044/jshr.2103.507. [DOI] [PubMed] [Google Scholar]
- Turner CW, Gantz BJ, Vidal C, Behrens A, Henry BA. Speech recognition in noise for cochlear implant listeners: benefits of residual acoustic hearing. The Journal of the Acoustical Society of America. 2004;115:1729–1735. doi: 10.1121/1.1687425. [DOI] [PubMed] [Google Scholar]