Abstract
We investigated whether sexual activity was associated with reproductive function in the BioCycle Study, a prospective cohort study that followed 259 regularly menstruating women aged 18 to 44 years for one (n=9) or two (n=250) menstrual cycles in 2005–2007. Women were not attempting pregnancy nor using hormonal contraceptives. History of ever having been sexually active was assessed at baseline and frequency of sexual activity, defined as vaginal-penile intercourse, was self-reported daily throughout the study. Serum concentrations of estradiol, luteinizing hormone (LH), follicle-stimulating hormone (FSH), progesterone, and testosterone were measured up to 8 times/cycle. Sporadic anovulation was identified using peak progesterone concentration. Linear mixed models were used to estimate associations between sexual activity and reproductive hormone concentrations and generalized linear models were used to estimate associations with sporadic anovulation. Models were adjusted for age, race, body mass index, perceived stress, and alcohol consumption and accounted for repeated measures within women. Elevated concentrations of estrogen (+14.6%, P<.01), luteal progesterone (+41.0%, P<.01) and mid-cycle LH (+23.4%, P<.01), but not FSH (P=.33) or testosterone (P=.37), were observed in sexually active women compared with sexually inactive women (no prior and no study-period sexual activity); sexually active women had lower odds of sporadic anovulation (adjusted odds ratio= 0.34, 95% confidence interval: 0.16–0.73). Among sexually active women, frequency of sexual activity was not associated with hormones or sporadic anovulation (all P>.23). Findings from our study suggest that ever having been sexually active is associated with improved reproductive function, even after controlling for factors such as age.
Keywords: ovulation, reproductive hormones, sexual activity
Introduction
Ovulation in women is thought to occur spontaneously during each menstrual cycle, regardless of sexual behavior, as a result of positive and negative feedback mechanisms of the hypothalamic-pituitary-ovarian axis (Adams and Ratto, 2013). However, given the potential evolutionary benefits of sexual activity influencing ovulatory function and consequent pregnancy success (Wilcox et al., 2004) and the evidence that sexual activity induces ovulation in other species (Bakker and Baum, 2000; Jöchle, 1975; Pfaus et al., 2003; Wu et al., 1977), it remains to be established whether or not humans have similar biological mechanisms in place to induce or augment ovulatory function (Stanislaw and Rice, 1987; Wilcox et al., 2004).
Female- versus male-initiated sexual activity has been shown to be greater around the time of ovulation (Adams et al., 1978; Gangestad et al., 2002; Harvey, 1987; Wallen, 2001) and numerous studies have found that sexual activity among women in general peaks during the time of ovulation (Bullivant et al., 2004; Caruso et al., 2014; Pagidas et al., 2010; Van Goozen et al., 1997; Wilcox et al., 2004) as does the desirability of a male partner (Larson et al., 2013). Together these studies suggest that peri-ovulatory hormonal patterns may cause an increase in female libido, which together with their partner’s desire and the couple’s intentions regarding pregnancy, can affect sexual behavior (Wallen, 2001).
Further characterization of the relationship between sexual activity and reproductive function has the potential to improve our understanding of couples’ fecundity (Buck Louis, 2011). A recent review concluded that although the relationship between sexual activity and menstrual cycle function has been studied, several conflicting results and methodological differences make it difficult to draw definitive conclusions (Brown et al., 2011). Previous studies have evaluated sexual activity patterns across the menstrual cycle, but have been limited in their examination of sexual activity and ovulatory function, have not included a comparison of reproductive function between sexually active women and women reporting no history of sexual activity, nor have evaluated the effect of reproductive function during one cycle on sexual activity in a subsequent cycle.
Therefore, we investigated the association between sexual activity and reproduction function, examining both the effect of sexual intercourse on reproductive function and the effect of reproductive function on sexual intercourse using longitudinally collected data. Our hypotheses were that sexually active women would have higher reproductive hormone levels and be less likely to experience anovulatory cycles compared with sexually inactive women and that reproductive hormones would be associated with sexual activity patterns. We investigated these hypotheses in a cohort of healthy premenopausal women, both with or without a history of sexual activity, who were not attempting pregnancy nor using hormonal contraceptives.
Materials and Methods
Study Population
The BioCycle Study was a prospective cohort study that included 259 healthy, regularly menstruating women aged 18 to 44 years from western New York State during 2005–2007 and followed them for up to two menstrual cycles. Details of the study population, materials and methods have been previously described (Wactawski-Wende et al., 2009). Briefly, exclusion criteria included use of oral contraceptives within the past 3 months; a history of pregnancy, breastfeeding, or attempting a pregnancy within the past 6 months; and any recent history of infection or diagnosis of a chronic medical condition, including menstrual and ovulatory disorders, or psychiatric condition, including premenstrual dysphoric disorder. In addition, women with a self-reported body mass index (BMI) of < 18 or > 35 kg/m2 at screening were excluded. The University at Buffalo Health Sciences Institutional Review Board (IRB) approved the study and served as the IRB designated by the National Institutes of Health for this study under a reliance agreement. All participants provided written informed consent. The main findings of the study concerning reproductive hormones and oxidative stress have been previously published (Schisterman et al., 2010).
Fasting blood samples were scheduled to be collected in the morning (between 7 and 8:30 am) at up to 8 visits per cycle planned to occur during menses; the mid-follicular phase; three days around the time of the luteinizing hormone (LH) surge; and the early, mid, and late luteal phases. The timing of visits was facilitated by the use of home fertility monitors (Clearblue Easy Fertility Monitor; Inverness Medical, Waltham, Massachusetts) (Howards et al., 2009). Nearly all women (94%) provided 7 or 8 blood specimens per cycle and 100% provided at least 5 specimens per cycle.
Blood collection and handling protocols were designed to minimize variability (Wactawski-Wende et al., 2009). All samples were processed and frozen at −80°C within 90 minutes of phlebotomy and analytes were measured in participant-specific batches within a single run to limit analytical variability. Estradiol, LH, follicle-stimulating hormone (FSH), and progesterone concentrations were measured in serum samples using solid-phase competitive chemiluminescent enzymatic immunoassays (DPC Immulite 2000 analyzer, Siemens Medical Solutions Diagnostics, Deerfield, IL) at the Kaleida Health Center for Laboratory Medicine (Buffalo, NY). Serum testosterone was measured by liquid chromatography/tandem mass spectrometry (Shimadzu Prominence Liquid Chromatogram with an ABSceix 5500 tandem mass spectrometer) by the Advanced Research and Diagnostic Laboratory, Minneapolis, MN. Increased sensitivity was achieved by using 100% acetonitrile mobile phase B as the solvent gradient elution and adding a low standard of 4 ng/dL. The interassay maximum coefficients of variation reported by the laboratory were ≤10% for estradiol; ≤5% for LH and FSH; ≤14% for progesterone; and ≤7% for testosterone. All hormone measurements were log-transformed for normality before statistical analysis and then transformed by exponentiation for table display. In addition, LH and progesterone measurements were restricted in the analysis to mid-cycle (three days around the LH surge) and luteal phase (early, mid and late), respectively, as these are the phases with the greatest biological variance for these hormones. For certain analyses, progesterone measurements from mid-cycle visits were also analyzed for comparison.
Sporadic anovulatory cycles were defined as cycles with peak serum progesterone concentrations ≤5 ng/mL and no observed serum LH peak during the mid or late luteal phase visits (Gaskins et al., 2009). These cycles were considered to reflect sporadic rather than chronic anovulation, as study participants were healthy women without reported gynecological or menstrual disorders.
Sexual Activity and Covariate Assessment
Participant characteristics, such as race, age, and smoking status, were assessed during a baseline visit via questionnaire. Trained study staff measured height and weight, from which BMI was calculated. Perceived stress level was measured using the 14-item Cohen Perceived Stress Scale (PSS) (Cohen et al., 1983). In addition, prior sexual history was ascertained by the question: “Have you ever been sexually active? (Y/N)”. Among sexually active women, contraceptive use history was obtained.
Sexual activity during the study period was assessed prospectively using a daily diary where participants reported whether they had engaged in sexual intercourse, defined as vaginal-penile intercourse, that day (Y/N), with instructions that participants should consider that each day ends at midnight. Alcohol intake was also assessed via the daily diary and was averaged over the study period and subsequently categorized as: low (≤ 0.5 drinks/day), moderate (0.5 to 1 drinks/day), or high (≥ 1 drinks/ day). The daily diary also captured information on medication use, hours of sleep, and minutes of vigorous physical activity.
Statistical Analysis
Baseline characteristics, sexual history, and contraceptive use history were compared among participants grouped into four sexual activity categories: (1) no prior and no current sexual activity during the study period (henceforth labeled “sexually inactive”), (2) history of sexual activity but not during the study-period (“not sexually active during study”), (3) weekly or less during the study-period (“weekly or less”), (4) greater than weekly during the study-period (“greater than weekly”). Fisher’s exact tests were used to test for differences among sexual activity categories for categorical variables and analysis of variance was used for continuous variables. In addition, pair-wise comparisons were performed between sexual activity categories on mean reproductive hormone concentrations, with the Tukey method used to account for multiple comparisons.
Variation in reported daily sexual activity across the menstrual cycle among women sexually active during the study was assessed using linear mixed models to account for repeated measures within women. Days were aligned in relation to the day of ovulation, which was estimated based on dates and levels of LH peak from the fertility monitor compared with the observed LH maximum value in serum and the first day of progesterone rise (Mumford et al., 2012). If the cycle was classified as anovulatory, cycle day 14 was assigned as the estimated day of ovulation for comparison purposes. Pair-wise comparisons were made between cycle days using the Tukey method. In addition, average frequency of sexual activity was compared between Mid-Cycle (7 days prior through 2 days after estimated date of ovulation) and Early Cycle (8 or more days prior to ovulation, including menses) and Late Cycle (3 or more days after ovulation), respectively. The “Early cycle” window was chosen as 8 or more days prior to ovulation because this captured the days during menstruation, when women are thought to be less likely to have intercourse (Bullivant et al., 2004). The “Mid-Cycle” window was chosen 7 days prior to through 2 days after ovulation to be generously inclusive of the fertile window (Lynch et al., 2006; Wilcox et al., 2004; Wilcox et al., 1995) and the “sexual phase” of the menstrual cycle (Bullivant et al., 2004). The “Late Cycle” was chosen at 3 or more days after ovulation to capture the remaining part of the cycle.
Linear mixed models were also used to estimate the association between sexual activity category and reproductive hormone concentrations. A first-order autoregressive moving-average correlation structure was specified for the correlation between hormone measurements within women. For these analyses, the four level sexual activity category was examined as well as a collapsed two level variable (sexually active vs. inactive [no prior and no study-period sexual activity]). In addition, we restricted the analyses to sexually active women to examine the association between average frequency of sexual activity and hormone concentrations.
Generalized linear models were used to estimate odds ratios (OR) for the association between categorized sexual activity (both four level and two level categories) and the likelihood of experiencing an anovulatory cycle during the study, while accounting for the repeated measurements within women. These models were also run after restricting the analyses to sexually active women.
In terms of the direction of effect, we took advantage of the longitudinal nature of our data to examine both the effect of sexual intercourse on reproductive function and, conversely, the effect of reproductive function on sexual intercourse. For the effect of sexual intercourse on reproductive function, we used linear mixed models (described above) to evaluate the association between previous-day sexual intercourse (any vs. none) and next-day hormone concentrations. We also examined sexual intercourse prior to the day of expected ovulation (any vs. none since first day of cycle) on the likelihood of anovulation in that cycle using generalized linear models (also described above). For the effect of reproductive function on sexual intercourse, we evaluated associations between previous-day hormone levels and report of next-day sexual intercourse (any vs. none). In addition, we examined anovulation in the first study cycle in relation to average frequency of sexual intercourse in the second study cycle. And lastly, we examined the association between hormone concentrations and same-day sexual intercourse. As blood draws were scheduled first thing in the morning, this analysis likely reflects the effect of reproductive function on sexual intercourse because sexual intercourse reported for that day presumably occurred later in the day or evening.
All models were run unadjusted and adjusted for age (continuous), race (white/black/other), BMI (continuous), PSS score (continuous), and alcohol intake (low/medium/high). Where appropriate we also adjusted for the effect of time-varying confounders using inverse probability weighting (Robins et al., 2000). All analyses were carried out using SAS version 9.3 (SAS Institute Inc., Cary, NC).
Results
Study participants had a mean (± standard deviation) age of 27.3 ± 8.2 (range: 18 to 44 years), BMI of 24.1 ± 3.9 (16 to 34 kg/m2), and were mostly white (60%, 154/258) (Table 1). Of the 258 women whose sexual activity category could be determined, there were 59 (23%) who were sexually inactive, 64 (25%) who were not sexually active during the study-period, 71 (28%) who were sexually active weekly or less, and 64 (25%) who were sexually active greater than weekly. Age, BMI, race, marital status, parity, and alcohol consumption differed by sexual activity category (all P <.05). Mean concentrations of estradiol, mid-cycle LH, FSH, and luteal progesterone differed by sexual activity category (all P <.05), with significantly higher levels observed between select categories of sexually activity women in comparison to sexually inactive women (Tukey P <.05). Mid-cycle progesterone did not differ among sexual activity categories: sexually inactive (median=1.1, interquartile range 0.7–1.7 ng/mL), not sexually active during study-period (1.0, 0.7–1.4), sexually active weekly or less (1.0, 0.6–1.5), or sexually active greater than weekly (0.9, 0.7–1.5) (P=0.90).
Table 1.
Characteristics of BioCycle study participants by sexual activity category
| Sexual Activity Category | ||||||
|---|---|---|---|---|---|---|
| Total Cohort | Sexually inactive | Not sexually active during study | Weekly or less | Greater than weekly | p-valuea | |
| Number of women (%) | 258 | 59 (23) | 64 (25) | 71 (28) | 64 (25) | ------ |
| Demographics | ||||||
| Mean ± SD | ||||||
| Age, yrs | 27.3±8.2 | 21.7±3.6 | 27.5±8.2 | 29.2±8.4 | 30.1±8.8 | <0.01 |
| BMI, kg/m2 | 24.1±3.9 | 23.5±3.7 | 25.5±4.0 | 23.5±3.6 | 23.8±3.8 | <0.01 |
| Age at menarche, yrs* | 12.5±1.2 | 12.4±1.4 | 12.3±1.3 | 12.6±1.1 | 12.6±1.2 | 0.45 |
| Menses length, days* | 5.3±1.5 | 5.3±1.3 | 5.2±1.3 | 5.4±1.5 | 5.5±1.9 | 0.32 |
| Total blood loss, g* | 65.8±56.0 | 55.5±43.3 | 65.7±56.3 | 68.5±54.1 | 71.7±65.8 | 0.17 |
| Stress (PSS score)b* | 20.2±6.8 | 20.4±6.8 | 19.8±7.4 | 21.5±6.1 | 18.9±6.8 | 0.14 |
| n (%): | ||||||
| Race | <0.01 | |||||
| White | 154 (60) | 25 (16) | 37 (24) | 44 (29) | 48 (31) | |
| Black | 51 (20) | 13 (25) | 16 (31) | 17 (33) | 5 (10) | |
| Other | 53 (20) | 21 (40) | 11 (21) | 10 (19) | 11 (21) | |
| Education | 0.54 | |||||
| Post-secondary | 225 (87) | 53 (24) | 53 (24) | 61 (27) | 58 (26) | |
| Marital status | <0.01 | |||||
| Married | 66 (26) | 0 (0) | 8 (12) | 25 (38) | 33 (50) | |
| Parity* | <0.01 | |||||
| Parous | 66 (26) | 0 (0) | 14 (21) | 24 (36) | 28 (42) | |
| Smoking | 0.28 | |||||
| Current smoker | 10 (4) | 0 (0) | 4 (40) | 3 (30) | 3 (30) | |
| Alcohol consumptionc | 0.02 | |||||
| Low | 180 (70) | 52 (29) | 43 (24) | 47 (26) | 38 (21) | |
| Moderate | 41 (16) | 4 (10) | 11 (27) | 12 (29) | 14 (34) | |
| High | 37 (14) | 3 (8) | 10 (27) | 12 (32) | 12 (32) | |
| Median (IQR) | ||||||
| Reproductive hormones, averaged | ||||||
| E2 (pg/mL) | 83.2 (65.4, 107.5) | 76.2 (54.1, 98.8) | 86.4 (65.8, 113.9) | 85.2 (74.3, 111.1)e | 85.1 (64.6, 97.8) | 0.03 |
| Mid-cycle LH (ng/mL) | 10.3 (7.7, 13.3) | 9.2 (6.2, 11.9) | 11.3 (8.3, 13.4) | 10.0 (7.8, 13) | 10.9 (8.2, 14.6)e | 0.02 |
| FSH (MIU/mL) | 5.3 (4.5, 6.2) | 4.9 (4.1, 5.9) | 5.4 (4.5, 6.2) | 5.4 (4.9, 6.6)e | 5.4 (4.6, 6.3) | 0.04 |
| Luteal Progesterone (ng/mL) | 5.3 (3.5, 7.3) | 4.5 (1.7, 6.2) | 5.8 (3.5, 8.1)e | 5.7 (3.6, 8.1)e | 5.5 (4.4, 7.4)e | <0.01 |
| Testosterone (ng/dL) | 28.5 (22.3, 34.6) | 30.0 (23.5, 35.7) | 28.9 (21.9, 39.1) | 26.5 (21.9, 34.8) | 28.0 (21.9, 32.3) | 0.28 |
Abbreviations: SD, standard deviation; BMI, body mass index; IQR, interquartile range; PSS, perceived stress scale; E2, estradiol; LH, luteinizing hormone; FSH, follicle-stimulating hormone
P-values calculated using Fisher’s exact test or analysis of variance where appropriate.
Perceived Stress Scale is a validated questionnaire to gauge stress levels.
Alcohol levels: low (≤ 0.5 drinks/day), moderate (0.5 to 1 drinks/day), high (≥1 drinks/day).
Testing performed on log hormone mean, then transformed by exponentiation for table display.
Pair-wise comparison of mean with sexually inactive group was statistically significant (Tukey P<0.05). No other pair-wise comparisons of means were statistically significant.
Missing values (N): age at menarche (3), menses length (7), total blood loss (7), stress (1), parity (5).
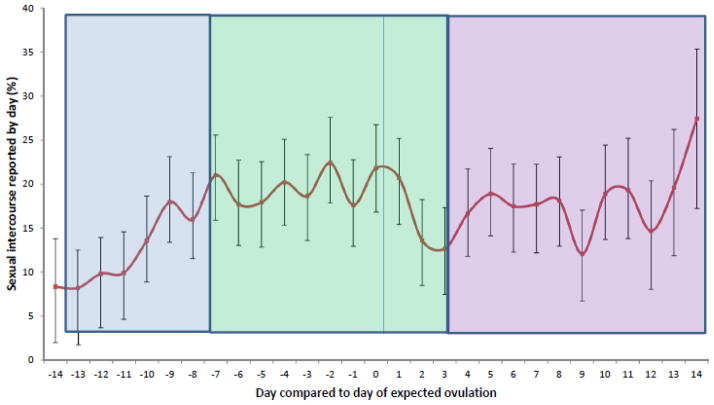
Days of reported sexual activity per cycle during the study-period ranged from 0 to 18 and daily sexual activity varied across the menstrual cycle (P<.01), with the average reported daily sexual activity during Mid-Cycle (19.0%) being significantly higher than that during Early-Cycle (12.1%, P<.01), but not different from that during Late Cycle (16.9%, P=.12) (Figure 1). Sexual activity was significant different (Tukey P<.05) between the following days: days -6, -4 and 1 were each different from day -13; day -2 was different from days -11, -12, -13 and -14; day 0 was different from days -14 and -12.
Figure 1.
Average daily sexual intercourse relative to day of expected ovulation among women sexually active during the study (n= 135)
P<.001 for overall variation in sexual intercourse across the menstrual cycle. Mixed model analyses took into account repeated measures from the same woman.
P<.05 for the following pairwise comparisons used the Tukey method to account for multiple comparisons: days -6, -4 and 1 were each different from day -13; day -2 was different from days -11, -12, -13 and -14; day 0 was different from days -14 and -12.
Average daily sexual intercourse during Mid-Cycle (19.0%) was significantly higher than the frequency of intercourse during Early Cycle (12.1%, P<.01), but not different from that during the Late Cycle (16.9%, P=.12).
Among 192 (74%) women who reported ever being sexually active, 177 (92%) reported a history of contraceptive use. The most commonly reported current method of contraception was male condoms (38%, 68/177) (supplementary table). For approximately half (52%, 92/177) of sexually active women with a history of contraceptive use, contraception was not currently being used or the method was unspecified.
In terms of sexual activity category and hormone concentrations, we observed higher estrogen, mid-cycle LH and luteal progesterone among sexually active women when compared with sexually inactive women after adjustment for confounders (all P<.05); FSH and testosterone did not differ by sexual activity status (P =.33 and P =.37, respectively) (Table 2). Among sexually active women, hormone concentrations did not differ between those sexually active weekly or less or greater than weekly in comparison to women not sexually active during the study (both P >.23) (Table 3). In terms of pair-wise comparisons of adjusted mean hormone concentrations, higher levels were observed between select categories of sexually activity women in comparison to sexually inactive women (Tukey P <.05) but were not different between the categories of sexually active women.
Table 2.
Percent difference in reproductive hormone concentrations by sexual activity category
| Percent Difference (95% Confidence Interval) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hormones | Sexually inactive | Not sexually active during study | Weekly or less | Greater than weekly | Sexually activea | |
| E2 (pg/mL) | Unadjusted | Reference | 18 (7, 30) | 20 (9, 32) | 11 (1, 23) | 16 (7, 26) |
| Adjusted | Reference | 15 (4, 28)b | 16 (4, 28)b | 12 (1, 25) | 15 (5, 25) | |
| Mid-cycle LH (ng/mL) | Unadjusted | Reference | 20 (4, 37) | 17 (2, 33) | 27 (11, 46) | 21 (8, 35) |
| Adjusted | Reference | 22 (5, 41)b | 21 (5, 40) | 29 (11, 50)b | 23 (9, 40) | |
| FSH (mIU/mL) | Unadjusted | Reference | 9 (1, 19) | 16 (8, 26) | 14 (5, 24) | 13 (6, 21) |
| Adjusted | Reference | 4 (−4, 13) | 5 (−4, 14) | 1 (−7, 11) | 4 (−4, 11) | |
| Luteal Progesterone (ng/mL) | Unadjusted | Reference | 53 (24, 90) | 56 (27, 93) | 59 (28, 97) | 56 (31, 86) |
| Adjusted | Reference | 43 (14, 79)b | 39 (11, 75)b | 41 (11, 78)b | 41 (16, 71) | |
| Testosterone (ng/dL) | Unadjusted | Reference | −4 (−13, 5) | −10 (−18, −1) | −9 (−18, 0) | −8 (−15, 0) |
| Adjusted | Reference | 6 (−3, 15) | 0 (−9, 9) | 5 (−4, 15) | 3 (−4, 12) | |
Abbreviations: E2, estradiol; LH, luteinizing hormone; FSH, follicle-stimulating hormone; BMI, body mass index Adjusted for age, race, BMI, stress, and alcohol intake
Sexually active includes not sexually active during study, weekly or less, and greater than weekly.
Pair-wise comparison of adjusted mean with sexually inactive group was statistically significant (Tukey P<0.05). No other pair-wise comparisons of adjusted means were statistically significant.
Table 3.
Percent difference in reproductive hormone concentrations by sexual activity level among sexually active women
| Percent Difference (95% Confidence Interval) | ||||
|---|---|---|---|---|
|
| ||||
| Hormones | Not sexually active during study | Weekly or less | Greater than weekly | |
| E2 (pg/mL) | Unadjusted | Reference | 2 (−7, 12) | −5 (−14, 4) |
| Adjusted | Reference | 1 (−9, 11) | −3 (−12, 7) | |
| Mid-cycle LH (ng/mL) | Unadjusted | Reference | −2 (−14, 11) | 6 (−7, 22) |
| Adjusted | Reference | 0 (−13, 15) | 6 (−8, 21) | |
| FSH (mIU/mL) | Unadjusted | Reference | 6 (−2, 15) | 4 (−4, 13) |
| Adjusted | Reference | 1 (−7, 9) | −2 (−10, 6) | |
| Luteal Progesterone (ng/mL) | Unadjusted | Reference | 2 (−15, 23) | 3 (−14, 24) |
| Adjusted | Reference | −4 (−20, 16) | −3 (−20, 17) | |
| Testosterone (ng/dL) | Unadjusted | Reference | −6 (−14, 3) | −5 (−14, 5) |
| Adjusted | Reference | −5 (−12, 3) | −1 (−9, 8) | |
Abbreviations: E2, estradiol; LH, luteinizing hormone; FSH, follicle-stimulating hormone; BMI, body mass index Adjusted for age, race, BMI, stress, and alcohol intake
Sexually inactive women experienced a greater percentage of sporadic anovulatory cycles compared with sexually active women (19% [22/117] vs. 5% [19/391] P<.01) (Table 4). In accord, sexually active women had a lower likelihood of sporadic anovulation during the study compared with sexually inactive women (adjusted OR=0.34, 95% confidence interval [CI] 0.16, 0.73). Among sexually active women, higher frequencies of sexual activity were not associated with likelihood of sporadic anovulation in comparison to women not sexually active during the study (both P>.83).
Table 4.
Association between sexual activity level and sporadic anovulation
| Anovulatory cycles/total cycles (%) | Odds Ratio (95% Confidence Interval) for Sporadic Anovulation | ||
|---|---|---|---|
| Unadjusted | Adjusted | ||
| Among all women | |||
|
| |||
| Sexual activity categorya | 41/508 (8) | ||
| Sexually inactive | 22/117 (19) | Reference | Reference |
| Not sexually active during study | 7/125 (6) | 0.26 (0.10, 0.65) | 0.38 (0.15, 0.95) |
| Weekly or less | 6/141 (4) | 0.19 (0.06, 0.57) | 0.29 (0.10, 0.85) |
| Greater than weekly | 6/125 (5) | 0.22 (0.08, 0.58) | 0.34 (0.11, 1.04) |
| Sexually activea | 41/508 (8) | ||
| Sexually inactive | 22/117 (19) | Reference | Reference |
| Sexually active | 19/391 (5) | 0.22 (0.11, 0.46) | 0.34 (0.16, 0.73) |
| Sexual activity prior to the day of expected ovulationb | 39/506 (8) | ||
| No sexual intercourse | 32/298 (11) | Reference | Reference |
| Any sexual intercourse | 7/208 (3) | 0.29 (0.12, 0.67) | 0.37 (0.15, 0.93) |
|
| |||
| Among sexually active women | |||
|
| |||
| Sexual activity categorya | 19/391 (5) | ||
| Not sexually active during study | 7/125 (6) | Reference | Reference |
| Weekly or less | 6/141 (4) | 0.75 (0.23, 2.46) | 0.88 (0.28, 2.75) |
| Greater than weekly | 6/125 (4) | 0.85 (0.28, 2.55) | 1.05 (0.30, 3.65) |
| Sexual activity prior to the day of expected ovulationb | 19/390 (5) | ||
| No sexual intercourse | 11/182 (6) | Reference | Reference |
| Any sexual intercourse | 7/208 (3) | 0.54 (0.22, 1.34) | 0.65 (0.22, 1.90) |
FSH, follicle-stimulating hormone; LH, luteinizing hormone; BMI, body mass index; E2, estradiol Adjusted for age, race, BMI, stress, and alcohol intake
Sexually active includes not sexually active during study, weekly or less, and greater than weekly. Among all women, 508 cycles were available for analysis after excluding 1 anovulatory cycle with missing information on sexual activity level, an additional 2 ovulatory cycles were excluded from the adjusted models due to missing perceived stress scale score; among sexually active women, 391 cycles were available for both the unadjusted and adjusted models.
Sexual activity was cycle-specific. Among all women, 506 cycles were available for analysis after excluding 3 anovulatory cycles with missing information on sexual intercourse leading up to ovulation, an additional 2 ovulatory cycles were excluded from the adjusted models due to missing perceived stress scale score; among sexually active women, 390 cycles were available for both the unadjusted and adjusted models.
In terms of the effect of sexual intercourse on reproductive function, hormone concentrations did not vary by previous day sexual intercourse (any vs. none) among all women or among only sexually active women (all P>.19) (Table 5). Sexual intercourse prior to the day of expected ovulation (any vs. none since first day of cycle) was associated with a decreased likelihood of sporadic anovulation in that cycle (adjusted OR=0.37, 95% CI 0.15, 0.93), but this association was not statistically significant when the analysis was restricted to sexually active women (Table 4).
Table 5.
Association between previous-day and same-day sexual intercourse and reproductive hormone concentrations
| Percent Difference (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
|
| |||||
| Hormones
|
Among all women in study | Among sexually active women | |||
| Previous-day sexual intercourse Any vs. None | Same-day sexual intercourse Any vs. None | Previous-day sexual intercourse Any vs. None | Same-day sexual intercourse Any vs. None | ||
| E2 (pg/mL) | Unadjusted | 5 (−3, 14) | 13 (4, 24) | 3 (−5, 12) | 12 (3, 23) |
| Adjusted I | 5 (−3, 14) | 14 (4, 24) | 4 (−4, 13) | 13 (3, 23) | |
| Adjusted II | 4 (−4, 13) | 15 (5, 27) | 3 (−5, 12) | 14 (4, 26) | |
| Mid-cycle LH (ng/mL) | Unadjusted | 11 (−5, 28) | 13 (−3, 32) | 7 (−8, 25) | 9 (−7, 28) |
| Adjusted I | 9 (−7, 26) | 11 (−5, 30) | 6 (−9, 23) | 8 (−8, 27) | |
| Adjusted II | 10 (−5, 26) | 13 (−7, 37) | 7 (−7, 23) | 10 (−10, 33) | |
| FSH (mIU/mL) | Unadjusted | 2 (−4, 8) | 6 (−1, 13) | 1 (−5, 8) | 5 (−2, 12) |
| Adjusted I | 0 (−5, 6) | 4 (−2, 12) | 0 (−6, 6) | 4 (−2, 11) | |
| Adjusted II | 1 (−5, 8) | 4 (−3, 11) | 1 (−5, 8) | 4 (−3, 11) | |
| Luteal Progesterone(ng/mL) | Unadjusted | −3 (−23, 22) | −14 (−34, 13) | −7 (−25, 15) | −19 (−38, 5) |
| Adjusted I | −9 (−27, 15) | −16 (−36, 9) | −11 (−28, 10) | −20 (−38, 4) | |
| Adjusted II | −9 (−29, 16) | −9 (−30, 17) | −12 (−31, 11) | −15 (−33, 8) | |
| Testosterone (ng/dL) | Unadjusted | 1 (−1, 4) | 6 (3, 9) | 1 (−1, 4) | 6 (3, 9) |
| Adjusted I | 1 (−1, 4) | 6 (3, 9) | 1 (−1, 4) | 6 (3, 9) | |
| Adjusted II | 2 (−1, 4) | 8 (4, 11) | 1 (−1, 4) | 8 (4, 11) | |
FSH, follicle-stimulating hormone; LH, luteinizing hormone; BMI, body mass index; E2, estradiol
Adjusted I: age, race, BMI, stress, and alcohol intake; Adjusted II: age, race, BMI, stress, alcohol intake and previous reproductive hormones using inverse probability of exposure weights
With respect to reproductive function affecting sexual intercourse, higher mid-cycle LH levels were associated with an increased likelihood of next-day sexual intercourse among all women and among sexually active women (Table 6). Among all women, a log increase in mid-cycle LH concentration was associated with a 28% increased odds of next-day sexual intercourse (P=.02), which is equivalent to an LH increase from approximately 10.3 ng/mL (the median) to 27.9 ng/mL. Although the adjusted ORs for the other reproductive hormones also indicated a positive relationship, they were not statistically different from the null (all P>.14). As for the association between hormone concentrations and same-day sexual intercourse, estradiol and testosterone levels were 14–15% and 8% higher, respectively, for women reporting any vs. no sexual intercourse (Table 5). For all other hormones, including mid-cycle progesterone, there was no association between hormone concentration and same-day sexual intercourse (all P> .21). In terms of reproductive function in one cycle affecting sexual intercourse in a subsequent cycle, women with an anovulatory cycle during their first study cycle were less likely to report greater than weekly sexual activity in the second study cycle, but this finding was not significant (among all women, P=.13) (Table 6).
Table 6.
Association between A) previous-day hormone concentrations and sexual intercourse and B) anovulation in first study cycle and sexual activity level in second study cycle
| A) | Odds Ratio (95% Confidence Interval) | |||||
|---|---|---|---|---|---|---|
| per log increase in previous-day hormone concentration | ||||||
| Sexual intercourse (any vs. none) | E2 (pg/mL) | Mid-cycle LH (ng/mL) | FSH (mIU/mL) | Luteal Progesterone(ng/mL) | Testosterone (ng/dL) | |
| Among all women | ||||||
| Unadjusted | 1.11 (0.95, 1.30) | 1.29 (1.05, 1.59) | 1.26 (0.98, 1.62) | 1.15 (0.95, 1.40) | 0.93 (0.62, 1.40) | |
| Adjusted I | 1.15 (0.99, 1.34) | 1.28 (1.04, 1.57) | 1.13 (0.88, 1.46) | 1.13 (0.91, 1.39) | 1.21 (0.78, 1.87) | |
| Adjusted II | 1.12 (0.96, 1.30) | 1.28 (1.04, 1.58) | 1.13 (0.88, 1.44) | 1.16 (0.94, 1.42) | 1.17 (0.76, 1.80) | |
| Among sexually active womena | ||||||
| Unadjusted | 1.06 (0.91, 1.23) | 1.23 (1.00, 1.52) | 1.16 (0.91, 1.49) | 1.07 (0.87, 1.30) | 1.04 (0.70, 1.54) | |
| Adjusted I | 1.10 (0.94, 1.28) | 1.23 (1.00, 1.52) | 1.13 (0.88, 1.46) | 1.06 (0.86, 1.31) | 1.09 (0.71, 1.67) | |
| Adjusted II | 1.08 (0.92, 1.26) | 1.23 (1.00, 1.52) | 1.13 (0.88, 1.45) | 1.09 (0.89, 1.35) | 1.05 (0.69, 1.60) | |
| B) | Odds Ratio (95% Confidence Interval) | ||
|---|---|---|---|
| Anovulation in first study cycle | |||
| Sexual activity level in second study cycle | Ovulation | Anovulation | |
| Among all women | |||
| Greater than weekly/all cycles (%)b | 65/248 (26) | 63/228 (28) | 2/20 (10) |
| Unadjusted | Reference | 0.29 (0.07, 1.29) | |
| Adjusted I | Reference | 0.36 (0.09, 1.36) | |
| Among sexually active women | |||
| Greater than weekly/all cycles (%)c | 65/192 (34) | 63/185 (34) | 2/7 (3) |
| Unadjusted | Reference | 0.77 (0.15, 4.11) | |
| Adjusted I | Reference | 0.82 (0.19, 3.46) | |
FSH, follicle-stimulating hormone; LH, luteinizing hormone; BMI, body mass index; E2, estradiol
Adjusted I: age, race, BMI, perceived stress level, and alcohol intake; Adjusted II: age, race, BMI, stress, alcohol intake and previous sexual activity level using inverse probability of exposure weights
Sexually active includes not sexually active during study, weekly or less, and greater than weekly.
Among all women, 248 second cycles were available for analysis excluding 2 second cycles missing sexual activity level, an additional cycle (with less than weekly sexual activity) was excluded in the adjusted analysis due to missing information on stress.
Among sexually active women, 192 second cycles were available for unadjusted and adjusted analyses.
Discussion
In a cohort of healthy, premenopausal women not taking hormonal contraceptives or attempting pregnancy, we observed that sexually active women had significantly higher serum concentrations of reproductive hormones (estradiol, mid-cycle LH, and luteal progesterone) and lower odds of sporadic anovulation in comparison with sexually inactive women (i.e. no prior and no study-period sexual activity). Among sexually active women, who included a group of women who did not report sexual intercourse during the 2 month study-period, frequency of sexual intercourse was not associated with reproductive function. We also found higher mid-cycle LH, estradiol and testosterone were associated with increased likelihood of subsequent sexual intercourse, while previous-day sexual intercourse was not associated with subsequent mid-cycle LH or other reproductive hormones. In addition, sexual intercourse since the first day of the cycle up until the expected day of ovulation was not associated with anovulation in that cycle among sexually active women, and anovulation in one cycle was not associated with sexual activity level in a subsequent cycle. Taken together, the findings from our study suggest that sexual activity status is related to hormone concentrations and ovulation in women, hormones are associated with likelihood of subsequent sexual intercourse, and there may exist a bi-directional relationship between sexual activity and reproductive function.
Our study found an increase in sexual activity around the time of ovulation among sexually active women. This is consistent with previous studies showing a peri-ovulatory increase in female sexual desire (Bancroft et al., 1983; Harvey, 1987; Stanislaw and Rice, 1988) and sexual activity (Brown et al., 2011; Caruso et al., 2014; Wilcox et al., 2004), specifically female-initiated sexual activity (Adams et al., 1978; Gangestad et al., 2002; Harvey, 1987; Van Goozen et al., 1997). The present study was not designed to evaluate whether the peri-ovulatory sexual activity was female or male-initiated; however, the higher mid-cycle LH, estradiol and testosterone associated with subsequent sexual intercourse suggests an intrinsically female-driven factor, which could result in pregnancy among at-risk cycles. Mechanisms of this increase in sexual activity could include an increase in self-perceived attractiveness (Schwarz and Hassebrauck, 2008) and/or an increase in libido (Dennerstein et al., 1994). Our finding of a similar frequency of sexual activity in the luteal phase compared to around the time of ovulation may be the result of partner-initiated sexual activity, which may not be menstrual phase specific (Caruso et al., 2014), or could be due to the fact that study participants were actively preventing pregnancy.
Few existing studies have been able to examine associations between sexual activity and reproductive hormone concentrations across either a single menstrual cycle or across multiple cycles. A recent study among 52 women (1–2 cycles each) not using hormonal contraceptives reported increased daily salivary estradiol, but not testosterone, concentrations on the days during which sexual intercourse was reported (Roney and Simmons, 2013). We also observed an acute association between sexual intercourse and estradiol, as well as a positive association between estradiol and sexual activity status (active vs. inactive). Unlike Roney and Simmons, we did observe a positive association between testosterone and same-day sexual intercourse, which agrees with another recent study of 1180 women (1 cycle each) that found frequency of sexual activity positively correlated with serum testosterone across the menstrual cycle (Caruso et al., 2014). In addition, we observed a higher frequency of sexual intercourse during the peri-ovulatory phase in this analysis, which corresponds with peak testosterone concentrations observed around ovulation as described in a previously published manuscript from this study (Sjaarda et al., 2014). Therefore, our findings support the association between testosterone and sexual activity (Pluchino et al., 2013; Turna et al., 2005).
Most previous studies on sexual behavior and menstrual cycle phase have excluded sexually inactive women and have assumed that hormones affect sexual behavior, as evidenced from their study design, analysis and interpretation of findings. We were able to make unique comparisons in our study as our cohort included a group of women (aged 18–37 years) who reported no prior and no study-period activity. Given the complex socio-cultural components involved in the occurrence of coital debut (Halpern et al., 2006), it is plausible that sexual activity status may affect hormone concentrations and ovulatory function rather than exclusively vice versa. Taking advantage of the longitudinal nature of our data, we were able to explore both directions of effect, finding evidence supporting a bi-directional effect of sexual activity and reproductive function. In this way our study provides a novel contribution to the study of female sexual behavior and menstrual cycle phase.
Our finding of sexual activity status possibly affecting reproductive function is unique, as our current understanding of the biological relationship between sexual activity and reproductive function is largely based on animal studies of induced (or “reflex”) ovulators and spontaneous ovulators (including humans). Though spontaneous ovulators are thought to ovulate according to an internal rhythmic cycle independent from copulatory behavior, it is feasible that sexual activity could modulate ovulation in these animals as well, perhaps due to exposure to “ovulation-inducing factor” protein in seminal plasma (Adams and Ratto, 2013; Ratto et al., 2012) or through genital stimulation causing the release of gonadotrophin releasing hormone (GnRH) (Huynh et al., 2013; Kruger et al., 2012; Zarrow and Clark, 1968). In agreement with copulatory behavior affecting ovulation even in spontaneous ovulators, we observed elevated mid-cycle LH and lower incident sporadic anovulation among sexually active women compared with the sexually inactive group. The mechanism behind these associations could be barrier-free frequent sexual intercourse (Stanislaw and Rice, 1987; Wilcox et al., 2004), as a large percentage of women in our study reported not using a form of contraception or it was not specified. However, the associations could also be due to the effects of hormones on subsequent sexual activity, as we described above.
Strengths of our study include the prospective capture of sexual activity, hormone concentrations and ovulation which are further strengthened by the measurement of these variables across two menstrual cycles using well-established laboratory protocols. Moreover, we were able to adjust for several factors, including age, race, BMI, alcohol intake and perceived stress levels, which may impact the relationships between sexual activity and reproductive function.
Still, our findings need to be interpreted in the context of important study limitations, most notably our measure of daily intercourse without further information regarding sexual libido, motivation, female orgasm, self- or partner-initiated activities or non vaginal-penile intercourse. Moreover, the possibility of residual confounding cannot be completely ruled out. In particular, we were unable to adjust for physical attractiveness, which was not captured in our assessment tools, and could be both an indicator of fecundity and predict sexual activity level. While we identified anovulatory cycles using serum hormone levels, we did not have radiological or direct visualization evidence to confirm ovulation. As such, we cannot eliminate the possibility of misclassification of ovulation status. In addition, our findings may have limited external validity as we cannot generalize them to reproductive-aged women using hormonal contraceptives or to those without regular menstrual cycles. Moreover, our study population is unique in that 23% reported no prior or current sexual activity, a proportion substantially higher than the <8% we might expect given the average age of study participants was 27 years (Mosher et al., 2005), which may further limit the generalizability of our findings. Lastly, though we utilized the temporal relationships between reproductive function and sexual intercourse available in our data to examine the direction of effects, the relationships between these factors may be bi-directional precluding our ability to infer causality. Additional studies are needed to help tease apart the directionality of effects.
In conclusion, we observed that sexual activity was associated with higher concentrations of estrogen, mid-cycle LH, and luteal progesterone and lower odds of sporadic anovulation among a population of young, healthy, regularly menstruating women not on hormonal contraceptives who were not seeking pregnancy. Importantly, the frequency of sexual activity did not seem to play as important of a role as whether the participant had ever had been sexually active. In addition, we found that higher mid-cycle LH, estradiol and testosterone levels were associated with increased likelihood of same or next-day sexual intercourse. While there are a few suggested mechanisms for the association between sexual activity and ovulation in animals, further studies are needed to characterize these relationships in humans and understand the direction of both long and short term effects. Improved understanding of the complicated association between sexual activity, reproductive hormones and ovulation has the potential to significantly increase our understanding of couples’ fecundity and the natural history of human ovulation.
Supplementary Material
Highlights.
Ovulation in women is thought to occur spontaneously during each menstrual cycle
Reproductive hormones likely affect sexual activity
However, sexual activity may affect reproductive hormones and ovulation
We examined women with and without a history of sexual activity
Women with a history of sexual activity had higher reproductive hormones
Women with a history of sexual activity had lower odds of anovulatory cycles
Acknowledgments
Funding: This study was funded by Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health, Bethesda, Maryland (Contract # HHSN275200403394C). The sources of funding had no role in study design, data collection, analysis, interpretation, writing the report, or in the decision to submit the article for publication.
We are indebted to all the investigators and staff at the University at Buffalo and we would like to especially recognize the BioCycle participants for their extraordinary commitment to the study.
Footnotes
Contributors: AP, SLM, GMBL, KAA, LAS, KCS, NJP, KK, JWW, and EFS contributed substantially to the conception and design of the study and analysis and interpretation of the data. AP wrote the first draft of the manuscript, which was critically revised by SLM, GMBL, KAA, LAS, KCS, NJP, KK, JWW, and EFS. AP, SLM and EFS had full access to all data and take responsibility for the integrity of the data and the accuracy of data analysis, and all authors approved the final manuscript. EFS is the guarantor.
Competing interests: All authors declare no conflicts of interest.
Ethical approval: The University at Buffalo Health Sciences Institutional Review Board (IRB) approved the study and served as the IRB designated by the National Institutes of Health for this study under a reliance agreement. All participants provided written informed consent.
Data sharing: Statistical code is available from the corresponding author.
Presentation Information: This analysis was presented at the 26th annual meeting of the Society for Pediatric and Perinatal Epidemiologic Research (SPER) in Boston, MA on June 17, 2013.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ankita Prasad, Email: aprasad128@gmail.com.
Sunni L. Mumford, Email: mumfords@mail.nih.gov.
Germaine M. Buck Louis, Email: louisg@mail.nih.gov.
Katherine A. Ahrens, Email: ahrenska@mail.nih.gov.
Lindsey A. Sjaarda, Email: lindsey.sjaarda@nih.gov.
Karen C. Schliep, Email: schliepkc2@mail.nih.gov.
Neil J. Perkins, Email: perkinsn@mail.nih.gov.
Kerri A. Kissell, Email: kissellka@mail.nih.gov.
Jean Wactawski-Wende, Email: jww@buffalo.edu.
Enrique F. Schisterman, Email: schistee@mail.nih.gov.
Reference list
- Adams DB, Gold AR, Burt AD. Rise in female-initiated sexual activity at ovulation and its suppression by oral contraceptives. N Engl J Med. 1978;299:1145–1150. doi: 10.1056/NEJM197811232992101. [DOI] [PubMed] [Google Scholar]
- Adams GP, Ratto MH. Ovulation-inducing factor in seminal plasma: a review. Anim Reprod Sci. 2013;136:148–156. doi: 10.1016/j.anireprosci.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Bakker J, Baum MJ. Neuroendocrine regulation of GnRH release in induced ovulators. Front Neuroendocrinol. 2000;21:220–262. doi: 10.1006/frne.2000.0198. [DOI] [PubMed] [Google Scholar]
- Bancroft J, Sanders D, Davidson D, Warner P. Mood, sexuality, hormones, and the menstrual cycle. III. Sexuality and the role of androgens. Psychosom Med. 1983;45:509–516. doi: 10.1097/00006842-198312000-00005. [DOI] [PubMed] [Google Scholar]
- Brown SG, Calibuso MJ, Roedl AL. Women’s sexuality, well-being, and the menstrual cycle: methodological issues and their interrelationships. Arch Sex Behav. 2011;40:755–765. doi: 10.1007/s10508-010-9630-3. [DOI] [PubMed] [Google Scholar]
- Buck Louis G. Fecundity and fertility. In: Buck Louis G, Platt R, editors. Reproductive and perinatal epidemiology. Oxford University Press; New York City, NY: 2011. pp. 30–54. [Google Scholar]
- Bullivant SB, Sellergren SA, Stern K, Spencer NA, Jacob S, Mennella JA, McClintock MK. Women’s sexual experience during the menstrual cycle: identification of the sexual phase by noninvasive measurement of luteinizing hormone. J Sex Res. 2004;41:82–93. doi: 10.1080/00224490409552216. [DOI] [PubMed] [Google Scholar]
- Caruso S, Agnello C, Malandrino C, Lo Presti L, Cicero C, Cianci S. Do hormones influence women’s sex? Sexual activity over the menstrual cycle. J Sex Med. 2014;11:211–221. doi: 10.1111/jsm.12348. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Dennerstein L, Gotts G, Brown JB, Morse CA, Farley TM, Pinol A. The relationship between the menstrual cycle and female sexual interest in women with PMS complaints and volunteers. Psychoneuroendocrinology. 1994;19:293–304. doi: 10.1016/0306-4530(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Gangestad SW, Thornhill R, Garver CE. Changes in women’s sexual interests and their partners’ mate-retention tactics across the menstrual cycle: evidence for shifting conflicts of interest. Proc Biol Sci. 2002;269:975–982. doi: 10.1098/rspb.2001.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Mumford SL, Zhang C, Wactawski-Wende J, Hovey KM, Whitcomb BW, Howards PP, Perkins NJ, Yeung E, Schisterman EF, Group BS. Effect of daily fiber intake on reproductive function: the BioCycle Study. American Journal of Clinical Nutrition. 2009;90:1061–1069. doi: 10.3945/ajcn.2009.27990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern CT, Waller MW, Spriggs A, Hallfors DD. Adolescent predictors of emerging adult sexual patterns. J Adolesc Health. 2006;39:926.e921–910. doi: 10.1016/j.jadohealth.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Harvey SM. Female sexual behavior: fluctuations during the menstrual cycle. J Psychosom Res. 1987;31:101–110. doi: 10.1016/0022-3999(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. American Journal of Epidemiology. 2009;169:105–112. doi: 10.1093/aje/kwn287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh H, Willemsen A, Holstege G. Female orgasm but not male ejaculation activates the pituitary. A PET-neuro-imaging study. Neuroimage. 2013;76:178–182. doi: 10.1016/j.neuroimage.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Jöchle W. Current research in coitus-induced ovulation: a review. J Reprod Fertil Suppl. 1975:165–207. [PubMed] [Google Scholar]
- Kruger TH, Leeners B, Naegeli E, Schmidlin S, Schedlowski M, Hartmann U, Egli M. Prolactin secretory rhythm in women: immediate and long-term alterations after sexual contact. Hum Reprod. 2012;27:1139–1143. doi: 10.1093/humrep/des003. [DOI] [PubMed] [Google Scholar]
- Larson CM, Haselton MG, Gildersleeve KA, Pillsworth EG. Changes in women’s feelings about their romantic relationships across the ovulatory cycle. Horm Behav. 2013;63:128–135. doi: 10.1016/j.yhbeh.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Lynch CD, Jackson LW, Buck Louis GM. Estimation of the day-specific probabilities of conception: current state of the knowledge and the relevance for epidemiological research. Paediatr Perinat Epidemiol. 2006;20(Suppl 1):3–12. doi: 10.1111/j.1365-3016.2006.00765.x. [DOI] [PubMed] [Google Scholar]
- Mosher WD, Chandra A, Jones J. Sexual behavior and selected health measures: men and women 15–44 years of age, United States, 2002. Adv Data. 2005:1–55. [PubMed] [Google Scholar]
- Mumford SL, Steiner AZ, Pollack AZ, Perkins NJ, Filiberto AC, Albert PS, Mattison DR, Wactawski-Wende J, Schisterman EF. The utility of menstrual cycle length as an indicator of cumulative hormonal exposure. Journal of Clinical Endocrinology and Metabolism. 2012;97:E1871–1879. doi: 10.1210/jc.2012-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagidas K, Carson SA, McGovern PG, Barnhart HX, Myers ER, Legro RS, Diamond MP, Carr BR, Schlaff WD, Coutifaris C, Steinkampf MP, Cataldo NA, Nestler JE, Gosman G, Giudice LC N.I.o.C.H.a.H.D.-R.M Network. Intercourse compliance, ovulation, and treatment success in the National Institute of Child Health and Human Development-Reproductive Medicine Network’s Pregnancy in Polycystic Ovary Syndrome (PPCOS) Trial. Fertil Steril. 2010;94:1444–1446. doi: 10.1016/j.fertnstert.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaus JG, Kippin TE, Coria-Avila G. What can animal models tell us about human sexual response? Annu Rev Sex Res. 2003;14:1–63. [PubMed] [Google Scholar]
- Pluchino N, Carmignani A, Cubeddu A, Santoro A, Cela V, Errasti T. Androgen therapy in women: for whom and when. Arch Gynecol Obstet. 2013;288:731–737. doi: 10.1007/s00404-013-2969-7. [DOI] [PubMed] [Google Scholar]
- Ratto MH, Leduc YA, Valderrama XP, van Straaten KE, Delbaere LT, Pierson RA, Adams GP. The nerve of ovulation-inducing factor in semen. Proc Natl Acad Sci U S A. 2012;109:15042–15047. doi: 10.1073/pnas.1206273109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Roney JR, Simmons ZL. Hormonal predictors of sexual motivation in natural menstrual cycles. Horm Behav. 2013;63:636–645. doi: 10.1016/j.yhbeh.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Gaskins AJ, Mumford SL, Browne RW, Yeung E, Trevisan M, Hediger M, Zhang C, Perkins NJ, Hovey K, Wactawski-Wende J, Group BS. Influence of endogenous reproductive hormones on F2-isoprostane levels in premenopausal women: the BioCycle Study. Am J Epidemiol. 2010;172:430–439. doi: 10.1093/aje/kwq131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz S, Hassebrauck M. Self-perceived and observed variations in women’s attractiveness throughout the menstrual cycle—a diary study. Evolution and Human Behavior. 2008;29:282–288. [Google Scholar]
- Sjaarda LA, Mumford SL, Kissell K, Schliep KC, Hammoud AO, Perkins NJ, Weck J, Wactawski-Wende J, Schisterman EF. Increased androgen, anti-Mullerian hormone and sporadic anovulation in healthy, eumenorrheic women: a mild PCOS-like phenotype? J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2013-3781. jc20133781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw H, Rice FJ. Acceleration of the menstrual cycle by intercourse. Psychophysiology. 1987;24:714–718. doi: 10.1111/j.1469-8986.1987.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Rice FJ. Correlation between sexual desire and menstrual cycle characteristics. Arch Sex Behav. 1988;17:499–508. doi: 10.1007/BF01542338. [DOI] [PubMed] [Google Scholar]
- Turna B, Apaydin E, Semerci B, Altay B, Cikili N, Nazli O. Women with low libido: correlation of decreased androgen levels with female sexual function index. Int J Impot Res. 2005;17:148–153. doi: 10.1038/sj.ijir.3901294. [DOI] [PubMed] [Google Scholar]
- Van Goozen SH, Wiegant VM, Endert E, Helmond FA, Van de Poll NE. Psychoendocrinological assessment of the menstrual cycle: the relationship between hormones, sexuality, and mood. Arch Sex Behav. 1997;26:359–382. doi: 10.1023/a:1024587217927. [DOI] [PubMed] [Google Scholar]
- Wactawski-Wende J, Schisterman EF, Hovey KM, Howards PP, Browne RW, Hediger M, Liu A, Trevisan M, Group BS. BioCycle study: design of the longitudinal study of the oxidative stress and hormone variation during the menstrual cycle. Paediatric and Perinatal Epidemiology. 2009;23:171–184. doi: 10.1111/j.1365-3016.2008.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K. Sex and context: hormones and primate sexual motivation. Horm Behav. 2001;40:339–357. doi: 10.1006/hbeh.2001.1696. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Dunson DB, McConnaughey DR, Kesner JS, Weinberg CR. On the frequency of intercourse around ovulation: evidence for biological influences. Hum Reprod. 2004;19:1539–1543. doi: 10.1093/humrep/deh305. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333:1517–1521. doi: 10.1056/NEJM199512073332301. [DOI] [PubMed] [Google Scholar]
- Wu CH, Blasco L, Flickinger GL, Mikhail G. Ovarian function in the preovulatory rabbit. Biol Reprod. 1977;17:304–308. doi: 10.1095/biolreprod17.2.304. [DOI] [PubMed] [Google Scholar]
- Zarrow MX, Clark JH. Ovulation following vaginal stimulation in a spontaneous ovulator and its implications. J Endocrinol. 1968;40:343–352. doi: 10.1677/joe.0.0400343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.