Abstract
Although sex differences and hormone effects on spatial cognition are observed in humans and animals, consensus has not been reached regarding exact impact on spatial working or reference memory. Recent studies in rats suggest that stress and/or reward, which are often different in tasks used to assess spatial cognition, can contribute to the inconsistencies in the literature. To minimize the impact of these sex- and sex hormone-sensitive factors, we used the Barnes maze to compare spatial working memory, spatial reference memory and spatial learning strategy in adult male, female, gonadectomized (GDX) male, and GDX male rats supplemented with 17β-estradiol (E) or testosterone propionate (TP). Rats received four acquisition trials, four trials 24 h later, and a single retention trial one week after. Males and females acquired the task during the first four trials and retained the task thereafter. In contrast, GDX rats took longer to acquire the task and showed retention deficits at 1 week. All deficits were attenuated similarly by TP and E. Assessment of search patterns also showed that strategies in the males transitioned from random to spatially focused and eventually direct approaches to the goal. However, this transition was faster in control and GDX-TP than in GDX and GDX-E rats. In contrast, the females almost invariantly followed the maze edge in thigmotactic, serial searches. Thus, while Barnes maze reveals activational, in part estrogenic effects on spatial cognition in males, its amenability to animals' use of multiple strategies may limit its ability to resolve mnemonic differences across sex.
Keywords: spatial working memory, spatial reference memory, Barnes maze, sex difference, gonadal hormones
Introduction
In humans, sex differences have been identified for a number of cognitive functions, e.g., superiority of adult females in verbal fluency (Herlitz et al., 2013; Mann et al., 1990; Weiss et al., 2003), superiority of males in spatial working memory (Lejbak et al., 2011; Talarowska et al., 2013). Sex differences have also been documented in the cognitive dysfunction that characterizes neurological and psychiatric disorders such as Parkinson's disease and schizophrenia wherein males are more often and more severely affected than females (Miller and Cronin-Golomb, 2010) (Han et al., 2012; Leung and Chue, 2000; Vaskinn et al., 2011). Particularly robust are the consensus findings for a male advantage in spatial tasks that range from mental object rotation (Hampson, 1990; Kaufman, 2007; Moffat and Hampson, 1996; Parsons et al., 2004) to virtual Morris water maze and radial arm maze tasks (Astur et al., 1998; Astur et al., 2004; Coluccia and Louse, 2004; Moffat et al., 1998; Woolley et al., 2010). Further, positive correlations have been identified between measures of spatial ability and circulating testosterone levels in men and women (Christiansen and Knussmann, 1987; Duff and Hampson, 2000; Gordon and Lee, 1986; Janowsky et al., 1994; Silverman et al., 1999), while negative correlations have been reported between estrogen levels and spatial cognition in women across the menstrual cycle (Hausmann et al., 2000; Simic and Santini, 2012).
While findings from human studies suggest that both organizational and activational hormone actions influence spatial ability, the exact natures of these actions have yet to be fully resolved. However, the numerous studies in animal and especially rodent models that have sought to clarify these issues have yet to reach complete consensus. For example, while a recent meta analysis supports a male over female advantage in spatial working and spatial reference memory in rats (Jonasson, 2005), the extant literature also includes studies finding no sex differences (Faraji et al., 2010; Healy et al., 1999; Juraska et al., 1984; Kolb and Cioe, 1996) or more infrequently, superior spatial performance in females (Gibbs and Johnson, 2008) on spatial tasks. Findings related to activational hormone effects from studies of gonadectomized (GDX) and hormone-supplemented rats span a similar gamut from no effects (Gibbs and Johnson, 2008; Luine et al., 1998; Sandstrom et al., 2006; Singh et al., 1994; Spritzer et al., 2011; Spritzer et al., 2008; Ziegler and Gallagher, 2005), to those that differentially identify spatial constructs as estrogen vs. androgen sensitive (Gibbs, 2005; Kritzer et al., 2007; Kritzer et al., 2001; McConnell et al., 2012; Sandstrom et al., 2006; Spritzer et al., 2011).
Several non-mnemonic factors are known to influence outcome measures in studies of sex and/or sex hormone effects on performance in spatial tasks. These include animals' ages (Bimonte-Nelson et al., 2003; Kanit et al., 2000) and hormone status, including the duration and dose of hormone deprivation and replacement (Bimonte and Denenberg, 1999; Daniel et al., 2006; Galea et al., 2001; Goudsmit et al., 1990; Spritzer et al., 2011; Spritzer et al., 2013). In addition, evidence for a male preference in utilization of hippocampal-dependent place strategies (Blokland et al., 2006; Hawley et al., 2012) and for high levels of testosterone in males and high levels of estrogen in proestrus females in biasing animals to use place rather than response strategies in solving spatial mazes (Korol et al., 2004; Spritzer et al., 2013) identify animals' approaches as yet another factor likely to influence outcomes, particularly across studies using mazes and testing contingencies where advantage is differentially conferred for place, response, or other strategies (Faraji et al., 2010; Gibbs and Johnson, 2008; Healy et al., 1999; Lund and Lephart, 2001; Ruprecht et al., 2013).
More recently, it has been suggested that task-related variables of stress and/or reward can also impact outcomes in studies of sex and sex hormone effects on spatial cognition (Hawley et al., 2013; McConnell et al., 2012). Both factors are known to differentiate and differentially influence behavior in gonadally intact and castrated male and female rats (Beiko et al., 2004; Belviranli et al., 2012; Conrad et al., 2004; Heinsbroek et al., 1987; Kritzer et al., 2007; Luine, 2007; Osborne et al., 2009). Thus, differences in sensitivity to stress and/or reward contingencies could help explain: the negative impact of GDX in appetitively motivated radial arm maze tasks but not aversely motivated Morris water maze tasks (Spritzer et al., 2011; Spritzer et al., 2008); the enhancement of male over female sex differences in spatial navigation in the dry-land ziggurat compared to Morris water maze (Faraji et al., 2010); and the dampening effects that pre-training has on the expression of male over female sex differences in the Morris water maze (Bucci et al., 1995; Faraji et al., 2010; Healy et al., 1999; Lukoyanov et al., 1999; Perrot-Sinal et al., 1996).
To minimize potentially confounding sex- and sex hormone-sensitive factors of stress and reward, a recent study compared short term spatial memory in extensively habituated control, GDX, and hormone-replaced male rats using a non-rewarded object in place memory task (McConnell et al., 2012). While findings of GDX-induced spatial working memory deficits were similar to those of previous studies (Gibbs and Johnson, 2008; Kritzer et al., 2007; Kritzer et al., 2001; Sandstrom et al., 2006; Spritzer et al., 2008), their attenuation by estrogen as well as by testosterone and dihydrotestosterone differs from the estrogen-insensitivity that has been found for GDX-induced spatial working memory deficits in rewarded tasks (Kritzer et al., 2007; Kritzer et al., 2001). This raises new questions about how activational hormone actions may influence rats' performances on spatial cognitive tasks and underscores the need for utilization of relatively stress- and reward-neutral testing conditions. Accordingly, we used the Barnes maze, a spatial memory paradigm where behavior is motivated by rodents' natural agoraphobia to search among holes to locate a recessed goal chamber (Barnes, 1979). While holding several advantages for the study of sex and sex hormone impact on spatial cognition, the Barnes maze has rarely been used for these purposes (Barrett et al., 2009; Berry et al., 2008; O'Leary et al., 2011; Ryan and Vandenbergh, 2006). Here the Barnes maze was used to compare multiple measures of performance related to task acquisition, spatial working memory, spatial reference memory, and spatial learning strategies in adult male, adult female, GDX males, and GDX male rats supplemented with testosterone propionate (TP) or 17β estradiol (E).
Materials and Methods
Animals
A total of 30 male and 8 female Sprague-Dawley rats (Taconic Farms, Germantown, NY) were used. Of the male rats, 8 were gonadectomized (GDX), 8 were GDX and supplemented with testosterone propionate (GDX-TP), 7 were GDX and supplemented with 17β-estradiol (GDX-E), and 7 received sham surgeries (CTRL) 28 days prior to behavioral testing. To allow similar habituation to housing conditions, female rats were housed in the Stony Brook University animal facility for similar lengths of time as the male subjects used in this study prior to the commencement of behavioral testing. All were housed in same sex/same treatment pairs under a 12 h, non-reversed light-dark cycle, in standard-sized cages. Cages and water bottles are purchased from Lab Products, Inc (Seaford, DE) and are made of Zyfone-a bisphenol –free plastic. Ground corncob bedding (Bed O' Cobs, Anderson) was provided and animals had free access to food (Purina PMI LabDiet: ProLab RMH 3000) and water. All animals were roughly 3 months of age and weighed 275-350g at time of testing. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Stony Brook University and were designed to minimize animal use and discomfort.
Surgeries
Twenty-eight days prior to maze habituation and behavioral testing male rats underwent GDX or sham surgery. Both surgical procedures were performed under aseptic conditions using intraperitoneal injections of ketamine (0.9 mg/kg) and xylazine (0.5 mg/kg) as anesthesia. For sham and GDX surgeries, an incision was made into the scrotum. For GDX, the vas deferens was bilaterally ligated (sterile non-absorbable, 6-0 silk sutures) and both testes were removed. For hormone-supplemented animals, slow-release pellets containing either testosterone propionate or 17β-estradiol (Innovative Research of America, Sarasota, FL) were implanted within the tunica. All incisions were closed with wound clips, which were removed after 10 days. Rats were given subcutaneous injections of buprenorphine (0.03 mg/kg) post operatively before being returned to home cages.
Hormone Replacement and Estrous Cycle Determination
Male rats were implanted with slow release pellets at the time of GDX. The testosterone propionate (TP) pellets used released 3-4 ng of TP per milliliter of blood per day and the 17β-estradiol (E) pellets used released 25 pg of E per milliliter of blood per day; both have been used previously in this and other labs and have been shown to produce sustained plasma hormone levels falling within physiological ranges (Adler et al., 1999; Collins et al., 1992; Kritzer, 2000). The efficacies of GDX and hormone replacement were verified in quantitative analyses of the weights of animals' androgen-sensitive bulbospongiosus muscles (BSMs) (Wainman and Shipounoff, 1941). The estrous cycle stage of female rats was assessed each day following maze testing via vaginal lavage and vaginal cytology (Goldman et al., 2007; Marcondes et al., 2002). Lavage samples were collected in saline using a fire-polished, sterile glass pipette. The sampled fluid was immediately placed on a slide, a coverslip was gently placed over it, and cytology was evaluated using light microscopy, differential interference contrast (DIC) optics, and a 20x objective. Estrous smears were identified by the predominance of cornified epithelial cells; proestrus smears were identified by the abundance of nucleated, non-cornified epithelial cells; diestrus smears were identified by the predominance of leukocytes along with cornified (diestrus I) or nucleated epithelial cells (diestrus II). On testing Day 1 four rats were in diestrus and four were in proestrus. Twenty-four hours later on testing Day 2, one rat transitioned from proestrus to estrus, two rats transitioned from diestrus to proestrus, and two rats transitioned from proestrus to diestrus (Table 1). Two rats remained in diestrus and one in proestrus from Day 1 to Day2 (Table 1). On testing Day 7, one week later, five rats were in diestrus and three were in proestrus (Table 1).
Table 1.
Number of rats in each estrous cycle stage across testing days as confirmed via vaginal lavage and vaginal cytology.
| Cycle Stage | Testing Day 1 | Testing Day 2 | Testing Day 3 |
|---|---|---|---|
| Estrus (low estrogen) | 0 | 1 | 0 |
| Diestrus | 4 | 4a | 5 |
| Proestrus (high estrogen) | 4 | 3b | 3 |
Two rats remained in diestrus from Day 1 to Day 2.
One rat remained in proestrus from Day 1 to Day 2.
Behavioral Testing
Apparatus
The Barnes maze consisted of a white, laminate, circular platform, 122 cm in diameter that was mounted on a rotatable pedestal 78 cm above the ground. Along its perimeter were 12 evenly spaced holes, each 10 cm in diameter that were separated 17 cm apart and were located 1.3 cm from the platform edge. The maze also had one removable black acrylic goal box (23 × 17 × 10.2 cm) that could be fitted under any of these holes. The maze pedestal was wrapped in black draping to make all holes, whether or not they contained the goal box, appear dark from the maze surface to minimize visual cues. A removable opaque open cylinder (20.3 cm in diameter, 17.8 cm high) was also used as a start box to position animals in the center of the maze for the start of each trial.
The maze was located in the center of a 12 ft square, sound-attenuated, evenly illuminated room. Bright light, which is sometimes used to motivate rats to locate the goal box was avoided. One wall of the room had fixed high contrast spatial cues (2 large, 10 inch, black triangles). During testing, the experimenter stood in a fixed position in one corner of the room 1.5 m away from the maze. A video camera (Logitech High Definition Webcam) was suspended 144.8 cm above the center of the maze and a laptop computer that operated the video camera was located next to the investigator. The testing room was located within a larger behavioral suite that included a separate, central room where animals were kept in home cages before and between trials.
Testing procedures
Testing began 28 days after GDX or sham surgery in the males and after a 28 day wait following purchase of the females. All animal handling and testing was done by a single, experienced individual (MNL).
1. Maze Habituation
Habituation to the maze was a step-wise process carried out in a single session. On the day before formal testing began, rats were transported in their home cages to the central room of the testing suite and were allowed to acclimate for approximately 1 h. After acclimatization, rats were moved (in their home cages) into the room containing the maze with the goal box placed in a specified position. Rats were removed from home cages, placed directly into the recessed goal box and were allowed to remain there undisturbed for 2 min. Rats were then returned to their home cages for a 15 min waiting period. Next, rats were placed on the maze surface adjacent to the goal hole; if they did not spontaneously enter the goal box, they were gently guided into it and were allowed to remain there undisturbed for 2 min. After being returned to the home cage for 15 min, rats were placed at the maze center within a temporarily constructed, opaque walkway that led directly to the goal hole; if rats did not spontaneously approach and enter the goal box, they were gently guided into it and allowed to remain undisturbed for a final 2 min.
2. Behavioral Testing
Behavioral testing began 24 h after maze habituation. The goal box was positioned under the hole located 180 degrees from its site during habituation. After rats were acclimated to the testing suite (1 h), rats were taken in home cages into the testing room. Testing began by placing rats in the start box (cylinder) located at the center of the maze. After a 10 s delay, the cylinder was lifted away and rats were given 3 min to explore. Upon entering the goal box, rats were allowed to stay undisturbed for 2 min. If rats failed to find the goal location within the 3 min trial, they were gently guided into it, and allowed to remain for 2 min. Rats were then returned to their home cages for a 15 min inter-trial interval. This testing process was then repeated for 3 additional trials to conclude Testing Day 1. Following the same procedures and using the same location of the goal box, rats received an additional 4 trials 24 h later (Testing Day 2) and one additional trial one week later (Testing Day 7). All maze and goal box surfaces were cleaned with 10% ethanol between each trial and the maze itself was also rotated between trials (but the goal box remained in the same relative position in space) to minimize olfactory cues.
Analysis
Behavior was scored off-line from archived video-recorded trials a by a single observer (MFK) who was blind to animal group. Videos were scored for the quantitative measures listed and described in Table 2.
Table 2.
Definitions of the quantitative measures of Barnes maze performance.
| Outcome Measure | Units | Description |
|---|---|---|
| Latency to Initiate Search | Seconds | Total time between start cylinder removal and first hole investigation. |
| Total Errors | Number | Total number of incorrect hole location investigations per trial. |
| Primary Errors | Number | Total numbers of first-time investigations of incorrect holes per trial. |
| Secondary Errors | Number | Total numbers of reinvestigations of previously visited incorrect holes per trial. |
| Consecutive Errors | Number | Total numbers of consecutive reinvestigations of incorrect holes. Rats must have stepped away from the hole in between investigations. |
| Latency to Find Goal | Seconds | Total time between start cylinder removal and front paws touching down in the goal box. |
Rats' paths were also digitized using the nose as a reference point and sampling video-recorded trials every 0.17 s (Tracker 4.62, Open Source Physics). These digitized paths were exported into ImageJ (open source, NIH) as black and white images. Black pixels were quantified, converted into centimeters and used to calculate the following:
Total path length: Total lengths of rats' paths (in centimeters).
Peripheral maze path length: Lengths of rats' paths within 20cm of the maze edge (in centimeters).
Central maze path length: Lengths of rats' paths within a 40cm radius from the center of the maze (in centimeters).
Rats' search strategies were also categorized according to quantitative criteria designed to be non-overlapping and applicable/sensitive to trials in which rats' made either large number, small numbers or no errors. These categorical variables were statistically compared across group using Fisher's Exact Test on per trial bases (see below). For each trial, rats' searches were assigned to one of the following categories:
Direct to Goal: Scored when all investigations involved holes that were within three holes from the goal on Day 1 and fewer than 3 errors were made; or within two holes from the goal on Days 2 and 7 and fewer than 2 errors were made.
Serial Search: Scored when 80% of holes investigated were adjacent or within 1 hole of each other, when animals made no changes in direction and remained in peripheral portions of the maze.
Random Search: Scored when 60% of holes investigated were non-adjacent and when animals made more than 2 changes of direction and/or traverses across central portions of the maze.
Statistics
Statistical analyses were conducted using SPSS, Version 22. Bulbospongiosus weights were compared across groups using a one-way analysis of variance (ANOVA). Quantitative Barnes maze data were compared across groups (per testing day) using two-way ANOVAs with repeated measures designs, where sex and hormone treatments (group) served as the independent factor and trials served as the repeated measure. When significant group, trial, or group by trial interactions were found, post hoc Bonferroni analyses were used to identify groups that over or underperformed (collapsing across trial), changes in trial-to-trial performance indicative of learning (collapsing across groups) or trials in which groups' performances diverged, respectively. Paired t-tests were also used in within groups comparisons of outcome measures between i) last trial of Day 1 and the first trial of Day 2 and ii) the last trial of Day 2 and the single Day 7 trial. Effect sizes were determined via η2 analyses. Fisher's exact tests were used to compare groups in terms of categorical variables of search strategy. In all cases p < 0.05 was accepted as significant and 0.05 < p <0.10 was defined as near significant.
Results
Effectiveness of Hormone Treatments in Males
The weights of the androgen sensitive bulbospongiosus muscles (BSM) in the male rats showed group differences that paralleled expected differences in circulating androgen levels. Thus, muscle weights in the CTRL and GDX-TP groups were on average 1.8g and 1.6g respectively, while in GDX and GDX-E rats, average muscle weights were 0.3g and 0.5g respectively (Fig 1). Statistical comparisons of individual rats' muscle weights (one-way ANOVA) identified significant main effects of group [F (3,24) = 19.73, p < 0.001, η2 = 0.71] on muscle mass and allowed post hoc comparisons confirmed that BSM weights of CTRL and GDX-TP rats were similar to each other; that the BSM weights of GDX and GDX-E rats were similar to each other; and that mean muscle weights of the CTRL and GDX-TP rats were significantly larger than those of both the GDX and GDX-E groups (p < 0.001, see Fig 1).
Figure 1.
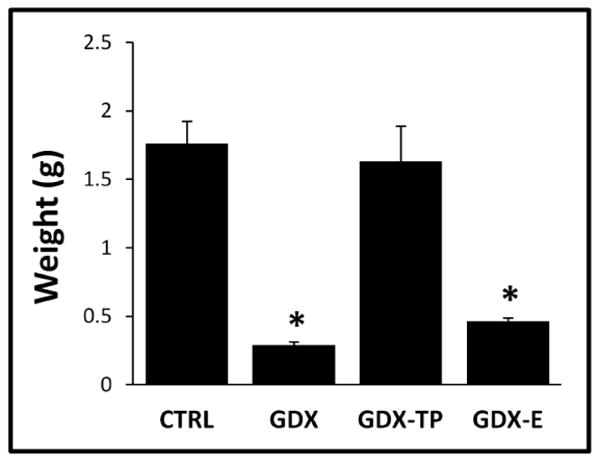
Bar graphs showing average bulbospongiosus muscle weights in grams (g) for gonadally intact control (CTRL), gonadectomized (GDX), and gonadectomized male rats supplemented with testosterone propionate (GDX-TP) or estradiol (GDX-E). Muscle weights of CTRL and GDX-TP rats were similar to each other and were significantly greater than those of GDX and GDX-E rats. Muscle weights of GDX and GDX-E rats were also similar to each other. Error bars represent standard errors of the mean. Asterisks denote significant differences from CTRL for post-hoc testing at the p < 0.05 level; η2 = 0.71.
Barnes Maze Testing
Task Acquisition: Day 1 Testing
During Trial 1, on the first day of testing, all groups showed similar, variable levels of performance. Thus, regardless of sex or hormone status, it took rats fewer than 10 sec to initiate hole searches but from 1-2 min to locate the goal (Fig 2). The total path lengths used in explorations were also lengthy and ranged from roughly 250 to 350 cm; for most groups one third or less of the path length involved traversing the maze center (Fig 3). Finally, in locating the goal, rats investigated an average of 8-18 incorrect locations. For most groups, this included substantial numbers of re-investigations of incorrect sites (Fig 4).
Figure 2.
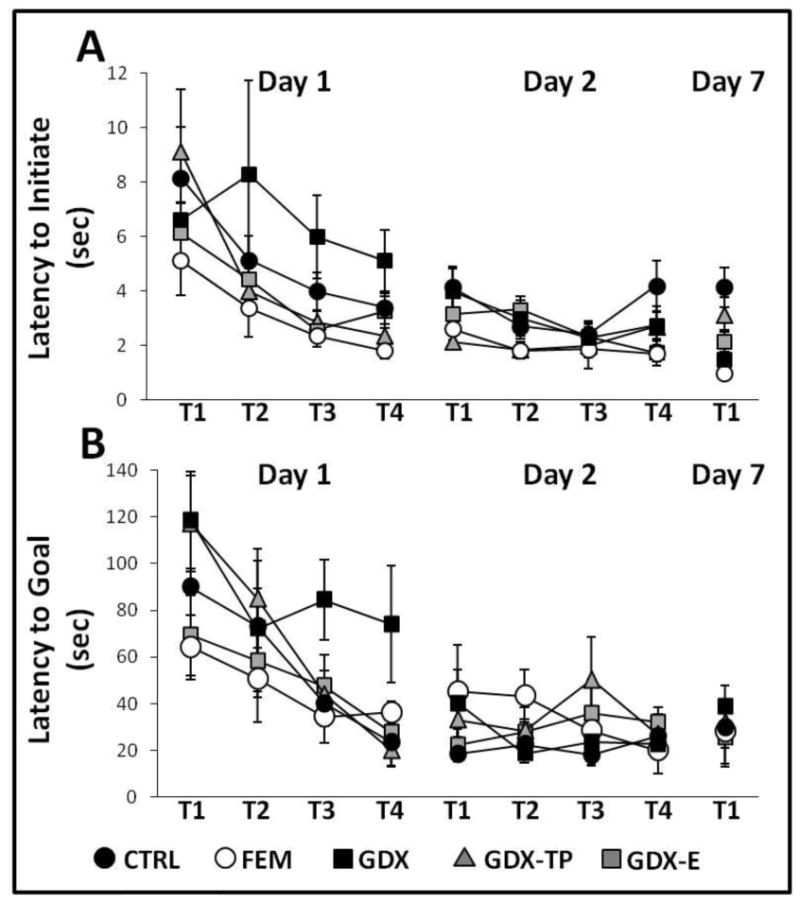
Line graphs showing (A) average latencies to initiate search and (B) average latencies to locate the goal across the four trials (T1-T4) of Day 1 (acquisition), the four trials of Day 2 (retention), and single trial of Day 7 (retention) testing for females (FEM, white circles), gonadally intact control (CTRL, black circles), gonadectomized (GDX, black squares), and gonadectomized male rats supplemented with testosterone propionate (GDX-TP (gray triangles) or estradiol (GDX-E, gray squares). Error bars represent standard errors of the mean. For all but the GDX group, latencies measured on Day 1 were similar and decreased systematically across trials. In the GDX rats, latencies were significantly longer than those of the other groups, with these differences being most marked on Trials 3 and 4. During Day 2 and Day 7 testing latencies were consistent and short across all trials in all groups. For latencies to initiate search, measures of effect sizes were η2 = 0.13-0.36 for Trials 1-4 of Day 1; η2 = 0.18-0.25 for Trials 1-4 of Day 2; and η2 = 0.46 on Day 7. For latencies to locate the goal, η2 = 0.20-0.28 for Trials 1-4 of Day 1; η2 = 0.09-0.17 for Trials 1-4 of Day 2; and η2 = 0.05 on Day 7.
Figure 3.
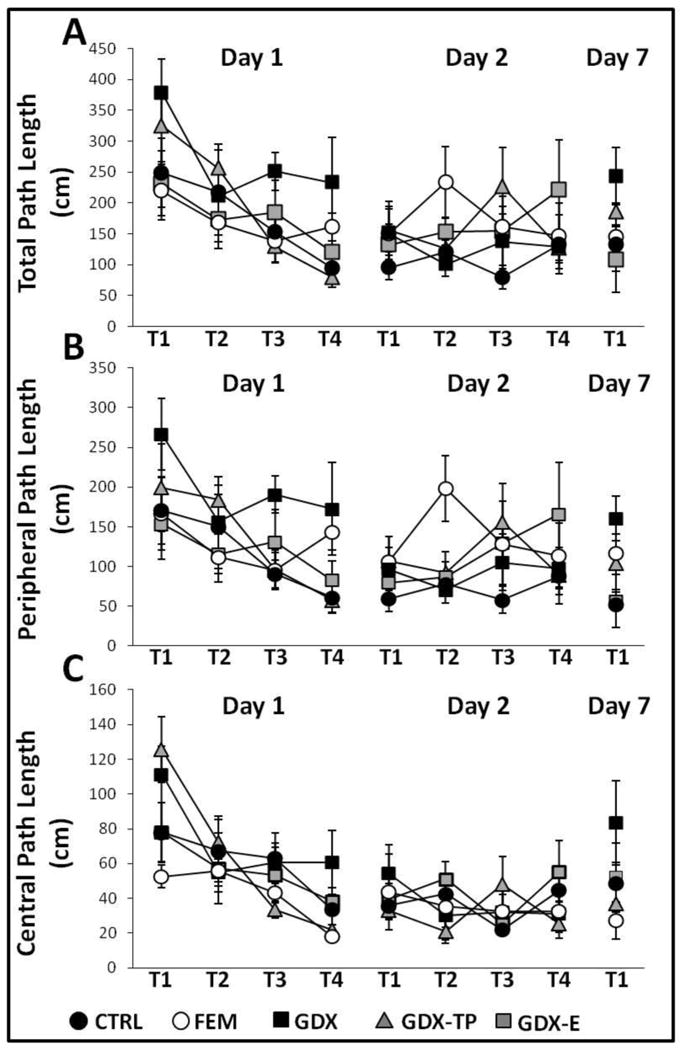
Line graphs showing (A) average total path lengths, (B) average peripheral maze path lengths and (C) average central maze path lengths -- all measured in centimeters (cm), during the four trials (T1-T4) of Day 1 (acquisition), the four trials of Day 2 (retention), and single trial of Day 7 (retention) testing for females (FEM, white circles), gonadally intact control (CTRL, black circles), gonadectomized (GDX, black squares), and gonadectomized male rats supplemented with testosterone propionate (GDX-TP, gray triangles) or estradiol (GDX-E, gray squares). Error bars represent standard errors of the mean. On Day 1 testing, all but the GDX and FEM groups showed similar, consistently shortened (A) total, (B) peripheral, and (C) center maze path lengths across trials. For the GDX rats (black squares) on all measures except central path length (C) were significantly longer compared to all other groups, with groups differences reaching their largest for T3 and/or T4. The FEM rats (white circles) were distinguished by relatively long peripheral maze paths (B) that were most noticeable in T4. During Day 2 and Day 7 testing, all path length metrics were similar and consistently short across trials in all groups. Measures of effect sizes for Trials 1-4 of Day 1 were as follows: Total path length, η2 = 0.16-0.24; Peripheral path length, η2 = 0.10-0.25; Center path length, η2 = 0.30-0.25. Effect sizes for Trials 1-4 of Day 2 were: Total path length, η2 = 0.05-0.07; Peripheral path length, η2 = 0.10-0.25; Center path length, η2 = 0.04-0.10. Day 7 effect sizes were: Total path length, η2 = 0.15; Peripheral path length, η2 = 0.25; Center path length, η2 = 0.10.
Figure 4.
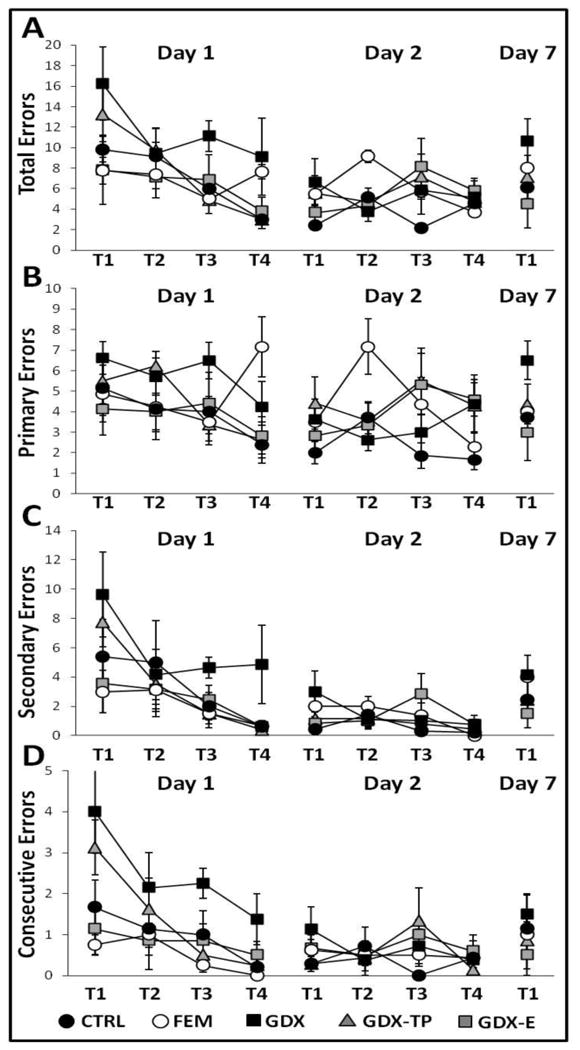
Line graphs showing (A) average numbers of total errors, (B) average numbers of primary errors, (C) average numbers of secondary errors, and (D) average numbers of consecutive errors committed during the four trials (T1-T4) of Day 1 (acquisition), the four trials of Day 2 (retention), and the single trial of Day 7 (retention) testing for females (FEM, white circles), gonadally intact control (CTRL, black circles), gonadectomized (GDX, black squares), and gonadectomized male rats supplemented with testosterone propionate (GDX-TP, gray triangles) or estradiol (GDX-E, gray squares). Error bars represent standard errors of the mean. For all but the GDX group, total (A) and primary (B) errors made during Day 1 testing were similar and decreased consistently across trials; the numbers of secondary (C) and consecutive errors (D) were also similar, were lower than primary errors, and dropped to near zero levels by T3 and T4. The GDX rats (black squares) made significantly more errors of all types compared to all other groups; the FEM rats (white circles) were also made noticeably more primary errors than the other groups during later trials. During Day 2 testing, error rates of all kinds were similar and consistently low across trials in all groups. On Day 7 testing, though no group differences were found, GDX rats (black squares) made significantly more total, primary, and secondary errors compared to T4 of Day 2 testing. Measures of effect sizes for Day 1 Trials 1-4 were as follows: Total errors, η2 = 0.14-0.23; Primary errors, η2 = 0.08-0.34; Secondary errors, η2 = 0.08-0.20; Consecutive Errors, η2 = 0.33-0.27. Effect sizes for Day 2 Trials 1-4 were as follows: Total errors, η2 = 0.11-0.26; Primary errors, η2 = 0.10-0.25; Secondary errors, η2 = 0.13-0.13; Consecutive Errors, η2 = 0.12-0.15. Day 7 effect sizes were: Total errors, η2 = 0.11; Primary errors, η2 = 0.17; Secondary errors, η2 = 0.07; Consecutive Errors, η2 = 0.11.
Over the subsequent three trials, latencies to initiate search and locate the goals (Fig 2), path lengths (Fig 3), and errors of all types (Fig 4) progressively decreased in every group. These observations were borne out in a series of two-way ANOVAs with repeated measures designs that identified significant main effects of trial for all outcome measures except Primary Errors (Table 3) and in post hoc comparisons that identified significant differences in each outcome measure for trials 3 and/or 4 compared to trials 1 and 2 (p = 0.0001-0.05).
Table 3.
Ranges of effect sizes (η2) across the four Day 1 trials and results of 2-way analyses of variance (ANOVA) with repeated measures design for each measure across the four Day 1 acquisition trials.
| BEHAVIOR | EFFECT SIZES | MAIN EFFECTS OF GROUP | MAIN EFFECTS OF TRIAL | GROUP × TRIAL INTERACTIONS |
|---|---|---|---|---|
| Latency to Search | η2 = 0.13-0.36 | (F4,33 = 2.43, p=0.07) | (F1.839,60.682 = 13.25, p<0.01) | (F7,60.682 = 1.20, p=0.32) |
| Latency to goal | η2 = 0.06-0.28 | (F4,33 = 4.76, p<0.01) | (F2.402,79.282, = 11.64, p<0.01) | (F9.610,79.282 = 0.77, p=0.65) |
| Total path length | η2 = 0.74-0.24 | (F4,33 = 3.74, p=0.01) | (F3,99 = 10.70, p<0.01) | (F12,99 = 1.02, p=0.45) |
| Peripheral path length | η2 = 0.09-0.25 | (F4,33 = 4.01, p=0.01) | (F2.229,73.573 = 6.49, p<0.01) | (F8.918,73.573 = 6.49, p<0.01) |
| Center path length | η2 = 0.03-0.30 | (F4,33 = 1.86, p=0.14) | (F2.425,80.038 = 16.65, p<0.01) | (F9.702,80.038 = 0.83, p=0.60) |
| Total Errors | η2= 0.03-0.23 | (F4,33 = 4.07, p=0.01) | (F2.169,71.574 = 5.23, p=0.01) | (F8.676,71.574 = 0.63, p=0.74) |
| Primary Errors | η2 = 0.08-0.34 | (F4,33 = 3.08, p=0.03) | (F3,99 = 2.12, p=0.01) | (F12,99 = 1.48, p=0.14) |
| Secondary Errors | η2 = 0.08-0.20 | (F4,33 = 4.05, p=0.01) | (F1.875,61.883 = 6.34, p<0.01) | (F7.501,61.883 = 0.62, p=0.75) |
| Consecutive errors | η2 = 0.07-0.36 | (F4,33 = 8.79, p<0.01) | (F2.002,66.069 = 9.32, p<0.01) | (F8.006,66.069 = 0.85, p=0.57) |
With the exception of Center Path Length, it was further evident that learning curves for all behavioral measures were relatively smooth, steep and overlapping for CTRL, GDX-E, GDX-TP, and FEM rats, but were noticeably flatter for the GDX group. Thus, as trials proceeded and as all other groups improved, GDX rats continued to take longer (Fig 2) and commit more errors (Fig 4) especially of the secondary and consecutive type in locating the goal, creating a divergence in the data that was especially evident over the final two trials. These differences were supported by two-way ANOVAs with repeated measures designs that identified significant main effects of Group for all measures except Center Path Length (Table 3), by post hoc comparisons that identified performance in the GDX group as significantly different from all others for every measure except Center Path Length (p = 0.0001-0.05) and by analyses of effect size where η2 increased across successive trials for all outcome measures (Figs 2-4).
Repeated Testing 24 h Later: Day 2 Testing
Four trials were conducted on Day 2, 24 h after Day 1 acquisition trials. Retention of the goal location was evident in all groups in performance in Trial 1 that equaled or bettered that recoded on the final trial from the previous day. This was confirmed in within-groups comparisons (paired t-tests) that found no significant differences in Day 1/Trial 4 compared to Day 2/Trial 1 data for any group on any outcome measure. Day 2 performance was also largely overlapping and asymptotic for all groups; although some variance was seen in the FEM group for path length and error measures (Fig 3A, B; 4A, B), for the most part, similar levels of peak performance were rapidly achieved and sustained for all groups. This was supported by a series of two-way ANOVAs with repeated measures design that found no significant or near-significant main effects of Group or Trial and no significant or near-significant interactions between these two for any performance measure. Analyses of effect sizes also revealed similar and similarly low η2 values across successive trials (Figs 2-4).
One-Week Retention Trial: Day 7 Testing
A single retention trial was conducted one week after task acquisition. For all groups latencies to initiate search and to locate the goal were similar to each other and similar to the peak performance observed one week prior on Testing Day 2. This was confirmed in within-groups comparisons that found no significant differences in Day 1/Trial 4 compared to Day 2/Trial 1 data for these measures in any of these groups. For all but the GDX rats, path length measures (Fig 3) and numbers of errors (all types, Fig 4) were largely overlapping across groups and were not significantly different (paired t-tests) from testing Day 2 values. In contrast, the GDX group made more errors (all types, Fig 4) and followed longer path lengths (all types, Fig 2) in locating the goal compared to the other groups. Although these group differences were not significant, within-groups comparisons identified significant differences within the GDX cohort for Day 2 vs. Day 7 measures of total, primary, and secondary errors [t(7) =2.35-3.67, p = 0.008-0.05] and measures of total and peripheral maze path lengths [t (7) =3.46-5.70, p =0.001-0.011] (Fig 4).
Maze Solving Strategy
Traces of animals' paths and quantitative assessments of the sequences of their hole searches were used to characterize solving strategies as either random, serial, or spatially constrained/direct. All groups began testing using searches that were random. The FEM group was distinctive in rapidly replacing the random approach to one wherein the edge of the maze and thigmotaxis were used to serially investigate holes (Figs 5, 6). In contrast, the CTRL and GDX-TP rats developed serial (non-thigmotatic) and finally spatially limited/direct approaches to the locating the goal (Figs 5, 6). Finally, the GDX and GDX-E rats also progressed from an initially random to more systematic, spatially focused searches. However, it was not until the final few trials on Day 2 that GDX and GDX-E rats began to reliably navigate more directly to the goal location (Figs 5, 6). The observed transitioning from group-wide utilization of random to group specific use of serial and/or direct modes of search strategy across trials was supported in statistical comparisons of categorical values of group and search strategy (Fishers Exact text) on per trial bases that progressively approached and reached significance (p = 0.001) during Day 1 testing by the last trial.
Figure 5.
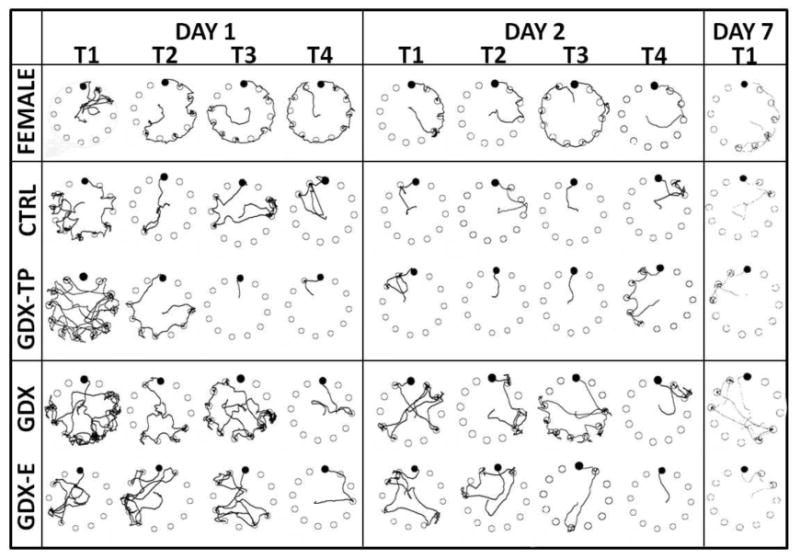
Digitized traces of paths followed by representative group subjects across Day 1, Day 2, and Day 7 trials. Open circles mark maze holes and the black circle marks the goal location. The traces illustrate the unique serial, thigmotactic hole investigation strategy that was consistently used by female rats; animals were remarkably invariant in following the physical edge of the maze in either a clockwise or counterclockwise direction to locate the goal. In contrast, control (CTRL) rats and gonadectomized rats supplemented with testosterone propionate (GDX-TP) rapidly transitioned across trials from random to serial to direct navigational strategies, while GDX and GDX rats supplemented with estradiol (GDX-E) rats needed a second testing day to transition from random to more systematic and spatially localized means to locate the goal. On Day 7 testing, female, CTRL, GDX-TP, GDX-E rats retained their strategies while GDX rats showed slightly less navigational retention.
Figure 6.
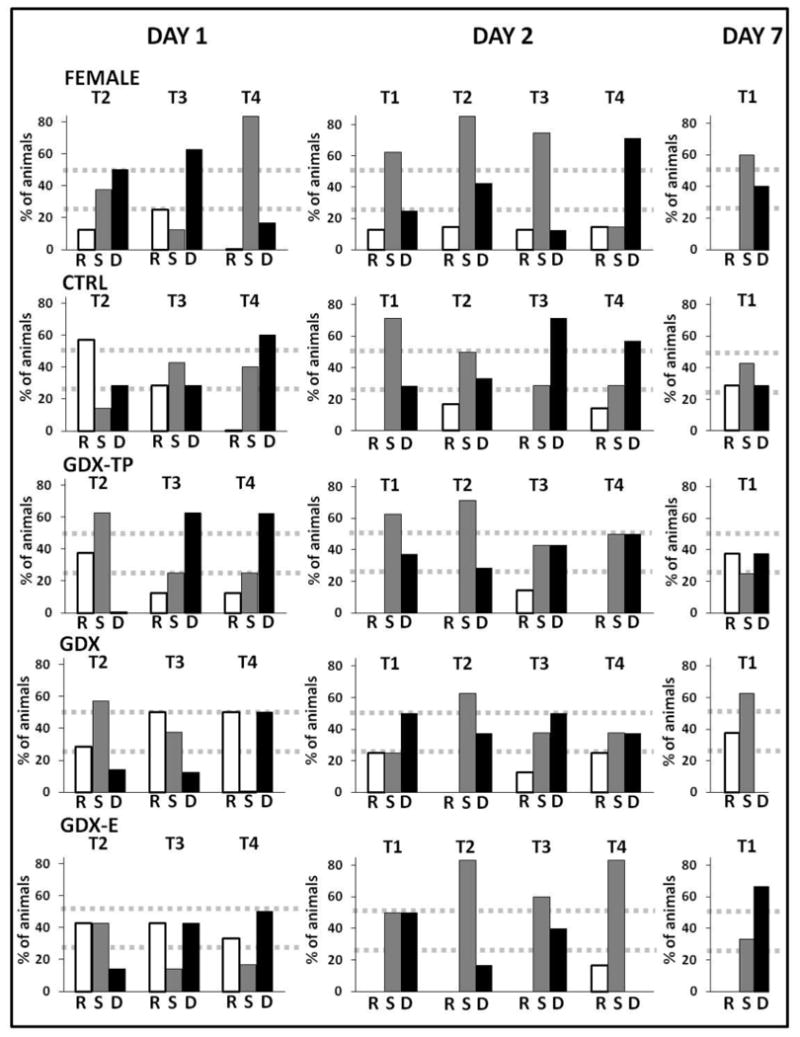
Bar graphs depicting percentage of animals utilizing random, serial, or direct searches for the goal across the last three trials (T2-T4) of Day 1 (acquisition), the four trials of Day 2 (retention), and the single trial of Day 7 (retention) testing. From T2 to T4 on Day 1, the percentage of control male rats (CTRL) and gonadectomized male rats supplemented with testosterone propionate (GDX-TP) utilizing primarily random searches (white bars) consistently decreased while those using serial (gray bars) or direct (black bars) searches progressively increased. By T4, the majority of CTRL and GDX-TP rats were moving directly to the goal with some rats continuing to employ serial searches and few to none performing random searches. Conversely, a large number of gonadectomized rats (GDX) and gonadectomized rats supplemented with estradiol (GDX-E) continued to demonstrate random searches from T2 through T4 with the percentage of animals doing so showing no appreciable decrease across acquisition trials. Additionally, while the number of GDX and GDX-E rats using direct searches progressively increased across trials and the number of rats performing serial searches decreased, the overall number of rats employing either of these types of searches did not change from T2 to T4. Finally, from T2 to T4, very few female rats demonstrated random searches. Most female rats used either serial or direct searches for the goal with the majority performing serial searches by T4. Across Day 2 trials, the majority of CTRL, GDX-TP, GDX, and GDX-E rats utilized either serial or direct searches with very few animals using a random search strategy while females continued to prefer serial searches. On Day 7, female and GDX-E rats maintained their navigational strategies while CTRL, GDX-TP, and GDX rats showed less retention with more rats using random searches compared to other groups. Fisher's exact tests only reached significance on Day 1, Trial 4 (p < 0.01).
Discussion
Sex differences and activational hormone effects have been identified for diverse aspects of learning, memory, and cognition in humans and animals (Christiansen and Knussmann, 1987; Daniel et al., 2003; Dohanich, 2002; Gouchie and Kimura, 1991; Luine, 2007; van Haaren et al., 1990). While spatial cognition and spatial learning appear to be sensitive to both biological sex and sex hormones in humans (Astur et al., 2004; Driscoll et al., 2005; Hampson, 1990; Linn and Petersen, 1985; Voyer et al., 1995), the literature, while generally supportive of a male advantage in human and in animal models alike, is not completely consistent. For animal studies, differences in the duration of hormone deprivation, the doses of hormone replacement, the paradigms used in behavioral testing and in the mnemonic constructs measured are likely to contribute to study-to-study differences (Daniel et al., 2006; Dohanich, 2002; Dohanich et al., 2009; Galea et al., 2001; Jonasson, 2005; Sandstrom et al., 2006; Spritzer et al., 2011; Spritzer et al., 2008). More recently, however, specific attention has been paid to the sex-specific sensitivity of outcome measures to task-related factors of stress and reward contingencies (Hawley et al., 2013; McConnell et al., 2012). Building on recent studies using an object in place spatial memory task (McConnell et al., 2012), the studies presented here used non-rewarded, relatively stress-neutral conditions of Barnes maze testing (Harrison et al., 2009). This task enabled us to compare multiple aspects of spatial cognition, e.g. spatial learning, working and reference memory, in gonadally intact male, gonadally intact female, GDX male, and in GDX male rats supplemented with TP or E. The group differences that are discussed further below are first demonstrations of such effects using Barnes maze testing. They also confirm and extend demonstrations of sex hormone impact on spatial cognition under testing conditions that deliberately minimize other potentially confounding, hormone-sensitive non-mnemonic influences on behavior (Hawley et al., 2013; McConnell et al., 2012).
Effects of GDX in Adult Male Rats: Task Acquisition, Spatial Learning and Spatial Working Memory
In Barnes maze testing, rats progressively navigate the apparatus using shorter, more direct routes to the goal while exploring fewer and fewer incorrect hole locations along the way. During Day 1 testing, CTRL (gonadally intact male) rats acquired the task rapidly as evinced by trial-to-trial reductions in all outcome measures. This improvement was accompanied by a shift in search strategy from random to more organized serial investigations of adjacent holes that were closer and closer to the goal and by the final trials, most CTRL rats navigated directly to the goal. Evidence of patent spatial working memory was also reflected in the group's diminishing number of primary errors and near elimination of secondary and consecutive type errors. In contrast, GDX rats showed less improvement across Day 1 trials and continued to commit relatively high numbers of secondary and consecutive type errors. The GDX rats also persisted in random hole searches. These deficits resembled those reported in earlier studies using other spatial mazes and paradigms. For example, GDX-induced deficits in task acquisition have been shown for T-maze delayed alternation (Kritzer et al., 2001), radial arm maze (Daniel et al., 2003) and operant delayed spatial alternation tasks (Kritzer et al., 2007; van Hest et al., 1988). Similar to their commission of secondary and consecutive Barnes maze errors, GDX rats have also been shown to re-enter previously visited arms significantly more often than controls in radial arm maze tasks (Gibbs and Johnson, 2008; Hasegawa and Mochizuki, 2009; Spritzer et al., 2008) and show impaired retention in a delayed match-to place version of the Morris water maze (Sandstrom et al., 2006). However, a critical difference is that the present studies demonstrate GDX-induced deficits under non-rewarded contingencies whereas earlier evidence of GDX-induced acquisition and spatial working memory deficits were gleaned from behaviors motivated by either food (Hasegawa and Mochizuki, 2009), palatable food (Daniel et al., 2003; Spritzer et al., 2008), or by food or water under conditions of food or water restriction (Gibbs and Johnson, 2008; Kritzer et al., 2007). This is significant as GDX rats show significantly reduced sucrose preference (Carrier and Kabbaj, 2012), increased novelty–induced hypophagia (Carrier and Kabbaj, 2012), lower rewarded response rates in operant random ratio acquisition tasks (Heinsbroek et al., 1987) and earlier break-points in operant progressive reward ratio tasks (Kritzer et al., 2007) compared to controls. Differences in stress associated with the different tasks may also be relevant given that GDX rats show greater anxiety and greater behavioral impairment in response to stress and circulating stress hormones (Carrier and Kabbaj, 2012; Khakpai, 2014; Osborne et al., 2009) compared to controls. With GDX effects on stress response and reward sensitivity poised to contribute to the poor performance reported among GDX groups in earlier studies, it is important that two studies have now shown significant GDX-induced impairment in spatial working memory under testing conditions wherein reward contingencies and stressors were relatively mitigated (McConnell et al., 2012). Further, by using the Barnes maze protocol, additional aspects of spatial cognition were also measured from behaviors motivated by rats' preferences for dark places. Given that dark preference has been shown to be potentiated by GDX in male rats compared to controls (Carrier and Kabbaj, 2012), the present study not only confirms and extends findings of GDX-induced deficits on spatial cognition but also shows them to be robust to testing conditions where motivation to perform the task may in fact be enhanced in GDX rats relative to CTRLS.
Effects of Hormone Replacement in Adult Male GDX Rats: Acquisition, Spatial Learning and Spatial Working Memory
The GDX-induced behavioral deficits observed during Barnes maze Day 1 testing were fully attenuated by giving GDX rats either E or TP. Thus, across all trials, latencies to find the goal, path lengths and numbers of primary, secondary, and consecutive type errors were overlapping in the CTRL, GDX-TP, and GDX-E groups. The comparable effects of supplementing GDX rats with TP or E suggest that the significant behavioral deficits induced in all of these domains by GDX stem at least in part from a loss of estrogen and/or estrogen metabolites. These findings contradict earlier studies from this lab where GDX-induced deficits in acquisition of T-maze (Kritzer et al., 2001) and operant spatial lever press tasks (Kritzer et al., 2007) were attenuated in GDX rats supplemented with TP but not with E. Although the dose and duration of hormone replacement have been shown to impact spatial cognition in GDX and gonadally intact male rats (Clark et al., 1995; Gibbs, 2005; Goudsmit et al., 1990; Spritzer et al., 2013), these factors were similar across studies and are thus unlikely to underlie the disparities in outcomes. Similarly, while Barnes maze testing has been shown to have minimal effects on rats' corticosterone levels (Harrison et al., 2009), rats in the prior studies were acclimated to handling, apparatus, and were subject to weeks-long testing, suggesting that cross-study differences in animals' stress responses may also have been minimal. In contrast, differences in reward contingencies do parse along lines of the effectiveness of TP vs. E-replacement to attenuate behavioral deficits in GDX rats. Thus, in both the T-maze and operant tasks -- where replacing GDX rats with TP but not E replacement effectively rescued GDX-induced acquisition deficits, subjects worked for water reward under similar protocols for water restriction (Kritzer et al., 2007; Kritzer et al., 2001). However, in the present study where GDX-induced deficits in acquisition and spatial cognition were equally and fully attenuated by both TP and E, all measured behaviors were spontaneous. These findings have parallels in GDX-induced deficits observed in a non-rewarded, object location memory paradigm which were also shown to be attenuated by supplementing GDX rats with E, T, or with dihydrotestosterone (DHT) (McConnell et al., 2012). Because DHT can be metabolized to estrogen receptor agonist compounds (3α and 3β-diol derivatives) (Jin and Penning, 2001; Torn et al., 2003; Wang et al., 2009), the data may suggest selective benefits of androgenic over estrogenic replacement in enhancing motivation to acquire tasks, and benefits of estrogenic over androgenic replacement in stimulating spatial cognition per se. Such a division of labor fits with evidence for reinforcing properties of androgens (Wood, 2004), and underscores the need for further assessment using paradigms that can effectively isolate the influences of gonadal steroids on cognitive, mnemonic, and non-mnemonic elements of the task.
Effects of GDX and Hormone Replacement in Adult Male Rats: Task Retention and Spatial Reference Memory
In contrast to the significant negative effects of GDX on Barnes task acquisition and spatial working memory, only subtle, non-significant GDX effects were observed in behaviors measured during retention testing 24 h later. Thus, while the GDX and GDX-E groups showed some initial improvement during Day 2 testing, asymptotic, peak performance that was similar in all groups emerged within a trial or two. Interestingly, the non-significant ‘catch-up’ observed in the GDX and GDX-E groups coincided with their transitioning from random to serial to spatially focused, direct navigations to the goal —similar to the transitions observed in the CTRL and GDX-TP groups during the first few acquisition trials. When tested on a single trial one week later, it was also evident that all groups retained the task. However, GDX rats made more errors of all types than the other groups, and significantly more errors than this same group made on the final trial of Day 2 testing.
Findings of no group differences in Barnes maze retention testing at a 24 h and 1 week delay add to consensus views of minimal to no effects of GDX on spatial reference memory. However, findings that after a week interval GDX rats made significantly more errors and followed longer pathways significantly more than they had previously does suggest some GDX influence on spatial reference memory. This may be in line with findings that T-replacement in GDX rats improves and T administration in gonadally intact rats impairs Morris Water maze spatial reference memory in adult but not aged rats (Goudsmit et al., 1990; Khalil et al., 2005; Spritzer et al., 2011). More recently, GDX-induced and T-attenuated deficits in spatial reference memory evaluated in Y-maze testing under non-rewarded contengencies were found for 48h but not 24h inter-trial intervals (Hawley et al., 2013). Taken together with the present findings, these data sum to underscore the potential complexity of hormone effects on spatial reference memory in male rats and suggest that rigorous experimental control and more demanding memory tasks are necessary to fully characterize them.
Sex and Sex Hormone Effects on Barnes Maze Strategies in Adult Male and Female Rats
All female subjects rapidly adopted and utilized a thigmotactic serial search strategy; within one or two trials nearly every member of the group followed the outer edge of the maze to investigate each hole encountered until arriving at the goal. These strategies had distinctive proprioceptive elements; subjects consistently followed the maze edge in either the clockwise or counter clockwise direction, and did not deviate in terms of approach or preferred direction across trials or testing days. These observations in the Barnes maze are thus commensurate with a female preference for response strategies in solving spatial mazes (Blokland et al., 2006; Hawley et al., 2012; Jonasson, 2005; Kanit et al., 2000).
The thigmotactic response strategies used by the females contrasted sharply with the spatial navigation that eventually was used by all male groups. This made it difficult to decipher comparative meaning from many of the other performance measures. For example, although most other behaviors measured, e.g., latency to find goal, commission of secondary and consecutive type errors, were similar in CTRL and female rats, it is far from clear that these reflect group similarities in task acquisition, spatial working, or spatial reference memory. In fact, those instances where scores between males and females diverged, i.e., the increased commission of primary errors and longer peripheral maze path lengths followed by females, suggest that their preferred spatial solving strategies enable them to locate the goal without tapping spatial working memory. Thus, the Barnes maze holds important advantages in meeting necessary challenges of parsing the effects of gonadal steroids on stress response and motivation to advance understanding of their impact on spatial cognition (Bowman et al., 2003; Bowman et al., 2009; Luine, 2002; McHenry et al., 2014; ter Horst et al., 2012). However, the degrees of freedom with which animals can solve the task may complicate the resolution of sex differences among behavioral domains of spatial working memory.
Highlights.
We test sex and hormone effects on spatial working and spatial reference memory.
Potential reward and stress confounds were minimized by using the Barnes maze.
Gonadectomy impairs spatial working but not spatial reference memory in male rats.
Both testosterone propionate and 17β-estradiol rescue gonadectomy-induced deficits.
Sex differences were observed in search strategy preference.
Acknowledgments
The authors would like to thank Surya Bhamidipaty for her assistance during the Barnes maze testing.
Funding: This work was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (R01-NS41966 to M.F.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler A, Vescovo P, Robinson JK, Kritzer MF. Gonadectomy in adult life increases tyrosine hydroxylase immunoreactivity in the prefrontal cortex and decreases open field activity in male rats. Neuroscience. 1999;89:939–954. doi: 10.1016/s0306-4522(98)00341-8. [DOI] [PubMed] [Google Scholar]
- Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behav Brain Res. 1998;93:185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Astur RS, Tropp J, Sava S, Constable RT, Markus EJ. Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav Brain Res. 2004;151:103–115. doi: 10.1016/j.bbr.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barrett GL, Bennie A, Trieu J, Ping S, Tsafoulis C. The chronology of age-related spatial learning impairment in two rat strains, as tested by the Barnes maze. Behav Neurosci. 2009;123:533–538. doi: 10.1037/a0015063. [DOI] [PubMed] [Google Scholar]
- Beiko J, Lander R, Hampson E, Boon F, Cain DP. Contribution of sex differences in the acute stress response to sex differences in water maze performance in the rat. Behav Brain Res. 2004;151:239–253. doi: 10.1016/j.bbr.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Belviranli M, Atalik KE, Okudan N, Gokbel H. Age and sex affect spatial and emotional behaviors in rats: the role of repeated elevated plus maze test. Neuroscience. 2012;227:1–9. doi: 10.1016/j.neuroscience.2012.09.036. [DOI] [PubMed] [Google Scholar]
- Berry A, Tomidokoro Y, Ghiso J, Thornton J. Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases brain amyloid-beta levels in female rats. Horm Behav. 2008;54:143–152. doi: 10.1016/j.yhbeh.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm AC. Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Exp Neurol. 2003;181:301–312. doi: 10.1016/s0014-4886(03)00061-x. [DOI] [PubMed] [Google Scholar]
- Blokland A, Rutten K, Prickaerts J. Analysis of spatial orientation strategies of male and female Wistar rats in a Morris water escape task. Behav Brain Res. 2006;171:216–224. doi: 10.1016/j.bbr.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Beck KD, Luine VN. Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm Behav. 2003;43:48–59. doi: 10.1016/s0018-506x(02)00022-3. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Micik R, Gautreaux C, Fernandez L, Luine VN. Sex-dependent changes in anxiety, memory, and monoamines following one week of stress. Physiol Behav. 2009;97:21–29. doi: 10.1016/j.physbeh.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Chiba AA, Gallagher M. Spatial learning in male and female Long-Evans rats. Behav Neurosci. 1995;109:180–183. doi: 10.1037//0735-7044.109.1.180. [DOI] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M. Testosterone and imipramine have antidepressant effects in socially isolated male but not female rats. Horm Behav. 2012;61:678–685. doi: 10.1016/j.yhbeh.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen K, Knussmann R. Sex hormones and cognitive functioning in men. Neuropsychobiology. 1987;18:27–36. doi: 10.1159/000118389. [DOI] [PubMed] [Google Scholar]
- Clark AS, Mitre MC, Brinck-Johnsen T. Anabolic-androgenic steroid and adrenal steroid effects on hippocampal plasticity. Brain Res. 1995;679:64–71. doi: 10.1016/0006-8993(95)00202-2. [DOI] [PubMed] [Google Scholar]
- Collins WF, 3rd, Seymour AW, Klugewicz SW. Differential effect of castration on the somal size of pudendal motoneurons in the adult male rat. Brain Res. 1992;577:326–330. doi: 10.1016/0006-8993(92)90292-h. [DOI] [PubMed] [Google Scholar]
- Coluccia E, Louse G. Gender differences in spatial orientation: A review. Journal of environmental psychology. 2004;24:329–340. [Google Scholar]
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL. Acute stress impairs spatial memory in male but not female rats: influence of estrous cycle. Pharmacol Biochem Behav. 2004;78:569–579. doi: 10.1016/j.pbb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Moerschbaecher JM. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmacology (Berl) 2003;170:294–300. doi: 10.1007/s00213-003-1537-4. [DOI] [PubMed] [Google Scholar]
- Dohanich G. Gonadal steroids, learning and memory. Hormones, brain and behavior. 2002;1:265–327. [Google Scholar]
- Dohanich G, Korol D, Shors T. Steroids, learning and memory. Hormones, brain and behavior. 2009;1:265–327. [Google Scholar]
- Driscoll I, Hamilton DA, Yeo RA, Brooks WM, Sutherland RJ. Virtual navigation in humans: the impact of age, sex, and hormones on place learning. Horm Behav. 2005;47:326–335. doi: 10.1016/j.yhbeh.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Duff SJ, Hampson E. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Horm Behav. 2000;38:262–276. doi: 10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- Faraji J, Metz GA, Sutherland RJ. Characterization of spatial performance in male and female Long-Evans rats by means of the Morris water task and the ziggurat task. Brain Res Bull. 2010;81:164–172. doi: 10.1016/j.brainresbull.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav Brain Res. 2001;126:115–126. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48:268–277. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–3183. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Gordon HW, Lee PA. A relationship between gonadotropins and visuospatial function. Neuropsychologia. 1986;24:563–576. doi: 10.1016/0028-3932(86)90100-4. [DOI] [PubMed] [Google Scholar]
- Gouchie C, Kimura D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology. 1991;16:323–334. doi: 10.1016/0306-4530(91)90018-o. [DOI] [PubMed] [Google Scholar]
- Goudsmit E, Van de Poll NE, Swaab DF. Testosterone fails to reverse spatial memory decline in aged rats and impairs retention in young and middle-aged animals. Behav Neural Biol. 1990;53:6–20. doi: 10.1016/0163-1047(90)90729-p. [DOI] [PubMed] [Google Scholar]
- Hampson E. Variations in sex-related cognitive abilities across the menstrual cycle. Brain Cogn. 1990;14:26–43. doi: 10.1016/0278-2626(90)90058-v. [DOI] [PubMed] [Google Scholar]
- Han M, Huang XF, Chen da C, Xiu MH, Hui L, Liu H, Kosten TR, Zhang XY. Gender differences in cognitive function of patients with chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:358–363. doi: 10.1016/j.pnpbp.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa N, Mochizuki M. Improved effect of Pycnogenol on impaired spatial memory function in partial androgen deficiency rat model. Phytother Res. 2009;23:840–843. doi: 10.1002/ptr.2702. [DOI] [PubMed] [Google Scholar]
- Hausmann M, Slabbekoorn D, Van Goozen SH, Cohen-Kettenis PT, Gunturkun O. Sex hormones affect spatial abilities during the menstrual cycle. Behav Neurosci. 2000;114:1245–1250. doi: 10.1037//0735-7044.114.6.1245. [DOI] [PubMed] [Google Scholar]
- Hawley WR, Grissom EM, Barratt HE, Conrad TS, Dohanich GP. The effects of biological sex and gonadal hormones on learning strategy in adult rats. Physiol Behav. 2012;105:1014–1020. doi: 10.1016/j.physbeh.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Hawley WR, Grissom EM, Martin RC, Halmos MB, Bart CL, Dohanich GP. Testosterone modulates spatial recognition memory in male rats. Horm Behav. 2013;63:559–565. doi: 10.1016/j.yhbeh.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Healy SD, Braham SR, Braithwaite VA. Spatial working memory in rats: no differences between the sexes. Proc Biol Sci. 1999;266:2303–2308. doi: 10.1098/rspb.1999.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek RP, van Haaren F, Zantvoord F, van de Poll NE. Sex differences in response rates during random ratio acquisition: effects of gonadectomy. Physiol Behav. 1987;39:269–272. doi: 10.1016/0031-9384(87)90020-5. [DOI] [PubMed] [Google Scholar]
- Herlitz A, Reuterskiold L, Loven J, Thilers PP, Rehnman J. Cognitive sex differences are not magnified as a function of age, sex hormones, or puberty development during early adolescence. Dev Neuropsychol. 2013;38:167–179. doi: 10.1080/87565641.2012.759580. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behav Neurosci. 1994;108:325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- Jin Y, Penning TM. Steroid 5alpha-reductases and 3alpha-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism. Best Pract Res Clin Endocrinol Metab. 2001;15:79–94. doi: 10.1053/beem.2001.0120. [DOI] [PubMed] [Google Scholar]
- Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Biobehav Rev. 2005;28:811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Henderson C, Muller J. Differential rearing experience, gender, and radial maze performance. Dev Psychobiol. 1984;17:209–215. doi: 10.1002/dev.420170302. [DOI] [PubMed] [Google Scholar]
- Kanit L, Taskiran D, Yilmaz OA, Balkan B, Demirgoren S, Furedy JJ, Pogun S. Sexually dimorphic cognitive style in rats emerges after puberty. Brain Res Bull. 2000;52:243–248. doi: 10.1016/s0361-9230(00)00232-x. [DOI] [PubMed] [Google Scholar]
- Kaufman SB. Sex differences in mental rotation and spatial visualization ability: Can they be accounted for by differences in working memory capacity? Intelligence. 2007;35:211–223. [Google Scholar]
- Khakpai F. The effect of opiodergic system and testosterone on anxiety behavior in gonadectomized rats. Behav Brain Res. 2014;263C:9–15. doi: 10.1016/j.bbr.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Khalil R, King MA, Soliman MR. Testosterone reverses ethanol-induced deficit in spatial reference memory in castrated rats. Pharmacology. 2005;75:87–92. doi: 10.1159/000087188. [DOI] [PubMed] [Google Scholar]
- Kolb B, Cioe J. Sex-related differences in cortical function after medial frontal lesions in rats. Behav Neurosci. 1996;110:1271–1281. doi: 10.1037//0735-7044.110.6.1271. [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45:330–338. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kritzer MF. Effects of acute and chronic gonadectomy on the catecholamine innervation of the cerebral cortex in adult male rats: insensitivity of axons immunoreactive for dopamine-beta-hydroxylase to gonadal steroids, and differential sensitivity of axons immunoreactive for tyrosine hydroxylase to ovarian and testicular hormones. J Comp Neurol. 2000;427:617–633. [PubMed] [Google Scholar]
- Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK. Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav. 2007;51:183–194. doi: 10.1016/j.yhbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirlis T, Robinson JK. Gonadectomy impairs T-maze acquisition in adult male rats. Horm Behav. 2001;39:167–174. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Lejbak L, Crossley M, Vrbancic M. A male advantage for spatial and object but not verbal working memory using the n-back task. Brain Cogn. 2011;76:191–196. doi: 10.1016/j.bandc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Leung A, Chue P. Sex differences in schizophrenia, a review of the literature. Acta Psychiatr Scand Suppl. 2000;401:3–38. doi: 10.1111/j.0065-1591.2000.0ap25.x. [DOI] [PubMed] [Google Scholar]
- Linn MC, Petersen AC. Emergence and characterization of sex differences in spatial ability: A meta-analysis. Child development. 1985:1479–1498. [PubMed] [Google Scholar]
- Luine V. Sex differences in chronic stress effects on memory in rats. Stress. 2002;5:205–216. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- Luine VN. The prefrontal cortex, gonadal hormones and memory. Horm Behav. 2007;51:181–182. doi: 10.1016/j.yhbeh.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Lukoyanov NV, Andrade JP, Dulce Madeira M, Paula-Barbosa MM. Effects of age and sex on the water maze performance and hippocampal cholinergic fibers in rats. Neurosci Lett. 1999;269:141–144. doi: 10.1016/s0304-3940(99)00442-5. [DOI] [PubMed] [Google Scholar]
- Lund TD, Lephart ED. Manipulation of prenatal hormones and dietary phytoestrogens during adulthood alter the sexually dimorphic expression of visual spatial memory. BMC Neurosci. 2001;2:21. doi: 10.1186/1471-2202-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann VA, Sasanuma S, Sakuma N, Masaki S. Sex differences in cognitive abilities: a cross-cultural perspective. Neuropsychologia. 1990;28:1063–1077. doi: 10.1016/0028-3932(90)90141-a. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- McConnell SE, Alla J, Wheat E, Romeo RD, McEwen B, Thornton JE. The role of testicular hormones and luteinizing hormone in spatial memory in adult male rats. Horm Behav. 2012;61:479–486. doi: 10.1016/j.yhbeh.2012.01.003. [DOI] [PubMed] [Google Scholar]
- McHenry J, Carrier N, Hull E, Kabbaj M. Sex differences in anxiety and depression: Role of testosterone. Front Neuroendocrinol. 2014;35:42–57. doi: 10.1016/j.yfrne.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller IN, Cronin-Golomb A. Gender differences in Parkinson's disease: clinical characteristics and cognition. Mov Disord. 2010;25:2695–2703. doi: 10.1002/mds.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Hampson E. A curvilinear relationship between testosterone and spatial cognition in humans: possible influence of hand preference. Psychoneuroendocrinology. 1996;21:323–337. doi: 10.1016/0306-4530(95)00051-8. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Hampson E, Hatzipantelis M. Navigation in a ,Äúvirtual&,Äù maze: Sex differences and correlation with psychometric measures of spatial ability in humans. Evolution and Human Behavior. 1998;19:73–87. [Google Scholar]
- O'Leary TP, Savoie V, Brown RE. Learning, memory and search strategies of inbred mouse strains with different visual abilities in the Barnes maze. Behav Brain Res. 2011;216:531–542. doi: 10.1016/j.bbr.2010.08.030. [DOI] [PubMed] [Google Scholar]
- Osborne DM, Edinger K, Frye CA. Chronic administration of androgens with actions at estrogen receptor beta have anti-anxiety and cognitive-enhancing effects in male rats. Age (Dordr) 2009;31:191–198. doi: 10.1007/s11357-009-9114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons TD, Larson P, Kratz K, Thiebaux M, Bluestein B, Buckwalter JG, Rizzo AA. Sex differences in mental rotation and spatial rotation in a virtual environment. Neuropsychologia. 2004;42:555–562. doi: 10.1016/j.neuropsychologia.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Kostenuik MA, Ossenkopp KP, Kavaliers M. Sex differences in performance in the Morris water maze and the effects of initial nonstationary hidden platform training. Behav Neurosci. 1996;110:1309–1320. doi: 10.1037//0735-7044.110.6.1309. [DOI] [PubMed] [Google Scholar]
- Ruprecht CM, Taylor CD, Wolf JE, Leising KJ. Task Complexity Modifies the Search Strategy of Rats. Behav Brain Res. 2013 doi: 10.1016/j.bbr.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm Behav. 2006;50:85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kim JH, Wasserman MA. Testosterone modulates performance on a spatial working memory task in male rats. Horm Behav. 2006;50:18–26. doi: 10.1016/j.yhbeh.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Silverman I, Kastuk D, Choi J, Phillips K. Testosterone levels and spatial ability in men. Psychoneuroendocrinology. 1999;24:813–822. doi: 10.1016/s0306-4530(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Simic N, Santini M. Verbal and spatial functions during different phases of the menstrual cycle. Psychiatr Danub. 2012;24:73–79. [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Daviau ED, Coneeny MK, Engelman SM, Prince WT, Rodriguez-Wisdom KN. Effects of testosterone on spatial learning and memory in adult male rats. Horm Behav. 2011;59:484–496. doi: 10.1016/j.yhbeh.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Fox EC, Larsen GD, Batson CG, Wagner BA, Maher J. Testosterone influences spatial strategy preferences among adult male rats. Horm Behav. 2013;63:800–812. doi: 10.1016/j.yhbeh.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Gill M, Weinberg A, Galea LA. Castration differentially affects spatial working and reference memory in male rats. Arch Sex Behav. 2008;37:19–29. doi: 10.1007/s10508-007-9264-2. [DOI] [PubMed] [Google Scholar]
- Talarowska M, Florkowski A, Chamielec M, Galecki P. Are there any differences in the working memory of men and women? Pol Merkur Lekarski. 2013;34:29–32. [PubMed] [Google Scholar]
- ter Horst JP, de Kloet ER, Schachinger H, Oitzl MS. Relevance of stress and female sex hormones for emotion and cognition. Cell Mol Neurobiol. 2012;32:725–735. doi: 10.1007/s10571-011-9774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torn S, Nokelainen P, Kurkela R, Pulkka A, Menjivar M, Ghosh S, Coca-Prados M, Peltoketo H, Isomaa V, Vihko P. Production, purification, and functional analysis of recombinant human and mouse 17beta-hydroxysteroid dehydrogenase type 7. Biochem Biophys Res Commun. 2003;305:37–45. doi: 10.1016/s0006-291x(03)00694-6. [DOI] [PubMed] [Google Scholar]
- van Haaren F, van Hest A, Heinsbroek RP. Behavioral differences between male and female rats: effects of gonadal hormones on learning and memory. Neurosci Biobehav Rev. 1990;14:23–33. doi: 10.1016/s0149-7634(05)80157-5. [DOI] [PubMed] [Google Scholar]
- van Hest A, van Kempen M, van Haaren F, van de Poll NE. Memory in male and female Wistar rats: effects of gonadectomy, and stimulus presentations during the delay interval. Behav Brain Res. 1988;29:103–110. doi: 10.1016/0166-4328(88)90057-5. [DOI] [PubMed] [Google Scholar]
- Vaskinn A, Sundet K, Simonsen C, Hellvin T, Melle I, Andreassen OA. Sex differences in neuropsychological performance and social functioning in schizophrenia and bipolar disorder. Neuropsychology. 2011;25:499–510. doi: 10.1037/a0022677. [DOI] [PubMed] [Google Scholar]
- Voyer D, Voyer S, Bryden MP. Magnitude of sex differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull. 1995;117:250–270. doi: 10.1037/0033-2909.117.2.250. [DOI] [PubMed] [Google Scholar]
- Wang H, Cheng JD, Montgomery D, Cheng KC. Evaluation of the binding orientations of testosterone in the active site of homology models for CYP2C11 and CYP2C13. Biochem Pharmacol. 2009;78:406–413. doi: 10.1016/j.bcp.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Siedentopf C, Hofer A, Deisenhammer EA, Hoptman MJ, Kremser C, Golaszewski S, Felber S, Fleischhacker WW, Delazer M. Brain activation pattern during a verbal fluency test in healthy male and female volunteers: a functional magnetic resonance imaging study. Neurosci Lett. 2003;352:191–194. doi: 10.1016/j.neulet.2003.08.071. [DOI] [PubMed] [Google Scholar]
- Wood RI. Reinforcing aspects of androgens. Physiol Behav. 2004;83:279–289. doi: 10.1016/j.physbeh.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Woolley DG, Vermaercke B, Op de Beeck H, Wagemans J, Gantois I, D'Hooge R, Swinnen SP, Wenderoth N. Sex differences in human virtual water maze performance: novel measures reveal the relative contribution of directional responding and spatial knowledge. Behav Brain Res. 2010;208:408–414. doi: 10.1016/j.bbr.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Gallagher M. Spatial memory in middle-aged female rats: assessment of estrogen replacement after ovariectomy. Brain Res. 2005;1052:163–173. doi: 10.1016/j.brainres.2005.06.006. [DOI] [PubMed] [Google Scholar]


