Abstract
Background
Breath testing is becoming an important diagnostic method to evaluate many disease states. In light of rising healthcare costs, is important to develop a simple non-invasive tool to potentially identify pediatric patients who need endoscopy for suspected inflammatory bowel disease (IBD).
Aim
The primary aim of this study was to analyze exhaled volatile organic compounds (VOCs) to evaluate for the presence of a unique breath pattern to differentiate pediatric patients with (IBD) from healthy controls.
Methods
A cross-sectional, single-center study included pediatric IBD patients and healthy controls (age range, 5-21 years). The diagnosis of IBD was confirmed by endoscopic, histologic, and radiographic data. Exhaled breath was collected and analyzed using a selective ion flow tube mass spectroscopy (SIFT-MS) to identify new markers or patterns of IBD.
Results
117 patients (62 with IBD and 55 healthy controls) were included in the study. Linear discriminant analysis and principle component analysis of mass scanning ion peak data demonstrated 21 pre-selected VOCs correctly classify patients with IBD or as healthy controls; p < 0.0001. Multivariable logistic regression analysis further showed 3 specific VOCs (1-octene, 1-decene, (E)-2-nonene) had excellent accuracy for predicting the presence of IBD with an area under the curve (AUC) of 0.96 (95% CI: 0.93, 0.99). No significant difference in VOCs was found between patients with Crohn's disease or ulcerative colitis and no significant correlation was seen with disease activity.
Conclusion
This pilot data supports the hypothesis that a unique breathprint potentially exists for pediatric IBD in the exhaled metabolome.
Keywords: Breath testing, volatile organic compounds, oxidative stress, pediatrics, inflammatory bowel disease
INTRODUCTION
Inflammatory bowel diseases (IBD) are among the top five chronic gastrointestinal diseases in the United States and account for rising healthcare costs exceeding $1.7 billion annually. It is estimated that approximately 1.4 million Americans suffer from IBD and about 10% of them are children under the age of 18 years (1).
The pathogenesis of IBD remains poorly understood. Current understanding involves the interaction between a genetic predisposition, a defective host mucosal immune system, and an imbalance of intestinal microbiota and environmental factors which lead to chronic intestinal inflammation. The diagnosis of IBD requires the detection of intestinal inflammation to distinguish from non-organic causes of abdominal pain, such as irritable bowel syndrome. Diagnosis of IBD often times poses a challenge to the clinician and requires an extensive workup.
In an effort to minimize cost and exposure to invasive procedures, multiple biomarkers have been evaluated to screen patients for suspected IBD. Commonly used non-specific markers of inflammation include erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), which do not always correlate with disease activity and are not specific to IBD inflammation (2). In addition, currently available IBD serology panels are very costly and can be inconclusive. On the other hand, fecal biomarkers, such as calprotectin and lactoferrin, are markers of bowel inflammation that have demonstrated utility in differentiating active from inactive IBD, as well as differentiating IBD from other diseases (3). A recent meta-analysis of studies of adults and children reported that fecal calprotectin is useful for identifying patients most likely to need endoscopy for suspected IBD (4). However, this testing is also associated with high cost at certain centers in the United States and requires patients to properly collect, store, and drop off stool specimens to a laboratory. Hence, more specific, rapid, relatively inexpensive, and non-invasive biomarkers are urgently needed to more accurately identify patients who need endoscopy for suspected IBD.
Breath testing is becoming an increasingly important non-invasive diagnostic method and safer alternative for diagnosing or screening children for many gastrointestinal diseases, including carbohydrate intolerance, Helicobacter pylori infection, small bowel bacterial overgrowth, and liver dysfunction. Human breath is a complex mixture containing hundreds of volatile organic compounds (VOCs). With recent advancements in mass spectrometry techniques such as selected ion flow tube mass spectrometry (SIFT-MS), it is possible to precisely identify trace gases in the human breath in the parts-per-billion range that correlate with different disease states.(5-10).
The primary aims of this study were to analyze VOCs using SIFT-MS in the breath of children with IBD and healthy controls and evaluate for the presence of a unique breath pattern of pediatric IBD that may help elucidate new pathways involved in disease pathogenesis. The secondary aim was to assess the ability of this technique to distinguish between Crohn's disease (CD) and ulcerative colitis (UC) and between active and inactive IBD.
METHODS
Study Population and Clinical Data
We conducted a cross-sectional, single center study that was approved by the Institutional Review Board at the Cleveland Clinic, in Cleveland, OH. Patients with documented IBD between the ages of 5 and 21 years were recruited from the Pediatric Gastroenterology Clinic and healthy controls between the ages of 5 and 21 years were recruited from the General Pediatric Clinic. All patients were recruited from March of 2012 to November of 2012. The healthy controls were recruited during their annual well child visit and their primary care provider verified that the patient had no identifiable active disease state or concurrent medication use. The diagnosis of IBD was confirmed by endoscopic, histologic, and radiographic data. Disease activity was determined according to the Pediatric Crohn's Disease Activity Index (PCDAI) (11) or the Pediatric Ulcerative Colitis Activity Index (PUCAI) (12). Active CD or UC was defined as a PCDAI >10 or PUCAI >10. CD or UC in remission was defined as a PCDAI ≤ 10 or PUCAI ≤ 10, respectively.
The patients with IBD were grouped using the Paris Classification which classified CD patients based on age, disease location, behavior and growth (13). The locations were designated as terminal ileum, colonic, ileocolonic, and upper gastrointestinal tract involvement. The CD phenotypes were designated as inflammatory, stricturing, penetrating, or both penetrating and stricturing disease. Presence of perianal disease was documented. Growth parameters were assessed for the absence of presence of growth delay. The UC patients were classified based on disease extent as proctitis, left sided (distal) disease, extensive (hepatic flexure distally) disease and pancolitis. Severe disease was defined as PUCAI ≥ 65.
Exclusion criteria included the following: a history of alcohol consumption or smoking, known diagnosis of carbohydrate intolerance, Helicobacter pylori infection or small bowel bacterial overgrowth, use of oral or intravenous antibiotics within a two week period prior to breath testing, colonic bowel preparation within in the preceding two weeks, prior resection of the ileo-cecal valve, diverting ileostomy and/or total abdominal colectomy, a current respiratory tract infection or known diagnosis of asthma.
Exhaled Breath Collection
After all patients/parents rendered informed consent and assent, exhaled breath samples were collected as previously described (9,14). Patients were not required to be fasting prior to the breath collection or required to provide a 24 hour dietary recall. All samples were collected during routine well child visits or follow up visits, between the hours of 8:00 AM and 5:00 PM. Briefly, study participants were asked to complete a mouth rinse with water before the breath sample was collected to decrease endogenous VOCs produced by oral bacteria. After normal exhalation to release residual air from the lungs, participants were asked to inhale to total lung capacity through a disposable mouth filter. The filter was used to eliminate exogenous VOCs and potential viral or bacterial agents from the inhaled air. The inhaled ambient air was also filtered through an attached N7500-2 acid gas cartridge (North Safety Products, Smithfield, Rhode Island, USA). The participants then proceeded to exhale through the mouth filter against 10 cm of water pressure into the attached Mylar bag (Convertidora Industrial, Jalisco, Mexico) at a constant flow rate until the lungs were emptied. The exhaled breath sample was retained in the Mylar bag, capped and stored in an incubator set to body temperature at 37.0 °C (98.6 °F). This method allowed the VOCs to remain in vapor state and prevented condensation onto the surface of the Mylar bag. All samples were analyzed within 4 hours. Mylar bags were cleaned by flushing with nitrogen between participants.
Selected-ion flow-tube mass spectrometry
The VOICE200 SIFT-MS instrument (Syft Technologies Ltd, Christchurch, New Zealand) was used to analyze breath samples in this study. The principle of SIFT-MS technology has been previously well described in the literature (15-17). Mass scans of the product ions generated in the chemical ionization mass spectrum from each reagent ion (H3O+, NO+, and O2+) were obtained in the mass scanning mode. MS between 14–200 amu was used to identify significant peaks at product ion masses representing unknown breath volatiles relating to IBD. More accurate concentration data was obtained by selected ion monitoring of product ions of 21 preselected compounds: 2-propanol, acetaldehyde, acetone, acrylonitrile, benzene, carbon disulfide, dimethyl sulfide, ethanol, isoprene, pentane, 1-decene, 1-heptene, 1-nonene, 1-octene, 3-methylhexane, (E)-2-nonene, ammonia, ethane, hydrogen sulfide, triethylamine and trimethylamine. These compounds have been previously identified as common constituents of the exhaled human breath metabolome (14,18).
Statistical Analysis
Descriptive statistics were computed for all factors. Data are presented as mean ± standard deviation, median [25th, 75th percentiles] or N (%). Univariable analysis was done to assess differences between IBD and healthy controls group. Analysis of variance (ANOVA) or the non-parametric Kruskal-Wallis tests was used to compare continuous variables. The Pearson's chi-square test was used for categorical factors. Classification was performed using linear discriminant analysis (LDA) and principal components analysis (PCA) of the mass scanning ion peak data. In addition, analysis of covariance (ANCOVA) was used to assess differences while adjusting for possible age, gender, race and BMI percentile differences. The logarithm of each VOC was modeled as the outcome variable. Multivariable logistic regression analysis was performed to assess if a combination of different VOCs or the addition of clinical characteristics improved prediction of IBD. Receiver operating characteristic analysis was used to study the role of VOCs in the diagnosis of IBD and the area under the receiver operating characteristic curve (AUC) with (95% CI) was reported. A p-value less than 0.05 was considered statistically significant. In addition, Spearman's correlation coefficient was used to assess correlations between clinical characteristics of IBD and VOCs. For this pilot study, the sample size was chosen by the authors based on previous data in different disease states. SAS (version 9.2, The SAS Institute, Cary, NC) and R (version 2.15.2, The R Foundation for Statistical Computing, Vienna, Austria) were used for all analyses.
RESULTS
Patient Characteristics
The study included 117 patients (62 with IBD and 55 healthy controls). The IBD cohort was significantly older (15.7 ± 3.3 vs. 12.1 ± 3.0 years) and more likely to be Caucasian (88.7% vs. 36.4%); p < 0.001 for all (Table 1). Of the 62 IBD patients, 51 were diagnosed with CD and 11 were diagnosed with UC. The predominant location for CD involvement was ileocolonic with upper gastrointestinal tract disease in 34 patients (66.7%). The predominant phenotype was inflammatory, occurring in 37 patients (72.5%). The predominant distribution for UC was extensive disease distal to the hepatic flexure in 6 patients (54.5%). Approximately 80% of the IBD cohort was in remission based on standardized pediatric IBD activity index tools (PCDAI and PUCAI). Maintenance medications for the IBD cohort were documented and 44 patients (70.9%) were on 5-aminosalicylate (5-ASA), 23 patients (37.1%) were on immunomodulator therapy, 36 patients (58%) were on biologics and 9 patients (14.5%) were on corticosteroids at the time of breath testing (Table 2). The youngest patient to successfully complete breath testing was 7 years old.
Table 1.
Demographic Characteristics
| Factor | Control (N=55) | IBD (N=62) | p-value |
|---|---|---|---|
| Age (years) | 12.1±3.0 | 15.7±3.3 | <0.001 |
| Male | 35(63.6) | 33(53.2) | 0.25 |
| Race | <0.001 | ||
| . Caucasian | 20(36.4) | 55(88.7) | <0.001 |
| . Black | 23(41.8) | 6(9.7) | NS |
| . Other | 12(21.8) | 1(1.6) | NS |
| BMI percentile | 55.8±24.6 | 53.7±30.5 | 0.7 |
Values presented as Mean ± SD with ANOVA or N (%) with Pearson's chi-square test.
Table 2.
IBD Clinical Characteristics
| Factor | CD (N=51) | UC (N=11) |
|---|---|---|
| Age at Diagnosis (years) | 12.4±3.5 | 13.2±3.7 |
| IBD Duration (years) | 2.9[1.09,4.4] | 6.6[1.6,7.3] |
| IBD surgery | 9(17.6) | 0(0.0) |
| Family history of IBD | 22(43.1) | 5(45.5) |
| Medications | ||
| 5-aminosalicylate (5-ASA) | 34(66.7) | 10(90.9) |
| Azathioprine/Mercaptopurine | 14(27.5) | 3(27.3) |
| Methotrexate | 6(11.8) | 0(0.0) |
| Anti-TNF | 29(56.9) | 7(63.6) |
| Prednisone | 7(13.7) | 2(18.2) |
| Hemoglobin (g/dL) | 12.8[11.7,13.9] | 12.2[11.5,14.1] |
| Hematocrit (%) | 38.7[35.6,40.6] | 37.7[35.5,42.2] |
| ESR (mm/hr) | 4.0[1.00,9.0] | 5.0[1.00,10.0] |
| CRP (mg/dL) | 0.10[0.10,0.10] | 0.10[0.10,0.10] |
| WBC (k/uL) | 6.9[5.7,9.0] | 7.5[5.7,8.5] |
| Platelets (k/uL) | 286.0[230.0,350.0] | 246.0[206.0,370.0] |
| Albumin (g/dL) | 4.2[4.1,4.5] | 4.4[3.5,4.5] |
| PCDAI Score | 4.8 ± 8.4 | --- |
| PUCAI Score | --- | 14.2 ± 15.5 |
Values presented as Mean ± SD, Median [P25, P75] or N (%).
Predicting the presence of IBD using a unique breathprint
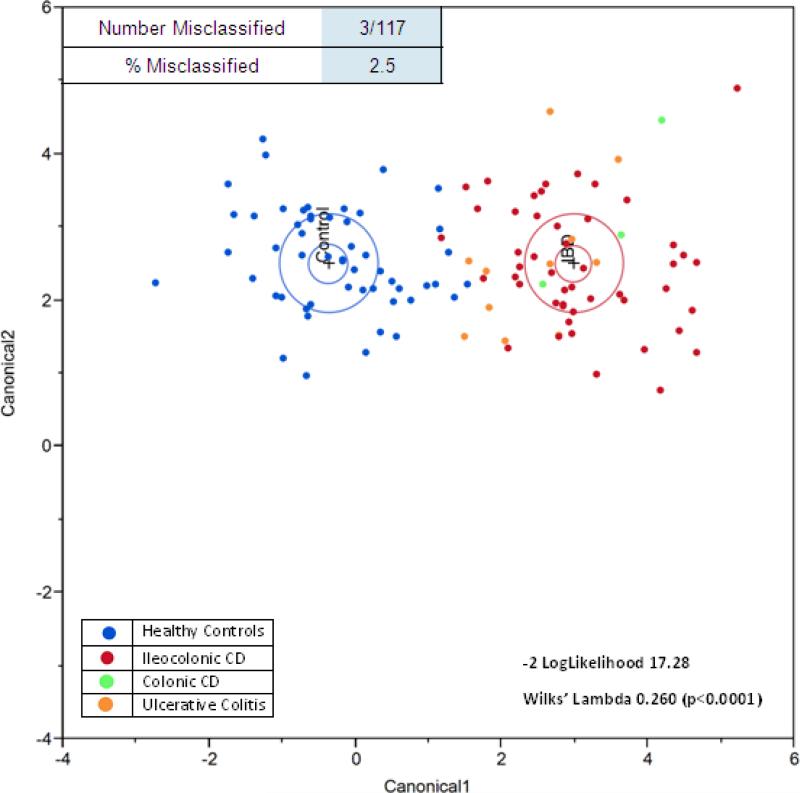
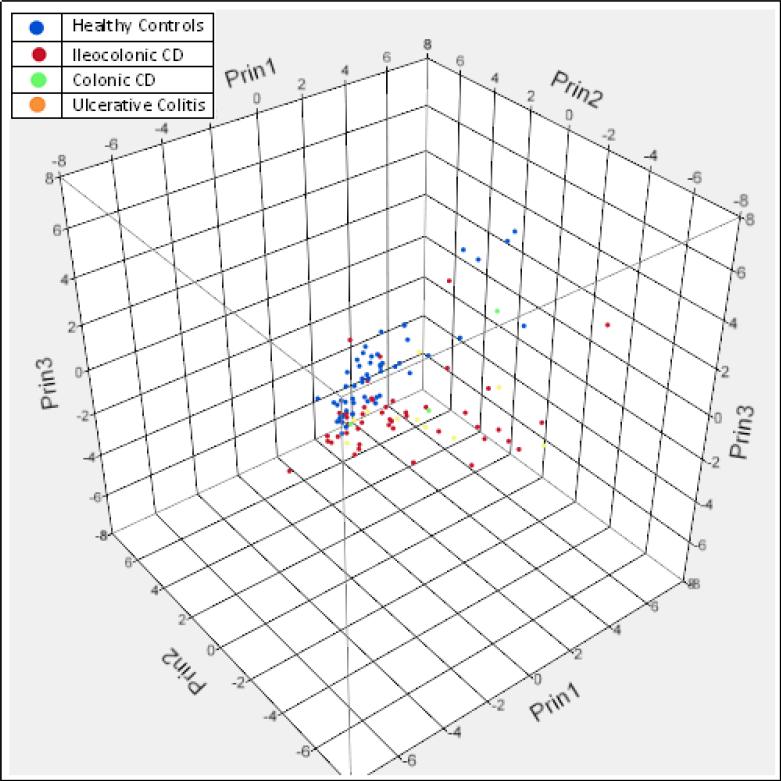
SIFT-MS data analysis was undertaken using discriminant analysis and results are shown in Figure 1. Linear discriminant analysis (LDA) of mass scanning ion peak data demonstrated 21 preselected VOCs can classify patients with IBD or as healthy controls with only three misclassifications (-2 log likelihood 17.28; Wilks’ Lambda 0.260 (p < 0.0001)) (Figure 1a). The discriminant analysis model correctly classified 97.5% of subjects with only three misclassifications. Only 1 of 62 patients (1.6%) with IBD was misclassified as being healthy. Whereas, 2 of 55 healthy controls (3.6%) were misclassified as having IBD. Principal components analysis (PCA) of the 21 preselected VOCs confirmed the accuracy of classifying patients with IBD or as healthy controls with a reclassification of 97% (Figure 1b).
Figure 1a.
Linear Discriminant Analysis (LDA) of VOCs in healthy controls vs. patients with IBD. Canonical discriminant analysis demonstrated 21 preselected VOCs can classify patients with IBD or as healthy controls. The model correctly classified 97.5% of patients with IBD with only three misclassifications. Only 1 of 62 patients (1.6%) with IBD was misclassified as being healthy.
Figure 1b.
Principal Components Analysis (PCA) of the 21 preselected VOCs confirmed the accuracy of classifying patients with IBD or as healthy controls with a reclassification of 97%.
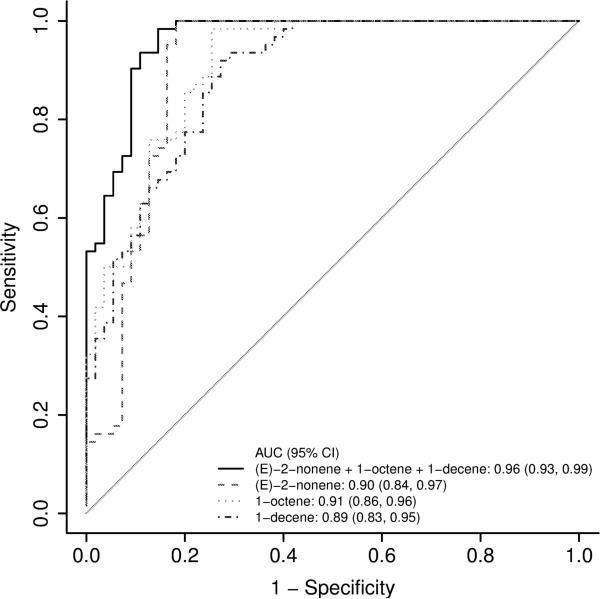
A comparison of the SIFT-MS results of patients with IBD to healthy controls revealed differences in concentration of 9 out of 21 routinely analyzed VOCs (Table 3a). After adjusting for age, gender, race and BMI, multivariable analysis showed 6 out 9 VOCs (1-octene, 1-decene, (E)-2-nonene, 1- nonene, 3-methylhexane and hydrogen sulfide) to be significantly different between the two groups (Table 3b). Logistic regression analysis further revealed three specific VOCs (1-octene, 1-decene, (E)-2-nonene) improved the accuracy of classifying a patient as healthy or as having IBD. Receiver operating characteristic (ROC) curve analysis demonstrated the accuracy for predicting the presence of IBD with an area under the curve (AUC) of 0.91 (95% CI: 0.86, 0.96) for 1-octene, 0.89 (95% CI: 0.83, 0.95) for 1-decene, and 0.90 (95% CI: 0.84, 0.97) for (E)-2- nonene. A model that combines all three compounds had a better accuracy for predicting IBD with an AUC of 0.96 (95% CI: 0.93, 0.99) (Figure 2).
Table 3a.
VOCs in IBD: Unadjusted analysis of routinely analyzed VOCs for SIFT-MS quantification showed significant differences in 9 VOCs including isoprene, 1-decene, 1-heptene, 1-nonene, 1-octene, 3- methylhexane, (E)-2-nonene, ammonia, and hydrogen sulfide. The unadjusted values are presented as median [P25,P75] as the data was skewed.
| Factor (ppb) | Control (N=55) | IBD (N=62) | p-value |
|---|---|---|---|
| 2-propanol | 97.4[75.0,129.5] | 115.6[84.6,138.8] | 0.096 |
| acetaldehyde | 29.7[22.0,39.4] | 27.1[20.1,44.2] | 0.95 |
| acetone | 50.1[37.2,82.6] | 41.7[29.1,69.5] | 0.055 |
| acrylonitrile | 0.59[0.46,0.70] | 0.57[0.44,0.71] | 0.64 |
| benzene | 1.8[1.5,2.5] | 2.1[1.7,2.6] | 0.057 |
| carbon disulfide | 1.6[1.3,2.2] | 1.7[1.2,2.3] | 0.71 |
| dimethyl sulfide | 1.2[1.07,1.5] | 1.2[0.99,1.4] | 0.79 |
| ethanol | 171.2[104.5,259.9] | 167.9[108.1,413.8] | 0.37 |
| isoprene | 5.9[3.9,8.2] | 8.4[4.5,12.1] | 0.037 |
| pentane | 11.4[9.4,15.0] | 12.9[8.8,19.0] | 0.49 |
| 1-decene | 1.9[1.5,3.4] | 5.8[4.0,13.6] | <0.001 |
| 1-heptene | 5.8[4.2,8.3] | 7.9[6.0,10.5] | 0.009 |
| 1-nonene | 6.8[4.8,10.1] | 3.9[3.1,5.9] | <0.001 |
| 1-octene | 4.2[3.0,7.6] | 13.9[10.4,28.0] | <0.001 |
| 3-methylhexane | 13.3[10.2,20.5] | 16.4[12.4,42.5] | 0.004 |
| (E)-2-nonene | 12.7[10.2,18.9] | 1.8[1.4,2.6] | <0.001 |
| ammonia | 52.4[39.4,69.3] | 42.4[31.0,50.7] | 0.004 |
| ethane | 63.4[56.6,69.2] | 62.2[59.2,68.0] | 0.94 |
| hydrogen sulfide | 0.36[0.28,0.45] | 0.28[0.23,0.37] | <0.001 |
| triethyl amine | 0.63[0.49,0.90] | 0.62[0.50,0.78] | 0.57 |
| trimethyl amine | 3.6[2.8,4.7] | 3.3[2.5,4.1] | 0.32 |
Values presented as Median [P25, P75] with Kruskal-Wallis test.
Table 3b.
VOCs in IBD: After adjusting for age, gender, race and BMI, results showed significant differences in 6 of the 9 VOCs including 1-decene, 1-nonene, 1-octene, 3- methylhexane, (E)-2-nonene and hydrogen sulfide. The adjusted data was calculated using a regression model (ANCOVA analysis), but given asymmetrical distribution of values, a log transformation was performed for each VOC and presented as Mean (95% CI).
| Factor (ppb) | Control (N=55) | IBD (N=62) | p-value |
|---|---|---|---|
| 2-propanol | 90.4 (78.9, 103.7) | 105.4 (90.2, 123.2) | 0.19 |
| acetaldehyde | 29.2 (24.6, 34.5) | 32.6 (26.9, 39.5) | 0.44 |
| acetone | 50.0 (38.8, 64.4) | 51.2 (38.3, 68.2) | 0.92 |
| acrylonitrile | 0.58 (0.53, 0.64) | 0.56 (0.51, 0.63) | 0.78 |
| benzene | 1.8 (1.6, 2.1) | 2.1 (1.8, 2.4) | 0.26 |
| carbon disulfide | 1.7 (1.5, 2.0) | 1.6 (1.4, 1.9) | 0.72 |
| dimethyl sulfide | 1.3 (1.1, 1.4) | 1.2 (1.03, 1.4) | 0.6 |
| ethanol | 172.9 (127.8, 233.8) | 253.9 (180.0, 358.1) | 0.14 |
| isoprene | 5.9 (4.8, 7.2) | 7.8 (6.1, 9.8) | 0.11 |
| pentane | 11.6 (10.1, 13.3) | 14.1 (12.0, 16.6) | 0.1 |
| 1-decene | 2.1 (1.7, 2.6) | 8.3 (6.5, 10.7) | <0.001 |
| 1-heptene | 6.4 (5.3, 7.9) | 8.5 (6.7, 10.7) | 0.11 |
| 1-nonene | 8.3 (6.6, 10.4) | 3.8 (2.9, 4.9) | <0.001 |
| 1-octene | 4.4 (3.7, 5.3) | 18.4 (14.9, 22.6) | <0.001 |
| 3-methylhexane | 14.3 (11.6, 17.7) | 23.6 (18.6, 30.0) | 0.006 |
| (E)-2-nonene | 11.7 (9.3, 14.8) | 1.5 (1.2, 2.0) | <0.001 |
| ammonia | 48.4 (42.8, 54.9) | 47.7 (41.3, 54.9) | 0.88 |
| ethane | 63.6 (61.2, 66.1) | 63.1 (60.4, 66.0) | 0.81 |
| hydrogen sulfide | 0.36 (0.33, 0.40) | 0.28 (0.25, 0.32) | 0.003 |
| triethyl amine | 0.66 (0.58, 0.76) | 0.63 (0.54, 0.73) | 0.66 |
| trimethyl amine | 3.4 (2.9, 4.0) | 4.1 (3.4, 4.9) | 0.19 |
Values presented as Mean (95% CL) and were obtained using ANCOVA analysis. The logarithm of each VOC was modeled as the outcome variable with age, gender, Caucasian race and BMI percentile as the independent variables.
Figure 2.
Receiver operating characteristic (ROC) curve analysis demonstrated the accuracy for predicting the presence of IBD with an area under the curve (AUC) of 0.91 (95% CI: 0.86, 0.96) for 1-octene, 0.89 (95% CI: 0.83, 0.95) for 1-decene, and 0.90 (95% CI: 0.84, 0.97) for (E)-2-nonene. A model that combines all three compounds (1-octene, 1-decene, (E)-2-nonene) had a better accuracy for predicting IBD with an AUC of 0.96 (95% CI: 0.93, 0.99).
Additional sensitivity analyses were performed and similar results were obtained for distinguishing patients with CD from healthy controls with an AUC of 0.96 (95% CI: 0.93, 0.99) and patients with UC from healthy controls with an AUC of 0.97 (95% CI: 0.94, 1.0). This suggests that the model is not sensitive to type of IBD and can only be used to differentiate children with IBD from healthy subjects.
In addition, as previously mentioned, the IBD cohort in our study was significantly older and comprised of a higher percentage of Caucasians. While multivariable analysis should have corrected for these differences, further subgroup analysis of the control group was performed to determine if the three VOCs (1-octene, 1-decene, (E)-2-nonene) varied according to age or race. No correlation was identified between patient age and any of the VOCs in the control group. However, subgroup analysis within the controls showed (E)-2-nonene to be significantly higher in Caucasians compared to blacks (median 14.9 ppb [12.3,20.0] vs. 11.7 [2.5,15.9], p-value 0.045). This is of particular interest given patients in the IBD group, who were primarily Caucasians, were found to have significantly lower levels of (E)-2-nonene than healthy controls (median 1.8 ppb [1.4,2.6] vs. 12.7 [10.2,18.9], p-value <0.001) (Table 3a). This inverse relationship we identified between the level of (E)-2-nonene and the presence of IBD may warrant further investigation. No difference was seen in 1-decene or 1-octene with regards to race.
Volatile organic compounds in the breath of children with IBD
Subgroup analysis of CD vs. UC showed there was no evidence to suggest significant difference in VOCs between CD and UC patients. Of interest, patients with known CD had significantly higher levels of pentane if they had ileocolonic disease versus colonic disease alone (median 14.2 ppb [9.9,23.0] vs. 8.7 [7.1,10.1], p-value 0.022). There was no correlation between VOCs and IBD activity as assessed by the PCDAI and PUCAI, albumin, ESR, CRP, hematocrit, platelets, and white blood cell count. Current medication use was also correlated with VOC concentrations to assess for any interactions or confounders. Forty-four of 62 patients with IBD patients were taking a 5-ASA and found to have significantly lower concentrations of 1-octene (median 12.3 ppb [9.5,26.0] vs. 17.3 [12.9,37.3], p-value 0.027) and (E)-2-nonene (median 1.6 ppb [1.3,2.3] vs. 2.2 [1.8,2.9], p-value 0.014) compared to the 18 patients who were not taking 5-ASA. No significant difference in VOC concentration was seen with being on immunomodulator therapy or biologics.
DISCUSSION
To our knowledge, this represents the first study to analyze human breath utilizing SIFT-MS in a pediatric IBD population. The principal finding of this study was that children with IBD have a unique breathprint that can separate them from healthy controls. Breath testing via SIFT-MS can potentially identify pediatric patients most likely to need endoscopy for suspected IBD and potentially reduce the number of invasive and costly procedures.
There have only been a few studies published utilizing metabolomic analysis in adult IBD patients to date. These studies utilized different technology to analyze VOCs in the headspace (the air above the sample) of urine and feces. Arasaradnam et al. recently reviewed the existing technologies (electronic nose, ion mobility spectroscopy and GC-MS) for detection of airborne gas phase biomarkers that emanate from biological samples like urine, breath and faeces which may herald a new age of non-invasive diagnostics in the field of gastroenterology. The authors also concluded that portable near-patient devices that can accurately detect disease specific VOCs is the non-invasive diagnostic goal (19).
Arasaradnam et al. previously demonstrated the usefulness of measuring VOCs in the headspace of urine using the electronic nose technology to profile adult patients with IBD (20). Similar to a human nose, the electronic nose can recognize a smell print associated with a certain individual or disease by using an array of gas sensors. However, a major limitation for the electronic nose and pattern recognition for use in clinical breath analysis is the inability to identify the specific volatile compounds. In contrast, SIFT-MS utilizes three precursor ions (H3O+, NO+ or O2+ which react with the breath sample to produce ionized products characteristic of the volatile chemicals. It has the ability to both recognize volatile compounds in the exhaled breath and quantify to the parts-per-trillion levels for several VOCs (21). In addition, SIFT-MS has unique analytical capabilities, such as real-time accurate analysis of single exhalations, even when there is an abundance of atmospheric gases (22).
A review of a study recently published by Walton et al. (23) demonstrated that a number of VOCs in the headspace of feces differ markedly between patients with Crohn's disease and other gastrointestinal conditions including UC and irritable bowel syndrome. In this study, 87 patients (19 healthy volunteers, 22 with CD, 20 with UC, and 26 individuals with IBS) were recruited. Fecal samples in patients with GI disorders were collected before and after treatment. The authors utilized gas chromatography-mass spectrometry to determine the concentrations of a selection of high abundance compounds. They found that patients with CD had significant elevations in the concentrations of ester and alcohol derivates of short-chain fatty acids and indole compared to patients in the other groups. After therapy, the levels of many of the VOCs significantly decreased and were similar to healthy controls. The authors concluded that intestinal dysbiosis in IBD may contribute to different fecal metabolite profiles. They also concluded that the normalization of the fecal VOC profile following therapy suggests re-establishment of relatively normal microbiotia.
Additionally, several adult studies in the past have demonstrated an association of elevated breath pentane levels and IBD. Dryahina et al. utilized SIFT-MS to quantify pentane levels in the exhaled breath of adult patients with CD, UC and healthy volunteers. The authors found pentane to be significantly elevated in the breath of both the CD (mean 114 ppb) and the UC patients (mean 84 ppb) relative to the healthy controls (mean 40 ppb). They concluded SIFT-MS can quantify pentane levels from human breath in real time and this technology can form the basis of non-invasive screening of inflammatory processes, including IBD (24). Kokoszka et al. utilized gas chromatography to assess the ability of exhaled breath pentane to determine the presence of active inflammation in 33 adults with known or suspected IBD. They used an indium-111-labeled leukocyte imaging to assess the presence of active inflammation. In the patients with a positive scan for active inflammation, the mean exhaled breath pentane level was significantly higher (4.3 nmol/l) when compared to those with intermediate scans (3.1 nmol/l) and those with negative scans (2.1 nmol/l) (25). Interestingly, our subgroup analysis showed patients with known ileocolonic CD had significantly higher levels of pentane (median 14.2 ppb [9.9,23.0]) when compared to those with isolated colonic disease alone (median 8.7 ppb [7.1,10.1]); p-value 0.022. Although pentane was not significant for distinguishing patients with IBD from healthy children, it was significant for distinguishing disease location in patients with known CD.
These studies demonstrate the usefulness of metabolomic analysis in elucidating components of disease pathophysiology and pathways leading to the development of IBD. We can extrapolate from these studies and suggest that gut microbiotia dysbiosis may explain some of the differences seen in the breath profile in children with IBD. Gevers et al. recently published the largest study to date detailing the patterns of gut microbiome dysbiosis in treatment-naïve pediatric Crohn's disease patients. The authors found an increased abundance in bacteria which include Enterobacteriaceae, Pasteurellacaea, Veillonellaceae, and Fusobacteriaceae, and decreased abundance in Erysipelotrichales, Bacteroidales, and Clostridiales, which correlated with degree of intestinal inflammation (26). These authors and Morita et al (27) found that the species increased in CD uniquely contributed pathway components of glycerophospholipid and lipopolysaccharide metabolism, which instigate inflammation. Additionally, the adherent and invasive properties likely alter host innate immunity, drive proinflammatory cytokines, and alter the intestinal microbiota in favor of dysbiosis, which ultimately leads to chronic inflammation and development of IBD. It is recognized that bacteria produce VOCs as metabolic by-products. The exhaled breath of humans is comprised in part of these VOCs produced by gut bacteria. Differences in gut microbiotia may result in differences in VOC patterns and may also be suggestive of different disease processes.
Our breath analysis revealed a unique pattern composed of three VOCs (1-octene, 1-decene, (E)-2-nonene) can distinguish healthy children from patients with IBD. However, we were not able to distinguish between active and inactive IBD using the SIFT-MS technology. The subgroup analysis was likely underpowered to distinguish active disease from remission given only 20% of patients had active disease at the time of the study. Also, subgroup analysis showed no significant differences in VOCs between patients with CD and UC. However, this subgroup analysis was also likely underpowered given only 11 patients had UC, while 51 had CD. Also, as no patients were treatment naïve, this may have also limited the ability of SIFT-MS to distinguish between the two forms of IBD.
There were several limitations to our study as noted earlier. Most importantly, we were not able to control factors that may influence VOC concentrations such as environmental exposures, diet, and fasting prior to the exhaled breath collection. In addition, similar to the studies by Arasaradnam et al and Walton et al, patients in our study were not treatment naïve. Concurrent medication use may have altered breath VOC concentrations. A study of newly diagnosed pediatric IBD patients and those with functional gastrointestinal disorders may provide additional clinical insight. Lastly, this study was cross-sectional and the VOCs were determined at a single-time point.
In conclusion, our study shows exhaled breath analysis is a promising noninvasive method to distinguish children with IBD from healthy children. This form of breath testing is feasible in pediatric patients as young as 7 years of age. We provide pilot data to support the hypothesis that a unique breathprint can be demonstrated for IBD in the exhaled metabolome. The results of this study are preliminary and need to be confirmed before they can be applied in clinical practice. The differences in the VOC pattern may one day help provide biologic insight into the mechanisms of IBD. Our pilot results indicate further studies should be pursued. The potential of this new application of SIFT-MS technology lies in possible point of care screening or outpatient ambulatory monitoring of IBD patients with a hand held device, as mass spectroscopy is not widely available. Once further validation studies confirm that this unique pattern of VOCs is highly sensitive and specific, solid-state sensors can be integrated into portable hand held, cost effective, point of care devices. The ultimate goal is to minimize unnecessary, expensive, and invasive testing of pediatric patients.
ACKNOWLEDGEMENTS
Declaration of funding interests: The funding for this research was supported by the BRCP 08-049 Third Frontier Program grant from the Ohio Department of Development. Dr. Raed Dweik was also supported by the following grants: HL107147, HL081064, HL103453, HL109250, 1U01AA021890 and RR026231 from the National Institutes of Health (NIH).
Footnotes
Conflict of Interest: No conflict of interest exists for any of the authors.
This study was presented as a poster presentation at Digestive Disease Week. May 18-21, 2013.
Author contributions: Nisha Patel: Study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
Naim Alkhouri: Study design, acquisition and analysis of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content.
Katharine Eng: Study design, acquisition and analysis of data.
Frank Cikach: Study design, acquisition, analysis and interpretation of data.
Lori Mahajan: Study design, analysis of data and critical revision of the manuscript for important intellectual content.
Chen Yan: Study design, acquisition and analysis of data.
David Grove: Acquisition of data; analysis and interpretation of data.
Ellen S. Rome: Study design, acquisition of data.
Rocio Lopez: Statistical analysis and interpretation of data
Raed A. Dweik: Study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content; obtained funding.
References
- 1.Loftus EV., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004 May;126(6):1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006 Mar;55(3):426–431. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008 Jan;103(1):162–169. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 4.van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010 Jul 15;341:c3369. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips M, Herrera J, Krishnan S, Zain M, Greenberg J, Cataneo RN. Variation in volatile organic compounds in the breath of normal humans. J Chromatogr B Biomed Sci Appl. 1999 Jun 11;729(1-2):75–88. doi: 10.1016/s0378-4347(99)00127-9. [DOI] [PubMed] [Google Scholar]
- 6.Hunter GW, Dweik RA. Applied breath analysis: an overview of the challenges and opportunities in developing and testing sensor technology for human health monitoring in aerospace and clinical applications. J Breath Res. 2008 Sep;2(3) doi: 10.1088/1752-7155/2/3/037020. 037020-7155/2/3/037020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mashir A, Dweik RA. Exhaled breath analysis: The new interface between medicine and engineering. Adv Powder Technol. 2009 Sep;20(5):420–425. doi: 10.1016/j.apt.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Braun P, Gmachl C, Dweik R. Bridging the Collaborative Gap: Realizing the Clinical Potential of Breath Analysis for Disease Diagnosis and Monitoring-Tutorial. Sensors Journal, IEEE. 2012;11:3258–3270. [Google Scholar]
- 9.Paschke KM, Mashir A, Dweik RA. Clinical applications of breath testing. F1000 Med Rep. 2010 Jul 22;2:56–56. doi: 10.3410/M2-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dweik RA. The great challenge for exhaled breath analysis: embracing complexity, delivering simplicity. J Breath Res. 2011 Sep;5(3) doi: 10.1088/1752-7155/5/3/030201. 030201-7155/5/3/030201. Epub 2011 Sep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyams JS, Ferry GD, Mandel FS, Gryboski JD, Kibort PM, Kirschner BS, et al. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr. 1991 May;12(4):439–447. [PubMed] [Google Scholar]
- 12.Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007 Aug;133(2):423–432. doi: 10.1053/j.gastro.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 13.Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011 Jun;17(6):1314–1321. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 14.Samara MA, Tang WH, Cikach F, Jr, Gul Z, Tranchito L, Paschke KM, et al. Single exhaled breath metabolomic analysis identifies unique breathprint in patients with acute decompensated heart failure. J Am Coll Cardiol. 2013 Apr 2;61(13):1463–1464. doi: 10.1016/j.jacc.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S, Huang J, Abbassi-Ghadi N, Spanel P, Smith D, Hanna GB. Selected ion flow tube mass spectrometry analysis of exhaled breath for volatile organic compound profiling of esophago-gastric cancer. Anal Chem. 2013 Jun 18;85(12):6121–6128. doi: 10.1021/ac4010309. [DOI] [PubMed] [Google Scholar]
- 16.Prince BJ, Milligan DB, McEwan MJ. Application of selected ion flow tube mass spectrometry to real-time atmospheric monitoring. Rapid Commun Mass Spectrom. 2010 Jun 30;24(12):1763–1769. doi: 10.1002/rcm.4574. [DOI] [PubMed] [Google Scholar]
- 17.Smith D, Spanel P. Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom Rev. 2005 Sep-Oct;24(5):661–700. doi: 10.1002/mas.20033. [DOI] [PubMed] [Google Scholar]
- 18.Risby TH, Sehnert SS. Clinical application of breath biomarkers of oxidative stress status. Free Radic Biol Med. 1999 Dec;27(11-12):1182–1192. doi: 10.1016/s0891-5849(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 19.Arasaradnam RP, Covington JA, Harmston C, Nwokolo CU. Review article: next generation diagnostic modalities in gastroenterology - gas phase volatile compound biomarker detection. Aliment Pharmacol Ther. 2014 Apr;39(8):780–789. doi: 10.1111/apt.12657. [DOI] [PubMed] [Google Scholar]
- 20.Arasaradnam RP, Ouaret N, Thomas MG, Quraishi N, Heatherington E, Nwokolo CU, et al. A novel tool for noninvasive diagnosis and tracking of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013 Apr;19(5):999–1003. doi: 10.1097/MIB.0b013e3182802b26. [DOI] [PubMed] [Google Scholar]
- 21.Ross BM. Sub-parts per billion detection of trace volatile chemicals in human breath using selected ion flow tube mass spectrometry. BMC Res Notes. 2008 Jul 10;1:41–0500-1-41. doi: 10.1186/1756-0500-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith D, Spanel P, Herbig J, Beauchamp J. Mass spectrometry for real-time quantitative breath analysis. J Breath Res. 2014 Mar 28;8(2):027101. doi: 10.1088/1752-7155/8/2/027101. [DOI] [PubMed] [Google Scholar]
- 23.Walton C, Fowler DP, Turner C, Jia W, Whitehead RN, Griffiths L, et al. Analysis of volatile organic compounds of bacterial origin in chronic gastrointestinal diseases. Inflamm Bowel Dis. 2013 Sep;19(10):2069–2078. doi: 10.1097/MIB.0b013e31829a91f6. [DOI] [PubMed] [Google Scholar]
- 24.Dryahina K, Spanel P, Pospisilova V, Sovova K, Hrdlicka L, Machkova N, et al. Quantification of pentane in exhaled breath, a potential biomarker of bowel disease, using selected ion flow tube mass spectrometry. Rapid Commun Mass Spectrom. 2013 Sep 15;27(17):1983–1992. doi: 10.1002/rcm.6660. [DOI] [PubMed] [Google Scholar]
- 25.Kokoszka J, Nelson RL, Swedler WI, Skosey J, Abcarian H. Determination of inflammatory bowel disease activity by breath pentane analysis. Dis Colon Rectum. 1993 Jun;36(6):597–601. doi: 10.1007/BF02049868. [DOI] [PubMed] [Google Scholar]
- 26.Gevers D, Kugathasan S, Denson LA, Vazquez-Baeza Y, Van Treuren W, Ren B, et al. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe. 2014 Mar 12;15(3):382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita H, Nakanishi K, Dohi T, Yasugi E, Oshima M. Phospholipid turnover in the inflamed intestinal mucosa: arachidonic acid-rich phosphatidyl/plasmenyl-ethanolamine in the mucosa in inflammatory bowel disease. J Gastroenterol. 1999 Feb;34(1):46–53. doi: 10.1007/s005350050215. [DOI] [PubMed] [Google Scholar]