Abstract
Although tyrosine kinase inhibitors (TKI) are the most common first-line therapy for metastatic renal cell carcinoma, high-dose interleukin-2 (HD-IL2) remains the only agent that provides durable complete responses. The optimal sequence of these agents remains uncertain. This retrospective multi-institutional study examined the safety and efficacy of HD-IL2 following TKI therapy. After IRB approval at 7 HD-IL2 centers, data relating to patient, disease, and treatment characteristics among 40 consecutive patients with metastatic renal cell carcinoma who were treated with HD-IL2 after at least 1 prior TKI therapy were retrospectively collected. The most common cardiac adverse events were grade 3 hypotension and vascular leak syndrome. Six patients (15%) experienced other grade ≥3 cardiac adverse events. There were 2 treatment-related deaths due to congestive heart failure, occurring in 1 patient with short TKI to HD-IL2 interval and another patient with an abnormal baseline cardiac stress test. Best responses included 2 CRs (5%, duration 40+ and 62+ mo), 3 PRs (8%, duration 6, 11, and 24 mo), 13 SD (32%, median duration 12 mo), 20 PD (50%), and 2 not evaluable patients. Median overall survival was 22 months. Administration of HD-IL2 could be safe and effective after TKI therapy; however, careful selection of patients is critical. We recommend baseline cardiac risk factor assessment, screening with both cardiac stress test and echocardiogram, and allowing a TKI to HD-IL2 interval of at least 2 months.
Key Words: high-dose interleukin-2, tyrosine kinase inhibitor (TKI), renal cell carcinoma, sunitinib, sorafenib
Renal cell carcinoma (RCC) accounted for nearly 64,000 new cases and over 13,000 deaths in the United States in 2014.1 One third of patients present with primary metastatic disease and 20%–40% of patients have recurrent metastatic disease after primary nephrectomy with curative intent.2 Therefore, approximately 50% of all patients diagnosed with RCC will require systemic therapy during the course of their disease. Interleukin-2 [(IL2) Proleukin; Prometheus Laboratories Inc., San Diego, CA)] was approved in 1992 for the treatment of relapsed mRCC based on an objective response rate (ORR) of 14% and, more importantly, a durable complete response (CR) rate of 5%.3,4 However, high-dose IL2 (HD-IL2) is associated with moderate to severe acute toxicity and requires intensive inpatient supportive management, limiting its use to major centers. Although most IL2-related toxicities reverse rapidly after therapy is completed, the cardiovascular treatment-related mortality has been reported to be as high as 4% in the early phase II studies of HD-IL2.3 In more recent series, the HD-IL2 treatment-related mortality rate was around 1%–2% in patients who were carefully screened and did not have coronary artery disease (CAD), and the CR, PR, and ORR rates were 5%–9%, 12%–23%, and 21%–28%, respectively.5,6 In 1 retrospective analysis of 259 patients treated with HD-IL2 at the National Cancer Institute (NCI), in which patients above 50 years old underwent cardiac stress testing and those with ischemic heart disease, significant arrhythmias, or significant comorbidities were specifically excluded, only 2 treatment-related deaths were observed.5 In the Cytokine Working Group (CWG) Select trial, there were 2 deaths in approximately 120 HD-IL2-treated patients in which CAD was an exclusion.6
Multitargeted tyrosine kinase inhibitors (TKIs) such as sunitinib, sorafenib, pazopanib, and axitinib have demonstrated improved ORR and/or survival compared with interferon therapy and/or supportive care.7–12 Because of oral administration, higher response rates, and more favorable toxicity profiles, TKIs have largely replaced cytokine therapies in the first-line treatment of mRCC. Nevertheless, HD-IL2 remains the only available treatment option that produces durable CRs.
The safety and efficacy of TKIs after prior cytokine therapy have been previously demonstrated in large prospective trials of these agents in the second-line setting.7–10,13,14 However, whether HD-IL2 can be given safely and effectively after prior TKI therapy has not been investigated prospectively. In a retrospective analysis of 23 patients who received salvage HD-IL2 therapy after prior VEGF-targeted therapy, there were no responses, and the incidence of severe cardiovascular toxicity was high, including 1 sudden cardiac death during HD-IL2 therapy.15 The authors suggested that better patient selection with rigorous cardiovascular screening and increasing the time interval between TKI and HD-IL2 therapy may allow safer administration of HD-IL2.15
Reports of toxicity and futility for HD-IL2 therapy post-TKIs potentially affect the delivery of beneficial, possibly curative, therapy to mRCC patients. Thus, this collaborative effort was mounted to evaluate the safety and efficacy of HD-IL2 therapy in an expanded cohort of such patients, and to explore the potential patient-related, disease-related, and drug-related factors that may predict safety and efficacy.
The therapeutic sequence of a TKI followed by HD-IL2 may occur in a number of settings. A patient may have a recent diagnosis of mRCC and be anxious to initiate any therapy. Once on medical therapy, the disease may stabilize. Then, as the patient learns more about their disease they may realize the potential for durable response with HD-IL2 therapy. Alternatively, the patient at diagnosis may not want to accept the potential toxicity associated with HD-IL2, but later discover the treatment is more tolerable than anticipated. Finally the patient may have rapidly progressive disease that is stabilized by a TKI and later becomes a good candidate for HD-IL2.
PATIENTS AND METHODS
IRB approval or exemption was obtained at all of the participating institutions. We retrospectively collected information regarding patient-related, disease-related, and treatment-related variables among patients with mRCC who were treated with HD-IL2 [600,000 IU/kg intravenously every 8 h for up to 14 doses (in 36 patients) or 720,000 IU/kg every 8 h for up to 12 doses per treatment week (in 4 patients)] between January 2005 and December 2010 after at least 1 prior TKI therapy (sunitinib, sorafenib, or both). Patient-related variables included age, sex, performance status, cardiac risk factors, personal history of CAD, and baseline cardiac evaluation if available. Cardiac risk factors were chosen based on the Framingham/Adult Treatment Panel III and the American Heart Association guidelines on assessment and management of cardiovascular risk and included age above 55 for men or above 65 for women, current or former tobacco use, diabetes, hypertension, dyslipidemia, and family history of CAD.16–20 For the majority of patients, primary lipid data were not available and dyslipidemia status was inferred based on statin use. Disease-related variables included histology, number and sites of metastatic disease, Memorial Sloan Kettering Cancer Center (MSKCC) prognostic stratification,21,22 and prior treatments. Primary data were too sparse to stratify by the Heng criteria.23 HD-IL2 treatment-related variables collected included time from initial diagnosis to start of HD-IL2 therapy, interval from discontinuation of TKI to start of HD-IL2, duration and amount of HD-IL2 therapy received, and grade 3–5 cardiac and noncardiac adverse events (AEs). Outcome variables included best response (RECIST v1.0),24 duration of response, time from end of HD-IL2 therapy to start of subsequent therapy, progression-free survival (PFS), and overall survival (OS). For OS and PFS, we evaluated the time from start of HD-IL2 to time of death or data cutoff in December 2010.
In this retrospective analysis, we used descriptive statistics including estimates of proportions, as well as, means, medians, and interquartile range, and range. We estimated the proportion of patients with significant cardiac toxicity and the proportion with any or ≥2 cardiac risk factors. Relationships between 2 factors were explored using the Fisher exact test. Quantitative outcomes such as the number of cardiac risk factors were explored, especially in relation to incidence of severe cardiac toxicity, and analyzed using the Wilcoxon rank-sum tests. However, given the sample size limitations, any formal comparisons were truly exploratory and hypothesis generating. A Kaplan-Meier survival curve was used to display the OS function. Cox proportional hazards regression models were used to estimate the hazard ratios of potential prognostic factors.
RESULTS
Forty patients treated consecutively at 7 HD-IL2 centers [Roswell Park Cancer Institute (9 patients), University of Colorado (8 patients), University of Utah Huntsman Cancer Institute (7 patients), University of Michigan (6 patients), Feinberg School of Medicine of Northwestern University (6 patients), Indiana University Melvin and Bren Simon Cancer Center (3 patients), and The Ohio State University Wexner Medical Center (1 patient)] met criteria for analysis.
Baseline patient and disease characteristics are described in Table 1. A total of 28 men and 12 women were treated. The median age was 53.5 years (range, 40–72 y). Thirty-nine patients (98%) had prior nephrectomy. All but 1 patient had clear cell histology (subclassification data not available). Twenty-two patients had primary metastatic disease, and 18 patients had recurrent metastatic disease. Thirty-one patients had multiple sites of metastases, most commonly involving lymph node, lung, bone, and liver. Three, 25, and 2 patients had MSKCC good-risk, intermediate-risk, and poor-risk disease, respectively. Data were not available to categorize 10 patients using this model. Best response to prior TKI therapy was stable disease (SD) in 50% of patients and partial response (PR) in 20% of patients. The median interval between TKI and HD-IL2 therapies was 6 weeks (range, 10 d–52 wk).
TABLE 1.
Patient Characteristics
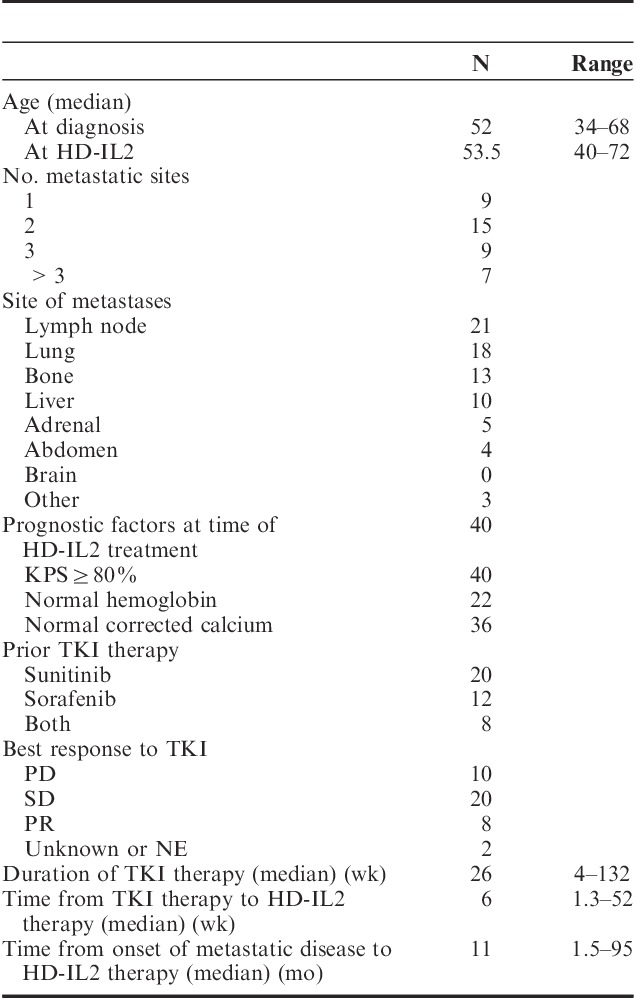
Baseline cardiac risk factors and baseline cardiac evaluations are listed in Table 2. Two patients had personal history of CAD. Thirty-four patients (85%) had at least 1 cardiac risk factor. Twenty-seven patients (68%) had ≤2 cardiac risk factors and 13 patients had ≥3 risk factors. Thirty-three patients (83%) underwent baseline cardiac evaluation with ≥1 modalities (7 with transthoracic echocardiogram or MUGA alone, 26 with nuclear medicine stress test or stress echocardiogram), including all 13 patients with ≥3 cardiac risk factors. Median ejection fraction (EF) was 65% (range, 50%–79%). Left ventricular (LV) hypertrophy and valvular abnormalities were seen in 2 patients each. Wall motion abnormality and ischemic changes were seen in 1 patient.
TABLE 2.
Cardiac Risk Factors
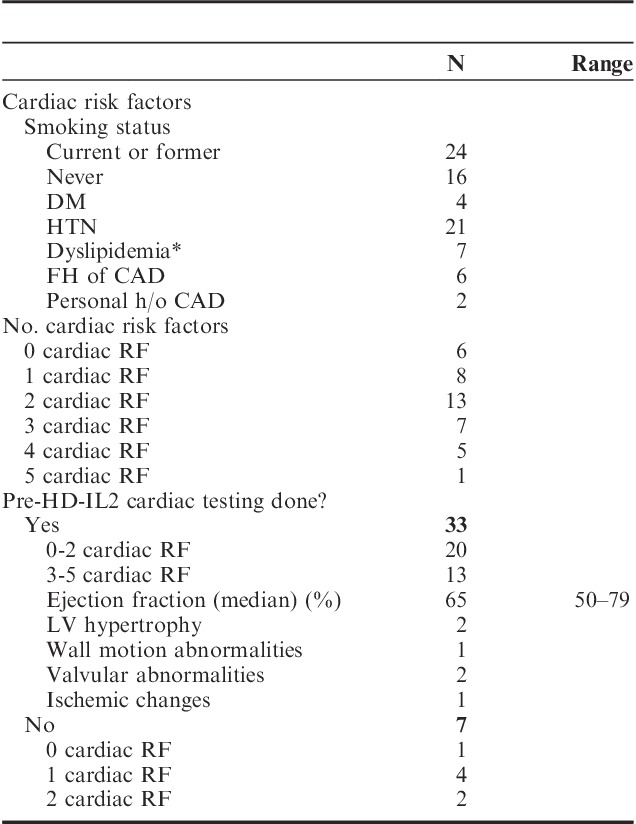
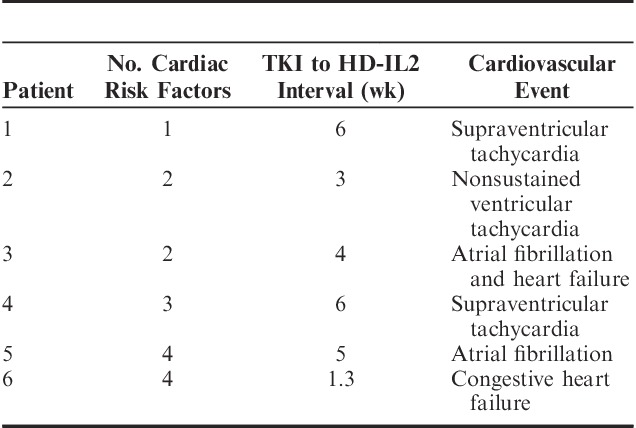
The most common cardiac AEs while on HD-IL2 therapy were grade 3 hypotension and vascular leak syndrome. Six patients (15%) experienced other grade ≥3 cardiac AEs, as noted in Table 3. Grade 1 creatine kinase or troponin elevations were reported in 3 patients. Two patients had reversible noncardiac respiratory failure. Two patients died within 1 month of receiving HD-IL2 therapy. The first patient (age 58 y, 2 cardiac risk factors, baseline EF 55%–70%, no wall motion abnormalities, and a 4 wk TKI to HD-IL2 interval) died 1 month after week 1 of cycle 2 of HD-IL2 due to new-onset atrial fibrillation and congestive heart failure (CHF). The second patient (age 72 y, positive history of CAD, EF 50%–65%, apical hypokinesis and small area of reversible ischemia in distribution of a stented mid-left anterior descending coronary artery on pre-HD-IL2 nuclear stress test, and a 10 d TKI to HD-IL2 interval) died 1 week after week 1 cycle 1 of HD-IL2 with severe CHF. Although the numbers are small, in our analysis, the median time from TKI to HD-IL2 was 8 weeks in patients who did not experience grade 3–5 AEs, and 4.5 weeks in patients who experienced grade 3–5 AEs (P=0.1 by Wilcoxon rank-sum test). The association between number of doses received and cardiac toxicity did not reach statistical significance, with P=0.064 for all-grade cardiac toxicity, and P=0.076 for grades ≥3 cardiac toxicity.
TABLE 3.
Patients With Severe (Grade 3–5) Cardiovascular Adverse Events
Twenty-seven, 12, and 1 patient(s) received 1, 2, and 3 courses of HD-IL2, respectively. The median number of doses of HD-IL2 received was 17 (range, 2–49). The clinical benefit rate was 45% [CR in 2 patients (5%), PR in 3 patients (8%), and SD in 13 patients (32%)]. Twenty patients had disease progression (PD) and 2 patients were not evaluable. The median duration of SD was 12 months (range, 1.5–41 mo). The duration of response was 6, 11, and 24 months for the 3 patients with PR. The 2 patients (5%) who achieved CR had 40+ and 62+ months’ duration of response. There was a trend noted between dose intensity and response (Table 4). The median OS from time of starting HD-IL2 therapy was 22 months (95% CI, 9, 36), as shown in Figure 1.
TABLE 4.
Dose Intensity and Response

FIGURE 1.
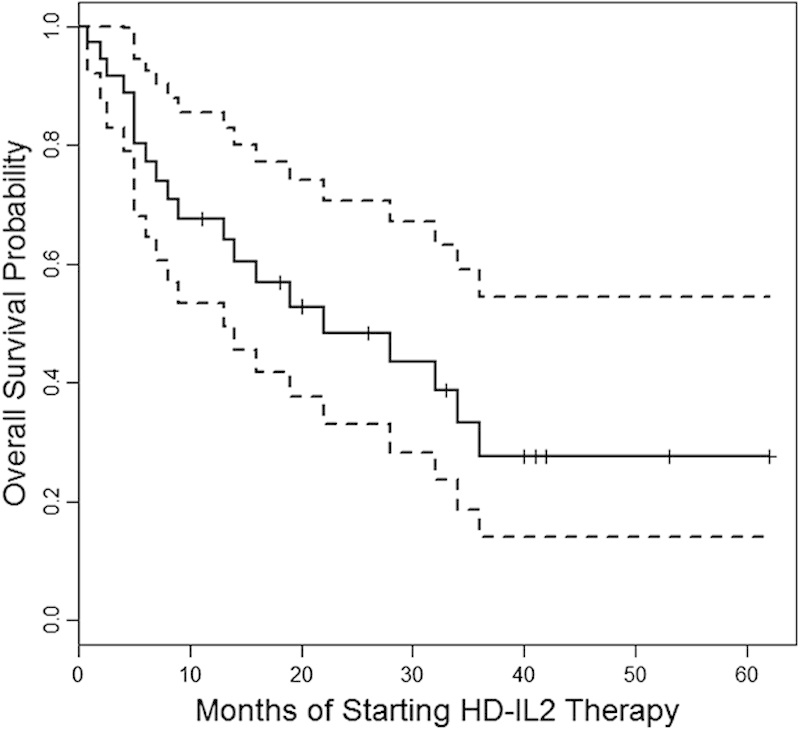
Median overall survival (mo) from time of starting HD-IL2 therapy. Dotted lines represent 95% confidence limits.
There was a skewed distribution of the MSKCC prognostic category among the patients, with only 2 patients each having good and poor prognoses, and the majority of patients (25 patients, 86%) having intermediate prognosis. Thus, the association of MSKCC prognostic category on survival, either from the time of HD-IL2 or from time of metastatic disease, cannot be adequately estimated.
DISCUSSION
To the best of our knowledge, this study is the largest series of patients treated with HD-IL2 after prior TKI therapy. Most importantly, we found that prior TKI did not preclude these patients from benefitting from HD-IL2 therapy. Two patients (5%) achieved CR with continuing response at 40+ and 62+ months at the time of data cutoff. The clinical benefit rate was 45%, with a median duration of SD or better of 18 months (range, 1.5–>62). Although patient numbers were small and it is difficult to compare findings of small studies years apart, our observed median OS was 22 months, compared with 15.8 months in the original studies of HD-IL2.25 There were no sudden cardiac deaths due to arrhythmias. There were 2 deaths due to CHF thought to be treatment related. In comparison, the previously discussed Cho et al15 report noted severe cardiac events in 6/15 patients (40%), no responses, and 3 patients (13%) with SD with median duration of 9 months. The differences in toxicity outcomes may reflect a more stringent patient selection process that has already been implemented at the major HD-IL2 institutions in this study. Our severe cardiac toxicity rate of 15% is higher than the previously reported rate of 8.5% in mRCC patients receiving HD-IL2 therapy.26 In addition, the treatment-related mortality (5%) in our series is higher compared with the contemporary trials of HD-IL2 therapy.5,6 However, 1 death occurred in a patient with known CAD and findings of reversible ischemia on pre-HD-IL2 cardiac testing. This patient may well not have been eligible for treatment in the CWG or NCI trials. This highlights the importance of excluding CAD with cardiac testing before initiating HD-IL2.
This retrospective study design has several limitations. First, we did not examine outcomes for any patients who received HD-IL2 before TKI for comparison. Second, the reliance on chart review may underestimate cardiac-related AEs if they were not noted formally as in prospective therapeutic trials. However, we believe that this would mostly affect reporting of lower grade events, and that all clinically significant, especially higher grade (grades 3–5) cardiac events, would have been documented in the clinical chart and would have been captured in our data collection.
In our study, the ORR of HD-IL2 after prior TKI was 13%, which is similar to historical ORR of 14% in the pre-TKI era3; therefore, prior TKI therapy did not seem to compromise response from HD-IL2. Nevertheless, the response rate was less than that reported in several contemporary trials of HD-IL2 given before TKI. In the Select study, the ORR was 28%, mostly due to a PR rate of 23%.6 In another study evaluating first-line HD-IL2 therapy of mRCC patients, Shablak et al27 found an ORR of 49%, including CR of 25%.
Other small studies in the post-TKI setting have now been reported. In an abstract of 16 mRCC patients receiving HD-IL2 after prior TKI (8 after TKI failure and 8 for consolidation after TKI), Hawkins et al28 reported 9 objective responses (56%), including 6 CRs (38%), with toxicity profile indistinguishable from those without prior TKI treatment.
To the best of our knowledge, our study is the first series to examine potential associations between number of cardiac risk factors and incidence of severe cardiac toxicities. Multiple traditional cardiovascular risk factors are used for risk assessment of CAD.16–19,29,30 However, whether these same factors can be used to predict the risk of cardiovascular toxicity from HD-IL2 has not been evaluated. In this study, we found that patients with ≥3 cardiac risk factors seemed to have higher incidence of grade 3–5 cardiac AEs, compared with patients with ≤2 risk factors, although this was not statistically significant due to the low incidence of severe cardiac toxicity overall. On the basis of our findings, and the assumption that the low overall incidence of cardiac toxicity is the result of a careful patient selection process already in place at major HD-IL2 institutions, we recommend risk-adapted cardiac evaluation before HD-IL2 therapy, similar to standard preoperative cardiac risk evaluation.19 For all patients with any known cardiac risk factors, we would recommend cardiac stress testing to rule out ischemic heart disease and echocardiogram to rule out cardiomyopathy before initiating HD-IL2.
The optimal interval between TKI and HD-IL2 therapy requires further investigation. The half-life of sunitinib is 2.5 days. With repeated daily administration, sunitinib accumulates 3- to 4-fold, whereas the primary active metabolite accumulates 7- to 10-fold, with a metabolite half-life of 96 hours [Sunitinib (Sutent) prescribing information, Pfizer Labs, 2010]. In this study, the 2 patients with treatment-related deaths had TKI to HD-IL2 intervals of 10 days and 4 weeks. All observed grade ≥3 cardiac toxicities occurred in patients whose TKI to HD-IL2 interval was ≤6 weeks, although there was no statistical correlation due to the small number of patients and cardiac events overall.
The cardiotoxicity, in particular CHF, associated with sunitinib and sorafenib, has been previously reported.31–34 Sunitinib-induced pericyte depletion and coronary microvascular dysfunction has also been reported.35 In the majority of patients who develop LV dysfunction, symptoms improve after dose interruption, dose modification, and/or initiation of heart failure therapy. The reported time to improvement of LV EF was between 1 and 9.6 weeks.31 Although CHF is not a common toxicity reported for HD-IL2, myocardial dysfunction as a result of myocarditis is a known event.36 Some institutions incorporate cardiac enzyme testing as a standard during and around HD-IL2 therapy.
Cho et al15 suggested that patients should be off TKIs at least 1–2 months before initiation of HD-IL2. On the basis of the pharmacologic half-lives of sunitinib and its active metabolites, complete clearance of the drug may require at least 3–4 weeks off therapy. However, the time to complete resolution of other clinical drug effects is not known. On the basis of these considerations, we would recommend an interval of at least 2 months between completion of a TKI and initiation of HD-IL2.
Sorafenib and sunitinib were the dominant TKIs in use during the time period encompassed by this study. With the approval of pazopanib and axitinib, we now have TKIs that are associated with a lower incidence of cardiomyopathy overall. Their effect on subsequent salvage IL2 therapy is unknown and requires further investigation. In the meantime, it would be prudent to exercise the same degree of caution in the selection of patients for HD-IL2 therapy after prior treatment with these agents.
Newer immunotherapies (nivolumab, ipilimumab, AGS-003, MPDL3280A, and others) are in development for mRCC.37–40 Long-term benefit and toxicity of these newer therapies remain to be determined. For the foreseeable future, HD-IL2 therapy remains the only modality with reproducible long-lasting CRs. Although limited by its retrospective nature and limited patient numbers, the strength of this study is that it incorporates data from multiple experienced investigators and HD-IL2 centers and reflects a diverse cross section of this patient population. We conclude that administration of HD-IL2 could be safe and effective after TKI therapy; however, careful selection of patients is critical. We recommend baseline cardiac risk factor assessment, screening with both cardiac stress test and echocardiogram, and allowing a TKI to HD-IL2 interval of at least 2 months.
ACKNOWLEDGMENT
The authors thank Dr Steven Clinton and Dr Ted Fraker for their critical review of this manuscript.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
Supported by NCI Cancer Center Support Grant P30CA046934.
All authors have declared that there are no financial conflicts of interest with regard to this work.
Footnotes
Preliminary results of this study were presented in part as a publication-only abstract in the Journal of Clinical Oncology [J Clin Oncol 29:2011 (suppl; abst e15079)], in conjunction with the 2011 ASCO Annual Meeting.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. [DOI] [PubMed] [Google Scholar]
- 2.Molina AM, Motzer RJ. Current algorithms and prognostic factors in the treatment of metastatic renal cell carcinoma. Clin Genitourin Cancer. 2008;6suppl 1S7–S13. [DOI] [PubMed] [Google Scholar]
- 3.Fyfe G, Fisher RI, Rosenberg SA, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6suppl 1S55–S57. [PubMed] [Google Scholar]
- 5.Klapper JA, Downey SG, Smith FO, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDermott DF, Ghebremichael M, Signoretti S, et al. The high-dose aldesleukin (HD IL-2) Select trial in patients with metastatic renal cell carcinoma (mRCC): Preliminary assessment of clinical benefit. J Clin Oncol. 2010;28:15s, (suppl; abstr 4514). [Google Scholar]
- 7.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. [DOI] [PubMed] [Google Scholar]
- 11.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14:552–562. [DOI] [PubMed] [Google Scholar]
- 13.Hutson TE, Davis ID, Machiels JP, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2010;28:475–480. [DOI] [PubMed] [Google Scholar]
- 14.Ratain MJ, Eisen T, Stadler WM, et al. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:2505–2512. [DOI] [PubMed] [Google Scholar]
- 15.Cho DC, Puzanov I, Regan MM, et al. Retrospective analysis of the safety and efficacy of interleukin-2 after prior VEGF-targeted therapy in patients with advanced renal cell carcinoma. J Immunother. 2009;32:181–185. [DOI] [PubMed] [Google Scholar]
- 16.Gotto AM, Jr, Kuller LH. Eligibility for lipid-lowering drug therapy in primary prevention: how do the Adult Treatment Panel II and Adult Treatment Panel III Guidelines compare?Circulation. 2002;105:136–139. [PubMed] [Google Scholar]
- 17.Expert Panel Recommendations.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 18.Expert Panel Recommendations.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 19.Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:1971–1996. [DOI] [PubMed] [Google Scholar]
- 20.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–e103. [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Bacik J, Mariani T, et al. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20:2376–2381. [DOI] [PubMed] [Google Scholar]
- 22.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–463. [DOI] [PubMed] [Google Scholar]
- 23.Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. [DOI] [PubMed] [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. [DOI] [PubMed] [Google Scholar]
- 25.Fyfe GA, Fisher RI, Rosenberg SA, et al. Long-term response data for 255 patients with metastatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1996;14:2410–2411. [DOI] [PubMed] [Google Scholar]
- 26.McDermott DF, Regan MM, Clark JI, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. [DOI] [PubMed] [Google Scholar]
- 27.Shablak A, Sikand K, Shanks JH, et al. High-dose interleukin-2 can produce a high rate of response and durable remissions in appropriately selected patients with metastatic renal cancer. J Immunother. 2011;34:107–112. [DOI] [PubMed] [Google Scholar]
- 28.Hawkins RE, Galvis V, Shanks J, et al. Treatment of metastatic renal cancer with high-dose interleukin-2 after targeted therapy. J Clin Oncol. 2012;30:(suppl 5; abstr 439). [Google Scholar]
- 29.Ridker PM, Buring JE, Rifai N, et al. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–619. [DOI] [PubMed] [Google Scholar]
- 30.D’Agostino RB, Sr, Grundy S, Sullivan LM, et al. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–187. [DOI] [PubMed] [Google Scholar]
- 31.Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khakoo AY, Kassiotis CM, Tannir N, et al. Heart failure associated with sunitinib malate: a multitargeted receptor tyrosine kinase inhibitor. Cancer. 2008;112:2500–2508. [DOI] [PubMed] [Google Scholar]
- 33.Mego M, Reckova M, Obertova J, et al. Increased cardiotoxicity of sorafenib in sunitinib-pretreated patients with metastatic renal cell carcinoma. Ann Oncol. 2007;18:1906–1907. [DOI] [PubMed] [Google Scholar]
- 34.Telli ML, Witteles RM, Fisher GA, et al. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol. 2008;19:1613–1618. [DOI] [PubMed] [Google Scholar]
- 35.Chintalgattu V, Rees ML, Culver JC, et al. Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci Transl Med. 2013;5:187ra69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong MK, Kaufman HL, Morse M, et al. The transient nature of significant toxicities associated with high dose interleukin (HD IL-2): Preliminary data from the Proclaim study. (Abstract from the 27th Annual Scientific Meeting of the Society of Immunotherapy of Cancer). J Immunother. 2012;35:753. [Google Scholar]
- 37.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366:2517–2519. [DOI] [PubMed] [Google Scholar]
- 38.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amin A, Dudek A, Logan T, et al. Prolonged survival with personalized immunotherapy (AGS-003) in combination with sunitinib in unfavorable risk metastatic RCC (mRCC). J Clin Oncol. 2013;31:(suppl 6; abstr 357). [Google Scholar]


