Abstract
Repeated social subjugation in early puberty lowers testosterone levels. We used hamsters to investigate the effects of social subjugation on male sexual behavior and metabolic activity within neural systems controlling social and motivational behaviors. Subjugated animals were exposed daily to aggressive adult males in early puberty for postnatal days 28 to 42, while control animals were placed in empty clean cages. On postnatal day 45, they were tested for male sexual behavior in the presence of receptive female. Alternatively, they were tested for mate choice after placement at the base of a Y-maze containing a sexually receptive female in one tip of the maze and an ovariectomized one on the other. Social subjugation did not affect the capacity to mate with receptive females. Although control animals were fast to approach females and preferred ovariectomized individuals, subjugated animals stayed away from them and showed no preference. Cytochrome oxidase activity was reduced within the preoptic area and ventral tegmental area in subjugated hamsters. In addition, the correlation of metabolic activity of these areas with the bed nucleus of the stria terminalis and anterior parietal cortex changed significantly from positive in controls to negative in subjugated animals. These data show that at mid-puberty, while male hamsters are capable of mating, their appetitive sexual behavior is not fully mature and this aspect of male sexual behavior is responsive to social subjugation. Furthermore, metabolic activity and coordination of activity in brain areas related to sexual behavior and motivation was altered by social subjugation.
Keywords: behavioral development, cytochrome oxidase activity, preoptic area, ventral tegmental area, appetitive male sexual behavior, social defeat, social subjugation
Introduction
Exposure to social stress or social subjugation during puberty impacts the development of social behavior (Delville et al., 2003). Hamsters exposed to daily attacks by adults in early puberty exhibit an accelerated maturation of agonistic behavior from play fighting to aggression (Wommack et al., 2003). In a range of species from hamsters and rats to Nazca boobies and rainbow trout, animals with similar experience become particularly aggressive towards younger individuals (Delville et al., 1998; Wommack et al., 2003; Øverli et al., 2004; Müller et al., 2011; Márquez et al., 2013), though they avoid and are cautious of larger individuals (Bastida, et al., 2009). However, these studies did not address sexual behavior development in males, though it is clear that social exposure can affect puberty in males. In mice, exposure to bedding from adult males delays masculine puberty (Vandenbergh, 1971). Hamsters exposed to repeated social subjugation in early puberty have decreased testosterone levels at mid puberty, though their gonadal weights are not affected (Wommack et al., 2004). Consequently, it would not be surprising to observe alterations in the maturation of sexual behavior in animals exposed to repeated social subjugation during puberty.
Sexual behavior and aggression in males share a common neural circuitry consisting of interconnected areas, known as the Vertebrate Social Behavior Network (Newman, 1999; Goodson, 2005). This network includes the lateral septum, preoptic area, parts of the hypothalamus, medial amygdala and midbrain central gray. As social stress during puberty affects agonistic behavior development and enhances avoidance it also impacts the expression of multiple systems in this network. The effects are widespread, impacting the activity of neurotransmitter systems, their capacity and connectivity between cells and the activity of these cells (Blanchard et al., 2001; McEwen and Gianaros, 2011). For example, chronic social stress enhances serotonin availability and dopamine expression within parts of the network in rats and hamsters (Delville et al., 1998; Wommack et al., 2004, Watt et al., 2009). Social stress affects BDNF expression and its receptors in the medial amygdala, ventral tegmental area or hippocampus (Arendt et al., 2012; Nikulina et al., 2012, Taylor et al., 2011). In rats and hamsters, acute social stress enhances the expression of c-fos, a neural marker of cellular activity in multiple brain areas, though it only remains elevated in several parts of the network after chronic social stress (Martinez et al., 1998; Kollack-Walker et al., 1999). These data point to substantial neural plasticity and adaptation in response to chronic social stress. As such it is likely that social stress will also lead to long term changes in metabolic capacity in multiple areas within the vertebrate social behavior network as well as other brain areas associated with stress or avoidance.
In the present study we decided to address changes in metabolic capacity within the vertebrate social behavior network in response to social stress as an index of long term plastic changes in the brain. We also looked at early male sexual behavior at mid puberty after two weeks of daily social subjugation. However, our studies were not limited to the consummation of male sexual behavior, but also included aspects of appetitive male sexual behavior, such as mate choice.
Methods
Animals
Male and Female golden hamsters were bred in the laboratory from a colony originally obtained from Harlan Sprague–Dawley (Indianapolis, IN). Each litter was culled to six animals (males and females) at approximately postnatal day 7 (P-7). On P-25, all animals were weaned and singly housed in Plexiglas cages in a separate room from stimulus animals. Animals were provided food and water provided ad libitum housed under a reversed light-day cycle (14L:10D, lights off at 10:00 am). All behavior tests and training procedures were conducted around the middle of the dark phase. Body weights were taken twice a week to monitor subjects' development. Animals were kept at the University of Texas at Austin in the Animal Resource Center, an AALAC-certified facility, and all procedures were carried according to relevant NIH guidelines and approved by the Institutional Animal Care and Use Committee.
Experimental Animals
On P-27, male hamsters were pre-tested for fearfulness in the presence of an adult male individual. Hamsters found to be inherently fearful (about 1:12) were not used in this study. Remaining animals were distributed between subjugated or control groups and balanced for litter origin and body weight.
Training and Stimulus Animals
Males used in this experiment to subjugate juveniles were experienced adult fighters. Stimulus females were ovariectomized at least one week before experimental use. Sexual receptivity was facilitated by two daily subcutaneous injections of 5 μg of estradiol benzoate (EB) in 2.5 ml sesame oil, followed by one injection of 500 mg progesterone in 0.1 ml sesame oil approximately three hours before experimental use (Carter and Porges, 1974). Non-receptive stimuli females received vehicle injections. Receptivity was confirmed before use by observing lordosis in response to a non-experimental male. Males were prevented from mounting during this confirmation. All females were at least P-60 at the time of the experiment.
Social Subjugation
Animals were subjugated using a previously established resident-intruder paradigm (Delville et al., 1998; Wommack, et al., 2003; Bastida, et al., 2009). From P-28 through P-42 experimental males (Subjugated) were placed into the home cage of an unknown adult male hamster for 20 minutes daily while naïve animals (Controls) were placed into a clean empty cage for the same duration of time. Behavioral testing occurred on P-45 and behavior in the presence of an aggressive adult hamster was videotaped and later scored for naïve and experimental animals. Submissive behaviors (animals showing tail ups displays, or remaining on their backs for extended periods) along with the number of attacks and bites inflicted by the adult males were recorded during each encounter. Any animal found bleeding or showing injuries was immediately removed from the study. Typically hamsters do not cause bite marks on the skin (Blanchard et al., 2003).
Consummation of Sexual Behavior
Consummatory sexual behavior of socially subjugated (N=12) and control male (N=10) hamsters was assessed at P-45 (mid-puberty). A sexually receptive stimulus female was introduced into the home cage of the male and recorded for 20 min. Videos were then coded using iMovie (Apple, Inc. Cupertino, CA) and EventCoder 1.0b10 software (generously made freely available by Dr. M. Goldstein, Cornell University) for the following sexual behaviors: latency to contact the female, duration of contact, duration of ano-genital investigations, latency to mount, latency to intromit, latency to ejaculate, counts and total duration of mounts, and counts and total duration of intromissions.
Appetitive Sexual Behavior
Appetitive sexual behavior of socially subjugated (N=17) and control males (N=15) was assessed using a Y-maze containing a stimulus female hamster in one arm of the maze. This behavioral apparatus allowed for the examination of motivational processes through stimulus preference. The Y-maze has previously been used to evaluate odor discrimination in female voles, individual recognition in male and female hamsters, and risk avoidance in hamsters (Johnston et al., 1997; Petrulis and Johnston, 1999; Lai and Johnston, 2002; Lai et al., 2005; Bastida, et al., 2009).
The Y-maze is constructed of clear polycarbonate and consists of a stem and two arm sections set 45° from each other (20cm high × 16cm wide each, approximately 170cm from base of stem to end of arms). Stimulus compartments (20cm long) are located at the end of each arm, with a similarly sized start box located at the base of the stem. The arm compartments are separated from the body of the maze by removable black polycarbonate screens with narrow perforations, allowing the transmission of olfactory cues. Lines are marked every 12 cm on the maze floor to assist with quantification of locomotor activity. A fan outside the start box facilitates airflow through the maze and draws odors from the arm compartments toward the start box.
All subjects were habituated to an empty Y-maze for 10 minutes daily for five days starting on P-40. On P-45, a receptive female (R) was placed into one arm compartment while a non-receptive female (NR) was placed into the second compartment. Stimulus females were allowed to habituate to the compartment for 10 minutes before testing began. Males were placed in the start box and then allowed to explore the maze for 10 minutes. The Y-maze was cleaned with 70%–95% ethanol between subjects during both habituation and testing. Behavior was assessed and videotaped on P-44 (no social stimuli) and P-45 (females present)(see Fig.1 for study timeline). The following observations were recorded and scored from observation of videos: latency to leave the start box, latency to enter each arm, durations of time spent in the start box, stem, each arm, and near the screen, and a count of the lines crossed in the start box, stem, and each arm. We also observed the duration of olfactory investigations as time spent at the screens and oriented toward the females. In addition, we recorded the number of alternations between arms of the maze.
Figure 1.
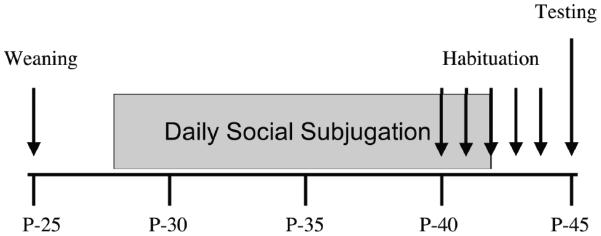
Timeline of events from postnatal day 25 (P-25) to P-45 during appetitive behavior testing. The animals were weaned on P-25, exposed to daily social subjugation from P-28 to P-42, habituated to the Y-maze daily from P-40 to P-44 and tested with females on P-45.
Quantitative Cytochrome Oxidase Histochemistry
On P-45, hamsters (n=10 subjugated and n=10 control) were sacrificed by rapid decapitation during the second half of the dark phase. Their brains were quickly extracted, flash frozen in isopentane cooled in dry ice, wrapped in Parafilm, and stored at −80°C. Later, the brains were cut into 40μm coronal sections using a Reichert-Jung cryostat set to −20°C. Sections were stored in a −40°C freezer until processed for histochemistry for cytochrome oxidase activity as previously described (Gonzalez-Lima and Garrosa, 1991, Gonzalez-Lima and Cada, 1994). These brains originated from a previous experiment (Wommack et al., 2004).
Cytochrome oxidase staining consisted of placing tissue in several incubation media. First, sections were placed in 0.1 M phosphate buffer (PB) with 10% w/v sucrose and 0.5% v/v glutaraldehyde for 5 min. After three washes in PB with 10% w/v sucrose to remove red blood cells for 5 min each, sections were incubated in Tris buffer (0.05 M Tris buffer solution, pH 7.6, with 275 mg/l cobalt chloride, 10% w/v sucrose, and 0.5% v/v dimethylsulfoxide) for 10 min in order to enhance tissue staining contrast and reduce the time spent in the diaminobenzidine (DAB) incubation procedure. After another PB wash, sections were then incubated in a solution of 350 mg diaminobenzidine tetrahydrochloride, 52.5 mg cytochrome c, 35 g sucrose, 14 mg catalase, and 1.75 ml dimethylsulfoxide in 700 ml of oxygen-saturated PB for one hour at 37°C followed by a formalin solution (10% w/v sucrose and 4% v/v formalin) to stop the incubation chemical reaction. Finally, slides were dehydrated in a series of ethanol baths (30%, 50%, 75%, 95%, 100%, and 100% v/v ethanol), cleaned with Xylene, and coverslipped with Permount.
Anatomically matched sections from all groups were stained in the same batch to remove the possibility of inter-batch variability as a possible confound between groups. Sets of homogenized brain tissue standards were also included in each batch of slides. These standards were used to quantify enzymatic activity and control for staining variability across different batches of cytochrome oxidase staining (Gonzalez-Lima and Cada, 1994).
As previously described (Gonzalez-Lima and Garrosa, 1991), optical density was sampled from each region of interest using JAVA version 1.4 (Jandel Scientific Corte Madera, CA) and were later converted to cytochrome oxidase units. The sampling area was adjusted for each region of interest in order to allow for four non-overlapping samples from each region. Three sections for each region were sampled per subject. Data was averaged for each animal and group means were compared. Brain regions and sub-regions were sampled, including those in the vertebrate social behavior network, as well as a number of other areas associated (Table 1).
Table 1.
List of areas or regions of interest (ROIs) sampled for cytochrome oxidase activity
| Social Behavior Network | ||
| LS | lateral septum | |
| BST | bed nucleus of the stria terminalis, medial division | |
| MeA | medial amygdala | |
| MPA | medial preoptic area | |
| MPO | medial preoptic nucleus | |
| AH | anterior hypothalamic area | |
| VMH | ventromedial hypothalamic nucleus | |
| DR | dorsal raphe nucleus | |
| MnR | median raphe nucleus | |
| CGD | central gray, dorsal | |
|
| ||
| Other Areas | ||
| Cortex | IL | infralimbic cortex, lateral |
| ILM | infralimbic cortex, medial | |
| ACg | anterior cingulate cortex | |
| AParS | anterior parietal cortex, superficial | |
| AParD | anterior parietal cortex deep | |
| MFrD | medial frontal cortex, deep | |
| MFrS | medial frontal cortex, superficial | |
| LFrS | lateral frontal cortex, superficial | |
| LFrD | lateral frontal cortex, deep | |
| PrLS | prelimbic cortex, superficial | |
| PrLD | prelimbic cortex, deep | |
| LOS | lateral orbital cortex, superficial | |
| LOD | lateral orbital cortex, deep | |
| MOS | medial orbital cortex, superficial | |
| MOD | medial orbital cortex, deep | |
| Sub-Cortical Areas | CA1 | CA1 field of the hippocampus |
| CA3 | CA3 field of the hippocampus | |
| Sub | Subiculum | |
| AcbSh | nucleus accumbens, shell | |
| AcbC | nucleus accumbens, core | |
| CPuDL | caudate putamen, dorsolateral | |
| CPuVL | caudate putamen, ventrolateral | |
| HDB | nucleus of the horizontal limb of the diagonal band of Broca | |
| BLA | basolateral amygdala | |
| Diencephalon | MS | medial septum |
| PVH | paraventricular hypothalamic nucleus | |
| MHb | medial habenular nucleus | |
| LHb | lateral habenular nucleus | |
| LH | lateral hypothalamic area | |
| Midbrain | SN | substantia nigra |
| CGV | central gray, ventral posterior | |
| VTA | ventral tegmental area | |
| IP | interpeduncular nucleus | |
| Hindbrain | SolM | nucleus of the solitary tract, medial part |
| SolVL | nucleus of the solitary tract, ventrolateral part | |
| LPBC | lateral parabrachial nucleus, central part | |
Data Analysis
Behavioral Analysis
For sexual behavior and preference tests, paired and unpaired Student's t- tests were used to compare each variable recorded in subjugated and control groups from tests with social stimuli present (P-45). Alpha levels were set at p <0.05, two-tailed.
Regional Cytochrome Oxidase Activity
A measure of global brain cytochrome oxidase activity was obtained by averaging intensity readings from all the brain sections used in each subject. This global measure of enzymatic activity was used as a reference for group differences in overall metabolic capacity, as well as for normalization of the cytochrome oxidase activity data of each region of interest for each subject (i.e., region of interest/brain) to minimize inter-subject staining differences unrelated to treatment group effects. Normalization ratios were multiplied by the average mean cytochrome oxidase activity of the naïve group to convert the units into those of enzymatic activity (μmol/min/g wet tissue). Regional cytochrome oxidase activity was averaged across subjects for each group to obtain mean ± standard error of mean. Analysis of cytochrome oxidase activity was performed using PASW® Statistics 18. Two-tailed Student's t-tests were used to compare the activity of each region between the subjugated and non-subjugated group. A stepwise discriminant analysis (Wilks' Lambda followed by Exact F tests for each step) was used as a multivariate statistical method that allowed analysis of all the brain regions together to avoid Type I errors associated with multiple univariate group comparisons. The purpose of the discriminant analysis was to identify the network of brain regions allowing a classification of subjects into two mutually exclusive groups: subjugated and control animals. In addition, Pearson's r correlations were calculated on the areas uncovered by both the t-tests between groups and the stepwise discriminate analysis. Differences in coordination of metabolic activity between regions in subjugated and control animals were assessed by comparing correlations using a Fisher's Z transformation on Fisher's Z scores of regions that showed significant correlations in activity in at least one group (p < 0.05). The equation for the Fisher's Z transformation is as follows:
where Zij is the Fisher Z transformation value for the correlation coefficient between regions i and j, ng1 is the sample size in group one, and ng2 is the sample size in group two.
Results
Consummatory behavior
The consummation of sexual behaviors was compared between subjugated and control male hamsters in the presence of a sexually receptive female. Overall, no statistically significant differences were found between groups. Animals in both groups spent equal amount of time in anogenital investigation (Subjugated: 140.1±54.0; Control: 154.8±36.8) of the females and remained in close contact with them for about 60% of the duration of the tests. Eventually most animals attempted to mount the females after approximately 50 seconds, copulated with them after about 100 seconds and ejaculated. Mount, intromission and ejaculation frequencies were similar between groups (Subjugated: 36.7±18.9 mounts, 35.2±20.2 intromissions, and 3.2±2.7 ejaculations; Control: 37.5±17.5 mounts, 38.0±18.0 intromissions, and 3.3±2.9 ejaculations). Few ectopic ejaculations were recorded in both groups.
Appetitive behavior
No differences between groups were observed in any of the measures recorded when no social stimulus was present in the Y-maze. However, differences between groups were evident in the presence of a stimulus. These affected most variables recorded in the maze, including time spent in various sections, latencies to reach each arm, line crossings, and durations of olfactory investigations near the stimuli.
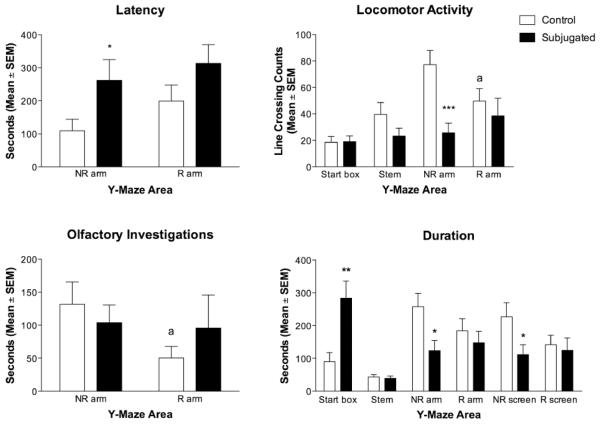
Differences between groups in time spent in the different parts of the maze were visible in the start box and the arms (Fig. 1). Subjugated animals spent half of the test in the start box, while controls spent most of their time near the females. Duration of time spent in the start box was 3 times higher in subjugated individuals [t(30) = 3.12, p < 0.01]. However, subjugated hamsters spent significantly less time in the NR arm [t(30) = 2.66, p < 0.05] and by the NR screen [t(30) = 2.24, p < 0.05]. Subjugated animals spent equal amount of time by the R and NR females (screen and arms), while controls showed a small but not statistically significant preference for NR females.
Just as they spent half of the test in the start box, subjugated animals also took nearly five minutes to reach the arms of the maze (Fig. 2). Subjugated hamsters took a significantly longer time to reach the NR arm of the maze than control animals [t(30) = 2.04, p = 0.05], though there was no difference between groups in latency to reach the R arm of the maze.
Figure 2.
Comparison between control and subjugated hamsters for different behavioral measures (Mean ± SEM) performed in each section of the Y-maze: start box, stem, arm with non- receptive female (NR Arm), arm with the receptive female (R Arm), proximity of the screen by the non receptive female (NR Screen), and proximity of screen by the receptive female (R Screen). The behaviors included duration of time in section of the maze, latency to enter the arms of the maze, locomotor activity (expressed as lines crossed), and duration of time spent performing olfactory investigations. Subjugated hamsters were exposed daily to aggressive males from postnatal day 28 (P-28) to P-42, while Controls were placed in empty clean cages. All tests were conducted at mid-puberty on P-45. Groups were compared for each section of the maze (Student t-tests; * p<0.05, ** p<0.01, *** p<0.001). Comparisons were also made between R and NR arms within the groups (Paired t-tests, a p<0.05).
Locomotor activity during the tests varied between groups, as controls animals crossed nearly twice as many lines in the Y-maze during the tests [t(24) = 2.21, p < 0.05]. The difference was statistically significant in the NR arm where control hamsters crossed about three times as many lines [t(24) = 4.07, p < 0. 001] (Fig. 3). There were no group differences in any other arm of the maze. A small difference was also visible in control animals between the NR and R arms, as they walked more lines closer to the NR females [t(11) = 2.82, p < 0. 05]. There was no difference between groups in line crossing counts in the R arm of the maze. We also compared the frequency of alternations between arms to determine whether it was associated with increased line crossings in controls. Both groups performed similar numbers of alternations [controls: 4.76 ± 6.06; subjugated: 6.33 ± 4.73; p > 0.1).
The analysis of duration of olfactory investigations at end of the arms near the females did not show any difference between groups. However, control males spent more than twice as much time investigating the NR females and they did with the R females [t(12) = 2.26, p < 0.05]. No such arm difference was visible in subjugated males.
Quantitative Cytochrome Oxidase Histochemistry
Cytochrome oxidase histochemistry resulted in a golden-brown colored staining that was darker in regions of the brain that have higher metabolic capacity. The Hamster Brain Atlas and the Rat Atlas of Cytochrome Oxidase and Cresyl Violet Staining were used to determine the sampled regions (Morin and Wood, 2001; Gonzalez-Lima and Cada, 1998). Only a few areas showed statistically significant differences between groups. Metabolic activity was significantly lower in subjugated animals in the medial preoptic area [MPA; Control: 199.16 ± 3.7, Subjugated: 191.69 ± 6.7, t (18) = 3.10, p < 0.01], medial preoptic nucleus [MPO; Control: 199.12 ± 4.1, Subjugated: 192.22 ± 7.9, t (18) = 2.45, p < 0.05], and ventral tegmental area [VTA; Control: 149.07 ± 21.2, Subjugated: 132.49 ± 13, t (18) = 2.11, p < 0.05]. The calculated effect size for the MPA, MPO and VTA were respectively 1.39, 1.10 and 0.94 (Cohen's d), which correspond approximately to 68, 59 and 53% of non-overlap between the groups.
The analysis of data using step-wise discriminant analysis (Wilks' Lambda) identified the VTA and MPA among a set of 6 significant areas (Table 2) whose variation in metabolic activity was classified into two mutually exclusive groups. These areas also included the infralimbic cortex (IL), ventral part of midrain central gray (CGV), medial frontal cortex (MFrD), and vental lateral part of the caudate putamen (CPuVL). The eigenvalue indicating the proportion of variance explained by the analysis was elevated (eigenvalue for 100% of variance: 8.693) with a canonical correlation of 0.948, indicative of a strong well-discriminated function. The Wilk's lambda for the function was low (Wilk's lambda = 0.10), thus indicating that the proportion of the total variance not explained by the comparison between groups was low. This lambda value was statistically significant [chi-square (6) = 34.484, p<0.001], strongly supporting a difference between control and subjugated animals. This suggests that of the regions sampled the differences in metabolic activity found in the MPA and VTA are likely to be reliable mean differences between subjugated and control animals, and not significantly different due to Type I errors. The CGV, IL, MFrD, and CPuVLwere also found to be discriminating variables in this analysis, but they were not found to be significantly different between groups in our original Student's t tests.
Table 2.
Wilks' Lambda in stepwise discriminant analysis between groups.
| Step | Brain Region | Lambda | Exact F | Significance | Coefficient |
|---|---|---|---|---|---|
| 1 | MPA | 0.653 | 9.582 | 0.006 | 1.971 |
| 2 | IL | 0.443 | 10.695 | 0.001 | 1.222 |
| 3 | CGV | 0.335 | 10.599 | <0.001 | −1.598 |
| 4 | LFrD | 0.247 | 11.429 | <0.001 | 1.964 |
| 5 | VTA | 0.143 | 16.806 | <0.001 | 1.416 |
| 6 | CPuVL | 0.100 | 19.421 | <0.001 | 0.842 |
The table includes the top 6 brain regions pulled by the analysis, the Wilks' lambda value, the Exact F value, the statistical significance of the Exact F value based on relevant degrees of freedom, and the standardized canonical discriminant function coefficient.
In this study, the MPA and VTA came up as the only areas picked up by the discriminate analysis and the t-test comparisons between groups, thus only these 2 areas were correlated with the rest of the sampled areas (Table 3). In control animals, cytochrome activity within the MPA was positively correlated with MPO, anterior parietal cortex (AParS and AParD) and caudate putamen (dorsolateral part, CPuDL). While metabolic activity within the VTA was correlated positively with the subiculum (Sub) and bed nucleus of the stria terminalis (medial division, BST), and negatively with the medial orbital, prelimbic and medial frontal cortices (MOD, PrLS, PrLD, MFrS and MFrD). In subjugated hamsters, cytochrome oxidase activity within the MPA was positively correlated with the lateral septum (LS), MPO, and midbrain central gray (dorsal and ventral parts, CGD and CGV), and negatively with the anterior parietal cortex (AParD), the medial orbital cortex (MOS), lateral orbital cortex (LOD), lateral prefrontal cortex (LFrD), and infralimbic cortices (IL and ILM). While metabolic activity within the VTA was positively correlated with the interpeduncular nucleus (IP) and negatively with the BST and ventrolateral hypothalamus (VLH). The differences in correlations with the MPA between groups were statistically significant for the anterior parietal cortex (AParS and AParS), from a positive to a negative relationship between controls and subjugated animals. Similarly, the change in correlation in metabolic activity between the VTA and BST from positive in controls to negative in subjugated animals was also statistically significant.
Table 3.
Comparison of correlations of cvtochrome oxidase activity between areas for different regions of interest (ROIs) between control and subjugated hamsters.
| ROIs | r (Control) | r (Subjugated) | Z score | p-value | q-value | |
|---|---|---|---|---|---|---|
| VTA | ||||||
| BST | 0.675 | −0.646 | 2.971 | 0.0030 | 0.027 | |
| VLH | −0.013 | −0.711 | 1.639 | 0.1012 | 0.328 | |
| Sub | 0.685 | 0.127 | 1.330 | 0.1835 | 0.328 | |
| IP | 0.443 | 0.733 | −0.859 | 0.3903 | 0.390 | |
| MOD | −0.719 | −0.243 | −1.23 | 0.2187 | 0.328 | |
| PrLS | −0.663 | −0.265 | −0.986 | 0.3242 | 0.328 | |
| PrLD | −0.643 | −0.284 | −0.882 | 0.3778 | 0.390 | |
| MFrS | −0.74 | −0.176 | −1.445 | 0.1485 | 0.328 | |
| MFrD | −0.693 | −0.035 | −1.532 | 0.1255 | 0.328 | |
| MPA | ||||||
| LS | 0.107 | 0.66 | −1.283 | 0.1995 | 0.239 | |
| MPO | 0.793 | 0.959 | −1.597 | 0.1103 | 0.212 | |
| CGD | 0.083 | 0.749 | −1.660 | 0.0969 | 0.212 | |
| CGV | −0.052 | 0.693 | −1.695 | 0.0901 | 0.212 | |
| CPuDL | 0.635 | −0.272 | 1.925 | 0.0542 | 0.212 | |
| AParS | 0.723 | −0.589 | 2.976 | 0.0029 | 0.017 | |
| AParD | 0.759 | −0.698 | 3.475 | 0.0005 | 0.006 | |
| MOS | −0.263 | −0.744 | 1.290 | 0.1971 | 0.239 | |
| LOD | −0.062 | −0.64 | 1.301 | 0.1933 | 0.239 | |
| LFrD | −0.150 | −0.658 | 1.195 | 0.2321 | 0.253 | |
| IL | −0.236 | −0.683 | 1.112 | 0.2661 | 0.266 | |
| ILM | −0.080 | −0.718 | 1.540 | 0.1236 | 0.212 |
This table lists areas significantly correlated (Pearson's r coefficients in bold) with the MPA and VTA in each group (Controls and Subjugated). The table also shows the final calculated Z score for each area correlated with either the MPA or VTA, as well as calculated p-values (2-tailed) and derived q-values (false discovery rates calculated from the p-values using the Benjamini-Hochberg method). Areas with a statistically significant correlation coefficient and Z scores are in bold.
Discussion
The purpose of the present study was to address whether social stress in early puberty can alter the development of male sexual behavior and affect metabolic capacity within neural systems mediating social and motivational behavior. The study was based on earlier reports showing avoidance and lower testosterone levels at mid-puberty (Bastida et al., 2009; Wommack et al., 2004). Our results indicate limited but specific effects in both behavior and metabolic capacity. Repeated social stress did not alter the consummatory component of male sexual behavior, as subjugated males were as capable of mating with receptive females as their controls when given an opportunity. However, subjugated males showed an avoidance of NR females and differential preferences as compared to their controls. These data were associated with decreased cytochrome oxidase activity within the MPA, a key area in the control of appetitive and consummatory male sexual behavior (Powers et al., 1987; Wood, 1997; Paredes, 2003; Dominguez and Hull, 2005; Been and Petrulis, 2010), and the VTA which is critical for reward and motivation of sexual behavior (van Furth and van Ree, 1996; Aron et al., 2005). As such, these data could be interpreted as a delay in the maturation of appetitive male sexual behavior and its underlying neural systems during puberty.
The lack of effect on the consummation of male sexual behavior is consistent with recent studies in adult hamsters (Jeffress and Huhman, 2013). In adults, conditioned defeat which is characterized by a long-lasting inhibition of aggression toward smaller intruders (Huhman et al., 2003) and lowered testosterone levels (Huhman et al., 1991) does not affect male sexual behavior, as these animals are still capable of mating with females. These recent data were obtained after a single defeat. Our data show that even repeated social subjugation in puberty which is also associated with reduced testosterone levels (Wommack et al., 2004) fails to affect mating. These findings are interesting as they suggest that the maturation of the consummatory aspect of male sexual behavior in hamsters does not depend on elevated levels of testosterone levels. Instead, the hormone has permissive effects on behavioral development. Previous studies showed a role for testosterone on the maturation of male sexual behavior after castration experiments in puberty (Meek et al., 1997), but simply lowering levels is not enough to prevent the developmental activation of the behavior.
In this study, appetitive sexual behavior was affected by social subjugation, as subjugated animals were more likely to stay in their start box than reach the females. It could be argued that they are simply more avoidant, and may be afraid of the females as they would avoid adult males (Bastida et al., 2009). In particular, subjugated males avoided NR females compared to controls, further generalizing these previous observations. This avoidance of females is unlikely to be associated with stress-induced hypoactivity even though subjugated males crossed fewer lines overall in the Y-maze in the presence of females. Their behavior did not differ in the absence of females. Furthermore, in a previous study on this model, subjugated hamsters were specifically tested for activity in two different ways and showed no impairments (Bastida et al., 2009). However, there was also a difference in preferences for females. While control males spent more time than subjugated animals in the proximity of NR females, they also appeared to prefer NR females, as evidenced by their olfactory investigations. In contrast, subjugated animals showed no preference. No animals in the study showed preference for sexually receptive females. This is not entirely surprising as preference for female vaginal secretions develops during puberty in sexually naïve males and is influenced by testosterone (Gregory et al., 1975; Johnston and Coplin, 1979; Murphy, 1980; Ballard and Wood, 2007; Bell et al., 2013). Nevertheless, this preference for NR females in control males was not expected and may reflect the maturation of appetitive sexual behavior. Animals in mid-puberty presented with a choice of females prefer ovariectomized individuals, although they may be more likely to attack them (Floody and Pfaff, 1977). This observation indicates that appetitive sexual behavior develops after consummatory aspects of the behavior during puberty. The reasons for the preference of possibly aggressive ovariectomized females are unclear at the moment. Perhaps sexually receptive females displaying lordosis responses in the presence of males are not particularly interesting to animals that have not completely maturated out of play fighting activity. Hamsters subjugated in early puberty undergo a faster maturation of agonistic behavior from play fighting to aggression (Wommack et al., 2003), thus they would be less likely to seek play fighting partners and showed reduced preference for ovariectomized females. Overall, our data show that repeated social subjugation in puberty has selective effects on appetitive male sexual behavior.
In this study, control and subjugated hamsters performed similar numbers of alternations between the NR and R arms of the maze. Alternation behavior is a measure of working memory and exploratory behavior (Lalonde, 2002). However, this does not necessarily mean an absence of effect on working memory or anxiety in subjugated animals. After all subjugated animals spent more time in the start box, particularly at the beginning of the test and took longer to reach the arms, thus they had less time for alternating between them. Perhaps a more specific test could point to a selective alteration in working memory or anxiety after social subjugation in this species.
At the level of the brain, we expected enhanced cytochrome oxidase activity in subjugated animals suggesting that stress enhances neural activity in a variety of regions, particularly areas associated with agonistic behavior and avoidance. Indeed, social defeat or subjugation results in enhanced c-fos expression in a variety of areas, as well as enhanced serotonin innervation or production of neurotrophic factors (Delville et al., 1998; Martinez et al., 1998; Kollack-Walker et al., 1999; Arendt et al., 2012; Nikulina et al., 2012). The discriminant analysis showed strong group differences. Cytochrome oxidase activity was decreased in subjugated animals within areas such as the MPA, VTA and parts of the frontal cortex. This decrease in cytochome oxidase activity contrasts with enhanced c-fos expression after social stress. However, changes in c-fos expression reflect evoked activity, while changes in cytochrome oxidase activity represent long-term effects of stress on metabolic activity within the brain (Sakata et al., 2002). As cytochrome oxidase activity reflects cumulative neural activity (Puga et al., 2007), the data suggest that repeated social subjugation persistently reduces neural activity in these areas.
The MPA is included within areas showing reduced cytochrome oxydase activity. In hamsters and rats, this area is critical to expression of male sexual behavior (Christensen et al., 1977; Malsbury, 1977; Powers et al., 1987; Wood, 1997; Paredes, 2003; Dominguez and Hull, 2005; Been and Petrulis, 2010). The appetitive and consummatory aspects of the behavior are integrated and mediated through neural activity in this area. The area is also rich in androgen and estrogen receptors (Wood et al., 1992; Wood and Newman, 1995), and it is possible that this reduction in activity is caused by lower testosterone levels in subjugated animals (Wommack et al., 2004). Testosterone treatment increase cytochrome oxidase activity in the neural song system of white crowned sparrows (Wennstrom et al., 2001) and repeated sociosexual interactions with females elevate cytochrome oxidase activity in the medial preoptic area of male rats (Sakata et al., 2002). Though subjugated animals were able to mate similarly to their controls, they did show altered appetitive male sexual behavior. Perhaps, this reduction in cytochrome oxidase activity in the MPA is associated with impaired appetitive male sexual behavior.
The decreased cytochrome activity within the VTA in subjugated animals could also be associated with impaired appetitive male sexual behavior. The ventral tegmental area is one of the sources of dopaminergic innervation to the MPA (Miller and Lonstein, 2009). Dopamine release in this area mediates appetitive male sexual behavior and copulation (Hull et al., 1986; Bitran et al., 1988; Hull et al., 1995; Dominguez and Hull, 2005). Alternatively, these changes in cytochrome oxidase activity within the VTA may also reflect aspects of fear such as the enhanced avoidance previously reported in subjugated animals (Bastida et al., 2009) and the avoidance of NR females in this study. Dopamine neurons in the VTA also participate in aversive or fear responses (Brischoux et al., 2009; Bromberg-Martin et al., 2010). These changes could also be associated to an adaptation to repeated social stress, such as long-lasting decreased cortisol peak responses (Wommack et al., 2004). The observation of decreased cytochrome oxidase activity within the VTA of congenitally helpless rats would be consistent with our data (Shumake et al., 2003), although subjugated hamsters will escape an attack if given a chance (Arendt et al., 2012).
Cytochrome oxidase activity within the MPA was significantly correlated with various areas within the vertebrate social behavior network such as the midbrain central gray, lateral septum and medial preoptic nucleus (Newman, 1999; Goodson, 2005). Interestingly, it was also correlated with a number of cortical areas. Most correlations with the MPA remained similar between subjugated and control animals, with the exception of the anterior parietal cortex. There, the correlations with the MPA were positive in controls and negative in subjugated animals. The change was statistically significant, suggesting a change in coordination of metabolic activity between these areas. In rodents, the parietal cortex has a dual sensory/motor function (Hall and Lindholm, 1974). At this point is it not clear which body parts were connected the sampled sections of the anterior parietal cortex in this study, and it is unclear how this change would relate to male sexual behavior. Perhaps this changing correlation reflects the social experience of the subjugated animals, having been attacked and bitten by residents. Perhaps this reversed correlation reflects a behavioral inhibition caused by repeated attacks.
The VTA also showed change in correlation of cytochrome oxidase activity with other areas, most noticeably the medial division of the BST. Correlations of activity with this area switched from positive in controls to negative in subjugated hamsters. This finding is interesting considering the roles and connections of these areas. The BST integrates inputs from the infralimbic cortex (one of the site of decreased cytochrome oxidase activity in our discriminant analysis) and the subiculum (Wood and Swann, 2005; Massi et al., 2008; Northcutt and Lonstein, 2011). The BST also integrate vomeronasal inputs with lateral septal afferents, suggesting an additional social memory regulation (Kevetter and Winans, 1981; Dantzer et al., 1988; Wood and Swann, 2005). Moreover, the anterior part of the medial division of the BST is capable of modulating directly VTA neurons (Georges and Aston-Jones, 2001; Wood and Swann, 2005; Northcutt and Lonstein, 2011). Finally, the medial division of the BST is also capable of regulating the activity of the HPA axis (Choi et al., 2007). As such, the BST has been proposed to integrate emotional-dependent (infralimbic inputs) and context-dependent (subiculum inputs) modulation of motivated behavior (Jalabert et al., 2009). Thus, some of the behavioral and endocrine characteristics of subjugated animals, such as decreased sexual appetitive behavior, learned avoidance and decreased cortisol responses, could be in response to a reversal of coordination of metabolic activity between these two areas. In this context, it is worth nothing that social subjugation in hamsters is associated with exceptional expression of tyrosine hydroxylase immunoreactivity in the anterior parts of the medial division on the BST (Wommack et al., 2004).
In summary, at mid-puberty hamsters are quite capable of mating with sexually receptive females, but their appetitive male sexual behavior is not fully mature. Sexually inexperienced juvenile hamsters prefer NR females. This finding is consistent with the concept that sexual experience during puberty triggers anxiety- and depressive-like behaviors in Siberian hamsters (Morris et al., 2013). Perhaps juvenile males prefer NR females because they are more likely to interact with them, as these animals are still engaging in play fighting (Wommack et al., 2003, Taravosh-Lahn and Delville, 2004). Thus, appetitive male sexual behavior in hamsters appears to mature from seeking females for play fighting. In this context, it makes sense for social subjugation to impact appetitive behavior at mid-puberty. Social subjugation accelerates the maturation of agonistic behavior from play fighting to aggression (Wommack et al., 2003). Subjugated animals would be less motivated in play fighting with females. These animals would be less interested in NR females and more likely to stay away from them. In the brain, changes in metabolic activity were selective. Social subjugation was associated with decreased metabolic activity in the preoptic area, which is involved in the control of male sexual behavior (Christensen et al., 1977; Malsbury, 1977; Powers et al., 1987; Wood, 1997; Paredes, 2003; Dominguez and Hull, 2005; Been and Petrulis, 2010). Decreased metabolic activity in the VTA in subjugated animals could also related to impaired appetitive sexual behavior, though it may also related to learned avoidance. Together, the limited breath of behavioral effects on sexual behavior development and metabolic activity in the brain support the concept of the resilience of juvenile hamsters to social stress. On an ecological point of view, it is likely that juvenile hamsters would encounter territorial adults after weaning and it makes sense that these experiences would not impair their reproductive fitness.
Highlights.
Social stress during puberty inhibits appetitive male sexual behavior
Social stress during puberty does not affect mating and copulation
Juvenile male copulate before preferring sexually receptive females
Social stress during puberty decreases metabolic activity in the MPA and VTA
Social stress during puberty alters the VTA-BST coordination of metabolic activity
Acknowledgements
These studies were supported by NSF IOB 0518272 awarded to YD, MH65728 and 076847 from NIH to FG-L, T32-MH65728 from NIH to FP, and T32-DA018926 from NIH to CCB. The authors are grateful to Dr. Kereshmeh Taravosh-Lahn for helpful discussions during the studies. The authors are also grateful Drs. Christine Duvauchelle, Andrea Gore, Theresa Jones and Juan Dominguez for helpful comments on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arendt DH, Smith JP, Bastida CC, Oliver KD, Eyster KM, Summers TR, Delville y., Summers CH. Contrasting hippocampal and amygdalar expression of genes related to neural plasticity during escape from social aggression. Physiol. Behav. 2012;107:670–679. doi: 10.1016/j.physbeh.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Brown LL. Reward, motivation, and emotional systems associated with early-stage intense romantic love. J. Neurophysiol. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Ballard CL, Wood RI. Partner preference in male hamsters: steroids, sexual experience and chemosensory cues. Physiol. Behav. 2007;91:1–8. doi: 10.1016/j.physbeh.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastida CC, Puga F, Delville Y. Risk assessement and avoidance in juvenile golden hamsters exposed to repeated stress. Horm. Behav. 2009;55:158–162. doi: 10.1016/j.yhbeh.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Been LE, Petrulis A. The role of the medial preoptic area in appetitive and consummatory reptroductive behaviors depends on sexual experience and odor volatility in male Syrian hamsters. Neuroscience. 2010;170:1120–1132. doi: 10.1016/j.neuroscience.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, Meerts SH, Sisk CL. Adolescent brain maturation is necessary for adult-typical mesocorticolimbic responses to a rewarding social cue. Dev. Neurobiol. 2013;73:856–869. doi: 10.1002/dneu.22106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, McKrittick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol. Behav. 2001;73:261–271. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Wall PM, Blanchard DC. Problems in the study of rodent aggression. Horm. Behav. 2003;44:161–170. doi: 10.1016/s0018-506x(03)00127-2. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hull EM, Holmes GM, Lookingland KJ. Regulation of male rat copulatory behavior by preoptic incertohypothalamic dopamine neurons. Brain Res. Bull. 1988;20:323–331. doi: 10.1016/0361-9230(88)90062-7. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. USA. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Porges SW. Ovarian hormones and the duration of sexual receptivity in the female golden hamster. Horm. Behav. 1974;5:303–315. doi: 10.1016/0018-506x(74)90017-8. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai Y, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: Implications for the integration of limbic inputs. J. Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen LW, Nance DM, Gorski RA. Effects of hypothalamic and proeptic lesions on reproductive behavior in male rats. Brain Res. Bull. 1977;2:137–141. doi: 10.1016/0361-9230(77)90010-7. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Koob GF, Bluthé RM, Le Moal M. Septal vasopressin modulates social memory in male rats. Brain Res. 1988;457:143–147. doi: 10.1016/0006-8993(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Delville Y, Melloni RH, Jr., Ferris CF. Behavioral and neurobiological consequences of social subjugation during puberty in golden hamsters. J. Neurosci. 1998;18:2667–2672. doi: 10.1523/JNEUROSCI.18-07-02667.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delville Y, David JT, Taravosh-Lahn K, Wommack JC. Stress and the development of agonistic behavior in golden hamsters. Horm. Behav. 2003;44:263–270. doi: 10.1016/s0018-506x(03)00130-2. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiol. Behav. 2005;86:356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Floody OR, Pfaff DW. Aggressive behavior in female hamsters: the hormonal basis for fluctuations in female aggressiveness correlated with estrous state. J. Comp. Physiol. Psychol. 1977;91:443–464. doi: 10.1037/h0077341. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J. Neurosci. 2001;21(1–6):RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Garrosa M. Quantitative histochemistry of cytochrome oxidase in rat brain. Neurosci. Lett. 1991;123:251–253. doi: 10.1016/0304-3940(91)90943-n. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Cada A. Cytochrome oxidase atlas of rat brain. In: Gonzalez-Lima F, editor. Cytochrome Oxidase in Neuronal Metabolism and Alzheimer's Disease. Plenum; New York: 1998. pp. 263–280. [Google Scholar]
- Gonzalez-Lima F, Cada A. Cytochrome oxidase activity an the auditory system of the mouse: a qualitative and quantitative histochemical study. Neuroscience. 1994;63:559–578. doi: 10.1016/0306-4522(94)90550-9. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E, Engel K, Pfaff DW. Male hamster preference for odors of female vaginal discharges: studies of experiential and hormonal determinants. J. Comp. Physiol. Psychol. 1975;89:442–446. doi: 10.1037/h0077043. [DOI] [PubMed] [Google Scholar]
- Hall RD, Lindholm EP. Organization of motor and somatosensory neocortex in the albino rat. Brain Res. 1974;66:23–38. [Google Scholar]
- Huhman KL, Moore TO, Ferris CF, Moughey EH, Meyerhoff JL. Acute and repeated exposure to social conflict in male golden hamsters: increases in plasma POMC-peptides and cortisol and decreased in plasma testosterone. Horm. Behav. 1991;25:206–216. doi: 10.1016/0018-506x(91)90051-i. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm. Behav. 2003;44:293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Hull EM, Bitran D, Pehec EA, Warner RK, Band LC, Holmes GM. Dopaminergic control of male sex behavior in rats: effects of an intracerebraly-infused agonist. Brain Res. 1986;370:73–81. doi: 10.1016/0006-8993(86)91106-6. [DOI] [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: Implications for sexual motivation and hormonal control of copulation. J. Neurosci. 1995;15:7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalabert M, Aston-Jones G, Herzog E, Manzoni O, Georges F. Role of the bed nucleus of the stria terminalis in the control of ventral tegmental area dopamine neurons. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:1336–1346. doi: 10.1016/j.pnpbp.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffress EC, Huhman KL. Copulatory and agonistic behavior in Syrian hamsters following social defeat. Aggress. Behav. 2013;39:239–245. doi: 10.1002/ab.21465. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Coplin B. Development of responses to vaginal secretions and other substances in golden hamsters. Behav. Neural Biol. 1979;25:473–489. doi: 10.1016/s0163-1047(79)90242-5. [DOI] [PubMed] [Google Scholar]
- Johnston RE, Sorokin ES, Ferkin MH. Female voles discriminate males' over-marks and prefer top-scent males. Anim. Behav. 1997;54:679–690. doi: 10.1006/anbe.1997.0471. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala”. J. Comp. Neurol. 1981;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Don C, Watson SJ, Akil H. Differential expression of c-fos mRNA within neurocircuits of male golden hamsters exposed to acute or chronic defeat. J. Neuroendocrinol. 1999;11:547–559. doi: 10.1046/j.1365-2826.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- Lai WS, Johnston RE. Individual recognition after fighting by golden hamsters: a new method. Physiol. Behav. 2002;76:225–239. doi: 10.1016/s0031-9384(02)00721-7. [DOI] [PubMed] [Google Scholar]
- Lai WS, Ramiro LL, Yu HA, Johnston RE. Recognition of familiar individuals in golden hamsters: a new method and functional neuroanatomy. J. Neurosci. 2005;25:11239–11247. doi: 10.1523/JNEUROSCI.2124-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci. Biobehav. Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Malsbury CW. Facilitation of male rat copulatory behavior by electrical stimulation of the medial preoptic area. Physiol. Behav. 1977;7:797–805. doi: 10.1016/0031-9384(71)90042-4. [DOI] [PubMed] [Google Scholar]
- Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur. J. Neurosci. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- Márquez C, Poirier GL, Cordero MI, Larsen MH, Groner A, Marquis J, Magistretti PJ, Trono D, Sandi C. Peripubertal stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl. Psychiatry. 2013;3:e216. doi: 10.1038/tp.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi L, Elezgarai I, Puente N, Reguero L, Grnades P, Manzoni OJ, Georges F. Cannabinoid receptors in the bed nucleus of the stria terminalis control cortical excitation of midbrain dopamine cells in vivo. J. Neurosci. 2008;28:10496–10598. doi: 10.1523/JNEUROSCI.2291-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Ann. Rev. Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek LR, Romeo RD, Novak CM, Sisk CL. Actions of testosterone in prepubertal and postpubertal male hamsters: dissociation of effects on reproductive behavior and brain androgen receptor immunoreactivity. Horm. Behav. 1997;31:75–88. doi: 10.1006/hbeh.1997.1371. [DOI] [PubMed] [Google Scholar]
- Miller SM, Lonstein JS. Dopaminergic projections to the medial preoptic area of postpartum rats. Neuroscience. 2009;159:1384–1396. doi: 10.1016/j.neuroscience.2009.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MR. Sexual preferences of male hamsters: importance of preweaning and adult experience, vaginal secretion, and olfactory or vomeronasal sensation. Behav. Neural Biol. 1980;30:323–340. doi: 10.1016/s0163-1047(80)91210-8. [DOI] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A Stereotaxic Atlas of the Golden Hamster Brain. Academic Press; San Diego: 2001. [Google Scholar]
- Morris JS, Weil ZM, Nelson RJ. Sexual experience and testosterone during adolescence alter neuronal morphology and behavior. Horm. Behav. 2013;64:454–460. doi: 10.1016/j.yhbeh.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Müller MS, Porter ET, Grace JK, Awkerman JA, Birchler KT, Gunderson AR, Schneider EG, Westbrook MA, Anderson DJ. Maltreated nestlings exhibit correlated maltreatment as adults: evidence of a “cycle of violence” in Nazca boobies (Sula granti) The Auk. 2011;128:615–619. [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann. NY Acad. Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Lacagnina MJ, Fanous S, Wang J, Hammer RP., Jr. Intermittent social defeat stress enhances mesocorticolimbic ΔFosB/BDNF co-expression and persistently activates corticotegmental neurons: implication for vulnerability to psychostimulants. Neuroscience. 2012;14:38–48. doi: 10.1016/j.neuroscience.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt KV, Lonstein JS. Neuroanatomical projectionsof the species-specific tyrosine hydroxylase-immunoreactive cells of the male prairie vole bed nucleus of the stria terminalis and medial amygdala. Brain Behav. Evol. 2011;77:176–192. doi: 10.1159/000326618. [DOI] [PubMed] [Google Scholar]
- Øverli Ø, Korzan WJ, Larson ET, Winberg S, Lepage O, Pottinger TG, Renner KJ, Summers CH. Behavioral and neuroendocrine correlates of displaced aggression in trout. Horm. Behav. 2004;45:324–329. doi: 10.1016/j.yhbeh.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Puga F, Barrett DW, Bastida CC, Gonzalez-Lima F. Functional networks underlying latent inhibition learning in the mouse brain. NeuroImage. 2007;38:171–183. doi: 10.1016/j.neuroimage.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RG. Medial preoptic area/anterior hypothalamus and sexual motivation. Scand. J. Psychol. 2003;44:203–212. doi: 10.1111/1467-9450.00337. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Johnston RE. Lesions centered on the medial amygdala impair scent-marking and sex-odor recognition but spare discrimination of individual odors in female golden hamsters. Behav. Neurosci. 1999;113:345–357. doi: 10.1037//0735-7044.113.2.345. [DOI] [PubMed] [Google Scholar]
- Powers JB, Newman SW, Bergondy ML. MPOA and BNST lesions in male Syrian hamsters: differential effects on copulatory and chemoinvestigatory behaviors. Behav. Brain Res. 1987;23:181–195. doi: 10.1016/0166-4328(87)90019-2. [DOI] [PubMed] [Google Scholar]
- Sakata J, Gonzalez-Lima F, Gupta A, Crews D. Repeated interactions with females elevate metabolic capacity in the limbic system of male rats. Brain Res. 2002;936:27–37. doi: 10.1016/s0006-8993(02)02491-5. [DOI] [PubMed] [Google Scholar]
- Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003;963:274–281. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- Taravosh-Lahn K, Delville Y. Aggressive behavior in female hamsters: development and the effect of repeated social stress. Horm. Behav. 2004;46:428–435. doi: 10.1016/j.yhbeh.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Taylor SL, Stanek LM, Ressler KJ, Huhman KL. Differential brain-derived neurotrphic factor expression in limbic brain regions following social defeat or territorial aggression. Behav. Neurosci. 2011;125:911–920. doi: 10.1037/a0026172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh JG. The influence of the social environment on sexual maturation in male mice. J. Reprod. Fertil. 1971;24:383–390. doi: 10.1530/jrf.0.0240383. [DOI] [PubMed] [Google Scholar]
- van Furth WR, van Ree JM. Sexual motivation: involvement of endogenous opioids in the ventral tegmental area. Brain Res. 1996;729:20–28. doi: 10.1016/s0006-8993(96)00225-9. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav. Neurosci. 2009;123:564–576. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennstrom KL, Reeves BJ, Brenowitz EA. Testosterone treatment increases the metabolic capacity of adult avian song control nuclei. J. Neurobiol. 2001;48:256–264. doi: 10.1002/neu.1055. [DOI] [PubMed] [Google Scholar]
- Wommack JC, Taravosh-Lahn K, David JT, Delville Y. Repeated exposure to social stress alters the development of agonistic behavior in male golden hamsters. Horm. Behav. 2003;43:229–236. doi: 10.1016/s0018-506x(02)00029-6. [DOI] [PubMed] [Google Scholar]
- Wommack JC, Salinas A, Melloni RH, Jr., Delville Y. Behavioural and neuroendocrine adaptations to repeated stress during puberty in male golden hamsters. J. Neuroendocrinol. 2004;16:767–775. doi: 10.1111/j.1365-2826.2004.01233.x. [DOI] [PubMed] [Google Scholar]
- Wood RI. Thinking about networks in the control of male hamster sexual behavior. Horm. Behav. 1997;32:40–45. doi: 10.1006/hbeh.1997.1403. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Androgen and estrogen receptors coexist within individual neurons in the brain of the Syrian hamster. Neuroendocrinology. 1995;62:487–497. doi: 10.1159/000127039. [DOI] [PubMed] [Google Scholar]
- Wood RI, Swann JM. The bed nucleus of the stria terminalis in the Syrian hamster: subnuclei and connections of the posterior division. Neuroscience. 2005;135:155–179. doi: 10.1016/j.neuroscience.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Wood RI, Brabec RK, Swann JM, Newman SW. Androgen and estrogen concentrating neurons in chemosensory pathways of the male Syrian hamster brain. Brain Res. 1992;596:89–98. doi: 10.1016/0006-8993(92)91536-n. [DOI] [PubMed] [Google Scholar]