SUMMARY
Studies of the identity and physiological function of mesenchymal stromal cells (MSCs) have been hampered by a lack of markers that permit both prospective identification and fate mapping in vivo. We found Leptin Receptor (LepR) is a marker that highly enriches bone marrow MSCs. Approximately 0.3% of bone marrow cells were LepR+, 10% of which were CFU-F, accounting for 94% of bone marrow CFU-F. LepR+ cells formed bone, cartilage, and adipocytes in culture and upon transplantation in vivo. LepR+ cells were Scf-GFP+, Cxcl12-DsRedhigh, and Nestin-GFPlow, markers which also highly enriched CFU-F, but negative for Nestin-CreER and NG2-CreER, markers which included few CFU-F. Fate-mapping showed LepR+ cells arose postnatally and gave rise to most bone and adipocytes formed in adult bone marrow, including bone regenerated after irradiation or fracture. LepR+ cells were quiescent but proliferated after injury. LepR+ cells are the major source of bone and adipocytes in adult bone marrow.
INTRODUCTION
Multipotent mesenchymal stromal cells (MSCs) have been defined in culture as non-hematopoietic, plastic-adherent, colony-forming cells, referred to as colony-forming unit-fibroblasts (CFU-F), that can differentiate into osteogenic, chondrogenic, and adipogenic progeny (Bianco et al., 2008; Friedenstein et al., 1970; Horwitz et al., 2005; Pittenger et al., 1999). A fundamental question concerns the identity of the cells that form CFU-F in culture and their physiological function in vivo.
In vivo, MSCs are often perivascular (Crisan et al., 2008; Sacchetti et al., 2007). In human tissues, MSCs have been prospectively identified based on the lack of expression of endothelial and hematopoietic markers along with positive expression for CD146 (Sacchetti et al., 2007) or CD146 with platelet-derived growth factor receptor β (PDGFRβ), CD106 and/or NG2 (Chou et al., 2012; Crisan et al., 2008; Schwab and Gargett, 2007). CD146+ MSCs from human bone marrow form osteogenic, chondrogenic, and adipogenic cells in culture and give rise to bone upon transplantation in vivo, forming bony ossicles that become invested with hematopoietic bone marrow (Sacchetti et al., 2007). The CD146+ cells persist around sinusoidal blood vessels in the ossicles and express HSC niche factors. Ectopic bones that become invested with bone marrow can also be formed by CD105+Thy1− mesenchymal cells from fetal mouse bones (Chan et al., 2009). Although much has been learned about the localization and developmental potential of MSCs, limitations in the ability to fate-map these cells in vivo have hindered our understanding of their normal physiological function.
Mouse MSCs have been prospectively identified based on the lack of expression of hematopoietic and endothelial markers and positive expression of PDGFRα (Morikawa et al., 2009; Omatsu et al., 2010; Park et al., 2012). The PDGFRα+Sca-1+CD45−Ter119− subset of cells appears to reside primarily around arterioles but does not express the hematopoietic stem cell (HSC) niche factor Cxcl12 while PDGFRα+Sca-1−CD45−Ter119− cells that express high levels of Cxcl12, also known as CXCL12-abundant reticular (CAR) cells, reside primarily around sinusoids (Morikawa et al., 2009; Omatsu et al., 2010). CAR cells include bi-potent progenitors of osteoblasts and adipocytes (Omatsu et al., 2010). Ablation of CAR cells with diphtheria toxin depletes not only adipogenic and osteogenic progenitors as well as HSCs (Omatsu et al., 2010). These data suggest MSCs within the bone marrow are a cellular component of the HSC niche.
Cells that express the Nestin-GFP transgene contain all of the CFU-F in mouse bone marrow (Kunisaki et al., 2013; Mendez-Ferrer et al., 2010). These cells also express high levels of the HSC niche factors Cxcl12 and Scf (Kunisaki et al., 2013; Mendez-Ferrer et al., 2010). Nestin-CreER-expressing cells in adult bone marrow can contribute to osteoblasts and chondrocytes, though no quantification was provided regarding their CFU-F content or their level of contribution to new bone (Mendez-Ferrer et al., 2010). Nestin-GFP is widely expressed by perivascular stromal cells in the bone marrow but they express little endogenous Nestin, Nestin-creER, or other Nestin transgenes (Ding et al., 2012). Furthermore, Nestin-GFP+ cells are heterogeneous, including both Nestin-GFPhigh cells that localize mainly around arterioles and Nestin-GFPlow cells that localize mainly around sinusoids, raising questions about the distribution of HSC niche factors and MSCs among these cell populations (Kunisaki et al., 2013).
During fetal development, Osterix (Osx) is expressed by cells that form bone marrow stroma, including perivascular, osteogenic, and adipogenic cells (Liu et al., 2013; Maes et al., 2010). In adult bone marrow, Osx is expressed by a subset of CAR cells (Omatsu et al., 2010), and fate mapping of these cells using Osx-CreER revealed a transient contribution to osteoblasts (Park et al., 2012). In contrast, Mx-1-Cre recombined widely among hematopoietic cells, PDGFRα+ stromal cells, and CFU-F in the bone marrow and these cells gave rise to most of the osteoblasts formed in adult bone marrow (Park et al., 2012). The widespread marking of both hematopoietic and stromal cells by Mx-1-Cre meant this marker could not be used to prospectively identify osteogenic progenitors.
Recently, we identified Leptin Receptor+ (LepR+) perivascular stromal cells that are the major source of Stem cell factor (Scf) and Cxcl12 in the bone marrow (Ding and Morrison, 2013; Ding et al., 2012). Conditional deletion of Scf with Lepr-Cre led to the depletion of quiescent HSCs (Ding et al., 2012; Oguro et al., 2013) and conditional deletion of Cxcl12 with Lepr-Cre led to HSC mobilization (Ding and Morrison, 2013). Here we show that LepR+ cells are highly enriched for CFU-F and uniformly express Prx1-Cre, PDGFRα and CD51, markers expressed by bone marrow MSCs. LepR+ cells were the main source of new osteoblasts and adipocytes in adult bone marrow and could form bony ossicles that support hematopoiesis in vivo. In contrast, Wnt1-Cre-expressing neural crest-derived cells and Nestin-CreER-expressing cells included few CFU-F and made little contribution to adult osteogenesis.
RESULTS
LepR+ bone marrow stromal cells are around sinusoids and arterioles
Sections from wild-type mice exhibited perivascular LepR staining throughout the bone marrow, around both sinusoids and arterioles (Figure 1A, 1C and 1D). The staining was in perivascular stromal cells that expressed the HSC niche factor Scf (Figure 1C). An antibody against the LepR extracellular domain stained in a pattern very similar to Tomato expression in Lepr-cre; tdTomato conditional reporter mice (Figure 1D). Ubc-creER; Leprfl/fl mice that had been treated with tamoxifen for a month to conditionally delete Lepr had little staining with the antibody in sections (Figure 1B) or in PDGFRα+CD45−Ter119−CD31− bone marrow stromal cells analyzed by flow cytometry (Figure S1A).
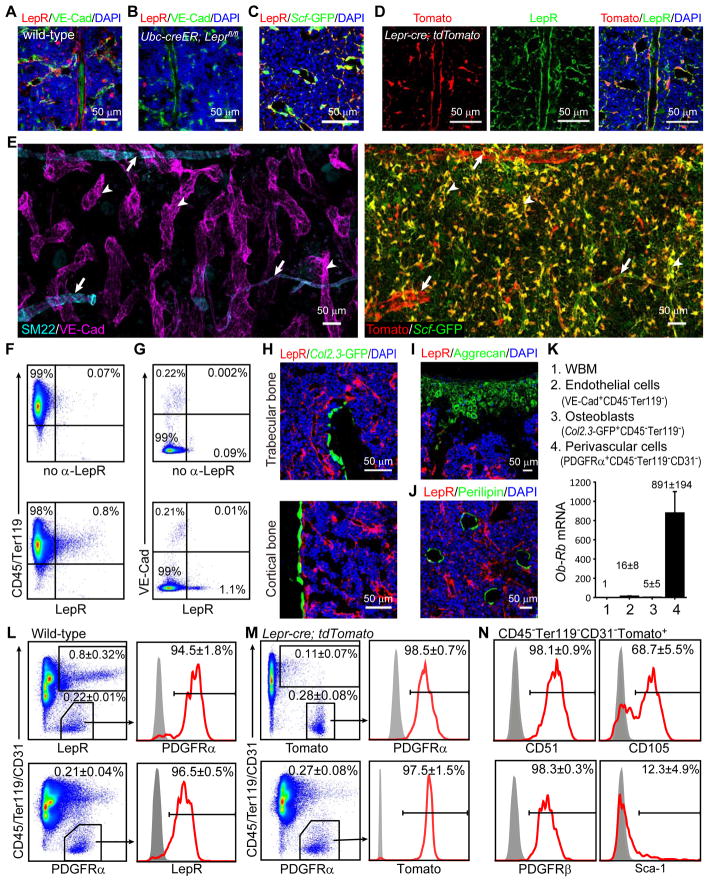
Figure 1. LepR and Scf-GFP expressing cells are abundant around sinusoids throughout the bone marrow and are distinct from other stromal cells.
(A and B) Representative femur sections from 3~4-month-old wild-type (A) and Ubc-creER; Leprfl/fl mice (B). The anti-LepR antibody stained perivascular cells in wild-type (A) but not Ubc-creER; Leprfl/fl (B) bone marrow (unless otherwise indicated, each panel reflects data from 3 mice/genotype from 3 independent experiments).
(C) Staining with anti-LepR antibody and Scf-GFP.
(D) Staining with anti-LepR antibody strongly overlapped with Tomato expression around sinusoids and arterioles in the bone marrow of Lepr-cre; tdTomato mice.
(E) Three dimensional reconstruction of a Z stack of tiled confocal images of femur bone marrow from a Lepr-cre; tdTomato; Scf-GFP mouse. Anti-VE-Cad staining marked sinusoids (arrowheads, left panel) and arterioles while anti-SM22 staining specifically marked arterioles (arrows, left panel). Left and right panels represent images from the same field of view. LepR was expressed by perivascular cells around sinusoids and arterioles but LepR+Scf-GFP+ cells were most abundant around sinusoids. Note that most Scf-GFP staining that did not overlap with Tomato staining represented the processes of perivascular cells that had Tomato staining in their cell body (see Figure S1B). The frequency of Scf-GFP+ cells appears high in this image because it represents a Z stack of images from a thick section, not a single optical section.
(F and G) Flow cytometry analysis showed that CD45/Ter119+ hematopoietic cells (F) and VE-cadherin+ endothelial cells (G) rarely stained positively for LepR. The bone marrow was dissociated mechanically in F (thus lacking stroma) or enzymatically in G (including stroma).
(H–J) Col2.3-GFP+ osteoblasts (H), Aggrecan+ chondrocytes (I) and Perilipin+ adipocytes (J) did not stain with anti-LepR antibody. (n=3–5 mice from at least 3 independent experiments)
(K) Quantitative RT-PCR of Ob-Rb transcript levels (normalized to β-Actin). Data represent mean±SD (standard deviation) from 4 independent experiments.
(L and M) In 2–4 month old mice, nearly all LepR+ bone marrow cells stained positively for PDGFRα, and vice versa, irrespective of whether the LepR+ cells were identified by antibody staining (L) or Tomato expression in Lepr-cre; tdTomato mice (M). The data represent mean±SD from 3–5 mice from at least 3 independent experiments.
(N) Marker expression by Tomato+ bone marrow cells from Lepr-cre; tdTomato mice.
We identified sinusoids and arterioles based on VE-Cadherin staining, which bound endothelial cells in both sinusoids and arterioles, and SM22 staining, which specifically marked vascular smooth muscle around arterioles. Sinusoids were typically larger in diameter, less uniform and thinner walled as compared to arterioles (Figure 1E). We observed LepR+ cells around both sinusoids and arterioles throughout the bone marrow, though LepR+ cells were much more prominent around some arterioles than others (Figure 1E). Nearly all the perisinusoidal LepR+ cells were Scf-GFP+; however, the periarteriolar LepR+ cells, especially those densely surrounding larger arterioles expressed less Scf-GFP (Figure 1E). The LepR+ cells around arterioles were negative for SM22+ or αSMA+ (Figure 1E and S1C).
We observed little LepR antibody staining in CD45+ or Ter119+ hematopoietic cells (Figure 1F) or in VE-Cadherin+ bone marrow endothelial cells (Figure 1A, 1E and 1G). The rare LepR antibody staining that was observed in these cells may reflect the expression of short isoforms of Lepr that lack the intracellular signaling domain (Ob–Ra, Ob–Rc, Ob–Rd, and Ob–Re). These isoforms are somewhat more broadly expressed than the Ob–Rb isoform, which encodes full-length LepR, including the intracellular signaling domain. It is this full-length isoform whose expression is marked by Lepr-cre. Little recombination was observed in macrophages (Figure S1D) or in other hematopoietic cells (Figure 1M) using Lepr-cre.
Consistent with the lack of EYFP expression in bone-lining cells from 2-month-old Lepr-cre; EYFP reporter mice (Ding et al., 2012), we were unable to detect LepR antibody staining in Col2.3-GFP+ osteoblastic cells from cortical or trabecular bone in 2-month-old mice (Figure 1H). Neither Aggrecan+ articular cartilage cells in the femur (knee joint; Figure 1I) nor Perilipin+ fat cells in the bone marrow (Figure 1J) exhibited LepR antibody staining. Thus, at 2 months of age, LepR expression was largely restricted to perivascular stromal cells and not to more differentiated mesenchymal derivatives in bone marrow.
Consistent with the LepR antibody staining and Lepr-cre; tdTomato conditional reporter expression pattern, quantitative real time-PCR (qPCR) showed that full length Ob-Rb transcripts were at 100- to 1000-fold higher levels in PDGFRα+CD45−Ter119−CD31− perivascular stromal cells as compared to unfractionated bone marrow cells, Col2.3-GFP+CD45−Ter119− osteoblastic cells, and VE-Cad+CD45−Ter119− bone marrow endothelial cells (Figure 1K). Virtually all LepR+ cells expressed Scf-GFP and nearly all Scf-GFP+ cells expressed LepR (Figure S1E). LepR+ Scf-GFP negative cells around certain arterioles appeared to represent less than 1% of LepR+ cells in bone marrow (Figure 1E). Nearly all LepR+ cells expressed high levels of Cxcl12-DsRed and nearly all cells that expressed high levels of Cxcl12-DsRed expressed LepR (Figure S1F). Most LepR+ cells expressed low levels of Nestin-GFP (Figure S1G and S1H), consistent with a recent report (Kunisaki et al., 2013). Cells that expressed high levels of Nestin-GFP did not stain with either LepR or PDGFRα (Figure S1H). The vast majority of bone marrow cells that express high levels of HSC niche factors and the bone marrow MSC marker PDGFRα are thus LepR+.
LepR+CD45−Ter119− bone marrow stromal cells accounted for 0.2% to 0.3% of enzymatically dissociated bone marrow cells, irrespective of whether these cells were identified by LepR antibody staining (Figure 1L) or Tomato expression in Lepr-cre; tdTomato conditional reporter mice (Figure 1M). Nearly all LepR+CD45−Ter119−CD31− bone marrow stromal cells were positive for PDGFRα and nearly all PDGFRα+CD45−Ter119−CD31− bone marrow cells were LepR+ (Figure 1L and 1M). These data suggested that LepR+ bone marrow stromal cells might be highly enriched for MSCs. Consistent with this possibility, we found that LepR+CD45−Ter119−CD31− bone marrow stromal cells were uniformly positive for the MSC markers CD51 (Pinho et al., 2013) and PDGFRβ (Komada et al., 2012) (Figure 1N). Approximately 68% of LepR+CD45−Ter119− cells were positive for the MSC marker CD105 (Chan et al., 2009; Park et al., 2012) (Figure 1N). LepR+CD45−Ter119− cells were heterogeneous for Sca-1 (Figure 1N), which is expressed by a subset of MSCs (Morikawa et al., 2009; Omatsu et al., 2010).
LepR+ cells are the main source of CFU-F in bone marrow
To assess CFU-F activity we enzymatically dissociated bone marrow cells and added them to adherent cultures at clonal density. Figure 2B shows the percentage of cells in each cell population sorted from unfractionated bone marrow cells (including both hematopoietic and stromal elements) that formed CFU-F colonies in culture. Figure 2C shows the percentage of cells in each cell population sorted from non-hematopoietic (CD45−Ter119−) bone marrow stromal cells that formed CFU-F colonies in culture. In our experiments, 0.012±0.002% of all enzymatically dissociated bone marrow cells (Figure 2B) or 1.3±0.5% of CD45−Ter119− bone marrow stromal cells formed CFU-F colonies (Figure 2C).
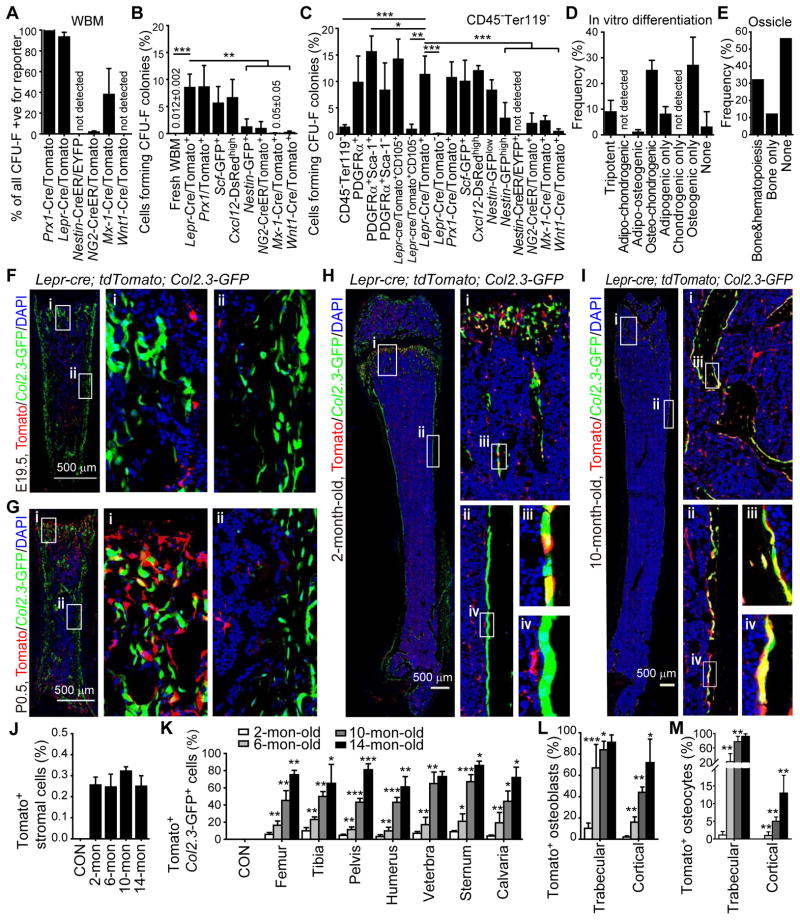
Figure 2. LepR+ cells contain most of the CFU-F in adult bone marrow and are the major source of new bone in adult mice.
(A) Percentage of all CFU-F colonies that were labeled by conditional reporter expression when cultured from enzymatically dissociated bone marrow of the indicated genotypes. Macrophage colonies were excluded by staining with anti-CD45 antibody in all experiments. (n=3–11 mice/genotype from at least 3 independent experiments).
(B) Percentage of bone marrow cells expressing each marker that formed CFU-F colonies in culture (n=3–5 mice/genotype from at least 3 independent experiments).
(C) Percentage of non-hematopoietic (CD45−Ter119−) bone marrow cells expressing each marker that formed CFU-F colonies in culture (n=3–11 mice/genotype from at least 3 independent experiments). Two-tailed Student’s t-tests were used to assess statistical significance. *P<0.05, **P<0.01, ***P<0.001.
(D) The percentage of CFU-F colonies that arose from Tomato+CD45−Ter119−CD31− cells from Lepr-cre; tdTomato mice that gave rise to Oil-red O+ adipocytes, Toluidine blue+ chondrocytes, and/or Alizarin-red S+ osteoblasts (n=3 mice from 3 independent experiments).
(E) Development of bone and hematopoiesis in ossicles formed by individual CFU-F colonies that arose from LepR+ stromal cells from 4 Col2.3-GFP; Lepr-cre; tdTomato mice.
(F–I) Representative femur sections from Lepr-cre; tdTomato; Col2.3-GFP mice of different ages showing the increasing generation of Tomato+Col2.3-GFP+ osteoblasts with age (3–5 mice/age from at least 4 independent experiments).
(J) The frequency of Tomato+CD45−Ter119−CD31−Col2.3-GFP− bone marrow stromal cells in the femurs of Lepr-cre; tdTomato; Col2.3-GFP mice did not change with age. Cells from ~6-month-old Col2.3-GFP; tdTomato mice were negative controls (CON). Data in all remaining panels represent mean±SD from 3–5 mice/age from at least 3 independent experiments.
(K) Percentage of Col2.3-GFP+ osteoblasts that were also Tomato+ in enzymatically dissociated bone from Lepr-cre; tdTomato; Col2.3-GFP mice of different ages. Osteoblasts from age-matched Col2.3-GFP or Col2.3-GFP; tdTomato mice were used as negative controls in each experiment (CON). Two-tailed Student’s t-tests were used to assess statistical significance among consecutive ages. *P<0.05, **P<0.01, ***P<0.001.
(L and M) Percentage of osteoblasts (L) and osteocytes (M) that were Tomato+ in bone sections from Lepr-cre; tdTomato; Col2.3-GFP mice.
Consistent with a prior study (Morikawa et al., 2009), 9.8±5.0% of PDGFRα+CD45−Ter119− bone marrow stromal cells, 15.6±3.0% of PDGFRα+Sca-1+CD45−Ter119− bone marrow stromal cells, and 8.3±5.2% of PDGFRα+Sca-1−CD45−Ter119− bone marrow stromal cells formed CFU-F colonies (Figure 2C). Although PDGFRα+Sca-1+ stromal cells were more highly enriched for CFU-F activity than PDGFRα+Sca-1− cells (Figure 2C), PDGFRα+Sca-1− stromal cells contained most of the CFU-F activity in the bone marrow (Figure S2A) because they were much more abundant than PDGFRα+Sca-1+ cells (0.22±0.08% versus 0.03±0.02% of bone marrow cells; Figure 1N and S1J). PDGFRα+ bone marrow cells are thus highly enriched for CFU-F. but we could not fate map PDGFRα+ cells because Pdgfra-CreER (Rivers et al., 2008) recombined poorly in bone marrow PDGFRα+ cells (data not shown).
The vast majority of bone marrow CFU-F derive from LepR+ cells. 94±4% of CFU-F colonies formed by bone marrow cells from Lepr-cre; tdTomato mice were Tomato+ (Figure 2A, S2B–S2E). In 2- to 4-month-old Lepr-cre; tdTomato mice, 8.5±2.5% of all Tomato+ bone marrow cells (Figure 2B) and 11±3.5% of Tomato+CD45−Ter119− bone marrow stromal cells formed CFU-F colonies (Figure 2C) whereas only 0.10±0.08% of Tomato negative CD45−Ter119− bone marrow stromal cells formed CFU-F colonies (Figure 2C). Consistent with a previous study (Park et al., 2012), almost all of the Tomato+ CFU-F in Lepr-cre; tdTomato bone marrow were CD105+ (Figure 2C). Cells derived from full-length LepR+ cells in the bone marrow were thus as highly enriched for CFU-F as PDGFRα+CD45−Ter119− bone marrow cells.
We split cells obtained from individual CFU-F colonies formed by LepR+ cells into three aliquots and subcloned into cultures that promoted bone, cartilage, or fat cell differentiation. 8.9±4.5% of CFU-F colonies formed by Tomato+ bone marrow cells from Lepr-cre; tdTomato mice underwent multilineage differentiation (Figure 2D), giving rise to Alizarin red S stained osteoblastic cells (Figure S2F and S2G), Oil red O stained adipocytes (Figure S2H and S2I), and Toluidine blue stained chondrocytic cells (Figure S2J and S2K). Most of the remaining CFU-F colonies formed by Tomato+ cells differentiated to osteoblastic cells with or without chondrocytic cells or fat cells. Overall, 58±17% of all CFU-F colonies formed by Tomato+ cells formed osteoblastic cells in culture.
We also sorted individual LepR+ cells from Lepr-cre; tdTomato; Col2.3-GFP mice into culture, allowed them to form CFU-F colonies, then expanded individual colonies, implanted the cells into denatured collagen sponges, transplanted subcutaneously, and assessed the development of bony ossicles and hematopoiesis eight weeks later. The presence of hematopoiesis was determined based on the presence of undifferentiated and differentiated erythroid, myeloid, and lymphoid cells in ossicle sections. Fourteen of 25 sponges did not form bone. Of the 11 that did form bone, we detected hematopoiesis in 8 (Figure 2D and S2L-S2N), 3 of which had abundant hematopoiesis that resembled bone marrow and 5 of which had smaller foci of hematopoietic cells distributed throughout each ossicle. In each case, we observed donor-derived (Tomato+) bone, stromal cells, and adipocytes in addition to host-derived CD45+ hematopoietic cells (Figure S2L and S2M). Cells from the 3 ossicles that contained the most hematopoietic cells gave multilineage reconstitution of irradiated mice, demonstrating the presence of primitive hematopoietic progenitors (data not shown).
When bone marrow cells were obtained from the femurs and tibias of Prx1-cre; tdTomato conditional reporter mice, all CFU-F colonies formed by unfractionated bone marrow cells were Tomato+ (Figure 2A). In 2- to 4-month-old Prx1-cre; tdTomato mice, 8.6±4.0% of all Tomato+ bone marrow cells (Figure 2B) and 10±3% of Tomato+CD45−Ter119− bone marrow stromal cells formed CFU-F colonies (Figure 2C). Nearly all LepR+ antibody stained cells were Tomato+ in Prx1-cre; tdTomato mice and nearly all Tomato+ cells were LepR+ (Figure S2P and S2Q). Thus, LepR+ cells in the marrow of limb bones uniformly express the MSC marker Prx1.
Consistent with the conclusion that MSCs secrete factors that promote HSC maintenance (Mendez-Ferrer et al., 2010; Omatsu et al., 2010; Sacchetti et al., 2007) both Scf-GFP+ bone marrow cells and Cxcl12-DsRedhigh bone marrow cells were highly enriched for CFU-F (Figure 2B and 2C). The observation that these cells are similarly enriched for CFU-F as LepR+ cells is consistent with our data demonstrating that nearly all cells that express Scf-GFP or high levels of Cxcl12-DsRed are LepR+ (Figure S1D and S1E). These data suggest a strong overlap between LepR+ stromal cells and CAR cells in the bone marrow.
CFU-F often expressed Mx1-Cre, consistent with a recent study (Park et al., 2012). Bone marrow sections from Mx-1-cre; tdTomato mice showed widespread Tomato expression among hematopoietic cells, vascular cells, perivascular cells and bone-lining osteoblastic cells (Figure S4B–S4D). We found that 37±25% of all CFU-F colonies formed by Mx-1-cre; tdTomato bone marrow cells were Tomato+ (Figure 2A) and 46±23% of all CD45−Ter119−CD31−PDGFRα+ stromal cells were Tomato+ (Figure S4A). Only rare Tomato+ bone marrow cells formed CFU-F colonies (Figure 2B and 2C), due to widespread recombination among hematopoietic cells and other stromal cells (Figure S4A).
CFU-F rarely expressed NG2-CreER. Only 1.8±1.0% of CFU-F colonies formed by bone marrow cells from NG2-creER; tdTomato reporter mice were Tomato+ (Figure 2A). Tomato+ cells accounted for 0.0026±0.007% of all bone marrow cells in NG2-creER; tdTomato mice and 8.8±3.6% of these cells stained positively for the MSC marker PDGFRα (Figure S3A). Only 9.8±3.4% of Tomato+ bone marrow cells in NG2-creER; tdTomato; Scf-GFP mice were positive for Scf-GFP (Figure S3A). We observed almost no NG2-CreER-expressing cells among PDGFRα+ cells or Scf-GFP+ cells in the bone marrow (Figure S3B–S3D).
In the bone marrow NG2-creER labeled vascular smooth muscle cells (Figure S3E), GFAP+ glia associated with nerve fibers (Figure S3F), chondrocytes, osteocytes, and rare osteoblasts (data not shown). NG2 antibody stained cells also included Aggrecan+ chondrocytes (Figure S3G), Col2.3-GFP+ osteoblasts, and osteocytes (Figure S3H) in 2-month-old mice. Smooth muscle cells (Murfee et al., 2005), peripheral nerve Schwann cells (Schneider et al., 2001), cartilaginous cells and osteoblasts (Fukushi et al., 2003) have all been previously reported to express NG2.
CFU-F also rarely expressed Nestin-creER. We did not detect any EYFP+ CFU-F colonies formed by bone marrow cells from Nestin-creER; loxp-EYFP mice (Figure 2A). We observed labeling of rare cells associated with some arterioles in the bone marrow of Nestin-creER; loxp-EYFP mice (Figure S4F) consistent with previously published images (Mendez-Ferrer et al., 2010). EYFP+ cells accounted for 0.0012%±0.0005% of bone marrow cells and were nearly uniformly negative for PDGFRα (Figure S4E). When we aged Nestin-creER; loxp-EYFP mice for 11 months we only detected rare EYFP+ osteoblasts (Figure S4F), demonstrating Nestin-CreER-expressing cells are not a significant source of bone in vivo.
The observation that Nestin-GFP+ cells included CFU-F but Nestin-CreER-expressing cells did not (Figure 2C and S1H) is consistent with our observation that different Nestin transgenes exhibit different expression patterns in the bone marrow (Ding et al., 2012). Neither Nestin-GFPlow nor Nestin-GFPhigh bone marrow cells appear to express endogenous Nestin (see microarray data in (Mendez-Ferrer et al., 2010) and RNAseq data in (Kunisaki et al., 2013)).
CFU-F were not neural crest-derived based on fate mapping with Wnt1-Cre (Chai et al., 2000; Echelard et al., 1994; Joseph et al., 2004). We observed Tomato+ nerve fibers and glia in the bone marrow of Wnt1-cre; tdTomato mice (Figure S4H), confirming that neural crest-derived cells were marked in the bone marrow. In 2- to 4-month-old Wnt1-cre; tdTomato mice, 0.17±0.29% of all Tomato+ bone marrow cells (Figure 2B) and 0.5±0.5% of Tomato+CD45−Ter119− cells (Figure 2C) formed CFU-F colonies. However, none of the CFU-F colonies formed by unfractionated bone marrow cells from Wnt1-cre; tdTomato reporter mice were Tomato+ (Figure 2A). Tomato+ cells in the bone marrow of 2-month-old Wnt1-cre; tdTomato reporter mice accounted for only 0.0017±0.001% of bone marrow cells and were mostly negative for the MSC marker PDGFRα (Figure S4G). We aged Wnt1-cre; tdTomato mice for 5 months and observed rare Tomato+ osteocytes (Figure S4H) but no Tomato+ osteoblasts, indicating that these cells are not a significant source of bone-forming progenitors in young adult mice.
LepR+ cells are a major source of bone in adult mice
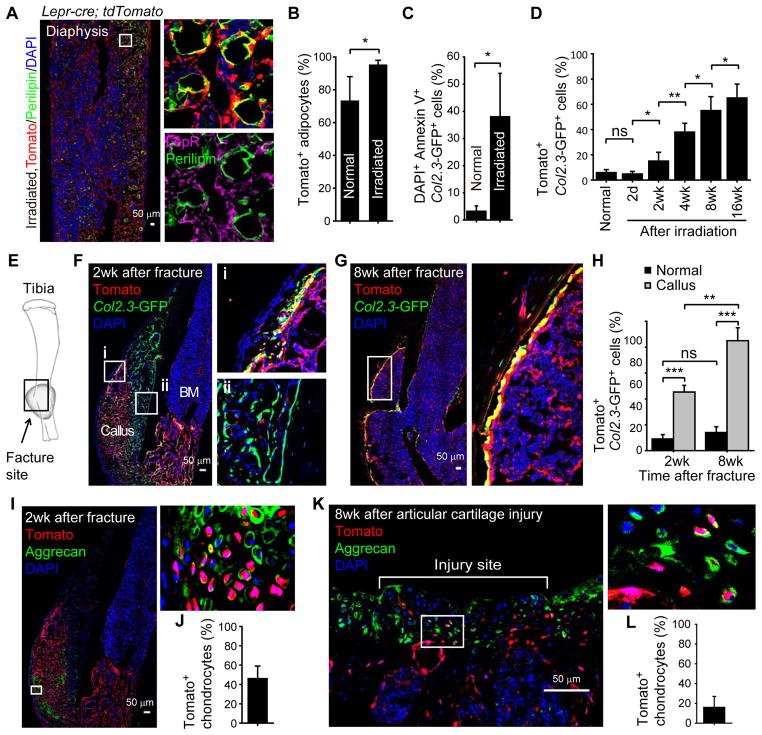
LepR+ cells arose perinatally in bone marrow and made little bone before two months of age. We fate mapped the LepR+ cells in vivo using Lepr-cre; tdTomato; Col2.3-GFP mice in which osteoblastic bone-lining cells can be unambiguously identified based on Col2.3-GFP expression (Kalajzic et al., 2002). At embryonic day (E) 19.5 Tomato+ cells were rare in the bone marrow of Lepr-cre; tdTomato; Col2.3-GFP mice and we observed no contribution of these cells to bone (Figure 2F). By postnatal day (P) 0.5, there was a sharp increase in the number of Tomato+ cells in metaphyseal bone marrow, though Tomato+ cells remained rare in the diaphyseal bone marrow and only rare Tomato+Col2.3-GFP+ osteoblasts were observed in trabecular bone (Figure 2G). By two months of age, Tomato+ cells were visible throughout the bone marrow in both metaphysis and diaphysis but Tomato+Col2.3-GFP+ cells remained infrequent (Figure 2H), accounting for 3–10% of Col2.3-GFP+ cells in several bones (Figure 2K). However, the contribution of LepR+ cells to bone increased sharply with age. Tomato+ cells accounted for 10–23% of Col2.3-GFP+ cells in several bones at 6 months of age, 43–67% of Col2.3-GFP+ cells at 10 months of age, and 61–81% of Col2.3-GFP+ cells at 14 months of age (Figure 2K and S2R). The increased frequency of Tomato+Col2.3-GFP+ cells was not caused by the induction of Lepr expression within Col2.3-GFP+ osteoblastic cells (Figure S2S and S2T).
By 10 months of age Tomato+ cells contributed not only to bone-lining osteoblastic cells but also to osteocytes within the bone matrix (Figure 2Iiii). The percentage of osteocytes that were Tomato+ increased significantly with age, but much more rapidly within trabecular bone (Figure 2M). Tomato+ cells represented 92±7% of osteocytes in trabecular bone and 13±5% of osteocytes in cortical bone at 14 months of age.
LepR+ cells are a major source of adipocytes in adult bone marrow
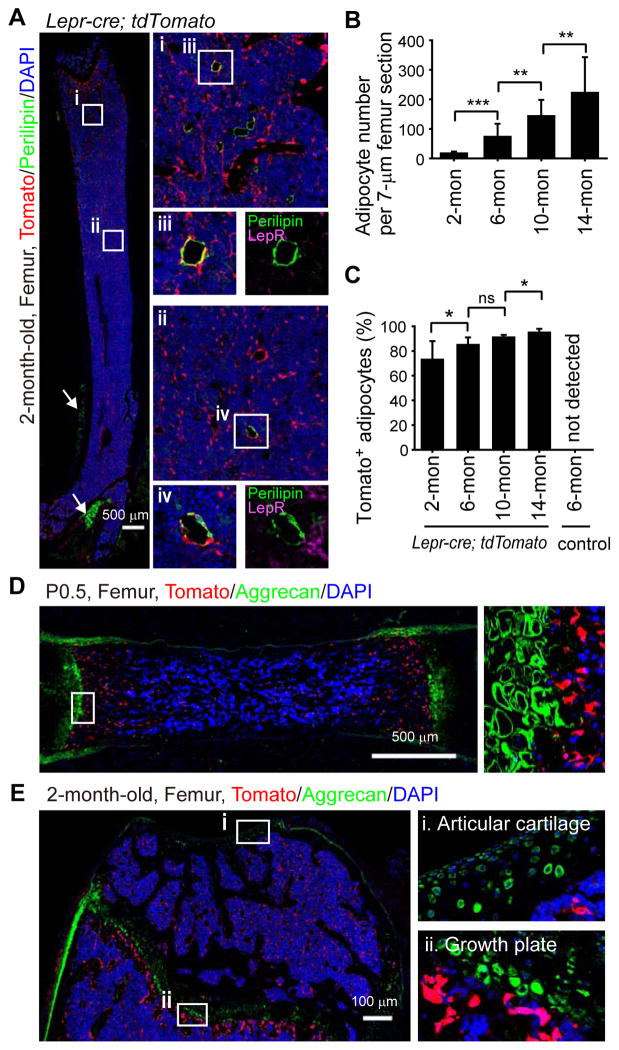
Although LepR antibody did not detectably stain Perilipin+ adipocytes (Figure 1J and 3A) most Perilipin+ adipocytes in the bone marrow of 2- to 14-month-old Lepr-cre; tdTomato mice were Tomato+ (Figure 3A–3C). The number of adipocytes in the bone marrow increased dramatically with age (Figure 3B). In contrast to those in the bone marrow, periosteal adipocytes (outside of the marrow cavity) were all negative for Tomato (Figure 3A), suggesting a distinct cellular origin. LepR+ cells are thus the major source of adipocytes in adult bone marrow.
Figure 3. LepR+ cells give rise to most bone marrow adipocytes but to few chondrocytes.
(A) Representative femur sections from a 2-month-old Lepr-cre; tdTomato mouse. Perilipin+ adipocytes in the bone marrow did not stain with an anti-LepR antibody (purple) but were Tomato+ (red). Periosteal adipocytes (arrows) were uniformly Tomato negative (representative of 4 mice from 4 independent experiments).
(B and C) Quantification of adipocyte number per 7 μm femur section (B) and the percentage of adipocytes that were Tomato+ at each age (C) in Lepr-cre; tdTomato mice. Two-tailed Student’s t-tests were used to assess statistical significance. ns, not significant, *P<0.05, **P<0.01, ***P<0.001. (n=3–5 mice/age from at least 3 independent experiments).
(D and E) Aggrecan+ chondrocytes were not Tomato+ in P0.5 (D) or 2-month-old (E) Lepr-cre; tdTomato mice (n=3–5 mice/age from at least 3 independent experiments).
Chondrogenesis is active during fetal development but largely inactive throughout adulthood (Raghunath et al., 2005). At P0.5 and at 2 months of age we detected no Tomato expression among Aggrecan+ chondrocytes in the femurs of Lepr-cre; tdTomato mice (Figure 3D) in spite of Tomato+ osteoblasts and perivascular stromal cells adjacent to the growth plate (Figure 3E). In 6-, 10-, and 14-month-old mice we remained unable to detect Tomato+ chondrocytes in articular cartilage or growth plate cartilage associated with femurs and tibias (Figure S5A and data not shown). We did observe an increasing contribution with age of Tomato+ cells to a layer of Aggrecan negative cells on the cartilage surface that appeared to be part of the synovial membrane (Figure S5Ai and S5B). We do not know whether Tomato+ synovial cells are lineally related to LepR+ bone marrow stromal cells. Overall, we observed little contribution of LepR+ cells to cartilage.
LepR+ bone marrow stromal cells are quiescent
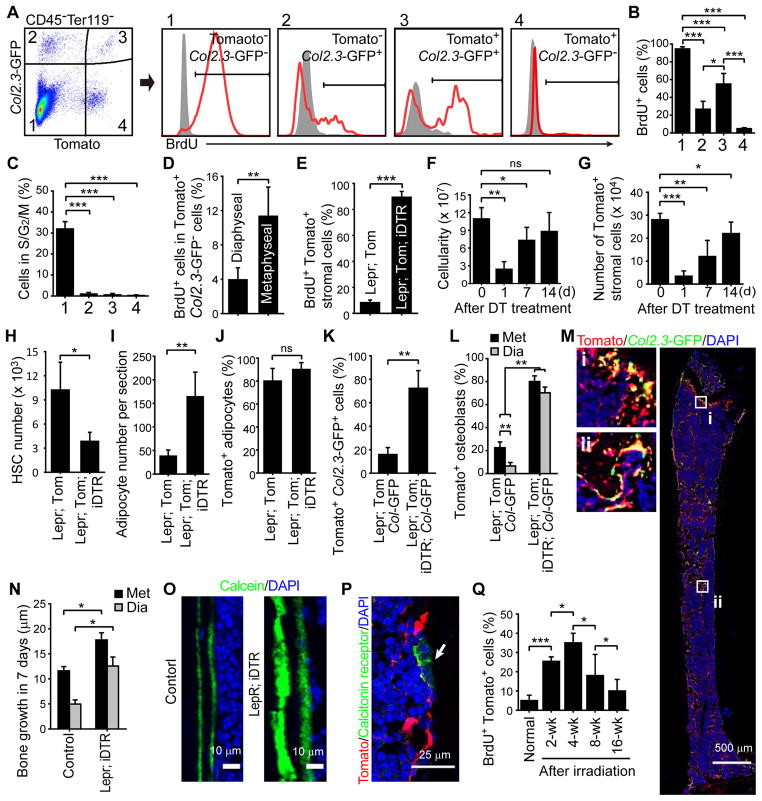
We administered bromo-deoxyuridine (BrdU) to 2-month-old Lepr-cre; tdTomato; Col2.3-GFP mice for 14 days. We found that 94±2.4% of Tomato−Col2.3-GFP− stromal cells were BrdU+, 27±8.9% of Tomato−Col2.3-GFP+ osteoblasts were BrdU+, and 55±12% of Tomato+Col2.3-GFP+ osteoblasts were BrdU+, but only 4.8±1.1% of Tomato+Col2.3-GFP− stromal cells were BrdU+ (Figure 4A and 4B). Consistent with this, only 0.23±0.3% of Tomato+Col2.3-GFP− stromal cells had greater than 2N DNA content by Hoechst staining (Figure 4C). LepR+ stromal cells are thus largely quiescent in normal adult bone marrow.
Figure 4. LepR+Col2.3-GFP− cells are quiescent under normal physiological conditions in adult bone marrow but go into cycle to regenerate bone after injury.
(A–C) BrdU incorporation (14 day pulse) (A and B) or Hoechst staining (C) by various stromal cell fractions from enzymatically dissociated bone and bone marrow obtained from 2-month-old Lepr-cre; tdTomato; Col2.3-GFP mice. Unless otherwise indicated, data in all remaining panels represent mean±SD from 3–4 mice in 3 independent experiments, with statistical significance assessed by two-tailed Student’s t-tests. ns, not significant, *P<0.05, **P<0.01, ***P<0.001.
(D) Percentage of Tomato+Col2.3-GFP−CD45−Ter119−CD31− bone marrow stromal cells that incorporated a 14 day pulse of BrdU.
(E) Percentage of Tomato+CD45−Ter119−CD31− bone marrow stromal cells that incorporated a 14 day pulse of BrdU in femurs and tibias from Lepr-cre; tdTomato mice and Lepr-cre; tdTomato; iDTR mice two weeks after diphtheria toxin (DT) treatment.
(F) Bone marrow cellularity in the femurs and tibias of Lepr-cre; tdTomato; iDTR mice at the indicated time points after DT treatment.
(G) Number of Tomato+CD45−Ter119−CD31− stromal cells in bone marrow from Lepr-cre; tdTomato; iDTR mice after DT treatment.
(H) Number of CD150+CD48−Lineage−Sca-1+c-kit+ HSCs in the femurs and tibias 7 days after DT treatment.
(I and J) Quantification of adipocyte number per 7 μm femur section (I) and the percentage of adipocytes that were Tomato+ (J) 14 days after DT treatment.
(K) Percentage of Col2.3-GFP+ osteoblasts that were also Tomato+ in enzymatically dissociated bone 2 weeks after DT treatment.
(L) Percentage of Col2.3-GFP+ osteoblasts that were also Tomato+ at metaphyseal (Met) and diaphyseal (Dia) bones 2 weeks after diphtheria toxin treatment.
(M) Representative femur sections from Lepr-cre; tdTomato; Col2.3-GFP; iDTR mice at 2 weeks after DT treatment. Note the formation of ectopic trabecular bone by Tomato+Col2.3-GFP+ cells in the diaphyseal bone marrow cavity (ii).
(N and O) Bone formation rate in Lepr-cre; iDTR and control mice after DT treatment. Two doses of calcein were injected at day 0 and 7 after DT treatment then the distance between calcein bands was measured at 14 days after DT treatment.
(P) Osteoclasts (arrow) were not labeled by Tomato in Lepr-cre; tdTomato mice.
(Q) Percentage of Tomato+CD45−Ter119−CD31− bone marrow stromal cells that incorporated a 14 day pulse of BrdU in Lepr-cre; tdTomato mice at various times after irradiation. (n=3–5 mice/time point from 3 independent experiments)
At 2 months of age the LepR+ cells gave rise to osteoblasts and adipocytes to a much greater extent in metaphyseal bone marrow than in diaphyseal bone marrow (Figure 2H, 2L and 3A). Tomato+Col2.3-GFP− cells from the metaphysis incorporated a 14-day pulse of BrdU at nearly three times the rate of Tomato+Col2.3-GFP− cells from the diaphysis (Figure 4D). Thus, there is regional regulation of LepR+ stromal cells in the bone marrow.
We ablated LepR+ bone marrow cells by administering diphtheria toxin to Lepr-cre; tdTomato; Rosa26-loxP-stop-loxP-iDTR (inducible diphtheria toxin receptor) mice and littermate controls. In contrast to DT-treated controls (lacking iDTR), in which only 8.2±2.0% of Tomato+Col2.3-GFP− bone marrow stromal cells incorporated a 14-day pulse of BrdU, 89±4.5% of Tomato+CD45−Ter119−CD31− cells from Lepr-cre; tdTomato; iDTR mice incorporated BrdU (Figure 4E). Bone marrow cellularity and the number of Tomato+ stromal cells in the bone marrow declined significantly one day after DT treatment but rebounded within two weeks (Figure 4F and 4G). Consistent with our demonstration that LepR+ stromal cells are critical for the maintenance of quiescent HSCs (Ding and Morrison, 2013; Ding et al., 2012; Oguro et al., 2013), DT treatment depleted CD150+CD48−LSK HSCs (Figure 4H).
Within 14 days of DT treatment adipogenesis (Figure 4J) and osteogenesis (Figure 4K) increased profoundly in the bone marrow. Trabecular bone began filling up the marrow cavity, including the diaphysis (Figure 4M). Virtually all of the bone marrow adipocytes as well as the new Col2.3-GFP+ osteoblasts were Tomato+ (Figure 4J–4L). The rate of bone formation, as measured by calcein labeling, was significantly higher in Lepr-cre; tdTomato; iDTR mice as compared to controls in both the metaphysis and the diaphysis (Figure 4N and 4O). LepR+ cells did not give rise to Calcitonin+ osteoclasts (Figure 4P). The regeneration of LepR+ cells after ablation is thus associated with increased adipogenesis and osteogenesis.
LepR+ cells are activated by irradiation to form osteoblasts and adipocytes
We found that 25±2.3% of the Tomato+ stromal cells incorporated BrdU over a 2 week period after irradiation of Lepr-cre; tdTomato mice (Figure 4Q). In the next 2 weeks after irradiation the percentage of Tomato+ stromal cells that incorporated BrdU significantly increased to 35±5.0% (Figure 4Q). At subsequent time points the percentage of Tomato+ stromal cells that incorporated BrdU significantly declined (Figure 4Q). LepR+ cells therefore divide transiently after irradiation. We observed a substantial increase in adipocyte frequency within the bone marrow after irradiation (Figure 5A) and 95±3.0% of the Perilipin+ adipocytes were Tomato+ (Figure 5B). The frequency of osteoblasts undergoing cell death significantly increased 2 days after irradiation (Figure 5C). By 16 weeks after irradiation, most (65±11%) osteoblasts derived from LepR+ cells (Figure 5D and Figure S6A). Irradiation did not activate LepR expression in osteoblasts (Figure S6B and S6C). We remained unable to detect any contribution of LepR+ cells to chondrocytes after irradiation (Figure S6D and data not shown).
Figure 5. LepR+ cells are the major source of new osteoblasts and adipocytes during tissue regeneration and can also form chondrocytes after subchondral perforation.
(A and B) Representative femur section from a 2-month-old Lepr-cre; tdTomato mouse 14 days after lethal irradiation and transplantation of wild-type bone marrow cells. Perilipin+ adipocytes in the bone marrow did not stain with an anti-LepR antibody (purple) but were Tomato+ (red) demonstrating they derived from endogenous radio-resistant LepR+ cells (mean±SD from 5 mice in 4 independent experiments).
(C) Percentage of Col2.3-GFP+ osteoblasts that were also DAPI+Annexin V+ in enzymatically dissociated bone 2 days after irradiation. Data in all remaining panels represent mean±SD from 3–4 mice (per time point) in 3 independent experiments.
(D) Percentage of Col2.3-GFP+ osteoblasts that were also Tomato+ in enzymatically dissociated bone from Lepr-cre; tdTomato; Col2.3-GFP mice at various time points after irradiation.
(E) Schematic of experimental fracture site. The black rectangle depicts the region shown in images G, H and J.
(F and G) Tomato expression by Col2.3-GFP+ osteoblasts at the fracture site in Lepr-cre; tdTomato; Col2.3-GFP mice at 2 weeks (F) or 8 weeks (G) after fracture.
(H) Percentage of Col2.3-GFP+ osteoblasts that were also Tomato+ in unfractured tibias from control mice (normal) as well as bone callus from Lepr-cre; tdTomato; Col2.3-GFP mice.
(I and J) Percentage of Aggrecan+ chondrocytes that were also Tomato+ at the fracture site 2 weeks after the fracture.
(K and L) Percentage of Aggrecan+ chondrocytes that were also Tomato+ 8 weeks after subchondral perforation of articular cartilage in Lepr-cre; tdTomato mice.
Statistical significance was always assessed using two-tailed Student’s t-tests. ns, not significant, *P<0.05, **P<0.01, ***P<0.001.
Regeneration of fractured bone by LepR+ cells
Two weeks after a break was created in the tibia of 2-month-old Lepr-cre; tdTomato; Col2.3-GFP mice (Figure 5E) a substantial increase of Tomato+ stromal cells was observed in the marrow cavity adjacent to the fracture site (Figure 5F). Col2.3-GFP+ cells close to periosteum were mostly negative for Tomato expression but much of the callus was composed of Tomato+ cells (Figure 5F). Tomato+ cells accounted for 46±13% of Aggrecan+ chondrocytes in the soft callus 2 weeks following the fracture (Figure 5I and 5J). At 2 and 8 weeks after the fracture, 45±5% and 85±10% of Col2.3-GFP+ osteoblasts were Tomato+ (Figure 5F–5H). In contrast, only 9–14% of Col2.3-GFP+ osteoblasts were Tomato+ in the undamaged tibia of the same mice (Figure 5H). We confirmed by qRT-PCR that Ob-Rb mRNA was not expressed by newly generated osteoblasts at the tibia fracture site (Figure S6E). Most of the osteoblasts and osteocytes that persisted long-term at the fracture site thus arose from LepR+ cells.
To investigate the contribution of LepR+ cells to the repair of perforated cartilage we performed a subchondral perforation in articular cartilage associated with the femur in the knee of Lepr-cre; tdTomato mice. Eight weeks following perforation, the injured region of the cartilage was covered by Aggrecan+ fibrocartilaginous tissues (Figure 5K) and 16±11% of the Aggrecan+ cells were positive for Tomato expression (Figure 5K and 5L). Thus, LepR+ cells form cartilage after injury even though they contribute little to the formation of cartilage during development.
LepR+ cells form bone, cartilage, and adipocytes after transplantation
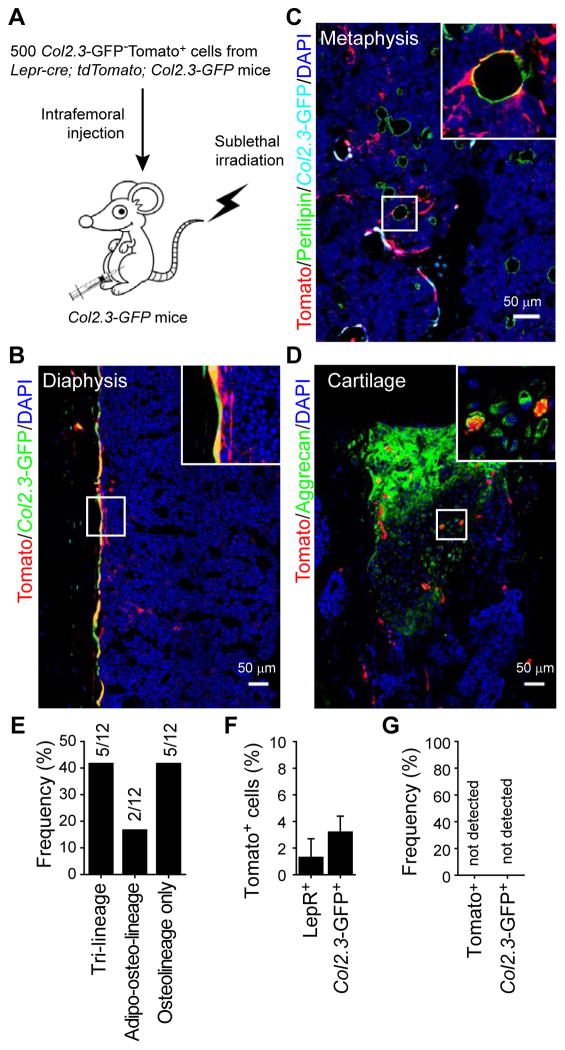
We transplanted 500 Tomato+Col2.3-GFP− cells from Lepr-cre; tdTomato; Col2.3-GFP mice into sublethally irradiated Col2.3-GFP mice by intrafemoral injection (Figure 6A). Four weeks later, Tomato expression was observed in Col2.3-GFP+ osteoblasts (Figure 6B), Perilipin+ adipocytes (Figure 6C), and Aggrecan+ chondrocytes in articular cartilage (which had been perforated by the injection; Figure 6D). Of 12 recipient femurs, five contained tri-lineage reconstitution by transplanted Tomato+ cells, two contained Tomato+ osteolineage cells and adipocytes but not cartilage, and five contained only Tomato+ osteolineage cells (Figure 6E). In an independent experiment in which engraftment was analyzed by flow cytometry, Tomato+ cells accounted for 1.3±1.4% of LepR+ cells and 3.2±1.2% of Col2.3-GFP+ cells in recipient femurs (Figure 6F). In contrast, transplantation of 105 Tomato−Col2.3-GFP−CD45−Ter119− stromal cells did not generate any Tomato+ or Col2.3-GFP+ cells (Figure 6G), suggesting that LepR− bone marrow cells have little capacity to form mesenchymal derivatives.
Figure 6. LepR+ cells give rise to osteoblasts, adipocytes, and chondrocytes after intrafemoral transplantation.
(A) Experimental design.
(B–D) Representative femur sections from Col2.3-GFP mice transplanted with 500 Tomato+Col2.3-GFP− cells as described in (A) (n=5). Note that the transplanted Tomato+Col2.3-GFP− cells gave rise to Col2.3-GFP+ osteoblasts (B), Perilipin+ adipocytes (C), and Aggrecan+ cartilage cells (at the injection site, D).
(E) Fraction of recipient mice in which Tomato+ cells were observed to contribute to each of the indicated mesenchymal lineages (n=12 mice).
(F) The percentage of LepR+ bone marrow stromal cells or Col2.3-GFP+ osteoblasts that were also Tomato+ (donor-derived) in the femurs of recipient mice (mean±SD from 3 mice in 3 independent experiments).
(G) No Tomato+LepR+ cells or Tomato+Col2.3-GFP+ cells were observed in the femurs of mice transplanted with 105 non-hematopoietic Col2.3-GFP−Tomato− cells from Lepr-cre; tdTomato; Col2.3-GFP mice (n=3).
PTEN regulates quiescence, maintenance, and differentiation of LepR+ cells
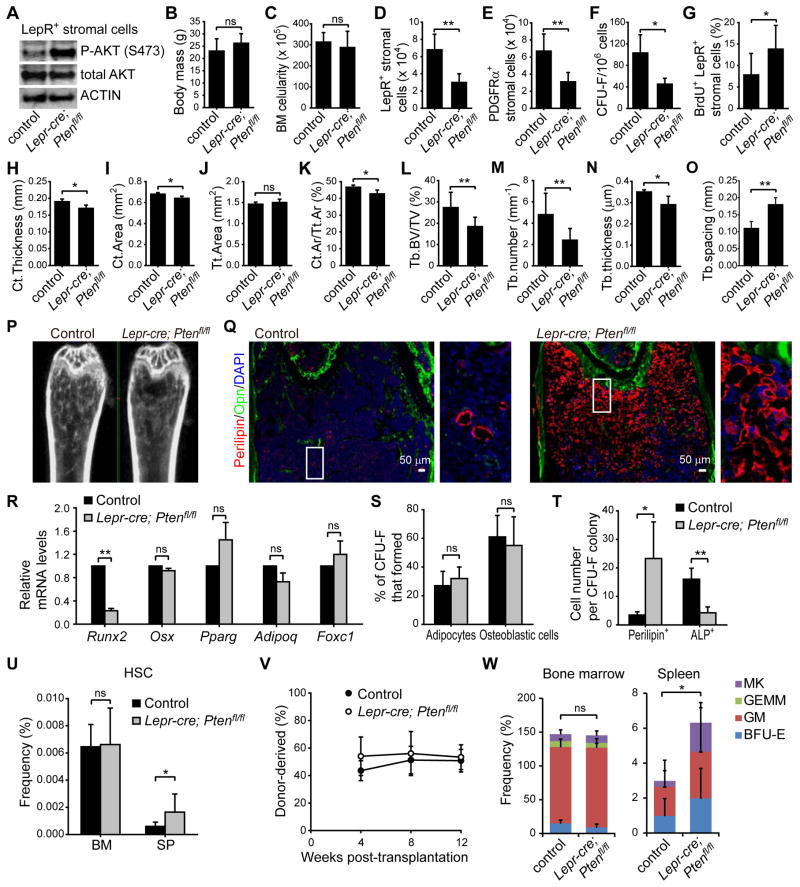
Pten is cell-autonomously required for the maintenance of HSCs (Kalaitzidis et al., 2012; Lee et al., 2010; Magee et al., 2012; Yilmaz et al., 2006; Zhang et al., 2006) and neural stem cells (Bonaguidi et al., 2011). To assess the consequences of Pten deletion from MSCs we generated Lepr-cre; Ptenfl/fl mice. As expected, AKT (S473) phosphorylation increased in LepR+ stromal cells isolated from Lepr-cre; Ptenfl/fl mice (Figure 7A). Body mass and bone marrow cellularity were normal in Lepr-cre; Ptenfl/fl mice (Figure 7B and 7C). However, we observed a more than 2-fold reduction in the frequencies of LepR+CD45−Ter119−CD31− stromal cells (Figure 7D), PDGFRα+CD45−Ter119−CD31− stromal cells (Figure 7E), and CFU-F (Figure 7F) in Lepr-cre; Ptenfl/fl mice relative to littermate controls. Based on BrdU incorporation, LepR+ cells divided more frequently in Lepr-cre; Ptenfl/fl mice relative to littermate controls (Figure 7G). Pten is thus cell-autonomously required to negatively regulate AKT activation and to maintain normal numbers of quiescent LepR+ MSCs in adult bone marrow.
Figure 7. PTEN regulates quiescence, maintenance, and differentiation in LepR+ stromal cells.
(A) Representative western-blots of flow cytometrically isolated LepR+CD45−Ter119−CD31− stromal cells from 4-month-old Lepr-cre; Ptenfl/fl mice and littermate controls.
(B) Body mass (Unless otherwise indicated, all remaining panels show mean±SD from 3–5 mice in 3–4 independent experiments, with statistical significance assessed by two-tailed Student’s t-tests, ns, not significant, *P<0.05, **P<0.01, ***P<0.001).
(C) Bone marrow cellularity in the femurs of 4-month-old Lepr-cre; Ptenfl/fl mice and littermate controls
(D) Number of LepR+CD45−Ter119−CD31− cells.
(E) Number of PDGFRα+CD45−Ter119−CD31− cells.
(F) CFU-F per million bone marrow cells.
(G) Percentage of LepR+CD45−Ter119−CD31− bone marrow stromal cells that incorporated a 14 day pulse of BrdU.
(H–O) MicroCT measurement of cortical (Ct) thickness (H), cortical area (I), total (Tt) area (J), cortical area/total area (Ct.Ar/Tt.Ar) (K), trabecular (Tb) bone volume/total volume (BV/TV) (L), trabecular number (M), trabecular thickness (N) and trabecular spacing (O) of femurs.
(P) Representative microCT images of femurs.
(Q) Representative femur sections showing excessive adipogenesis at metaphyseal bone marrow in Lepr-cre; Ptenfl/fl mice.
(R) qPCR analysis of transcript levels for genes associated with osteogenic or adipogenic differentiation in LepR+ stromal cells. Transcript levels were normalized based on β-actin amplification then set to 1 in control samples for comparison purposes.
(S) The percentage of CFU-F colonies that contained adipocytes (Oil red O+) and/or osteoblastic cells (Alzarin red S+) after culture in differentiation medium for 3 weeks.
(T) The average number of Perilipin+ adipocytes or Alkaline phosphatase+ (ALP+) osteogenic cells that spontaneously differentiated per CFU-F colony after culture for 1 week in standard medium.
(U) Number of CD150+CD48−Lineage−Sca-1+c-kit+ HSCs in the bone marrow and spleen.
(V) Donor-cell engraftment when 3×105 donor bone marrow cells were transplanted along with 3×105 recipient bone marrow cells into irradiated recipient mice (n=11–12 recipient mice per genotype in 3 experiments).
(W) Frequencies of myeloerythroid-colony-forming progenitors in the bone marrow and spleen.
Four month old Lepr-cre; Ptenfl/fl mice had a decreased volume of trabecular and cortical bone (Figure 7H–7P) but increased adipogenesis in the metaphysis relative to littermate controls (Figure 7Q). The expression of Runx2, a transcription factor required for osteogenesis (Komori et al., 1997; Otto et al., 1997), was markedly reduced in LepR+ stromal cells from Lepr-cre; Ptenfl/fl as compared to control mice (Figure 7R). Pten deletion did not affect the percentage of CFU-F colonies that contained adipocytes versus osteoblastic cells (Figure 7S) but significantly increased the number of Perilipin+ adipocytes and significantly reduced the number of alkaline phosphatase+ osteogenic cells that spontaneously differentiated within these colonies (Figure 7T). Pten is thus required by LepR+ cells to promote osteogenesis and to restrain adipogenesis.
We did not detect a decline in the frequency of HSCs (Figure 7U) or colony-forming hematopoietic progenitors (Figure 7W and Figure S7B–S7F) in the bone marrow of Lepr-cre; Ptenfl/fl mice. Bone marrow cells from Lepr-cre; Ptenfl/fl and control mice gave similar levels of donor cell reconstitution upon transplantation into irradiated mice (Figures 7V and S7A). Nonetheless, we did observe significant increases in the frequencies of HSCs (Figure 7U) and colony-forming hematopoietic progenitors (Figure 7W) in the spleen. These data suggest that Pten deletion from LepR+ bone marrow stromal cells changed the bone marrow niche in a way that led to the mobilization of HSCs and colony-forming progenitors.
DISCUSSION
Our data support the conclusion that MSCs are an important component of the HSC niche (Kunisaki et al., 2013; Mendez-Ferrer et al., 2010; Omatsu et al., 2010; Sacchetti et al., 2007) as LepR+ stromal cells are a major source of HSC niche factors in addition to MSC activity. Nearly all Scf-GFP+ bone marrow stromal cells and Cxcl12-DsRedhigh bone marrow stromal cells were LepR+ (Figures 1C, 1E, S1E and S1F). The high expression of Cxcl12 (Figure S1E) and PDGFRα (Figure 1L and 1M) by LepR+ stromal cells indicates that these cells overlap strongly with CAR cells, which are also found primarily around sinusoids throughout the bone marrow (Omatsu et al., 2010; Sugiyama et al., 2006). However, our data indicate that NG2-CreER-expressing cells are not a significant source of MSCs in the bone marrow. NG2-CreER-expressing cells were much more rare than Scf-GFP+ cells or Cxcl12-DsRed+ cells (Figure S1E, S1F and S3A–S3C) and we observed little PDGFRα or Scf-GFP expression by these cells (Figure S3A–S3D). While Kunisaki et al. (2013) concluded that NG2+NestinhighLepR− periarteriolar cells express high levels of Scf and Cxcl12, their RNAseq analysis showed that the “NestinhighLepR−” cells they analyzed were negative for Nestin and positive for Lepr expression (see GSE48764 in the Gene Expression Omnibus, referenced by Kunisaki et al., 2013). Thus, the data from Kunisaki et al. are consistent with our data indicating that cells with high levels of Scf and Cxcl12 in the bone marrow are marked by LepR.
EXPERIMENTAL PROCEDURES
Mice
All mice were maintained in a C57BL/6 background, including Lepr-cre (DeFalco et al., 2001), Leprfl/fl (Cohen et al., 2001), Ptenfl/fl (Groszer et al., 2001), Scf-GFP (Ding et al., 2012), Cxcl12-DsRed (Ding and Morrison, 2013), Ubc-creER (Ruzankina et al., 2007), Rosa26-CAG-loxp-stop-loxp-tdTomato (Madisen et al., 2010), Rosa26-loxp-stop-loxp-EYFP (Srinivas et al., 2001), Rosa26-loxp-stop-loxp-iDTR (Buch et al., 2005), NG2-creERTM (Zhu et al., 2011), Wnt-1-cre (Danielian and McMahon, 1996), Nestin-GFP (Mignone et al., 2004), Nestin-creER (Balordi and Fishell, 2007), Mx-1-cre (Kuhn et al., 1995), and Col2.3-GFP (Kalajzic et al., 2002) mice. To induce CreER activity, male mice (>2-month-old) were injected with 1 mg tamoxifen (Sigma) daily for 5 consecutive days followed by feeding ab libidum with chow containing 400 mg/kg tamoxifen for at least 2 weeks. To induce Mx-1-Cre expression, 2-month-old mice were injected with 10 μg poly-inosine:poly-cytosine (pIpC; Amersham)/20 g body mass every other day for 10 days. To treat mice with diphtheria toxin (DT), mice were intraperitoneally injected with 100 ng of DT for 7 consecutive days. All mice were housed in the Animal Resource Center at the University of Texas Southwestern Medical Center (UTSW). All procedures were approved by the UTSW Institutional Animal Care and Use Committee.
For methods related to flow cytometry, bone sectioning, qPCR, culture assay conditions, ossicle formation, irradiation, cell cycle analysis, calcein labeling, micro CT, western analysis, bone fracturing, intrafemoral injection, and subchondral perforation see Supplementary Methods.
Supplementary Material
Highlights.
LepR+ cells account for 0.3% of cells and 94% of CFU-F in adult bone marrow
LepR+ cells form osteoblasts, chondrocytes, and adipocytes in culture and in vivo
LepR+ cells give rise to most of the bone and adipocytes formed in adult marrow
LepR+ cells are normally quiescent but proliferate after injury to regenerate bone
Acknowledgments
S.J.M. is a Howard Hughes Medical Institute Investigator, the Mary McDermott Cook Chair in Pediatric Genetics, and the director of the Hamon Laboratory for Stem Cells and Cancer. This work was supported by the National Heart, Lung and Blood Institute (HL097760) and the Cancer Prevention and Research Institute of Texas. B.O.Z. was supported by a fellowship from the Leukemia and Lymphoma Society. R.Y. was supported by a fellowship from the Damon Runyon Foundation. J.P. was supported by a fellowship from the National Science Foundation. We thank Dr. Hung Luu in Children’s Medical Center for his expert analysis of hematopoiesis in ossicles and Dr. Aktar Ali in the Mouse Metabolic Phenotyping Core at UT Southwestern for microCT analysis of bone. B.O.Z. performed most of the experiments. R.Y. analyzed the hematopoietic phenotype of Lepr-cre; Ptenfl/fl mice. M.M.M. obtained most of the three dimensional images of bone marrow. J.P. analyzed the bone formation rate of Lepr-cre; iDTR mice. B.O.Z. and S.J.M. designed and interpreted all experiments and wrote the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci. 2007;27:5936–5947. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K, Helms JA, Kuo CJ, Kraft DL, Weissman IL. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou DB, Sworder B, Bouladoux N, Roy CN, Uchida AM, Grigg M, Robey PG, Belkaid Y. Stromal-derived IL-6 alters the balance of myeloerythroid progenitors during Toxoplasma gondii infection. J Leukoc Biol. 2012;92:123–131. doi: 10.1189/jlb.1011527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Zhao C, Cai X, Montez JM, Rohani SC, Feinstein P, Mombaerts P, Friedman JM. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. doi: 10.1172/JCI13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Danielian PS, McMahon AP. Engrailed-1 as a target of the Wnt-1 signalling pathway in vertebrate midbrain development. Nature. 1996;383:332–334. doi: 10.1038/383332a0. [DOI] [PubMed] [Google Scholar]
- DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echelard Y, Vassileva G, McMahon AP. Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development. 1994;120:2213–2224. doi: 10.1242/dev.120.8.2213. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Fukushi J, Inatani M, Yamaguchi Y, Stallcup WB. Expression of NG2 proteoglycan during endochondral and intramembranous ossification. Dev Dyn. 2003;228:143–148. doi: 10.1002/dvdy.10359. [DOI] [PubMed] [Google Scholar]
- Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- Joseph NM, Mukouyama YS, Mosher JT, Jaegle M, Crone SA, Dormand EL, Lee KF, Meijer D, Anderson DJ, Morrison SJ. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development. 2004;131:5599–5612. doi: 10.1242/dev.01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaitzidis D, Sykes SM, Wang Z, Punt N, Tang Y, Ragu C, Sinha AU, Lane SW, Souza AL, Clish CB, et al. mTOR complex 1 plays critical roles in hematopoiesis and Pten-loss-evoked leukemogenesis. Cell Stem Cell. 2012;11:429–439. doi: 10.1016/j.stem.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalajzic Z, Liu P, Kalajzic I, Du Z, Braut A, Mina M, Canalis E, Rowe DW. Directing the expression of a green fluorescent protein transgene in differentiated osteoblasts: comparison between rat type I collagen and rat osteocalcin promoters. Bone. 2002;31:654–660. doi: 10.1016/s8756-3282(02)00912-2. [DOI] [PubMed] [Google Scholar]
- Komada Y, Yamane T, Kadota D, Isono K, Takakura N, Hayashi S, Yamazaki H. Origins and properties of dental, thymic, and bone marrow mesenchymal cells and their stem cells. PLoS One. 2012;7:e46436. doi: 10.1371/journal.pone.0046436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Nakada D, Yilmaz OH, Tothova Z, Joseph NM, Lim MS, Gilliland DG, Morrison SJ. mTOR activation induces tumor suppressors that inhibit leukemogenesis and deplete hematopoietic stem cells after Pten deletion. Cell Stem Cell. 2010;7:593–605. doi: 10.1016/j.stem.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Strecker S, Wang L, Kronenberg MS, Wang W, Rowe DW, Maye P. Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One. 2013;8:e71318. doi: 10.1371/journal.pone.0071318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JA, Ikenoue T, Nakada D, Lee JY, Guan KL, Morrison SJ. Temporal changes in PTEN and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell. 2012;11:415–428. doi: 10.1016/j.stem.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murfee WL, Skalak TC, Peirce SM. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation. 2005;12:151–160. doi: 10.1080/10739680590904955. [DOI] [PubMed] [Google Scholar]
- Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13:102–116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, Lin CP, Kronenberg HM, Scadden DT. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10:259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y, Frenette PS. PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210:1351–1367. doi: 10.1084/jem.20122252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Raghunath J, Salacinski HJ, Sales KM, Butler PE, Seifalian AM. Advancing cartilage tissue engineering: the application of stem cell technology. Curr Opin Biotechnol. 2005;16:503–509. doi: 10.1016/j.copbio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Schneider S, Bosse F, D’Urso D, Muller H, Sereda MW, Nave K, Niehaus A, Kempf T, Schnolzer M, Trotter J. The AN2 protein is a novel marker for the Schwann cell lineage expressed by immature and nonmyelinating Schwann cells. J Neurosci. 2001;21:920–933. doi: 10.1523/JNEUROSCI.21-03-00920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–2911. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138:745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.