Abstract
Objectives
Cotrimoxazole prophylactic treatment (CPT) prevents opportunistic infections in HIV-infected or HIV-exposed children, but estimates of the effectiveness in preventing malaria vary. We reviewed studies that examined the effect of CPT on malaria incidence in children in sub-Saharan Africa.
Methods
We searched PubMed and EMBASE for randomized controlled trials (RCTs) and cohort studies of the effect of CPT on malaria incidence and mortality in children, and extracted data on the prevalence of sulfadoxine-pyrimethamine resistance–conferring point mutations. Incidence rate ratios (IRR) from individual studies were combined using random-effects meta-analysis; confounder-adjusted estimates were used for cohort studies. The importance of resistance was examined in meta-regression analyses.
Results
Three RCTs and four cohort studies with 5,039 children (1,692 HIV-exposed; 2,800 HIV-uninfected; 1,486 HIV-infected) were included. Children on CPT were less likely to develop clinical malaria episodes than those without prophylaxis (combined IRR 0.37, 95% confidence interval: 0.21–0.66) but there was substantial between-study heterogeneity (l-squared=94%, p < 0.001). The protective efficacy of CPT was highest in an RCT from Mali, where the prevalence of antifolate resistant plasmodia was low. In meta-regression analyses there was some evidence that the efficacy of CPT declined with increasing levels of resistance. Mortality was reduced with CPT in an RCT from Zambia, but not in a cohort study from Côte d'Ivoire.
Conclusions
CPT reduces malaria incidence and mortality in children in sub-Saharan Africa, but study designs, settings and results were heterogeneous. CPT appears to be beneficial for HIV-infected and HIV-exposed as well as HIV-uninfected children.
Keywords: Cotrimoxazole prophylaxis, Malaria, children, sub-Saharan Africa
INTRODUCTION
According to the latest WHO estimates, there were about 219 million cases of malaria in 2010 and an estimated 660,000 deaths, with the great majority of malaria deaths occurring in sub-Saharan Africa in children under five years of age (WHO 2012b). HIV-infected children with malaria have a higher parasite density than HIV-uninfected ones and HIV-1 infection is associated with severe and fatal malaria, suggesting that HIV might impede the age-related acquisition of malaria specific immunity (Berkley 2009). Independently of parasite density, both exposure to HIV-1 in children born to HIV-infected mothers and HIV infection are associated with severe malarial anemia during acute P. falciparum infection (Otieno 2006).
Cotrimoxazole (CTX) is an antimicrobial drug containing a fixed dose combination of sulfamethoxazole and trimethoprim. The combination of these drugs produces a synergistic effect against a variety of bacterial and protozoal infections (Wormser et al. 1982), as well as fungal infections such as Pneumocystis jirovecii pneumonia (PCP). Cotrimoxazole prophylactic treatment (CPT) is recommended for children infected with HIV and should be continued until immune recovery is observed on antiretroviral therapy (ART) (WHO 2006). For HIV-exposed uninfected (HEU) children (children born to mothers living with HIV) CPT is recommended from the age of six weeks until they stop breastfeeding and HIV infection is ruled out (WHO 2006).
Randomized clinical trials (RCTs), observational studies and economic analyses have shown that CPT is cost-effective in reducing morbidity and mortality among infants and children living with or exposed to HIV (Chintu et al. 2004, Ryan et al. 2008). Estimates of the effectiveness of CPT for preventing malaria, however, vary widely. For example, the incidence of malaria was reduced by 99% in a clinical trial in Mali (Thera et al. 2005) but only by 39% in another clinical trial in Uganda (Sandison et al. 2011). Furthermore, the uptake of CPT by national programs has been slow and CPT continues to be underused (Date et al. 2010, Hutchinson et al. 2011). A major concern with CPT is that its widespread use in high malaria transmission areas may favour cross resistance to sulphadoxine-pyrimethamine (SP), a drug used for intermittent preventive therapy (IPT) in pregnant women, for seasonal malaria chemoprophylaxis in children (as SP-AQ) and for intermittent preventive therapy in children (IPTi) (Sridaran et al. 2010, WHO 2012a, WHO 2011). However, it remains to be determined if the presence of antifolate resistant mutants affects the protective efficacy of CPT against malaria.
We conducted a systematic review of the literature and a meta-analysis to explore the effect of CPT on malaria incidence and mortality in HIV positive and HEU children in different settings in sub-Saharan Africa.
METHODS
A protocol for this systematic review was written and registered with the International prospective register of systematic reviews (PROSPERO) (Booth et al. 2013). The reporting of the review followed the PRISMA guidelines (Liberati et al. 2009). The PRISMA checklist is given in Appendix 1.
Search strategy
We searched PubMed and EMBASE on May 29, 2013 for RCTs and prospective cohort studies assessing the effect of CPT on the incidence of malaria and mortality in children aged 0–15 years in sub-Saharan Africa. In PubMed we combined free text words and medical subject headings (MESH) describing the age group, the intervention and the outcome. The detailed PubMed search, which was developed in collaboration with an expert librarian, is given in Appendix 2. The PubMed search was adapted for EMBASE. Additionally, we searched relevant articles using the reference lists of all identified publications.
Study Selection
We included all RCTs and cohort studies published between January 1990 and June 2013 that compared the incidence of malaria and all-cause mortality in children receiving and not receiving CPT. No language restrictions were applied. We excluded studies in adult populations and studies from regions other than sub-Saharan Africa. Two reviewers (N.M.) and (G.W.) independently selected studies first based on titles and abstracts, and, in a second step, based on the full text of potentially eligible articles.
Data Extraction
Two independent reviewers (N.M.) and (G.W.) extracted data on the study design and setting, the characteristics of study populations, malaria incidence and mortality in children receiving CPT and control groups. We also extracted data on the prevalence of sulfadoxine-pyrimethamine resistance–conferring point mutations in the dihydrofolate reductase (DHFR) and dihydropteroate synthase (DHPS) genes. Discrepancies were resolved through discussions with a third reviewer (M.E.).
Assessment of risk of bias
For both RCTs and cohort studies, N.M. and G.W. independently examined each included study for risk of bias using a standard form. The form for RCTs included information on the sequence generation, allocation concealment, blinding (participants, personnel and outcome assessor), incomplete outcome data, selective outcome reporting and other sources of bias. The methodological components of the trials were assessed and classified as high, low or unclear risk of bias as recommended by the Cochrane collaboration (Higgins et al. 2011). Where differences arose, these were resolved by discussions with the third reviewer (M.E). For cohort studies we developed our own checklist, which covered the selection of comparison groups, confounding, the assessment of exposures and outcomes, the completeness of follow-up and the reporting of outcomes. The checklist is reproduced in Appendix 3.
Malaria transmission
Malaria transmission intensity data was obtained by matching, by latitude and longitude, the published Malaria Atlas Project estimates for 2007 and 2010 of the P. falciparum parasite prevalence in children aged 2–10 years (PfPR2–10) (Hay et al. 2009, Gething et al. 2011) to the observational studies or trials. The PfPR2–10 data from 2007 were used for studies conducted between 2000 and 2008, and the 2010 estimates were used for the more recent studies.
Statistical Analysis
The primary outcome of interest was incidence of malaria and the secondary outcome all-cause mortality. We combined incidence rate ratios (IRR) for malaria and hazard ratios (HR) for mortality using inverse-variance random-effects meta-analysis. The RCT by Thera et al. (2005) recorded only one episode of clinical malaria in the CPT arm. We calculated exact confidence intervals for the IRR and obtained an estimate of the standard error of the IRR by dividing the difference of the log rate ratio and the upper confidence interval by 1.96. For cohort studies, we used results from analyses maximally adjusted for potential confounders. We used random-effects meta-regression to assess the association between antifolate resistance and the effectiveness of CPT on malaria incidence. The markers used for resistance were the dhps A437G and the K540E mutations. As dhps mutations have started to occur more recently and show a more geographically heterogeneous distribution than the major dhfr mutations (S108N, N51I and C59R), they were expected to have a stronger spatio-temporal correlation with SP efficacy. The dhps A437G mutation was used because our analysis included a study from West Africa, where the dhps K540E mutation is largely absent. For the two studies that did not report any resistance data, we used modeled data provided by the World Wide Antimalarial Resistance Network. For details of the data used in the model see WWARN's Molecular Surveyor (http://www.wwarn.org/surveyor/) and http://www.drugresistancemaps.org (Flegg et al. 2013, Naidoo and Roper 2013). We also used meta-regression to examine whether the effectiveness of CPT depended on the study design (RCT or cohort study), the duration of the study, whether the children on CPT group were HIV-infected, or on the annualized parasite prevalence. All statistical analyses were performed using Stata software version 12.1 (College Station, TX).
RESULTS
Study and participant characteristics
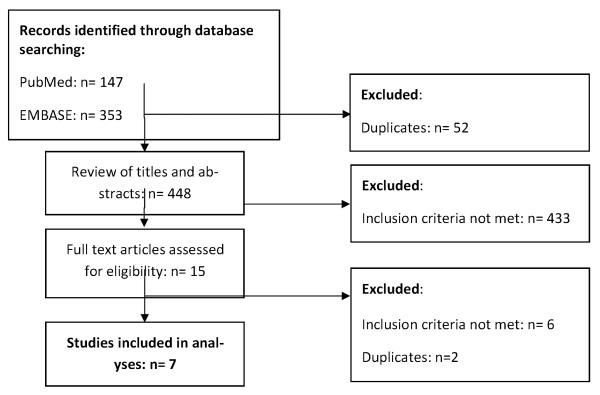
The searches of PubMed and EMBASE identified 500 publications. Screening of titles and abstracts resulted in 15 potentially eligible studies. Nine studies, including three RCTs and six observational studies met our inclusion criteria (Figure 1) (Sandison et al. 2011, Thera et al. 2005, Chintu et al. 2004, Gasasira et al. 2010, Dow et al. 2012, Ezeamama et al. 2012, Desmonde et al. 2011, Kamya et al. 2007, Arinaitwe et al. 2012). Two reports (Kamya et al. 2007, Arinaitwe et al. 2012) were excluded because they were based on study populations already included in the analysis (Gasasira et al. 2010, Sandison et al. 2011). In total 1,692 HIV-exposed, 2,800 HIV-uninfected and 1,486 HIV-infected children aged between six weeks (Dow et al. 2012) and 15 years (Thera et al. 2005) were included in the meta-analyses. The studies assessing malaria incidence originated from four countries in sub-Saharan Africa: Uganda, Mali, Malawi, Tanzania. Those evaluating mortality were from Zambia and Côte d'Ivoire. The number of children in each study ranged from 170 to 2,298 and the duration of follow-up ranged from 3 to 28 months. Transmission intensity at the time of the study was highest in the rural study site in Mali (Pfpr: 0.49) and in urban Côote d'Ivoire (0.68) and lowest in the urban sites in Tanzania and Zambia (0.07) (Table 1).
Figure 1.
Flow Chart of identification of eligible studies
Table 1.
Characteristics of studies and children included in the systematic review and meta-analysis of cotrimoxazole prophylactic treatment (CPT) and malaria incidence or mortality.
|
Author (year) |
Country (Setting) |
Pfpr |
Months of fup |
Design | CPT regimen |
Children in CPT group
|
Children in control group
|
Adherence monitoring |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age (years) |
HIV status | No. | Age (years) |
HIV sta- tus |
|||||||
| Malaria incidence | ||||||||||||
| Thera (2005) | Mali (rural) | 0.49 | 3 | RCT | TMP 150 mg/m2 SMX 750 mg/m2 Thrice-weekly | 160 | 10 (2.9) | Healthy children | 80 | 10 (2.8) | Healthy children | No |
| Gasasira (2010) | Uganda (urban) | 0.20 | 28 | Cohort | Once-daily | 292 | 6.0 (2.6) | HIV- infected | 517 | 7.4 (2.7) | Healthy children | Yes (self-reported) |
| Sandison (2011) | Uganda (rural) | 0.38 | 24 | RCT | TMP 40–80 mg/kg SMX 200–400 mg/kg Once-daily | 90 | 9.6 (8.3–12.4) | HIV- exposed | 80 | 10.0 (8.9–13.5) | HIV-exposed | Yes (self-reported) |
| Dow (2012) | Malawi (urban) | 0.34 | 6 | Cohort | TMP 40mg/kg SMX 200mg/kg | 1239 | NR | HIV-exposed | 283 | NR | HIV-exposed | No |
| Ezeamama (2012) | Tanzania (urban) | 0.07 | 27 | Cohort | NR | * | NR | HIV-infected and HIV-exposed | * | NR | HIV-infected and HIV-exposed | Yes (self-reported) |
| Mortality | ||||||||||||
| Chintu (2004) | Zambia (urban) | 0.07 | 18.9 | RCT | TMP 40–80 mg SMX 200–400 mg Once-daily | 265 | 4.2 (2.8–8.3) | HIV- infected | 269 | 4.5 (2.1–8.2) | HIV-infected | No |
| Desmonde (2011) | Cote d'Ivoire (urban) | 0.68 | 12 | Cohort | NR | 271 | NR | HIV- infected | 134 | NR | HIV-infected | No |
NR, not reported; RCT, randomized controlled trial, Pfpr: annualized parasite prevalence in children 2–10 years of age in X-survey
The study by Ezeamama et al included 255 HIV-infected and 2043 HIV-exposed uninfected children. Cotrimoxazole prophylaxis was analyzed as a time-varying covariate, based on whether or not the mother reported giving cotrimoxazole to her child over the past month.
Risk of bias
All three RCTs reported adequate generation of random allocation sequences, whereas only Chintu et al. (2004) and Sandison et al. (2011) reported allocation concealment. Chintu et al. (2004) used double-blinding whereas the two others were open-label studies. The three trials adequately addressed incomplete outcome data: proportions of drop-outs were low and outcome data was missing for less than 3% of children. The meta-analysis included only five studies which precluded a meaningful graphical or statistical examination of publication or other reporting biases.
In each of the four observational studies included in this review, the CPT and non-CPT groups were selected from the same population and followed up for the same calendar period. The only exception was the study by Dow et al. (2012), which compared the incidence of malaria between HIV-exposed children before and after 2006, the year CPT guidelines were introduced in Malawi. All studies adjusted for potential confounders at the analysis stage; however, the variables included in the multivariable models differed across studies (Appendix 4). Finally, patient retention was over 80% in all four cohort studies.
Malaria incidence
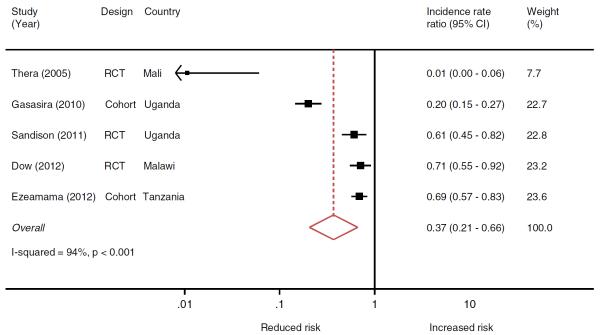
Five studies including 2,036 children on CPT and 3,003 not on CPT evaluated the association between CPT use and malaria incidence. Children on CPT were less likely to develop malaria episodes than those without prophylaxis (IRR 0.37, 95% confidence interval (CI): 0.21–0.66) but there was substantial between-study heterogeneity (I-squared= 94%, p < 0–001) (Figure 2). The RCT by Thera et al. (2005) received little weight in the meta-analysis (8%). Its exclusion from the analysis decreased the efficacy of CPT (IRR 0.50; 95% CI: 0.30,0.84) but between study heterogeneity remained similar (I-squared=94%, p<0.001). The four remaining studies included in this analysis all received weights between 23% and 24%.
Figure 2.
Meta-analysis of randomized clinical trials (RCTs) and cohort studies of the effect of cotrimoxazole prophylactic treatment on the incidence of malaria in children.
Prevalence of resistance mutations
Three studies analyzing the association between CPT and malaria incidence also reported on the prevalence of antifolate resistance mutations (Table 2) (Thera et al. 2005, Sandison et al. 2011, Gasasira et al. 2010). Gasasira et al. and Sandison et al. compared the presence of resistance mutations in children receiving and those not receiving CPT in moderate and high transmission areas in Uganda (Sandison et al. 2011, Gasasira et al. 2010). The prevalence of the dhfr/dhps quintuple mutant, composed of the dhfr 51I, 59R, and 108N and dhps 437G and 540E mutations, was between 86% and 95% and there was no significant difference between comparison groups. Thera et al. reported on antifolate drug resistance in a moderate transmission rural area in Mali. In their study, 14% of the children had the triple dhfr mutant at baseline. Double dhps mutants were not found (Thera et al. 2005).
Table 2.
Prevalence of antifolate resistance mutations in studies on the effect of cotrimoxazole prophylactic treatment (CPT) on malaria incidence.
| Study | Thera et al. | Gasasira et al. | Sandison et al. | Dow et al.* | Ezeamama et al.* |
|---|---|---|---|---|---|
| Country | Mali | Uganda | Uganda | Malawi | Tanzania |
| Study period | 2000 | 2005–06 | 2007–08 | 2004–09 | 2004–08 |
| DHPS gene mutations | |||||
| Gly-437 (437G) | 38% | 96% | 100% | 96% | 77% |
| Glu-540 (540E) | 0% | 97% | 100% | 98% | 71% |
Estimates for Dow et al. and Ezeamama et al. were obtained from modeled data provided by the World Wide Antimalarial Resistance Network. For details of the data used in the model see WWARN's Molecular Surveyor (http://www.wwarn.org/surveyor/) and http://www.drugresistancemaps.org.
DHPS: dihydropteroate synthase
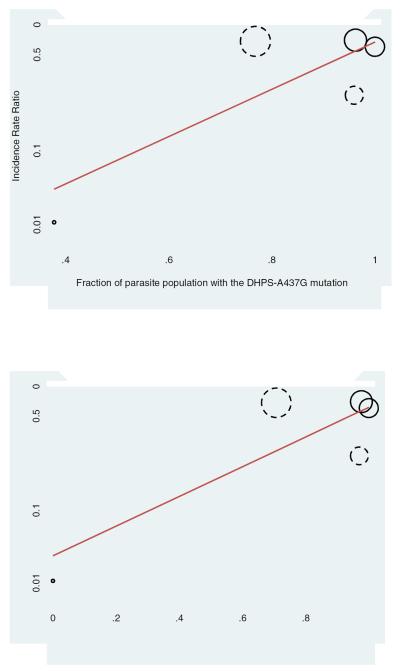
There was some evidence that the effectiveness of CPT decreased with increasing prevalence of the dhps A437G and dhps K540E resistance mutations (Figure 3), but this did not reach conventional levels of statistical significance (p=0.11 and p=0.09, respectively). There was little evidence that the reduction in malaria incidence was related to the study design (p=0.42), the duration of the study (p=0.42), whether the children on CPT were HIV-infected (p=0.75), or on the annualized parasite prevalence (p=0.35).
Figure 3.
Results from meta-regression on the impact of the antifolate resistance mutations dhps A437G (top panel) and dhps K540E (lower panel) on the efficacy of CPT in preventing malaria in children. Solid circles denote randomized controlled trials, dashed circles cohort studies. The size of the circle is proportional to the inverse of the variance of the incidence rate ratio. The regression line is shown in red.
Mortality
One RCT from Zambia (Chintu et al. 2004) and one observational study from Cote d'Ivoire (Desmonde et al. 2011) reported on the association between CPT and all-cause mortality. 536 participants on CPT and 403 not on CPT were followed for 12 – 19 months. CPT reduced mortality by 43% (HR: 0.57, 95% CI: 0.43–0.77) in the RCT. The crude estimate from the observational study was similar (0.53, 0.23–1.25), however the protective effect of CPT disappeared after adjustment for age, sex and immunodeficiency (1.13, 0.37–3.43).
DISCUSSION
Our systematic review and meta-analysis of the impact of CPT on the incidence of malaria included children from four countries in sub-Saharan Africa. We found that CPT reduced malaria incidence in children by 63%. The protective efficacy of CPT seemed to depend on the population prevalence of resistance mutations in the parasite genes Pfdhfr and Pfdhps but remained important in areas with very high levels of antifolate resistance. The evidence on the role of CPT in reducing mortality among children relied on two studies: an RCT from Zambia (Chintu et al. 2004) showing a statistically significant reduction of mortality by 43% with CPT and an observational study from Côte d'Ivoire (Desmonde et al. 2011) reporting no difference between children receiving and not receiving CPT.
Although the studies included in the meta-analysis of CPT and malaria were different in their design, study population and geographical setting, all showed a protective effect of CPT on the incidence of malaria. The reduction in malaria incidence was less (50% reduction) when the outlier (Thera et al. 2005) was excluded from the analysis, but the association between CPT and malaria incidence remained statistically significant. The study by Thera et al. (Thera et al. 2005) was the only one in HIV-negative children, in whom the protective efficacy of CTX might be different than in HIV-infected children. Two cohort studies compared HIV-infected children on CPT with HIV-exposed or healthy children not on CPT (Gasasira et al. 2010, Ezeamama et al. 2012). HIV infection increases the risk of malaria and the protective effect of CPT might therefore have been underestimated in these studies. Interestingly, the impact of CPT on malaria incidence seemed to depend on the geographical area where the study was performed, possibly in relation with the prevalence of resistance mutations in Pfdhfr and Pfdhps genes. The meta-regression showed that there was a trend towards a higher protective efficacy of CPT on malaria in regions where the prevalence of the resistance mutation dhps A437G was lowest, but this was essentially driven by one study (Thera et al. 2005). Other factors including geographical differences in transmission intensity may also have played a role in generating this association. Our results underline the paucity of data from regions other than East Africa and the lack of large studies in HIV-uninfected children. Of note, a study from Uganda showed that CPT was not associated with a higher prevalence of resistance mutations in HIV-infected children and adults with P. falciparum parasitemia (Malamba et al. 2010).
Our study confirms the substantial impact of CPT on malaria incidence and mortality in African children. WHO has recommended CPT for all HIV-infected children for many years. However, despite the low price and relative safety of the drug (Mermin et al. 2004), this preventive strategy has not been widely used for HIV-uninfected children. In a sub-study of the ARROW trial, in which children older than 3 years and on ART for longer than 96 weeks were randomized to continue or stop CPT, those who discontinued the drug were more likely to be hospitalized, including for malaria (Bwakura-Dangarembizi et al. 2014). The fact that the protective effect of CPT was most important in patients with high CD4 cell counts supports the view that this might be a valid approach for malaria prevention in immune-competent children. However, there have been concerns that the widespread use of CPT would lead to an increase in antifolate resistance in sub-Saharan Africa (Lynen et al. 2007). These resistance mutations also have an impact on the efficacy of sulfadoxine-pyrimethamine (Naidoo and Roper 2013), which is still the only drug recommended for intermittent preventive treatment (IPT) in pregnant women (Garner and Gulmezoglu 2006, Menendez et al. 2007, Kayentao et al. 2013, ter Kuile et al. 2007). Only one study included in this meta-analysis assessed the prevalence of antifolate resistance mutations before and after the use of CPT and did not show an increase in the prevalence of these mutations over time (Thera et al. 2005), consistent with other CPT studies in HIV infected adults (Malamba et al. 2010, Malamba et al. 2006).
Although the evidence on the protective efficacy of CPT on malaria has been reviewed (Kamya et al. 2012, Manyando et al. 2013), this is the first report to include a meta-analysis and a thorough assessment of the risk of bias of both RCTs and observational studies. The most important limitation of this report is the small number of studies which could be included in the analyses. Only five studies were eligible for the meta-analysis assessing the impact of CPT on malaria incidence. As a consequence, it was not possible to conduct sub-group analyses to effectively assess sources of heterogeneity. Furthermore, as the publications originated from only four countries, our results are not representative of the overall situation in sub-Saharan Africa. Importantly, only one study reported on the impact of CPT on malaria incidence in a region with a low prevalence of antifolate resistance mutations, as illustrated by the isolated, small bubble in the left lower quadrant of Figure 3. The association between CPT and mortality was only evaluated in one RCT and one observational study, which can be explained by the convincing results shown in the trial by Chintu et al. (2004). An additional trial evaluating the use of CPT in HIV-infected children would not be possible for ethical reasons. We therefore did not perform a meta-analysis for this outcome. Finally, only one study evaluated the protective efficacy of CPT on malaria in HIV-uninfected children (Thera et al. 2005). The many advantages of CPT prophylaxis in HIV-infected children justify its recommendation in routine clinical care. However, CPT is not widely prescribed to children who are HIV-negative, partly due to the lack of evidence and because, in contrast to HIV-infected children who are in regular medical care, the delivery of this treatment is not straightforward in this population.
Conclusion
To our knowledge, this is the first meta-analysis assessing the effect of cotrimoxazole prophylaxis on the prevention of malaria in children in SSA. Although highly heterogeneous, the findings have clinical implications. Our study shows that CPT has a strong protective effect against malaria despite widespread resistance to it. Since this study only focused on children, future research should investigate whether the effect is the same in adults and HIV-infected pregnant women. More evidence from HIV-uninfected populations is needed in order to answer the question whether cotrimoxazole should become an option in the control of malaria among the general population in sub-Saharan Africa.
Acknowledgements
We would like to thank Dr David Larsen (Syracuse University, NY, US) for support with extracting malaria transmission intensity data, and Dr Cally Roper (London School of Hygiene and Tropical Medicine, UK) and Jennifer Flegg (University of Oxford, UK) from the World Wide Antimalarial Resistance Network for providing SP resistance data. This study was supported by the National Institutes of Health, through the leDEA Southern Africa collaboration and by the Malaria Capacity Development Consortium which is funded by The Wellcome Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Appendix 1.
PRISMA guidelines checklist
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. |
|
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). |
|
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g.. Web address), and, if available, provide registration information including registration number. |
|
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. |
|
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. |
|
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. |
|
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). |
|
| Data collection process | 10 | Describe method of data extraction from reports (eg., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. |
|
| Data items | 11 | List and defne all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. |
|
| Risk of bias in individual studies |
12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. |
|
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. |
|
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). |
|
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. |
|
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. |
|
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. |
|
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. |
|
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). |
|
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias)r and at review-level (e.g., incomplete retrieval of identified research, reporting bias). |
|
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. |
|
From: Moher D. Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/Journal.pmed1000097
Appendix 2. The search Strategy for PubMed
((((child OR children OR childhood OR infant* OR newborn* OR neonate* OR adolescen*)) AND (cotrimoxazole OR co-trimoxazole OR co trimoxazole OR trimethoprim-sulphamethoxazole OR trime-thoprim sulphamethoxazole OR tmp-smx OR tmp smx OR bactrim)) AND (((malaria OR plasmodium OR plasmodium infection* OR paludis* OR malarial fever OR marsh fever)) AND ((((((therapy OR therapeut* OR treatment* OR pharmacotherap*)) OR (diagnosis OR diagnosed)) OR (epidemiol* OR preval* OR inciden*)) OR (mortalit* OR death* OR surviv* OR case fatalit*)) OR (prevention OR preventative OR preventive OR protect* OR prophyla* OR control OR controlled)))) OR (((“Malaria/diagnosis”[Mesh] OR “Malaria/drug therapy”[Mesh] OR “Malaria/epidemiology”[Mesh] OR “Malaria/mortality”[Mesh] OR “Malaria/prevention and control”[Mesh])) OR (“Mortality”[Mesh]))) AND (((((“Child”[Mesh]) OR “Child, Preschool”[Mesh]) OR “lnfant”[Mesh]) OR “Infant, Newborn”[Mesh]) OR “Adolescent”[Mesh])) AND (“Trimethoprim-Sulfamethoxazole Combination”[Mesh])
Appendix 3.
Checklist for the assessment of the risk of bias in cohort studies
| Domain | Description | Reviewer's assessment |
|---|---|---|
| Selection bias |
Were groups selected from the same underlying source population? |
Yes No Unclear |
| Were the data collected for the purpose of this study? | Yes No Unclear | |
|
Were the calendar periods of follow up the same in the two groups? |
Yes No Unclear | |
| Was the comparability of groups assessed? | Yes No Unclear | |
| If yes describe the variables compared: | ||
| Potential confounders? | Yes No Unclear | |
| Outcome variables before intervention? | Yes No Unclear | |
| Confounding |
Did the researchers describe how they decided which po tential confounding domains should be considered? |
Yes No Unclear |
| If yes describe the method used: | ||
|
Did the authors control for confounding at the design stage? |
Yes No Unclear | |
| If yes list on which domains participants were matched: | ||
|
Did the researchers adjust for confounding at the analy sis stage? |
Yes No Unclear | |
| If yes, which method was used: | ||
| Stratification | Yes No Unclear | |
| Multivariable regression | Yes No Unclear | |
| Propensity scores (matching) | Yes No Unclear | |
| Propensity scores (multivariable regression) | Yes No Unclear | |
| Causal modelling (IPTW, g estimation) | Yes No Unclear | |
| What potential confounders were considered? | ||
| Please provide list below: | ||
| Information bias |
Were the defintions / approaches to assess exposures and outcomes the same in the comparison groups? |
Yes No Unclear |
| Loss to follow up | Was follow-up near complete (>90%)? | Yes No Unclear |
| Outcome reporting bias |
Did the study have a protocol pre-specifying the out come? |
|
| Malaria | Yes No Unclear | |
| Mortality | Yes No Unclear | |
|
Was investigation of the effect of the intervention on the outcome a pre-specified objective of the primary study? |
||
| Malaria | Yes No Unclear | |
| Mortality | Yes No Unclear | |
| Overall assessment | What is the risk of bias in this study? | High Low |
Appendix 4.
Variables included in adjusted analyses from individual cohort studies
| Study | Country | Variables adjusted for |
|---|---|---|
| Gasasira et al. | Uganda | Age |
| Stratification by time periods | ||
| Dow et al. | Malawi | Age, rainy season, ART status, first pregnancy |
| Ezeamama et al. | Tanzania | Season |
| Mother: | ||
| Age, CD4, education, marital status, Socio-economic status, Bed-net use, history of neonatal mortality | ||
| Child: | ||
| CD4, sex, low birth-weight, CTX compliance, breastfeed ing, intervention status | ||
| Desmonde et al. | Ivory Coast | Age, sex, CD4, PMTCT, mother dead, follow-up center |
References
- ARINAITWE E, GASASIRA A, VERRET W, et al. The association between malnutrition and the incidence of malaria among young HIV-infected and -uninfected Ugandan children: a prospective study. Malar J. 2012;11:90. doi: 10.1186/1475-2875-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERKLEY JA, BEJON P, MWANGI T, et al. HIV infection, malnutrition and invasive bacterial infection among children with severe malaria. Clin Infect Dis. 2009;49:336–343. doi: 10.1086/600299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOOTH A, CLARKE M, DOOLEY G, et al. PROSPERO at one year: an evaluation of its utility. Syst Rev. 2013;2:4. doi: 10.1186/2046-4053-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BWAKURA-DANGAREMBIZI M, KENDALL L, BAKEERA-KITAKA S, et al. A randomized trial of prolonged co-trimoxazole in HIV-infected children in Africa. N Engl J Med. 2014;370:41–53. doi: 10.1056/NEJMoa1214901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHINTU C, BHAT GJ, WALKER AS, et al. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet. 2004;364:1865–71. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- DATE AA, VITORIA M, GRANICH R, BANDA M, FOX MY, GILKS C. Implementation of co-trimoxazole prophylaxis and isoniazid preventive therapy for people living with HIV. Bull World Health Organ. 2010;88:253–9. doi: 10.2471/BLT.09.066522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESMONDE S, COFFIE P, AKA E, et al. Severe morbidity and mortality in untreated HIV-infected children in a paediatric care programme in Abidjan, Cote d'Ivoire 2004–2009. BMC Infect Dis. 2011;11:182. doi: 10.1186/1471-2334-11-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOW A, KAYIRA D, HUDGENS M, et al. The Effects of Cotrimoxazole Prophylactic Treatment on Adverse Health Outcomes among Human Immunodeficiency Virus-Exposed, Uninfected Infants. Pediatr Infect Dis J. 2012 doi: 10.1097/INF.0b013e31825c124a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EZEAMAMA AE, SPIEGELMAN D, HERTZMARK E, et al. HIV infection and the incidence of malaria among HIV-exposed children from Tanzania. J Infect Dis. 2012;205:1486–94. doi: 10.1093/infdis/jis234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEGG JA, PATIL AP, VENKATESAN M, et al. Spatiotemporal mathematical modelling of mutations of the dhps gene in African Plasmodium falciparum. Malar J. 2013;12:249. doi: 10.1186/1475-2875-12-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARNER P, GULMEZOGLU AM. Drugs for preventing malaria in pregnant women. Cochrane Database Syst Rev. 2006:CD000169. doi: 10.1002/14651858.CD000169.pub2. [DOI] [PubMed] [Google Scholar]
- GASASIRA AF, KAMYA MR, OCHONG EO, et al. Effect of trimethoprimsulphamethoxazole on the risk of malaria in HIV-infected Ugandan children living in an area of widespread antifolate resistance. Malar J. 2010;9:177. doi: 10.1186/1475-2875-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GETHING PW, PATIL AP, SMITH DL, et al. A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J. 2011;10:378. doi: 10.1186/1475-2875-10-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAY SI, GUERRA CA, GETHING PW, et al. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;6:el000048. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGGINS JP, ALTMAN DG, GOTZSCHE PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTCHINSON E, PARKHURST J, PHIRI S, et al. National policy development for cotrimoxazole prophylaxis in Malawi, Uganda and Zambia: the relationship between Context, Evidence and Links. Health Res Policy Syst. 2011;9(Suppl 1):S6. doi: 10.1186/1478-4505-9-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMYA MR, BYAKIKA-KIBWIKA P, GASASIRA AF, HAVLIR D, ROSENTHAL PJ, DORSEY G, ACHAN J. The effect of HIV on malaria in the context of the current standard of care for HIV-infected populations in Africa. Future Virol. 2012;7:699–708. doi: 10.2217/FVL.12.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMYA MR, GASASIRA AF, ACHAN J, et al. Effects of trimethoprim-sulfamethoxazole and insecticide-treated bednets on malaria among HIV-infected Ugandan children. AIDS. 2007;21:2059–66. doi: 10.1097/QAD.0b013e3282ef6da1. [DOI] [PubMed] [Google Scholar]
- KAYENTAO K, GARNER P, VAN EIJK AM, et al. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: systematic review and meta-analysis. Jama. 2013;309:594–604. doi: 10.1001/jama.2012.216231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIBERATI A, ALTMAN DG, TETZLAFF J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYNEN L, JACOBS J, COLEBUNDERS R. Co-trimoxazole prophylaxis in tropical countries in the era of highly active antiretroviral therapy: do we know enough? Trans R Soc Trop Med Hyg. 2007;101:1059–60. doi: 10.1016/j.trstmh.2007.07.001. [DOI] [PubMed] [Google Scholar]
- MALAMBA S, SANDISON T, LULE J, REINGOLD A, WALKER J, DORSEY G, MERMIN J. Plasmodium falciparum dihydrofolate reductase and dihyropteroate synthase mutations and the use of trimethoprim-sulfamethoxazole prophylaxis among persons infected with human immunodeficiency virus. Am J Trop Med Hyg. 2010;82:766–71. doi: 10.4269/ajtmh.2010.08-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAMBA SS, MERMIN J, REINGOLD A, et al. Effect of cotrimoxazole prophylaxis taken by human immunodeficiency virus (HIV)-infected persons on the selection of sulfadoxine-pyrimethamine-resistant malaria parasites among HIV-uninfected household members. Am J Trop Med Hyg. 2006;75:375–80. [PubMed] [Google Scholar]
- MANYANDO C, NJUNJU EM, D'ALESSANDRO U, VAN GEERTRUYDEN JP. Safety and efficacy of co-trimoxazole for treatment and prevention of Plasmodium falciparum malaria: a systematic review. PLoS One. 2013;8:e56916. doi: 10.1371/journal.pone.0056916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENENDEZ C, D'ALESSANDRO U, TER KUILE FO. Reducing the burden of malaria in pregnancy by preventive strategies. Lancet Infect Dis. 2007;7:126–35. doi: 10.1016/S1473-3099(07)70024-5. [DOI] [PubMed] [Google Scholar]
- MERMIN J, LULE J, EKWARU JP, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–34. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- NAIDOO I, ROPER C. Mapping 'partially resistant', 'fully resistant', and 'super resistant' malaria. Trends Parasitol. 2013 doi: 10.1016/j.pt.2013.08.002. [DOI] [PubMed] [Google Scholar]
- OTIENO JA, OUMA C, ONG'ECHA J, et al. Increased severe anemia in HIV-1 exposed and HIV-1 positive infants and children during acute malaria. AIDS. 2006;20 doi: 10.1097/01.aids.0000200533.56490.b7. [DOI] [PubMed] [Google Scholar]
- RYAN M, GRIFFIN S, CHITAH B, et al. The cost-effectiveness of cotrimoxazole prophylaxis in HIV-infected children in Zambia. AIDS. 2008;22:749–57. doi: 10.1097/QAD.0b013e3282f43519. [DOI] [PubMed] [Google Scholar]
- SANDISON TG, HOMSY J, ARINAITWE E, et al. Protective efficacy of co-trimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: A randomised clinical trial. BMJ. 2011;342 doi: 10.1136/bmj.d1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRIDARAN S, MCCLINTOCK SK, SYPHARD LM, HERMAN KM, BARNWELL JW, UDHAYAKUMAR V. Anti-folate drug resistance in Africa: meta-analysis of reported dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) mutant genotype frequencies in African Plasmodium falciparum parasite populations. Malar J. 2010;9:247. doi: 10.1186/1475-2875-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TER KUILE FO, VAN EIJK AM, FILLER SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA. 2007;297:2603–16. doi: 10.1001/jama.297.23.2603. [DOI] [PubMed] [Google Scholar]
- THERA MA, SEHDEV PS, COULIBALY D, et al. Impact of trimethoprim-sulfamethoxazole prophylaxis on falciparum malaria infection and disease. J Infect Dis. 2005;192:1823–9. doi: 10.1086/498249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Recommendations for a public health approach. World Helath Organization; Geneva: 2006. Guidelines on Co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults. [Google Scholar]
- WHO . Intermittent preventive treatment for infants using sulfadoxine-pyrimethamine (SP-IPT) for malaria control in Africa: Implementation field Guide. World Health Organization; 2011. [Google Scholar]
- WHO . WHO policy Recommendation: Seasonal malaria Chemoprevention (SMC) for Plasmodium falciparum malaria control in highly seasonal transmission areas of the Sahel sub-region in Africa. World Health Organization; 2012a. [Google Scholar]
- WHO . World Malaria Report 2012. World Health Organization; Geneva: 2012b. [Google Scholar]
- WORMSER GP, KEUSCH GT, HEEL RC. Co-trimoxazole (trimethoprim-sulfamethoxazole): an updated review of its antibacterial activity and clinical efficacy. Drugs. 1982;24:459–518. doi: 10.2165/00003495-198224060-00002. [DOI] [PubMed] [Google Scholar]