SUMMARY
Background
Factors associated with post-thrombotic syndrome are known clinically, but the underlying cellular processes at the vein wall are not well-delineated. Prior work suggests that vein wall damage does not correlate with thrombus resolution, but rather with plasminogen activator-1 (PAI-1) and matrix metalloproteinase (MMP) activity.
Objective
We hypothesized that PAI-1 would confer post venous thrombosis (VT) vein wall protection via a Vitronectin (Vn) dependent mechanism.
Methods
A stasis model of VT was used with harvest over 2 weeks, in wild type (WT), Vn−/−, and PAI-1 overexpressing mice (PAI-1 Tg).
Results
PAI-1 Tg mice had larger VT at 6 and 14 days, compared to controls, but Vn−/−mice had no alteration of VT resolution. Gene deletion of Vn resulted in increased, rather than expected decrease in circulating PAI-1 activity. While both Vn−/− and PAI-1 Tg had attenuated intimal fibrosis, PAI-1 Tg had significantly less vein wall collagen and a compensatory increase in collagen III gene expression. Both Vn−/− and PAI-1 Tg vein wall had less monocyte chemotactic factor-1, and fewer macrophages (F4/80), with significantly less MMP-2 activity and decreased TIMP-1 antigen. Ex vivo assessment of TGFβ mediated fibrotic response showed that PAI-1 Tg vein walls had increased profibrotic gene expression (collagen I, III, MMP-2 and α-SMA) as compared with controls, opposite of the in vivo response.
Conclusions
The absence of Vn increases circulating PAI-1, which positively modulates vein wall fibrosis in a dose-dependent manner. Translationally, PAI-1 elevation may decrease vein wall damage after DVT, perhaps by decreasing macrophage-mediated activities.
Keywords: fibrosis, PAI-1, postthrombotic syndrome, venous thrombosis, vitronectin
INTRODUCTION
Deep venous thrombosis (DVT) is a significant health care problem in the United States, with 250,000 to 900,000 patients affected yearly.[1],[2] An additional 200,000 patients are affected by the late sequelae of post thrombotic syndrome (PTS), characterized by leg pain, sensations of heaviness, limb edema, discoloration and occasionally ulceration.[3] The end result after DVT is the conversion of a compliant, thin walled vein with functional valves to a thick walled vessel, often with nonfunctioning valves.[4] The physiological result of damaged veins is pooling of blood in the dependent extremities, producing venous hypertension and pain, fluid transudation, edema, and in severe cases, ulceration.
Despite effective anticoagulant therapy treatments for DVT, there are no therapies that specifically target the PTS remodeling processes.[5] While surgical and interventional therapy may be beneficial for selected patients, treatment for PTS is mainly supportive, consisting of compression and wound care. While compression therapy may provide symptomatic relief, it does not correct the fundamental changes that occur in the venous system. Indeed, recent data from a randomized controlled trial of compression for preventing PTS did not show a benefit as compared with placebo.[6] An improved understanding of the biology of PTS, including potential therapeutic targets, is crucial in the effort to develop effective treatments.
We have recently shown the importance in plasminogen activator-1 (PAI-1) in post-DVT vein wall remodeling.[7] Gene-deletion of PAI-1 hastened venous thrombus resolution in a mouse model of stasis DVT, but resulted in a thickened and collagen-rich vessel wall. Thrombin-antithrombin levels were not elevated in the thrombi of PAI-1 null mice, suggesting that the mechanism of increased fibrosis was not due to PAI-1's thrombin inhibitory activity, but possibly its role in binding Vitronectin (Vn), an abundant plasma glycoprotein.
Vitronectin is produced by the liver and found circulating at appreciable levels in the plasma, in the α-granules of platelets and in the extracellular matrix.[8] It plays a multifaceted role in vascular biology, particularly with regards to thrombogenesis, peri-cellular proteolysis, leukocyte recruitment and cellular adhesion/migration.[8, 9] Vn has been associated with fibrosis of organ systems such as liver[10, 11] and lung[12, 13] and implicated as a mediator of neointima formation in the arterial vasculature.[14-16] To date, its effect on post thrombotic venous remodeling is unknown.
Experimental models of arterial thrombosis differ with regards to the role of Vn, with some data suggesting that it plays a key role in promoting arterial thrombogenesis[17] and stabilization of thrombi,[18] while other data suggesting an inhibitory role in thrombogenesis.[19] However, little is understood about how or if Vn contributes to venous thrombosis (VT), with only one study to date demonstrating increased time to occlusion in Vn−/− mice undergoing photochemically induced thrombosis of the jugular vein.[17] In this study, we hypothesize: (1) Vn inhibits thrombus resolution via stabilization of PAI-1 and (2) Vn contributes to vein wall fibrosis via increased monocyte vein wall infiltration.
METHODS
Animals
Male C57BL/6 (WT) mice (Jackson Laboratory, Farmington, CT), Vitronectin gene-deleted (Vn−/−) mice[20] (backcrossed >10 generations on C57BL/6 mice) and PAI-1 over-expressing mice (PAI-1 Tg, backcrossed > 10 generations on C57BL/6 background)[21] were utilized in this study. Initial pilot studies comparing homozygous littermates without transgene (PAI-1 Tg littermates) to C57BL/6 controls demonstrated identical phenotype with regards to venous thrombosis (similar size and cellular morphology). Thus, in the interest of humane and responsible animal use, C57BL/6 mice were utilized as controls (rather than homozygous littermates) for all experiments. . Animals ranged from 8-10 weeks of age with average weight of 24.2g (WT), 24.4g (Vn), and 24.6g (PAI-1 Tg). All work was approved by the University of Michigan, University Committee on Use and Care of Animals and was performed in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Model of Deep Venous Thrombosis
A DVT model of IVC ligation, or stasis thrombosis, was performed as described.[22] Briefly, mice were anesthetized via 2% inhaled isoflurane. IVC exposure was gained via midline laparotomy and dorsal branches were interrupted with electrocautery. Side branches and the infrarenal IVC were ligated with 7-0 prolene (Ethicon, Inc., Somerville, NJ) to generate blood stasis and ultimately thrombosis. Fascial closure was performed with 5-0 Vicryl suture and skin closure with Vetbond tissue adhesive (3M Animal Care Products, St. Paul, MN). Mice were euthanized at 2, 6 and 14 days post-thrombosis with collection of plasma, vein wall, and thrombus. The vein wall and the thrombus were harvested separately at all time points.[22, 23] Prior to processing, the IVC and its associated thrombus were weighed (grams) and measured (centimeters). Thrombus weight, encompassing the thrombus and the vein wall, was used as a measure of thrombosis resolution.[22, 24]
Gelatin Zymography of MMP-2 and MMP-9
Gelatin substrate zymography was performed using pre-cast 10% SDS-polyacrylamide gels containing 1 mg/ml of gelatin (unless otherwise stated, all zymography supplies from Novex, San Diego, CA).[25-27] Vein wall tissue was homogenized in SDS, centrifuged for 10 minutes at 2000g. Supernatants were aliquoted equally and diluted with tris-glycine-SDS sample buffer and electrophoretically separated under non-reducing conditions. Separated proteins were re-natured in 2.7% Triton X-100 for 2 hours to induce gelatin lysis by re-natured MMPs. The gels were developed for 48 hours at 37°C in 50 mM Tris-HCL, 5 mM CaCl2 and 0.2% Brij 35 followed by staining with Coomassie Blue and de-staining in 10% acetic acid. MMP activity was evident by clear bands against a darkly stained background where the substrate has been degraded by the enzyme. Samples containing human recombinant MMP-2 and MMP-9 (Oncogene, Boston, MA) were included as standards. Concentrations of the Densitometry analysis were performed using a FOTO/Analyst CCD CAMERA (Fotodyne, Hartland, WI) and GEL-Pro Analyzer software version 3.1 (Media Cybernetics, Silver Springs, MD). Ratios of optical densities/total protein were calculated. Results were normalized to total protein using bicinchoninic acid protein assay kit, assessing colorimetric detection and quantification of total protein, read between 560 and 590 nm (Pierce, Rockford, Ill).
Antigen determination
Antigen determination via enzyme linked immunosorbent assay (ELISA) was performed on vein wall homogenate for MCP-1, Interleukin (IL)1β, and IL-13 (R&D Systems, Minneapolis, MN). Samples were aliquoted in duplicate on plates pre-coated with appropriate capture antibody and then detected by a biotinylated antibody, followed by tetramethylbenzadine (TMB) substrate according to the manufacturer's protocol. The results were read on an Elx808 plate reader (Biotek, Winooski, VT) at a 450-nm wavelength. Total protein was measured by using a bicinchoninic acid protein assay kit, as detailed in previous section. Results were normalized to total protein (mg).
PAI-1 activity assay
To measure active murine PAI-1 concentrations in plasma samples, human uPA (rheotromb) was coupled to carboxylated beads (Luminex) and used for capture active PAI-1. A standard curve was generated using known concentrations of murine PAI-1 in PAI-1 depleted mouse plasma (Molecular Innovations, Novi, MI). The sample, 10uL PBS-1% BSA and 5000 beads in 30mL of PBS-1% BSA were incubated overnight at 4°C. The beads were mixed with biotin-labeled rabbit anti-mouse PAI-1 (Molecular Innovations) followed by addition of streptavidin-R-phycoerythrin (Molecular Probes). The beads were then read with the Luminex 100 (median setting, 100 ml sample size, 100 events/bead).
Real-time quantitative polymerase chain reaction (RT-PCR)
Immediately upon harvest, vein wall segments were preserved in TRIzol reagent. The samples underwent reverse transcription with addition of Oligo(dT) primer and dNTP at 65°C for 5 minutes followed by first strand buffer, dithiothreitol and ribonucleoside inhibitor at 37°C for 2 minutes. M-MLV Reverse Transcriptase (Life Technologies, Carlsbad, CA) was added and RNA heated to 37°C for 50 minutes then 70°C for 20 minutes. The resultant cDNA was amplified by Taq polymerase in the Rotogene quantitative polymerase chain reaction system (Qiagen Inc., Valencia, CA). Rotogene quantification utilizes the cycle threshold (Ct) for the gene of interest normalized to the housekeeping gene β-actin. Relative mRNA expression is calculated by the formula 2-(Ct target gene-Ct reference gene) and cycle lengths used are within the exponential phase of the polymerase chain reaction.[28] These primers included β-actin, procollagen (Col)1a2, Col3a, MMP-2 and α-SMA.
Histology / Immunohistochemistry/ Collagen Staining
Inferior vena cava and associated thrombi were harvested en bloc, fixed in formalin, embedded in paraffin and cut into 5 m sections as previously described.[29, 30] Vein wall monocyte cell counts were performed following staining with F4/80 antibody (1:100; Abcam, Cambridge, MA). Briefly, non-specific binding sites were blocked with normal serum, primary antibody was added, followed by biotin-labeled secondary antibody (Rat IgG). An avidin, biotin, peroxidase complex was performed according to manufacturer's instructions (Vector Laboratories Inc., Burlingame, California) and the slides were counterstained with hematoxylin. Monocyte cell counts of five representative high power fields of both vein wall and thrombus were performed in a blinded fashion.[31]
Collagen content was quantified with Picrosirius red stain as previously described.[7, 32, 33] These sections were then analyzed in crossed-plane polarized light from a monochromatic source to assess cross linked collagen. Two images for each were obtained using a Zeiss Axio M1 scope and Zeiss AxioVision software (Carl Zeiss Microimaging GmbH, Göttingen, Germany) at 0 and 90 degrees to the plane of polarization, in order to capture the birefringence of fibers extinguished in one direction. The images were analyzed blindly utilizing NIH Image J software. The inferior vena cava wall was outlined as a region of interest, and then the image underwent threshold segmentation to differentiate collagen from other (primarily cellular and empty space) components of the vein wall. A vein wall collagen score was assigned by the formula [(% birefringent area) × (measured vein wall area)] / (total specimen area).
To account for non-collagen vein wall changes, intimal thickness and fibrosis scoring assessed from H and E-sections were scored by a board-certified veterinary pathologist in a blinded manner as previously described.[7, 22, 31] Briefly, the scoring system ranges from a score of 0 (no evidence of fibrosis) to 3 (intima contains numbers fibroblasts and is irregularly thickened by large amounts of collagenous connective tissue). A consistent mid-section thrombosed IVC segment was used for all histological analysis.[34-36]
Ex vivo methods vein wall assessment
Inferior vena cava was harvested in control mice (WT and PAI-1 Tg), placed in cold Dulbecco phosphate buffered saline with 2× penicillin/streptomycin/glutamine (Gibco, Invitrogen, Carlsbad) and cleaned using a dissecting microscope.[24, 34, 37] The vein wall was divided lengthwise, weighed and each half further divided into 3-4 pieces. The vein was incubated in Dulbecco Modified Eagle Medium (Gibco) containing 5% FCS and 1% PSG with or without transforming growth factor β1 (10mcg/ml, mouse recombinant, R&D Systems, Minneapolis, MN) in a 12-well plate. [38] The tissue culture was incubated for 24 hours at 37° in 5% carbon dioxide. The wet weight of the vein wall was recorded, the samples were gently washed in DMEM, placed in Trizol and frozen at −80° for RT-PCR processing.
Statistical analyses
All statistical analyses were performed using Graph Pad Prism version 6.01 for Windows (Graph Pad Software, San Diego, CA). The statistical differences between groups were determined by an unpaired t-test with Welch's correction, one-way ANOVA analysis with Tukey's multiple comparison test, or Dunnett's multiple comparison test as deemed appropriate. Data is reported as mean ± standard error of the mean (SEM) and differences considered significant at a p value of ≤.05. Animal numbers (n) for experiments were pre-determined based upon independent statistical review, protocols and numbers needed for significance. For an alpha=0.5 and power of 80%, n=5 is sufficient for immunological tests and n=10 sufficient for zymography.
RESULTS
Vn−/− mice have paradoxically increased circulating PAI-1 activity
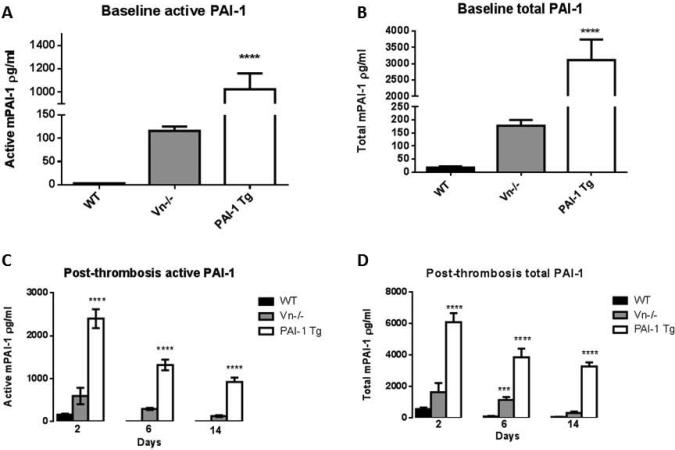
Despite the recent commercial availability of Vn−/− mice and subsequent increase in use, no studies have characterized levels of circulating active PAI-1 in these animals. PAI-1 activity as measured in control mice and at three time points post-thrombosis (2, 6 and 14 days) is demonstrated in Figure 1. Both total and active PAI-1 levels were elevated in the Vn−/− mice when compared to WT. Contrary to expectations, we found active PAI-1 elevated 36-fold compared to wild-type (Figure 1A) and total circulating PAI-1 to be elevated 10-fold in Vn−/− mice (Figure 1B). Active PAI-1 remained elevated in the Vn−/− mice following thrombosis with significant elevations at days 6 and 14, with 82-fold and 39-fold increase over WT respectively, (Figure 1C), with similar elevations seen of total PAI-1 (Figure 1D). To determine whether the results we found with regards to thrombus resolution and vein wall fibrosis were truly due to alterations in PAI-1 activity in these animals or rather a secondary function of Vn, we included a comparison cohort of PAI-1 overexpressing transgenic mice (PAI-1 Tg) with normal endogenous Vn. Levels of active circulating PAI-1 in these animals were markedly elevated at baseline (137-fold) and post-thrombosis (15 to 412 fold) compared to WT (Figure 1A and C). Similar elevations were seen in total PAI-1 at baseline (172 fold) and post-thrombosis compared to WT (10 to 54 fold) (Figure 1B and D).
Figure 1.
Both active and total PAI-1 were elevated in transgenic PAI-1 overexpressing mice (PAI-1 Tg) compared to WT (n=5 for all groups) at baseline (A, B) and following induction of deep venous thrombosis (C, D), with intermediate levels of PAI-1 in the Vn−/− depleted mice (*** p<0.001, **** p<0.0001).
Venous thrombogenesis and thrombus resolution are unaffected by Vn−/−
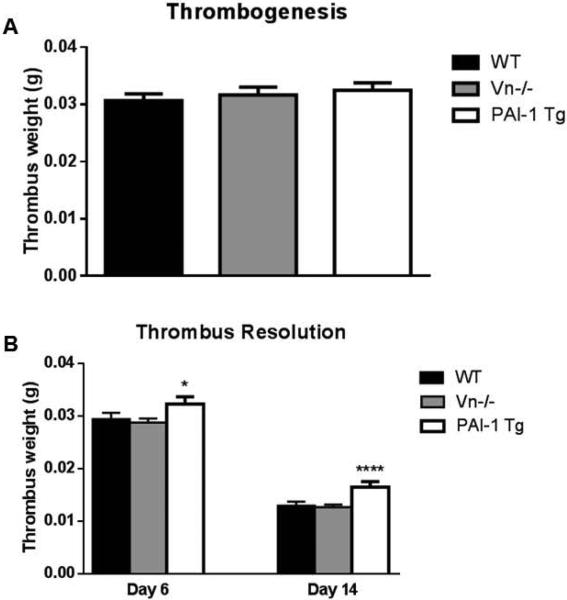
Thrombus weights during acute thrombogenesis, as measured at day 2, were similar between WT and Vn−/− mice (Figure 2A). Thrombus resolution, as measured by thrombus weight at 6 and 14 days post-thrombosis (Figure 2B), was also unaffected by Vn −/−. Transgenic PAI-1 overexpressing mice exhibited impairment of thrombus resolution, with increased residual thrombus weight at 6 and 14 days post thrombosis (Figure 2B). However, thrombogenesis (2d) was not affected in these animals (Figure 2A).
Figure 2.
Neither Vn−/− gene deletion nor PAI-1 over-expression affected thrombogenesis, as measured by thrombus weight 2 days post thrombosis, in the deep venous system (A, all n>25). Thrombus resolution was impaired in the PAI-1 Tg mice but not in the Vn−/− mice (B, all n>25, *p<0.05, ****p<0.0001).
Vein wall fibrosis is attenuated by excess PAI-1
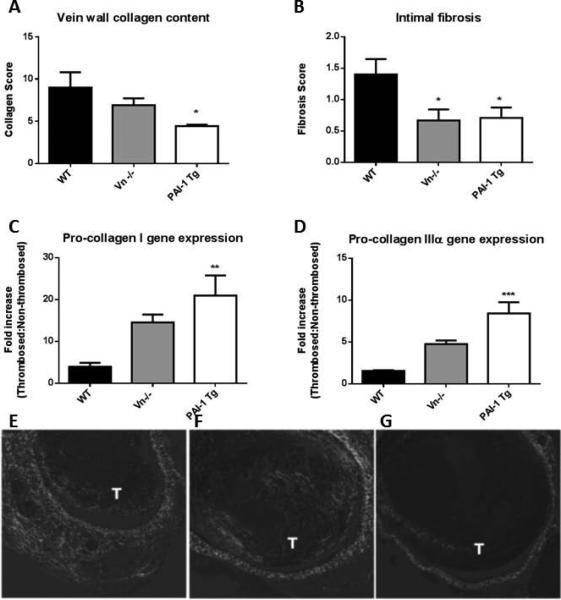
Vein wall fibrosis, intimal thickness and collagen content were assessed at day 14 and collagen gene expression assessed at days 6 and 14. Vein wall fibrosis was significantly diminished in mice with elevated PAI-1 (Vn−/− mice and PAI-1 Tg) at day 14 compared to WT (Figure 3B). Total vein wall collagen content, as measured by Picrosirius red staining,[7] demonstrated a stepwise reduction with increasing levels of circulating PAI-1 (Figure 3A, E-F). Decreases in vein wall fibrosis at day 14 were accompanied by a paradoxical increase in pro-collagen I and IIIα gene expression in Vn−/− (3.7-fold and 2.1-fold) and PAI-1 Tg (5.3-fold and 4.7-fold) mice compared to controls (Figures 3C,D).
Figure 3.
At two weeks post-thrombosis collagen content was inversely related to PAI-1 activity with PAI-1 Tg mice exhibiting nearly a 50% reduction compared to WT at day 14 (A, E-G, n=5, *p<0.05). Cellular fibrotic changes (B, n=5) were inversely associated with levels of PAI-1. Compared to WT, collagen I and IIIa gene expression was increased in mice with lowest vein wall collagen deposition (C,D, n=5-10, **p<0.01, ***p<0.001). Decreased collagen content is evident in vein wall stained with Sirius Red in Vn (F) and PAI-1 Tg (G) compared to WT (E, T=thrombus).
PAI-1 inhibits monocyte recruitment and influx
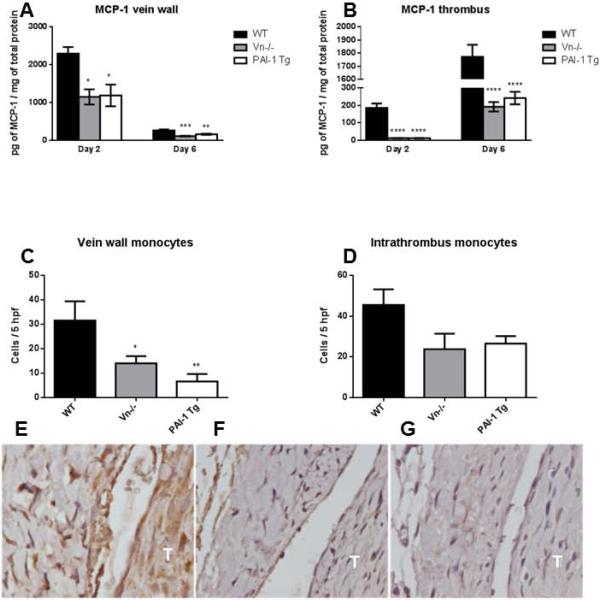
Monocyte chemotactic protein 1 (MCP-1) peaks during the early and middle stages of thrombus resolution, drives monocytes chemotaxis, and is implicated in clearance of residual thrombus.[39-41] At these time-points (days 2 and 6), MCP-1 levels were suppressed by ≥50% in mice with elevated PAI-1 (Vn−/− and PAI-Tg), both in the vein wall (Figure 4A) and in the thrombus (Figure 4B). This correlated with diminished vein wall (Figures 4C, E-G) and thrombus (Figures 4D-G) monocyte influx at day 14 as measured by positively staining F4/80 cells and impaired thrombus resolution (Figure 2B) in mice with markedly elevated circulating PAI-1 (PAI-1 Tg).
Figure 4.
MCP-1 was significantly decreased in Vn−/− and PAI-1 Tg mice in both the vein wall (A) and thrombus at days 2 and 6 (B, n=5-10). Vein wall monocyte influx (C) but not intrathrombus monocyte influx (D) at day 14 was significantly suppressed in Vn−/− and PAI-1 Tg mice (n=4-5). E-G, representative photomicrogaphs at 100x of WT (E), Vn−/− (F) and PAI-1 Tg (G) mice stained with monocyte specific antibody F4/80. T, thrombus, *p≤0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001 by analysis of variance with Dunnett's multiple comparison test.
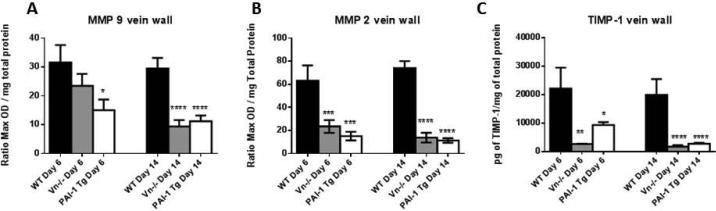
Vein wall MMP activity is inversely related to PAI-1 activity
The target of PAI-1 inhibition, plasmin, can serve as an activator of MMP-2 and MMP-9.[42-44] Furthermore, monocytes represent a source of MMPs in the post thrombotic vein wall[29] and recruitment is altered in the presence of excess PAI-1 (Figure 4C). In mice with PAI-1 (Vn−/− and PAI-1 Tg) elevation, total MMP-9 and -2 levels were diminished in the post-thrombotic vein wall (Figures 5A and B). Vein wall TIMP-1 antigen did not account for inhibition of MMPs, and was actually decreased at 6 and 14 days in Vn−/− and PAI-1 Tg mice compared to WT (Figure 5C).
Figure 5.
Vein wall MMP-9 (A) and -2 (B, all n=8-12, ***p<0.001, ****p<0.0001) activity is diminished during thrombus resolution (6d and 14d) in Vn−/− and PAI-1 Tg mice compared to WT controls. TIMP-1 levels were similarly decreased in PAI-1 Tg mice and Vn−/− vein wall (C, n=5-10 *p<0.05, **p<0.01).
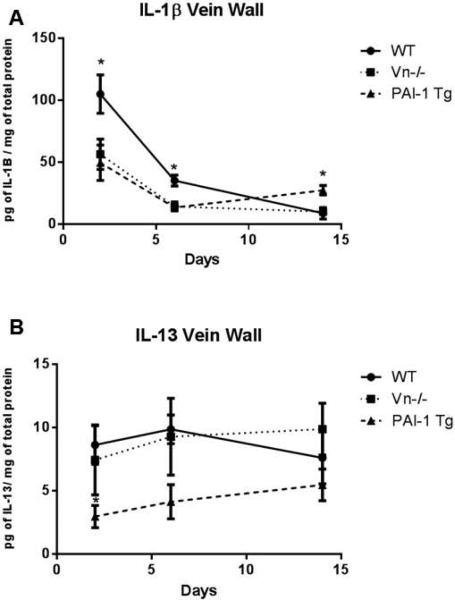
Genetic alterations in Vn and PAI-1 result in altered inflammatory cytokine milieu
Pro-inflammatory cytokine IL-1β is upregulated in the post-thrombotic vein wall[41] and augments PAI-1 production by endothelial cells.[45-47] We found that in animals with very high levels of PAI-1, peak vein wall IL-1β upregulation was attenuated (Figure 6A). Consistent with the anti-fibrotic phenotype of the PAI-1 Tg mice, we found a reduction of IL-13 in the vein wall at early and mid-time points post-thrombosis (Figure 6B). The Vn−/− mice exhibited an intermediate vein wall cytokine profile: IL-1β levels approximated those of the PAI-1 Tg mice and IL-13 levels approximated the WT.
Figure 6.
IL-1β was reduced in the vein wall at early and mid-time points post thrombosis (A, all n=5-10, p*<0.05) in Vn−/− and PAI-1 Tg mice. PAI-1 Tg but not Vn−/−mice demonstrated a reduction in IL-13 at 2 days post thrombosis (B).
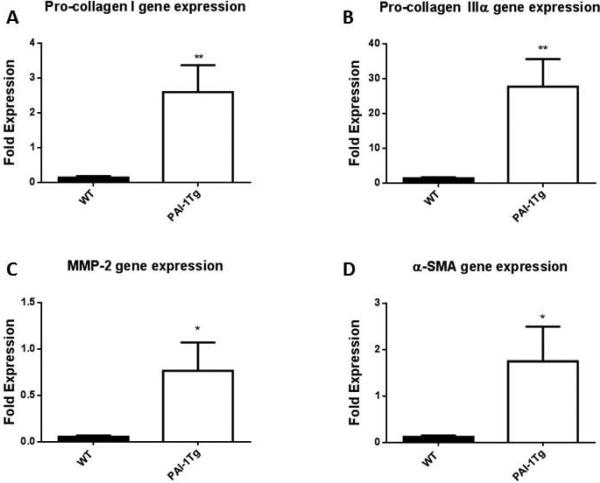
PAI-1 Tg vein walls are not anti-fibrotic ex vivo
Given that PAI-1 over expressing vein wall fibrosis was significantly reduced, we asked if the altered genotype affected the fibrotic response ex vivo. We used our organ culture model[37] to assess the response to fibrotic mediator TGF-β (Figure 7). Consistent with the increases seen in vivo with increased pro-collagen I and IIIα gene expression in response to thrombosis compared to WT (Figure 3C,D), the ex vivo cultured vein wall demonstrated an increase in pro-collagen gene expression in response to fibrotic stimulation with TGF-β (Figure 7A,B). Additional proteins associated with fibrotic injury, MMP-2 (Figure 7C) and α-SMA[37] (Figure 7D), demonstrated increased relative gene expression in PAI-1 Tg mice vein wall, suggesting that the anti-fibrotic effect of PAI-1 over expression was not due to fundamental changes in vein wall gene expression, but rather the anti-fibrotic effect of circulating PAI-1.
Figure 7.
Ex vivo vein wall response to pro-fibrotic stimuli with TFG-β demonstrates divergent response between WT and PAI-1 Tg mice. As compared with saline treated controls,, TGF-β administration was associated with a significant increase in pro-collagen I, IIIα,MMP-2 and α-SMA gene expression in PAI-1Tg vein wall compared to WT (A-D, all n=5, *p<0.05, **p<0.01).
DISCUSSION
Vein wall fibrosis after DVT, clinically manifested as PTS, is a problem with no known direct therapy. Thus, defining the mechanisms of vein wall fibrosis is important translationally. In this study, we have shown the following: (1) contrary to findings in arterial injury models[17-19], the presence of Vn does not influence the amplitude of thrombogenesis or thrombus resolution; (2) Vn−/− mice have increased circulating active and total PAI-1; (3) elevation of PAI-1 is protective against vein wall fibrosis and inversely correlates with vein wall MMP activity; (4) elevation of PAI-1 is associated with decreased monocyte infiltrate and altered cytokine profile; (5) these effects do not appear to be due to genotype specific alterations in the vein wall but rather to the anti-fibrotic effect of circulating PAI-1.
A significant role of PAI-1 in vascular thrombosis and thrombus resolution has been confirmed in animal and human studies alike.[48] Consistent with previous data suggesting that PAI-1 gene deletion does not affect venous thrombogenesis, we found here that PAI-1 over-expression failed to impact initial thrombus weight.[49] However, the role of PAI-1's “cofactor” Vn remains understudied, with few animal studies evaluating thrombotic potential in Vn−/− mice, and none specifically evaluating the deep venous system.[17-19] In a small vessel model of photochemically-induced VT, thrombogenesis was impaired in Vn−/− mice.[17] This contrasts to our data which demonstrates that in the deep vein system, at the time of peak thrombogenesis, there was no difference between thrombus weight and size in Vn−/− and WT mice. The discrepancy between these results suggest that Vn may function very early in thrombogenesis, as the photochemical model was used to study early thrombus formation at a very early time point (3-24 hours), or that Vn is less essential to stasis-induced thrombus formation. Furthermore, Vn deletion does not hasten thrombus resolution, in contrast with PAI-1−/− mice.[7] These findings are supported by elevation of active PAI-1 levels in Vn −/− mice, perhaps due to inability of PAI-1 to remain localized to site of fibrin formation or extracellular matrix via its interaction with Vn. Transgenic overexpression of PAI-1, which produced levels 3 to 17 times higher than the Vn−/−mice, resulted in incremental inhibition of thrombus resolution with larger VT, suggesting that the effect of PAI-1 on thrombus resolution is likely dose dependent.
Although genetic deletion of PAI-1 is associated with increased fibrinolysis by secondary increasing plasmin activity and smaller VT, an increase in vein wall fibrosis was observed.[7] PAI-1's role in organ fibrosis is complex, and dependent on the animal model. For example, divergent results with PAI-1 deletion exist in arterial injury models, with increased medial hyperplasia after wire injury[50] or decreased medial hyperplasia after ferric chloride injury.[51] From the current study, the mechanisms of PAI-1 anti-fibrotic effect is likely two-fold; first, significantly decreased MMP-2 and -9 activity in the post-thrombotic vein wall was found. Elevation of both MMPs is associated with post-thrombotic vein wall fibrosis, [37, 52, 53] and a reduction is consistent with attenuated fibrotic injury. Second, fewer macrophages (and consistently, decreased MCP-1) were found in the vein wall and thrombus of PAI-1 Tg and Vn−/− mice. While macrophages may have pro-fibrotic or anti-fibrotic activities[54], it is likely in the current experiments that less pro-fibrotic signaling was present in the PAI-1 Tg mice. In lung injury models, IL-1β[55] and IL-13[56] are pro-fibrotic. Consistently, in the current model, IL-1β and IL-13 were significantly elevated in WT mice post-thrombosis, and reduction of these pro-fibrotic mediators may also account for the observed reduction in fibrotic injury.
Binding of PAI-1 to its “cofactor” Vn stabilizes and confers PAI-1 to a longer half-life, contributing to local anti-fibrinolytic activity but also indirectly enhancing the anti-adhesive functions of PAI-1. PAI-1 competes with monocyte surface receptor urokinase plasminogen activator receptor (u-PAR) for the NH2 terminus of Vn.[8] Binding of PAI-1 displaces u-PAR and inhibits monocyte adhesion.[57] Vn also exhibits a variety of PAI-1-independent activities, including increasing cellular migration[13] and vascular permeability.[58] Interestingly, the vein walls from PAI-1 Tg mice were not intrinsically resistant pro-fibrotic stimulus. Indeed, using a low TGF-β dose, we found PAI-1 Tg vein walls were more likely to have increased collagen gene expression and fibrotic gene markers of MMP-2 and α-SMA. This suggests that the anti-fibrotic phenotype associated with elevated PAI-1 in our model was not the result of genotype specific decreased vein wall susceptibility to fibrosis , but more likely the anti-fibrotic effect of circulating PAI-1.
Post thrombotic vein wall injury is complex, and new data suggests that compression therapy may not be truly effective.[59] New and specific therapies are needed, particularly since aggressive thrombolysis may be limited to specific good risk patients and carries a risk of life threatening hemorrhage.[60] In this study we demonstrated that increased circulating PAI-1, either from gene deletion of PAI-1 ligand Vn or in setting of transgenic over-expression, is associated with attenuated fibrosis of the post-thrombotic vein wall. While it is unlikely that exogenous PAI-1 would be practical as it would impair thrombus resolution, interruption of PAI-1-fibrin interaction via specific targeting of vitronectin[6161] has the potential to decrease vein wall fibrotic response without affecting thrombus resolution, and represents a future area of study.
Acknowledgments
Supported by: HL092129 (PKH), NIH 5 T32 HL076123-09 (TW and AO), and HL089407 (DL and TW)
Footnotes
Presented in part at the 25th Meeting of the American Venous Forum, Phoenix, AZ, February 2013
Conflicts of Interest and Source of Funding: None of the authors report conflicts of interest.
ADDENDUM
Conception and Design: P. K. Henke, T. W. Wakefield, D. A. Lawrence
Data collection and experimentation: N. L. Ballard-Lipka, K. J. Roelofs, D. M. Farris, A. T. Obi
Analysis and Interpretation: A. T. Obi, P. K. Henke, T. W. Wakefield, D. A. Lawrence, J. A. Diaz
Writing the article: A. T. Obi, P. K. Henke
Critical revision of the article: P. K. Henke, T. W. Wakefield, D. A. Lawrence, J. A. Diaz
Obtained funding: P. K. Henke, T. W. Wakefield, D. A. Lawrence
Overall responsibility: P. K. Henke
References
- 1.Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol. 2011 Feb;86:217–20. doi: 10.1002/ajh.21917. PubMed PMID: 21264912. Epub 2011/01/26. eng. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, et al. Heart Disease and Stroke Statistics--2010 Update. A Report From the American Heart Association. Circulation. 2009 Dec 17; doi: 10.1161/CIRCULATIONAHA.109.192667. PubMed PMID: 20019324. Epub 2009/12/19. Eng. [DOI] [PubMed] [Google Scholar]
- 3.Bergan JJ, Schmid-Schonbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006 Aug 3;355:488–98. doi: 10.1056/NEJMra055289. PubMed PMID: 16885552. Epub 2006/08/04. eng. [DOI] [PubMed] [Google Scholar]
- 4.Johnson BF, Manzo RA, Bergelin RO, Strandness DE., Jr. Relationship between changes in the deep venous system and the development of the postthrombotic syndrome after an acute episode of lower limb deep vein thrombosis: a one- to six-year follow-up. J Vasc Surg. 1995 Feb;21:307–12. doi: 10.1016/s0741-5214(95)70271-7. discussion 13. PubMed PMID: 7853603. eng. [DOI] [PubMed] [Google Scholar]
- 5.Henke PK, Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. Feb;53:500–9. doi: 10.1016/j.jvs.2010.08.050. PubMed PMID: 21129900. Epub 2010/12/07. eng. [DOI] [PubMed] [Google Scholar]
- 6.Kahn SR, Shapiro S, Wells PS, Rodger MA, Kovacs MJ, Anderson DR, Tagalakis V, Houweling AH, Ducruet T, Holcroft C, Johri M, Solymoss S, Miron MJ, Yeo E, Smith R, Schulman S, Kassis J, Kearon C, Chagnon I, Wong T, et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014 Mar 8;383:880–8. doi: 10.1016/S0140-6736(13)61902-9. PubMed PMID: 24315521. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin JF, Sood V, Elfline MA, Luke CE, Dewyer NA, Diaz JA, Myers DD, Wakefield T, Henke PK. The role of urokinase plasminogen activator and plasmin activator inhibitor-1 on vein wall remodeling in experimental deep vein thrombosis. J Vasc Surg. 2012 Oct;56:1089–97. doi: 10.1016/j.jvs.2012.02.054. PubMed PMID: 22796119. Pubmed Central PMCID: 3463752. Epub 2012/07/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Preissner KT, Reuning U. Vitronectin in vascular context: facets of a multitalented matricellular protein. Semin Thromb Hemost. 2011 Jun;37:408–24. doi: 10.1055/s-0031-1276590. PubMed PMID: 21805447. Epub 2011/08/02. eng. [DOI] [PubMed] [Google Scholar]
- 9.Stefansson S, Haudenschild CC, Lawrence DA. Beyond fibrinolysis: the role of plasminogen activator inhibitor-1 and vitronectin in vascular wound healing. Trends Cardiovasc Med. 1998 May;8:175–80. doi: 10.1016/S1050-1738(98)00003-6. PubMed PMID: 21235930. Epub 1998/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 10.Patsenker E, Popov Y, Wiesner M, Goodman SL, Schuppan D. Pharmacological inhibition of the vitronectin receptor abrogates PDGF-BB-induced hepatic stellate cell migration and activation in vitro. J Hepatol. 2007 May;46:878–87. doi: 10.1016/j.jhep.2006.11.011. PubMed PMID: 17258347. Epub 2007/01/30. eng. [DOI] [PubMed] [Google Scholar]
- 11.Koukoulis GK, Shen J, Virtanen I, Gould VE. Vitronectin in the cirrhotic liver: an immunomarker of mature fibrosis. Hum Pathol. 2001 Dec;32:1356–62. doi: 10.1053/hupa.2001.29675. PubMed PMID: 11774169. Epub 2002/01/05. eng. [DOI] [PubMed] [Google Scholar]
- 12.Courey AJ, Horowitz JC, Kim KK, Koh TJ, Novak ML, Subbotina N, Warnock M, Xue B, Cunningham AK, Lin Y, Goldklang MP, Simon RH, Lawrence DA, Sisson TH. The vitronectin-binding function of PAI-1 exacerbates lung fibrosis in mice. Blood. 2011 Aug 25;118:2313–21. doi: 10.1182/blood-2010-12-324574. PubMed PMID: 21734232. Pubmed Central PMCID: 3162358. Epub 2011/07/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazar MH, Christensen PJ, Du M, Yu B, Subbotina NM, Hanson KE, Hansen JM, White ES, Simon RH, Sisson TH. Plasminogen activator inhibitor-1 impairs alveolar epithelial repair by binding to vitronectin. Am J Respir Cell Mol Biol. 2004 Dec;31:672–8. doi: 10.1165/rcmb.2004-0025OC. PubMed PMID: 15308506. Epub 2004/08/17. eng. [DOI] [PubMed] [Google Scholar]
- 14.Peng L, Bhatia N, Parker AC, Zhu Y, Fay WP. Endogenous vitronectin and plasminogen activator inhibitor-1 promote neointima formation in murine carotid arteries. Arterioscler Thromb Vasc Biol. 2002 Jun 1;22:934–9. doi: 10.1161/01.atv.0000019360.14554.53. PubMed PMID: 12067901. Epub 2002/06/18. eng. [DOI] [PubMed] [Google Scholar]
- 15.Coleman KR, Braden GA, Willingham MC, Sane DC. Vitaxin, a humanized monoclonal antibody to the vitronectin receptor (alphavbeta3), reduces neointimal hyperplasia and total vessel area after balloon injury in hypercholesterolemic rabbits. Circ Res. 1999 Jun 11;84:1268–76. doi: 10.1161/01.res.84.11.1268. PubMed PMID: 10364564. Epub 1999/06/12. eng. [DOI] [PubMed] [Google Scholar]
- 16.Dufourcq P, Couffinhal T, Alzieu P, Daret D, Moreau C, Duplaa C, Bonnet J. Vitronectin is up-regulated after vascular injury and vitronectin blockade prevents neointima formation. Cardiovasc Res. 2002 Mar;53:952–62. doi: 10.1016/s0008-6363(01)00547-8. PubMed PMID: 11922905. Epub 2002/03/30. eng. [DOI] [PubMed] [Google Scholar]
- 17.Eitzman DT, Westrick RJ, Nabel EG, Ginsburg D. Plasminogen activator inhibitor-1 and vitronectin promote vascular thrombosis in mice. Blood. 2000 Jan 15;95:577–80. PubMed PMID: 10627465. Epub 2000/01/11. eng. [PubMed] [Google Scholar]
- 18.Konstantinides S, Schafer K, Thinnes T, Loskutoff DJ. Plasminogen activator inhibitor-1 and its cofactor vitronectin stabilize arterial thrombi after vascular injury in mice. Circulation. 2001 Jan 30;103:576–83. doi: 10.1161/01.cir.103.4.576. PubMed PMID: 11157725. [DOI] [PubMed] [Google Scholar]
- 19.Fay WP, Parker AC, Ansari MN, Zheng X, Ginsburg D. Vitronectin inhibits the thrombotic response to arterial injury in mice. Blood. 1999 Mar 15;93:1825–30. PubMed PMID: 10068653. Epub 1999/03/09. eng. [PubMed] [Google Scholar]
- 20.Zheng X, Saunders TL, Camper SA, Samuelson LC, Ginsburg D. Vitronectin is not essential for normal mammalian development and fertility. Proc Natl Acad Sci U S A. 1995 Dec 19;92:12426–30. doi: 10.1073/pnas.92.26.12426. PubMed PMID: 8618914. Pubmed Central PMCID: 40370. Epub 1995/12/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eitzman DT, McCoy RD, Zheng X, Fay WP, Shen T, Ginsburg D, Simon RH. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest. 1996 Jan 1;97:232–7. doi: 10.1172/JCI118396. PubMed PMID: 8550840. Pubmed Central PMCID: 507084. Epub 1996/01/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wojcik BM, Wrobleski SK, Hawley AE, Wakefield TW, Myers DD, Jr., Diaz JA. Interleukin-6: a potential target for post-thrombotic syndrome. Ann Vasc Surg. 2011 Feb;25:229–39. doi: 10.1016/j.avsg.2010.09.003. PubMed PMID: 21131172. Epub 2010/12/07. eng. [DOI] [PubMed] [Google Scholar]
- 23.Myers D, Jr., Farris D, Hawley A, Wrobleski S, Chapman A, Stoolman L, Knibbs R, Strieter R, Wakefield T. Selectins influence thrombosis in a mouse model of experimental deep venous thrombosis. J Surg Res. 2002 Dec;108:212–21. doi: 10.1006/jsre.2002.6552. PubMed PMID: 12505044. [DOI] [PubMed] [Google Scholar]
- 24.Henke PK, Varma MR, Moaveni DK, Dewyer NA, Moore AJ, Lynch EM, Longo C, Deatrick CB, Kunkel SL, Upchurch GR, Jr., Wakefield TW. Fibrotic injury after experimental deep vein thrombosis is determined by the mechanism of thrombogenesis. Thromb Haemost. 2007;98:1045–55. PubMed PMID: 18000610. Eng. [PubMed] [Google Scholar]
- 25.Eagleton MJ, Peterson DA, Sullivan VV, Roelofs KJ, Ford JA, Stanley JC, Upchurch GR. Nitric oxide inhibition increases aortic wall matrix metalloproteinase-9 expression. J Surg Res. 2002;104:15–21. doi: 10.1006/jsre.2002.6396. 01/15/04. [DOI] [PubMed] [Google Scholar]
- 26.Dorffler-Melly J, Schwarte LA, Ince C, Levi M. Mouse models of focal arterial and venous thrombosis. Basic Res Cardiol. 2000 Dec;95:503–9. doi: 10.1007/s003950070028. PubMed PMID: 11192373. [DOI] [PubMed] [Google Scholar]
- 27.Upchurch GR, Ford JW, Weiss SJ, Knipp BS, Peterson DA, Thompson RW, Eagleton MJ, Broady AJ, Proctor MC, Stanley JC. Nitric oxide inhibition increases matrix metalloproteinase-9 expression by a rat aortic smooth muscle cells in vitro. J Vasc Surg. 2001;34:76–83. doi: 10.1067/mva.2001.115598. 01/15/04. [DOI] [PubMed] [Google Scholar]
- 28.Ginzinger DG. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol. 2002 Jun;30:503–12. doi: 10.1016/s0301-472x(02)00806-8. PubMed PMID: 12063017. Epub 2002/06/14. eng. [DOI] [PubMed] [Google Scholar]
- 29.Henke PK, Varga A, De S, Deatrick CB, Eliason J, Arenberg DA, Sukheepod P, Thanaporn P, Kunkel SL, Upchurch GR, Jr., Wakefield TW. Deep vein thrombosis resolution is modulated by monocyte CXCR2-mediated activity in a mouse model. Arterioscler Thromb Vasc Biol. 2004 Jun;24:1130–7. doi: 10.1161/01.ATV.0000129537.72553.73. PubMed PMID: 15105284. [DOI] [PubMed] [Google Scholar]
- 30.Henke PK, Pearce CG, Moaveni DM, Moore AJ, Lynch EM, Longo C, Varma M, Dewyer NA, Deatrick KB, Upchurch GR, Jr., Wakefield TW, Hogaboam C, Kunkel SL. Targeted deletion of CCR2 impairs deep vein thombosis resolution in a mouse model. J Immunol. 2006 Sep 1;177:3388–97. doi: 10.4049/jimmunol.177.5.3388. PubMed PMID: 16920980. Epub 2006/08/22. eng. [DOI] [PubMed] [Google Scholar]
- 31.Diaz JA, Ballard-Lipka NE, Farris DM, Hawley AE, Wrobleski SK, Myers DD, Henke PK, Lawrence DA, Wakefield TW. Impaired fibrinolytic system in ApoE gene-deleted mice with hyperlipidemia augments deep vein thrombosis. J Vasc Surg. 2012 Mar;55:815–22. doi: 10.1016/j.jvs.2011.08.038. PubMed PMID: 22119245. Pubmed Central PMCID: 3289767. Epub 2011/11/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979 Jul;11:447–55. doi: 10.1007/BF01002772. PubMed PMID: 91593. eng. [DOI] [PubMed] [Google Scholar]
- 33.Cuttle L, Nataatmadja M, Fraser JF, Kempf M, Kimble RM, Hayes MT. Collagen in the scarless fetal skin wound: detection with picrosirius-polarization. Wound Repair Regen. 2005 Mar-Apr;13:198–204. doi: 10.1111/j.1067-1927.2005.130211.x. PubMed PMID: 15828945. eng. [DOI] [PubMed] [Google Scholar]
- 34.Moaveni DK, Lynch EM, Luke C, Sood V, Upchurch GR, Wakefield TW, Henke PK. Vein wall re-endothelialization after deep vein thrombosis is improved with low-molecular-weight heparin. J Vasc Surg. 2008 Mar;47:616–24. doi: 10.1016/j.jvs.2007.11.040. PubMed PMID: 18295113. Pubmed Central PMCID: 2350236. Epub 2008/02/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henke PK, Mitsuya M, Luke CE, Elfline MA, Baldwin JF, Deatrick KB, Diaz JA, Sood V, Upchurch GR, Wakefield TW, Hogaboam C, Kunkel SL. Toll-like receptor 9 signaling is critical for early experimental deep vein thrombosis resolution. Arterioscler Thromb Vasc Biol. Jan;31:43–9. doi: 10.1161/ATVBAHA.110.216317. PubMed PMID: 20966396. Epub 2010/10/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sood V, Luke CE, Deatrick KB, Baldwin J, Miller EM, Elfline M, Upchurch GR, Jr., Wakefield TW, Henke PK. Urokinase plasminogen activator independent early experimental thrombus resolution: MMP2 as an alternative mechanism. Thromb Haemost. Dec;104:1174–83. doi: 10.1160/TH10-03-0184. PubMed PMID: 20886179. Epub 2010/10/05. eng. [DOI] [PubMed] [Google Scholar]
- 37.Laser A, Elfline M, Luke C, Slack D, Shah A, Sood V, Deatrick B, McEvoy B, Ostra C, Comerota A, Kunkel S, Hogaboam C, Henke PK. Deletion of cysteine-cysteine receptor 7 promotes fibrotic injury in experimental post-thrombotic vein wall remodeling. Arterioscler Thromb Vasc Biol. 2014 Feb;34:377–85. doi: 10.1161/ATVBAHA.113.302428. PubMed PMID: 24311382. Epub 2013/12/07. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007 Aug;13:952–61. doi: 10.1038/nm1613. PubMed PMID: 17660828. Epub 2007/07/31. eng. [DOI] [PubMed] [Google Scholar]
- 39.Hogaboam CM, Steinhauser ML, Chensue SW, Kunkel SL. Novel roles for chemokines and fibroblasts in interstitial fibrosis. Kidney Int. 1998 Dec;54:2152–9. doi: 10.1046/j.1523-1755.1998.00176.x. PubMed PMID: 9853282. Epub 1998/12/16. eng. [DOI] [PubMed] [Google Scholar]
- 40.Humphries J, McGuinness CL, Smith A, Waltham M, Poston R, Burnand KG. Monocyte chemotactic protein-1 (MCP-1) accelerates the organization and resolution of venous thrombi. J Vasc Surg. 1999 Nov;30:894–9. doi: 10.1016/s0741-5214(99)70014-5. PubMed PMID: 10550187. Epub 1999/11/05. eng. [DOI] [PubMed] [Google Scholar]
- 41.Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol. 2008 Mar;28:387–91. doi: 10.1161/ATVBAHA.108.162289. PubMed PMID: 18296594. Epub 2008/02/26. eng. [DOI] [PubMed] [Google Scholar]
- 42.Legrand C, Polette M, Tournier JM, de Bentzmann S, Huet E, Monteau M, Birembaut P. uPA/plasmin system-mediated MMP-9 activation is implicated in bronchial epithelial cell migration. Exp Cell Res. 2001 Apr 1;264:326–36. doi: 10.1006/excr.2000.5125. PubMed PMID: 11262189. Epub 2001/03/23. eng. [DOI] [PubMed] [Google Scholar]
- 43.Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest. 2008 Sep;118:3012–24. doi: 10.1172/JCI32750. PubMed PMID: 18677407. Pubmed Central PMCID: 2491456. Epub 2008/08/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takano A, Hirata A, Inomata Y, Kawaji T, Nakagawa K, Nagata S, Tanihara H. Intravitreal plasmin injection activates endogenous matrix metalloproteinase-2 in rabbit and human vitreous. Am J Ophthalmol. 2005 Oct;140:654–60. doi: 10.1016/j.ajo.2005.04.017. PubMed PMID: 16140249. Epub 2005/09/06. eng. [DOI] [PubMed] [Google Scholar]
- 45.Bevilacqua MP, Schleef RR, Gimbrone MA, Jr., Loskutoff DJ. Regulation of the fibrinolytic system of cultured human vascular endothelium by interleukin 1. J Clin Invest. 1986 Aug;78:587–91. doi: 10.1172/JCI112613. PubMed PMID: 3090105. Pubmed Central PMCID: 423598. Epub 1986/08/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schleef RR, Bevilacqua MP, Sawdey M, Gimbrone MA, Jr., Loskutoff DJ. Cytokine activation of vascular endothelium. Effects on tissue-type plasminogen activator and type 1 plasminogen activator inhibitor. J Biol Chem. 1988 Apr 25;263:5797–803. PubMed PMID: 3128548. Epub 1988/04/25. eng. [PubMed] [Google Scholar]
- 47.Libby P, Simon DI. Inflammation and thrombosis: the clot thickens. Circulation. 2001 Apr 3;103:1718–20. doi: 10.1161/01.cir.103.13.1718. PubMed PMID: 11282900. Epub 2001/04/03. eng. [DOI] [PubMed] [Google Scholar]
- 48.Van De Craen B, Declerck PJ, Gils A. The Biochemistry, Physiology and Pathological roles of PAI-1 and the requirements for PAI-1 inhibition in vivo. Thromb Res. 2012 Oct;130:576–85. doi: 10.1016/j.thromres.2012.06.023. PubMed PMID: 22801256. Epub 2012/07/18. eng. [DOI] [PubMed] [Google Scholar]
- 49.Obi AT, Diaz JA, Ballard-Lipka NL, Roelofs KJ, Farris DM, Lawrence DA, Henke PK, Wakefield TW. Low-molecular-weight heparin modulates vein wall fibrotic response in a plasminogen activator inhibitor 1-dependent manner. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2014 doi: 10.1016/j.jvsv.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carmeliet P, Moons L, Lijnen R, Janssens S, Lupu F, Collen D, Gerard RD. Inhibitory role of plasminogen activator inhibitor-1 in arterial wound healing and neointima formation: a gene targeting and gene transfer study in mice. Circulation. 1997 Nov 4;96:3180–91. doi: 10.1161/01.cir.96.9.3180. PubMed PMID: 9386191. [DOI] [PubMed] [Google Scholar]
- 51.Zhu Y, Farrehi PM, Fay WP. Plasminogen activator inhibitor type 1 enhances neointima formation after oxidative vascular injury in atherosclerosis-prone mice. Circulation. 2001 Jun 26;103:3105–10. doi: 10.1161/01.cir.103.25.3105. PubMed PMID: 11425776. [DOI] [PubMed] [Google Scholar]
- 52.Deatrick KB, Luke CE, Elfline MA, Sood V, Baldwin J, Upchurch GR, Jr., Jaffer FA, Wakefield TW, Henke PK. The effect of matrix metalloproteinase 2 and matrix metalloproteinase 2/9 deletion in experimental post-thrombotic vein wall remodeling. J Vasc Surg. 2013 Nov;58:1375–84. e2. doi: 10.1016/j.jvs.2012.11.088. PubMed PMID: 23490298. Pubmed Central PMCID: 3688659. Epub 2013/03/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deatrick KB, Obi A, Luke CE, Elfline MA, Sood V, Upchurch GR, Jr., Jaffer F, Wakefield TW, Henke PK. Matrix metalloproteinase-9 deletion is associated with decreased mid-term vein wall fibrosis in experimental stasis DVT. Thromb Res. 2013 Sep;132:360–6. doi: 10.1016/j.thromres.2013.06.027. PubMed PMID: 23978304. Epub 2013/08/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. PubMed PMID: 17981560. Epub 2007/11/06. eng. [DOI] [PubMed] [Google Scholar]
- 55.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010 Mar 15;207:535–52. doi: 10.1084/jem.20092121. PubMed PMID: 20176803. Pubmed Central PMCID: 2839145. Epub 2010/02/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999 Mar;103:779–88. doi: 10.1172/JCI5909. PubMed PMID: 10079098. Pubmed Central PMCID: 408149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fogo AB. Renal fibrosis: not just PAI-1 in the sky. J Clin Invest. 2003 Aug;112:326–8. doi: 10.1172/JCI19375. PubMed PMID: 12897200. Pubmed Central PMCID: 166301. Epub 2003/08/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li R, Ren M, Chen N, Luo M, Zhang Z, Wu J. Vitronectin increases vascular permeability by promoting VE-cadherin internalization at cell junctions. PLoS One. 2012;7:e37195. doi: 10.1371/journal.pone.0037195. PubMed PMID: 22606350. Pubmed Central PMCID: 3350505. Epub 2012/05/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kahn SR, Shapiro S, Wells PS, Rodger MA, Kovacs MJ, Anderson DR, Tagalakis V, Houweling AH, Ducruet T, Holcroft C, Johri M, Solymoss S, Miron MJ, Yeo E, Smith R, Schulman S, Kassis J, Kearon C, Chagnon I, Wong T, et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2013 Dec 5; doi: 10.1016/S0140-6736(13)61902-9. PubMed PMID: 24315521. Epub 2013/12/10. Eng. [DOI] [PubMed] [Google Scholar]
- 60.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012 Feb;141:e419S–94S. doi: 10.1378/chest.11-2301. PubMed PMID: 22315268. Pubmed Central PMCID: 3278049. Epub 2012/02/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Podor TJ, Peterson CB, Lawrence DA, Stefansson S, Shaughnessy SG, Foulon DM, Butcher M, Weitz JI. Type 1 plasminogen activator inhibitor binds to fibrin via vitronectin. J Biol Chem. 2000 Jun 30;275:19788–94. doi: 10.1074/jbc.M908079199. PubMed PMID: 10764803. [DOI] [PubMed] [Google Scholar]