Abstract
Background
Despite the efficacy of ceftriaxone (CTX) in animal models of CNS diseases, including drug addiction, its utility as a CNS-active therapeutic may be limited by poor brain penetrability and cumbersome parenteral administration. An alternative is the β-lactamase inhibitor clavulanic acid (CA), a constituent of Augmentin that prevents antibiotic degradation. CA possesses the β-lactam core necessary for CNS activity but, relative to CTX, possesses: 1) oral activity; 2) 2.5-fold greater brain penetrability; and 3) negligible antibiotic activity.
Methods
To compare the effectiveness of CA (10 mg/kg) and CTX (200 mg/kg) against centrally-mediated endpoints, we investigated their effects against morphine’s rewarding, hyperthermic, and locomotor-sensitizing actions. Endpoints were based on prior evidence that CTX attenuates morphine-induced physical dependence, tolerance, and hyperthermia.
Results
As expected, rats treated with morphine (4 mg/kg) displayed hyperthermia and conditioned place preference (CPP). Co-treatment with CTX or CA inhibited development of morphine-induced CPP by approximately 70%. Morphine’s hyperthermic effect was also suppressed, with CTX and CA producing 57% and 47% inhibition, respectively. Locomotor sensitization induced by repeated morphine exposures was inhibited by CA but not CTX.
Conclusions
The present findings are the first to suggest that CA disrupts the in vivo actions of morphine and point toward further studying CA as a potential therapy for drug addiction. Further, its ability to disrupt morphine’s rewarding effects at 20-fold lower doses than CTX identifies CA as an existing, orally-active alternative to direct CTX therapy for CNS diseases.
Keywords: Clavulanic acid, ceftriaxone, β-lactam antibiotic, conditioned place preference, β-lactamase inhibitor, morphine, glutamate transporter, sensitization
1. INTRODUCTION
The β-lactam antibiotic ceftriaxone (CTX; Rocephin®) can improve symptoms of CNS diseases in multiple animal models including amyotrophic lateral sclerosis, multiple sclerosis, stroke, seizure, Huntington’s disease, depression, and drug addiction (Rao and Sari, 2012; Ward et al., 2011; Knackstedt et al., 20e10; Rawls et al., 2007, 2010; Miller et al., 2008; Sari et al., 2009; Lipski et al., 2007; Rothstein et al., 2005). Despite promising preclinical results with CTX, its clinical utility as a therapeutic for CNS diseases may be limited by unfavorable pharmacokinetic and pharmacodynamic properties. One drawback is poor blood-brain barrier penetrability that requires administration of high doses (i.e., 200 mg/kg for 5-10 days in rodents extrapolating to 13 g/day in humans) to achieve CNS efficacy, thus increasing the risk of adverse effects such as toxicity and diarrhea (Rothstein et al., 2005).
An alternative to CTX is clavulanic acid (CA), which is normally administered in combination with amoxicillin (Augmentin) to overcome resistance in bacteria that secrete the β-lactamase enzyme which otherwise inactivates most penicillins. CA is structurally similar to CTX. Both compounds contain a central β-lactam ring required for enhancement of cellular glutamate reuptake through activation of glutamate transporter subtype 1 (GLT-1), which is thought to be a primary reason for the efficacy of CTX in animal models of CNS diseases (Rothstein et al., 2005). Relative to CTX, CA displays a number of desirable properties related to CNS activity, including negligible antibacterial activity, oral bioavailability (64-75%), and enhanced brain penetrability evident from a cerebrospinal fluid/plasma ratio of around 0.25 in patients with intact meninges (Kim et al., 2009; Nakagawa et al., 1994; Bolton et al., 1986).
Based on their structural commonality and differences in brain penetrability, we hypothesized that CA would display comparable efficacy to CTX, but at much lower doses, against three effects of morphine that are predominantly CNS-mediated: 1) rewarding effects assessed by the conditioned place preference (CPP) assay; 2) hyperthermia produced by acute morphine exposure; and 3) locomotor sensitization in rats exposed to repeated morphine, withdrawn from morphine, and then reintroduced to morphine following a period of forced abstinence. We selected these endpoints because of the ability of CTX to inhibit in vivo effects of morphine (Rawls et al., 2007; 2010a, b; Rao and Sari, 2012) and other drugs of abuse including psychostimulants and alcohol (Knackstedt et al. 2010; Sondheimer and Knackstedt, 2011; Trantham-Davidson et al. 2012; Sari et al., 2009; Alajaji et al. 2013; Fischer et al. 2013).
2. METHODS
2.1. Animals, chemicals and dosing schedule
Male Sprague-Dawley rats (225-250 g) were pair-housed, maintained on a 12-hr light/dark cycle, and provided ad libitum access to food and water. Procedures were approved by the Institutional Animal Care and Use Committees. Ceftriaxone sodium (CTX) and potassium clavulanate (CA) were injected intraperitoneally (i.p.) at 200 and 10 mg/kg, respectively. Morphine sulfate was injected subcutaneously (s.c.) and provided by the National Institute on Drug Abuse (NIDA). Morphine was injected at doses of either 4 mg/kg (CPP, body temperature experiments) or 20 mg/kg (locomotor experiments). All drugs were dissolved in saline.
Dosing schedules for CTX and CA utilized repeated injections. This paradigm was based on consistent evidence across multiple laboratories showing that CTX has to be given repeatedly, for at least 5 days, and at a dose of 200 mg/kg, to detect significant efficacy in animal models of CNS diseases (Rothstein et al., 2005). For comparative purposes, CA was administered under the same schedule, and its dose of 10 mg/kg was estimated from prior in vivo work (Shanna et al., 2013; Kost et al., 2009).
2.2. Effects of CA and CTX on morphine-induced conditioned place preference (CPP)
For CPP experiments a two-sided Stoelting chamber (40 × 45 × 35 cm) separated by a removable partition was used. Rats were handled for 4 days and then received three consecutive daily injections of CTX or CA prior to conditioning. Rats continued to receive daily injections of CTX, saline or CA (following chamber exposure) during conditioning (but not on the post-test day). A biased-CPP procedure consisting of three phases was employed. During bias testing (day 1) each rat was allowed free access to both sides of the chamber for 30 min and individual preferences were determined. The non-preferred side was used as the drug-paired environment. During conditioning (days 2-7) rats were injected with morphine or saline on alternating days and were confined to the appropriate side for 30 min. A counterbalanced design was used such that half of the rats received morphine and were confined to the non-preferred side on days 2, 4 and 6 while the other half of the subjects received morphine on days 3, 5 and 7. During testing (day 8) rats did not receive an injection and were allowed to freely explore both sides of the chamber for 30 min. Preference score was determined by subtracting time spent in the non-preferred side during the pretest from time spent in the drug-paired side during testing.
2.3. Effects of CA and CTX on morphine-induced hyperthermia
For body temperature experiments rats were randomly divided into 6 groups and injected for 7 days with CTX, CA, or saline. One day after the last injection rats were placed individually into a temperature-controlled room (21 ± 0.3°C) and allowed to acclimatize for 60 min (Rawls et al., 2007). Baseline rectal temperatures were then taken every 30 min for 90 min using a thermistor probe and digital thermometer. A mean baseline temperature was determined from the 3 baseline temperatures. Rats were then injected with morphine or saline, and body temperature was measured 60 min after the injection of morphine or saline. To test for acute effects of CA or CTX, another set of experiments were conducted as described above except that CA or CTX was injected only once, 30 min prior to morphine.
2.4. Effects of CA and CTX on morphine-induced locomotor sensitization
The dosing schedule used to induce sensitization of locomotor activity to morphine was based on a paradigm previously used to demonstrate the presence of morphine physical dependence in rats (Rawls et al., 2010b). In that study, repeated injections of CTX during chronic morphine exposure reduced the severity of the withdrawal syndrome precipitated by naloxone. In the present experiment, morphine (20 mg/kg) or saline was injected twice daily for 10 days (07.00 and 19.00 h). Thirty min before each morphine injection, rats were injected with saline, CTX or CA. Following the last day of repeated treatments, rats underwent 10 days of forced abstinence in which they did not receive any injection. Following the 10-day abstinence interval, rats were challenged with morphine (20 mg/kg) or saline. Injections were conducted in home cages except for the day of morphine challenge on which behavioral experimentation was conducted.
For assessment of locomotor activity, rats were placed individually into activity chambers and allowed to acclimate for 60 min. Basal activity was recorded for 60 min prior to morphine injection, followed by recording of activity for 60 min. The Digiscan DMicro system (Accuscan, Inc., Columbus, OH) was used to measure locomotor activity as described (Tallarida et al., 2013; Lisek et al., 2012; Rasmussen et al., 2011). Chambers consisted of transparent plastic boxes (45 cm × 20 cm × 20 cm) set inside metal frames equipped with 16 infrared light emitters and detectors. The beam height was 4.5 cm, and the space between beams was 2.5 cm. The number of photocell beam breaks was recorded by a computer interface.
2.5. Data analysis
Data were analyzed by two-way ANOVA (pretreatment, treatment), and a Bonferroni test was used to identify differences between individual treatment groups. In all cases, P < 0.05 was considered statistically significant.
3. RESULTS
3.1. CA and CTX attenuate rewarding effects of morphine
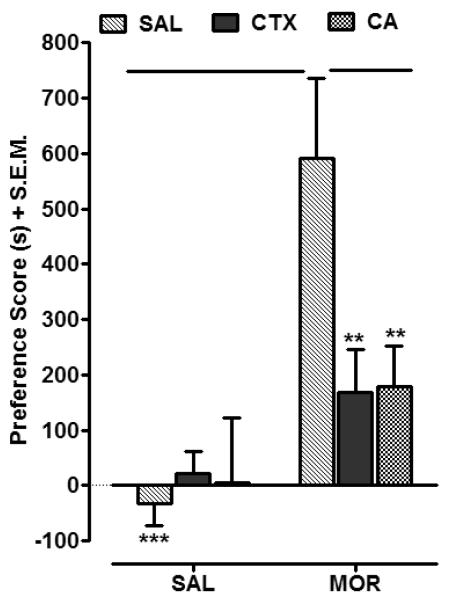
Two-way ANOVA conducted on the data set presented in Fig. 1 revealed significant treatment, pretreatment and interaction effects (Treatment, [F (1, 50) = 157.84, P < 0.0001]; Pretreatment, [F (2, 50) = 24.45, P < 0.0001]; and Interaction, [F (2, 50) = 38.31, P < 0.0001]). Morphine-treated rats displayed a significant preference shift relative to saline controls (P < 0.001). Preference for the cocaine-paired side was significantly reduced in rats pretreated with CTX compared to rats pretreated with saline (P < 0.01). Similarly, in rats pretreated with CA, the preference for the cocaine-paired side was significantly less than in rats pretreated with saline (P < 0.01). Pretreatment with CTX inhibited morphine-induced CPP by 72% whereas pretreatment with CA produced a 70% inhibition of the morphine preference. No significant CPP was observed in rats pretreated with CTX or CA followed by saline (P > 0.05).
Fig. 1.
CTX (200 mg/kg) and CA (10 mg/kg) attenuated CPP to morphine (4 mg/kg) (MOR). During conditioning, rats were pretreated with saline (SAL), CTX, or CA followed by the injection of either SAL or MOR. Data are expressed as mean preference score (s) + S.E.M. N=8-10 rats/group. **P < 0.01, ***P < 0.01 compared to SAL MOR group.
3.2. CA and CTX attenuate the hyperthermic effect of morphine
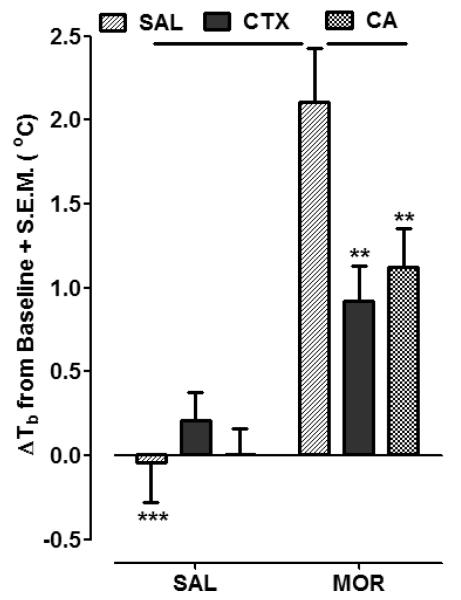
Effects of CTX and CA on morphine-induced hyperthermia are presented in Fig. 2. Mean baseline temperature values for the groups presented in Fig. 2 were: (SAL + SAL, 37.8 ± 0.22; CTX + SAL, 37.6 ± 0.17; CA + SAL, 37.6 ± 0.12; SAL+ MOR, 37.8 ± 0.25; CTX + MOR, 37.5 ± 0.21; and CA + MOR, 37.3 ± 0.27). Two-way ANOVA conducted on the data set presented in Fig. 2 revealed significant treatment and interaction effects (Treatment, [F (1, 30) = 51.11, P < 0.0001]; Pretreatment, [F (2, 30) = 2.87, P = 0.0727]; and Interaction, [F (2, 30) = 5.37, P < 0.05]). In saline-pretreated rats, the administration of morphine produced significant hyperthermia (2.11 ± 0.32 oC) compared to saline administration (P < 0.001). The hyperthermic response to morphine was attenuated in rats pretreated with CTX (0.92 ± 0.21 oC) (P < 0.01) or CA (1.12 ± 0.23 oC) (P < 0.01). In rats pretreated with CTX or CA, the change in body temperature following saline injection was not significantly different than in rats pretreated with saline (P > 0.05). The hyperthermic effect of morphine was not impacted by a single, acute injection of either CA or CTX (P > 0.05) (data not shown). In these acute experiments, the hyperthermia produced by morphine was not significantly different in rats pretreated with saline (1.78 ± 0.31 oC) than in rats pretreated with CTX (1.64 ± 0.28 oC) or CA (2.01 ± 0.45 oC) (P > 0.05, n=6 rats per group).
Fig. 2.
CTX (200 mg/kg) and CA (10 mg/kg) attenuated the hyperthermic effect of an acute injection of morphine (4 mg/kg) (SAL MOR). Rats were pretreated with saline (SAL), CTX, or CA followed by the injection of either SAL or MOR. Data are expressed as mean change in body temperature (60 min post-SAL or MOR injection) from baseline (ΔTb oC) + S.E.M. N=6 rats/group. **P < 0.01, ***P < 0.001 compared to SAL MOR group.
3.3. CA and CTX attenuate morphine-induced locomotor sensitization
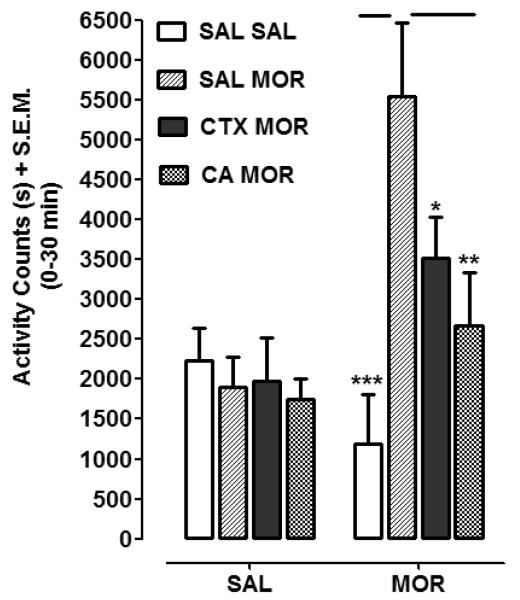
Effects of CTX and CA on sensitization of locomotor activity produced by repeated morphine injections are shown in Fig. 3. Two-way ANOVA conducted on the data set revealed significant treatment, pretreatment and interaction effects (Treatment, [F (1, 50) = 8.60, P < 0.01]; Pretreatment, [F (3, 50) = 3.97, P < 0.05]; and Interaction, [F (3, 50) = 5.00, P < 0.01]). Post-hoc analysis revealed that a challenge injection of morphine produced greater locomotor activity in rats with prior morphine experience (SAL-MOR + MOR) than in rats previously naïve to morphine (SAL-SAL + MOR) (P < 0.001). In rats pretreated with a combination of morphine and CA (CA-MOR + MOR), a challenge injection of morphine produced less locomotor activity than in rats pretreated with only morphine (SAL-MOR + MOR) (P < 0.05). In rats pretreated with a combination of morphine and CTX (CTX-MOR + MOR), locomotor activity produced by morphine challenge was also less than in rats pretreated with only morphine (SAL-MOR + MOR) (P < 0.01). In rats entirely naïve to morphine, a saline injection following pretreatment with CTX (CTX SAL) or CA (CA SAL) did not produce locomotor activation that was significantly different from rats pretreated with only saline (SAL SAL) (P > 0.05).
Fig. 3.
CTX (200 mg/kg) and CA (10 mg/kg) pretreatment attenuated locomotor sensitization produced by morphine (MOR) (20 mg/kg). Rats were pretreated with saline/saline (SAL SAL), saline/morphine (SAL MOR), CTX/morphine (CTX MOR), or CA/morphine (CA MOR). Following 10 days of drug absence, rats from each group were treated with either saline (SAL) or morphine (MOR) and locomotor activity was measured. Data are expressed as total activity counts (0-30 min post-injection) following MOR or SAL challenge. N=6-8 rats/group. *P < 0.05, **P < 0.01, ***P < 0.001 compared to SAL MOR group.
4. DISCUSSION
CA displayed efficacy against the rewarding, hyperthermic, and locomotor-sensitizing effects of morphine at 20-fold lower doses than CTX. Prior work has shown that CTX disrupts pharmacological effects of different classes of drugs of abuse. Some of the effects of CTX are prevention of analgesic tolerance and physical dependence that develops during chronic morphine exposure (Rawls et al., 2007, 2010a, b), inhibition of opioid-induced hyperalgesia (Chen et al., 2012), inhibition of relapse to cocaine seeking (Knackstedt et al., 2010; Sari et al., 2009), inhibition of the direct reinforcing effects of cocaine in mice maintained under a progressive-ratio responding schedule (Ward et al., 2011), inhibition of locomotor sensitization produced by cocaine and amphetamine (Tallarida et al., 2013; Sondheimer and Knackstedt, 2011; Rasmussen et al., 2011), prevention of methamphetamine-induced CPP (Abulseoud et al., 2013), and reduction of alcohol consumption (Sari et al., 2013; Rao and Sari, 2012). Despite structural similarities between CA and CTX, and the more favorable pharmacokinetic and physiochemical properties of CA as related to CNS activity (Nakagawa et al., 1994; Bolton et al., 1986), the potential effectiveness of CA as a therapeutic for CNS diseases is limited to studies that have examined neuroprotection, anxiety, and erectile dysfunction (Kim et al., 2009; Kost et al., 2011, 2012; Sanna et al., 2013). To our knowledge, the present results are the first evidence that CA can disrupt rewarding and sensitizing effects of an addictive substance.
Doses of 10 mg/kg of CA and 200 mg/kg of CTX were approximately equi-effective in reducing CPP produced by morphine. One explanation for CA displaying efficacy at a 20-fold lower dose than CTX is enhanced brain penetrability, particularly since the CSF/plasma ratios of CA and CTX are 0.25 and 0.1, respectively, in subjects with intact meninges (Nakagawa et al., 1994). Involvement of additional factors, such as differences in catabolism or pharmacological mechanism of action, cannot be discounted. The mechanism most commonly linked to the CNS efficacy of CTX is enhancement of cellular glutamate uptake through activation of GLT-1 transporters (Rothstein et al., 2005), although additional mechanisms, including chelation of calcium ions, impairment of antigen-specific T cell migration into the CNS and enhancement of cystine-glutamate exchange (Lipski et al., 2007; Melzer et al., 2008; Lewerenz et al., 2009), have been identified. In keeping with the GLT-1 mechanism, prior work has shown that CTX inhibits morphine-induced hyperthermia and analgesic tolerance through GLT-1 transporter activation, evident from the finding that CTX efficacy is abolished by the selective GLT-1 transporter inhibitor dihydrokainate (Rawls et al., 2007, 2010a). Although effects of CTX on CPP produced by morphine or other opioids have not been reported, it should be noted that morphine-induced CPP is reduced by MS-153, a neuroprotective agent with multiple mechanisms of action, including enhancement of glutamate reuptake and blockade of voltage-gated channels (Nakagawa et al., 2005). More recently, it was demonstrated that CTX inhibits the development of methamphetamine-induced CPP (Abulseoud et al., 2012).
The mechanism of action underlying CA efficacy against morphine’s rewarding effects was not investigated in the present experiments but will be the focus of future studies. On the basis of its neuroprotective action and structural similarity to CTX (Kost et al., 2012), one possibility is that CA, similar to CTX, increases GLT-1 transporter activity, perhaps due to an elevation in GLT-1 protein expression (Rothstein et al., 2005). Indeed, as noted above, pharmacological strategies that enhance GLT-1 activity are thought to inhibit two of the morphine-induced effects (CPP, hyperthermia) that were attenuated by CA in the present experiments (Nakagawa et al., 2005; Rawls et al., 2010a; 2007). Furthermore, the third effect, behavioral sensitization, is highly dependent on increased glutamate transmission and blocked by antagonism of NMDA and AMPA receptors (Vanderschuren and Kalivas, 2000; Jeziorski et al., 1994; Carlezon et al., 1999). The ability of CA and CTX to attenuate morphine-induced behavioral sensitization in the present experiments is consistent with a glutamate-based mechanism. It is also in accordance with earlier work demonstrating that CTX inhibits locomotor sensitization produced by amphetamine (Rasmussen et al., 2011) and cocaine (Tallarida et al., 2013; Sondheimer and Knackstedt, 2011) and MS-153 inhibits behavioral sensitization produced by phencyclidine (Abekawa et al., 2002). Additional mechanisms of action that may have contributed to the efficacy of CA against morphine’s rewarding effects include enhancement of monoamine transmission and inhibition of glutamate carboxypeptidase II (GCPII), also known as NAALADase (Sanna et al., 2013; Banani et al., 2012; Kost et al., 2011). A role for 5-HT systems in the conditioned rewarding effects of morphine have been demonstrated previously, with activation of 5-HT2 receptors producing inhibition and blockade of 5-HT3 receptors causing enhancement (Nomikos and Spyraki, 1988; Acquas et al., 1988; Carboni et al., 1989; Higgins et al., 1992). GCPII (NAALADase) is a predominantly astrocytic enzyme that catalyzes the conversion of NAAG (N-Acetylaspartylglutamic acid), an endogenous mGluR2/3 agonist, into glutamate (Slusher et AL., 1999). Pharmacological inhibition of GCPII is known to attenuate morphine tolerance and reward, perhaps through endogenous activation of presynaptic mGluR2/3 autoreceptors (Popik et al., 2003; Bossert et al., 2005).
CA has also been shown to increase dopamine transmission (Kost et al., 2011; Sanna et al., 2013). While the possibility that CA increased dopamine transmission in the present experiments cannot be excluded, it is unlikely that such an increase, if it did occur, could have accounted for the efficacy of CA against morphine’s pharmacological effects. This is because enhanced dopamine transmission would not be expected to inhibit the rewarding effects of morphine. Further, the development of behavioral sensitization to morphine is thought to be more dependent on glutamate transmission than on dopamine transmission (Vanderschuren and Kalivas, 2000). Although it is somewhat difficult to reconcile the behavioral effects of CA observed here with its dopamine-enhancing effects (Sanna et al., 2013; Kost et al., 2011, 2012), it should be noted that our study used a dosing paradigm in which CA was administered repeatedly. This dosing paradigm was used because multiple laboratories have demonstrated that the preclinical activity of CTX in reducing the symptoms of CNS diseases is dependent on repeated administration (Rothstein et al., 2005; Sari et al., 2009, 2013; Rawls et al., 2007, 2010a, b; Tallarida et al., 2013; Trantham-Davidson et al., 2012). In contrast to our dosing schedule, the in vivo studies showing that CA increases dopamine transmission have used a design in which CA was injected once (Kim et al., 2009; Sanna et al., 2013). Though speculative, it could be that monoamine transmission is augmented following acute CA exposure and that glutamate uptake is enhanced following chronic exposure to CA. Alternatively, the neuroprotective and anti-opioid effects of CA that are potentially dependent on a reduction in glutamate transmission could occur subsequent to direct impacts of CA on monoamine transmission. Regardless, now that the efficacy of CA has been shown against morphine, future studies will turn toward delineating the mechanism of action of CA to identify its potential impacts on glutamatergic, dopaminergic, and 5-HT systems that contribute to the addictive process.
The selection of CTX and CA doses, as well as the dosing schedule, was based on evidences across multiple laboratories that reliable CNS activity requires repeated exposure to doses of 100-200 mg/kg, a feature that may be related to its poor brain penetrability (Ward et al., 2011; Knackstedt et al., 2010; Rawls et al., 2007, 2010a,b; Miller et al., 2008; Sari et al., 2009; Lipski et al., 2007; Rothstein et al., 2005). Thus, at doses below 200 mg/kg, it is unlikely that CTX would have exerted significant inhibition of morphine-induced responses. Furthermore, in a case in which a higher dose of CTX (400 mg/kg) was tested against naloxone-precipitated withdrawal responses in morphine-dependent rats, it was no more effective than 200 mg/kg but did increase the prevalence of diarrhea (Rawls et al., 2010b). For CA it will be important in future experiments to conduct dose-effect experiments to determine if CNS activity can be detected at doses lower than the10 mg/kg used here.
In summary, the present findings suggest that CA has the ability to disrupt in vivo actions of morphine. Our results point to studying CA as a potential therapy for drug addiction and, most importantly, an attractive alternative to direct CTX therapy for management of CNS diseases. Possible benefits of repositioning CA for new indications are that it has been vetted in diverse patient populations, displays oral activity, is brain penetrable, possesses only negligible antibacterial activity, and potentially acts through multiple mechanisms of action.
Acknowledgements
None.
Author Disclosures Role of Funding Source This study was funded and supported by National Institute on Drug Abuse grants DA030676, DA025314, and DA01342. NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors Authors Scott M. Rawls and Joseph Schroeder designed the studies. Authors Nicholas G. Tolman, Faye F. McKenna, Kelly L. Watkins, Sara M. Passeri, Alexander H. Hsu, and Brittany R. Shinn conduced conditioned place preference experiments. Author Scott M. Rawls conducted body temperature and locomotor experiments. Authors Scott M. Rawls and Joseph Schroeder conducted the statistical analyses for the experiments and managed the literature searches and summaries of previous related work. Author Scott M. Rawls wrote drafts of the manuscript, which were subsequently circulated to all authors for their comments, critiques and suggestions. All authors contributed to and have approved the final manuscript.
Conflict of Interest All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abekawa T, Honda M, Ito K, Inoue T, Koyama T. Effect of MS-153 on the development of behavioral sensitization to locomotion- and ataxia-inducing effects of phencyclidine. Psychopharmacology. 2002;160:122–131. doi: 10.1007/s00213-001-0958-1. [DOI] [PubMed] [Google Scholar]
- Abulseoud OA, Miller JD, Wu J, Choi DS, Holschneider DP. Ceftriaxone upregulates the glutamate transporter in medial prefrontal cortex and blocks reinstatement of methamphetamine seeking in a condition place preference paradigm. Brain Res. 2012;1456:14–21. doi: 10.1016/j.brainres.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alajaji M, Bowers MS, Knackstedt L, Damaj MI. Effects of the beta-lactam antibiotic ceftriaxone on nicotine withdrawal and nicotine-induced reinstatement of preference in mice. Psychopharmacology. 2013;228:419–426. doi: 10.1007/s00213-013-3047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acquas E, Carboni E, Leone P, Di Chiara G. 5-HT3 receptors antagonists block morphine- and nicotine- but not amphetamine-induced place-preference conditioning. Pharmacol. Res. Commun. 1988;20:1113–1114. doi: 10.1016/s0031-6989(88)80752-5. [DOI] [PubMed] [Google Scholar]
- Banani A, Maleki-Dizaji N, Garjani A, Soraya H, Mostafalou S, Ziaee M. Clavulanic acid exhibits anti-inflammatory effects on carrageenan-induced paw edema model of inflammation in rats. Ann. Biol. Res. 2012;3:3312–3320. [Google Scholar]
- Bolton GC, Allen GD, Davies BE, Filer CW, Jeffery DJ. The disposition of CA in man. Xenobiotica. 1986;16:853–863. doi: 10.3109/00498258609038967. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Busch RF, Gray SM. The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport. 2005;16:1013–1016. doi: 10.1097/00001756-200506210-00026. [DOI] [PubMed] [Google Scholar]
- Carboni E, Acquas E, Leone P, Di Chiara G. 5HT3 receptor antagonists block morphine- and nicotine- but not amphetamine-induced reward. Psychopharmacology. 1989;97:175–178. doi: 10.1007/BF00442245. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Rasmussen K, Nestler EJ. AMPA antagonist LY293558 blocks the development, without blocking the expression, of behavioral sensitization to morphine. Synapse. 1999;31:256–262. doi: 10.1002/(SICI)1098-2396(19990315)31:4<256::AID-SYN3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Chen Z, He Y, Wang ZJ. The beta-lactam antibiotic, ceftriaxone, inhibits the development of opioid-induced hyperalgesia in mice. Neurosci. Lett. 2012;509:69–71. doi: 10.1016/j.neulet.2011.12.029. [DOI] [PubMed] [Google Scholar]
- Fischer KD, Houston AC, Rebec GV. Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. J. Neurosci. 2013;33:9319–9327. doi: 10.1523/JNEUROSCI.3278-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Joharchi N, Nguyen P, Sellers EM. Effect of the 5-HT3 receptor antagonists, MDL72222 and ondansetron on morphine place conditioning. Psychopharmacology. 1992;106:315–320. doi: 10.1007/BF02245411. [DOI] [PubMed] [Google Scholar]
- Jeziorski M, White FJ, Wolf ME. MK-801 prevents the development of behavioral sensitization during repeated morphine administration. Synapse. 1994;16:137–147. doi: 10.1002/syn.890160207. [DOI] [PubMed] [Google Scholar]
- Kim DJ, King JA, Zuccarelli L, Ferris CF, Koppel GA, Snowdon CT, Ahn CH. CA: a competitive inhibitor of !-lactamases with novel anxiolytic-like activity and minimal side effects. Pharmacol. Biochem. Behav. 2009;93:112–120. doi: 10.1016/j.pbb.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. CTX restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol. Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost GC, Selvaraj S, Lee YB, Kim DJ, Ahn CH, Singh BB. Clavulanic acid increases dopamine release in neuronal cells through a mechanism involving enhanced vesicle trafficking. Neurosci. Lett. 2011;504:170–175. doi: 10.1016/j.neulet.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost GC, Selvaraj S, Lee YB, Kim DJ, Ahn CH, Singh BB. CA inhibits MPP(+)-induced ROS generation and subsequent loss of dopaminergic cells. Brain Res. 2012;1469:129–35. doi: 10.1016/j.brainres.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, Albrecht P, Tien ML, Henke N, Karumbayaram S, Kornblum H.I, Wiedau-Pazos, M., Schubert D, Maher P, Methner A. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. J. Neurochem. 2009;111:332–343. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- Lipski J, Wan CK, Bai JZ, Pi R, Li D, Donnelly D. Neuroprotective potential of CTX in in vitro models of stroke. Neuroscience. 2007;146:617–629. doi: 10.1016/j.neuroscience.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Melzer N, Meuth SG, Torres-Salazar D, Bittner S, Zozulya AL, Weidenfeller C, Kotsiari A, Stangel M, Fahlke C, Wiendl H. A beta-lactam antibiotic dampens excitotoxic inflammatory CNS damage in a mouse model of multiple sclerosis. PLoS One. 2008;3:e3149. doi: 10.1371/journal.pone.0003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Dorner JL, Shou M, Sari Y, Barton SJ, Sengelaub DR, Kennedy RT, Rebec GV. Up-regulation of GLT1 expression increases glutamate uptake and attenuates the Huntington’s disease phenotype in the R6/2 mouse. Neuroscience. 2008;153:329–337. doi: 10.1016/j.neuroscience.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Yamada M, Tokiyoshi K, Miyawaki Y, Kanayama T. Penetration of potassium clavulanate/ticarcillin sodium into cerebrospinal fluid in neurosurgical patients. Jpn. J. Antibiot. 1994;47:93–101. [PubMed] [Google Scholar]
- Nomikos GG, Spyraki C. Effects of ritanserin on the rewarding properties of d-amphetamine, morphine and diazepam revealed by conditioned place preference in rats. Pharmacol. Biochem. Behav. 1988;30:853–858. doi: 10.1016/0091-3057(88)90110-4. [DOI] [PubMed] [Google Scholar]
- Popik P, Kozela E, Wróbel M, Wozniak KM, Slusher BS. Morphine tolerance and reward but not expression of morphine dependence are inhibited by the selective glutamate carboxypeptidase II (GCP II, NAALADase) inhibitor, 2-PMPA. Neuropsychopharmacology. 2003;28:457–467. doi: 10.1038/sj.npp.1300048. [DOI] [PubMed] [Google Scholar]
- Rao PS, Sari Y. Glutamate transporter 1: target for the treatment of alcohol dependence. Curr. Med. Chem. 2012;19:5148–5156. doi: 10.2174/092986712803530511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen B, Unterwald EM, Rawls SM. Glutamate transporter subtype 1 (GLT-1) activator ceftriaxone attenuates amphetamine-induced hyperactivity and behavioral sensitization in rats. Drug Alcohol Depend. 2011;118:484–488. doi: 10.1016/j.drugalcdep.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Tallarida R, Robinson W, Amin M. The !-lactam antibiotic, ceftriaxone, attenuates morphine-evoked hyperthermia in rats. Br. J. Pharmacol. 2007;151:1095–1102. doi: 10.1038/sj.bjp.0707309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Zielinski M, Patel H, Sacavage S, Baron DA, Patel D. !-lactam antibiotic reduces morphine analgesic tolerance in rats through GLT-1 transporter activation. Drug Alcohol Depend. 2010;107:261–263. doi: 10.1016/j.drugalcdep.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. !-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sanna F, Melis MR, Angioni L, Argiolas A. Clavulanic acid induces penile erection and yawning in male rats: comparison with apomorphine. Pharmacol. Biochem. Behav. 103:750–755. doi: 10.1016/j.pbb.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN, Lee MR, Choi DS. Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. J. Mol. Neurosci. 2013;51:779–787. doi: 10.1007/s12031-013-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J. Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusher BS, Vornov JJ, Thomas AG, Hurn PD, Harukuni I, Bhardwaj A, Traystman RJ, Robinson MB, Britton P, Lu XC, Tortella FC, Wozniak KM, Yudkoff M, Potter BM, Jackson PF. Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat. Med. 1999;5:1396–1402. doi: 10.1038/70971. [DOI] [PubMed] [Google Scholar]
- Sondheimer I, Knackstedt LA. Ceftriaxone prevents the induction of cocaine sensitization and produces enduring attenuation of cue- and cocaine-primed reinstatement of cocaine-seeking. Behav. Brain Res. 2011;225:252–258. doi: 10.1016/j.bbr.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida CS, Corley G, Kovalevich J, Yen W, Langford D, Rawls SM. Ceftriaxone attenuates locomotor activity induced by acute and repeated cocaine exposure in mice. Neurosci. Lett. 2013;556:155–159. doi: 10.1016/j.neulet.2013.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trantham-Davidson H, LaLumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA. Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J. Neurosci. 2012;32:12406–12410. doi: 10.1523/JNEUROSCI.1976-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Kim J, Rasmussen BA, Corley G, Henry C, Walker EA, Rawls SM. !-lactam antibiotic decreases acquisition of and motivation to respond for cocaine, but not sweet food, in C57Bl/6 mice. Behav. Pharmacol. 2011;22:370–373. doi: 10.1097/FBP.0b013e3283473c10. [DOI] [PMC free article] [PubMed] [Google Scholar]