Abstract
Background
Drug dependent individuals often make drug-taking decisions when they do not feel well. Yet, few studies have examined the influence of an aversive state on decision-making related neural processing.
Methods
We investigate brain activation to decision-making during an aversive interoceptive challenge in methamphetamine users using functional magnetic resonance imaging (fMRI). Recently abstinent inpatients with methamphetamine use disorder (METH; n=20) and healthy comparison subjects (CTL; n=22) performed a two-choice prediction task at three fixed error rates (ER; 20%=reward, 50%=uncertainty, 80%=punishment) while anticipating and experiencing episodes of inspiratory breathing load during fMRI.
Results
METH exhibited higher trait anxiety in conjunction with lower anterior insula (AI) and inferior frontal gyrus (IFG) activation than CTL across trials. METH also showed lower posterior insula (PI) and anterior cingulate cortex (ACC) activation than CTL during breathing load independent of ER. For the crucial ER by interoception interaction, METH displayed lower ACC activation to punishment/loss than CTL during breathing load. Within METH, lower trait anxiety was linked to bilateral AI/IFG attenuation across trials.
Conclusions
AI/IFG attenuations in METH are suggestive of an executive functioning deficit, particularly in users with low anxiety, reflecting reduced resources allocated to choice selection. In contrast, PI/ACC reductions in METH appear specific to impairments in registering and evaluating interoceptive experiences. Taken together, inadequate activation of brain areas that are important for regulating when one does not feel well may be the neural basis for poor decision-making by METH.
Keywords: functional magnetic resonance imaging (fMRI), methamphetamine, decision-making, error processing, interoception, breathing load
1. Introduction
Methamphetamine addiction constitutes a major public health issue, linked to cardiac, pulmonary, and neurological impairments, heightened depression and anxiety, compromised decision-making, and poor quality of life (Gonzales et al., 2010). Identifying components of neural, behavioral, physiological, and experiential systems that are compromised in methamphetamine dependent individuals (METH) can facilitate innovative treatment interventions to reduce future harm to the individual and society (Shoptaw, 2014). The study of interoception, the physiological condition of the body (Craig, 2003), may aid in the search for markers of impairment. Brain regions involved in interoception may be dysregulated in addiction, contributing to the maintenance, escalation, and/or relapse of substance use (Gray and Critchley, 2007; Naqvi and Bechara, 2010; Paulus et al., 2009; Stewart and Paulus, 2013; Verdejo-Garcia et al., 2012).
Addiction may reflect a discrepancy between an individual's predicted versus actual internal state known as the bodily prediction error, an imbalance that could in turn adversely influence the degree of future drug-related approach versus avoidance behavior (Paulus and Stewart, 2014; Paulus et al., 2009). Researchers assert that the insular cortex coordinates with other brain regions to process and integrate somatosensory feeling states in order to guide future decisions (Bechara, 2005; Damasio et al., 1996). It has been argued (Blomqvist et al., 2000; Craig, 2003, 2014; Damasio and Carvalho, 2013) that thalamic regions deliver sensory information to posterior insula (PI) and then to anterior insula (AI), resulting in bodily feeling states that are registered by anterior cingulate cortex (ACC), initiating motivated action to regain internal homeostasis and minimize bodily prediction error (Critchley et al., 2004). METH may possess inadequate function in this relay system in response to bodily signals, which limits engagement in adaptive behavior (Paulus and Stewart, 2014).
Decision-making may be impaired in METH, particularly during conditions of interoceptive distress. Therefore, it is critical to understand the interaction between compromised interoception and cognitive control systems involving regions of the prefrontal cortex (PFC) and how this may lead to suboptimal decision-making in METH (e.g., using drugs despite negative outcomes). METH exhibit lower thalamic, AI and PFC activation than healthy comparison subjects (CTL) while making decisions within the context of pleasant interoceptive touch via soft bristle brush (May et al., 2013). Although no studies have examined decision-making in METH during the experience of aversive interoceptive stimuli, young adults transitioning to stimulant dependence exhibit lower thalamic, ACC, and PFC activation than stimulant-naïve CTL while making choices during an aversive inspiratory breathing load (Stewart et al., 2013). Moreover, AI, ACC, and PFC hypoactivations are often linked to aversive outcomes in METH. For instance, METH display lower ACC activation than CTL in response to losses (Gowin et al., 2014b). Attenuated AI activation during risky behavior with a potential for high loss also predicts future relapse in recently abstinent METH (Gowin et al., 2014a). AI and ACC hypoactivations are both linked to heightened error rates (ER) in METH (London et al., 2005). METH also exhibit lower AI, ACC, and PFC activation than CTL in situations of response conflict (Nestor et al., 2011; Salo et al., 2012) as well as AI and PFC reductions to aversive images of human suffering (Kim et al., 2011). Taken together, findings support the hypothesis that METH may not process negative consequences of drug use as prediction errors to correct and optimize future behavior (Shoptaw, 2014). This prediction is consistent with a more general deficit in METH showing attenuated PFC activation when making decisions within the context of all types of feedback – rewarding, uncertain, and punishing outcomes (Paulus et al., 2003, 2005). Therefore, METH may exhibit a general neural processing deficit characterized by reduced resources devoted to the integration of decision-outcome contingencies. In other words, for METH an aversive outcome does not necessarily result in corrective behavior.
Although METH is linked to impaired decision-making, individual differences in comorbid psychopathology, particularly anxiety, adversely impact treatment outcomes in METH (Glasner-Edwards et al., 2010). Anxiety symptoms, often heightened in METH (Gonzales et al., 2010; London et al., 2004; Salo et al., 2011), are linked to greater AI activation to aversive stimuli and risky decisions (Hartley and Phelps, 2012; Paulus and Stein, 2010; Simmons et al., 2011; Stein et al., 2007). These findings are suggestive of heightened prediction error in high anxious individuals, in direct contrast with attenuated prediction error predicted for METH. Individual differences in anxiety symptoms may moderate the neural responses of METH to interoceptive contexts, which might lead to differential relapse prevention strategies as a function of stress responses in METH.
The present study examined these issues by utilizing functional magnetic resonance imaging (fMRI) to compare neural mechanisms of decision-making during an aversive interoceptive manipulation in METH and CTL. A two-choice prediction task with varying types of feedback (20%, 50%, and 80% ERs) was employed to examine decision-making in response to rewarding, uncertain, and punishing outcomes, respectively. In addition, an inspiratory breathing load shown to activate AI and PFC during decision-making was used as an aversive interoceptive manipulation during the two-choice prediction task (Paulus et al., 2012; Stewart et al., 2013). Breathing physiology (carbon dioxide levels), behavioral performance, and subjective experience of the aversive manipulation were also measured to examine multiple facets of interoceptive processing. Furthermore, measures of emotion and personality were collected to examine individual differences in neural interoceptive processing within METH. Of interest was whether anxiety moderated AI activation within the context of aversive stimuli in METH, given that heightened anxiety has previously been linked to exaggerated AI responses to threat (Paulus and Stein, 2006).
Three hypotheses were tested in the present investigation: Compared to CTL, we predicted that METH would exhibit: (1) lower PFC activation across all trials, suggestive of reduced resource allocation for executive functions more generally (Paulus et al., 2003, 2002); (2) lower thalamic and AI activation in response to the aversive breathing load manipulation, consistent with prior work showing neural attenuations in METH within the context of pleasant interoceptive stimuli (May et al., 2013); and (3) lower AI and ACC activation during the aversive interoceptive condition specifically within the context of punishment (80% ER and high losses), in line with studies showing links between attenuated AI/ACC with losses, errors, and response conflict in METH (Gowin et al., 2014a, 2014b; London et al., 2005; Nestor et al., 2011; Salo et al., 2012).
2. Methods
2.1. Subject Recruitment
The study protocol was approved by the local Human Subjects Review Board and individuals gave written informed consent prior to enrollment. METH were recruited from the inpatient Veterans Administration Alcohol and Drug Treatment Program and local recovery homes. CTL were recruited from posted fliers and internet advertisements. Individuals were phone-screened to rule out: (1) left-handedness, assessed by the Edinburgh Handedness Inventory (Oldfield, 1971); (2) fMRI contraindications (e.g., irremovable metal; pregnancy; claustrophobia); (3) traumatic head injury and loss of consciousness > 5m. Individuals who passed the phone screen were scheduled for an interview.
2.2. Clinical Interview Session
Participants completed a urine screen, questionnaires, and the Semi-Structured Assessment for Drug Dependence and Alcoholism (SSADDA; Pierucci-Lagha et al., 2005) to determine presence of DSM-V Axis I disorders, Axis II antisocial personality disorder (ASPD), and lifetime substance use. Experimenters administered the Wide Range Achievement Test (WRAT-4) to obtain verbal intelligence quotient (IQ; Wilkinson and Robertson, 2006). Subjects completed personality and symptom assessment questionnaires known to correlate with substance use disorders, including the Sensation Seeking Scale (SSS; Zuckerman, 2007), the Barratt Impulsiveness Scale (BIS; Patton et al., 1995), the Beck Depression Inventory II (BDI-II; Beck et al., 1996), and the State-Trait Anxiety Inventory (STAI) trait anxiety subscale (Spielberger et al., 1983). To assess trait interoceptive responses and coping responses to stressful events, participants completed the Body Awareness Questionnaire (BAQ; Shields et al., 1989) and the COPE scale (Carver and Scheier, 1989), respectively.
Individuals were excluded from either group if they met criteria for: (1) lifetime schizophrenia, bipolar disorder, or obsessive compulsive disorder; (2) current (past six months) anxiety disorders or unipolar depression; (3) diagnosed neurological disorders; (4) ASPD; and (5) a positive urine toxicology test. METH were required to be in treatment, between 15-120 days sober (M=45.47; SD=19.76; range=16-91; n=1 missing data). No METH reported symptoms of withdrawal. CTL could not meet criteria for lifetime substance use disorder. Upon consensus review of diagnoses confirmed by a psychiatrist (M.P.P.), eligible participants were scheduled for the neuroimaging session.
The final cohort (Table 1) consisted of 42 participants (n=20 METH; n=22 CTL). All METH met criteria for current DSM-V methamphetamine use disorder. Some METH met criteria for comorbid current DSM-V use disorders: (1) alcohol (n=8); (2) cocaine (n=2); (3) marijuana (n=2); (4) opiate (n=2) (n=2 METH had missing diagnosis sheets and were unable to be categorized). On average, METH reported 15967.26 lifetime sessions of methamphetamine use (SD=18941.32; range=414-74310) and began using methamphetamine at 22 years of age (SD=8.12; range=12-46) (n=1 had missing use/age of onset data). With respect to nicotine use in the past month, 35% (7/20) METH reported smoking at least 10 cigarettes daily, whereas no CTL reported smoking at that frequency.
Table 1. Self-Report, Physiological, and Behavioral Results.
| METH (14M, 6F) |
CTL (15M, 7F) |
Group Statistics | ||||||
|---|---|---|---|---|---|---|---|---|
| Demographics | M | SD | M | SD | df | t | p | Cohen's d |
|
| ||||||||
| Age (Years) | 38.45 | 8.81 | 34.55 | 8.78 | 40 | 1.44 | 0.16 | — |
| Education (Years)† | 13.25 | 1.16 | 14.59 | 1.84 | 40 | 2.85 | 0.01 | 0.89 |
| WRAT-4 Verbal IQ | 95.36 | 14.3 | 109.58 | 11.46 | 31 | 3.18 | 0.01 | 1.10 |
|
| ||||||||
| Race/Ethnicity | Percentage | Percentage | df | χ2 | p | Cohen's d | ||
|
| ||||||||
| African American | 15% | 14% | 3 | 3.78 | 0.29 | |||
| Asian/Pacific Islander | 5% | 9% | ||||||
| Caucasian | 65% | 77% | — | |||||
| Mixed | 15% | 0% | ||||||
| Hispanic | 15% | 18% | 1 | 0.08 | 0.78 | — | ||
|
| ||||||||
| Personality Measures | M | SD | M | SD | df | t | p | Cohen's d |
|
| ||||||||
| BIS Impulsivity Total | 72.81 | 10.83 | 54.09 | 10.01 | 38 | 5.62 | 0.01 | 1.80 |
| SSS Sensation Seeking Total | 20.14 | 5.98 | 18.41 | 6.29 | 38 | 0.89 | 0.38 | — |
| STAI Trait Anxiety | 43.58 | 10.6 | 30.41 | 7.17 | 36 | 5.50 | 0.01 | 1.48 |
| BAQ Body Awareness | 83.44 | 21.8 | 75.43 | 22.87 | 37 | 1.11 | 0.27 | — |
|
| ||||||||
| Coping with Stressful Events | M | SD | M | SD | df | t | p | Cohen's d |
|
| ||||||||
| Positive Reinterpretation/Growth | 12.63 | 3.18 | 12.77 | 2.25 | 39 | 0.17 | 0.87 | — |
| Mental Disengagement | 9.84 | 2.52 | 8.41 | 2.61 | 39 | 1.78 | 0.08 | — |
| Focus on Venting Emotions | 10.39 | 2.81 | 7.50 | 2.56 | 38 | 3.40 | 0.01 | 1.08* |
| Instrumental Social Support | 10.79 | 3.22 | 11.27 | 2.39 | 39 | 0.55 | 0.59 | — |
| Active Coping | 11.44 | 2.59 | 12.41 | 2.58 | 38 | 1.18 | 0.25 | — |
| Denial† | 6.67 | 2.87 | 4.86 | 1.32 | 38 | 2.46 | 0.02 | 0.86* |
| Religion | 9.65 | 4.39 | 7.27 | 4.36 | 37 | 1.68 | 0.10 | — |
| Humor | 9.61 | 4.03 | 8.86 | 3.54 | 38 | 0.63 | 0.54 | — |
| Behavioral Disengagement | 7.59 | 3.62 | 5.95 | 2.62 | 36 | 1.62 | 0.12 | — |
| Restraint | 9.72 | 2.08 | 10.05 | 2.38 | 38 | 0.45 | 0.65 | — |
| Emotional Social Support | 9.88 | 4.09 | 10.23 | 2.93 | 37 | 0.29 | 0.77 | — |
| Substance Use† | 12.00 | 4.37 | 4.55 | 1.30 | 38 | 7.00 | 0.01 | 2.63 |
| Acceptance | 11.44 | 3.20 | 11.05 | 2.48 | 38 | 0.44 | 0.66 | — |
| Suppression Competing Activities | 10.65 | 2.12 | 10.23 | 2.45 | 37 | 0.56 | 0.58 | — |
| Planning | 12.24 | 3.44 | 12.86 | 2.55 | 37 | 0.66 | 0.52 | — |
|
| ||||||||
| Substance Use (# in Past Month) | M | SD | M | SD | df | t | p | Cohen's d |
|
| ||||||||
| Alcoholic Drinks | 3.59 | 10.33 | 6.52 | 10.23 | 36 | 0.88 | 0.39 | — |
| Caffeinated Drinks | 44.12 | 32.03 | 31.43 | 25.94 | 36 | 1.35 | 0.19 | — |
| Cigarettes† | 168.11 | 203.77 | 2.73 | 12.79 | 38 | 3.44 | 0.01 | 1.53 |
|
| ||||||||
| Pre-Scan State Affect | M | SD | M | SD | df | t | p | Cohen's d |
|
| ||||||||
| PANAS PA | 32.3 | 9.38 | 32.45 | 7.46 | 40 | 0.06 | 0.95 | — |
| PANAS NA† | 12.45 | 2.84 | 10.55 | 1.26 | 40 | 2.76 | 0.01 | 0.93* |
| BDI-II Depression†¤ | 11.78 | 8.26 | 0.92 | 1.32 | 20 | 3.91 | 0.01 | 2.27 |
|
| ||||||||
| Physiology During Task | M | SD | M | SD | df | t | p | Cohen's d |
|
| ||||||||
| Average CO2† | 1.56 | .21 | 1.19 | 0.36 | 30 | 3.58 | 0.01 | 1.30 |
|
| ||||||||
| Behavioral Performance | M | SD | M | SD | df | t | p | Cohen's d |
|
| ||||||||
| RT 20% ER | 600.62 | 162.50 | 529.79 | 103.83 | 40 | 1.70 | 0.10 | — |
| RT 50% ER | 616.63 | 163.67 | 540.48 | 101.61 | 40 | 1.83 | 0.08 | — |
| RT 80% ER | 617.81 | 149.75 | 552.37 | 111.15 | 40 | 1.62 | 0.11 | — |
| Win-Stay 20% ER | 0.56 | 0.25 | 0.58 | 0.25 | 40 | 0.34 | 0.74 | — |
| Win-Stay 50% ER | 0.56 | 0.25 | 0.55 | 0.21 | 40 | 0.31 | 0.76 | — |
| Win-Stay 80% ER | 0.51 | 0.16 | 0.55 | 0.17 | 40 | 0.81 | 0.43 | — |
| Lose-Shift 20% ER | 0.53 | 0.17 | 0.65 | 0.11 | 40 | 2.81 | 0.01 | 0.86 |
| Lose-Shift 50% ER | 0.54 | 0.17 | 0.67 | 0.15 | 40 | 2.61 | 0.02 | 0.81 |
| Lose-Shift 80% ER | 0.59 | 0.20 | 0.71 | 0.16 | 40 | 2.03 | 0.04 | 0.67 |
|
| ||||||||
| VAS Breathing Load Ratings | M | SD | M | SD | df | t | p | Cohen's d |
|
| ||||||||
| Pleasantness | 4.19 | 3.21 | 3.50 | 2.75 | 34 | 0.69 | 0.49 | — |
| Unpleasantness | 2.88 | 2.94 | 4.15 | 3.47 | 34 | 1.18 | 0.25 | — |
| Intensity† | 1.72 | 1.72 | 3.12 | 3.02 | 34 | 1.68 | 0.10 | — |
| Tingling Sensations† | 1.05 | 2.24 | 0.38 | 0.71 | 34 | 1.24 | 0.23 | — |
| Fear of Losing Control | 0.42 | 1.07 | 0.79 | 1.78 | 33 | 0.73 | 0.47 | — |
| Faintness | 0.44 | 1.41 | 0.42 | 1.46 | 34 | 0.05 | 0.96 | — |
| Fear of Dying | 0.17 | 0.48 | 0.25 | 0.74 | 34 | 0.39 | 0.70 | — |
| Unreality | 0.49 | 1.27 | 0.81 | 2.32 | 34 | 0.51 | 0.61 | — |
| Hot and Cold Flashes† | 0.35 | 0.77 | 0.13 | 0.37 | 34 | 1.05 | 0.31 | — |
| Trembling | 0.48 | 1.00 | 0.45 | 1.72 | 34 | 0.07 | 0.94 | — |
| Choking | 0.37 | 1.12 | 0.38 | 0.93 | 34 | 0.06 | 0.96 | — |
| Fear of Going Crazy | 0.17 | 0.36 | 0.48 | 1.81 | 34 | 0.70 | 0.49 | — |
| Abdominal Distress | 0.61 | 1.86 | 0.53 | 1.99 | 33 | 0.11 | 0.91 | — |
| Chest Pain† | 0.78 | 2.04 | 0.11 | 0.30 | 34 | 1.36 | 0.19 | — |
| Heart Palpitations | 0.12 | 0.27 | 0.13 | 0.43 | 34 | 0.07 | 0.95 | — |
| Sweating | 0.35 | 0.89 | 0.12 | 0.25 | 34 | 1.11 | 0.27 | — |
| Dizziness | 0.37 | 0.92 | 0.24 | 0.52 | 34 | 0.50 | 0.62 | — |
Note:
Groups have unequal variances.
BDI-II was added to the study protocol midway through data collection, so only 9 METH and 13 CTL completed this measure.
Group differences were no longer significant for this measure after 8 METH with comorbid alcohol use disorder were removed from analysis. METH = methamphetamine use disorder subjects. CTL = healthy comparison subjects. WRAT-4 = Wide Range Achievement Test. BIS = Barratt Impulsiveness Scale. SSS = Sensation Seeking Scale. STAI = State Trait Anxiety Scale. BAQ = Body Awareness Questionnaire. PANAS = Positive and Negative Affect Scale. PA = Positive Affect. NA = Negative Affect. CO2 = Carbon Dioxide. RT = reaction time (ms). ER = ER. VAS = Visual Analog Scale. Degrees of freedom < 40 indicate missing data.
2.3. Neuroimaging Session
2.3.1. Overview
Subjects completed pre-scan urine tests and subjective reports of state affect using the Positive and Negative Affect Schedule (PANAS) (Watson et al., 1988), followed by a fMRI scan consisting of a two-choice prediction task with an added breathing load manipulation.
2.3.2. Breathing load apparatus
Subjects wore a nose clip and respired through a mouthpiece and non-rebreathing valve (2600 series, Hans Rudolph). The apparatus was attached to the fMRI head coil, eliminating the need for mouth muscle contraction while maintaining an airtight seal. The resistance load was a stainless steel screen mesh disk placed in a Plexiglas tube (loading manifold). Subjects were given a 40 cmH2O/L/sec inspiratory load applied to only the inspiratory port of the non-rebreathing valve for 40 seconds at a time. Prior to scanning, subjects were given instructions about the task and experienced three 1-minute breathing load segments. Subjects completed post-scan VAS questionnaires, wherein they rated the breathing load experience on a 10 cm scale anchored from “not at all” (0) to “extremely” (10) on 16 dimensions (see Table 1; Chan and Davenport, 2008).
2.3.3. Two-choice prediction task
For the present study, a task previously used to examine decision-making situations with uncertain outcomes (Paulus et al., 2003, 2002, 2005) was coupled with an aversive interoceptive breathing load manipulation. For each trial (fixed duration: 5000 ms), a house appeared in the center of the computer screen (variable duration: 416-797 ms), followed by an updated image of the house with two people: one to the left and one to the right (fixed duration: 1500 ms; Figure 1). Subjects were instructed to predict, at the beginning of each trial, which person would be picked up by the car by pressing the left or right button. If subjects did not respond, they automatically received negative feedback following response timeout. Participants were given no predictive information and had to make a choice based on the history of preceding responses and outcomes.
Figure 1.
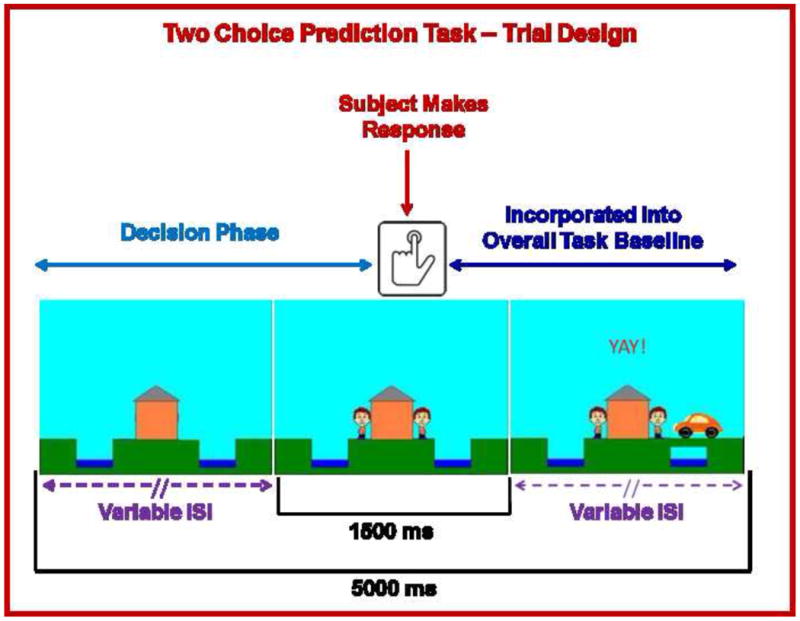
Illustration of two choice prediction task. For each trial, a house was shown in the center with two people: one on the left and one on the right of the house. Subjects pressed a button to predict whether a car would come by on the left or right side to pick up the person. After the subject made a decision, the car was presented on the left or right side of the screen. If the selected response matched the side where the car was presented, the person on the selected side met up with the car. Although each trial lasted 5000 ms and subjects were allowed to respond with a button press during a fixed 1500 ms period at the point when they saw the two people on the screen, the length of the beginning and ends of each trial were designed to have variable inter-stimulus intervals (ISI). Brain activation consisting of time from trial onset to the button press was included in fMRI analysis as a decision regressor of interest, wherein brain activation during the remainder of the trial was incorporated into the overall baseline regressor.
After the reaction time (RT) window ended, the car was presented on the far left or right side of the screen for the remainder of the trial (variable duration: 2703-3084 ms). If the selected response (left or right) matched the side where the car was presented, the person on the selected side met up with the car. Unbeknownst to the subject, the car was presented according to a predetermined schedule. A computer algorithm, which took each subject's response into account, determined whether a response would be ‘correct’ or ‘incorrect’. Correct responses consisted of “YAY!” presented in the top center of the computer screen for the remainder of the trial duration, while incorrect responses consisted of “BOO!” presented in the same location. Time of trial onset to the subject's button press was considered the decision phase of interest, whereas the remaining portion of the trial was incorporated into the overall baseline with which the decision phase was later compared.
Figure 2 shows that the two-choice prediction task was divided into three types of trials with differing ERs: (1) 20% ER = reward: “YAY” presented after 80% of responses and “BOO” presented for remaining 20%; (2) 50% ER = uncertainty: “YAY” presented after 50% of responses and “BOO” presented for remaining 50%; and (3) 80% ER = punishment: “YAY!” presented after 20% of responses and “BOO!” presented for remaining 80%. Two runs of 122 trials each were presented (total number of trials for each ER: 20%=80, 50%=84, 80%=80). Each ER was presented consecutively for 9-20 trials.
Figure 2.
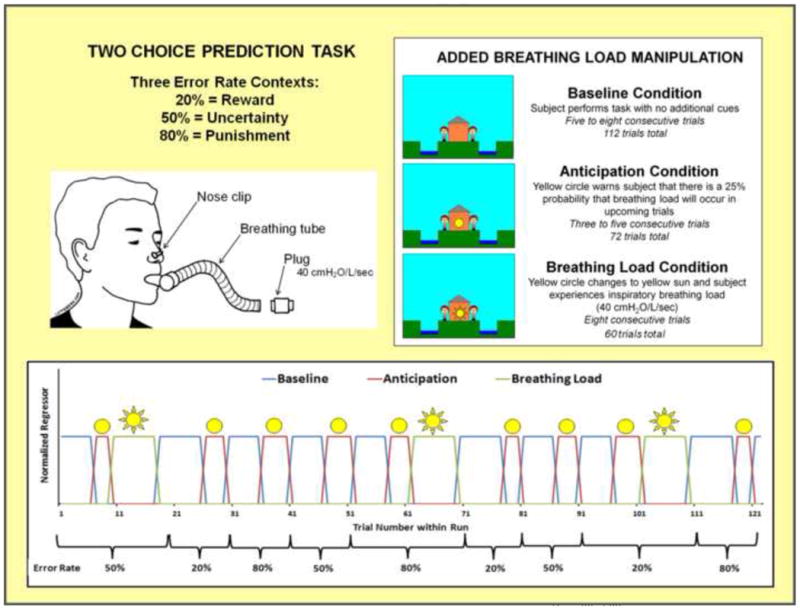
Illustration of two choice prediction task with added breathing load manipulation. Unbeknownst to subjects, the task was divided into three blocks of trials with differing reinforcement schedules: 20%, 50% and 80% error rates. Within the context of each error rate, subjects also experienced three interoception conditions: baseline, anticipation of breathing load, and experience of breathing load.
Within each ER, subjects also experienced an aversive interoceptive manipulation, involving three conditions: (1) baseline (6-8 consecutive trials): no additional cues presented; (2) anticipation (3-5 consecutive trials): a yellow circle shown in the center of the house, warning of a 25% chance of loaded breathing; and (3) breathing load (8 consecutive trials): a yellow sun shown in the center of the house, wherein the subject experienced an inspiratory 40 cmH2O/L/sec breathing load (40s duration). This paradigm was implemented using an event-related fMRI design, consisting of 2 runs with 306 repetition times (TR) each (TR=2000 ms; 2.5 TR per trial). The total number of trials presented for baseline, anticipation, and breathing load interoception conditions were 112, 72, and 60 respectively, with a total of 24 anticipation and 20 breathing load trials presented for each ER. RT was recorded for each trial. Order of conditions and ERs (see Figure 2) was kept fixed across subjects, although specific feedback given to each subject within each ER was contingent upon frequency of his/her responses to match up with the reinforcement determined by each ER.1
3. Results
3.1. Self-Report, Physiology, and Behavior
Table 1 depicts means and standard deviations as a function of group. METH had lower verbal IQ and education than CTL. METH also endorsed higher impulsivity, trait anxiety, state negative affect, denial/substance use coping, emotional venting, and higher past-month nicotine use than CTL. Although groups did not differ on VAS ratings, all participants rated the breathing load manipulation as moderately unpleasant and intense. METH exhibited higher CO2 levels than CTL across trials (F(1,30)=12.04, p<0.01; see Figure 3A). In addition, a main effect of interoception condition indicated that CO2 was lower during breathing load (M=1.28, SE=0.05) than baseline (M=1.43, SE=0.06) and anticipation (M=1.42, SE=0.06) across subjects (F(2,60)=25.94, p<0.01). A main effect of group emerged for lose-shift behavior (F(1,40)=9.46, p=.004), wherein METH made less lose-shift responses than CTL across all ERs (see Figure 3A).
Figure 3. Group Main Effect (A) Physiology and Behavior Results.
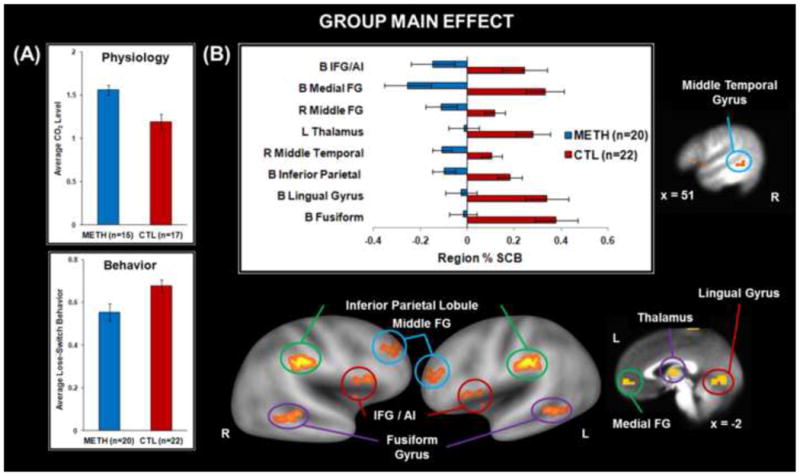
Methamphetamine use disorder (METH) subjects exhibited lower carbon dioxide (CO2) levels across interoception conditions/error rates and lower lose-switch behavior across error rates than healthy comparison subjects (CTL); (B) Neuroimaging Results. METH exhibited lower percent signal change from baseline (% SCB) than CTL in inferior frontal gyrus (IFG), anterior insula (AI), medial frontal gyrus (FG), middle frontal gyrus, thalamus, middle temporal gyrus, inferior parietal lobule, lingual gyrus, and fusiform gyrus. B = bilateral, L = left, R = right. Error bars indicate ±1 standard error of the mean.
3.2. Neuroimaging
Group differences in brain activation are illustrated in Tables 2-4. In addition, Figures 3-6 illustrate group differences in AFNI (Cox, 1996) and CARET (Van Essen et al., 2001), the latter used for mapping regions on the cortical brain surface.
Table 2.
Neuroimaging Results: Group Main Effect.
| Voxels | Vol | x | y | z | L/R | Regions within Cluster | BA | Group | Cohen's d |
|---|---|---|---|---|---|---|---|---|---|
| 18 | 1152 | -50 | 14 | 2 | L | Inferior Frontal Gyrus, Anterior Insula | 45,47 | CTL>METH | 0.92 |
| 13 | 832 | 45 | 11 | 4 | R | Inferior Frontal Gyrus, Anterior Insula | 45,47,13 | CTL>METH | 0.88 |
| 17 | 1088 | -4 | 52 | -3 | L/R | Medial Frontal Gyrus | 10 | CTL>METH | 1.41 |
| 19 | 1216 | 35 | 42 | 25 | R | Middle Frontal Gyrus | 9,10 | CTL>METH | 0.91 |
| 12 | 768 | -1 | -9 | 10 | L | Thalamus | - | CTL>METH | 0.93 |
| 157 | 10048 | -35 | -62 | -8 | L | Fusiform, Middle Occipital Gyrus, Culmen, Declive | 37 | CTL>METH | 1.24 |
| 29 | 1856 | 35 | -54 | -11 | R | Fusiform | 37 | CTL>METH | 1.07 |
| 38 | 2432 | -2 | -70 | -2 | L | Lingual Gyrus | 18 | CTL>METH | 1.06 |
| 23 | 1472 | 21 | -67 | -5 | R | Lingual Gyrus | 19 | CTL>METH | 0.92 |
| 26 | 1664 | -60 | -32 | 25 | L | Inferior Parietal Lobule | 40 | CTL>METH | 1.11 |
| 25 | 1600 | 59 | -22 | 35 | R | Inferior Parietal Lobule, Postcentral Gyrus | 2,40 | CTL>METH | 1.35 |
| 20 | 1280 | 51 | -39 | 0 | R | Middle Temporal Gyrus | 22 | CTL>METH | 1.10 |
Note: Vol = volume in microliters. METH = methamphetamine use disorder subjects. CTL = healthy comparison subjects. L = left hemisphere. R = right hemisphere. BA = Brodmann Area. Regions reflect significant clusters of at least 12 contiguous voxels meeting the F(1,40)=4.09 p<.05 threshold corrected for multiple comparisons. Coordinates reflect center of mass.
Table 4. Neuroimaging Results: Group x Error Rate x Interoception Interaction.
| Voxels | Vol | x | y | z | L/R | Regions within Cluster | BA | BL 20ER | BL 50ER | BL 80ER | Cohen's d |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 13 | 832 | -12 | 12 | 27 | L | Anterior Cingulate Gyrus | 24 | ns | ns | CTL>METH | 0.82 |
| 12 | 768 | 17 | -30 | 31 | R | Posterior Cingulate Gyrus | 31 | ns | ns | CTL>METH | 1.04 |
Note: Vol = volume in microliters. METH = methamphetamine use disorder subjects. CTL = healthy comparison subjects. ER = error rate. BL = breathing load. L = left hemisphere. R = right hemisphere. BA = Brodmann Area. Regions reflect significant clusters of at least 12 contiguous voxels meeting the F(4,320)=2.40 p<.05 threshold corrected for multiple comparisons. Coordinates reflect center of mass. ns = regions not significantly different between groups.
Figure 6. Neuroimaging Results, Group x Error Rate x Interoception Interaction.
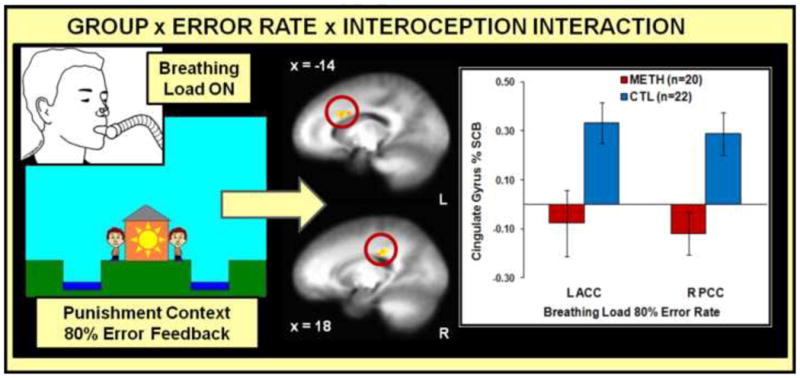
During the breathing load manipulation, methamphetamine use disorder (METH) subjects exhibited lower left (L) anterior cingulate (ACC) and right (R) posterior cingulate (PCC) percent signal change from baseline (% SCB) while making decisions within the context of punishment (80% error rate) than healthy comparison subjects (CTL). Error bars indicate ±1 standard error of the mean.
3.2.1. Group main effect
Figure 3B illustrates that METH exhibited lower lingual gyrus, medial frontal gyrus, fusiform gyrus, inferior parietal lobule, and AI/IFG activation than CTL. Furthermore, METH displayed attenuated middle frontal gyrus, middle temporal gyrus, and thalamus activation (see Table 2). Figure 4 demonstrates that within METH, higher trait anxiety was associated with higher left AI/IFG activation (Pearson's r=.48, p=.04; Spearman's ρ=.45, p=.05; Regression β=.02, t=1.99).
Figure 4. Insula-Anxiety Interaction.

Methamphetamine use disorder (METH) subjects endorsed higher State-Trait Anxiety Inventory (STAI) trait anxiety scores than healthy comparison subjects (CTL). Higher trait anxiety scores in METH were linked with higher left (L) inferior frontal gyrus (IFG) and anterior insula (AI) percent signal change from baseline (% SCB), comparable to % SCB of CTL. No relationship between trait anxiety and AI/IFG activation was evident within CTL. Error bars indicate ±1 standard error of the mean.
3.2.2. Group x interoception interaction
Similar patterns of activation emerged for baseline and anticipation conditions as a function of group. However, Figure 5 demonstrates that during breathing load, METH exhibited lower activation in striatum, thalamus, and ACC than CTL. In addition, Table 3 indicates that METH showed lower temporal gyrus/PI activation than CTL during breathing load.
Figure 5. Neuroimaging Results, Group x Interoception Interaction.
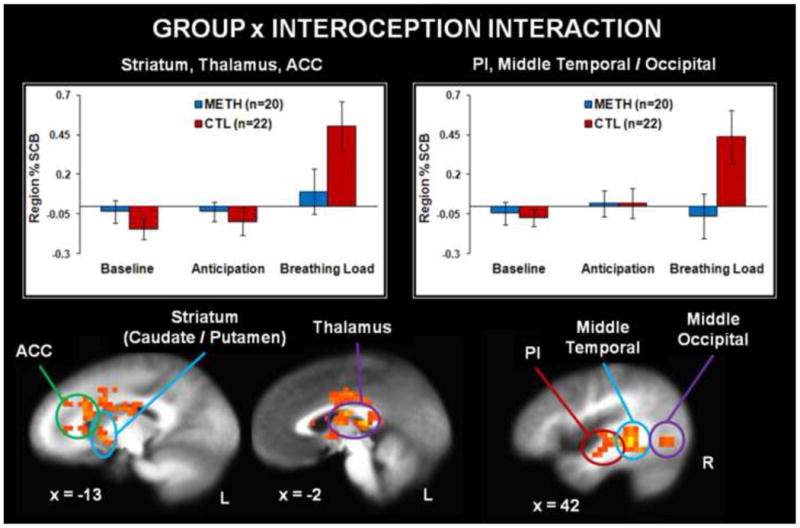
Methamphetamine use disorder (METH) subjects exhibited lower percent signal change from baseline (% SCB) than healthy comparison subjects (CTL) in posterior insula (PI), middle temporal gyrus, middle occipital gyrus, striatum, thalamus, and anterior cingulate cortex (ACC) in response to the aversive interoceptive stimulus (breathing load). Groups did not differ in % SCB during the baseline condition (consisting of the task alone with no additional cues) or the anticipation condition (cue warning of 25% chance of upcoming breathing load). L = left, R = right. Error bars indicate ±1 standard error of the mean.
Table 3.
Neuroimaging Results: Group x Interoception Interaction.
| Voxels | Vol | x | y | z | L/R | Regions within Cluster | BA | Breathing Load | Cohen's d |
|---|---|---|---|---|---|---|---|---|---|
| 532 | 34048 | -4 | 2 | 15 | L/R | Caudate, Thalamus, Lentiform Nucleus, Putamen, Anterior Cingulate | - | CTL>METH | 0.76 |
| 171 | 10944 | 45 | -43 | -2 | R | Middle/Inferior/Superior Temporal Gyrus, Posterior Insula, Middle Occipital Gyrus | 13, 22, 37 | CTL>METH | 0.90 |
| 103 | 6592 | -57 | -16 | 16 | L | Postcentral Gyrus, Transverse Temporal Gyrus, Inferior Parietal Lobe | 40, 41, 43 | CTL>METH | 0.87 |
| 42 | 2688 | 6 | -75 | 47 | R | Precuneus | 7 | CTL>METH | 0.86 |
| 20 | 1280 | 17 | -17 | 29 | R | Cingulate Gyrus | - | CTL>METH | 0.77 |
| 27 | 1728 | -3 | -36 | 71 | L | Paracentral Lobule | 4 | CTL>METH | 0.84 |
| 17 | 1088 | -1 | -68 | 8 | L | Cuneus | - | CTL>METH | 0.69 |
| 18 | 1152 | -36 | -74 | -15 | L | Declive, Fusiform | 19 | CTL>METH | 0.87 |
Note: Vol = volume in microliters. METH = methamphetamine use disorder subjects. CTL = healthy comparison subjects. L = left hemisphere. R = right hemisphere. BA = Brodmann Area. Regions reflect significant clusters of at least 12 contiguous voxels meeting the F(2,320)=3.03 p<.05 threshold corrected for multiple comparisons. Coordinates reflect center of mass.
3.2.3. Group x ER interaction
No significant effects emerged.
3.2.4. Group x ER x interoception interaction
Figure 6 illustrates that during breathing load within 80% ER, METH exhibited lower ACC and posterior cingulate gyrus (PCC) activation than CTL (see Table 4). In addition, during baseline within 20% ER, METH displayed lower ACC and PCC than CTL. No other group effects were significant.
3.3. Exploratory Analyses
Patterns of brain activation in METH were not related to verbal IQ, education, CO2, or lose-shift behavior, nor were they driven by METH participants with comorbid alcohol use disorder.2
4. Discussion
The present study examined three hypotheses focused on how METH process decisions differently when exposed to an aversive interoceptive stimulus, focusing on frontocingulate, thalamic, and insular regions.
4.1. PFC and Decision-Making
First, as predicted, METH exhibited lower PFC activation than CTL across trials, specifically in IFG and right middle frontal gyrus, consistent with prior work and suggestive of reduced attentional resources allocated to decision making (Paulus et al., 2003, 2002).
4.2. Thalamus, AI, and Aversive Interoception
Second, we hypothesized that METH would show lower thalamic/AI responding than CTL during breathing load. Although this prediction was confirmed for thalamic activation, METH exhibited lower AI than CTL across all trials, regardless of interoceptive condition. Similar to attenuated thalamic responding, METH displayed lower PI and ACC activation than CTL when experiencing an aversive interoceptive stimulus. AI/IFG attenuations in METH are suggestive of an executive functioning deficit to non-drug related stimuli, reflecting reduced resources allocated to choice selection (Paulus et al., 2004; Preuschoff et al., 2008). In contrast, PI/ACC reductions in METH appear specific to impairments in registering and evaluating aversive interoceptive experiences during decision-making, consistent with the purported roles of these regions in somatosensory processing and monitoring of homeostatic changes, respectively (Craig, 2003). These distinctions are consistent with differential connectivity patterns in healthy individuals as a function of subregions of the insular cortex (Chang et al., 2013; Deen et al., 2011), wherein dorsal AI is connected to anterior IFG and other regions of an executive control network involving switching and inhibition. In contrast, PI is more closely connected with pregenual ACC (Deen et al., 2011) and linked to pain and somatosensory processing (Chang et al., 2013). In addition to neural attenuation linked to interoception, METH exhibited higher CO2 levels than CTL, but this physiological difference was not specific to the breathing load condition, nor was it significantly correlated with brain activation in METH. Specifically in response to stress, METH show a pattern of externalization that may translate to maladaptive decision-making in everyday life: blunted somatosensory processing, reduced vigilance to negative consequences, and heightened employment of denial and emotional venting in order to cope.
4.3. AI, ACC, Aversive Interoception and Losses
Our third hypothesis stated that METH would demonstrate lower AI/ACC activation than CTL during decision-making paired with loaded breathing and punishing feedback (80% ER). This hypothesis was partially supported, in that METH exhibited lower ACC activation than CTL while making loaded breathing decisions within the context of high losses, in line with research linking attenuated ACC with losses, errors, and response conflict in METH (Gowin et al., 2014a, 2014b; London et al., 2005; Nestor et al., 2011; Salo et al., 2012). Similar to attenuated ACC activation to losses, METH employed a lose-switch response strategy less frequently than CTL across trials when confronted with negative feedback. METH may not accurately register losses or errors, thereby failing to effectively utilize error information to update future behavior.
4.4. Implications
The present study supports the assertion that when healthy individuals experience adverse interoceptive conditions, they tend to utilize additional neural resources to process available options, possibly to facilitate more optimal decisions when in distress. In contrast, METH deploy fewer neural resources when making decisions during aversive interoceptive conditions, perhaps resulting in sub-optimal decision-making. The role of insular cortex in substance use disorders is a complex one. First, insular hyperactivation has been observed in drug users and often reflects heightened urges and craving responses to drug cues. Second, insular hypoactivation has also been found in drug users and has been hypothesized to reflect reduced responsiveness to non-drug related stimuli (e.g., Naqvi and Bechara, 2010; Naqvi et al., 2014; Paulus and Stewart, 2014). Thus, stimulus- and behavior-appropriate insula activation is emerging as a possible “interoceptive tuning” mechanism to optimize an individual's response to his or her internal and external environment. This notion is consistent with the proposed primary function of the insular cortex in addiction as signaling interoceptive effects of drug consumption in order to prioritize goal-directed drug seeking at the expense of attention to non-drug related goals (Naqvi et al., 2014). Future studies of addiction need to determine the degree to which pairing of appetitive and/or aversive interoceptive stimuli with the presence and absence of stimulant-related drug cues affects this tuning mechanism.
Given that METH show reduced neural processing to aversive stimuli in this investigation, one important question is whether desensitization to interoceptive stress is a marker of predisposition to methamphetamine addiction, or a consequence of methamphetamine use because neural systems have habituated to chronic life stress? Recent work points to attenuated ACC deployment during aversive interoceptive conditions as a marker for the transition to problem stimulant use, suggestive of a pre-existing condition of reduced stress sensitivity (Stewart et al., 2013). Although the present investigation cannot conclude with certainty that attenuated brain activation during stress has adverse implications for METH, available research on METH links reduced neural resources to poorer behavioral indices of decision-making (Gowin et al., 2014b). Perhaps sensitizing METH to interoceptive stress may help them improve decision-making skills by bringing more ACC resources online.
4.5. Anxiety and AI/IFG Function
We explored individual differences in insular responding as a function of anxiety symptoms within METH, given prior work demonstrating positive correlations between anxiety and insular activation (Paulus and Stein, 2006, 2010). We replicated research showing elevated anxiety scores in METH compared to CTL, and within METH, higher trait anxiety was linked to left IFG/AI activation during decision-making across trials, independent of interoception or ER. METH with low anxiety may be more susceptible to compromised neural processing related to decision-making, although more research is needed to replicate this finding. METH also endorsed higher impulsivity than CTL, with higher impulsivity marginally linked to higher anxiety within METH, findings in line with prior work asserting that anxious-impulsive personality traits may represent markers of stimulant addiction vulnerability (Ersche et al., 2012).
4.6. Limitations
Despite the strengths of this study, our investigation possesses several limitations. First, we did not have the statistical power to determine whether AI/IFG correlations with anxiety were attributable to or replicable in comorbid depression symptoms in METH, given that less than half completed the BDI-II. Given the high co-occurrence of depression and anxiety symptoms in METH (Gonzales et al., 2010; London et al., 2004; Salo et al., 2011) this issue warrants further exploration. Second, several METH met criteria for comorbid substance use disorders. Although post-hoc analyses determined that group differences in brain activation remained significant when METH with alcohol use disorder were removed, due to the small number of individuals with comorbid cocaine and opiate use disorders, the influence of these disorders was not examined. Third, METH endorsed higher nicotine use than CTL, and although past-month nicotine use did not correlate with neural activation in METH, future studies should make attempts to recruit CTL matched on nicotine use to further rule out its effects on brain function. Fourth, groups did not differ on subjective responses of the aversive interoceptive manipulation, nor did they differ on overall BAQ. The lack of differences between groups is consistent with recent work showing no differences in VAS ratings of pleasant interoceptive stimuli in chronic METH (May et al., 2013) and aversive interoceptive stimuli in stimulant abusers (Stewart et al., 2013). Future studies may need to employ an approach that uses several levels of subjective reactions, such as self-report, changes in physiological responding, or changes in responses to affective stimuli, to better delineate the subjective effects of the aversive interoceptive experience.
4.7. Summary
This investigation is the first to demonstrate that METH exhibit attenuated neural responses during decision-making within the context of aversive interoceptive stimuli, demonstrating that reduced cingulate resources and less adaptive behavioral performance characterize responses to loss in METH, particularly during an aversive stressor. Overall, findings suggest that METH may not accurately register aversive feedback in order to update future behavior.
Supplementary Material
Acknowledgments
Data collection and analysis for the present study were funded by the National Institute on Drug Abuse (NIDA; grant #5P20DA027843-04 to Susan F. Tapert and Martin P. Paulus).
Role of Funding Source: NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Neuroimaging acquisition and statistical analyses appear as Supplementary Material and can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:….
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Contributors: Authors S.F. Tapert and M.P. Paulus designed the study. P.W. Davenport and M.P. Paulus designed the two choice prediction task with the additional breathing load manipulation. J.L. Stewart and A.C. May collected the data. A.C. May and T. Poppa processed the fMRI data. J.L. Stewart undertook statistical analysis, literature review, and first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest: All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bechara A. Decision-making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Blomqvist A, Zhang ET, Craig AD. Cytoarchitectonic and immunohistochemical characterization of a specific pain and temperature relay, the posterior portion of the ventral medial nucleus, in the human thalamus. Brain. 2000;123:601–619. doi: 10.1093/brain/123.3.601. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56:267–283. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- Chan PY, Davenport PW. Respiratory-related evoked potential measures of respiratory sensory gating. J Appl Physiol. 2008;105:1106–1113. doi: 10.1152/japplphysiol.90722.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobio. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. Topographically organized projection to posterior insular cortex from the posterior portion of the ventral medial nucleus in the long-tailed macaque monkey. J Comp Neurology. 2014;522:36–63. doi: 10.1002/cne.23425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio A, Carvalho GB. The nature of feelings: evolutionary and neurobiological origins. Nat Rev Neurosci. 2013;14:143–152. doi: 10.1038/nrn3403. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Everitt BJ, Bishop D. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011;21:1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Turton AJ, Chamberlain SR, Müller U, Bullmore ET, Robbins TW. Cognitive dysfunction and anxious-impulsive personality traits are endophenotypes for drug dependence. Am J Psychiatry. 2012;169:926–936. doi: 10.1176/appi.ajp.2012.11091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson R. Anxiety disorders among methamphetamine dependent adults: association with post-treatment functioning. Am J Addict. 2010;19:385–390. doi: 10.1111/j.1521-0391.2010.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales R, Mooney L, Rawson RA. The methamphetamine problem in the United States. Annu Rev Public Health. 2010;31:385–398. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Harlé KM, Stewart JL, Wittmann M, Tapert SF, Paulus MP. Attenuated insular processing during risk predicts relapse in early abstinent methamphetamine-dependent individuals. Neuropsychopharmacology. 2014a;39:1379–1387. doi: 10.1038/npp.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowin JL, Stewart JL, May AC, Ball TM, Wittmann M, Tapert SF, Paulus MP. Altered cingulate and insular cortex activation during risk-taking in methamphetamine dependence: losses lose impact. Addiction. 2014b;109:237–247. doi: 10.1111/add.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Critchley HD. Interoceptive basis to craving. Neuron. 2007;54:183–186. doi: 10.1016/j.neuron.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Anxiety and decision-making. Biol Psychiatry. 2012;72:113–118. doi: 10.1016/j.biopsych.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YT, Song HJ, Seo JH, Lee JJ, Lee J, Kwon DH, et al. The differences in neural network activity between methamphetamine abusers and healthy subjects performing an emotion-matching task: functional MRI study. NMR Biomed Res. 2011;24:1392–1400. doi: 10.1002/nbm.1702. [DOI] [PubMed] [Google Scholar]
- London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, Thompson PM, Brody AL, Geaga JA, Hong MS, Hayashi KM, Rawsron RA, Ling W. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry. 2005;58:770–778. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- May AC, Stewart JL, Migliorini R, Tapert SF, Paulus MP. Methamphetamine dependent individuals show attenuated brain response to pleasant interoceptive stimuli. Drug Alcohol Depend. 2013;131:238–246. doi: 10.1016/j.drugalcdep.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann NY Acad Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor LJ, Ghahremani DG, Monterosso J, London ED. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194:287–295. doi: 10.1016/j.pscychresns.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Tapert SF, Liu TT. Trend detection via temporal difference model predicts inferior prefrontal cortex activation during acquisition of advantageous action selection. Neuroimage. 2004;21:733–743. doi: 10.1016/j.neuroimage.2003.09.060. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Flagan T, Simmons AN, Gillis K, Kotturi S, Thom N, Johnson DC, Van Orden KF, Davenport PW, Swain JL. Subjecting elite athletes to inspiratory breathing load reveals behavioral and neural signatures of optimal performers in extreme environments. PLoS One. 2012;7:e29394. doi: 10.1371/journal.pone.0029394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision-making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biol Psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76:342–350. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision-making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schulteis G. The role of interoception and alliesthesia in addiction. Pharmacol Biochem Behav. 2009;94:1–7. doi: 10.1016/j.pbb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, Pollastri A, Farrer L, Kranzler HR. Diagnostic reliability of the Semi-structured Assessment for Drug Dependence and Alcoholism (SSADDA) Drug Alcohol Depend. 2005;80:303–312. doi: 10.1016/j.drugalcdep.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Fassbender C, Buonocore MH, Ursu S. Behavioral regulation in methamphetamine abusers: an fMRI study. Psychiatry Res. 2012;211:234–238. doi: 10.1016/j.pscychresns.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Flower K, Kielstein A, Leamon MH, Nordahl TE, Galloway GP. Psychiatric comorbidity in methamphetamine dependence. Psychiatry Res. 2011;186:356–361. doi: 10.1016/j.psychres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SA, Mallory ME, Simon A. The Body Awareness Questionnaire – reliability and validity. J Pers Assess. 1989;53:802–815. [Google Scholar]
- Shoptaw S, et al. Commentary on Gowin(2014): brain is behavior—methamphetamine dependence and recovery. Addiction. 2014;109:248–249. doi: 10.1111/add.12442. [DOI] [PubMed] [Google Scholar]
- Simmons AN, Stein MB, Strigo IA, Arce E, Hitchcock C, Paulus MP. Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Hum Brain Mapp. 2011;32:1836–1846. doi: 10.1002/hbm.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE, Vagg PR. State-Trait Anxiety Inventory (STAI) Consulting Psychologists Press; Palo Alto: 1983. [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164:318–327. doi: 10.1176/ajp.2007.164.2.318. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Parnass JM, May AC, Davenport PW, Paulus MP. Altered frontocingulate activation during aversive interoceptive processing in young adults transitioning to problem stimulant use. Front Sys Neurosci. 2013;7:89. doi: 10.3389/fnsys.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Paulus MP. Neural correlates of craving for psychoactive drugs. In: Miller P, editor. Principles of Addiction: Comprehensive Addictive Behaviors and Disorders. Academic Press; Waltham, Massachusetts: 2013. pp. 453–465. [Google Scholar]
- Van Essen DC, Dickson J, Harwell J, Hanlon D, Anderson CH, Drury HA. An integrated software system for surface-based analysis of cerebral cortex. J Am Med Inform Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neurosci Biobehav Rev. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. WRAT 4: Wide Range Achievement Test; Professional manual. Psychological Assessment Resources, Inc.; Lutz, FL: 2006. [Google Scholar]
- Zuckerman M. The sensation seeking scale V (SSS-V): still reliable and valid. Pers Indiv Diff. 2007;43:1303–1305. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


