Abstract
Background and aims
No effective pharmacotherapy for methamphetamine (MA) use disorder has yet been found. This study evaluated sustained-release methylphenidate (MPH-SR) compared with placebo (PLA) for treatment of MA use disorder in people also undergoing behavioural support and motivational incentives.
Design
This was a randomized, double-blind, placebo-controlled design with MPH-SR or PLA provided for 10 weeks (active phase) followed by 4 weeks of single-blind PLA. Twice-weekly clinic visits, weekly group counseling (CBT), and motivational incentives (MI) for MA-negative urine drug screens (UDS) were included.
Setting
Treatment sites were in Los Angeles, California (LA) and Honolulu, Hawaii (HH), USA.
Participants
110 MA-dependent (via DSM-IV) participants (LA = 90; HH = 20).
Measurements
The primary outcome measure is self-reported days of MA use during the last 30 days of the active phase. Included in the current analyses are drug use (UDS and self-report), retention, craving, compliance (dosing, CBT, MI), adverse events, and treatment satisfaction.
Findings
No difference was found between treatment groups in self-reported days of MA use during the last 30 days of the active phase (p=0.22). In planned secondary outcomes analyses, however, the MPH group had fewer self-reported MA use days from baseline through the active phase compared with the PLA group (p=0.05). The MPH group also had lower craving scores and fewer marijuana-positive UDS than the PLA group in the last 30 days of the active phase. The two groups had similar retention, other drug use, adverse events, and treatment satisfaction.
Conclusions
Methylphenidate may lead to a reduction in concurrent methamphetamine use when provided as treatment for patients undergoing behavioural support for moderate to severe methamphetamine use disorder but this requires confirmation.
INTRODUCTION
An estimated 52 million individuals worldwide have used methamphetamine (MA) and amphetamine-type stimulants such as MDMA within the past year for non-medical purposes, second only to marijuana, and more than heroin and cocaine combined.1 In the United States, admissions to publicly-funded treatment programs for amphetamine-related problems showed an overall increase from 3.7% to 5.7% between 2000 and 2010.2 To date, there are no FDA-approved pharmacotherapies to treat MA use disorders. Evidence-based behavioral approaches have proven only modestly effective in reducing MA use.
Prior clinical trials have investigated medications that target dysregulation among the various neurotransmitter systems affected by chronic MA use. Early studies documented some promise for bupropion as a treatment for MA dependence,3–5 given its ability to increase intrasynaptic dopamine and possibly ameliorate MA induced DA dysregulation. However, later studies failed to replicate these findings, although bupropion reduced MA use in those with mild to moderate levels of MA use.3,6 Other antidepressants, including fluoxetine, paroxetine, mirtazapine and sertraline, have also been investigated.7–10 Of these, only mirtazapine significantly reduced MA use.8 Antipsychotics (aripiprazole, risperidone), antiepileptics (topiramate, vigabatrin, gabapentin), and other agents (dextroamphetamine, odansetron, varenicline, baclofen, modafinil, N-acetyl cysteine+naltrexone, and the proprietary approach Prometa®) either failed to demonstrate efficacy or have yet to be studied in large placebo controlled clinical trials.11–23 Notably, methylphenidate demonstrated preliminary efficacy in reducing relapse among newly abstinent individuals who had been in residential treatment for amphetamine dependence.24
Methylphenidate (MPH) is a stimulant widely used to treat ADHD in adolescents and adults.25–29 Animal models show that MPH increases extracellular DA30 indicating that some of the effects produced by MPH in ADHD patients are related to amending brain DA deficiencies.31 Sustained-release MPH (MPH-SR) also decreased some positive subjective cocaine effects and cocaine choice in cocaine-dependent ADHD patients.32
Herin and colleagues33 report that clinical studies support the efficacy of MPH for the treatment of stimulant dependence. The sustained-release formulation of MPH appears to have lower abuse liability than immediate-release MPH, as assessed in clinical trials.34
The concept of MPH as a pharmacotherapy for MA use disorder is largely based on its mechanism of action. MPH binds to dopamine transporter (DAT) and norepinephrine transporter (NET) with modest potency, preventing reuptake of synaptic DA and NE.35–38 The medication blocks DAT with a potency similar to that of cocaine. Like cocaine, MPH can reduce the effects of concomitantly administered MA.36 Laboratory findings on MPH’s intracellular mechanism of action further provide a strong mechanism-based argument for its consideration as a treatment for MA use disorder.39
In a European study by Tiihonen et al.24 amphetamine-dependent patients (intravenous injection users) were randomly assigned to MPH-SR (54mg/day), aripiprazole (15mg/day), or placebo for 20 weeks. The MPH group had significantly fewer amphetamine-positive urine samples (67.3%) than the aripiprazole (90.7%) or placebo groups (82.0%), providing evidence of an effective pharmacotherapy for amphetamine dependence.
Other studies have documented positive results for MPH: A study of cocaine abusers with ADHD found that MPH-SR at 60mg/day reduced the positive and reinforcing effects of cocaine.32 In a study of MPH-SR (60mg/day) treatment of cocaine abusers with ADHD40, improvements were found in ADHD symptoms and reduced cocaine use. The dosage of 60mg/day MPH was well tolerated, with only one subject dropping out due to insomnia. Additional work to verify the safety of MPH for adults has confirmed the absence of safety or tolerability issues at various doses of sustained-release medication.33
The aim of this study is to evaluate MPH-SR compared to placebo in 110 individuals with methamphetamine use disorder participating at study sites in Los Angeles, California (LA), and Honolulu, Hawaii (HH).
METHODS
Design
This double-blind, placebo-controlled design included randomization to medication condition for 10 weeks of active treatment followed by a 4-week period of single-blind placebo. Twice-weekly clinic visits included dispensing observed in-clinic doses and take-home medication, assessments including urine drug screens (UDS), and provision of Motivational Incentives (MI). Group Cognitive Behavioral Therapy (CBT) was provided once weekly. Study procedures were standardized across sites except that the HH site conducted additional brain MRI studies and blood samples were collected for future genetic testing.
Participants
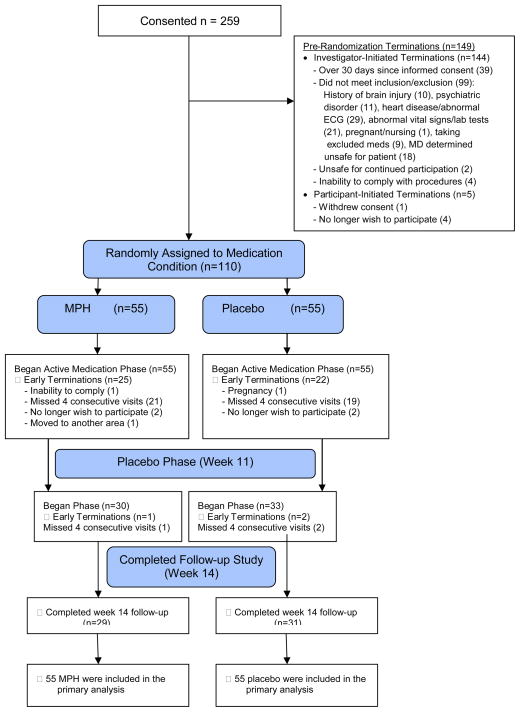
The informed consent process was completed for 259 participants, 149 dropped out or withdrew, and 110 participants were randomized to medication condition between November 2010 and March 2013 (Figure 1), including 90 in LA and 20 in HH, evenly distributed across conditions (MPH=55; PLA=55) Inclusion criteria included being 18–59 years old, and meeting DSM-IV-TR criteria for MA dependence. Exclusion criteria included a history of seizures or brain injury, a sensitivity or previous adverse reaction to MPH, any medical, neurological, or psychiatric disorder that would make study compliance difficult or unsafe, first-degree relatives with early cardiovascular morbidity or mortality, and being pregnant or nursing. Participants were also excluded if they were prescribed medications that could interact with the study medication (e.g. clonidine, Coumadin anticoagulants, anticonvulsants, vasopressor agents, some antidepressants, MAO inhibitor use in previous 14 days).
Figure 1.
Study Participant Flow (Consort Diagram)
Compensation was provided for time, travel, and related costs. A total of $40 at the LA site, and $50 at the HH site was provided for screening; and $20 was provided for each of the twice-weekly clinic visits. The maximum compensation was $600 at LA and $610 at HH.
Procedures
Recruitment, Consent and Screening
Recruitment methods included print and radio ads, internet postings, flyers posted in local community sites, and word of mouth. Interested individuals were prescreened via telephone before face-to-face informed consent interviews and screening visits. Screening assessments collected information to determine eligibility and to provide baseline data.
Randomization
Eligible participants were randomized to treatment condition on a 1:1 schedule (MPH, PLA) stratified by gender, and days of MA use in the last 30 days (<10 days, ≥10 days). The UCLA Integrated Substance Abuse Program (ISAP) Data Management Center (DMC) managed randomizations using an urn procedure with blocks of 8. Assignment logs were provided only to the study pharmacist and other unblinded study personnel for preparation of study medication.
Active Medication and Placebo Phases
The double-blind, Active Treatment Phase occurred from weeks 1–10; participants received either active (MPH) or placebo (PLA) drug. The single-blind Placebo Phase occurred in weeks 11–14. Assessments and MI were included at twice-weekly clinic visits, and group CBT was provided once weekly throughout the study.
Medication
MPH dosage was MPH-SR (Concerta®) 18mg daily for Week 1, 36mg for Week 2; and 54mg for Weeks 3–10, following the schedule utilized by Tiihonen et al (2007). The PLA group received matching capsules consisting of lactose. Study drugs were provided by Janssen Pharmaceuticals. An unblinded pharmacist or physician prepared medication for once-weekly dispensing in child-proof bottles. Dosing was observed by staff on clinic visit days. Participants were instructed to take medication once a day upon awakening and were warned not to take a double dose to make up for a missed dose.
Motivational Incentives (MI)
Participants earned draws for each MA-negative (MA−) UDS. Using a “fishbowl” method, 100 chips were marked to correspond to value ($1, $2, $5, $10). Participants drew chips starting with the first MA− UDS. The number of draws increased every two visits with continued consecutive MA− UDS, capped at a maximum of 10 draws per visit. A maximum of 28 UDS were possible across the study, with 190 draws possible for the 14-week period. Participants also earned a $5 bonus on the first occasion after four consecutive MA− UDS. No draws were provided for a MA-positive (MA+) UDS, a missed visit, or a visit in which a UDS was not tested, and the number of draws reset to one for the next MA− UDS.
Cognitive-Behavioral Therapy (CBT)
Participants were encouraged to attend a weekly 1–1½ hour group CBT session with a trained therapist and a treatment manual developed for this study. Therapists were trained and monitored by a senior therapist with extensive CBT expertise.
Measures
Measures used for the current analyses include: 1) Urine Drug Screen (UDS) for opiates, cocaine, amphetamine, MA, and marijuana (MJ) collected at each clinic visit. 2) Addiction Severity Index Lite41 (ASI) at screening, week 10, and study end. (3) Substance Use Report of self-reported drug use for 2 weeks before screening, and each day during the study. 4) Craving collected weekly: a) Visual Analog Craving Scale42–43 (VAS); b) Craving Questionnaire–Now version44 (CQ-Now). 5) Demographics. 6) Connors Adult ADHD Scale.45 7) Dose Log documented prescribed daily dose of study medication and dose reported as taken. 8) CBT and MI Logs collected weekly. 9) Treatment Satisfaction at study end. 10) Adverse Events (AEs) collected at each visit.
Outcomes
The primary outcome is days of MA use self-reported for the last 30 days of the 10-week active treatment phase. Planned secondary outcomes include reduction in self-reported MA use, MA use via UDS, retention, craving, adverse events, other drug use, treatment compliance, and treatment satisfaction.
Analyses
The intent-to-treat (ITT) analysis strategy retains all participants for the entire treatment period, independent of treatment response.46 Prior to outcome analyses, attrition analyses addressed whether screen-fail participants differ from those randomized. Baseline variables that differed significantly between conditions using univariate analyses were used as covariates in testing study hypotheses.
Primary analyses used longitudinal models47, including normal mixed-effects models48–49 MIXED; PROC MIXED50 for continuous measures and generalized linear mixed models48–49 GLMM; GLIMMMIX50 for categorical measures. Longitudinal models handle time-dependent covariates and complex correlation structures among observations within each participant to model time trends and yield estimates of treatment effects, as consistently as possible. Derived variable analyses,51 reduces the multiple during-treatment repeated measures into summaries, used to confirm results from longitudinal models. This applied statistical theory preserves the transitional probabilities inherent in the UDS data and partially accounts for the non-independence of repeated observations when estimating treatment effects.47 In the longitudinal models, random effects mixed models were used with participants as level 1 and site as level 2 to account for the data design where multiple observations are nested within participants and participants are nested within sites. Retention is the number of days from randomization to the last clinic visit compared between conditions using survival analysis. Counts and composites by treatment condition used t-tests and ANOVAs.
To address whether MPH reduces MA use compared to placebo, primary power analyses were conducted using all randomized participants in this ITT analysis. We estimated that 55 MPH and 55 placebo participants would be needed, assuming that 75% of the sample complete at least Week 4 of the medication phase, which can then be used for end-point analysis to provide at least 80% power to detect medication effects in the medium effect size range (d=0.40) with a two-tailed alpha value of less than 0.05. All analyses were performed using SAS50 version 9.3 and Stata 13.
RESULTS
Participants
Baseline characteristics by treatment group (MPH=55; PLA=55) are shown in Table 1. Demographic information was not collected on 5 participants, but no differences in baseline characteristics were found between participants who were (n=110) and were not (n=149) randomized.
Table 1.
Baseline Demographic and Drug Use Characteristics by Treatment Condition
| Baseline characteristics | MPH Group (N=55) | Placebo Group (N=55) |
|---|---|---|
| Age, years | 38.7 (9.8) | 39.5 (10.4) |
| Gender | ||
| Male | 45 (81.8%) | 45 (81.8%) |
| Female | 10 (18.2%) | 10 (18.2%) |
| Race/Ethnicity | ||
| African-American | 13 (23.6%) | 13 (23.6%) |
| White | 34 (61.8%) | 32 (58.2%) |
| Other race | 8 (14.6%) | 10 (18.2%) |
| Education, years | 12.4 (2.9) | 12.9 (1.6) |
| % ADHD | 18 (32.7%) | 14 (25.5%) |
| Drug use | ||
| Mean years MA use, lifetime (sd) | 10.8 (7.8) | 11.9 (9.9) |
| Mean days MA use - last 30 days (sd) | 13.1 (9.7) | 11.4 (9.8) |
| UA positive for MA, n (%) | 28 (50.9%) | 20 (36.4%) |
| Mean years Opioid use, lifetime (sd) | 0.47 (2.22) | 0.85 (3.11) |
| Mean days Opioid use - last 30 days (sd) | 0.47 (2.25) | 0.51 (2.25) |
| UA positive for Opioids, n (%) | 0 | 0 |
| Mean years Cocaine use, lifetime (sd) | 3.64 (5.21) | 3.57 (6.19) |
| Mean days Cocaine use - last 30 days (sd) | 0.85 (3.26) | 0.31 (0.11) |
| UA positive for Cocaine, n (%) | 3 (5.5%) | 3 (5.5%) |
| Mean years MJ use, lifetime (sd) | 9.67 (7.26) | 10.90 (11.57) |
| Mean days MJ use - last 30 days (sd) | 6.57 (10.33) | 7.07 (10.19) |
| UA positive for MJ, n (%) | 18 (32.7%) | 19 (34.6%) |
MA Use by Self-report
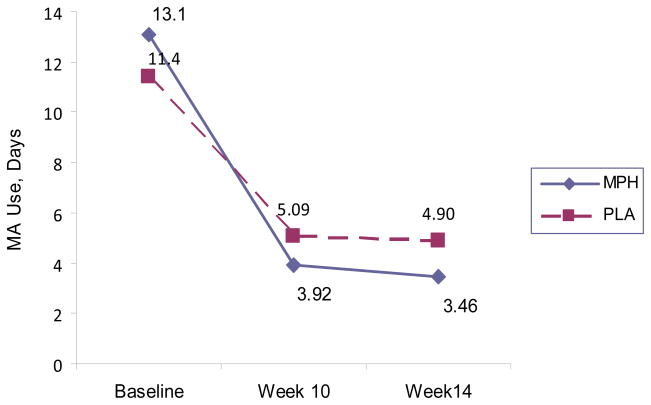
Table 2 shows no difference in self-reported days of MA use in the last 30 days (ASI) of the active phase (p=0.22). The reduction in MA use days from baseline to week 10, however, is statistically greater for the MPH group than the PLA group (6.56 vs. 3.82 days, p=0.05). Figure 2 shows self-reported MA use days in the last 30 days at Baseline, Week 10, and Week14.
Table 2.
MA and Other Drug Use Outcomes by treatment group
| MPH (n=55) | Placebo (n=55) | P | |
|---|---|---|---|
| Self-Reported MA Use | |||
| Mean Days of MA use in last 30 days at week 10 | 3.92 | 5.09 | 0.22 |
| Change in days of MA use (week 10 – baseline) | −6.56 | −3.82 | 0.05 |
| UDS MA Use | |||
| MA+ UDS at week 10, controlling for BL UDS and site | 18.5% | 27.1% | 0.12 |
| MA+ UDS at weeks 9 &10, controlling for BL UDS and site | 15.8% | 29.6% | 0.004 |
| MA+ UDS at week 14, controlling for BL UDS and site | 15.6% | 33.8% | 0.025 |
| % of group with ≥3 MA− UDS (weeks 1 to 10) | 63.6% | 61.8 | 0.84 |
| % of group with ≥3 MA− UDS (weeks 7 to 10) | 47.3% | 43.6% | 0.70 |
| TES | .69 | .68 | 0.85 |
| Longest period of continuous MA-abstinence, days (sd) | 39.8 (5.4) | 35.9 (4.8) | 0.30 |
| Other Drug Use | (n=30) | (n=33) | |
| Mean days of MJ use in last 30 days at week 10 | 4.06 | 6.88 | 0.13 |
| % MJ+ UDS at Week 10 | 20.0% | 36.7% | 0.05 |
| % MJ+ UDS at Week 14 | 26.0% | 40.0% | 0.13 |
| Mean days of Opioid use in last 30 days at week 10 | 0.07 | 0.58 | 0.14 |
| % Opioid+ UDS at Week 10 | 0% | 3.3% | 0.19 |
| % Opioid+ UDS at Week 14 | 2% | 0% | 0.29 |
| Mean days of Cocaine use in last 30 days at week 10 | 1.34 | 1.03 | 0.63 |
| % Cocaine+ UDS at Week 10 | 6.0% | 5.0% | 0.82 |
| % Cocaine+ UDS at Week 14 | 16.0% | 7.3% | 0.14 |
Figure 2.
Self-Reported Methamphetamine Use for the Last 30 Days at Study Time-points by Treatment Group
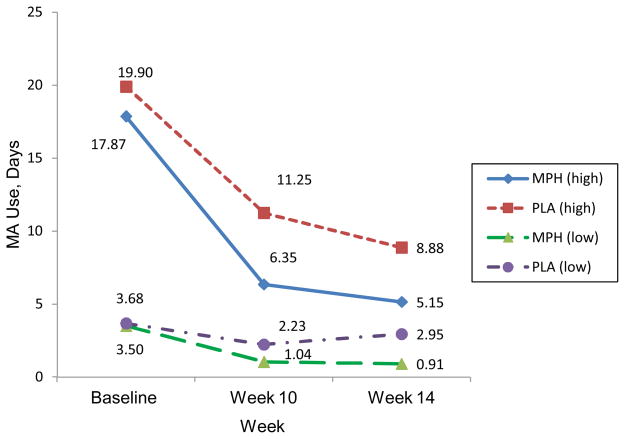
Significant differences are found by treatment group for the high MA use subgroup (≥10 days of MA use at baseline, n=17) who reported fewer days of MA use in the last 30 days of the active phase (6.35 days), compared to the PLA group (n=16, 11.25 days) (p=0.049). The low MA use subgroup (<10 days, n=14) showed a similar trend; reporting fewer MA-use days for the last 30 days of the active phase compared to the low-use PLA group (n=22)(p=0.16)(Figure 3).
Figure 3.
Self-Reported Methamphetamine Use for the last 30 Days at Study Time-points by High vs Low Baseline Methamphetamine Use
MA Use by Urine Drug Test Results
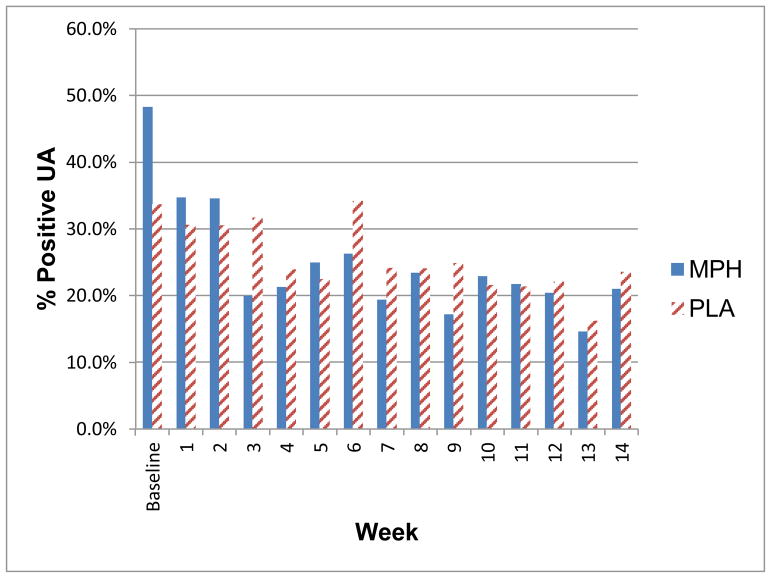
After controlling for baseline UDS and site, and with missing tests counted as positive, no difference in MA+ UDS was found between treatment groups (18.5% for MPH; 27.1% PLA, p=0.12), at week 10. At week 14, however, the MPH group was less likely to be MA+ (OR=0.18, p=0.025) compared to the PLA group. Figure 4 shows percent of MA+ UDS by study week. No difference was found between groups in the percentage who had 3 or more consecutive MA− UDS at any time-point (Table 2).
Figure 4.
Percent of UDS positive for MA at each study week by treatment group
Week 1 daily dose = 18mg,
Week 2 daily dose = 36mg;
Weeks 3–10 daily dose = 54mg;
Single-blind Placebo provided to all participants during Weeks 11–14
Table 2 also shows that the percentage of MA− UDS did not differ by treatment group (MPH=0.69; PLA=0.68; p=0.85) during the active phase as measured by the Treatment Effectiveness Score52 (TES). The TES computes a percentage by dividing the number of MA− UDS by the total number of UDS possible.
Concordance between self-reported MA use and UDS ranges from 86.4% (week 5) to 96.7% (week 9). Kappa measure of agreement ranges from .58 (week 10) to .90 (week 9).
Other Drug Use
Other drug use including cocaine, marijuana (MJ), and opioids was analyzed by both self-report (ASI) and UDS at Week 10 as presented in Table 2. Except for MJ use, no differences by treatment group were found for any drug.
Retention
Retention was measured as: 1) percent who completed the active medication phase; 2) mean number of weeks in treatment. Results show no difference in either measure of retention: 52.7% of the MPH group and 57.4% of the PLA group completed week 10 (p=0.62). The MPH group completed a mean of 7.6 weeks as compared to 7.8 for the PLA group (p=0.83).
Craving
Table 3 shows CQ-NOW craving scores were significantly greater in the PLA group at week 10 (p=0.03) and at week 14 (p=0.007) as compared to the MPH group. No significant group differences were found with the VAS.
Table 3.
Retention, Craving and Treatment Compliance by Treatment Group
| MPH (n=55) | Placebo (n=55) | P | |
|---|---|---|---|
| Retention | |||
| % completing active treatment phase | 52.7% | 57.4% | 0.62 |
| Mean # weeks in treatment (sd) | 7.63 (3.47) | 7.78 (3.39) | 0.83 |
| Craving | |||
| Mean CQ-Now Score at baseline (sd) | 3.85 (0.92) | 4.20 (1.12) | 0.08 |
| Mean CQ-Now Score, at week 10 (sd) | 4.46 (0.92) | 4.97 (0.85) | 0.03 |
| Mean CQ-Now Score at week 14 (sd) | 4.52 (0.96) | 5.22 (0.90) | 0.007 |
| Mean VAS Score at baseline | 48.25 (29.21) | 37.85 (29.19) | 0.07 |
| Mean VAS Score at week 10 (sd) | 23.90 (23.74) | 23.15 (25.33) | 0.90 |
| Mean VAS Score at week14 (sd) | 14.56 (16.85) | 14.83 (17.93) | 0.94 |
| Treatment Compliance | |||
| Medication Compliance | 95.23% | 95.34% | 0.77 |
| Mean # clinic visits for 10-week active phase | 12.98 (7.11) | 13.49 (6.99) | 0.71 |
| Mean # clinic visits for 14-week study | 16.27 (10.03) | 17.38 (10.24) | 0.57 |
| % CBT attendance | 49.04% | 56.36% | 0.03 |
| Mean # MI session attendance (sd) | 15.58 (9.72) | 16.65 (10.13) | 0.29 |
| % study drug compliance | 95.23% | 95.34% | 0.77 |
Treatment Compliance
The MPH group reported taking 95.23% of study drug prescribed, as compared to the PLA group with 95.34% (p=0.77). There were no significant differences in clinic visits for the 10-week active phase, and the entire 14-week study between groups (see Table 3). Attendance at CBT sessions was higher in the PLA group than in the MPH group (56.34% vs. 49.04%, p=0.03). For MI sessions, 15.58 sessions were attended by the MPH group, and 16.65 sessions were attended by the PLA group (p=0.28).
Adverse Events (AEs)
No differences in number or type of AEs were found by group, with 88 mild (MPH=21, PLA=37, p=0.23) and 10 moderate/severe (MPH=7; PLA=3, p=0.99) AEs deemed possibly or definitely study related. Mild AEs required no intervention; moderate or severe AEs resulted in some change to one’s usual routine (e.g., taking over-the-counter medications). No serious AEs occurred during the study (Table 4).
Table 4.
Adverse Events (in alphabetical order) deemed definitely/possibly related to study drug (n=98)
| Event Description | Severity | |
|---|---|---|
| Mild | Moderate/Severe | |
|
| ||
| Frequency | ||
|
| ||
| Aches/Pains/Injuries/fractures | 2 | - |
|
| ||
| Anxiety | 5 | 1 |
|
| ||
| Back Pain | 1 | - |
|
| ||
| Body aches | 1 | - |
|
| ||
| Cold, Flu, Allergy Symptoms: Other | 1 | - |
|
| ||
| Decreased appetite | 3 | - |
|
| ||
| Depression | 3 | 1 |
|
| ||
| Diarrhea | 3 | - |
|
| ||
| Gastrointestinal/Urinary: Other | 2 | - |
|
| ||
| Headache | 14 | 2 |
|
| ||
| Heartburn | - | 1 |
|
| ||
| Insomnia(difficulty getting to sleep) | 6 | 1 |
|
| ||
| Irritability | 5 | - |
|
| ||
| Itchiness | 3 | - |
|
| ||
| Lightheaded/dizzy | 7 | - |
|
| ||
| Miscellaneous: Others | 8 | - |
|
| ||
| Memory/concentration problems | 1 | - |
|
| ||
| Nausea/vomiting | 5 | 2 |
|
| ||
| Nightmares | 1 | - |
|
| ||
| Reduced quality of sleep | 2 | - |
|
| ||
| Respiratory/cardiac: Other | 1 | - |
|
| ||
| Restless legs | 1 | - |
|
| ||
| Restlessness | 1 | - |
|
| ||
| Runny nose | 2 | - |
|
| ||
| Sleepiness/drowsiness | 1 | - |
|
| ||
| Stomach/abdominal | 1 | - |
|
| ||
| Stuffy nose | 1 | - |
|
| ||
| Suicidal ideation/gesture/thoughts | - | 1 |
|
| ||
| Sweating | 2 | - |
|
| ||
| Upset stomach | 3 | - |
|
| ||
| Vivid dreams | 1 | 1 |
|
| ||
| Weakness | 1 | - |
|
| ||
| Total | 88 | 10 |
Treatment Satisfaction
No differences in satisfaction by treatment group were found, likely due to small cell sizes; 95.3% of the total sample reported being satisfied or very satisfied with treatment. A greater percentage of the MPH group reported medication as very effective (45.5%) compared to the PLA group (40.0%); 15.1% of the MPH group and 26.7% of the PLA group reported that medication was not effective. Both treatment groups reported that the treatment components were equally helpful; however, a larger percentage of the MPH group reported that medication was most helpful (23.4%) compared to PLA (18.2%).
DISCUSSION
No difference between groups was found for number of MA use days during the final 30 days of the active treatment phase. The MPH group, however, reduced MA use from baseline to end of the active phase significantly more than the PLA group (6½ vs. 3½ days). That finding suggests the possibility that a more clinically appropriate measure of the effectiveness of a pharmacotherapy would be a reduction in drug use assessed over the entire treatment period that takes into account level of drug use at baseline.
When this study was designed, the predominant measure used in NIH-sponsored treatment research for stimulant use disorders was self-reported days of use in the last 30 days of a study’s experimental condition. Thus, in accord with that prevailing convention, MA use in the final 30 days of the medication phase was adopted as the primary outcome of this trial. Subsequent analysis is consistent with prior research, such as the NIDA-sponsored bupropion study for MA use reported by Elkashef4. In that trial, the primary analysis did not reveal a treatment effect, but subsequent division of participants into two use-level groups did result in positive findings of treatment effect in the “lighter” user group. Furthermore, that same data set was subsequently re-analyzed by McCann and Li53 to use a non-binary evaluation of success and failure that created new endpoints and analytic approaches to determine that bupropion effectively facilitated abstinence.
Thus, in assessing MA use from baseline to the end of the active phase, our analysis found a significant difference in MA use between the groups (p=0.05). This was especially notable among moderate- to severe-level MA users (≥10 days of MA use in the month before baseline). Treatment groups also differed in self-reported MA craving assessed with the CQ-Now at Weeks 10 and 14, with the PLA group reporting significantly greater craving. The attenuation of craving in the MPH group is consistent with the observation by Tiihonen24 that a medication with at least some reinforcing effects is likely to be necessary to reduce craving in the early stages of recovery. Conversely, no difference in craving was found with the VAS, which may be due to less sensitivity of the measure. Against the background of many negative medication trials for MA use disorder, we believe that these findings are clinically important and relevant.
Recently, Miles and colleagues54 used a parallel group, double-blind, randomized placebo-controlled, 20-week trial set in Finland and New Zealand with 79 amphetamine/MA-dependent participants randomized to placebo or extended-release MPH. No difference was found in the proportion of positive UDS between treatment groups. Unlike our study, Miles et al found no difference in outcomes between subgroups of completers by treatment arm, whereas our results (not presented) show a difference in outcome when analyzing the completer group who remained in treatment through the end of the active phase, and assessing outcomes by level of baseline use. Similar to the Miles study, our UDS analyses did not demonstrate a difference by treatment group at Week 10. Controlling for baseline levels of MA use and site, however, we found a significant difference in UDS results at Week 14 (p=0.025) such that the MPH group was less likely to have a MA+ UDS result than the PLA group. Our results showed that the high-use MA group (≥10 days in the month before baseline) reported fewer days of MA use as compared with the PLA group. In contrast, the multi-site bupropion trial found that participants with low MA-use levels at baseline had better treatment response compared to placebo53 (p<0.0001). These results from two trials using two different medications support the importance of incorporating baseline level of MA use when assessing treatment effectiveness.
The PLA group attended a greater number of scheduled CBT sessions, and the treatment satisfaction survey documented that 66.7% of the PLA group reported that the CBT sessions were very effective as compared to only 50.0% of the MPH group. With higher rates of craving in this group, it may be that the PLA group relied more on the CBT sessions for treatment support.
Use of MJ was relatively high at about 7 days in the previous 30 days at baseline, with 33% of the sample having a positive MJ UDS. Interestingly, the MPH group had a significantly lower MJ-positive UDS at Week 10 compared to the PLA group. Specifically, in the MPH group, MJ-positive UDS decreased from 32.7% at baseline to 20.0% at week 10, whereas the PLA group had 34.6% MJ-positive UDS at baseline and 36.7% at week 10. Since MJ is often used to counteract the stimulant effects of MA, the concurrent decrease in MJ use may be expected in the MPH-treated group as they reported fewer days of MA use. MPH is also used extensively for treatment of ADHD symptoms, which is highly prevalent in MJ users.55 This finding may be related to improved ADHD symptoms.
Limitations of this study include concerns about standardizing procedures across multiple study sites; however, between-site analyses indicated few site-related differences. To address medication compliance, dosing was observed on scheduled clinic days, and participants were instructed to bring their medication bottles to clinic visits for pill counts. Significant participant dropout did occur but no differences by treatment condition were found, suggesting that the reasons for dropout were not related to study drug condition. However, since any medication must be acceptable to be considered a viable treatment option, high study drop-out may be an indication that medication or dose is not acceptable or adequate. Other limitations include the relatively small sample size, especially for female participants, inhibiting analyses to explore gender-related outcomes.
Conclusion
Study results suggest that methylphenidate may reduce methamphetamine use and craving, especially among moderate- and severe-level methamphetamine users. A future multi-center trial should incorporate approaches to enhance medication compliance including measuring MPH blood levels.
Table 5.
Treatment Satisfaction
| n = | MPH 30 | Placebo 33 | Total 63 |
|---|---|---|---|
| How satisfied are you with the treatment you received? | |||
| Very satisfied | 66.7% | 60.7% | 63.5% |
| Satisfied | 30.0% | 33.3% | 31.8% |
| Dissatisfied | 0% | 3.0% | 1.6.% |
| Very dissatisfied | 3.3% | 3.0% | 3.1% |
|
| |||
| How much do you think the treatment you received helped you? | |||
| Helped very much | 60.0% | 69.7% | 65.1% |
| Helped somewhat | 40.0% | 27.3% | 33.3% |
| Did not make a difference | 0% | 3.0% | 1.6% |
|
| |||
| How effective has the study medication been in treating your methamphetamine dependence? | |||
| Very effective | 45.5% | 40.0% | 42.9% |
| Somewhat effective | 39.4% | 33.3% | 36.5% |
| Not effective | 15.1% | 26.7% | 20.6% |
|
| |||
| Was the psychosocial treatment you received effective in treating your methamphetamine dependence? | |||
| Very effective | 50.0% | 66.7% | 58.7% |
| Somewhat effective | 46.7% | 27.3% | 36.5% |
| Not effective | 3.3% | 6.0% | 4.8% |
|
| |||
| Which treatment component do you think was most helpful? | |||
| Medication | 23.4% | 18.2% | 20.6% |
| Psychosocial | 23.3% | 21.2% | 22.2% |
| Both equally helpful | 53.3% | 57.6% | 55.6% |
| Neither helpful | 0% | 3.0% | 1.6% |
Acknowledgments
Funding from the National Institute on Drug Abuse (R01 DA 025084).
This study was supported by grants from the National Institute on Drug Abuse: R01-DA025-084 (WL); U01-DA013045 (WL); K24-DA16170 (LC), and study medication was provided by Janssen Pharmaceuticals. We wish to thank study team members at the UCLA Integrated Substance Abuse Programs: Sandy MacNicoll, Al Hasson, Claudia Gonzalez, Brian Perrochet, Christie Thomas, Mark Oyama, Jeff Annon, Matt Torrington, Daniel Dickerson, Karen Miotto, Shannon Schroeder, Jacqueline Fahey, Brittany Thornton, Elizabeth Nelson, Michelle Smith, and our data manager extraordinaire, Dave Bennett. At the University of Hawaii site, we thank Krista Bridges for nursing support, Kathryn Monsey for CBT, Michael Mueller and Michael Mau for Information Technology support, and Meredith Hermasura for administrative support. Also, sincere gratitude to Norris Turner and Hearee Chung from Janssen Pharmaceuticals, and Stephanie Befort from Quintiles for assistance with medication supply.
Footnotes
Registered at Clinicaltrials.com (NCT01044238).
No Author Declarations.
References
- 1.United Nations Office on Drugs and Crime. [Accessed September 2013];World Drug Report 2013. at: http://www.unodc.org/unodc/secured/wdr/wdr2013/World_Drug_Report_2013.pdf.
- 2.Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. [Google Scholar]
- 3.Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J. Pharmacotherapy of methamphetamine addiction: An update. Substance Abuse. 2008;29:31–49. doi: 10.1080/08897070802218554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33(5):1162–70. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- 5.Newton TF, Roache JD, De La Garza R, II, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31(7):1537–44. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- 6.Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, De La Garza R, Newton T, Ling W. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96(3):222–32. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batki SL, Moon J, Delucchi K, Bradley M, Hersh D, Smolar S, Mengis M, Lefkowitz E, Sexe D, Morello L, Everhart T, Jones RT, Jacob P., 3rd Methamphetamine quantitative urine concentrations during a controlled trial of fluoxetine treatment. Preliminary analysis. Ann N Y Acad Sci. 2000;909:260–3. doi: 10.1111/j.1749-6632.2000.tb06688.x. [DOI] [PubMed] [Google Scholar]
- 8.Colfax GN, Santos GM, Das M, Santos DM, Matheson T, Gasper J, Shoptaw S, Vittinghoff E. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168–75. doi: 10.1001/archgenpsychiatry.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piasecki MP, Steinagel GM, Thienhaus OJ, Kohlenberg BS. An exploratory study: the use of paroxetine for methamphetamine craving. J Psychoactive Drugs. 2002;34(3):301–4. doi: 10.1080/02791072.2002.10399967. [DOI] [PubMed] [Google Scholar]
- 10.Shoptaw S, Huber A, Peck J, Yang X, Liu J, Dang J, Roll J, Shapiro B, Rotheram-Fuller E, Ling W. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85(1):12–8. doi: 10.1016/j.drugalcdep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Anderson AL, Li SH, Biswas K, McSherry F, Holmes T, Iturriaga E, Kahn R, Chiang N, Beresford T, Campbell J, Haning W, Mawhinney J, McCann M, Rawson R, Stock C, Weis D, Yu E, Elkashef AM. Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2012;120(1–3):135–41. doi: 10.1016/j.drugalcdep.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodie JD, Figueroa E, Laska EM, Dewey SL. Safety and efficacy of gamma-vinyl GABA (GVG) for the treatment of methamphetamine and/or cocaine addiction. Synapse. 2005;55(2):122–5. doi: 10.1002/syn.20097. [DOI] [PubMed] [Google Scholar]
- 13.Coffin PO, Santos GM, Das M, Santos DM, Huffaker S, Matheson T, Gasper J, Vittinghoff E, Colfax GN. Aripiprazole for the treatment of methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108:751–61. doi: 10.1111/add.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkashef A, Kahn R, Yu E, Iturriaga E, Li SH, Anderson A, Chiang N, Ait-Daoud N, Weiss D, McSherry F, Serpi T, Rawson R, Hrymoc M, Weis D, McCann M, Pham T, Stock C, Dickinson R, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Li MD, Johnson BA. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297–306. doi: 10.1111/j.1360-0443.2011.03771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galloway GP, Buscemi R, Coyle JR, Flower K, Siegrist JD, Fiske LA, Baggott MJ, Li L, Polcin D, Chen CY, Mendelson J. A randomized, placebo-controlled trial of sustained-release dextroamphetamine for treatment of methamphetamine addiction. Clin Pharmacol Ther. 2011;89(2):276–82. doi: 10.1038/clpt.2010.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant JE, Odlaug BL, Kim SW. A double-blind, placebo-controlled study of N-acetyl cysteine plus naltrexone for methamphetamine dependence. Eur Neuropsychopharmacol. 2010;20(11):823–8. doi: 10.1016/j.euroneuro.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Heinzerling KG, Shoptaw S, Peck JA, Yang X, Liu J, Roll J, Ling W. Randomized, placebo-controlled trial of baclofen and gabapentin for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006;85(3):177–84. doi: 10.1016/j.drugalcdep.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Johnson BA, Ait-Daoud N, Elkashef AM, Smith EV, Kahn R, Vocci F, Li SH, Bloch DA Methamphetamine Study Group. A preliminary randomized, double-blind, placebo-controlled study of the safety and efficacy of ondansetron in the treatment of methamphetamine dependence. Int J Neuropsychopharmacol. 2008;11(1):1–14. doi: 10.1017/S1461145707007778. [DOI] [PubMed] [Google Scholar]
- 19.Ling W, Shoptaw S, Hillhouse M, Bholat MA, Charuvastra C, Heinzerling K, Chim D, Annon J, Dowling PT, Doraimani G. Double-blind placebo-controlled evaluation of the Prometa protocol for methamphetamine dependence. Addiction. 2012;107:361–369. doi: 10.1111/j.1360-0443.2011.03619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meredith CW, Jaffe C, Yanasak E, Cherrier M, Saxon AJ. An open-label pilot study of risperidone in the treatment of methamphetamine dependence. J Psychoactive Drugs. 2007;39(2):167–72. doi: 10.1080/02791072.2007.10399875. [DOI] [PubMed] [Google Scholar]
- 21.Meredith CW, Jaffe C, Cherrier M, Robinson JP, Malte CA, Yanasak EV, Kennedy A, Ferguson LC, Tapp AM, Saxon AJ. Open trial of injectable risperidone for methamphetamine dependence. J Addict Med. 2009;3(2):55–65. doi: 10.1097/ADM.0b013e31818e2185. [DOI] [PubMed] [Google Scholar]
- 22.Urschel HC, Hanselka LL, Baron M. A controlled trial of flumazenil, gabapentin and hydroxyzine in treatment of methamphetamine dependence. CPDD 70th annual scientific meeting; June 2008; Puerto Rico. [Google Scholar]
- 23.Zorick T, Sevak RJ, Miotto K, Shoptaw S, Swanson AN, Clement C, De La Garza R, 2nd, Newton TF, London ED. Pilot Safety Evaluation of Varenicline for the Treatment of Methamphetamine Dependence. J Exp Pharmacol. 2009;2010(2):13–18. [PMC free article] [PubMed] [Google Scholar]
- 24.Tiihonen J, Kuoppasalmi K, Föhr J, Tuomola P, Kuikanmäki O, Vorma H, Sokero P, Haukka P, Meririnne E. A Comparison of Aripiprazole, Methylphenidate, and Placebo for Amphetamine Dependence. American Journal of Psychiatry. 2007;164:160–2. doi: 10.1176/ajp.2007.164.1.160. [DOI] [PubMed] [Google Scholar]
- 25.Aron AR, Dowson J, Sahakian BJ, Robbins TW. Methylphenidate improves response inhibition in adults with Attention Deficit/Hyperactivity Disorder. Biological Psychiatry. 2003;54:1465–8. doi: 10.1016/s0006-3223(03)00609-7. [DOI] [PubMed] [Google Scholar]
- 26.Biederman J, Spencer T. Methylphenidate in treatment of adults with Attention-Deficit/Hyperactivity Disorder. Journal of Attention Disorders. 2002;6 (Suppl 1):S101–7. doi: 10.1177/070674370200601s12. [DOI] [PubMed] [Google Scholar]
- 27.Faraone SV. Etiology and path physiology of adult attention-deficit/hyperactivity disorder. Primary Psychiatry. 2004;11(7):28–40. [Google Scholar]
- 28.Faraone SV, Spencer T, Aleardi M, Pagano C, Biederman J. Meta-analysis of the efficacy of methylphenidate for treating adult attention deficit hyperactivity disorder. Journal of Clinical Psychopharmacology. 2004;54(1):24–9. doi: 10.1097/01.jcp.0000108984.11879.95. [DOI] [PubMed] [Google Scholar]
- 29.Wilens T, Pelham W, Stein M, Conners CK, Abikoff H, Atkins M, August G, Greenhill L, McBurnett K, Palumbo D, Swanson J, Wolraich M. ADHD Treatment With Once-daily OROS Methylphenidate: Interim 12-month Results From a Long-term Open-label Study. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42(4):424–33. doi: 10.1097/01.CHI.0000046814.95464.7D. [DOI] [PubMed] [Google Scholar]
- 30.Seeman P, Madras BK. Anti-hyperactivity medication: methylphenidate and amphetamine. Mol Psychiatry. 1998;3:386–96. doi: 10.1038/sj.mp.4000421. [DOI] [PubMed] [Google Scholar]
- 31.Dougherty DD, Bonab AA, Spencer TJ, Rauch SL, Madras BK, Fischman AJ. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet. 1999;354 (9196):2132–33. doi: 10.1016/S0140-6736(99)04030-1. [DOI] [PubMed] [Google Scholar]
- 32.Collins SL, Levin FR, Foltin RW, Kleber HD, Evans SM. Response to cocaine, alone and in combination with methylphenidate, in cocaine abusers with ADHD. Drug Alcohol Depend. 2006;82:158–67. doi: 10.1016/j.drugalcdep.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Herin DV, Rush CR, Grabowski J. Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. Annals of the New York Academy of Sciences. 2010;1187:76–100. doi: 10.1111/j.1749-6632.2009.05145.x. [DOI] [PubMed] [Google Scholar]
- 34.Spencer TJ, Adler LA, McGough JJ, Muniz R, Jiang H, Pestreich L. Efficacy and Safety of Capsules in Adults with Attention-Deficit/Hyperactivity Disorder. Biol Psychiatry. 2007;61:1380–7. doi: 10.1016/j.biopsych.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 35.Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- 36.Sandoval V, Riddle E, Hanson G, Fleckstein A. Methylphenidate alters vesicular monoamine transport and prevents methamphetamine-induced dopaminergic deficits. Journal of Pharmacology And Experimental Therapeutics. 2003;304:1181–7. doi: 10.1124/jpet.102.045005. [DOI] [PubMed] [Google Scholar]
- 37.Volkow ND, Wang GJ, Fowler JS, et al. Effects of methylphenidate on regional brain glucose metabolism in humans: relationship to dopamine D2 receptors. Am J Psychiatry. 1997;154:50–5. doi: 10.1176/ajp.154.1.50. [DOI] [PubMed] [Google Scholar]
- 38.Volkow ND, Wang GJ, Fowler JS, et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: Replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- 39.Hanson GR, Sandoval V, Riddle E, Fleckenstein AE. Psychostimulants and vesicle trafficking: a novel mechanism and therapeutic implications. Annals of the New York Academy of Sciences. 2004;1025:146–50. doi: 10.1196/annals.1316.019. [DOI] [PubMed] [Google Scholar]
- 40.Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. 2007;87(1):20–9. doi: 10.1016/j.drugalcdep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 41.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 42.Childress AR, McLellan AT, O’Brien CP. Conditioned responses in a methadone population: A comparison of laboratory, clinic, and natural settings. J Subst Abuse Treat. 1986;3:173–9. doi: 10.1016/0740-5472(86)90018-8. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan RF, Cooney NL, Baker LH, Gillespie RA, Meyer RE, Pomerlau OF. Reactivity to alcohol-related cues: Physiological and subjective responses in alcoholics and non-problem drinkers. J Stud Alcohol. 1985;46:267–72. doi: 10.15288/jsa.1985.46.267. [DOI] [PubMed] [Google Scholar]
- 44.Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- 45.Connors CK, Erhardt D, Epstein JN, Parker JDA, Sitarenios G, Sparrow E. Self-ratings of ADHD symptoms in Adults I: Factor structure and normative data. Journal of Attention Disorders. 1999;3:141–151. [Google Scholar]
- 46.Lavori PW. Clinical trials in psychiatry: Should protocol deviation censor patient data? Neuropsychopharmacology. 1992;6:39–48. [PubMed] [Google Scholar]
- 47.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. New York: Oxford University Press Inc; 1994. [Google Scholar]
- 48.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. SAS Institute Inc; Cary, NC, USA: 1996. [Google Scholar]
- 49.Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. J Am Stat Assoc. 1993;88:9–25. [Google Scholar]
- 50.SAS Institute. MIXED; PROC MIXED; GLMM: CLIMMIX macro. SAS Institute Inc; Cary, NC: 1996. [Google Scholar]
- 51.Diggle PJ. Testing for random dropouts in repeated measure data. Biometrics. 1989;45:1255–8. [Google Scholar]
- 52.Ling W, Shoptaw S, Rawson RA, Klett CJ. In: Harris LS, editor. A composite score for evaluating treatment response in cocaine pharmacotherapy trials (TES); Problems of Drug Dependence, 1994: Proceedings of the 56th Annual Scientific Meeting of the College on Problems of Drug Dependence, Inc. Vol. II. National Institute on Drug Abuse Research Monograph 153; Washington, DC: Supt. of Docs., U.S. Govt. Print. Off; 1995. p. 495. NIH Pub. No. (ADM) 95-3883. [Google Scholar]
- 53.McCann D, Li S. A novel nonbinary evaluation of success and failure reveals B=bupropion efficacy versus methamphetamine dependence: Re-analysis of a multisite trial. CNS Neurosci Ther. 2012;18:414–8. doi: 10.1111/j.1755-5949.2011.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miles SW, Sheridan J, Russell B, Kydd R, Wheeler A, Walters C, Gamble G, Hardley P, Jensen M, Kuoppasalmi K, Tuomola P, Föhr J, Kuikanmäki O, Vorma H, Salokangas R, Mikkonen A, Kallio M, Kauhanen J, Kiviniemi V, Tiihonen J. Extended-release methylphenidate for treatment of amphetamine/methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108:1279–86. doi: 10.1111/add.12109. [DOI] [PubMed] [Google Scholar]
- 55.Fergusson DM, Boden JM. Cannabis use and adult ADHD symptoms. Drug Alcohol Depend. 2008;95(1–2):90–6. doi: 10.1016/j.drugalcdep.2007.12.012. [DOI] [PubMed] [Google Scholar]