Abstract
We recently reported that microinjection of ethanol into the rostral ventrolateral medulla (RVLM) elicits modest increases in local extracellular signal-regulated kinase (ERK) and blood pressure (BP) in conscious normotensive rats. In this study, we tested the hypothesis that RVLM ser/thr phosphatases dampen the ERK-dependent pressor effect of ethanol in normotensive rats. We show that the pressor response elicited by intra-RVLM ethanol (10 μg) was: (i) abolished following local ERK inhibition with PD98059 (1 μg); (ii) associated with significant reduction in local phosphatase activity. Inhibition of the RVLM ser/thr phosphatase activity by okadaic acid (OKA, 0.4 μg) or fostriecin (15 pg) caused significant increases in blood pressure (BP) and potentiated the magnitude and duration of the pressor response as well as the phosphatase inhibition elicited by subsequent intra-RVLM administration of ethanol. Intra-RVLM acetaldehyde (2 μg), the main metabolic product of ethanol, caused no changes in BP or RVLM phosphatase activity but it produced significant increases in BP and inhibition of local phosphatase activity in rats treated with OKA or fostriecin. Together, the RVLM phosphatase activity acts tonically to attenuate the ERK-dependent pressor effect of ethanol or acetaldehyde in normotensive rats.
Keywords: Ethanol, hypertension, phosphatases, rostral ventrolateral medulla, extracellular signal-regulated kinase, normotensive rats
1. Introduction
The mitogen-activated protein kinases (MAPKs) are a group of serine/threonine kinases that convert extracellular stimuli into a wide range of cellular responses. They include the extracellular signal-regulated kinases (ERK), c-Jun amino (N)-terminal kinases, and p38 (English and Cobb, 2002; Cargnello and Roux, 2011). MAPKs have been implicated in many actions of ethanol such as hepatotoxicity, pancreatitis, neurotoxicity and increased cancer risk (Yang et al., 2001; Kalluri and Ticku, 2003; Aroor and Shukla, 2004). Reported studies suggest that ethanol may upregulate or downregulate the phosphorylation of MAPKs depending on the experimental settings including dose and duration of exposure, MAPK isoform under investigation, and cell or tissue type (Aroor and Shukla, 2004). Our previous reports showed that systemic (Mao et al., 2003) or intra-RVLM (Li et al., 2005) ethanol elicits sympathoexcitation and pressor response in conscious spontaneously hypertensive (SHR) but not in their normotensive counterparts, Wistar Kyoto rats (WKY). Acetaldehyde, the oxidative product of ethanol (Correa et al., 2003; Zhang et al., 2004), appears to mediate, at least partly, the pressor effect of ethanol (El-Mas and Abdel-Rahman, 2012). In a more recent study (El-Mas et al., 2013), we established evidence that implicated alterations in the phosphorylation/dephosphorylation profile of RVLM-MAPKs in ethanol- or acetaldehyde-evoked pressor response in SHRs because: (i) the latter response was associated with increase in the RVLM level of phosphorylated ERK, (ii) both the neurochemical and BP responses were abolished after pharmacologic inhibition of ERK, and (iii) the inhibition of local phosphatases by OKA increased the level of phosphorylated ERK in the RVLM and BP (El-Mas et al., 2013).
The reason for the reduced pressor effect of ethanol in normotensive compared with hypertensive rats (Li et al., 2005; El-Mas and Abdel-Rahman, 2012) is not clear. In the present study, we tested the hypothesis that a compromised phosphatase-dependent dephosphorylation of phospho-ERK accounts for the negligible pressor response caused by intra-RVLM ethanol or acetaldehyde in normotensive rats. To test this intriguing hypothesis, we investigated the effects of intra-RVLM ethanol or acetaldehyde on the BP and heart rate (HR) in conscious normotensive rats following the inhibition of local activity (i) ERK with PD98059, and (i) phosphatases with OKA or fostriecin. Further, we investigated the impact of intra-RVLM ethanol or acetaldehyde alone or in combination with OKA on the RVLM phosphatase activity.
2. Results
Baseline MAP and HR values in all rat groups prior to the RVLM microinjections of the vehicle or drug regimens were not statistically different (Table 1).
Table 1.
Average baseline values of mean arterial pressure (MAP, mmHg) and heart rate (HR, beats/min) prior to any drug or vehicle treatments.
| Groups | n | MAP | HR |
|---|---|---|---|
| DMSO/ACSF | 10 | 121±7 | 299±8 |
| DMSO/ethanol | 6 | 121±5 | 304±6 |
| DMSO/acetaldehyde | 6 | 125±3 | 311±10 |
| Okadaic acid/ACSF | 7 | 118±4 | 304±11 |
| Okadaic acid /ethanol | 10 | 121±3 | 326±15 |
| Okadaic acid /acetaldehyde | 7 | 124±3 | 301±30 |
| Fostriecin/ACSF | 5 | 122±3 | 273±12 |
| Fostriecin/ethanol | 7 | 117±3 | 300±6 |
| Fostriecin/acetaldehyde | 6 | 116±4 | 295±7 |
Values are mean±SEM of 5-10 observations.
2.1. The inhibitory effect of ethanol or acetaldehyde on RVLM phosphatase activity is potentiated by OKA
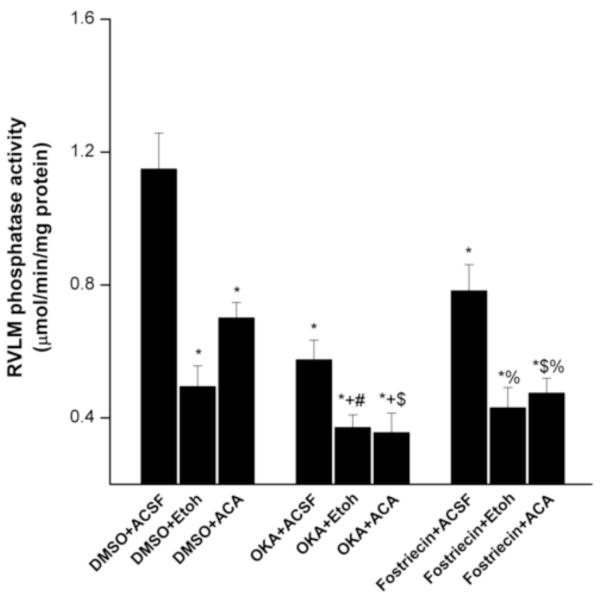
The effects of ethanol, acetaldehyde, or their vehicle (ACSF) on the RVLM serine/threonine phosphatase catalytic activity in the absence and presence of the phosphatase inhibitors (OKA or fostriecin) are shown in figure 1. The RVLM phosphatase activity was significantly reduced by microinjection of ethanol or acetaldehyde (Fig. 1). Phosphatase activity was also significantly reduced in RVLM neurons after microinjection of OKA or fostriecin. The inhibition of phosphatase activity was potentiated in rats treated with ethanol or acetaldehyde along with the phosphatase inhibitors (OKA or fostriecin) (Fig. 1).
Fig. 1.
Effects of intra-RVLM ethanol (10 μg) or acetaldehyde (2 μg) on local serine/threonine phosphatase activity in WKY rats in the absence and presence of okadaic acid or fostriecin (phosphatase inhibitors). Values are mean±S.E.M. of 5-10 observations. *P<0.05 vs. “DMSO+ACSF”, +P<0.05 vs. “OKA+ACSF”, #P<0.05 vs. “DMSO+Etoh”, $P<0.05 vs. “DMSO+acetaldehyde”, %P<0.05 vs. “fostriecin+ACSF”.
2.2. Exaggerated pressor effects for ethanol or acetaldehyde in OKA- or fostriecin-treated rats
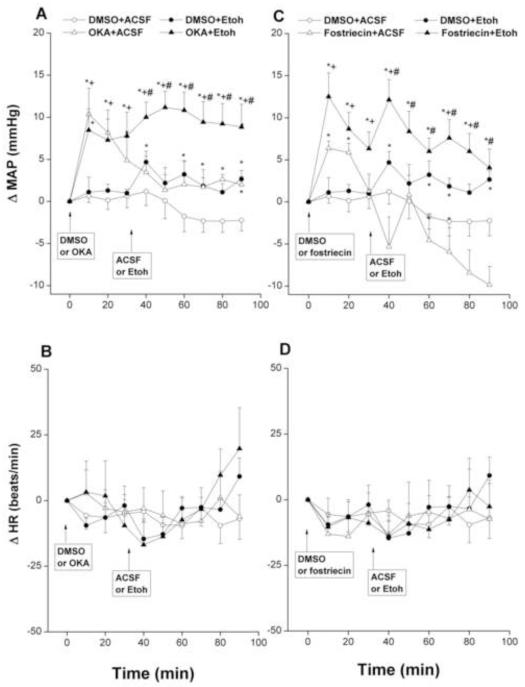
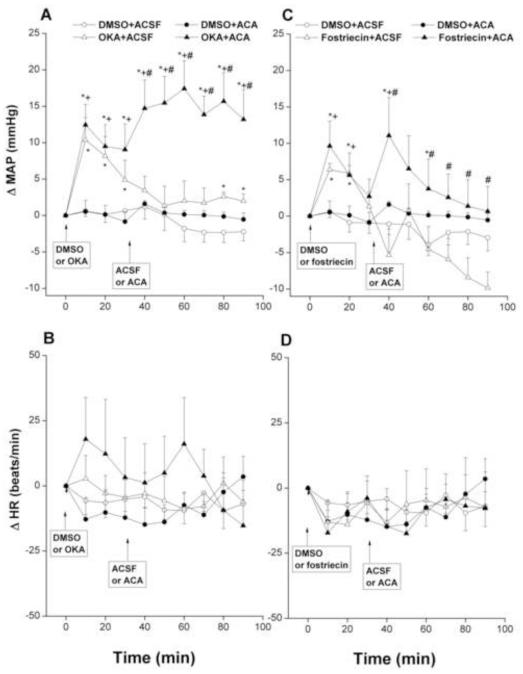
Intra-RVLM ethanol (10 μg) caused modest but significant increases in MAP compared with corresponding ACSF values (Fig. 2A). By contrast, no changes in MAP were demonstrated after intra-RVLM administration of acetaldehyde (2 μg, Fig. 3A). The inhibition of RVLM phosphatases by OKA (0.4 μg, Fig. 2A) or fostriecin (15 pg, Fig. 2C) significantly increased MAP compared with corresponding DMSO values. The increases in MAP caused by OKA appeared at 10 min, then MAP showed gradual decline but remained significantly higher than corresponding DMSO values throughout the 90-min observation period (Fig. 2A). Fostriecin increased MAP for 20 min, after which MAP returned to DMSO values (Fig. 2C). Additional and sustained increases in MAP were seen when OKA- or fostriecin-pretreated WKY rats subsequently (30 min later) received intra-RVLM ethanol (Fig. 2A-C) or acetaldehyde (Fig. 3A-C).
Fig. 2.
Changes in mean arterial pressure (MAP) and heart rate (HR) caused by intra-RVLM ethanol (Etoh, 10 μg) in the absence or presence of phosphatase inhibitors (0.4 μg okadaic acid, OKA, or 15 pg fostriecin). Values are mean±S.E.M. of 5-10 observations. *P<0.05 vs. “DMSO+ACSF”, +P<0.05 vs. “DMSO+Etoh”, #P<0.05 vs. “OKA+ACSF” or “fostriecin+ACSF”.
Fig. 3.
Changes in mean arterial pressure (MAP) and heart rate (HR) caused by intra-RVLM acetaldehyde (ACA, 2 μg) in the absence and presence of phosphatase inhibitors (0.4 μg okadaic acid, OKA, or 15 pg fostriecin). Values are mean±S.E.M. of 5-10 observations. *P<0.05 vs. “DMSO+ACSF”, +P<0.05 vs. “DMSO+ACA”, #P<0.05 vs. “OKA+ACSF” or “fostriecin+ACSF”.
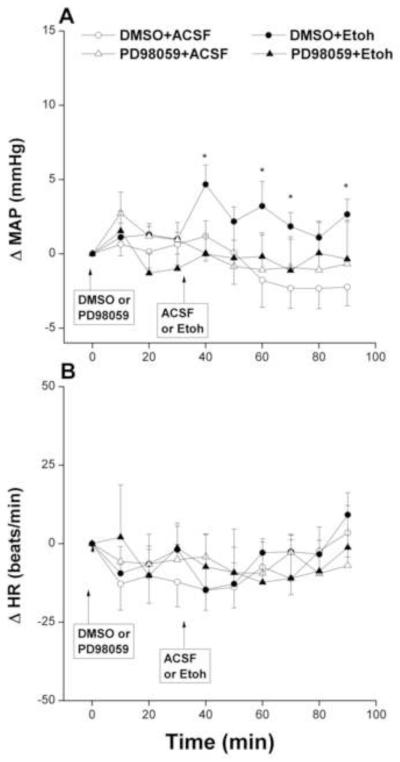
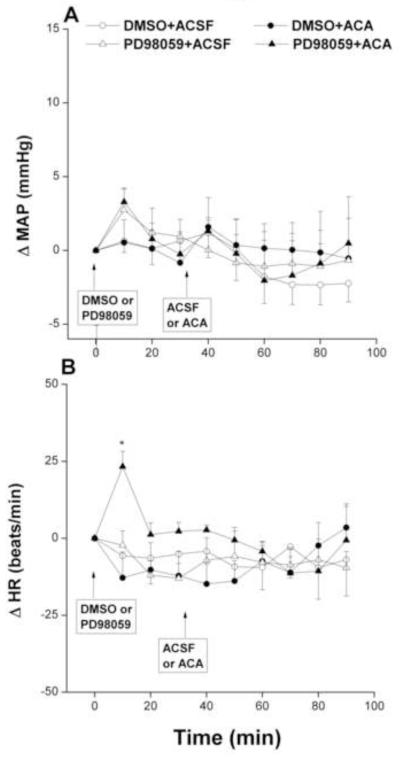
The inhibition of ERK by PD98059 (1 μg) in the RVLM neurons had no effect on baseline MAP, compared with corresponding vehicle (DMSO) values, but it virtually abolished the pressor effect of ethanol (Fig. 4A). On the other hand, changes in MAP caused by the PD98059/acetaldehyde regimen were not statistically different from those caused by their vehicle (Fig. 5A). Unlike MAP, HR was not significantly affected by any of the drug regimens employed in the present study (Figs. 2-5, bottom panels) except for a significant and brief (10 min) increase in HR after the administration of PD98059 in the PD98059/acetaldehyde group (Fig. 5, bottom panel).
Fig. 4.
Changes in mean arterial pressure (MAP) and heart rate (HR) caused by intra-RVLM ethanol (Etoh, 10 μg) in the absence and presence of PD98059 (1 μg, ERK1/2 inhibitor). Values are mean±S.E.M. of 6-10 observations. *P<0.05 vs. “DMSO+ACSF”.
Fig. 5.
Changes in mean arterial pressure (MAP) and heart rate (HR) caused by intra-RVLM acetaldehyde (ACA, 2 μg) in the absence and presence of PD98059 (1 μg, ERK1/2 inhibitor). Values are mean±S.E.M. of 6-10 observations. *P<0.05 vs. “DMSO+ACSF”.
3. Discussion
The current study extends our previous work in which we showed that intra-RVLM ethanol or acetaldehyde elicits substantially greater pressor effects in conscious SHRs compared with WKY rats (El-Mas and Abdel-Rahman, 2012) and that enhanced phosphorylation of the MAPK/ERK in tissues of the rostral medulla underlies the pressor action of ethanol in SHRs (El-Mas et al., 2013). Given the importance of phosphatases in the deactivation (dephosphorylation) of MAPKs (Cargnello and Roux, 2011), evidence was sought in this study to implicate the phosphatase/ERK cascade in the diminished pressor effect of intra-RVLM ethanol or acetaldehyde in WKY rats. Several new findings are reported in this study. First, in contrast to negligible effects of acetaldehyde, ethanol caused modest but significant elevations in BP and inhibition of the RVLM phosphatase activity. Second, additive increases in BP and reductions in RVLM phosphatase activity were observed upon intra-RVLM administration of phosphatase inhibitors (OKA or fostriecin) along with ethanol or acetaldehyde. Third, ERK inhibition abolished the pressor effect of ethanol. It is concluded that the RVLM phosphatases act tonically to dampen the ERK-dependent pressor effect of ethanol in WKY rats.
Comparison of our present data in WKY rats and previous studies in SHRs (El-Mas et al., 2013) reveals some important inter-strain similarities and differences with regards to the interaction of ethanol with the RVLM phosphatase/ERK cascade. Like its effects in SHRs (El-Mas et al., 2013), the inhibition of ERK1/2 by PD98059 abolished the pressor effect of intra-RVLM ethanol in WKY rats, suggesting a key role for ERK1/2 signaling in the BP response to ethanol. It is worth noting that the RVLM ERK1/2 signaling positively correlates with sympathetic activity in several rat models including stroke-prone SHRs (Kishi et al., 2010) and in heart failure (Gao et al., 2010). Moreover, the activation or inhibition of the angiotensin AT1 receptors in the RVLM is paralleled with qualitatively similar changes in BP and ERK1/2 signaling (Seyedabadi et al., 2001; Kishi et al., 2010). Considering the major role of RVLM neurons in controlling central sympathetic discharges (Turner et al., 2013) and in the sympathoexcitatory action of ethanol (Mao et al., 2003), the findings that pharmacologic inhibition of ERK1/2 abolished the pressor action of ethanol in WKY rats (this study) or SHRs (El-Mas et al., 2013), despite the exaggerated pressor effect of ethanol in the SHR, suggests a crucial role for ERK1/2 in the increased sympathetic activity, which led to the rise in BP. Nonetheless, these findings highlight a potential role for local regulation of ERK phosphorylation/dephosphorylation in the compromised pressor effect of ethanol in normotensive rats.
Reported neurobiological studies suggest a primary role for serine/threonine phosphatases in the deactivation (dephosphorylation) of MAPKs (Cargnello and Roux, 2011). In SHRs for example, pharmacologic inhibition of RVLM phosphatases (OKA) upregulates ERK1/2 and elevates BP (El-Mas et al., 2013). Also, estrogen protects cells against glutamate-induced oxidative stress through preserving phosphatase activity in the face of oxidative insults, resulting in attenuation of the persistent phosphorylation of ERK associated with neuronal death (Yi et al., 2008). With this idea in mind, we tested the hypothesis that RVLM phosphatases act tonically to offset the pressor effect of intra-RVLM ethanol in WKY rats.
The likelihood of this assumption was verified by examining the individual and combined effects of ethanol and the phosphatase inhibitors (OKA or fostriecin) on phosphatase activity and BP. The results showed that the combined microinjection of ethanol along with OKA or fostriecin caused significantly more inhibition of phosphatase activity compared with the inhibitory effects of the individual drugs (Fig. 1). Interestingly, the BP response followed a similar pattern as indicated by the greater and more sustained elevations in BP observed following intra-RVLM administration of the ethanol/OKA or ethanol/fostriecin regimen. This parallelism in the magnitudes of the pressor effect and RVLM phosphatase inhibition seems to favor a causal relationship between the two phenomena. Because OKA inhibits the catalytic activity of all ser/thr phosphatase isoforms (PP1 through PP6) whereas fostriecin selectively inhibits PP1 and PP2A (Swingle et al., 2007), it is conceivable to suggest that the 2 latter isoforms of phosphatases might be selectively involved in the modulation of the ERK-dependent pressor effect of ethanol. Notably, fostriecin also offers a better experimental tool for phosphatase inhibition because of the reported cytotoxicity associated with the use of nanomolar concentrations of OKA (Herschman et al., 1989; Yoshizawa et al., 1990). Notably, ethanol, compared with vehicle, inhibited RVLM phosphatase activity in WKY rats (0.5±0.1 vs. 1.2±0.1 mol/min/mg protein, P<0.05), but not in SHRs (1.5±0.3 vs. 1.4±0.3 mol/min/mg protein) in our previous study (El-Mas et al., 2013), reflecting a strain-dependent effect for ethanol on RVLM phosphatases.
The latter conclusion is further bolstered by data obtained from the acetaldehyde experiment. Acetaldehyde is the oxidative product of ethanol (Quertemont et al., 2005) and has been implicated in some of its biological actions including the ethanol-evoked pressor response (Pastor and Aragon, 2008; Karahanian et al., 2011; El-Mas and Abdel-Rahman, 2012). Here we report that although intra-RVLM acetaldehyde inhibited phosphatase activity by ~ 30% and caused 3-4 mmHg increase in BP, these changes were not statistically different from those of vehicle-treated values. More importantly, the two effects of acetaldehyde (BP elevation and RVLM phosphatase inhibition) were intensified when it was microinjected following OKA or fostriecin. In fact, comparison of the present findings (figures 1-3) revealed comparable pressor and phosphatase inhibitory profiles for the ethanol/OKA and acetaldehyde/OKA regimens. The data further corroborate our previous report concerning the role of acetaldehyde in mediating the pressor effect of ethanol (El-Mas and Abdel-Rahman, 2012) and highlight the capacity of local phosphatases to downregulate the pressor response elicited by intra-RVLM ethanol or its metabolic product acetaldehyde.
It is important to comment on the microinjected doses of ethanol and acetaldehyde in the current study. The 10 μg dose of ethanol has been used in reported studies from our laboratory (El-Mas and Abdel-Rahman, 1993b, 2012) and others (Varga and Kunos, 1990; 1992). This dose of ethanol interacts selectively with subsets of MAPKs (El-Mas et al., 2013) and central GABA and glutamate receptors (El-Mas and Abdel-Rahman, 1993b; Varga and Kunos, 1990) and its effects disappear within 1-2 hr. Such selectivity and reversibility eliminate the possibility of involvement of nonspecific neuronal damage in the ethanol effects. More importantly, the 10 μg intra-RVLM dose of ethanol elicits cardiovascular effects comparable to those caused by the 1 g/kg dose of systemic ethanol (El-Mas and Abdel-Rahman, 1993a, 1993b, 1998; Varga and Kunos, 1990, 1992). The 1 g/kg systemic dose of ethanol produces blood alcohol levels similar to those seen in humans after consumption of moderate to heavy amounts of alcohol (Ireland et al., 1984; Abdel-Rahman et al., 1987), which establishes the clinical relevance of our findings. The microinjected dose of acetaldehyde (2 μg) was selected based on reported relative potencies of acetaldehyde and ethanol in cardiovascular (El-Mas and Abdel-Rahman, 2012) and behavioral studies (Quertemont et al., 2005). A higher intra-RVLM dose of acetaldehyde (4 μg) increased BP to levels that were not different from those produced by the 2 μg dose (El-Mas et al., 2013).
Overall, the current data underscore the role of the RVLM phosphatases, particularly the PP1 and PP2A isoforms, in the ERK-dependent pressor action of ethanol in normotensive rats. We demonstrate an inverse relationship between BP and phosphatase activity in the RVLM tissues because (i) phosphatase inhibition by ethanol, OKA, or fostriecin was coupled with BP elevations, and (ii) greater increases in BP and decreases in phosphatase activity developed after combined RVLM administration of OKA or fostriecin along with ethanol or acetaldehyde. It is conceivable that the residual phosphatase activity in rats receiving the individual treatments (ethanol, acetaldehyde, or phosphatase inhibitor) might have acted to hamper the excessive rises in BP.
4. Materials and methods
Male WKY rats (12–13 weeks old, Charles River, Raleigh, NC) were used. All experiments were approved by the institutional animal care and use committee and carried out in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health.
4.1. Intracranial and intravascular cannulations
The methods described in our previous studies for the intra-RVLM (El-Mas and Abdel-Rahman, 2012; El-Mas et al., 2013) and femoral artery (El-Mas and Abdel-Rahman, 1995, 1997, 1999) cannulations and drug administration were employed.
4.2. Measurement of phosphatase activity
Micropunches were taken from the RVLM of frozen brains with a stainless steel bore (i.d. 0.94 mm, Stoelting Co, Wood Dale, IL) as reported in previous studies including ours (Bardgett et al., 2010; El-Mas et al., 2013). Tissues were mixed in 20 μl buffer (20 mM imidazole HCl, 2 mM EDTA, and 2 mM EGTA) and centrifuged at 2000 × g for 5 min. After quantification of the total protein content with the Bradford assay (Bradford, 1976), the ser/thr phosphatase activity in the protein extract of RVLM tissue was measured colorimetrically using the Ser/Thr Phosphatase Assay Kit 1 (Malachite Green Assay, Upstate Cell Signaling Solutions, Lake Placid, NY) according to the manufacturer’s instructions and as described in our previous studies (El-Mas et al., 2013). Briefly, 2 μl of the protein extract (~ 10 μg protein) was transferred into a 96-well microtiter plate and mixed with 25 μl assay buffer and threonine phosphopeptide. After 30 min, the enzymatic reaction was terminated by the addition of 100 μl of freshly prepared malachite green solution. The absorbance was measured at 640 nm and enzyme activity was estimated from a standard curve prepared with standard phosphate solutions.
4.3. Protocols and experimental groups
We investigated the effects of OKA (0.4 μg, nonselective ser/thr phosphatase inhibitor), fostriecin (15 pg, selective PP1/PP2A inhibitor), or PD98059 (1 μg, ERK inhibitor) on BP and HR responses elicited by intra-RVLM ethanol or acetaldehyde. The doses of OKA (Choe et al., 2004), fostriecin (Chao et al., 2007), and PD98059 (Gerdjikov et al., 2004; El-Mas et al., 2013) were selected based on reported studies. A total of 9 groups of conscious WKY rats (n=5-10 each), pre-instrumented with intra-RVLM and intra-arterial cannulas as indicated under methods, were used. A period of at least 60 min was allowed at the beginning of each experiment for BP stabilization. All injections (80 nl) were made unilaterally into the RVLM according to established protocol in our lab (El-Mas and Abdel-Rahman, 2012; El-Mas et al., 2013). Control rats received 80 nl of 5% DMSO, the vehicle for the pharmacologic inhibitors, followed by the artificial cerebrospinal fluid (ACSF) to control for ethanol/acetaldehyde microinjection. Treatment groups received one of the inhibitors or the vehicle followed 30 min later by 80 nl of 15.7% ethanol (10 μg), 2.5% acetaldehyde (2 μg) or ACSF. These selected doses of ethanol and acetaldehyde produce similar cardiovascular (El-Mas and Abdel-Rahman, 2012) and behavioral (Quertemont et al., 2005) effects. BP and HR were monitored for 60 min following intra-RVLM ethanol, acetaldehyde or ACSF.
At the conclusion of the experiments, rats were euthanized with an overdose of pentobarbital sodium (100 mg/kg) and their brains were rapidly removed and stored at −80°C until used for the determination of phosphatase activity in RVLM neurons as detailed above.
4.4. Drugs
PD98059, okadaic acid, fostriecin, acetaldehyde (Sigma Chemical Co., St. Louis, MO), ethanol (Midwest Grain Products Co., Weston, MO) were purchased from commercial vendors.
4.5. Statistical analysis
Values are expressed as means±S.E.M. Mean arterial pressure (MAP) was computed via integration of the area under the pulse pressure curve using the LabChart software. The analysis of variance (ANOVA) followed by a Newman-Keuls post-hoc analysis was used for multiple comparisons with the level of significance set at P<0.05.
Highlights.
Phosphatase activity in the RVLM is inversely related to BP
Phosphatase inhibition mediates the pressor effect of intra RVLM ethanol
Phosphatase inhibition by OKA or fostriecin exacerbates ethanol evoked pressor response Acetaldehyde mimics the BP and neurochemical effects of ethanol
Acknowledgments
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant 2R01 AA07839-19]. The authors thank Kui Sun for her technical assistance.
Abbreviations
- BP
blood pressure
- HR
heart rate
- OKA
okadaic acid
- ACSF
artificial cerebrospinal fluid
- MAPKs
mitogen-activated protein kinases
- ERK
extracellular signal-regulated kinase
- RVLM
rostral ventrolateral medulla
- SHRs
spontaneously hypertensive
- WKY
Wistar Kyoto rats
Footnotes
Dr. Mahmoud. M. El-Mas: Department of Pharmacology, Faculty of Pharmacy, Alexandria University, Egypt (mahelm@hotmail.com).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Rahman A-RA, Merrill RH, Wooles WR. Effect of acute ethanol administration on the baroreceptor reflex control of heart rate in normotensive human volunteers. Clin. Sci. 1987;72:113–122. doi: 10.1042/cs0720113. [DOI] [PubMed] [Google Scholar]
- Aroor AR, Shukla SD. MAP kinase signaling in diverse effects of ethanol. Life Sci. 2004;74:2339–2364. doi: 10.1016/j.lfs.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension. 2010;55:284–290. doi: 10.1161/HYPERTENSIONAHA.109.146605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Ma YL, Lee EH. Protein kinase CK2 impairs spatial memory formation through differential cross talk with PI-3 kinase signaling: activation of Akt and inactivation of SGK1. J. Neurosci. 2007;27:6243–6248. doi: 10.1523/JNEUROSCI.1531-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe ES, Parelkar NK, Kim JY, Cho HW, Kang HS, Mao L, Wang JQ. The protein phosphatase 1/2A inhibitor okadaic acid increases CREB and Elk-1 phosphorylation and c-fos expression in the rat striatum in vivo. J. Neurochem. 2004;89:383–390. doi: 10.1111/j.1471-4159.2003.02334.x. [DOI] [PubMed] [Google Scholar]
- Correa M, Arizzi MN, Betz A, Mingote S, Salamone JD. Open field locomotor effects in rats after intraventricular injections of ethanol and the ethanol metabolites acetaldehyde and acetate. Brain Res. Bull. 2003;62:197–202. doi: 10.1016/j.brainresbull.2003.09.013. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Direct evidence for selective involvement of aortic baroreceptors in ethanol-induced impairment of baroreflex control of heart rate. J. Pharmacol. Exp. Ther. 1993a;264:1198–1205. [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Upregulation of imidazoline receptors in the medulla oblongata accounts for the enhanced hypotensive effect of clonidine in aortic barodenervated rats. Brain Res. 1995;691:195–204. doi: 10.1016/0006-8993(95)00672-d. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Aortic barodenervation upregulates α2-adrenoceptors in the nucleus tractus solitarius and rostral ventrolateral medulla: an autoradiographic study. Neuroscience. 1997;79:581–590. doi: 10.1016/s0306-4522(96)00648-3. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Ovariectomy abolishes ethanol-induced impairment of baroreflex control of heart rate in conscious rats. Eur. J. Pharmacol. 1998;349:253–261. doi: 10.1016/s0014-2999(98)00202-7. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Role of the sympathetic control of vascular resistance in ethanol-clonidine hemodynamic interaction in SHRs. J. Cardiovasc. Pharmacol. 1999;34:589–596. doi: 10.1097/00005344-199910000-00017. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Enhanced catabolism to acetaldehyde in rostral ventrolateral medullary neurons accounts for the pressor effect of ethanol in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H837–844. doi: 10.1152/ajpheart.00958.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman A-RA. Role of NMDA and non-NMDA receptors in the nucleus tractus solitarius in the depressant effect of ethanol on baroreflexes. J. Pharmacol. Exp. Ther. 1993b;266:602–610. [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. Role of rostral ventrolateral medullary ERK/JNK/p38 MAPK signaling in the pressor effects of ethanol and its oxidative product acetaldehyde. Alcohol. Clin. Exp. Res. 2013;37:1827–1837. doi: 10.1111/acer.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English JM, Cobb MH. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol. Sci. 2002;23:40–45. doi: 10.1016/s0165-6147(00)01865-4. [DOI] [PubMed] [Google Scholar]
- Gao L, Li Y, Schultz HD, Wang WZ, Wang W, Finch M, Smith LM, Zucker IH. Downregulated Kv4.3 expression in the RVLM as a potential mechanism for sympathoexcitation in rats with chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H945–955. doi: 10.1152/ajpheart.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdjikov TV, Ross GM, Beninger RJ. Place preference induced by nucleus accumbens amphetamine is impaired by antagonists of ERK or p38 MAP kinases in rats. Behav. Neurosci. 2004;118:740–750. doi: 10.1037/0735-7044.118.4.740. [DOI] [PubMed] [Google Scholar]
- Herschman HR, Lim RW, Brankow DW, Fujiki H. The tumor promoters 12-O-tetradecanoylphorbol-13-acetate and okadaic acid differ in toxicity, mitogenic activity and induction of gene expression. Carcinogenesis. 1989;10:1495–1498. doi: 10.1093/carcin/10.8.1495. [DOI] [PubMed] [Google Scholar]
- Ireland MA, Vandongen R, Davidson L, Beilin LJ, Rouse IL. Acute effects of moderate alcohol consumption on blood pressure and plasma catecholamines. Clin. Sci. 1984;66:643–648. doi: 10.1042/cs0660643. [DOI] [PubMed] [Google Scholar]
- Kalluri HS, Ticku MK. Regulation of ERK phosphorylation by ethanol in fetal cortical neurons. Neurochem. Res. 2003;28:765–769. doi: 10.1023/a:1022822119560. [DOI] [PubMed] [Google Scholar]
- Karahanian E, Quintanilla ME, Tampier L, Rivera-Meza M, Bustamante D, Gonzalez-Lira V, Morales P, Herrera-Marschitz M, Israel Y. Ethanol as a prodrug: brain metabolism of ethanol mediates its reinforcing effects. Alcohol. Clin. Exp. Res. 2011;35:606–612. doi: 10.1111/j.1530-0277.2011.01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Hirooka Y, Konno S, Ogawa K, Sunagawa K. Angiotensin II type 1 receptor-activated caspase-3 through ras/mitogen-activated protein kinase/extracellular signal-regulated kinase in the rostral ventrolateral medulla is involved in sympathoexcitation in stroke-prone spontaneously hypertensive rats. Hypertension. 2010;55:291–297. doi: 10.1161/HYPERTENSIONAHA.109.138636. [DOI] [PubMed] [Google Scholar]
- Li G, Wang X, Abdel-Rahman AA. Brainstem norepinephrine neurons mediate ethanol-evoked pressor response but not baroreflex dysfunction. Alcohol. Clin. Exp. Res. 2005;29:639–647. doi: 10.1097/01.alc.0000160083.72579.ec. [DOI] [PubMed] [Google Scholar]
- Mao L, Li G, Abdel-Rahman AA. Effect of ethanol on reductions in norepinephrine electrochemical signal in the rostral ventrolateral medulla and hypotension elicited by I1-receptor activation in spontaneously hypertensive rats. Alcohol. Clin. Exp. Res. 2003;27:1471–1480. doi: 10.1097/01.ALC.0000086062.95225.0C. [DOI] [PubMed] [Google Scholar]
- Pastor R, Aragon CM. Ethanol injected into the hypothalamic arcuate nucleus induces behavioral stimulation in rats: an effect prevented by catalase inhibition and naltrexone. Behav. Pharmacol. 2008;19:698–705. doi: 10.1097/FBP.0b013e328315ecd7. [DOI] [PubMed] [Google Scholar]
- Quertemont E, Tambour S, Tirelli E. The role of acetaldehyde in the neurobehavioral effects of ethanol: a comprehensive review of animal studies. Prog. Neurobiol. 2005;75:247–274. doi: 10.1016/j.pneurobio.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Seyedabadi M, Goodchild AK, Pilowsky PM. Differential role of kinases in brain stem of hypertensive and normotensive rats. Hypertension. 2001;38:1087–1092. doi: 10.1161/hy1101.096054. [DOI] [PubMed] [Google Scholar]
- Swingle M, Ni L, Honkanen RE. Small-molecule inhibitors of ser/thr protein phosphatases: specificity, use and common forms of abuse. Methods Mol. Biol. 2007;365:23–38. doi: 10.1385/1-59745-267-X:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A, Kumar N, Farnham M, Lung M, Pilowsky P, McMullan S. Rostroventrolateral medulla neurons with commissural projections provide input to sympathetic premotor neurons: anatomical and functional evidence. Eur. J. Neurosci. 2013;38:2504–2515. doi: 10.1111/ejn.12232. [DOI] [PubMed] [Google Scholar]
- Varga K, Kunos G. Ethanol inhibition of baroreflex bradycardia: role of brainstem GABA receptors. Br. J. Pharmacol. 1990;101:773–775. doi: 10.1111/j.1476-5381.1990.tb14155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K, Kunos G. Inhibition of baroreflex bradycardia by ethanol involves both GABAA and GABAB receptors in the brainstem of the rats. Eur. J. Pharmacol. 1992;214:223–232. doi: 10.1016/0014-2999(92)90122-k. [DOI] [PubMed] [Google Scholar]
- Yang ZW, Wang J, Zheng T, Altura BT, Altura BM. Ethanol-induced contractions in cerebral arteries: role of tyrosine and mitogen-activated protein kinases. Stroke. 2001;32:249–257. doi: 10.1161/01.str.32.1.249. [DOI] [PubMed] [Google Scholar]
- Yi KD, Cai ZY, Covey DF, Simpkins JW. Estrogen receptor-independent neuroprotection via protein phosphatase preservation and attenuation of persistent extracellular signal-regulated kinase 1/2 activation. J. Pharmacol. Exp. Ther. 2008;324:1188–1195. doi: 10.1124/jpet.107.132308. [DOI] [PubMed] [Google Scholar]
- Yoshizawa S, Matsushima R, Watanabe MF, Harada K, Ichihara A, Carmichael WW, Fujiki H. Inhibition of protein phosphatases by microcystins and nodularin associated with hepatotoxicity. J. Cancer Res. Clin. Oncol. 1990;116:609–614. doi: 10.1007/BF01637082. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li SY, Brown RA, Ren J. Ethanol and acetaldehyde in alcoholic cardiomyopathy: from bad to ugly en route to oxidative stress. Alcohol. 2004;32:175–186. doi: 10.1016/j.alcohol.2004.01.005. [DOI] [PubMed] [Google Scholar]