Abstract
Background
The budding yeast S. cerevisiae possesses multiple glucose transporters with different affinities for glucose that enable it to respond to a wide range of glucose concentrations. The steady-state levels of glucose transporters are regulated in response to changes in the availability of glucose. This study investigates the glucose regulation of the low affinity, high capacity glucose transporter Hxt1.
Methods and results
Western blotting and confocal microscopy were performed to evaluate glucose regulation of the stability of Hxt1. Our results show that glucose starvation induces endocytosis and degradation of Hxt1 and that this event requires End3, a protein required for endocytosis, and the Doa4 deubiquitination enzyme. Mutational analysis of the lysine residues in the Hxt1 N-terminal domain demonstrates that the two lysine residues, K12 and K39, serve as the putative ubiquitin-acceptor sites by the Rsp5 ubiquitin ligase. We also demonstrate that inactivation of PKA (cAMP-dependent protein kinase A) is needed for Hxt1 turnover, implicating the role of the Ras/cAMP-PKA glucose signaling pathway in the stability of Hxt1.
Conclusion and general significance
Hxt1, most useful when glucose is abundant, is internalized and degraded when glucose becomes depleted. Of note, the stability of Hxt1 is regulated by PKA, known as a positive regulator for glucose induction of HXT1 gene expression, demonstrating a dual role of PKA in regulation of Hxt1.
1. Introduction
Glucose serves as the primary carbon and energy source of a multitude of cells, varying in complexity from unicellular microorganisms to higher eukaryotes [1, 2]. Glucose is by far the preferred energy source of the budding yeast S. cerevisiae, because glucose regulation of cellular function dictates the organism’s distinctive fermentative lifestyle [3, 4]. The yeast prefers to ferment rather than oxidize glucose even when oxygen is abundant [5, 6]. Because ATP production by fermentation is inefficient, the yeast cells metabolize the available glucose vigorously to meet cellular ATP demands [7]. They do so by increasing glucose uptake through glucose transporters (HXTs) [8–10].
S. cerevisiae copes with changes in glucose availability by expressing at least six members of the glucose transporter family with different affinities for glucose (Hxt1, 2, 3, 4, 6, and 7) [11–13]. The yeast cells detect extracellular glucose over a broad concentration range and express only those glucose transporters best suited for the amount of glucose available in the medium [14–17]: (1) Hxt1 is a low affinity glucose transporter with a Km value around 100 mM for glucose and expressed when glucose levels are high (> ~55 mM), (2) The HXT2 and HXT4 genes encode glucose transporters with moderate affinity for glucose (Km values around 10 mM) and their expression is induced in the presence of low levels of glucose (~11 mM) [12, 18], (3) Hxt3 has a low affinity for glucose (Km values around 30 – 60 mM) and induced by both low and high levels of glucose [9, 11, 12], and (4) The HXT6 and HXT7 genes encode high affinity glucose transporters with a Km value around 1 mM for glucose and their expression is induced by low concentrations of glucose or by non-fermentable carbon sources such as glycerol or ethanol [19–21].
Expression of several HXT genes (HXT1–4) is repressed by the Rgt1 repressor in the absence of glucose [22, 23]. Rgt1 does so by recruiting the general corepressor complex Ssn6-Tup1 to the HXT promoters in a manner that requires the HXT corepressor Mth1 [24–27]. High glucose induces expression of the HXT genes by inhibiting the function of Mth1 and Rgt1. Mth1 mRNA and protein levels are downregulated by high glucose via the Snf1 (AMPK)-Mig1 and Rgt2/Snf3 pathways, respectively [28–32]. Glucose-induced downregulation of Mth1 enables the phosphorylation of Rgt1 by PKA (cAMP-PKA pathway), leading to dissociation of Rgt1 from DNA and thereby expression of HXT genes [33–35]. Thus, three glucose signaling pathways converge at multiple points for fine-tuned regulation of HXT gene expression [36].
The steady-state levels of the yeast glucose transporters are also regulated posttranslationally. The high affinity glucose transporters such as Hxt2, Hxt6 and Hxt7 are internalized and targeted to the vacuole for degradation in cells grown in high glucose medium by a process, known as catabolite degradation [37–39]. Recently, it has been shown that the low affinity glucose transporter Hxt3 is endocytosed and degraded in the vacuole when glucose-fed cells are exposed to glucose-free medium [40]. These observations lead to the view that the stability of glucose transporters may be regulated by glucose concentration. In this study, we demonstrate that glucose starvation induces endocytosis and degradation of the low affinity glucose transporter Hxt1 and that ubiquitination is necessary for endocytosis of Hxt1. Furthermore, we show that the stability of Hxt1 is regulated by PKA, required for glucose induction of HXT gene expression, suggesting the role of the Ras/cAMP-PKA pathway in the transcriptional and posttranslational regulation of Hxt1.
2. Materials and methods
2.1. Yeast strains and growth conditions
The Saccharomyces cerevisiae strains used in this study are listed in Table 1. Cells were grown in YP (2% bacto-peptone, 1% yeast extract) and SC (synthetic yeast nitrogen base medium containing 0.17% yeast nitrogen base and 0.5% ammonium sulfate) media supplemented with the appropriate amino acids and carbon sources.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| BY4741 | Mata his3Δ1 leu2Δ0 ura3Δ0 met15Δ | [31] |
| KFY63 | Matα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 bcy1::KanMX | This study |
| KFY101 | Matα hxt1-17Δ gal2Δ agt1Δ stl1Δ his3Δ1 ura3Δ0 leu2Δ0 | [55] |
| KFY122 | Matα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 doa4::KanMX | This study |
| KFY123 | Mata his3-1 leu2-0 ura3-0 RSP5 | [50] |
| KFY124 | Mata his3-1 leu2-0 ura3-0 rsp5-1/smm1 | [50] |
| KFY127 | Matα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 end3::KanMX | This study |
2.2. Plasmid construction
The plasmids used in this study are listed in Table 2. JKP315 was constructed by gap repair, as described previously [25]. JKP323, JKP324 and JKP325 were constructed by fusing the designated HXT1 ORFs into pUG35 vector as XbaI-HindIII fragments. JKP315 was mutagenized by QuikChange Site-Directed Mutagenesis Kit (Stratagene) according to manufacturer’s protocol to produce JKP332, JKP333, JKP334 and JKP335.
Table 2.
Plasmids used in this study
| Name | Description | Source |
|---|---|---|
| JKP315 | Hxt1-GFP, Ura3, CEN | This study |
| JKP323 | Hxt1 (60–570)-GFP, Ura3, CEN | This study |
| JKP324 | Hxt1 (1–512)-GFP, Ura3, CEN | This study |
| JKP325 | Hxt1 (60–512)-GFP, Ura3, CEN | This study |
| JKP332 | Hxt1 (K12A)-GFP, Ura3, CEN | This study |
| JKP333 | Hxt1 (K27A)-GFP, Ura3, CEN | This study |
| JKP334 | Hxt1 (K35A)-GFP, Ura3, CEN | This study |
| JKP335 | Hxt1 (K39A)-GFP, Ura3, CEN | This study |
| KFP11 | Ras2Val19, Leu2, CEN | [53] |
2.3. Yeast membrane preparation, Western blotting and protein half-life measurement
Membrane enriched fractions were essentially prepared as described previously [41], with minor modifications. Briefly, after washing with 10 mM phosphate buffer at pH 7.4, the cell pellet was resuspended in membrane isolation buffer (100 mM Tris-Cl, pH 8, 150 mM NaCl and 5 mM EDTA) containing 10 mM sodium azide, protease and phosphatase inhibitors and vortexed with acid-washed glass beads. Membrane enriched fraction was collected by centrifugation at 12,000 rpm for 40 min at 4°C and suspended in the aforementioned buffer containing 5 M urea. The proteins were precipitated with 10% TCA, neutralized with 20 µl of 1 M Tris base and finally dissolved in 80 µl of SDS buffer (50 mM Tris-HCl, pH, 6.8, 10% glycerol, 2% SDS and 5% β-mercaptoethanol).
For Western blotting, proteins were resolved by SDS-PAGE (10%) and transferred to PVDF (Polyvinylidene fluoride) membrane (Millipore). The membranes were incubated with appropriate antibodies (anti-GFP or anti-Actin antibody, Santa Cruz) in TBST buffer (10 mM Tris-HCl, pH, 7.5, 150 mM NaCl and 0.1% Tween-20) and proteins were detected by the enhanced chemiluminescence (ECL) system (Pierce). The half-life of Hxt1-GFP was measured as described previously [58]. The band intensities were measured by densitometry using ImageJ v1.4r software (NIH) and normalized with the intensities of actin, and the values were plotted on a semi-logarithmic graph against time and further fitted to an exponential line.
2.4. Microscopy and image analysis
Yeast cells expressing Hxt1-GFP were stained with FM4-64 (lipophilic styryl dye to stain the vacuolar membrane, 1µg/ml) and analyzed with Olympus FluoView confocal microscope under 63× oil immersion objective lens using GFP or Texas Red filter. Images from confocal microscope were captured by FluoView software (Olympus). At least 200 cells showing the respective makers (e.g., FM6-64) were analyzed per each condition. Standard deviations were calculated from three or more independent experiments and are shown as error bars.
3. Results and Discussion
3.1. Hxt1 protein levels are posttranslationally downregulated in response to glucose starvation
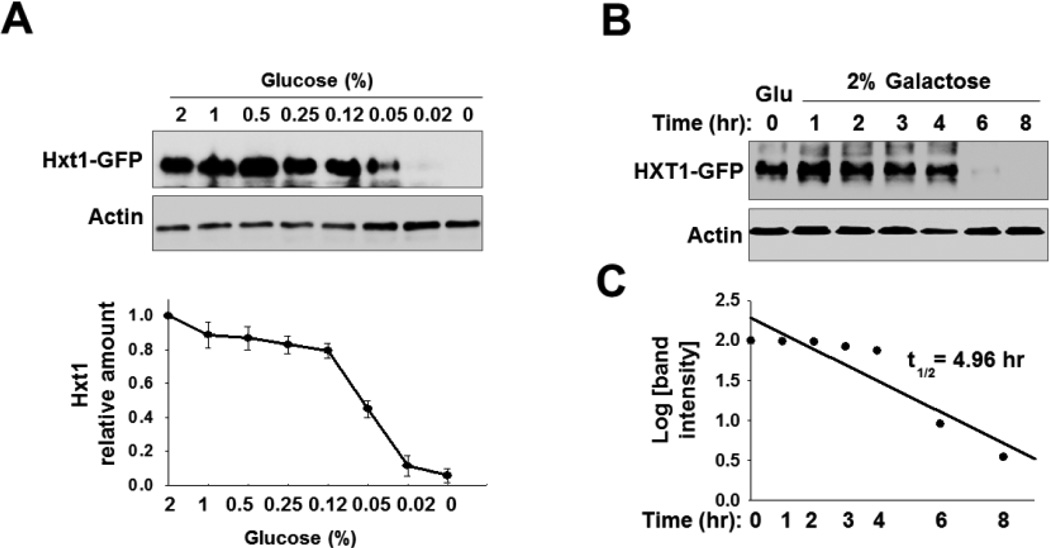
The expression levels of yeast nutrient transporters are regulated at both the transcriptional and posttranslational levels [42]. To understand glucose regulation of Hxt1 at a posttranslational level, we examined the abundance of Hxt1 at the plasma membrane by Western blotting. To this end, the HXT1 gene was fused to the GFP (green fluorescent protein) coding sequence on a centromeric plasmid (PMET25-HXT1-GFP). Because HXT1 gene expression is repressed by Rgt1 under glucose starvation conditions [22, 24], we interrupted glucose regulation of HXT1 expression by replacing its promoter with the promoter of MET25, which is not regulated by glucose [28, 43]. We found that the cell surface levels of Hxt1-GFP are greater in cells grown on high glucose (2%) than in cells grown in the low glucose (~0.05%) or glucose-free medium (Gal, as 0% glucose) (Fig. 1A). We also found that the amounts of immunodetected Hxt1-GFP in glucose-grown cells were reduced by ~50% within 5 h after the cells were shifted to galactose medium (Figs. 1B and 1C).
Fig. 1.
Hxt1 protein levels are posttranslationally downregulated in response to glucose starvation. (A) Western blotting analysis of the expression levels of Hxt1-GFP in the plasma membrane-enriched fraction. Yeast cells (WT) expressing Hxt1-GFP were grown in SC-2% glucose medium to mid log phase (O.D600nm = 1.2–1.5) and equal amounts of cells were shifted to SC medium containing different glucose concentrations. Membrane fractions were immunoblotted with anti-GFP antibody (top panel), and the intensity of each band on the blot was quantified by densitometric scanning (bottom panel). (B) Yeast cells (WT) expressing Hxt1-GFP were grown in SC-2% glucose (Glu) medium to mid log phase and equal amounts of cells were shifted to SC-2% galactose medium and incubated for indicated times. Membrane-enriched fractions were immunoblotted with anti-GFP antibody. (C) Determination of the half-life of Hxt1-GFP protein. The Western blot images in (B) were scanned and the half-life was determined, as described in the materials and methods.
3.2. Glucose starvation induces endocytosis and degradation of Hxt1
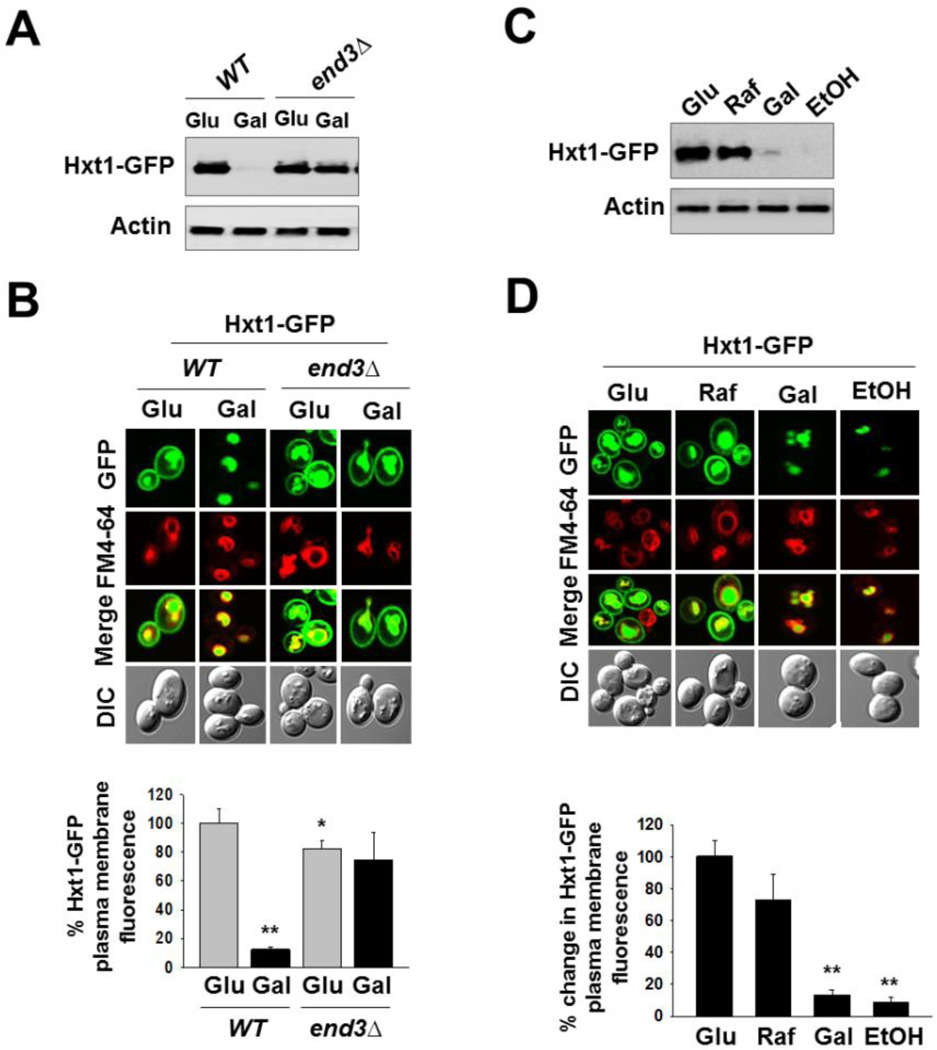
Given that Hxt1-GFP protein levels are markedly diminished in glucose-starved cells, we examined whether this downregulation occurs via endocytosis by examining the stability of Hxt1-GFP in the mutant lacking End3 involved in the internalization step of endocytosis. Western blotting analysis showed that glucose starvation-induced downregulation of Hxt1-GFP is observed in wild type cells but not in the end3Δ mutant, suggesting Hxt1 turnover by endocytosis (Fig. 2A). This was confirmed by confocal microscopy showing that ~90% of plasma membrane-localized Hxt1-GFP disappears when cells are exposed to glucose-free medium (Gal) (Fig. 2B) and that, however, Hxt1-GFP is localized at the plasma membrane constitutively in the end3Δ mutant (Fig. 2B). Of note, Hxt1-GFP seems to be constitutively localized to the vacuole, and this occurs even in the end3Δ mutant (Fig. 2B), suggesting that Hxt1 may be directly localized to the vacuole from the Golgi. These results are consistent with the vacuolar targeting of the uracil permease Fur4 and the maltose permease Mal61 in a constitutive manner [44, 45].
Fig. 2.
Glucose starvation induces endocytosis and subsequent degradation of Hxt1. (A) Yeast cells (WT and end3Δ) expressing Hxt1-GFP were grown in SC-2% glucose (Glu) medium to mid-log phase and shifted to SC medium containing 2% galactose (Gal) for 6 h. Membrane-enriched fractions were immunoblotted with anti-GFP antibody. (B) Yeast cells (WT and end3Δ) expressing Hxt1-GFP were grown as described in (A). Confocal microscope image (top panel) and quantification of relative GFP fluorescence in the plasma membrane (bottom panel, *P < 0.05, **P < 0.001) were shown. (C) Yeast cells (WT) expressing Hxt1-GFP were grown in SC-2% glucose (Glu) medium to mid-log phase and shifted to SC medium containing either 2% raffinose (Raf), 2% galactose (Gal) or 2% ethanol (EtOH) for 6 h. Membrane fractions were immunoblotted with anti-GFP antibody. (D) Yeast cells (WT) expressing Hxt1-GFP were grown as described in (C). Confocal microscope image (top panel) and quantification of relative GFP fluorescence in the plasma membrane (bottom panel, **P < 0.001) were shown. The FM6-64 dye was used to stain the vacuolar membrane (red), and actin was served as loading control in (A) and (C). Pma1 is frequently used as a loading control for membrane fractions; it, however, is not appropriate for this study because its expression is critically regulated by glucose [52].
We next determined whether Hxt1-GFP degradation is stimulated by glucose starvation or by specific carbon sources. Raffinose is a trisaccharide, consisting of fructose-glucose-galactose that is equivalent to low glucose, because yeast cells cleave the fructose-glucose bond by invertase inefficiently and thus eventually obtain low levels of glucose from it [9]. Glucose and galactose only differ with respect to C-4, yet galactose does not enter through glucose transporters, suggesting that the glucose transporters display remarkable substrate specificity. Both Western blotting and confocal microscopy analyses revealed that Hxt1-GFP levels are high in cells grown on glucose or raffinose but are very low in cells grown on galactose or ethanol (Fig. 2C and 2D). These results suggest that Hxt1 is subjected to endocytosis and degradation in the vacuole under glucose starvation conditions.
3.3. The amino-terminal cytoplasmic domain of Hxt1 regulates its glucose starvation-induced turnover
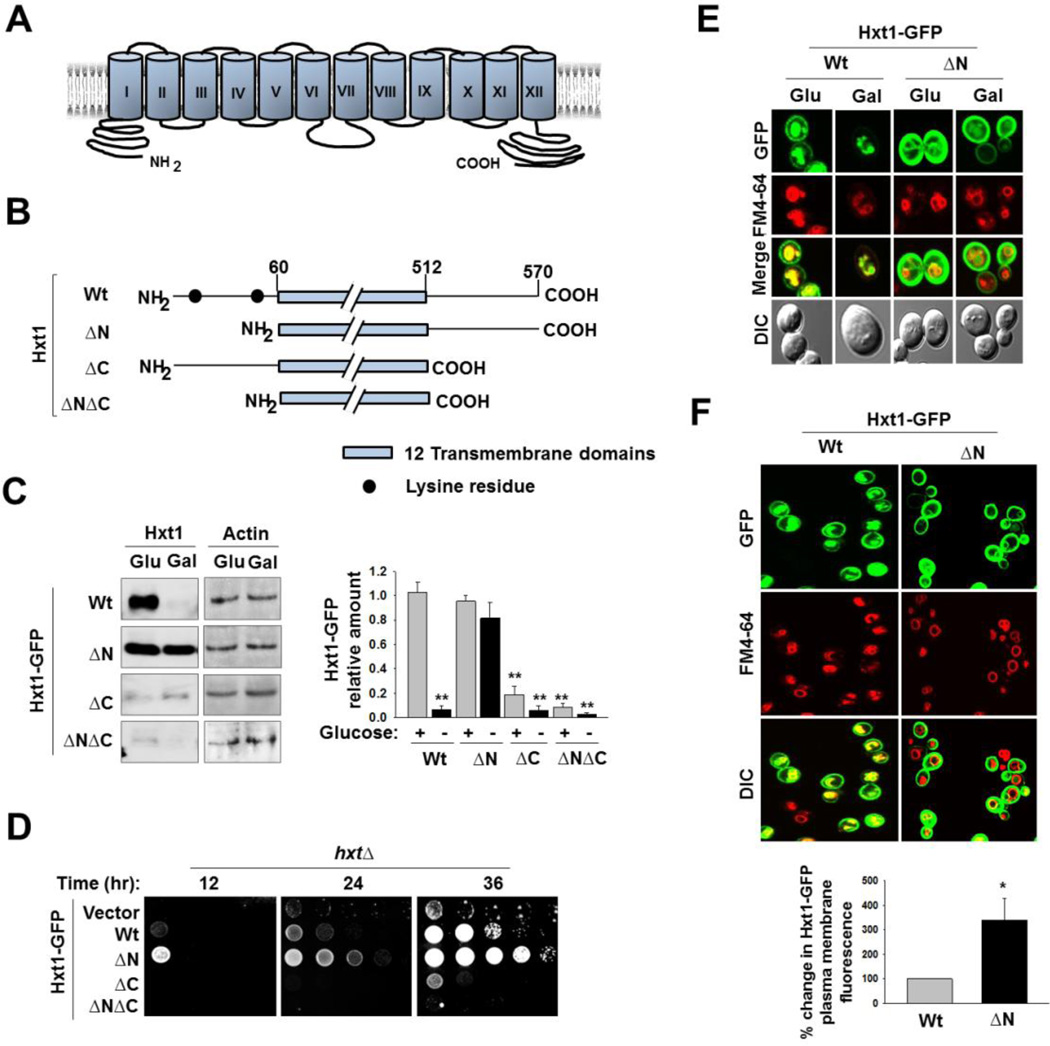
To identify the regions of Hxt1 that regulate its turnover, we constructed deletion mutants of Hxt1 that lack its amino (N)- or carboxy (C)-terminal cytoplasmic domain and examined their stability in glucose-free medium (Fig. 3A and 3B). We found that Hxt1 degradation in galactose-grown cells is abolished by the deletion of its entire N-terminal cytoplasmic domain (residues 1–59) (Fig. 3C, ΔN) and that, by contrast, the deletion of the C-terminal domain of Hxt1 (residues 513–570, Hxt1-GFP-ΔC) renders it unstable in both glucose and galactose-grown cells. Therefore, glucose starvation-induced Hxt1 turnover may be regulated by its N-terminal domain, raising the hypothesis that the C-terminal domain might play a role in the turnover of Hxt1 by regulating the N-terminal domain (Fig. 3C, ΔC). We tested this hypothesis by examining the stability of Hxt1-GFP-ΔNΔC that lacks both the N- and C-terminal domains and found that this protein, like Hxt1-GFP-ΔC, is inherently unstable (Fig. 3C, ΔNΔC). These results suggest that the C-terminal domain of Hxt1 may not be directly involved in its glucose starvation-induced degradation but may contribute to its structural stability.
Fig. 3.
The N-terminal cytoplasmic domain of Hxt1 is required for its turnover. (A) Schematic diagram of predicted secondary structure of Hxt1. Twelve transmembrane domains and cytosolic N- and C-terminal tails are shown. (B) Schematic maps of Hxt1 constructs (Wt, 60–570 aa (ΔN); 1–512 aa (ΔC); 60–512 aa (ΔNΔC)) showing lysine residues at its N-terminal domain. (C) Yeast cells (WT) expressing indicated Hxt1-GFP proteins were grown as described in Fig. 2A, and plasma membrane fractions were immunoblotted with anti-GFP antibody (left panel), and the intensity of each band on the blot was quantified by densitometric scanning (right panel, **P < 0.001). Actin was served as loading control. (D) Yeast cells (hxtΔ) expressing indicated Hxt1-GFP proteins were spotted on 2% glucose plate supplemented with Antimycin A (1µg/ml). The first spot of each row represents a count of 5 × 107 cell/ml, which is diluted 1:10 for each spot thereafter. The plate was incubated for indicated times and photographed. (E) Yeast cells (WT) expressing indicated Hxt1-GFP proteins were grown as described in Fig. 2A and analyzed by confocal microscopy. (F) Yeast cells (WT) expressing indicated Hxt1-GFP proteins were grown in SC-2% glucose medium to mid log phase and stained with FM6-64 (red). Confocal microscope images (top panel) and quantification of relative fluorescent intensity of Hxt1-GFP at the plasma membrane (bottom panel, * P <0.05) were shown.
To understand the effects of the N- or C-terminal deletion of Hxt1 on its function, we analyzed glucose transport activity of the Hxt1 deletion constructs. A yeast mutant lacking all 17 glucose transporters are unable to grow on glucose, and this growth defect was complemented by expression of any one of HXT genes [9]. The hxt null strain was transformed with plasmids encoding wild type or truncated Hxt-GFP proteins and scored for growth in glucose medium containing the respiratory inhibitor Antimycin A (Fig. 3D). The growth defect of the hxt null strain was restored by the expression of wild type Hxt1-GFP or Hxt1-GFP-ΔN, but not of GFP-Hxt1ΔC or GFP-Hxt1ΔNΔC (Fig. 3D). Of note, the growth rate of cells expressing Hxt1-GFP-ΔN was faster than that of cells expressing wild type Hxt1-GFP. Confocal microscopy showed that Hxt1-GFP-ΔN accumulates constitutively at the plasma membrane and that its localization ti the vacuole is markedly reduced, compared with that of wild type Hxt1-GFP1 (Fig. 3E). As a result, the plasma membrane levels of Hxt1-GFP-ΔN were 2–3 folds higher than those of wild type Hxt1-GFP, suggesting that Hxt1 localization to the vacuole is regulated by its N-terminal domain (Fig. 3F). Consequently, deletion of the N-terminal domain of Hxt1-GFP likely leads to its accumulation at the plasma membrane, enabling cells expressing Hxt1-GFP-ΔN to grow faster than cells expressing wild type Hxt1-GFP (Fig. 3D).
3.4. Ubiquitination is necessary for endocytosis of Hxt1
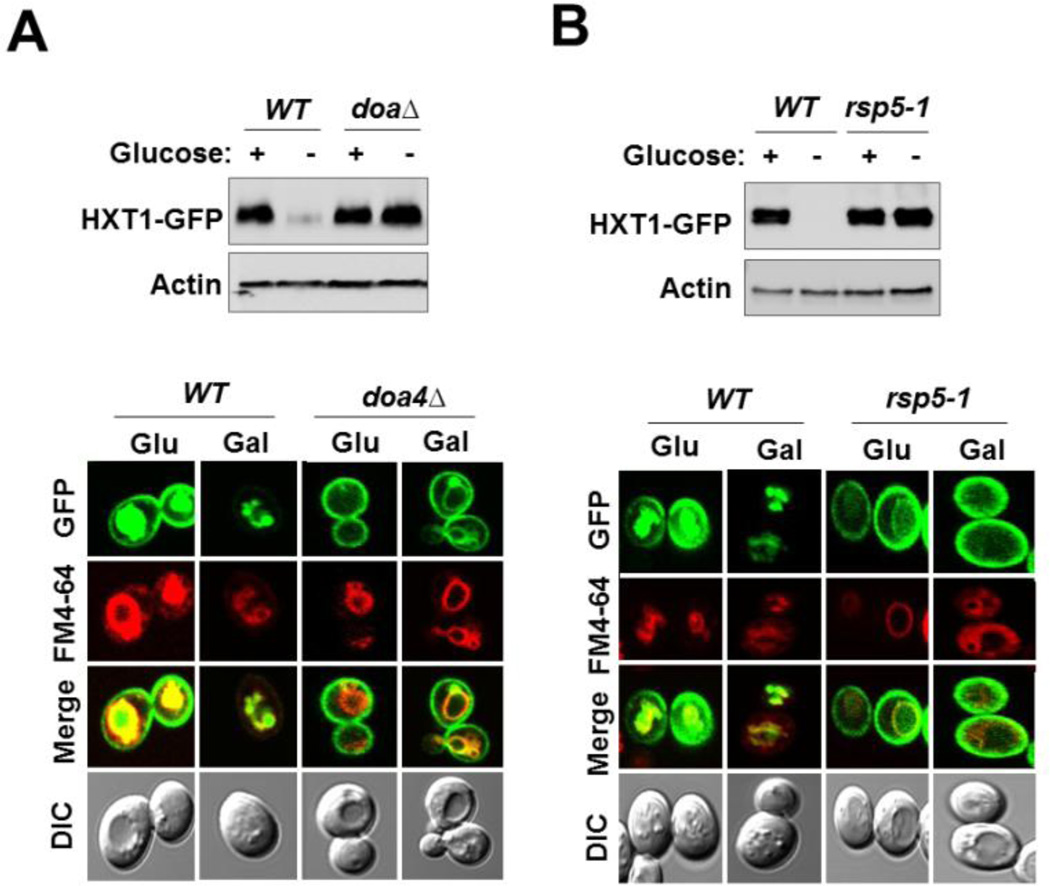
Ubiquitination is a common signal for endocytosis and subsequent degradation [46]. Because a number of yeast nutrient transporters are ubiquitinated by the ubiquitin ligase Rsp5 and this process is affected by the ubiquitin isopeptidase Doa4 which is required for recycling ubiquitin from ubiquitinated substrates [45, 47–49], we examined the stability of the Hxt1 transporter in the strain carrying the doa4Δ or rsp5-1ts mutation [50]. Western blotting analysis indicated that Hxt1-GFP levels are constitutively high in doa4Δ (Fig. 4A, top) and rsp5-1ts mutants (Fig. 4B, top). Consistently, Hxt1-GFP accumulates constitutively at the plasma membrane in those mutant strains (the bottom panels of Fig. 4A and 4B). We also observed the absence of intracellular GFP signal in the doa4Δ and rsp5-1 mutants, suggesting that ubiquitination plays a key role in the movement of Hxt1 transporter into intracellular compartments. Similar observations were made in cells expressing Hxt1-GFP-ΔN, in which the truncated Hxt1 transporter accumulates at the plasma membrane but its vacuolar localization is abolished, suggesting that vacuole trafficking of Hxt1-GFP from the Golgi may be a regulatory process (Fig. 3E and 3F). These results are consistent with previous findings that ubiquitin is involved in intracellular trafficking of plasma membrane proteins [51] and mediates vacuolar targeting of the maltose permease Mal61 [45].
Fig. 4.
Hxt1 is ubiquitinated by the ubiquitin ligase Rsp5. (A) and (B) Yeast cells of indicated genotypes expressing Hxt1-GFP were grown as described in Fig. 2A. Plasma membrane fractions were immunoblotted with anti-GFP antibody (A), and subcellular localization of Hxt1-GFP was analyzed by confocal microscopy (B). The FM6-64 dye was used to stain the vacuolar membrane (red), and actin was served as loading control.
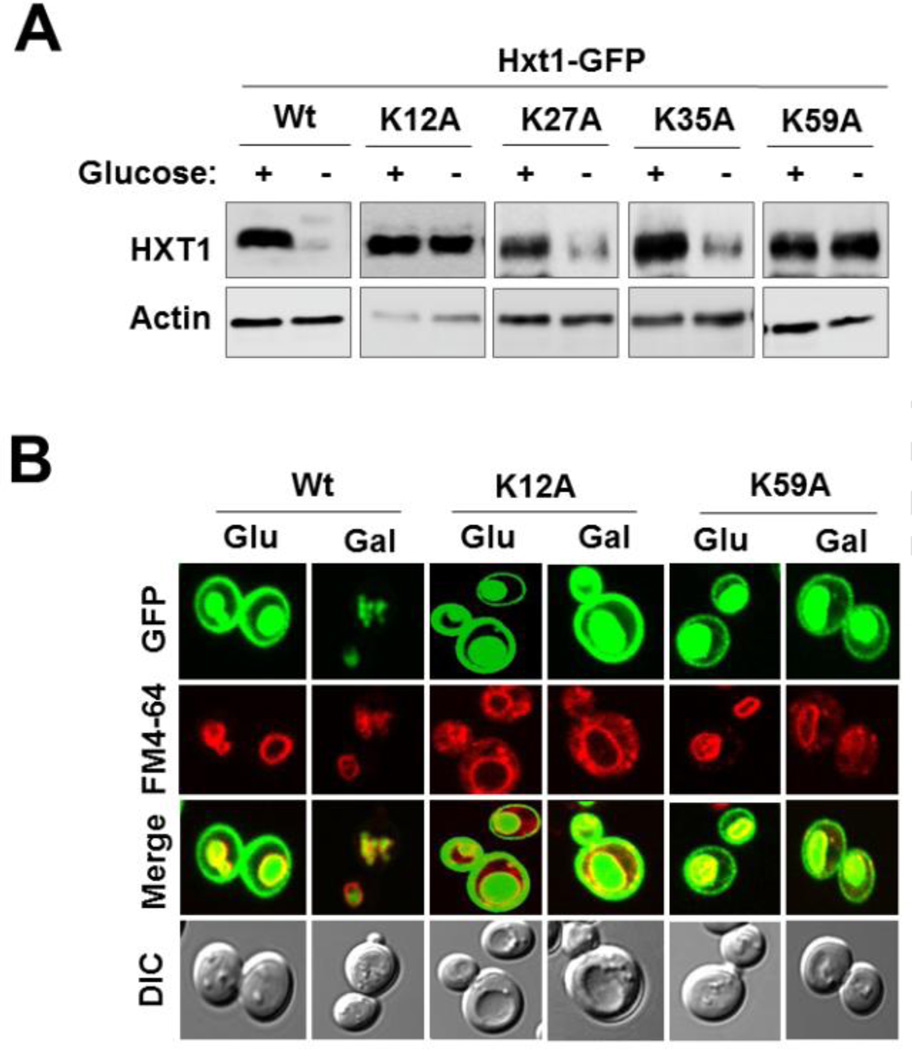
3.5. K12 and K39 at the N-terminal domain of Hxt1 serve as putative ubiquitination sites
Given that the N-terminal cytoplasmic domain of Hxt1 regulates its turnover, we examined whether the domain is responsible for its ubiquitination. We changed the four lysine residues present in the N-terminal domain of Hxt1—K12, K27, K35 and K39—to alanines and examined the stability of the resulting Hxt1 mutants. Substitutions of two of these residues (K12A and K39A) resulted in a marked increase of Hxt1 levels in galactose-grown cells, suggesting that the two lysine residues may serve as putative ubiquitination sites (Fig. 5A). Consistently, Hxt1K12A-GFP and Hxt1K39A-GFP were accumulated at the plasma membrane in both glucose and galactose-grown cells (Fig. 5B). Notably, these two Hxt1 mutants were resistant to degradation but were able to localize to the vacuole. Therefore, it is likely that ubiquitination of Hxt1 at K12 and/or K39 may be required for its glucose starvation-induced endocytosis but may not be involved in its trafficking from the Golgi to the vacuole. While the N-terminal domain of Hxt1 is important for its vacuolar accumulation (Fig. 3E and 3F), any of the four lysine residues in the domain are not involved in its localization to the vacuole (Hxt1K27A-GFP and Hxt1K35A-GFP are also localized to the vacuole, data not shown). These observations suggest that glucose starvation–induced downregulation of Hxt1 and its accumulation in the vacuole may occur by separate mechanisms.
Fig. 5.
K12 and K39 serve as putative ubiquitin-acceptor lysine residues. Yeast cells (WT) expressing indicated Hxt1-GFP proteins were grown as described in Fig. 2A. Plasma membrane fractions were immunoblotted with anti-GFP antibody (A), and subcellular localization of Hxt1-GFP (Wt, K12A and K39A) was analyzed by confocal microscopy (B).
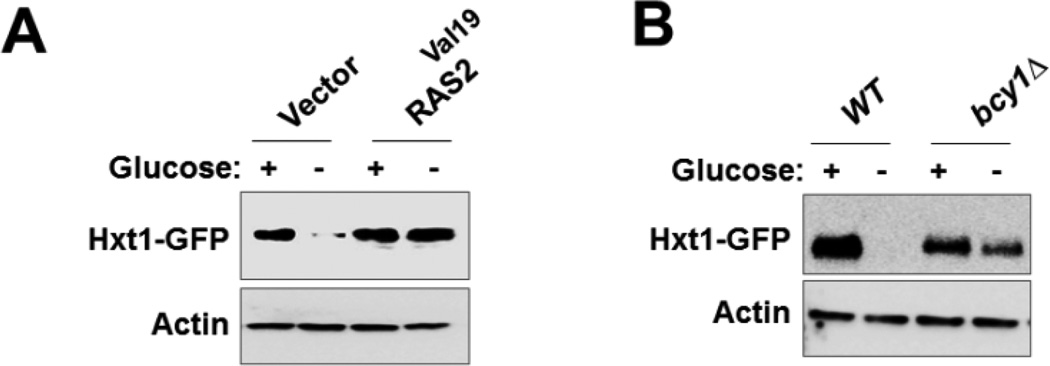
3.6. Activation of the RAS/cAMP-PKA pathway prevents glucose starvation-induced Hxt1 turnover
The RAS/cAMP-PKA glucose signaling pathway is involved in many different cellular processes including cell growth, stress resistance, and metabolism [15]. A previous work showed that rapamycin induces Hxt1 degradation and that this is prevented by expression of a constitutively active RAS2 (RAS2Val19) or deletion of BCY1, encoding the PKA regulatory subunit, which binds and inactivates PKA in the absence of glucose [53]. To understand whether glucose starvation-induced Hxt1 turnover is regulated by the RAS/cAMP-PKA pathway, we examined the expression of Hxt1-GFP in cells in which PKA is constitutively active. Western blotting analysis showed that Hxt1-GFP degradation under glucose starvation conditions is significantly inhibited in cells either expressing Ras2Val19 (Fig. 6A) or lacking Bcy1 (Fig. 6B). This finding is consistent with the previous report that inactivation of PKA is needed for Hxt3 turnover [54]. Therefore, these findings support the view that PKA acts to prevent turnover of Hxt1 and Hxt3.
Fig. 6.
Inactivation of RAS/cAMP-PKA pathway is required for glucose starvation-induced Hxt1 turnover. (A) Yeast cells (WT) coexpressing Hxt1-GFP in combination with either empty vector or Ras2Val19 were grown as described in Fig. 2A. Membrane fractions were immunoblotted with anti-GFP antibody. (B) Yeast cells (WT and bcy1Δ) expressing Hxt1-GFP were grown as described in Fig. 2A Membrane fractions were immunoblotted with anti-GFP antibody. Actin was served as loading control.
4. Conclusion
Yeast glucose transporters, like other cell surface nutrient transporters, are internalized and targeted for degradation in the vacuole when they are not needed. Recent studies have shown that the stability of glucose transporter isoforms is regulated by glucose concentration. High affinity glucose transporters such as Hxt2 [37] and Hxt6/Hxt7 [38] are turned over in high glucose conditions; low affinity glucose transporters Hxt3 [54], in glucose starvation conditions. Our results in this study show that the low affinity glucose transporter Hxt1, highly expressed when glucose is abundant, undergoes endocytosis and subsequent degradation when glucose becomes depleted. The molecular mechanism underlying this phenomenon is unclear but is proposed to be a signal-induced process in which glucose triggers the ubiquitination of glucose transporters that signals their endocytosis and subsequent degradation in the vacuole [17]. In this regard, previous studies have shown that the Ras/cAMP-PKA glucose signaling pathway may be involved in turnover of Hxt1, Hxt3 and Hxt7 [53, 54]. In agreement, active PKA appears to prevent Hxt1 turnover (Fig. 6), suggesting the role of PKA in the regulation of the stability of Hxt proteins and providing insights into the regulation of glucose transporters.
Highlights.
Glucose starvation induces endocytosis and degradation of Hxt1
Ubiquitination is necessary for endocytosis of Hxt1
K12 and K59 at the N-terminal domain of Hxt1 serve as putative ubiquitination sites
Inactivation of PKA is needed for Hxt1 turnover
Acknowledgements
We thank Mark Johnston for yeast strains and plasmids and Christopher Burd for providing the rsp5-1 mutant strain. This work was supported by NIH grant GM087470 to JHK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rolland F, Wanke V, Cauwenberg L, Ma P, Boles E, Vanoni M, de Winde JH, Thevelein JM, Winderickx J. The role of hexose transport and phosphorylation in cAMP signalling in the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 2001;1:33–45. doi: 10.1111/j.1567-1364.2001.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 2.Towle HC. Glucose as a regulator of eukaryotic gene transcription. Trends Endocrinol Metab. 2005;16:489–494. doi: 10.1016/j.tem.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Gancedo JM. Yeast carbon catabolite repression. Microbiol Mol Biol Rev. 1998;62:334–361. doi: 10.1128/mmbr.62.2.334-361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson M. Glucose repression in yeast. Curr Opin Microbiol. 1999;2:202–207. doi: 10.1016/S1369-5274(99)80035-6. [DOI] [PubMed] [Google Scholar]
- 5.Crabtree HG. Observations on the carbohydrate metabolism of tumours. Biochem J. 1929;23:536–545. doi: 10.1042/bj0230536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagunas R. Energetic irrelevance of aerobiosis for S. cerevisiae growing on sugars. Mol Cell Biochem. 1979;27:139–146. doi: 10.1007/BF00215362. [DOI] [PubMed] [Google Scholar]
- 7.Lagunas R. Misconceptions about the energy metabolism of Saccharomyces cerevisiae. Yeast. 1986;2:221–228. doi: 10.1002/yea.320020403. [DOI] [PubMed] [Google Scholar]
- 8.Johnston M, Kim JH. Glucose as a hormone: receptor-mediated glucose sensing in the yeast Saccharomyces cerevisiae. Biochem Soc Trans. 2005;33:247–252. doi: 10.1042/BST0330247. [DOI] [PubMed] [Google Scholar]
- 9.Ozcan S, Johnston M. Function and regulation of yeast hexose transporters. Microbiol Mol Biol Rev. 1999;63:554–569. doi: 10.1128/mmbr.63.3.554-569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rolland F, Winderickx J, Thevelein JM. Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem Sci. 2001;26:310–317. doi: 10.1016/s0968-0004(01)01805-9. [DOI] [PubMed] [Google Scholar]
- 11.Boles E, Hollenberg CP. The molecular genetics of hexose transport in yeasts. FEMS Microbiol Rev. 1997;21:85–111. doi: 10.1111/j.1574-6976.1997.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 12.Reifenberger E, Boles E, Ciriacy M. Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur J Biochem. 1997;245:324–333. doi: 10.1111/j.1432-1033.1997.00324.x. [DOI] [PubMed] [Google Scholar]
- 13.Reifenberger E, Freidel K, Ciriacy M. Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol Microbiol. 1995;16:157–167. doi: 10.1111/j.1365-2958.1995.tb02400.x. [DOI] [PubMed] [Google Scholar]
- 14.Gancedo JM. The early steps of glucose signalling in yeast. FEMS Microbiol Rev. 2008;32:673–704. doi: 10.1111/j.1574-6976.2008.00117.x. [DOI] [PubMed] [Google Scholar]
- 15.Rolland F, Winderickx J, Thevelein JM. Glucose-sensing and -signalling mechanisms in yeast. FEMS Yeast Res. 2002;2:183–201. doi: 10.1111/j.1567-1364.2002.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 16.Ozcan S, Johnston M. Three different regulatory mechanisms enable yeast hexose transporter (HXT) genes to be induced by different levels of glucose. Mol Cell Biol. 1995;15:1564–1572. doi: 10.1128/mcb.15.3.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horak J. Regulations of sugar transporters: insights from yeast. Curr Genet. 2013;59:1–31. doi: 10.1007/s00294-013-0388-8. [DOI] [PubMed] [Google Scholar]
- 18.Maier A, Volker B, Boles E, Fuhrmann GF. Characterisation of glucose transport in Saccharomyces cerevisiae with plasma membrane vesicles (countertransport) and intact cells (initial uptake) with single Hxt1, Hxt2, Hxt3, Hxt4, Hxt6, Hxt7 or Gal2 transporters. FEMS Yeast Res. 2002;2:539–550. doi: 10.1111/j.1567-1364.2002.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 19.Diderich JA, Schepper M, van Hoek P, Luttik MA, van Dijken JP, Pronk JT, Klaassen P, Boelens HF, de Mattos MJ, van Dam K, Kruckeberg AL. Glucose uptake kinetics and transcription of HXT genes in chemostat cultures of Saccharomyces cerevisiae. J Biol Chem. 1999;274:15350–15359. doi: 10.1074/jbc.274.22.15350. [DOI] [PubMed] [Google Scholar]
- 20.Ye L, Berden JA, van Dam K, Kruckeberg AL. Expression and activity of the Hxt7 high-affinity hexose transporter of Saccharomyces cerevisiae. Yeast. 2001;18:1257–1267. doi: 10.1002/yea.771. [DOI] [PubMed] [Google Scholar]
- 21.Liang H, Gaber RF. A novel signal transduction pathway in Saccharomyces cerevisiae defined by Snf3-regulated expression of HXT6. Mol Biol Cell. 1996;7:1953–1966. doi: 10.1091/mbc.7.12.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozcan S, Leong T, Johnston M. Rgt1p of Saccharomyces cerevisiae, a key regulator of glucose-induced genes, is both an activator and a repressor of transcription. Mol Cell Biol. 1996;16:6419–6426. doi: 10.1128/mcb.16.11.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JH. DNA-binding properties of the yeast Rgt1 repressor. Biochimie. 2009;91:300–303. doi: 10.1016/j.biochi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JH, Polish J, Johnston M. Specificity and regulation of DNA binding by the yeast glucose transporter gene repressor Rgt1. Mol Cell Biol. 2003;23:5208–5216. doi: 10.1128/MCB.23.15.5208-5216.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy A, Shin YJ, Cho KH, Kim JH. Mth1 regulates the interaction between the Rgt1 repressor and the Ssn6-Tup1 corepressor complex by modulating PKA-dependent phosphorylation of Rgt1. Mol Biol Cell. 2013 doi: 10.1091/mbc.E13-01-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakshmanan J, Mosley AL, Ozcan S. Repression of transcription by Rgt1 in the absence of glucose requires Std1 and Mth1. Curr Genet. 2003;44:19–25. doi: 10.1007/s00294-003-0423-2. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt MC, McCartney RR, Zhang X, Tillman TS, Solimeo H, Wolfl S, Almonte C, Watkins SC. Std1 and Mth1 proteins interact with the glucose sensors to control glucose-regulated gene expression in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:4561–4571. doi: 10.1128/mcb.19.7.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JH, Brachet V, Moriya H, Johnston M. Integration of transcriptional and posttranslational regulation in a glucose signal transduction pathway in Saccharomyces cerevisiae. Eukaryot Cell. 2006;5:167–173. doi: 10.1128/EC.5.1.167-173.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaniak A, Xue Z, Macool D, Kim JH, Johnston M. Regulatory network connecting two glucose signal transduction pathways in Saccharomyces cerevisiae. Eukaryot Cell. 2004;3:221–231. doi: 10.1128/EC.3.1.221-231.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moriya H, Johnston M. Glucose sensing and signaling in Saccharomyces cerevisiae through the Rgt2 glucose sensor and casein kinase I. Proc Natl Acad Sci U S A. 2004;101:1572–1577. doi: 10.1073/pnas.0305901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spielewoy N, Flick K, Kalashnikova TI, Walker JR, Wittenberg C. Regulation and recognition of SCFGrr1 targets in the glucose and amino acid signaling pathways. Mol Cell Biol. 2004;24:8994–9005. doi: 10.1128/MCB.24.20.8994-9005.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flick KM, Spielewoy N, Kalashnikova TI, Guaderrama M, Zhu Q, Chang HC, Wittenberg C. Grr1-dependent inactivation of Mth1 mediates glucose-induced dissociation of Rgt1 from HXT gene promoters. Mol Biol Cell. 2003;14:3230–3241. doi: 10.1091/mbc.E03-03-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JH, Johnston M. Two glucose-sensing pathways converge on Rgt1 to regulate expression of glucose transporter genes in Saccharomyces cerevisiae. J Biol Chem. 2006;281:26144–26149. doi: 10.1074/jbc.M603636200. [DOI] [PubMed] [Google Scholar]
- 34.Jouandot D, 2nd, Roy A, Kim JH. Functional dissection of the glucose signaling pathways that regulate the yeast glucose transporter gene (HXT) repressor Rgt1. J Cell Biochem. 2011;112:3268–3275. doi: 10.1002/jcb.23253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palomino A, Herrero P, Moreno F. Tpk3 and Snf1 protein kinases regulate Rgt1 association with Saccharomyces cerevisiae HXK2 promoter. Nucleic Acids Res. 2006;34:1427–1438. doi: 10.1093/nar/gkl028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JH, Roy A, Jouandot D, 2nd, Cho KH. The glucose signaling network in yeast. Biochim Biophys Acta. 2013;1830:5204–5210. doi: 10.1016/j.bbagen.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kruckeberg AL, Ye L, Berden JA, van Dam K. Functional expression, quantification and cellular localization of the Hxt2 hexose transporter of Saccharomyces cerevisiae tagged with the green fluorescent protein. Biochem J. 1999;339(Pt 2):299–307. [PMC free article] [PubMed] [Google Scholar]
- 38.Krampe S, Stamm O, Hollenberg CP, Boles E. Catabolite inactivation of the high-affinity hexose transporters Hxt6 and Hxt7 of Saccharomyces cerevisiae occurs in the vacuole after internalization by endocytosis. FEBS Lett. 1998;441:343–347. doi: 10.1016/s0014-5793(98)01583-x. [DOI] [PubMed] [Google Scholar]
- 39.Horak J. The role of ubiquitin in down-regulation and intracellular sorting of membrane proteins: insights from yeast. Biochim Biophys Acta. 2003;1614:139–155. doi: 10.1016/s0005-2736(03)00195-0. [DOI] [PubMed] [Google Scholar]
- 40.Snowdon C, Schierholtz R, Poliszczuk P, Hughes S, van der Merwe G. ETP1/YHL010c is a novel gene needed for the adaptation of Saccharomyces cerevisiae to ethanol. FEMS Yeast Res. 2009;9:372–380. doi: 10.1111/j.1567-1364.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- 41.Galan JM, Moreau V, Andre B, Volland C, Haguenauer-Tsapis R. Ubiquitination mediated by the Npi1p/Rsp5p ubiquitin-protein ligase is required for endocytosis of the yeast uracil permease. J Biol Chem. 1996;271:10946–10952. doi: 10.1074/jbc.271.18.10946. [DOI] [PubMed] [Google Scholar]
- 42.Kriel J, Haesendonckx S, Rubio-Texeira M, Van Zeebroeck G, Thevelein JM. From transporter to transceptor: signaling from transporters provokes re-evaluation of complex trafficking and regulatory controls: endocytic internalization and intracellular trafficking of nutrient transceptors may, at least in part, be governed by their signaling function. Bioessays. 2011;33:870–879. doi: 10.1002/bies.201100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasula S, Jouandot D, 2nd, Kim JH. Biochemical evidence for glucose-independent induction of HXT expression in Saccharomyces cerevisiae. FEBS Lett. 2007;581:3230–3234. doi: 10.1016/j.febslet.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volland C, Urban-Grimal D, Geraud G, Haguenauer-Tsapis R. Endocytosis and degradation of the yeast uracil permease under adverse conditions. J Biol Chem. 1994;269:9833–9841. [PubMed] [Google Scholar]
- 45.Gadura N, Michels CA. Sequences in the N-terminal cytoplasmic domain of Saccharomyces cerevisiae maltose permease are required for vacuolar degradation but not glucose-induced internalization. Curr Genet. 2006;50:101–114. doi: 10.1007/s00294-006-0080-3. [DOI] [PubMed] [Google Scholar]
- 46.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 47.Springael JY, Andre B. Nitrogen-regulated ubiquitination of the Gap1 permease of Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:1253–1263. doi: 10.1091/mbc.9.6.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amerik AY, Nowak J, Swaminathan S, Hochstrasser M. The Doa4 deubiquitinating enzyme is functionally linked to the vacuolar protein-sorting and endocytic pathways. Mol Biol Cell. 2000;11:3365–3380. doi: 10.1091/mbc.11.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rotin D, Staub O, Haguenauer-Tsapis R. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J Membr Biol. 2000;176:1–17. doi: 10.1007/s00232001079. [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Sitaram A, Burd CG. Regulation of copper-dependent endocytosis and vacuolar degradation of the yeast copper transporter, Ctr1p, by the Rsp5 ubiquitin ligase. Traffic. 2007;8:1375–1384. doi: 10.1111/j.1600-0854.2007.00616.x. [DOI] [PubMed] [Google Scholar]
- 51.Helliwell SB, Losko S, Kaiser CA. Components of a ubiquitin ligase complex specify polyubiquitination and intracellular trafficking of the general amino acid permease. J cell Biol. 2001;153:649–662. doi: 10.1083/jcb.153.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serrano R. In vivo glucose activation of the yeast plasma membrane ATPase. FEBS Lett. 1983;156:11–14. doi: 10.1016/0014-5793(83)80237-3. [DOI] [PubMed] [Google Scholar]
- 53.Schmelzle T, Beck T, Martin DE, Hall MN. Activation of the RAS/cyclic AMP pathway suppresses a TOR deficiency in yeast. Mol Cell Biol. 2004;24:338–351. doi: 10.1128/MCB.24.1.338-351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snowdon C, van der Merwe G. Regulation of Hxt3 and Hxt7 turnover converges on the Vid30 complex and requires inactivation of the Ras/cAMP/PKA pathway in Saccharomyces cerevisiae. PLoS One. 2012;7:e50458. doi: 10.1371/journal.pone.0050458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wieczorke R, Krampe S, Weierstall T, Freidel K, Hollenberg CP, Boles E. Concurrent knockout of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 1999;464:123–128. doi: 10.1016/s0014-5793(99)01698-1. [DOI] [PubMed] [Google Scholar]