Abstract
Introduction
Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults; it carries a dismal prognosis. Adenoviral vector (Ad)-mediated gene transfer is being developed as a promising therapeutic strategy for GBM. Preclinical studies have demonstrated safety and efficacy of adenovirus administration into the brain and tumor mass in rodents and into the non-human primates’ brain. Importantly Ads have been safely administered within the tumor resection cavity in humans.
Areas Covered
Background on GBM and Ad vectors; we describe gene therapy strategies for GBM and discuss the value of combination approaches. Finally we discuss the results of the human clinical trials for GBM that have used adenoviral vectors.
Expert Opinion
The transduction characteristics of Ad vectors, and their safety profile, added to their capacity to achieve high levels of transgene expression have made them powerful vectors for the treatment of GBM. Recent gene therapy successes in the treatment of retinal diseases and systemic brain metabolic diseases, encourages the development of gene therapy for malignant glioma. Exciting clinical trials are currently recruiting patients; although it is large randomized phase III controlled clinical trials that will provide the final decision on the success of gene therapy for the treatment of GBM.
Keywords: High-capacity adenovirus, dendritic cells, immunotherapy, glioblastoma multiforme, Flt3L, HSV1-TK
1 Introduction - Overview of Gliomas
The term “malignant glioma” encompasses a heterogeneous group of glial based, high-grade tumors including anaplastic astrocytoma, GBM, mixed oligoastrocytoma, and anaplastic oligodendroglioma. The World Health Organization (WHO) classifies all of these tumors as either grade III (anaplastic astrocytoma, oligoastrocytoma, and anaplastic oligodendroglioma) or grade IV (GBM). The WHO has adopted the St. Anne-Mayo system for histologic diagnosis. According to this system, two of the following four features must be present for the diagnosis of anaplastic astrocytoma: 1) nuclear atypia, 2) mitoses, 3) endothelial proliferation, and 4) necrosis. When at least three of these features are present, the diagnosis is glioblastoma multiforme. Of note, the grading system for these glial malignancies is purely histologic due to the high concordance of the prognosis with these histologic features. A systemic survey is not performed due to the extremely low risk of dissemination of this tumor outside the central nervous system.
Glioblastoma multiforme is the most common primary malignant tumor of the central nervous system. Between 2004 and 2008, the Central Brain Tumor Registry of the United States (CBTRUS) reported that glioblastoma multiforme accounted for 16.3 % of the primary central nervous system tumors. The only well-established risk factor for developing a glioblastoma multiforme is exposure to ionizing radiation, though GBMs occur more frequently in males, whites, and with increasing age (1). There is no pathognomonic presentation of the disease and most clinical presentations are due to irritation or destruction of local central nervous system structures or due to increased intracranial pressure. Thus, common clinical presentation includes focal neurologic deficits, altered mental status, seizures, personality changes, and headaches.
There has been extensive preclinical and clinical research into the pathophysiology of malignant gliomas, but at this point the prognosis remains extremely poor. Based on work published by Stupp and colleagues, the current standard of care includes maximal surgical resection, radiation therapy, and temozolomide chemotherapy (2). The median survival remains between 12 and 15 months (1). A wide variety of factors have been postulated to account for continued therapeutic failure, including neurotoxicity of therapeutic agents that is dose-limiting, difficulty accessing the central nervous system with therapeutic agents secondary to the blood-brain barrier, resistance of glioblastoma to radiotherapy, and the inability to achieve complete surgical resection due to the invasive nature of the disease.
Despite extensive research, little headway has been made in combating this devastating disease. Thus, novel therapeutic approaches are necessary to escape the current limitations to therapy. These novel therapeutic approaches include a wide variety of adenoviral-based therapies, including both the use of adenoviral-vectors for gene delivery and the use of modified oncolytic adenoviruses.
2. Adenoviral Vectors for Gene Therapy Applications
Adenoviruses are non-enveloped, icosahedral viruses approximately 90 nm in diameter. Complexes composed of the hexon protein, along with a number of hexon-associated proteins, comprise the 20 sides of the icosahedron (3). A complex called the penton base is located on the surface of the capsid at each of the 12 vertices, and another protein complex, the fiber, extends outward from the surface of the capsid (3). The end of the fiber complex, known as the knob domain, binds to the coxsackie and adenovirus receptor (CAR), thus mediating cell tropism (4,5). Also, the interaction of the adenoviral penton protein with cell surface integrins (INT), such as INT αvβ3 and αvβ5, leads to the internalization of the virus by endocytosis (6). Alternatively, the Major Histocompatibility Complex Class I (MHCI) α2 domain has been proposed to be involved in the initial binding of adenoviruses to cell membranes (7). The genome is a linear double stranded DNA, covalently bound on either end to a protein called the terminal protein; there are other numerous associated DNA-binding proteins (4). The total length of the genome is approximately 35 thousand bases. Upon infection, the genome does not integrate into the host genome, but remains episomal while expressing viral genes.
Gene therapy applications using adenoviruses have typically used serotypes 2 and 5, classified under adenovirus species type C (4). Progress in this area has proceeded in two stages. Initially, first-generation adenoviral vectors (Ads) were produced by deletion of the E1, and sometimes E3, region of the virus genome, thereby largely eliminating the ability of the virus to express viral genes within infected cells. This was a necessary step to reduce the toxicity associated with adenovirus infection. The therapeutic transgene was inserted into the E1 region. Drawbacks of first generation Ads included residual expression of viral proteins, which was associated with a significant immune response and loss of therapeutic transgene expression (4,8-15). Additionally, their insertion capacity for therapeutic transgenes is limited to approximately 8 kbp. The latest generation Ads, i.e. high-capacity helper dependent adenoviral vectors (HCAds), have been engineered to delete all endogenous viral coding regions from the vector's genome (16). The immune response generated by HC-Ads is not as significant as the first generation vectors, and they also allow for much larger inserts (~35 kbp maximum cloning capacity) (8,17). Importantly, HC-Ad are capable of eliciting long-term transgene expression, even in the presence of an anti-Ad systemic immune response, which has been shown to curtail transgene expression from first generation Ads (13,14,18). With established methods for modifying viral genomes along with the technology for cell culture-based production and amplification of the vectors, it is possible to remove nearly all native viral DNA and insert novel DNA sequences for therapeutic purposes. This flexibility allows for the production of non-replicating Ad viruses with completely engineered genomes, suitable for gene therapy applications for GBM in human patients (19-21).
Conditionally replicative adenoviruses (CRAds) were developed as an alternative therapeutic strategy. CRAds are recombinant adenoviruses that can selectively replicate within and kill tumor cells. CRAds have several advantages, such as: i) replication of the Ad itself allows amplification of the input dose of the virus, ii) high levels of expression of therapeutic transgenes, as a result of the replication of the virus DNA, iii) spreading of the therapeutic effect to other adjacent tumor cells. Oncolytic Ads can kill the cells by direct lysis as the result of the replicative cycle, but they can also express cytotoxic proteins, stimulate the production of inflammatory cytokines, activate T-cell-mediated immunity and sensitize tumor cells to chemotherapy. To specifically replicate in tumor cells, the E1A gene has been encoded under the control of tissue/tumor-specific promoters or enhancers (22). Transcriptional specificity has been achieved using C-X-C chemokine receptor 4 (CXCR4), which is active in human mesenchymal stem cells (hMSC) and human glioma cells. Thus, hMSC could provide replicative-oncolytic Ad to distant glioma cells (23). The oncolytic Ad delta-24 the E1A gene has a small deletion that restricts the interaction of E1A with the retinoblastoma protein (Rb). Thus, Delta-24 can only replicate in cancer cells that have disrupted Rb function, without affecting normal cells (24). Using a similar approach, ONYX-015 Ad has a deletion in the E1B gene, thus when the virus infects normal cells with active p53, it cannot replicate. However, when this virus infects tumor cells with an aberrant p53 function, it replicates and cause cellular lysis (25). The role of p53 in the ONYX-015 selectivity is not clearly understood as it has been shown that this vector could still replicate in tumor cells with wild type p53 (26). O'Shea et al demonstrated that the loss of E1B induces the expression of p53, but not its activation (27). Instead, the selective replication in tumor cells seems to be determined by the capacity of these cells to export late viral RNA in the absence of E1B (27). Another strategy to improve clinical efficacy of these vectors is to augment their infectivity. The oncolytic potency of the Ad5-delta24 adenovirus was enhanced by the incorporation into the HI loop of the fiber knob domain of a sequence encoding for Arg- Gly-Asp peptide, that its known to interact with αv integrins (28). This modification allows the virus to utilize αv integrins as an alternative receptor during the cell entry process (29) and has enhanced infectivity in in vitro and in vivo GBM models (30).
An important problem that remains to be solved for the use of these vectors in the clinical setting is the development of anti-Ad neutralizing antibodies that hampers the infectivity of replication competent vectors upon their repeated administration (31). Nevertheless, the replication competent adenoviruses have proven to be safe for the treatment of patients (32). The possibility of genetic manipulations of these vectors, joined with the accelerated increase in the knowledge of the mechanisms and the proteins involved in tumor progression will enable researchers to achieve more effective and specific cancer therapies.
3. Gene Therapeutic Targets for Glioblastoma
3.1 Conditional Cytotoxic Therapy
Suicide genes encode for enzymes that activate non-toxic compounds into cytotoxic molecules. When prodrugs are incorporated by transduced cells, they are converted into cytotoxic compounds by the conditionally cytotoxic enzymes encoded within Ads. These cytotoxic compounds can freely diffuse into neighboring cells or migrate through cell-to-cell contacts, amplifying the cytotoxic effect. Herpes Simplex Virus Type 1-Thymidine Kinase (TK) phosphorylates the prodrug ganciclovir (GCV) to GCV-monophosphate, which after conversion into a tri-phosphorylated GCV by cellular kinases becomes 2-deoxyguanosine triphosphate (33). When GCV-triphosphate is incorporated into duplicating DNA, it inhibits DNA polymerase and leads to DNA chain termination (Figure 1) (33). Other suicide gene/nucleoside analog systems include E. coli cytosine deaminase, which catalyzes the conversion of the prodrug 5-fluorocytosine to 5-fluorouracil, resulting in DNA breaks (34). Cytochrome P450 converts cyclophosphamide into a toxin that leads to DNA crosslinking and protein alkylation (35). E. coli purine nucleoside phosphorylase converts nontoxic purine nucleoside analogs, such as 6-methylpurine and F-araAMP into toxic adenine analogs that block mRNA and protein synthesis (36). Carboxypeptidase G2 is combined with the prodrug 4-benzoyl-L-glutamic acid to produce a mustard alkylating agent that does not require further enzymatic processing to promote cell-cycle independent DNA-crosslinking (37).
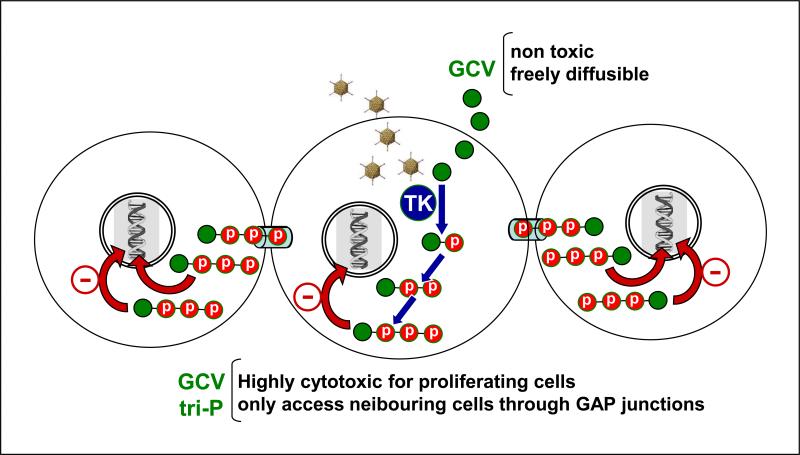
Figure 1. Mechanism of action and bystander effect of adenovirus vectors encoding HSV1-thymidine kinase.
Herpes Simplex Virus Type 1-thymidine kinase (TK) phosphorylates the non-toxic prodrug ganciclovir (GCV) to GCV-monophosphate, which after conversion into a triphosphorylated GCV by cellular kinases is incorporated into the duplicating DNA chain during the S phase of the cell cycle, leading to apoptotic cell death of proliferating cells. While GCV can freely diffuse through cells, triphosphate-GCV is a highly charged molecule and accesses neighboring cells through gap junctions, inducing apoptotic cell death in non-transduced proliferating cells surrounding the infected cell.
The suicide gene that has been exploited the most for the treatment of GBM is TK (38). TK mutants have also been developed that exhibit high affinity for the prodrugs GCV and acyclovir, SR39 and SR26, respectively (39,40). These transgenes allow for the use of suicide gene therapy with lower systemic concentrations of the prodrugs, reducing toxicity (41). A novel tomato plant-derived TK (toTK), which exhibits high affinity and specificity for the nucleoside analogue azidothymidine (AZT), shows a robust cytotoxic effect in human GBM cells in vitro (42). The advantages of this system are that AZT easily penetrates the blood-brain barrier and phosphorylates AZT to AZT-monophosphate (42).
TK+GCV sensitizes GBM cells to the cytotoxic effect of radiotherapy and chemotherapeutic agents (43). Treatment of intracranial human GBM xenografts with Ad-TK increased the efficacy of radiotherapy and reduced the occurrence of neurological side effects in irradiated mice (44). It has been suggested that synergistic effects of TK and radiation may also involve a mechanism of TK-mediated immune-stimulation (45). Considering the many preclinical and clinical studies that show TK synergism with cytotoxic agents as well as with immune-stimulants, it appears worth further developing this strategy.
3.2 Toxins
Genes encoding proteins that inhibit protein synthesis, such as Pseudomonas exotoxin A (PE), Diphtheria toxin (DT), and saporin have been encoded in gene therapy vectors to kill tumor cells. However, gene therapy-mediated delivery of these types of highly cytotoxic proteins in the brain may induce neurotoxic side effects, by inhibition of protein synthesis in non-neoplastic cells that can become infected in the tissue surrounding the brain tumor. Gene therapy vectors encoding these highly cytotoxic proteins can be more specific and less potentially neurotoxic if transgene expression is driven by inducible or tumor cell-specific promoters.
In order to control the expression of ribosome-inactivating toxins, inducible plasmids encoding native or attenuated DT have been constructed and tested for efficacy and expression in vitro (46,47). Native DT was examined using either the tetracycline-dependent Tet-Off promoter or a lactose-dependent promoter, and DT expression was found to be more tightly regulated by the Tet-Off system (47). Attenuated DT was tested using the Tet-Off system; however, toxicity was not totally abolished (46), perhaps due to the leakiness of the Tet-Off system (48).
Another strategy used to reduce the non-specific toxic effects of cytotoxins consists of fusing them with ligands that bind to receptors overexpressed specifically on the surface of GBM cells, such as receptors for cytokines, growth factors, ephrins, urokinase-type plasminogen activator, or transferrin. For example, IL13Rα2, a mutated receptor for IL-13 that is expressed in GBM specimens but is not present in normal brain tissue, has been targeted by using IL-13 fused to PE toxin (49,50). Unfortunately, a Phase III trial for GBM using intracranial delivery of hIL-13-PE showed dose-related neurotoxicity in several of the patients, possibly related to the expression in normal brain tissue of IL4αR, which binds native hIL-13 (51,52). In fact, findings from our lab indicate that a single injection of hIL-13-PE into the mouse brain leads to severe neurotoxicity (53). Additionally, intra-tumoral hIL-13-PE requires multiple injections or continuous infusion to be effective (54,55).
To overcome these limitations, we constructed an adenoviral vector (Ad.mIL-4.TRE.mIL-13-PE, Figure 2) encoding a mutated form of hIL13 that does not bind to the physiological IL-13/IL-4R fused to PE toxin (53,56). This vector also encoded for a mutated form of IL-4 (mIL4, IL-4.Y124D) that blocks the physiological IL13R/IL4R without binding to IL-13α2R (56,57). Intra-tumoral administration of Ad.mIL-4.TRE.mIL-13-PE provided long-term expression of mIL-13-PE and a robust cytotoxic response in IL-13Rα2 expressing-GBM cells, leading to tumor regression and long-term survival without neurotoxicity (53). Our findings indicate that Ads can be excellent tools to deliver cytotoxic genes to the brain.
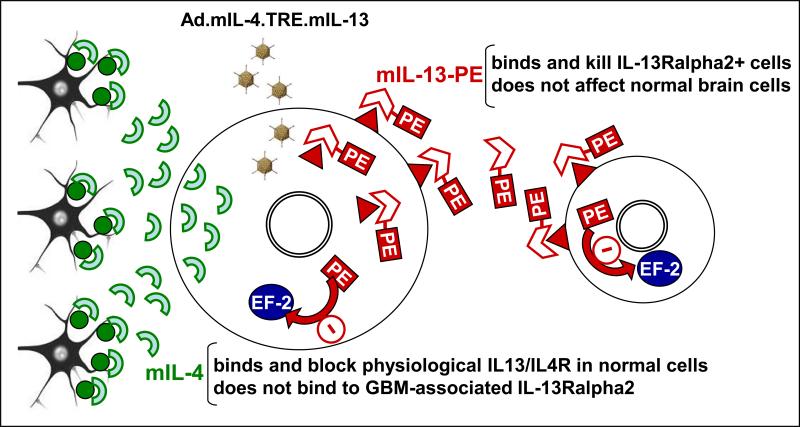
Figure 2. Mechanism of action and bystander effect of vectors encoding mIL-13-PE.
Ad.mIL-4.TRE.mIL13 vector encodes for chimeric toxin mIL-13-PE (mutated human interleukin-13), which targets and kills glioma cells expressing IL-13Rα2, absent in non-tumor cells. Upon binding to IL-13Rα2, PE toxin (Pseudomonas endotoxin) is internalized and inhibits elongating factor 2 (EF2), blocking protein synthesis and leading to tumor cell death. The release of mIL-13-PE from infected cells provides a strong bystander effect. As an extra safety feature, the adenovirus vector encodes for mIL-4 that binds and blocks physiological IL-13/IL-4R, present in normal brain cells.
3.3 Gene Therapy-Mediated Immune Stimulatory Strategies
Although the central nervous system (CNS) is an immune-privileged organ, immune responses against glioma cells can be effectively mounted as activated immune cells can traffic in and out of the central nervous system (58). A strategy to mediate dendritic cell (45) activation in the CNS is by intra-tumoral administration of cytokines to influence either (1) DC differentiation and recruitment to the tumor site or (59) increase DC activation and antigen presenting capacity. Key cytokines that act in these pathways are summarized below (Figure 3).
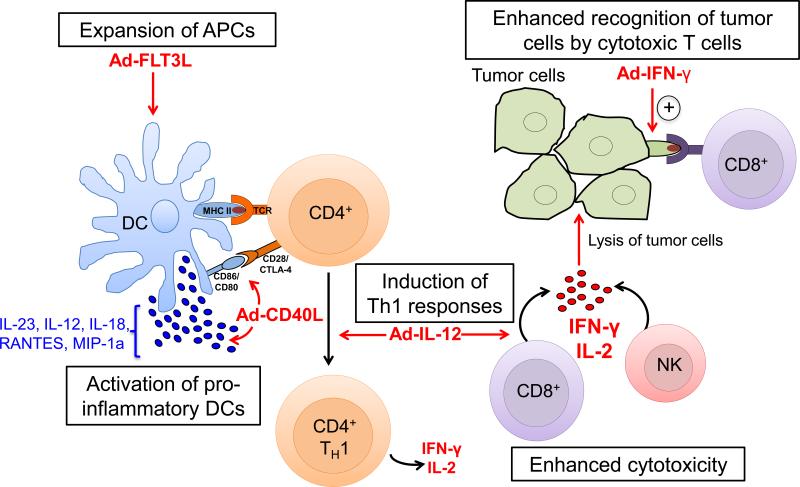
Figure 3. Therapeutic targets to enhance antitumor immunity using Ad vectors.
FLT3L drives the generation of myeloid and lymphoid derived DC populations. CD40L enhances the expression of co-stimulatory ligands such as CD86/CD80 on DCs and also stimulates the release of cytokines including IL-12, IL-18 and RANTES/CCL5. IL-12 promotes the development of CD4+ TH1 cells and the release of IFN-γ and IL-2 by CD8+ T cells and NK cells, thus increasing cytotoxicity against tumor cells. IFN-γ and IL-2 also play a role in the generation of memory T cells. Additionally, IFN-γ enhances MHC I expression on tumor cells and facilitates their recognition by CD8+ T cells. APC: antigen presenting cell, Ad: adenovirus, DC: dendritic cell, TCR: T cell receptor; Antigen.
3.3.1 DC differentiation and recruitment
3.3.1.1 Flt3L
Fms-like tyrosine kinase 3 ligand (Flt3L) was initially characterized as a cytokine that promoted myelopoiesis and B lymphopoiesis. Subsequently, it was shown that Flt3L promoted the proliferation of both myeloid- and lymphoid-derived DC populations in mice (60). The anti-tumor potential of Flt3L has been tested in murine models of breast cancer, prostate cancer, and metastatic lung carcinoma (61-63). In these studies, recombinant Flt3L was administered systemically and enhanced tumor regression with increased survival. Our laboratory has tested adenoviral delivery of Flt3L in rodent models of intracranial glioma (64-67). In these experiments, Flt3L administration inhibited tumor growth, increased survival, and increased the expression of the DC markers OX62 and MHC II in rats and CD11c, 33D1, MHC II and F4/80 in mice (68).
3.3.2 DC activation
3.3.2.1 CD40L
The CD40/CD40 ligand (CD40L) interaction plays an essential role in cell-mediated anti-tumor immune responses. Tada et al. showed that T-cell-dependent antitumor effects in a model of lung carcinoma were mediated through CD40L-induced maturation of DCs that upregulated CD86 expression and produced IL-23, IL-12p35 and IL-18, among others (69). In a model of CT-26 colon cancer, intra-tumoral administration of adenovirus expressing CD40L resulted in the production of IL-12 and IFN-γ and with an increased expression of chemokines such as MIP-1α, MIP-1β, MIP-2, RANTES and eotaxin, thus inducing tumor regression and protection against re-challenge. Therapeutic efficacy of CD40L administration was also observed in a murine melanoma model (70). In glioma however, the effect of CD40L cytokine therapy has not been extensively tested. Our data have shown that adenovirus expressing CD40L fails to show therapeutic efficacy in the RG2 glioma model, which is also refractory to several other therapies (40) .
3.3.2.2 IFNs
The contribution of IFN-γ was confirmed in primary tumorigenesis models, where mice that were either insensitive to or deficient in IFN-γ developed tumors more rapidly than wild type controls (71,72). Since then, other tumor models have confirmed the importance of IFN-γ in controlling tumor growth (73). Mice with syngeneic GL26 gliomas were treated with intratumoral administration of IFN-γ-expressing adenovirus (Ad-IFN-γ), which resulted in increased MHC I expression on tumor cells, enhanced infiltration of CD4+ and CD8+ T cells within the tumor, and increased survival (74). The role of Type I IFNs in tumor immunosurveillance was confirmed in IFNAR1-/- and IFNAR2-/- mice. In both cases, lack of Type I IFNs made the mice more susceptible to developing tumors, and the tumors also progressed at a faster rate (73). In studies using glioma cell lines and human glioma xenografts in mice, INF-β treatment induced tumor cell apoptosis and regression (75). To avoid the toxicity associated with the systemic administration of IFN-β, adenoviral vectors expressing IFN-β (Ad-IFN-β) were used to deliver IFN-β directly to the tumor site. Data from our lab has shown that rats treated with Flt3L/TK that have overexpression of IFN-γ within the tumor microenvironment show an enhanced anti-tumor immune response that is predominantly T cell mediated. Ultimately, these rats showed increased survival . Administration of an Ad vector encoding IFN-α in combination with Ad.TK led to tumor regression and long-term survival in rats bearing intracranial GBM (76). However, the neurotoxicity induced by Ad-IFN-α when injected in the normal rat brain makes this vector unsuitable for the treatment of GBM (76).
3.4 Stimulating T-cell Function
Glioma cells, like many tumor cells, have mechanisms to evade the immune system that affect several cell types, including CD4+ helper T cells and CD8+ T cells. Studies have shown that patients with malignant gliomas exhibit severe impairment of T cell-mediated immunity (77). T cells purified from glioma patients often have impaired mitogenic responses, including decreased IL-2 production and signaling and decreased IFN-γ production (78). Immune-stimulatory cytokines have thus been considered as adjuvant therapy for glioma in an effort to counter tumor-induced immunosuppression (Figure 4).
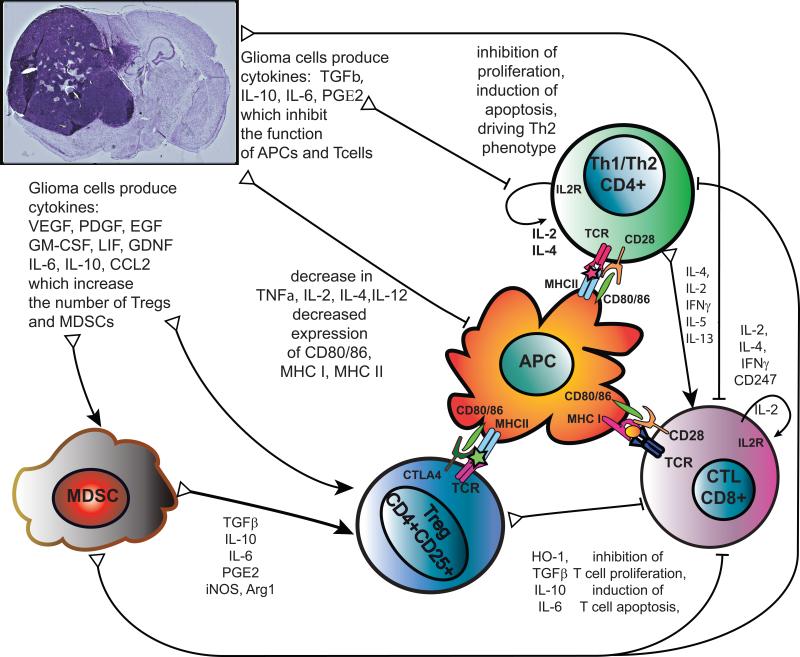
Figure 4. Mechanisms of tumor-induced immune suppression.
Glioma cells inhibit T cell function through cytokines that act directly on T cells, like: TGFβ, IL-10, IL-6, which inhibit T cell production of IL-2, IL-4, IFN-γ, expression of IL2R and CD247, signaling downstream of the T cell receptor and activation of CTLs by T helper cells. This results in inhibition of T cell activation and proliferation. Through enhanced production of reactive oxygen species by PGE2, glioblastomas also directly induce T cell apoptosis. IL-2, IL-4, IFN-γ can be used to stimulate T cell function and aid anti-glioma therapies. Glioma cells can also inhibit T cell function indirectly by impairing differentiation, maturation, activation and function of antigen presenting cells (APC), reducing the expression of co-stimulatory molecules (CD80/CD86), MHC-I and MHC-II, decreasing the production of immunostimulatory cytokines TNFα, IL-2, IL-4, IL-12. Therapies to increase production of CD80/86 and immunostimulatory molecules show promise as adjuvant therapies for glioma. In addition, glioma cells produce cytokines (VEGF, PDGF, LIF, GDNF, IL-6, IL-10, CCL2) which alter hematopoietic lineages to promote differentiation towards immunosuppressive phenotypes and increase the number of Myeloid Derived Suppressor Cells (MDSCs) and regulatory T cells (Tregs). These are recruited to the tumor microenvironment and circulate back to lymphoid organs to suppress anti-tumor immune responses further. Abbreviations used: Arg1, arginase 1; CCL2, Chemokine ligand 2 (also known as monocyte chemotactic protein -1 MCP-1); EGF, epidermal growth factor; GDNF, glial derived neurotrophic factor; GM-CSF, granulocyte macrophage colony stimulating factor; HO-1 heme oxygenase 1; iNOS, inducible nitric oxide synthase (also known as NOS2); LIF, leukemia inhibitory factor; PGE2, Prostaglandin E2; PDGF, platelet-derived growth factor; TGFβ, Transforming growth factor beta; TNFα, Tumor necrosis factor alpha, VEGF, vascular endothelial growth factor.
3.4.1 Costimulatory molecules
T cell activation proceeds first by binding of antigens on antigen presenting cells (APCs) and second by interaction of T cell CD28 with CD80 and CD86 on the APC. Tumor cells can express homologues of CD80 and CD86 to block T cell activation and induce apoptosis (79). Adenoviral vector expression of B7.1 in malignant astrocytomas increased survival in a mouse orthotopic xenograft model, and this was correlated with increased lymphocyte infiltration in the tumor (80). Viral administration of IL-18, IL-12, and B7.1 led to increased therapeutic efficacy and protective immunity attributed to T cells and possibly NK cells (81).
3.4.2 IL-2
IL-2 has been considered for therapeutic use as a cytokine to enhance proliferation of lymphocytes and induce the production of cells such as natural killer (NK) cells and cytotoxic T lymphocytes (CTLs). Studies in rodent models of glioma have shown that IL-2 can inhibit tumor formation and increase survival alone or in combination with other therapies (40,82), and increased numbers of cytotoxic T cells and memory T cells along with decreased numbers of Tregs within the tumor mass have been demonstrated (40).
3.4.3 IL-12
In a rat glioma model, administration of IL-12 with a tumor cell vaccine showed a significant decrease in tumor growth and provided protective immunity to tumor challenge (83). Intracerebral administration of a conditionally replication-competent HSV virus engineered to express IL-12 improved survival of a mouse model of glioblastoma, and this was correlated with increased intra-tumoral infiltration of CD4+ and CD8+ T cells and macrophages (84). Adenovirus-mediated transfer of IL-12 led to 50% of the animals surviving more than 60 days after tumor implantation, and this was accompanied by significant tumor infiltration with CD4+ and CD8+ lymphocytes (85). Intra-tumoral injection of mesenchymal stem cells expressing a more potent IL-12 led to increased survival and rejection of tumors when rechallenged (86). This study also showed pronounced tumor infiltration with CD4+ and CD8+ cells.
3.4 Combination Therapies
In an effort to overcome the shortcomings of single therapies, combination therapies have been developed. Our lab has pioneered the combination of Ad-TK and Ad-Flt3L. The theory behind this combination is that expression of TK results in phosphorylation of GCV ultimately resulting in tumor cell death (87). Cell death induces the release of tumor antigens into the tumor microenvironment and damage-associated molecular pattern molecules (DAMPs), which are nuclear or cytosolic molecules that when released or exposed in the cell membrane during apoptosis or necrosis trigger an immune response against self-antigens. Tumor cells release a wide variety of DAMPS, such as DNA, heat-shock proteins or (high-mobility group box 1, HMGB1) and ATP that could mediate antitumor immunity (88). Our results indicate that release of HMGB1 from Ad-TK infected tumor cells is required for the efficacy of Ad.TK+Ad.Flt3L-mediated immunotherapy (64,65). Flt3L increases the number of infiltrating dendritic cells into the tumor microenvironment (58,65,76), which are able to phagocytose the antigens that were released during the TK-induced cell death. Soluble HMGB1 activates DCs through a TLR2-mediated mechanism (65) and then, activated dendritic cells transport the antigens to the draining lymph nodes generating a T cell-mediated, antigen-specific cytotoxic immune response (65,66,76). This combination therapy elicits long-term survival and immunological memory in multiple rat and murine glioma models, including both unifocal and multifocal GBM as well as primary and recurrent GBM (Figure 5) (89-91). We have subsequently described the use of a novel bi-cistronic high-capacity Ad vectors (HC-Ad) driving expression of both thymidine kinase and inducible expression of Flt3L in a single vector platform (21,92). These studies suggest this may be a promising avenue for treatment of GBM in human patients. We recently assessed whether temozolomide (TMZ)—the current standard of care chemotherapeutic agent—affects the response to treatment with Ad-TK and Ad-Flt3L. We found that while TMZ reduced the number of T cells found within the tumor, the therapeutic efficacy remained the same (67). This finding makes translation into a clinical trial where Ad-TK and Ad-Flt3L are combined with current standard of care, very favorable.
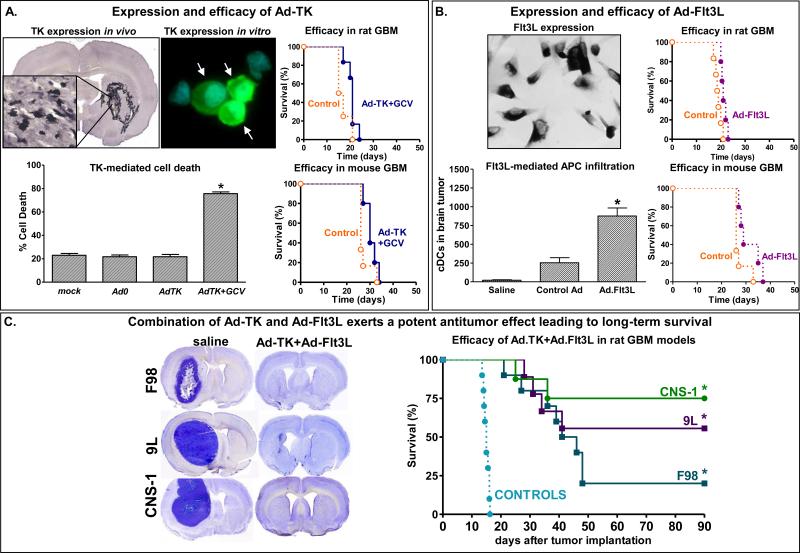
Figure 5. Efficacy of the combined conditional cytotoxic, immune stimulatory gene therapy strategy (Ad-TK and Ad-Flt3L) in murine models of GBM.
A. Expression of TK was assessed by immunostaining in CNS-1 rat GBM tumors in vivo and in vitro. Cell death was evaluated by flow cytometric analysis. The efficacy of Ad-TK+GCV was evaluated in mice and rats bearing syngeneic CNS-1 and GL26 tumors, respectively. B, Ad-mediated expression of Flt3L was assessed by immunostaining in J3T canine GBM cells. Rats bearing intracranial GBM were treated with Ad-Flt3L, control vector encoding b-Gal or saline. Infiltration of DCs in the brain tumor was assessed 5 days after treatment. The efficacy of Ad-Flt3L was evaluated in mice and rats bearing syngeneic CNS-1 and GL26 tumors, respectively. C, Rats were implanted with F98, 9L or CNS-1 tumors in the brain and treated with Ad-TK+Ad-Flt3L Microphotographs show the neuropathology (Nissl) of moribund saline-treated rats and of Ad-TK+Ad-Flt3L-treated long-term survivors. Graphs show Kaplan Meier survival curves of tumor-bearing rats (*p<0.05 vs saline, Long rank test).
In addition to combining adenoviral vectors, we have also combined adenoviral-vector mediated gene therapy with dendritic cell vaccination. We found that the combination of intratumoral Ad-Flt3L/Ad-TK with dendritic cell vaccination resulted in long-term survival in ~90% of animals. Compared to either therapy alone, this represented a significant increase in long-term survival (93). We believe that modification of the microenvironment by Ad-Flt3L/Ad-TK enhances the efficacy of dendritic cell vaccination by potentiating the anti-tumor immunity generated by dendritic cell vaccination. This promising finding is under further investigation with a view towards translation to a phase 1 clinical trial.
It has been previously shown that expression of human melanoma differentiation-associated gene-7 (MDA-7), also known as interleukin (IL)-24, can induce tumor cells’ death and Ad bearing the mda-7 gene (Ad-mda-7) produced antitumor effects to a number of human tumors, but not in non-transformed cells (94). Ad-mda-7-mediated cytotoxicity was attributable to various mechanisms including endoplasmic stresses-induced apoptosis, autophagy, anti-angiogenesis and immune stimulation (95). In GBM models, both in vitro and in vivo, the anti-proliferative effects of Ad.mda-7 were enhanced by radiation in a greater than additive fashion (96,97). Due partly to insufficient adenovirus serotype 5 gene delivery this therapeutic approach has shown limited success in GBM. To address this problem, a recombinant adenovirus that comprises the tail and shaft domains of a serotype 5 virus and the knob domain of a serotype 3 virus expressing MDA-7/IL-24 (Ad.5/3-mda-7) was generated. Ad.5/3-mda-7 more effectively infected and killed GBM cells in vitro and in vivo when compared to Ad.5-mda-7 (98). It has also recently been shown that histone deacetylase inhibitors (HDACIs) increase MDA-7/IL-24 lethality through mechanisms involving ER stress and activation of the extrinsic apoptosis pathway (94). Adenoviral delivery of mda-7/IL-24 to GBM cells and tumors can be enhanced by a serotype 3 tropism modification and by engineering of the virus to conditionally replicate in tumor cells (99). This approach constitutes an attractive adjuvant therapeutic strategy for GBM.
4. Clinical Trials for Gliomas using Adenoviral Vectors
A number of adenoviral-based therapies have been tested in clinical trials. Therapeutic approaches have included the use of oncolytic viruses as well as gene therapy with gene targets including TK, p53, and IFN-β. To date, there has been no significant extension of survival or progression-free survival using adenoviral-based therapies, but additional trials are forthcoming.
Table 1 shows an overview of adenovirus-based gene therapy clinical trials for glioma. The first trial was published in 2000 by Trask and colleagues (100). They performed a dose-escalation in 13 patients with recurrent malignant gliomas injecting replication-defective adenoviral vector carrying herpes simplex thymidine kinase (Ad-TK) via intratumoral injection. Four escalating doses were administered with the highest dose showing neurotoxicity. While this study was not powered for survival analysis and overall survival was similar to the historical norm, 3 of the 13 patients survived longer than 25 months, representing a significant survival advantage in those 3 patients. Thus, this initial Phase I study suggested that intratumoral injection of adenoviral vector-based therapy is safe but at higher doses does have dose-limiting toxicity and suggested that some patients may respond to therapy (100). Following publication of this initial study, four additional Phase I studies were published using Ad-TK vectors (101-105). These additional trials yielded similar results, finding that delivery of thymidine kinase is safe, with higher doses showing neurotoxicity. Similarly, while not powered to analyze survival, each study showed overall survival and progression-free survival similar to historical norms but had a small subset of longer-term survivors. The authors concluded that the safety and potential efficacy justified further trials. Three additional studies are either ongoing or completed but not published (106).
Table 1.
Summary of clinical trials to date utilizing adenoviral-based therapy for malignant gliomas.
| STUDY TYPE | VECTOR | GENE | PATIENTS | DELIVERY SITE | EFFICACY | YEAR | REF |
|---|---|---|---|---|---|---|---|
| Oncolytic Viruses | |||||||
| Phase I | AdV (ONYX-015) | NA | 24 | Resection Cavity | Recurrent GBM median survival 4.9 months | 2004 | (109) |
| Phase I | AdV (Ad5-Delta24RGD) | NA | Recruiting | Intratumoral | Ongoing | 2014 | (121) |
| Phase I/II | AdV (Ad5-Delta24RGD) | NA | Recruiting | Intratumoral CED | Ongoing | 2014 | (122) |
| Gene Vectors | |||||||
| Phase I | AdV | TK | 13 | Intratumoral | Recurrent GBM mean survival 9.2 months. 3 survivors > 25 months | 2000 | (100) |
| Phase I | AdV | TK | 11 | Resection Cavity | Mean survival 13.7 months | 2003 | (101) |
| Phase I | AdV | TK | 14 | Resection Cavity | Median survival 4 months | 2003 | (103) |
| Phase I | AdV | IFN-β | 11 | Intratumoral | Median survival 4.2 months | 2008 | (108) |
| Phase I | AdV | p53 | 15 | Intratumoral | Median survival 10 months. 1 survivor > 3 years | 2003 | (107) |
| Phase I | AdV | TK | 7 | Resection Cavity | Mean survival 15 months | 2000 | (102) |
| Phase IB | AdV | TK | 13 | Resection Cavity | Mean survival 12.4 months | 2005 | (45) |
| Phase III | AdV (ARK Ther.) | TK | 250 | Resection Cavity | Median survival 16.5 vs 15 months | 2013 | (117) |
| Phase I | AdV | TK+ Flt3L | Not Available | Resection Cavity | Ongoing | 2013 | (106) |
| Phase Ib | AdV (Advantagene) | TK | Not Available | Not Available | Ongoing | 2008 | (123) |
| Phase IIa | AdV (Advantagene) | TK | Not Available | Not Available | Ongoing | 2007 | (124) |
Another approach has been the delivery of p53 (107). This Phase I study was a dose escalation including 15 patients with recurrent GBM. During this study, a maximum tolerated dose was not reached. The authors found that intra-tumoral Ad-p53 led to expression of functional p53 protein but only within close proximity to the injection site. This phase I study was not powered to examine overall survival; however, survival in these patients was similar to historical norms. One patient remained alive at the time of publication, more than 3 years following treatment. Thus, Ad-p53 administration was shown to be safe with demonstrated expression near the injection site. The authors felt that future research should focus on generating more widespread expression (107).
The final gene therapy target that has made it to clinical trial is IFN-β (108). This Phase I trial involved 11 patients, and dose-limiting toxicity was reached. Detectable levels of IFN-β were found within the tumor, and at the highest dose the authors found induction of apoptosis within the resected tumor specimen. Overall and progression-free survival were similar to historical norms, although this study was not powered to compare survival. The reproducible induction of apoptosis within the tumor specimens was a promising finding and suggested that further research is warranted (108).
In addition to gene-based adenoviral therapies, one clinical trial has utilized an oncolytic adenovirus (109). Chiocca and colleagues generated a replication-conditional adenovirus mutant called ONYX-015. This replication-conditional virus is thought to replicate efficiently in cells with disruptions in the p53 tumor suppressor pathway, though the exact conditional mechanism is controversial (110). This Phase I, dose-escalation trial included 24 patients with recurrent malignant glioma. During this study, the maximum tolerated dose was not reached. Progression-free and overall survival was similar to historical norms. Similar to previous studies using other adenoviral-based therapies, a small subset of longer-term survivors was seen. The authors concluded that administration of ONYX-015 was safe at reported doses and that future research should focus on the efficacy of this treatment (109). The DNX-2401 or Delta-24-RGD-4C for recurrent malignant gliomas is currently being tested in an ongoing clinical trial.
Taken together, these trials suggest that administration of adenovirus-based gene therapy is safe, though there do appear to be dose-limiting side effects at higher doses. The main purpose of these Phase I trials was to establish a safety profile and to determine the maximum tolerated dose for each therapy. Though not powered to examine survival, no survival benefit was seen in any of the studies, although most of the studies had a small subset of longer-term survivors. Understanding why some patients enrolled in the trials responded very favorably to therapy and others did not will be important to improving these therapies in order to generate a survival advantage. Our team has also started enrolling patients in a phase I trial at our institution using Ad-CMV-Flt3L and Ad-CMV-TK co-administered to patients in the resection cavity (106). Additional trials are ongoing and we await the next set of results to aid in further development of promising adenovirus-mediated gene therapies for GBM.
5. Conclusions
In conclusion, GBM patients still have a dismal prognosis with current standard of care including resection, chemotherapy, and radiotherapy. Adenovirus-based gene transfer represents an attractive option for the delivery of a wide range of beneficial genes for therapeutic purposes, including conditional cytotoxic enzymes, toxins, cytokines, and shRNAs. Adenoviruses can also be engineered to selectively replicate within and kill tumor cells. Given the ability to engineer the adenovirus genome in addition to the demonstrated safety of adenoviral vector administration to GBM patients in the clinic, adenoviral-mediated gene therapy should prove to be a valuable therapeutic tool for the treatment of GBM.
6. Expert Opinion
Adv-mediated gene therapy, specifically, for the treatment of malignant glioma
The ability to utilize Ads to transfer genes followed studies on the capacity of adenoviruses to induce tumors in rodents. Of note, in humans the most common Ad serotypes are not oncogenic, even if the protein E1a encodes various functions that classify it as an oncogene. Deletion of genomic regions predicted to initiate the replication cycle led to the discovery of the early transcription region 1, which contains the E1a and E1b genes, which are expressed very early after viral infection (111,112) In the process of doing so, Frank Graham and colleagues developed the calcium-phosphate transfection method, a robust gene transfer method that ushered widespread genetic studies. Deletions in the adenoviral genome then led to their replacement by alternative genetic material, giving birth to Ad-mediated gene transfer (113). As the idea of gene transfer gained hold, a search for ideal vector systems began. Thirty years later, the practical outcome of this search can be gleaned from the frequency of viral systems within the current clinical trials’ database, ClinicalTrials.gov. Searching this data base for “gene therapy/transfer and the viral delivery system”, adenovirus returns 69 studies, AAV 41, Herpes Simplex virus 8, retrovirus 61, and lentivirus 20 trials; plasmid delivery returns 19 studies. When these current trials are compared to the number of historical trials, an interesting pattern appears with adenovirus being employed in 23% of trials, retrovirus in 20%, AAV in 5%, Herpes simplex virus in 3%, and lentivirus in 3% of trials overall. Thus, the use of AAV and lentiviral vectors is on the increase, while adenovirus’ use appears stable over time. The numbers give an overview of “winners” and “losers” in the translation to clinical trials. However, the trends noted need to be dissected to understand what the numbers actually mean, and where the largest successes can be found. While a handful of gene therapeutics have made it into the market in Asia, no gene therapeutic product is yet available for general patient use outside of their experimental use in North America and Europe.
AAV vectors have enjoyed recent success in the treatment of inherited eye diseases (114), while lentiviral vectors, building on the strong record of retrovirus, have made gene transfer into the bone marrow safer and more effective (115,116) . In summary, rather than winner takes all, vectors have found their ideal niches. Just like in experimental gene transfer, lentiviral vectors are most powerful when used to transduce cells with a high division rate, such as the bone marrow, while adenoviral and AAV vectors are more powerful when transducing non-dividing cells such as those found in the retina and the brain. Lentiviral vector gene transfer to the bone marrow and AAV gene delivery to the retina, have found their most efficient niche in treating inherited diseases of the bone marrow and retina, respectively. The high level and robust gene delivery achieved by adenovirus has found its niche in gene therapy for cancer.
Adenoviruses expressing various potentially therapeutic targets have been developed. In the clinic, this comes down to just a few approaches. While expression of interferon-ß was tried earlier, these studies did not progress to larger phase III clinical trials (108). Most trials using adenovirus have utilized the HSV1-TK + prodrug (i.e., ganciclovir, valganciclovir) approach. This has progressed to a Phase III randomized controlled clinical trial throughout Europe, which has been recently completed and published (117). Though safe, the efficiency in prolonging the life of patients was deemed too low to warrant commercial approval by the European Medicines Agency (European equivalent of FDA). Nevertheless, a general faith in this approach's robustness is leading other scientists and companies in the US to repeat these trials within a North American context (45,100-103).
An approach that has made much progress is the use of replication competent adenoviruses, with or without additional therapeutic payloads. Of significance, currently there are two trials open for the treatment of malignant gliomas with adenoviral gene therapy. Active studies (‘Safety Study of Replication-competent Adenovirus (Delta-24-rgd) in patients With Recurrent Glioblastoma’ [NCT01582516], and ‘Virus DNX2401 and Temozolomide in Recurrent Glioblastoma (D24GBM)’ [NCT01956734], are currently active for the treatment of patients with recurrent gliobalstoma, are complemented by the study ‘DNX-2401 (Formerly Known as Delta-24-RGD-4C) for Recurrent Malignant Gliomas’ [NCT00805376] which is currently analyzing data from another trial on the use of this same replicating adenovirus for recurrent glioma.
The other trial currently open, i.e., ‘Combined Cytotoxic and Immune-Stimulatory Therapy for Glioma’, sponsored by PRL at The University of Michigan [NCT01811992], will explore the use of two adenoviruses given in combination, as described above, to patients undergoing primary resection of malignant glioma grade IV. One adenovirus expresses the cytokine Flt3L which recruits immune cells, i.e., DCs to the tumor microenvironment, while the second virus, Ad-TK expresses the conditionally cytotoxic enzyme HSV1-TK which in combination with valganciclovir will kill tumor cells and release powerful TLR2 agonists, i.e., HMGB1(65). Pre-clinical work on this approach has been published over the last ten years, in over twenty publications, which have shown the overall efficiency of this approach. In this approach, the therapeutic intent is to reconstruct those aspects of the immune system normally not present in the brain, i.e., the capacity to stimulate immune responses against antigens located within the brain parenchyma proper.
It has been shown over the last fifty years that what is usually described as the brain's “immune privilege” is the selective inability for the immune system to recognize particulate (non-diffusible) antigens located within the brain parenchyma. In 2002 we postulated that these responses were absent due to the lack of a cell located within the brain that would be able to pick up foreign antigen, leave the brain parenchyma and migrate to the lymph nodes to carry out the antigen presentation and stimulation of the full blown adaptive immune response (118,119). The missing cell type was postulated to be the dendritic cells, and we developed the direct Ad-mediated delivery of Flt3L to the brain as a way to attract dendritic cells to the brain, and thus, reconstruct the immune circuits left behind during the co-evolution of the brain and the immune system (58,89). Regarding the safety of this approach, examination of all our models has failed to show the induction of brain autoimmunity (89,120). In summary, the transduction characteristics of adenoviral vectors, and their overall safety profile, added to their capacity to achieve high levels of transgene expression in the brain have made them the vector of choice to treat GBM. Exciting clinical trials are now recruiting patients, and we will look forward to the opportunity to continue to update the scientific community on their progress. In the long run, it is only large randomized phase III controlled clinical trials that will provide the final decision on the overall success of gene therapy for the treatment of patients suffering from malignant brain tumors.
Article Highlights.
Glioblastoma multiforme (GBM) is the most common primary brain tumor, and novel therapies are greatly needed for this disease.
Replication deficient first-generation or high-capacity adenoviruses can be used as gene therapy vectors to effectively deliver therapeutic genes to treat GBM.
Various therapeutic paradigms, including conditional cytotoxicity, toxins, immunostimulation, and RNA interference can be employed to treat GBM.
Replication-competent adenoviruses that directly lyse tumor cells can also be used for GBM treatment.
A number of clinical trials for GBM utilizing first-generation adenoviral vectors have been safely performed, demonstrating the feasibility of adenoviral vector administration in humans.
Acknowledgements
We are grateful to Dr. Karin Murasko for her academic leadership, to M. Dahlgren for superb administrative support, and for her exceptional editing skills, and to R. Lemons and M. Dzaman for superb technical assistance. This work was supported by National Institutes of Health/National Institute of Neurological Disorders & Stroke (NIH/NINDS) Grants U01-NS052465, U01-NS052465-S1, R01-NS074387, R01-NS057711, MICHR Pilot R14 U040007, and BioInterfaces Institute, University of Michigan U042841 to M.G.C.; NIH/NINDS Grants R01-NS054193, R01-NS061107, R01-NS082311, R21-NS084275, and M-Cube U036756 University of Michigan to P.R.L.; the Department of Neurosurgery, University of Michigan School of Medicine; the Michigan Institute for Clinical and Health Research, NIH UL1-TR000433; University of Michigan Cancer Biology Training Grant, NIH/NCI (National Cancer Institute) T32-CA009676; University of Michigan Training in Clinical and Basic Neuroscience, NIH/NINDS T32-NS007222; the University of Michigan Medical Scientist Training Program, NIH/NIGMS (National Institute of General Medicine Sciences) T32-GM007863, and the National Institutes of Health through the University of Michigan's Cancer Center Support Grant P30-CA046592. M.C. and M.A.M.A were supported by the Consejo Nacional de Ciencia y Tecnologia (CONICET PIP 114-201101-00353) and the Agencia Nacional de Promocion Cientifica y Tecnologica (PICT-2012-0830).
Footnotes
Declaration of Interest The authors declare no competing interests.
References
- 1.Fisher JL, Schwartzbaum JA, Wrensch M, et al. Epidemiology of brain tumors. Neurol Clin. 2007;25:867–890. vii. doi: 10.1016/j.ncl.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Rux JJ, Burnett RM. Adenovirus structure. Hum Gene Ther. 2004;15:1167–1176. doi: 10.1089/hum.2004.15.1167. [DOI] [PubMed] [Google Scholar]
- 4*.Volpers C, Kochanek S. Adenoviral vectors for gene transfer and therapy. J Gene Med. 2004;6(Suppl 1):S164–171. doi: 10.1002/jgm.496. Review of adenoviral vectors including high capacity, gutless vectors. [DOI] [PubMed] [Google Scholar]
- 5.Bergelson JM, Cunningham JA, Droguett G, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Bergelson JM. Adenovirus receptors. J Virol. 2005;79:12125–12131. doi: 10.1128/JVI.79.19.12125-12131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong SS, Karayan L, Tournier J, et al. Adenovirus type 5 fiber knob binds to MHC class I alpha2 domain at the surface of human epithelial and B lymphoblastoid cells. Embo J. 1997;16:2294–2306. doi: 10.1093/emboj/16.9.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowenstein PR, Thomas CE, Umana P, et al. High-capacity, helper-dependent, “gutless” adenoviral vectors for gene transfer into brain. Methods Enzymol. 2002;346:292–311. doi: 10.1016/s0076-6879(02)46062-4. [DOI] [PubMed] [Google Scholar]
- 9.Zirger JM, Puntel M, Bergeron J, et al. Immune-mediated loss of transgene expression from virally transduced brain cells is irreversible, mediated by IFNgamma, perforin, and TNFalpha, and due to the elimination of transduced cells. Mol Ther. 2012;20:808–819. doi: 10.1038/mt.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puntel M, Barrett R, Sanderson NS, et al. Identification and visualization of CD8+ T cell mediated IFN-gamma signaling in target cells during an antiviral immune response in the brain. PloS ONE. 2011;6:e23523. doi: 10.1371/journal.pone.0023523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barcia C, Gerdes C, Xiong WD, et al. Immunological thresholds in neurological gene therapy: highly efficient elimination of transduced cells might be related to the specific formation of immunological synapses between T cells and virus-infected brain cells. Neuron Glia Biol. 2006;2:309–322. doi: 10.1017/S1740925X07000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowenstein PR, Mandel RJ, Xiong WD, et al. Immune responses to adenovirus and adeno-associated vectors used for gene therapy of brain diseases: the role of immunological synapses in understanding the cell biology of neuroimmune interactions. Curr Gene Ther. 2007;7:347–360. doi: 10.2174/156652307782151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Barcia C, Jimenez-Dalmaroni M, Kroeger KM, et al. One-year expression from high-capacity adenoviral vectors in the brains of animals with pre-existing anti-adenoviral immunity: clinical implications. Mol Ther. 2007;15:2154–2163. doi: 10.1038/sj.mt.6300305. Long -term expression mediated by high capacity adenoviral vectors in the presence of pre-existing anti/adenoviral immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas CE, Schiedner G, Kochanek S, et al. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum Gene Ther. 2001;12:839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- 15**.Thomas CE, Schiedner G, Kochanek S, et al. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci USA. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. Brain immune responses elicited by first generation and high capacity adenovirus vectors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kochanek S. High-capacity adenoviral vectors for gene transfer and somatic gene therapy. Hum Gene Ther. 1999;10:2451–2459. doi: 10.1089/10430349950016807. [DOI] [PubMed] [Google Scholar]
- 17.Lowenstein PR, Castro MG. Genetic engineering within the adult brain: implications for molecular approaches to behavioral neuroscience. Physiol Behav. 2001;73:833–839. doi: 10.1016/s0031-9384(01)00520-0. [DOI] [PubMed] [Google Scholar]
- 18*.Xiong W, Goverdhana S, Sciascia SA, et al. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. High capacity adenoviral vectors mediated regulatable transgene expression in the presence of anti-adenoviral immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.VanderVeen N, Paran C, Krasinkiewicz J, et al. Effectiveness and preclinical safety profile of doxycycline to be used “off-label” to induce therapeutic transgene expression in a phase I clinical trial for glioma. Hum Gene Ther Clin Dev. 2013;24:116–126. doi: 10.1089/humc.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muhammad AK, Puntel M, Candolfi M, et al. Study of the efficacy, biodistribution, and safety profile of therapeutic gutless adenovirus vectors as a prelude to a phase I clinical trial for glioblastoma. Clin Pharmacol Ther. 2010;88:204–213. doi: 10.1038/clpt.2009.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puntel M, Muhammad AKMG, Farrokhi C, et al. Safety profile, efficacy, and biodistribution of a bicistronic high-capacity adenovirus vector encoding a combined immunostimulation and cytotoxic gene therapy as a prelude to a phase I clinical trial for glioblastoma. Toxicol Appl Pharmacol. 2013;268:318–330. doi: 10.1016/j.taap.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez R, Schuur ER, Lim HY, et al. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57:2559–2563. [PubMed] [Google Scholar]
- 23.Sonabend AM, Ulasov IV, Tyler MA, et al. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008;26:831–841. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 24*.Fueyo J, Gomez-Manzano C, Alemany R, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. Description of oncolytic adenoviral vectors targeting the Rb pathway. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Manzano C, Yung WK, Alemany R, et al. Genetically modified adenoviruses against gliomas: from bench to bedside. Neurology. 2004;63:418–426. doi: 10.1212/01.wnl.0000133302.15022.7f. [DOI] [PubMed] [Google Scholar]
- 26.Harada JN, Berk AJ. p53-Independent and -dependent requirements for E1B-55K in adenovirus type 5 replication. J Virol. 1999;73:5333–5344. doi: 10.1128/jvi.73.7.5333-5344.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Shea CC, Johnson L, Bagus B, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki K, Fueyo J, Krasnykh V, et al. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin Cancer Res. 2001;7:120–126. [PubMed] [Google Scholar]
- 29.Dmitriev I, Krasnykh V, Miller CR, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Lamfers ML, Idema S, Bosscher L, et al. Differential effects of combined Ad5- delta 24RGD and radiation therapy in in vitro versus in vivo models of malignant glioma. Clin Cancer Res. 2007;13:7451–7458. doi: 10.1158/1078-0432.CCR-07-1265. Radiation therapy used in combination with an oncolytic adenoviral vector in models of glioma. [DOI] [PubMed] [Google Scholar]
- 31.Hemminki O, Bauerschmitz G, Hemmi S, et al. Oncolytic adenovirus based on serotype 3. Cancer Gene Ther. 2011;18:288–296. doi: 10.1038/cgt.2010.79. [DOI] [PubMed] [Google Scholar]
- 32.Yu W, Fang H. Clinical trials with oncolytic adenovirus in China. Curr Cancer Drug Targets. 2007;7:141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 33**.Moolten FL. Tumor chemosensitivity conferred by inserted herpes thymidine kinase genes: paradigm for a prospective cancer control strategy. Cancer Res. 1986;46:5276–5281. Tumor specific cytotoxicity conferred by herpes thymidine kinase genes and ganciclovir. [PubMed] [Google Scholar]
- 34.Kurozumi K, Tamiya T, Ono Y, et al. Apoptosis induction with 5-fluorocytosine/cytosine deaminase gene therapy for human malignant glioma cells mediated by adenovirus. J Neuro-Oncol. 2004;66:117–127. doi: 10.1023/b:neon.0000013494.98345.80. [DOI] [PubMed] [Google Scholar]
- 35.Dachs GU, Tupper J, Tozer GM. From bench to bedside for gene-directed enzyme prodrug therapy of cancer. Anti-Cancer Drugs. 2005;16:349–359. doi: 10.1097/00001813-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Gadi VK, Alexander SD, Waud WR, et al. A long-acting suicide gene toxin, 6-methylpurine, inhibits slow growing tumors after a single administration. J Pharmacol Exp Ther. 2003;304:1280–1284. doi: 10.1124/jpet.102.044743. [DOI] [PubMed] [Google Scholar]
- 37.Springer CJ, Niculescu-Duvaz I. Prodrug-activating systems in suicide gene therapy. J Clin Invest. 2000;105:1161–1167. doi: 10.1172/JCI10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Maatta AM, Samaranayake H, Pikkarainen J, et al. Adenovirus mediated herpes simplex virus-thymidine kinase/ganciclovir gene therapy for resectable malignant glioma. Curr Gene Ther. 2009;9:356–367. doi: 10.2174/156652309789753365. Review on adenovirus mediated conditional cytotoxic gene therapy for glioma. [DOI] [PubMed] [Google Scholar]
- 39.Kokoris MS, Black ME. Characterization of herpes simplex virus type 1 thymidine kinase mutants engineered for improved ganciclovir or acyclovir activity. Protein Sci. 2002;11:2267–2272. doi: 10.1110/ps.2460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mineharu Y, Muhammad AK, Yagiz K, et al. Gene therapy-mediated reprogramming tumor infiltrating T cells using IL-2 and inhibiting NF-kappaB signaling improves the efficacy of immunotherapy in a brain cancer model. Neurotherapeutics. 2012;9:827–843. doi: 10.1007/s13311-012-0144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Black ME, Kokoris MS, Sabo P. Herpes simplex virus-1 thymidine kinase mutants created by semi-random sequence mutagenesis improve prodrug-mediated tumor cell killing. Cancer Res. 2001;61:3022–3026. Improved herpes simplex virus-1 thymidine kinase mutants for tumor killing. [PubMed] [Google Scholar]
- 42.Khan Z, Knecht W, Willer M, et al. Plant thymidine kinase 1: a novel efficient suicide gene for malignant glioma therapy. Neuro-Oncol. 2010;12:549–558. doi: 10.1093/neuonc/nop067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valerie K, Brust D, Farnsworth J, et al. Improved radiosensitization of rat glioma cells with adenovirus-expressed mutant herpes simplex virus-thymidine kinase in combination with acyclovir. Cancer Gene Ther. 2000;7:879–884. doi: 10.1038/sj.cgt.7700185. [DOI] [PubMed] [Google Scholar]
- 44.Nestler U, Wakimoto H, Siller-Lopez F, et al. The combination of adenoviral HSV TK gene therapy and radiation is effective in athymic mouse glioblastoma xenografts without increasing toxic side effects. J Neuro-Oncol. 2004;67:177–188. doi: 10.1023/b:neon.0000021897.53969.ca. [DOI] [PubMed] [Google Scholar]
- 45**.Chiocca EA, Aguilar LK, Bell SD, et al. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011;29:3611–3619. doi: 10.1200/JCO.2011.35.5222. Results from a Phase Ib study of gene therapy for malignant glioma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keyvani K, Baur I, Paulus W. Tetracycline-controlled expression but not toxicity of an attenuated diphtheria toxin mutant. Life Sci. 1999;64:1719–1724. doi: 10.1016/s0024-3205(99)00109-5. [DOI] [PubMed] [Google Scholar]
- 47.Paulus W, Baur I, Oberer DM, et al. Regulated expression of the diphtheria toxin A gene in human glioma cells using prokaryotic transcriptional control elements. J Neurosurg. 1997;87:89–95. doi: 10.3171/jns.1997.87.1.0089. [DOI] [PubMed] [Google Scholar]
- 48.Curtin JF, Candolfi M, Xiong W, et al. Turning the gene tap off; implications of regulating gene expression for cancer therapeutics. Mol Cancer Ther. 2008;7:439–448. doi: 10.1158/1535-7163.MCT-07-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Debinski W, Gibo DM. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med. 2000;6:440–449. [PMC free article] [PubMed] [Google Scholar]
- 50**.Debinski W, Obiri NI, Pastan I, et al. A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J Biol Chem. 1995;270:16775–16780. doi: 10.1074/jbc.270.28.16775. Cytotoxicity interleukin 13 - Pseudomonas exotoxin in human cancer cells. [DOI] [PubMed] [Google Scholar]
- 51.Kunwar S, Prados MD, Chang SM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25:837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 52.Liu H, Prayson RA, Estes ML, et al. In vivo expression of the interleukin 4 receptor alpha by astrocytes in epilepsy cerebral cortex. Cytokine. 2000;12:1656–1661. doi: 10.1006/cyto.2000.0773. [DOI] [PubMed] [Google Scholar]
- 53**.Candolfi M, Xiong W, Yagiz K, et al. Gene therapy-mediated delivery of targeted cytotoxins for glioma therapeutics. Proc Natl Acad Sci USA. 2010;107:20021–20026. doi: 10.1073/pnas.1008261107. Gene therapy mediated delivery of IL13 - Pseudomonas exotoxin in models of glioma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawakami K, Kawakami M, Kioi M, et al. Distribution kinetics of targeted cytotoxin in glioma by bolus or convection-enhanced delivery in a murine model. J Neurosurg. 2004;101:1004–1011. doi: 10.3171/jns.2004.101.6.1004. [DOI] [PubMed] [Google Scholar]
- 55.Mintz A, Gibo DM, Madhankumar AB, et al. Molecular targeting with recombinant cytotoxins of interleukin-13 receptor alpha2-expressing glioma. J Neuro-Oncol. 2003;64:117–123. doi: 10.1007/BF02700026. [DOI] [PubMed] [Google Scholar]
- 56.Debinski W, Gibo DM, Obiri NI, et al. Novel anti-brain tumor cytotoxins specific for cancer cells. Nat Biotechnol. 1998;16:449–453. doi: 10.1038/nbt0598-449. [DOI] [PubMed] [Google Scholar]
- 57.Madhankumar AB, Mintz A, Debinski W. Interleukin 13 mutants of enhanced avidity toward the glioma-associated receptor, IL13Ralpha2. Neoplasia. 2004;6:15–22. doi: 10.1016/s1476-5586(04)80049-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curtin JF, King GD, Barcia C, et al. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol. 2006;176:3566–3577. doi: 10.4049/jimmunol.176.6.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanchez-Perez L, Choi BD, Reap EA, et al. BLyS levels correlate with vaccine-induced antibody titers in patients with glioblastoma lymphodepleted by therapeutic temozolomide. Cancer Immunol Immunother. 2013;62:983–987. doi: 10.1007/s00262-013-1405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chakravarty PK, Alfieri A, Thomas EK, et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59:6028–6032. [PubMed] [Google Scholar]
- 62.Chen K, Braun S, Lyman S, et al. Antitumor activity and immunotherapeutic properties of Flt3-ligand in a murine breast cancer model. Cancer Res. 1997;57:3511–3516. [PubMed] [Google Scholar]
- 63.Somers KD, Brown RR, Holterman DA, et al. Orthotopic treatment model of prostate cancer and metastasis in the immunocompetent mouse: efficacy of flt3 ligand immunotherapy. Int J Cancer. 2003;107:773–780. doi: 10.1002/ijc.11464. [DOI] [PubMed] [Google Scholar]
- 64.Candolfi M, Yagiz K, Foulad D, et al. Release of HMGB1 in response to proapoptotic glioma killing strategies: efficacy and neurotoxicity. Clin Cancer Res. 2009;15:4401–4414. doi: 10.1158/1078-0432.CCR-09-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65**.Curtin JF, Liu N, Candolfi M, et al. HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med. 2009;6:e10. doi: 10.1371/journal.pmed.1000010. First report on HMGB1 as an endogenous TLR2 activator in cancer models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66*.Ghulam Muhammad AK, Candolfi M, King GD, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009;15:6113–6127. doi: 10.1158/1078-0432.CCR-09-1087. Conditional cytotoxic/immune stimulation elicits anti-tumor immunological memory in glioma models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67*.Candolfi M, Yagiz K, Wibowo M, et al. Temozolomide does not impair gene therapy-mediated antitumor immunity in syngeneic brain tumor models. Clin Cancer Res. 2014;20:1555–1565. doi: 10.1158/1078-0432.CCR-13-2140. Temozolomide does not impair conditional cytotoxic/immune-stimulatory gene therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ali S, Curtin JF, Zirger JM, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10:1071–1084. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tada Y, J OW, Yu L, et al. T-cell-dependent antitumor effects produced by CD40 ligand expressed on mouse lung carcinoma cells are linked with the maturation of dendritic cells and secretion of a variety of cytokines. Cancer Gene Ther. 2003;10:451–456. doi: 10.1038/sj.cgt.7700584. [DOI] [PubMed] [Google Scholar]
- 70.Peter I, Nawrath M, Kamarashev J, et al. Immunotherapy for murine K1735 melanoma: combinatorial use of recombinant adenovirus expressing CD40L and other immunomodulators. Cancer Gene Ther. 2002;9:597–605. doi: 10.1038/sj.cgt.7700475. [DOI] [PubMed] [Google Scholar]
- 71.Kaplan DH, Shankaran V, Dighe AS, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 73.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 74.Ehtesham M, Samoto K, Kabos P, et al. Treatment of intracranial glioma with in situ interferon-gamma and tumor necrosis factor-alpha gene transfer. Cancer Gene Ther. 2002;9:925–934. doi: 10.1038/sj.cgt.7700516. [DOI] [PubMed] [Google Scholar]
- 75.Arko L, Katsyv I, Park GE, et al. Experimental approaches for the treatment of malignant gliomas. Pharmacol Ther. 2010;128:1–36. doi: 10.1016/j.pharmthera.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Candolfi M, King GD, Yagiz K, et al. Plasmacytoid dendritic cells in the tumor microenvironment: immune targets for glioma therapeutics. Neoplasia. 2012;14:757–770. doi: 10.1593/neo.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dix AR, Brooks WH, Roszman TL, et al. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100:216–232. doi: 10.1016/s0165-5728(99)00203-9. [DOI] [PubMed] [Google Scholar]
- 78.Mahaley MS, Jr., Brooks WH, Roszman TL, et al. Immunobiology of primary intracranial tumors. Part 1: studies of the cellular and humoral general immune competence of brain-tumor patients. J Neurosurg. 1977;46:467–476. doi: 10.3171/jns.1977.46.4.0467. [DOI] [PubMed] [Google Scholar]
- 79.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 80.Morioka J, Kajiwara K, Yoshikawa K, et al. Adenovirus-mediated gene transfer of B7.1 induces immunological anti-tumor effects in a murine brain tumor. J Neuro-Oncol. 2002;60:13–23. doi: 10.1023/a:1020260822669. [DOI] [PubMed] [Google Scholar]
- 81.Ino Y, Saeki Y, Fukuhara H, et al. Triple combination of oncolytic herpes simplex virus-1 vectors armed with interleukin-12, interleukin-18, or soluble B7-1 results in enhanced antitumor efficacy. Clin Cancer Res. 2006;12:643–652. doi: 10.1158/1078-0432.CCR-05-1494. [DOI] [PubMed] [Google Scholar]
- 82.Chen B, Timiryasova TM, Haghighat P, et al. Low-dose vaccinia virus-mediated cytokine gene therapy of glioma. J Immunother. 2001;24:46–57. doi: 10.1097/00002371-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 83.Jean WC, Spellman SR, Wallenfriedman MA, et al. Interleukin-12-based immunotherapy against rat 9L glioma. Neurosurgery. 1998;42:850–856. doi: 10.1097/00006123-199804000-00097. discussion 856-857. [DOI] [PubMed] [Google Scholar]
- 84.Parker JN, Gillespie GY, Love CE, et al. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci USA. 2000;97:2208–2213. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Y, Ehtesham M, Samoto K, et al. In situ adenoviral interleukin 12 gene transfer confers potent and long-lasting cytotoxic immunity in glioma. Cancer Gene Ther. 2002;9:9–15. doi: 10.1038/sj.cgt.7700399. [DOI] [PubMed] [Google Scholar]
- 86.Ryu CH, Park SH, Park SA, et al. Gene therapy of intracranial glioma using interleukin 12-secreting human umbilical cord blood-derived mesenchymal stem cells. Hum Gene Ther. 2011;22:733–743. doi: 10.1089/hum.2010.187. [DOI] [PubMed] [Google Scholar]
- 87.Fischer U, Steffens S, Frank S, et al. Mechanisms of thymidine kinase/ganciclovir and cytosine deaminase/ 5-fluorocytosine suicide gene therapy-induced cell death in glioma cells. Oncogene. 2005;24:1231–1243. doi: 10.1038/sj.onc.1208290. [DOI] [PubMed] [Google Scholar]
- 88.Tang D, Kang R, Coyne CB, et al. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89**.Ali S, King GD, Curtin JF, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. Combined cytotoxic/immune-stimulatory gene therapy elicits long-term survival in glioma models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.King GD, Muhammad AK, Curtin JF, et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro-Oncol. 2008;10:19–31. doi: 10.1215/15228517-2007-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.King GD, Muhammad AK, Larocque D, et al. Combined Flt3L/TK gene therapy induces immunological surveillance which mediates an immune response against a surrogate brain tumor neoantigen. Mol Ther. 2011;19:1793–1801. doi: 10.1038/mt.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Puntel M, Muhammad AK, Candolfi M, et al. A novel bicistronic high-capacity gutless adenovirus vector that drives constitutive expression of herpes simplex virus type 1 thymidine kinase and tet-inducible expression of Flt3L for glioma therapeutics. J Virol. 2010;84:6007–6017. doi: 10.1128/JVI.00398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93**.Mineharu Y, King GD, Muhammad AK, et al. Engineering the brain tumor microenvironment enhances the efficacy of dendritic cell vaccination: implications for clinical trial design. Clin Cancer Res. 2011;17:4705–4718. doi: 10.1158/1078-0432.CCR-11-0915. Combined cytotoxic/immune-stimulatory gene therapy enhances dendritic cell vaccinaiton efficacy in glioma models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dent P, Yacoub A, Hamed HA, et al. MDA-7/IL-24 as a cancer therapeutic: from bench to bedside. Anti-Cancer Drugs. 2010;21:725–731. doi: 10.1097/CAD.0b013e32833cfbe1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Emdad L, Lebedeva IV, Su ZZ, et al. Historical perspective and recent insights into our understanding of the molecular and biochemical basis of the antitumor properties of mda-7/IL-24. Cancer Biol Ther. 2009;8:391–400. doi: 10.4161/cbt.8.5.7581. [DOI] [PubMed] [Google Scholar]
- 96.Yacoub A, Mitchell C, Lebedeva IV, et al. mda-7 (IL-24) Inhibits growth and enhances radiosensitivity of glioma cells in vitro via JNK signaling. Cancer Biol Ther. 2003;2:347–353. doi: 10.4161/cbt.2.4.422. [DOI] [PubMed] [Google Scholar]
- 97.Yacoub A, Mitchell C, Lister A, et al. Melanoma differentiation-associated 7 (interleukin 24) inhibits growth and enhances radiosensitivity of glioma cells in vitro and in vivo. Clin Cancer Res. 2003;9:3272–3281. [PubMed] [Google Scholar]
- 98.Hamed HA, Yacoub A, Park MA, et al. Inhibition of multiple protective signaling pathways and Ad.5/3 delivery enhances mda-7/IL-24 therapy of malignant glioma. Mol Ther. 2010;18:1130–1142. doi: 10.1038/mt.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Hamed HA, Yacoub A, Park MA, et al. Histone deacetylase inhibitors interact with melanoma differentiation associated-7/interleukin-24 to kill primary human glioblastoma cells. Mol Pharmacol. 2013;84:171–181. doi: 10.1124/mol.113.086553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100**.Trask TW, Trask RP, Aguilar-Cordova E, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000;1:195–203. doi: 10.1006/mthe.2000.0030. Results on a Phase I study using Ad-TK and ganciclovir in recurrent glioma. [DOI] [PubMed] [Google Scholar]
- 101.Germano IM, Fable J, Gultekin SH, et al. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas. J Neuro-Oncol. 2003;65:279–289. doi: 10.1023/b:neon.0000003657.95085.56. [DOI] [PubMed] [Google Scholar]
- 102*.Sandmair AM, Loimas S, Puranen P, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000;11:2197–2205. doi: 10.1089/104303400750035726. Comparison of adenoviral and retroviral vectors encoding HSV1-TK in human glioma. [DOI] [PubMed] [Google Scholar]
- 103.Smitt PS, Driesse M, Wolbers J, et al. Treatment of relapsed malignant glioma with an adenoviral vector containing the herpes simplex thymidine kinase gene followed by ganciclovir. Mol Ther. 2003;7:851–858. doi: 10.1016/s1525-0016(03)00100-x. [DOI] [PubMed] [Google Scholar]
- 104.Smith JG, Raper SE, Wheeldon EB, et al. Intracranial administration of adenovirus expressing HSV-TK in combination with ganciclovir produces a dose-dependent, self-limiting inflammatory response. Hum Gene Ther. 1997;8:943–954. doi: 10.1089/hum.1997.8.8-943. [DOI] [PubMed] [Google Scholar]
- 105.Eck SL, Alavi JB, Alavi A, et al. Treatment of advanced CNS malignancies with the recombinant adenovirus H5.010RSVTK: a phase I trial. Hum Gene Ther. 1996;7:1465–1482. doi: 10.1089/hum.1996.7.12-1465. [DOI] [PubMed] [Google Scholar]
- 106.Clinicaltrials.gov . Clinicaltrialsgov. US National Institutes of Health; 2013. Combined Cytotoxic and Immune-Stimulatory Therapy for Glioma ( NCT01811992). wwwclinicaltrialsgov. [Google Scholar]
- 107.Lang FF, Bruner JM, Fuller GN, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 108.Chiocca EA, Smith KM, McKinney B, et al. A phase I trial of Ad.hIFN-beta gene therapy for glioma. Mol Ther. 2008;16:618–626. doi: 10.1038/sj.mt.6300396. [DOI] [PubMed] [Google Scholar]
- 109*.Chiocca EA, Abbed KM, Tatter S, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004;10:958–966. doi: 10.1016/j.ymthe.2004.07.021. Effects of oncolytic adenoviruses in recurrent glioma. [DOI] [PubMed] [Google Scholar]
- 110.Edwards SJ, Dix BR, Myers CJ, et al. Evidence that replication of the antitumor adenovirus ONYX-015 is not controlled by the p53 and p14(ARF) tumor suppressor genes. J Virol. 2002;76:12483–12490. doi: 10.1128/JVI.76.24.12483-12490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111**.Hitt MM, Graham FL. Adv Virus Res. 2000;55:479–505. doi: 10.1016/s0065-3527(00)55014-3. Adenovirus vectors for human gene therapy. Development of adenoviruses as gene therapy vectors. [DOI] [PubMed] [Google Scholar]
- 112.Graham FL. Adenovirus vectors for high-efficiency gene transfer into mammalian cells. Immunol Today. 2000;21:426–428. doi: 10.1016/S0167-5699(00)01676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 114.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Biffi A, Montini E, Lorioli L, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 116**.Aiuti A, Biasco L, Scaramuzza S, et al. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. Lentiviral-mediated gene therapy in Wiskott-Aldrich syndrome patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117**.Westphal M, Yla-Herttuala S, Martin J, et al. Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:823–833. doi: 10.1016/S1470-2045(13)70274-2. Report of Phase 3 clinical trial results of Ad-TK mediated gene therapy for human glioma patients. [DOI] [PubMed] [Google Scholar]
- 118.Lowenstein PR. Immunology of viral-vector-mediated gene transfer into the brain: an evolutionary and developmental perspective. Trends Immunol. 2002;23:23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- 119.Lowenstein PR. Dendritic cells and immune responses in the central nervous system. Trends Immunol. 2002;23:70. doi: 10.1016/s1471-4906(01)02151-2. [DOI] [PubMed] [Google Scholar]
- 120.Larocque D, Sanderson NS, Bergeron J, et al. Exogenous fms-like tyrosine kinase 3 ligand overrides brain immune privilege and facilitates recognition of a neo-antigen without causing autoimmune neuropathology. Proc Natl Acad Sci USA. 2010;107:14443–14448. doi: 10.1073/pnas.0913496107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clinicaltrials.gov . Clinicaltrialsgov. US National Institutes of Health; 2008. DNX-2401 (Formerly Known as Delta-24-RGD-4C) for Recurrent Malignant Gliomas ( NCT00805376). wwwclinicaltrialsgov. [Google Scholar]
- 122.Clinicaltrials.gov . Clinicaltrialsgov. US National Institutes of Health; 2012. Safety Study of Replication-competent Adenovirus (Delta-24-rgd) in Patients With Recurrent Glioblastoma ( NCT01582516). wwwclinicaltrialsgov. [Google Scholar]
- 123.Clinicaltrials.gov . Clinicaltrialsgov. US National Institutes of Health; 2008. Phase 1b Study of AdV-tk + Valacyclovir Combined With Radiation Therapy for Malignant Gliomas ( NCT00751270). wwwclinicaltrialsgov. [Google Scholar]
- 124.Clinicaltrials.gov . Clinicaltrialsgov. US National Institutes of Health; 2007. Phase 2a Study of AdV-tk With Standard Radiation Therapy for Malignant Glioma (BrTK02) ( NCT00589875). wwwclinicaltrialsgov. [Google Scholar]