Abstract
The adverse effects of deep brain stimulation (DBS) surgery on swallowing could potentially exacerbate the natural deterioration of airway protection associated with Parkinson’s disease (PD) degeneration and increase the incidence of aspiration pneumonia and associated death. There are no studies that compare swallowing outcomes associated with subthalamic nucleus (STN) versus globus pallidus interna (GPi) DBS surgery; therefore, we completed a retrospective study comparing swallowing outcomes in a cohort of patients with PD who underwent unilateral DBS surgery in either the STN or GPi. A chart review was completed to identify all patients with a diagnosis of PD who received videofluoroscopic swallowing evaluations before DBS and after unilateral DBS in the STN or GPi. The retrospective search yielded 33 patients (STN = 14, GPi = 19) with idiopathic PD who met the inclusion criteria. Mean penetration–aspiration (PA) scores did not change significantly for participants who underwent GPi surgery (z = −.181, p = .857), but mean PA scores significantly worsened for participants who underwent STN DBS (z = −2.682, p = .007). There was a significant improvement in Unified PD Rating Scale (UPDRS) scores off medication before surgery, to off medication and on stimulation after surgery for both groups (F = 23.667, p < .001). Despite the limitations of a retrospective analysis, this preliminary study suggests that unilateral STN DBS may have an adverse effect on swallowing function, while unilateral GPi DBS does not appear to have a similar deleterious effect. This study and other future studies should help to elucidate the mechanisms underpinning the effects of DBS on swallowing function.
Keywords: Dysphagia, Parkinson’s disease, Aspiration, Deep brain stimulation surgery, Deglutition, Deglutition disorders
Introduction
Deep brain stimulation (DBS) surgery into the subthalamic nucleus (STN) and the globus pallidus interna (GPi) has been found to produce a similar therapeutic benefit for common motor impairments such as tremor, rigidity, and bradykinesia [1–4]. However, when considering the long-term outcomes of patients with Parkinson’s disease (PD) following DBS surgery, it is necessary to determine whether one DBS target is less disruptive to swallowing function than the other, or whether one might actually improve swallowing. This is of critical importance since any adverse effect of DBS on swallowing could actually exacerbate the natural deterioration of airway protection associated with PD degeneration and increase the incidence of aspiration pneumonia and associated death.
Studies comparing nonswallowing-specific outcomes after STN and GPi DBS in PD have suggested that axial functions are more negatively impacted with STN DBS than with GPi DBS. Specifically, a few studies have revealed the possibility that GPi DBS results in fewer adverse events (i.e., surgical, device-related, pneumonia, falls, and death) [4–6], an improved quality of life [7, 8], and a reduced frequency of postoperative neurocognitive and mood changes [2, 9–11]. Based on these data, some researchers have suggested that GPi DBS may be “safer” for swallowing function than STN DBS [5], but there have been no empirical studies comparing swallowing outcomes in patients treated with STN versus GPi DBS [12]. The aim of this retrospective study was to compare swallowing outcomes in a cohort of patients with PD who underwent unilateral DBS surgery in the STN versus GPi.
Methods
Participants
Participants included in this analysis provided informed consent and were enrolled in an Institutional Review Board (IRB)-approved DBS database (INFORM-PD). A chart review was completed to identify all participants with a diagnosis of PD who received videofluoroscopic swallowing (VFS) evaluations before DBS and after unilateral DBS in the STN or the GPi. All procedures were performed at the University of Florida Center for Movement Disorders and Neurorestoration (UF CMDNR) between August 2010 and June 2013. All potential DBS patients were evaluated by a multidisciplinary team (neurology, neurosurgery, neuropsychology, psychiatry, physical therapy, occupational therapy, and speech/swallow therapy) as part of a standardized protocol to determine candidacy for DBS. Within this protocol patients received a VFS before DBS, and then returned to the UF CMDNR 6 months after unilateral DBS implantation for a complete re-evaluation, including a repeat VFS. DBS target selection (STN vs. GPi) was not randomized but was selected by the multidisciplinary team based on the preoperative profile, the risk–benefit analysis, and the expectations of the individual patient for specific symptom improvement. At the UF CMDNR, DBS surgery is generally staged such that the benefits of the unilateral lead are assessed prior to bilateral lead implantation. Studies have identified bilateral improvement with unilateral lead placement; therefore, some patients do not require an immediate second lead. In addition, there is evidence of fewer adverse effects with unilateral DBS surgeries versus bilateral surgeries [11, 13–15].
Surgical Procedure
Frame-based, stereotactic DBS lead implantations were performed under local anesthesia. Targeting was performed using stereotactic computerized tomography (CT) fused to high-resolution gadolinium-enhanced MPRAGE plus FGATIR MR imaging, and deformable three-dimensional atlas matching to facilitate direct patient-specific target and trajectory selection, avoiding cortical and periventricular veins, sulci, and ventricles. Multiple-pass microelectrode mapping was used to verify and refine target selection physiologically. The DBS leads (model 3387, Medtronic, Minneapolis, MN) were implanted at the selected site, and intraoperative macrostimulation via the implanted lead was used as a final confirmation of appropriate lead position. Pulse generators were implanted and the DBS devices were activated 1 month after intracranial lead implantation. Following initial DBS activation, repeated follow-up evaluations were performed during the first 6 months to optimize chronic stimulation parameters and make appropriate medication adjustments. All postoperative adjustments of DBS parameter settings were performed while the participants were in an off-medication state (i.e., medications were held the night before programming sessions).
Swallowing Evaluations and Clinical Outcomes
Each patient received VFS evaluations before and after unilateral DBS implantation. Swallowing studies were completed by a licensed and certified speech-language pathologist (SLP) with clinical expertise in the evaluation of swallowing in people with PD. VFS evaluations are considered by most SLPs to be the gold standard for evaluation of swallowing, particularly in populations with silent aspiration and diffuse swallowing impairment. Pre-DBS swallowing studies were completed with the patients “on” PD medications. Post-DBS swallowing studies were completed with the patients “on” PD medications and “on” optimized DBS stimulation. Participants were seated upright and positioned in the lateral viewing plane using a properly collimated Siemens radiographic/fluoroscopic unit. The images were recorded at the standard 30 frames per second. The standardized swallowing evaluation consisted of swallowing barium contrast boluses of different consistencies: two trials of one teaspoon of thin liquid, one single bolus of thin liquid by cup, one sequential swallow of thin liquid by cup, one teaspoon of pudding, one teaspoon of pudding mixed with a solid (i.e., graham cracker), one single bolus of thin liquid by cup, and one barium tablet.
Several swallow-specific measures were obtained from VFS evaluations. Measurement of swallowing outcomes was completed by trained and certified SLPs. The primary swallowing outcome selected for this analysis was the penetration–aspiration (PA) scale score [16]. The PA scale is a clinically validated tool for assessing airway compromise during swallowing. A score of 1 indicates the safest swallow with no penetration or aspiration and a score of 8 indicates the most compromised swallowing safety, with contrast entering the airway without the presence of a cough response (i.e., silent aspiration).
The Swallowing Quality of Life Questionnaire (SWAL-QOL) [17] is a validated assessment tool used to determine a participant’s quality of life related to swallowing function. This measure consists of 44 questions, each rated on a 5-point Likert scale where a lower score indicates worse swallowing-related QOL and a higher score indicates better swallowing-related QOL. The items are categorized into ten subscales that address various domains, including fear of swallowing, burden of swallowing, social impact of swallowing dysfunction, and a symptom profile. Patients completed the SWAL-QOL at each of the two swallowing evaluation time points.
UPDRS Motor Part III scores were collected in an unblinded fashion by a trained neurologist, nurse practitioner, or physician’s assistant at the UF CMDNR during each clinical visit, before and 6 months after unilateral DBS surgery. Evaluations were conducted with participants on and off medication before DBS. Post-DBS evaluations were performed on medication while on and off stimulation, and off medication while on and off stimulation. Levodopa equivalent dose (LED) [18] was calculated before and after DBS.
Statistical Analysis
Descriptive statistics were utilized to compare the baseline characteristics of the STN and GPi groups. Statistical analysis was performed using a repeated-measures analysis of variance to compare swallowing and motor outcomes in the STN and GPi groups before and after DBS implantation. Time of evaluation (pre-DBS and post-DBS) was used as the repeated factor. The independent variable was the DBS target (STN or GPi) and the dependent variables included PA score, SWAL-QOL scores, UPDRS scores, and LED. Nonparametric statistics were applied for post hoc comparisons. Probability values <.05 were considered significant. Statistical analyses were performed using SPSS 21.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Results
Patient Characteristics
The retrospective search yielded 33 patients (STN = 14, GPi = 19) with idiopathic PD who met the inclusion criteria. Patients who underwent bilateral DBS surgeries, already had at least one lead in place, did not undergo both pre- and post-DBS swallowing evaluations, or experienced severe complications (e.g., intracerebral hemorrhage) were excluded from the analysis. Demographic information, including age at surgery, disease duration, UPDRS, and surgery-specific variables, is included in Table 1.
Table 1.
Participant demographics
| GPi | STN | |
|---|---|---|
| Participants | n = 19 | n = 14 |
| Gender | 16 M; 3F | 12 M; 2 F |
| Left vs. right | 8 left (7 M; 1F); 11 right (9 M; 2F) | 11 left (9 M; 2F); 3 right |
| Age at surgery (years) | 64.26 (8.79) | 66.5 (7.02) |
| Disease duration (years) | 12.11 (4.15) | 11.21 (5.21) |
| UPDRS-III on medication | 23.13 (6.73) | 23.43 (10.64) |
| UPDRS-III off medication | 39.89 (11.06) | 35.93 (8.98) |
Values are mean (standard deviation)
Effect of Surgery on Swallowing Outcomes
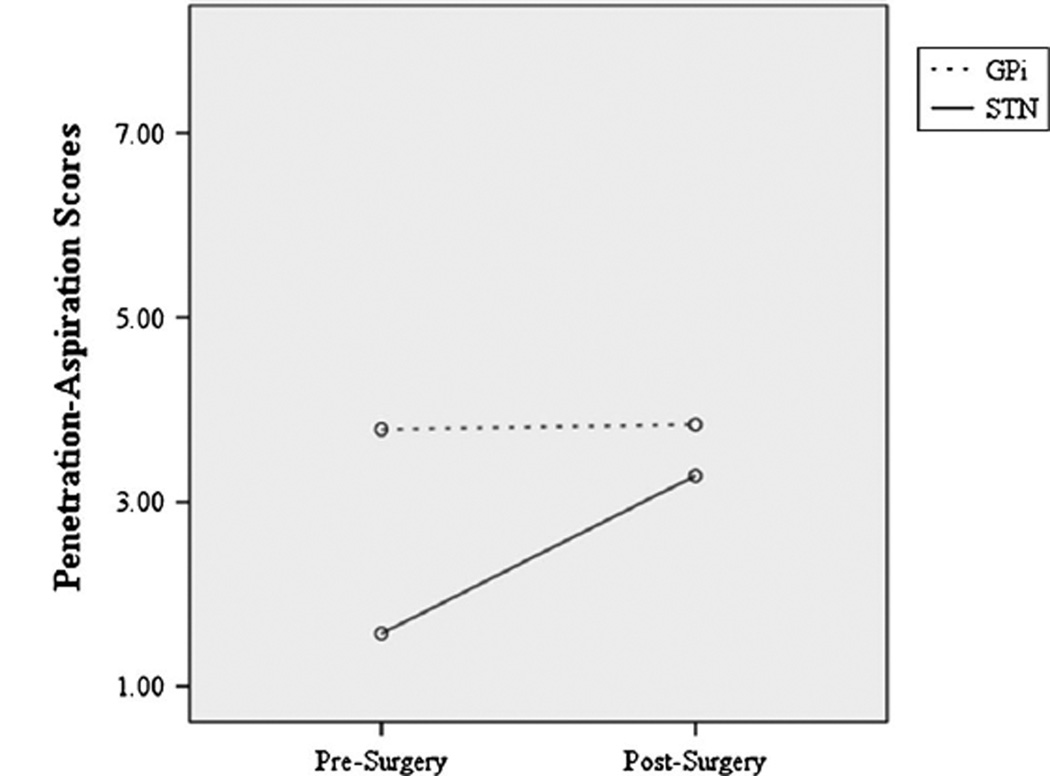
The repeated-measures ANOVA identified a statistically significant interaction that PA scores differed significantly between time points by surgery type (F = 4.545, p = 0.041; Fig. 1). Mean PA scores did not change significantly for participants who received GPi surgery (z = −.181, p = .857) but significantly worsened for participants who received STN DBS (z = −2.682, p = .007). Of note, the groups were significantly different at baseline (z = −2.924, p = .003) but not after DBS surgery (z = −.419, p = .675). Repeated-measures ANOVA identified no significant change in SWAL-QOL scores from before to after surgery for either group of patients (F = 1.466, p = .239).
Fig. 1.
The repeated-measures ANOVA identified a statistically significant interaction revealing that penetration–aspiration scores differed significantly between time points by surgery type, with mean penetration–aspiration scores not changing significantly for participants who received GPi surgery (dotted line), but mean penetration–aspiration scores significantly worsening for participants who received STN DBS (solid line)
Effect of Surgery on UPDRS and LED
There was a significant improvement in UPDRS off medication before surgery to UPDRS off medication and on stimulation after surgery for both groups (F = 23.667, p < .001). This was also the case for UPDRS on medication before surgery to UPDRS on medication and on stimulation after surgery (F = 4.806, p = .038). In addition, there was a trend toward a significant decrease in LED after surgery (F = 3.645, p = .067), which again was independent of lead location. Means and standard deviations for all outcomes are reported in Table 2.
Table 2.
Pre- and post-DBS for selected outcomes by lead location
| Unilateral GPi | Unilateral STN | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Penetration–aspiration scale score | 3.79 (2.27) | 3.84 (2.57) | 1.57 (1.16) | 3.28 (1.86) |
| SWAL-QOL | 112.13 (15) | 110.06 (15.88) | 112.3 (14.85) | 113.04 (11.87) |
| SWAL-QOL symptom profile | 56.13 (10.44) | 55.41 (10.22) | 59.7 (7.80) | 60.04 (5.87) |
| LED | 1,433.28 (593.13) | 1,360.66 (631.17) | 1,345.54 (811.20) | 1,180.18 (822.90) |
| UPDRS-III On Medication | 23.13 (6.73) | 23.43 (10.64) | ||
| UPDRS-III Off Medication | 39.89 (11.06) | 35.93 (8.98) | ||
| UPDRS-III On Meds/On Stim | 19.65 (9.25) | 21.43 (10.46) | ||
| UPDRS-III On Meds/Off Stim | 26.42 (11.29) | 23.31 (7.33) | ||
| UPDRS-III Off Meds/On Stim | 31.17 (9.11) | 28.5 (8.73) | ||
| UPDRS-III Off Meds/Off | 38.76 (9.16) | 38.29 (9.64) | ||
Values are mean (standard deviation)
Discussion
Deep brain stimulation (DBS) surgery is quickly becoming the management option of choice for persons with advanced Parkinson’s disease (PD). The two subcortical structures that are most commonly targeted in DBS surgery for the treatment of PD are the STN and the GPi [4]. Although STN and GPi DBS are generally considered safe and effective for the treatment of people with PD [1–4], important unanswered questions remain regarding the impact of DBS on less studied but important motor functions such as swallowing [12]. Understanding the effects of DBS on swallowing function is timely as DBS continues to gain traction as a management option in PD and is especially important considering that aspiration pneumonia, secondary to degenerative airway protective deficits, is a leading cause of death in the PD population [19].
The current retrospective analysis is the first study to compare swallowing outcomes associated with STN versus GPi DBS in patients with PD. There have been a few studies that investigated swallowing outcomes as related to STN surgery [20–25], but none have identified robust functional changes (either positive or negative) with surgery [12]. As is usually the case with DBS surgery, the participants in this study demonstrated an improvement in UPDRS scores independent of whether they received STN or GPi DBS. This finding was supported by the literature which has revealed comparable motor improvements in patients who receive STN versus GPi DBS in several randomized clinical trials [1–4]. In addition, there was a trend toward decreased LEDs in this group, again, independent of lead location. Interestingly, it was only the physiological measure of swallowing function that demonstrated a differential effect of lead location on the measurable outcome.
Results revealed a differential effect of DBS on PA scores as a function of lead location, whereby patients who underwent unilateral GPi DBS had no change in swallowing safety after DBS, but those who underwent STN DBS had a significant worsening of PA scores after surgery. Of concern is the obvious selection bias inherent in a retrospective study. In the case of these data, there were different levels of swallowing impairment in the STN versus GPi groups, with the GPi DBS group presenting with worse swallowing safety at baseline. At the UF CMDNR, the GPi target is selected more often for patients with more impaired swallowing and/or cognitive functioning at baseline. This approach to target selection is based on studies that have identified GPi DBS as resulting in fewer negative cognitive outcomes as compared to STN DBS, and results of large clinical trials which have reported fewer swallowing adverse events with GPi DBS as compared to STN DBS [1, 2, 4, 5, 9, 11].
At baseline, the GPi group had a median PA score of 5 (penetration to the level of the vocal folds with residue) and the STN group had a median PA score of 1 (the lowest score on the PA scale: no penetration or aspiration), but the median scores after surgery indicated that most patients who received STN DBS were then penetrating above the level of the vocal folds. This is a clinically significant change. Interestingly, the GPi group showed maintenance of swallowing function, i.e., no improvement or worsening of swallowing function. One explanation for this is that the patients who received STN DBS surgery did not respond as well to DBS as those who received GPi DBS, but this theory is not supported by the data. Patients in both groups demonstrated improvement in baseline UPDRS off-medication scores to UPDRS off-medication, on-stimulation scores. Participants also demonstrated a trend toward reductions in LED.
Patient reported swallowing-related quality of life was not significantly different between the groups. In fact, there was no significant change in SWAL-QOL scores after DBS in either group, despite the differential effects to swallowing safety in those who received STN versus GPi DBS. This finding is not completely surprising given the lack of awareness of swallowing dysfunction often associated with PD. In fact, silent aspiration (aspiration without appropriate cough response) occurs in one-third of patients with PD and lack of awareness of dysfunction has been identified in several other subsystems [26]. This highlights the need to closely monitor swallowing function in patients with PD, particularly post-DBS surgery, given that they may be unreliable swallowing historians.
Given that all swallowing evaluations were completed with the patients on medication at baseline and on medication and on stimulation after surgery, there is no way to determine whether the differences in swallowing function from before to after surgery were a result of DBS stimulation or the microlesion created during surgery [3]. Interestingly, comparisons of UPDRS scores at baseline (on medication) to UPDRS scores after surgery (on medication and on stimulation) (same conditions as for the swallowing studies) revealed that both groups (STN and GPi) had significant improvement in UPDRS scores independent of group membership. This further supports the hypothesis that DBS in the STN compared to the GPi has differential effects on corticospinal versus corticobulbar functions and on axial versus appendicular functions.
The mechanism to explain the differential effects of STN versus GPi DBS on swallowing motor outcomes is not entirely clear. It is possible that the differential effect is secondary to the differences in the reciprocal connections between the pedunculopontine nucleus (PPN) and the GPi or STN. The GPi inhibits the PPN, whereas the STN excites the PPN [27, 28]. In addition, the nucleus tractus solitarius, one of the nuclei that form part of the central pattern generator for swallowing, receives cholinergic input from the PPN [29]. It is not clear whether this same differential effect of GPi and STN DBS is seen on other corticobulbar-mediated functions such as speech and cough, but there is evidence to suggest that GPi DBS also results in fewer adverse effects to gait, an axial function [5, 30]. Another theory is that the small size of the STN as compared to the GPi results in greater spread of activation to adjacent neural structures that might adversely affect swallowing outcomes. Yet others might hypothesize that the effects are the result of simple capsular or corticobulbar spread of stimulation. Future studies planned by our laboratory will address these questions.
Conclusion
There are a very limited number of experimental studies that have evaluated the effects of DBS on swallowing function in patients with PD [12]. Despite the limitations of a retrospective analysis, this preliminary study suggests that STN DBS may have an adverse effect on swallowing function, while GPi DBS does not appear to have a similar deleterious effect. Our research group is currently conducting a study to prospectively assess changes in swallowing and cough functions resulting from DBS surgery. This study and other future studies should help to elucidate the mechanisms underpinning the effects of DBS on swallowing function and potentially lead to modification of DBS procedures that will minimize adverse effects and improve long-term outcomes for these patients. Swallowing dysfunction and failure of airway protection resulting in aspiration pneumonia is a leading cause of death in patients with PD, and since the use of DBS as an effective treatment modality for PD continues to increase, this information is critical to the field.
Acknowledgments
The authors thank the participants and their families. This work was funded in part by an National Institutes of Health (NCATS) CTSA through the University of Florida (UL1TR000064 and KL2TR000065).
Dr. Troche’s work is supported in part by an NIH (NCATS) CTSA through the University of Florida (UL1TR000064 and KL2TR000065). Ms. Brandimore’s work is supported in part by a predoctoral fellowship through the Department of Veterans Affairs. Dr. Morishita has been supported by Japan Society for Promotion of Science and St. Luke Life Science Institute. He has received honoraria from Otsuka pharmaceutical as a consultant within the past 12 months. Dr. Hegland’s work is supported in part by the American Heart Association and BAE defense systems. Dr. Okun serves as a consultant for the National Parkinson Foundation and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. Dr. Okun has previously received honoraria, but in the past >36 months has received no support from industry. Dr. Okun has received royalties for publications with Demos, Manson, Amazon, and Cambridge (movement disorders books). Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. Dr. Okun has participated in CME activities on movement disorders in the last 36 months sponsored by PeerView, Prime, and Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic and ANS/St. Jude and has no financial interest in these grants. Dr. Okun has participated as a site primary investigator and/or coinvestigator for several NIH-, foundation-, and industry-sponsored trials over the years but has not received honoraria.
Footnotes
Disclosures Dr. Foote and Mr. Chen have no disclosures to report.
Contributor Information
Michelle S. Troche, Email: michi81@ufl.edu, michi81@phhp.ufl.edu, Department of Speech, Language, and Hearing Sciences, University of Florida, PO Box 117420, Gainesville, FL 32611, USA; Center for Movement Disorders and Neurorestoration, University of Florida, 3450 Hull Rd, Gainesville, FL 32607, USA.
Alexandra E. Brandimore, Email: aessman@ufl.edu, Department of Speech, Language, and Hearing Sciences, University of Florida, PO Box 117420, Gainesville, FL 32611, USA; Center for Movement Disorders and Neurorestoration, University of Florida, 3450 Hull Rd, Gainesville, FL 32607, USA; Brain Rehabilitation Research Center, Malcom Randall VA, 1601 SW Archer Rd, Gainesville, FL 32608, USA.
Kelly D. Foote, Email: foote@neurosurgery.ufl.edu, Department of Neurosurgery, University of Florida, 1600 SW Archer Rd, Gainesville, FL 32610, USA; Center for Movement Disorders and Neurorestoration, University of Florida, 3450 Hull Rd, Gainesville, FL 32607, USA.
Takashi Morishita, Email: takashi.morishita@neurosurgery.ufl.edu, Department of Neurosurgery, University of Florida, 1600 SW Archer Rd, Gainesville, FL 32610, USA; Center for Movement Disorders and Neurorestoration, University of Florida, 3450 Hull Rd, Gainesville, FL 32607, USA.
Dennis Chen, Email: qicong123@ufl.edu, Center for Movement Disorders and Neurorestoration, University of Florida, 3450 Hull Rd, Gainesville, FL 32607, USA.
Karen W. Hegland, Email: kwheeler@ufl.edu, Department of Speech, Language, and Hearing Sciences, University of Florida, PO Box 117420, Gainesville, FL 32611, USA; Center for Movement Disorders and Neurorestoration, University of Florida, 3450 Hull Rd, Gainesville, FL 32607, USA.
Michael S. Okun, Email: okun@neurology.ufl.edu, Department of Neurology, University of Florida, 2000 SW Archer Rd, Gainesville, FL 32610, USA; Center for Movement Disorders and Neurorestoration, University of Florida, 3450 Hull Rd, Gainesville, FL 32607, USA.
References
- 1.Zahodne LB, Okun MS, Foote KD, Fernandez HH, Rodriguez RL, Kirsch-Darrow L, et al. Cognitive declines one year after unilateral deep brain stimulation surgery in Parkinson’s disease: a controlled study using reliable change. Clin Neuropsychol. 2009;23:385–405. doi: 10.1080/13854040802360582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol. 2005;62:554–560. doi: 10.1001/archneur.62.4.554. [DOI] [PubMed] [Google Scholar]
- 3.Okun MS, Gallo BV, Mandybur G, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomised controlled trial. Lancet Neurol. 2012;11:140–149. doi: 10.1016/S1474-4422(11)70308-8. [DOI] [PubMed] [Google Scholar]
- 4.Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362:2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- 5.Hariz MI, Rehncrona S, Quinn NP, Speelman JD, Wensing C, et al. Multicenter study on deep brain stimulation in Parkinson’s disease: an independent assessment of reported adverse events at 4 years. Mov Disord. 2008;23:416–421. doi: 10.1002/mds.21888. [DOI] [PubMed] [Google Scholar]
- 6.Rocchi L, Carlson-Kuhta P, Chiari L, Burchiel KJ, Hogarth P, Horak FB. Effects of deep brain stimulation in the subthalamic nucleus or globus pallidus internus on step initiation in Parkinson disease: laboratory investigation. J Neurosurg. 2012;117:1141–1149. doi: 10.3171/2012.8.JNS112006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues JP, Walters SE, Watson P, Stell R, Mastaglia FL. Globus pallidus stimulation improves both motor and nonmotor aspects of quality of life in advanced Parkinson’s disease. Mov Disord. 2007;22:1866–1870. doi: 10.1002/mds.21427. [DOI] [PubMed] [Google Scholar]
- 8.Zahodne LB, Okun MS, Foote KD, Fernandez HH, Rodriguez RL, Wu SS, et al. Greater improvement in quality of life following unilateral deep brain stimulation surgery in the globus pallidus as compared to the subthalamic nucleus. J Neurol. 2009;256:1321–1329. doi: 10.1007/s00415-009-5121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Videnovic A, Metman LV. Deep brain stimulation for Parkinson’s disease: prevalence of adverse events and need for standardized reporting. Mov Disord. 2008;23:343–349. doi: 10.1002/mds.21753. [DOI] [PubMed] [Google Scholar]
- 10.Drapier D, Drapier S, Sauleau P, Haegelen C, Raoul S, Biseul I, et al. Does subthalamic nucleus stimulation induce apathy in Parkinson’s disease? J Neurol. 2006;253:1083–1091. doi: 10.1007/s00415-006-0177-0. [DOI] [PubMed] [Google Scholar]
- 11.Funkiewiez A, Ardouin C, Caputo E, Krack P, Fraix V, Klinger H, et al. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. J Neurol Neurosurg Psychiatr. 2004;75:834–839. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troche MS, Brandimore AE, Foote KD, Okun MS. Swallowing and deep brain stimulation in Parkinson’s disease: a systematic review. Parkinsonism Relat Disord. 2013;19:783–788. doi: 10.1016/j.parkreldis.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung SJ, Jeon SR, Kim SR, Sung YH, Lee MC. Bilateral effects of unilateral subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Eur Neurol. 2006;56:127–132. doi: 10.1159/000095704. [DOI] [PubMed] [Google Scholar]
- 14.Taba HA, Wu SS, Foote KD, Hass CJ, Fernandez HH, Malaty IA, et al. A closer look at unilateral versus bilateral deep brain stimulation: results of the National Institutes of Health COMPARE cohort. J Neurosurg. 2010;113:1224–1229. doi: 10.3171/2010.8.JNS10312. [DOI] [PubMed] [Google Scholar]
- 15.Alberts JL, Hass CJ, Vitek JL, Okun MS. Are two leads always better than one: an emerging case for unilateral subthalamic deep brain stimulation in Parkinson’s disease. Exp Neurol. 2008;214:1–5. doi: 10.1016/j.expneurol.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood J. A penetration–aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 17.McHorney CA, Robbins J, Lomax K, Rosenbek JC, Chignell K, Kramer AE, et al. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III. Documentation of reliability and validity. Dysphagia. 2002;17:97–114. doi: 10.1007/s00455-001-0109-1. [DOI] [PubMed] [Google Scholar]
- 18.Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A, et al. Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol. 2011;68:165. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorell JM, Johnson CC, Rybicki BA. Parkinson’s disease and its comorbid disorders: an analysis of Michigan mortality data, 1970 to 1990. Neurology. 1994;44:1865–1868. doi: 10.1212/wnl.44.10.1865. [DOI] [PubMed] [Google Scholar]
- 20.Ciucci MR, Barkmeier-Kraemer JM, Sherman SJ. Subthalamic nucleus deep brain stimulation improves deglutition in Parkinson’s disease. Mov Disord. 2008;23:676–683. doi: 10.1002/mds.21891. [DOI] [PubMed] [Google Scholar]
- 21.Silbergleit AK, Lewitt P, Junn F, Schultz LR, Collins D, Beardsley T, et al. Comparison of dysphagia before and after deep brain stimulation in Parkinson’s disease. Mov Disord. 2012;27:1763–1768. doi: 10.1002/mds.25259. [DOI] [PubMed] [Google Scholar]
- 22.Kulneff L, Sundstedt S, Olofsson K, van Doorn J, Linder J, Nordh E, et al. Deep brain stimulation—effects on swallowing function in Parkinson’s disease. Acta Neurol Scand. 2012;127:329–336. doi: 10.1111/ane.12019. [DOI] [PubMed] [Google Scholar]
- 23.Lengerer S, Kipping J, Rommel N, Weiss D, Breit S, Gasser T, et al. Deep-brain-stimulation does not impair deglutition in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:847–853. doi: 10.1016/j.parkreldis.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Wolz M, Hauschild J, Fauser M, Klingelhofer L, Reichmann H, Storch A. Immediate effects of deep brain stimulation of the subthalamic nucleus on nonmotor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:994–997. doi: 10.1016/j.parkreldis.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Kitashima A, Umemoto G, Tsuboi Y, Higuchi MA, Baba Y, Kikuta T. Effects of subthalamic nucleus deep brain stimulation on the swallowing function of patients with Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:480–482. doi: 10.1016/j.parkreldis.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Ramig LO, Fox C, Sapir S. Parkinson’s disease: speech and voice disorders and their treatment with the Lee Silverman Voice Treatment. Semin Speech Lang. 2004;25:169–180. doi: 10.1055/s-2004-825653. [DOI] [PubMed] [Google Scholar]
- 27.Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 2004;27:585–588. doi: 10.1016/j.tins.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Benarroch EE. Pedunculopontine nucleus: functional organization and clinical implications. Neurology. 2013;80:1148–1155. doi: 10.1212/WNL.0b013e3182886a76. [DOI] [PubMed] [Google Scholar]
- 29.Carpenter MB. Core text of neuroanatomy. 3rd ed. Baltimore: Williams & Wilkins; 1985. [Google Scholar]
- 30.St George RJ, Carlson-Kuhta P, Burchiel KJ, Hogarth P, Frank N, Horak FB. The effects of subthalamic and pallidal deep brain stimulation on postural responses in patients with Parkinson disease. J Neurosurg. 2012;116:1347–1356. doi: 10.3171/2012.2.JNS11847. [DOI] [PMC free article] [PubMed] [Google Scholar]