Abstract
During cytokinesis the cytoplasm of a cell is divided to form two daughter cells. In animal cells, the existing plasma membrane is first constricted and then abscised to generate two individual plasma membranes. Plant cells on the other hand divide by forming an interior dividing wall, the so-called cell plate, which is constructed by localized deposition of membrane and cell wall material. Construction starts in the centre of the cell at the locus of the mitotic spindle and continues radially towards the existing plasma membrane. Finally the membrane of the cell plate and plasma membrane fuse to form two individual plasma membranes. Two microtubule-based cytoskeletal networks, the phragmoplast and the pre-prophase band (PPB), jointly control cytokinesis in plants. The bipolar microtubule array of the phragmoplast regulates cell plate deposition towards a cortical position that is templated by the ring-shaped microtubule array of the PPB. In contrast to most animal cells, plants do not use centrosomes as foci of microtubule growth initiation. Instead, plant microtubule networks are striking examples of self-organizing systems that emerge from physically constrained interactions of dispersed microtubules. Here we will discuss how microtubule-based activities including growth, shrinkage, severing, sliding, nucleation and bundling interrelate to jointly generate the required ordered structures. Evidence mounts that adapter proteins sense the local geometry of microtubules to locally modulate the activity of proteins involved in microtubule growth regulation and severing. Many of the proteins and mechanisms involved have roles in other microtubule assemblies as well, bestowing broader relevance to insights gained from plants.
Keywords: Microtubule, Phragmoplast, Cytoskeleton, Plant, Cortical array, Cytokinesis
Introduction
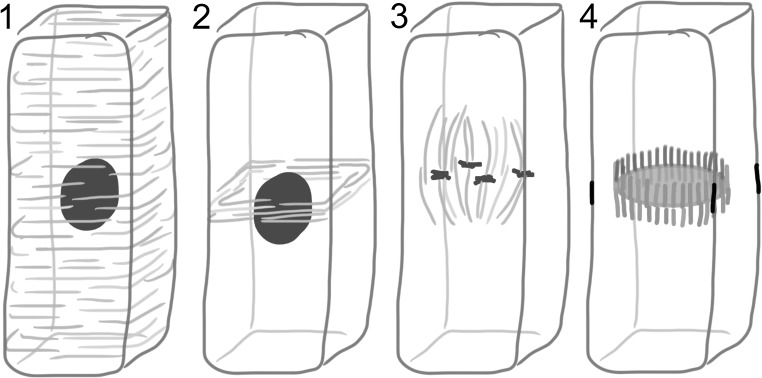
In the course of the cell cycle of higher plants, four microtubule networks in succession control plant cell growth and division (Wasteneys 2002) (Fig. 1). The location and orientation of the future division plane is established already before mitosis itself starts. First, microtubules associate to the plasma membrane and form an aligned cortical array (CA). Then, during the plant-specific pre-prophase a dense ring-like structure of microtubules develops out of the cortical array. The microtubule orientation of this pre-prophase band (PPB) is preserved from the CA (Cleary et al. 1992; Vos et al. 2004). The still intact nucleus is located at the ring’s centre and dictates the construction of the mitotic spindle during the disassembly of the PPB (Vos et al. 2008; Masoud et al. 2013). Finally the bipolar microtubule array of the spindle is reused in the phragmoplast (Lee and Liu 2013). The phragmoplast deposits wall material containing vesicles to construct a new membrane enclosed cross-wall, termed the cell plate, in the cell centre. The phragmoplast gradually expands in the direction of the plasma membrane and becomes ring-shaped after microtubules at completed sections in the middle of the cell plate disassemble. The locus of the connection of the cell plate to the plasma membrane coincides with the position of the erstwhile PPB that has left cortical markers, making the PPB therefore a 100 % marker for the division plane (reviewed in Van Damme et al. 2007; Van Damme 2009; Müller et al. 2009; Rasmussen et al. 2013; Smith 2001). Communication between the cytoplasmic phragmoplast and cortical remnants of the PPB allow for control over symmetric and asymmetric cell divisions (Gallagher and Smith 1999; Rasmussen et al. 2013). How the four networks exchange relevant positional and orientational information is summarized in Fig. 1.
Fig. 1.
Spatial relations between microtubule networks in plant cell division. The cortical microtubule array 1 develops into the dense ring-like pre-prophase band 2 whose location coincides with the cell nucleus blue. Other microtubules that are initially formed around the nuclear envelope develop into a mitotic spindle that segregates sister chromatids 3. The phragmoplast 4 forms out of the spindle remnant and expands radially towards the location of the erstwhile pre-prophase band black leaving a finished cell plate in its wake yellow. (Color figure online)
A striking feature of all microtubule networks in land plants, including the mitotic spindle, is that their organization occurs in absence of centrosome-like organelles (Murata et al. 2007). Most microtubules in animal cells first appear at centrosomes, typically associated with the nuclear envelope, and then grow outwards. In contrast, microtubule growth in plants is initiated exclusively at spatially dispersed sites. The initiation, or nucleation, is catalysed by specialized nucleation complexes, and is the first of a number of microtubule-associated activities that occur in almost all networks (Teixidó-Travesa et al. 2012). After nucleation, periods of microtubule growth and shrinkage succeed each other in a GTP-dependent process termed dynamic instability (Gardner et al. 2013). Switches between the two states are termed catastrophes and rescues respectively. Dynamic instability is most pronounced at the so-called plus-end of microtubules, but also the minus-end can have different states, being primarily static or shrinking (Ehrhardt and Shaw 2006). Throughout the eukaryotic kingdom, the dynamic state of microtubules is controlled by a host of conserved proteins (Subramanian and Kapoor 2012). Moreover, lateral associations between microtubules are facilitated by bundling proteins and specialized motor proteins. The latter activity can generate a force to slide microtubules along each other (Kapitein et al. 2005; Janson et al. 2007; Peterman and Scholey 2009). Finally, existing microtubules can become severed to form two new microtubules (Roll-Mecak and McNally 2010). To mechanistically understand the formation of ordered microtubule networks for plant cell division it will be key to investigate microtubule nucleation, dynamics, bundling, sliding, and severing in an integral manner. Here, we will focus on recent progress on two plant-specific networks, the CA and the phragmoplast and will discuss how feedback between different microtubule activities is organized.
Division plane selection
As already mentioned, the orientation of the division plane is determined by the PPB, which in turn inherits its orientation from the CA. The CA consists of microtubules associated to the inner face of the plasma membrane and is linked to the cell wall by an as yet unidentified molecular component. It is by now well established that microtubules of the CA are able to self-organize into a highly aligned state, whose default orientation is transverse to the growth direction of the cell (Ehrhardt and Shaw 2006). The driving force for this ordering process are collisions between growing plus-ends and other obstructing microtubules (Dixit and Cyr 2004). In such a collision the incoming growing microtubule can either bend and continue to grow alongside the obstructing microtubule—an event called zippering or entrainment—, switch to a shrinking state—an event called induced catastrophe or catastrophic collision—, or slip over the obstruction and continue to grow in the same direction—an event called cross-over —. A number of modelling studies have shown that, provided there are enough of these collisions, requiring e.g. a high enough rate of nucleation of new microtubules, spontaneous alignment of microtubules will occur (Allard et al. 2010b; Eren et al. 2010; Tindemans et al. 2010; Hawkins et al. 2010). There is still some debate on which of these collision outcomes is necessary and/or sufficient to explain the ordering process, but it is clear that induced catastrophes by themselves are a sufficient cause (Deinum and Mulder 2013). The relative probabilities of collision outcomes were shown to be dependent on the interaction angle and differ between cell types (Dixit and Cyr 2004; Wightman and Turner 2007). Control over collision outcomes may well involve microtubule bundling proteins and physical forces that are generated during collisions of cortically adhered microtubules (Allard et al. 2010a; Portran et al. 2013). There is also increasing evidence that selective severing of microtubules at cross-over sites by the severing protein katanin, in conjunction with adaptor proteins such as SPIRAL2, plays an important role in establishing and maintaining proper organization of the CA (Wightman and Turner 2007; Wightman et al. 2013; Zhang et al. 2013; Lindeboom et al. 2013).
The mechanism of relative alignment in itself is not sufficient to explain the lining up of microtubules in a particular direction for division plane selection. Here it turns out there is a key role for cell shape, and more specifically for the “edges’’ between the different cell faces in coaxing alignment into a particular direction. A notable physical difference between these edges is their radius of curvature. Cell edges that are generated when a new cell wall is constructed during cytokinesis are relatively sharp. Older edges are more rounded. Ambrose et al. (Ambrose et al. 2011) have shown that microtubules impinging on sharp edges have a very high probability (~90 %) of suffering a catastrophe. For the remaining edges these edge-induced catastrophes are more rare, occurring roughly in 25 % per cent of all cases. This difference is sufficient to ensure that the preferred orientation of the CA becomes parallel to the sharp edges (Deinum 2013). Clearly, to form a division plane in a perpendicular direction, as observed for cell patterning during plant development, this strong selection must be overridden. Here there appears to be a role for the conserved microtubule associated protein CLASP that promotes the microtubule growth phase. In this case CLASP is seen to be localized to specific edges and to decorate microtubule bundles that cross-over from one cell face onto an adjacent cell face (Ambrose et al. 2011). CLASP may therefore guide microtubule growth through hard-to-take corners and enable the formation of CAs that are perpendicular to sharp cell edges.
The orientation-maintaining transition from the ordered CA to the PPB is as yet still poorly understood. One possibility that has been suggested is a “search-and-capture” mechanism, by which a local propensity to bundle and stabilize microtubules at the locus of the PPB, possibly under control of the nucleus, serves to concentrate the microtubules to this region, either by depleting the free tubulin pool in the rest of the cortex, or by incorporating non-transverse microtubules through the zippering mechanism (Vos et al. 2004).
Cell plate formation
At the onset of mitosis a basket of microtubules appears around the still intact nuclear envelope and develops into a bipolar, noncentrosomal spindle after the nuclear envelope is broken down (Jensen and Bajer 1973; De Mey et al. 1982; Vos et al. 2008; Nakaoka et al. 2012; Masoud et al. 2013). The mechanism of centrosome-independent spindle formation in plants is very relevant to other eukaryotic cells since their spindles more generally contain a subset of microtubules that are generated independently of centrosomes (Wainman et al. 2009; Uehara and Goshima 2010). Spindles comprise two mirrored sets of polarized microtubules with plus-ends pointing towards the centre (Dhonukshe et al. 2006). Microtubule plus-ends either connect to their counterparts of the other spindle half or link each chromatid to the spindle apparatus via the multiprotein kinetochore complex. Whereas the latter microtubules shorten during sister chromatid segregation (Jensen and Bajer 1973), the former persist and form the template for the phragmoplast (Fig. 2). As such, phragmoplast position, orientation and bipolarity are inherited from the spindle apparatus. These events are very much reminiscent of the animal cytokinetic microtubule array which also develops from remaining spindle microtubules (Glotzer 2009). During cellularization of certain multinucleated plant cells, spindles do not precede phragmoplast assembly. However, here another bipolar microtubule array is reported to form between nuclear surfaces to act as a precursor for the phragmoplast (Van Lammeren et al. 1985; reviewed in Otegui and Staehelin 2000; Pickett-heaps et al. 1999; De Storme and Geelen 2013). Thus, in general, the phragmoplast maintains the bipolarity that is established by a preceding microtubule structure.
Fig. 2.
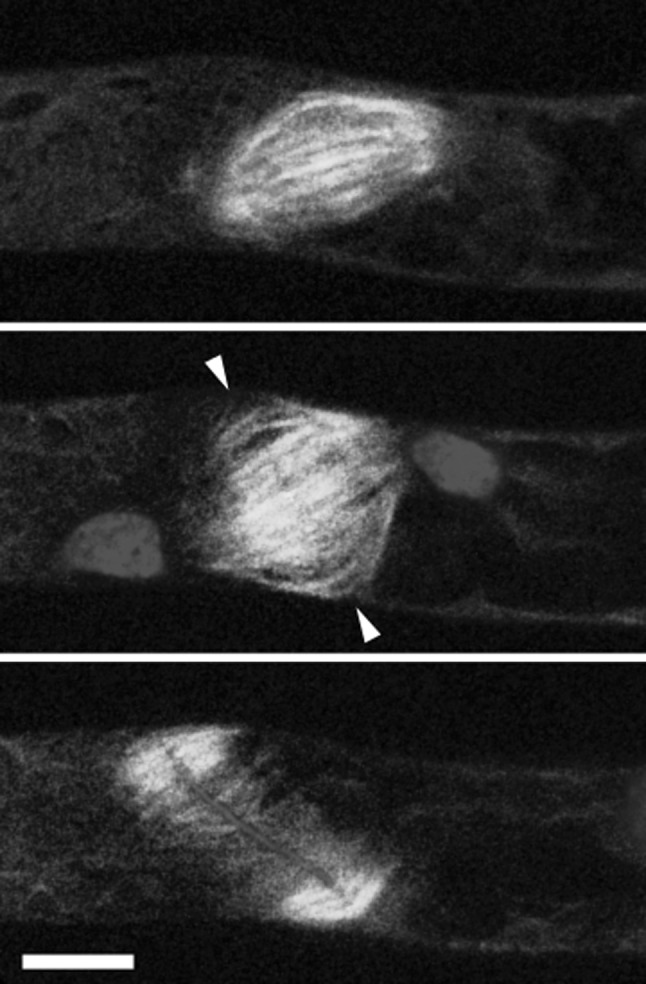
Cytokinesis visualized by live cell imaging in the moss Physcomitrella patens. Microtubules are shown in green and chromatin and cell-plate material in red. Chromatids are segregated towards spindle poles and the remaining microtubules of the mitotic spindle (top) are used as template for the initial phragmoplast (middle; 6 min later). The reforming daughter nuclei are seen at the phragmoplast poles while accumulation of cell plate material is visible in the central plane of the phragmoplast where microtubules of opposite poles form antiparallel overlaps (arrowheads). Fifteen minutes later the central region of the cell plate has matured and the phragmoplast has expanded laterally (bottom). Microtubules were visualized using GFP fused to α-tubulin (Hiwatashi et al. 2008), chromatin using mRFP fused to histone2B (Nakaoka et al. 2012) and the membranous material delivered to the cell plate with the lipophilic styryl dye FM4-64. The scale bar indicates 10 µm
A new cell wall section is constructed at the equatorial plane of the phragmoplast (Fig. 2), but how are the factors involved in cell wall assembly targeted to this site? Since microtubule plus-ends point towards the phragmoplast centre, an attractive targeting mechanism is directed transport of vesicular cargo along microtubules towards the division plane (Euteneuer and Mcintosh 1980; Lee et al. 2001; reviewed in Mcmichael and Bednarek 2013). A subset of microtubules not only terminate near the phragmoplast centre, but extend across the division plane and meet with the opposing set to form short, antiparallel overlaps that coincide with the site of cell plate assembly. A key player for proper formation of these overlaps is the microtubule cross-linker MAP65, without which the two opposing sets of microtubules are not properly engaged, ultimately leading to cytokinesis failure (Müller et al. 2004; Ho et al. 2011a; Kosetsu et al. 2013). Besides aiding in maintenance of the overall bipolar structure of the phragmoplast, these cross-linked antiparallel overlaps may provide a very localized scaffold for directing cytokinetic processes, much akin to the stem body/Flemming body in animal cells (Otegui et al. 2005; Fuller et al. 2008; Glotzer 2009). Such a notion is supported by the delayed cell plate build-up when MAP65 is down-regulated even though a seemingly normal, bipolar phragmoplast is present (Kosetsu et al. 2013). Microtubule overlaps therefore seem to be important for both the structural integrity of the phragmoplast and the proper recruitment of cell wall material.
To act as a robust scaffold and hence to facilitate building of a well-defined cell plate, the position and size of overlaps would require strict control. Recent work using various model systems has provided insight into possible mechanisms for length regulation of overlaps in bipolar microtubule structures (Subramanian and Kapoor 2012). A common concept encountered in these cases is that the dynamic parameters of microtubules engaged in an overlap are locally altered by an effector protein recruited to the overlap by microtubule cross-linkers of the Ase1/PRC1/MAP65 family. For instance, in yeast and human spindles the microtubule rescue factor CLASP is recruited (Bratman and Chang 2007; Liu et al. 2009; Al-Bassam et al. 2010) and in case of the animal midzone the growth-inhibiting kinesin KIF4 is recruited by PRC1 (Bieling et al. 2010; Hu et al. 2011; Nunes Bastos et al. 2013). Large fluctuations in microtubule length are thus generally supressed in overlaps. Plus-end-directed motors at overlaps, some of which are possibly also recruited by cross-linkers, may furthermore contribute to length control by sliding microtubules apart (Nislow et al. 1992; Kapitein et al. 2005; Fu et al. 2009). In plants, microtubule growth in overlaps in combination with sliding by unknown motor proteins forms a plausible explanation for the continuous outward-directed flux of microtubule mass observed in both spindles and phragmoplasts (Asada et al. 1991; Dhonukshe et al. 2006; Murata et al. 2013). In the phragmoplast several kinesin motors have been identified that reside at the overlap zone and function in phragmoplast assembly and maintenance (Lee and Liu 2000, 2013 Lee et al. 2007; Hiwatashi et al. 2008; Ho et al. 2011a). Yet, how they contribute to the forces at play in the antiparallel overlaps of the phragmoplast is still not as well conceived as in other systems. It will be intriguing to learn how microtubule sliding, bundling and dynamics jointly control overlap length. The manner in which multiple overlaps are aligned in space is yet another intriguing and open question.
What drives the radial expansion of the phragmoplast towards the maternal cell wall? Phragmoplast microtubules themselves exhibit no or very little lateral movement (Yasuhara et al. 1993). Instead, existing microtubules are thought to disassemble at completed cell plate sections while newly nucleated microtubules form new overlaps at the leading edge, propagating the bipolar configuration laterally (Murata et al. 2013). In agreement, genetic or antibody interference of components of the microtubule-nucleation pathway severely inhibits phragmoplast expansion (Ho et al. 2011b; Hotta et al. 2012; Nakaoka et al. 2012; Murata et al. 2013). Temporal control over phragmoplast expansion likely incorporates feedback from the state of cell plate assembly (Yasuhara and Shibaoka 2000) and may be achieved by a MAPK pathway that posttranslationally modifies MAP65 (Sasabe et al. 2006a, b; Sasabe and Machida 2012).
Aforementioned microtubule nucleation factors localize throughout the phragmoplast with the exception of the equatorial region (Liu et al. 1993; Zeng et al. 2009; Ho et al. 2011b; Nakaoka et al. 2012; Murata et al. 2013). Recent observations indicate that they may primarily reside on microtubule bundles that run perpendicular to the division plane (Murata et al. 2013). In the plant cortical array microtubule-dependent nucleation occurs at approximately 40° with respect to the template microtubule (Murata et al. 2005; Murata and Hasebe 2007; Chan et al. 2009). Though not yet directly shown, it is likely that microtubule branching angles in the phragmoplast also occur within a certain regime to reinforce its bipolar organization. Using meiotic frog extracts such a mechanism in which existing microtubules form a template for the ordered branching of new microtubules was elegantly demonstrated to operate in animal spindles (Petry et al. 2013). The ordered branching of microtubules may therefore be an important factor for setting up and maintaining network polarity.
Ultimately, the direction of expansion of the phragmoplast should be towards the sites on the parental cell membrane, occupied earlier by the PPB (reviewed in Van Damme et al. 2007; Van Damme 2009; Müller et al. 2009; Rasmussen et al. 2013; Smith 2001). Early centrifugation experiments have demonstrated that dislocated phragmoplasts are able to move towards the PPB-marked division zone, yet the nature of the driving forces remains elusive to date (Ota 1961; Mineyuki and Gunning 1990). To induce changes in phragmoplast position, the marked cortical area could function as a signaling beacon, producing a gradient of a certain signaling molecule that affects phragmoplast dynamics. Alternatively, cytoskeletal filaments could form a physical link between the marked cortex and the phragmoplast (Lloyd and Traas 1988; Sano et al. 2005; Van Damme et al. 2007). The selective presence or absence of various cytoskeletal-related proteins at the PPB-marked cortex is in support of such a linking mechanism. Proteins that are differentially located at the PPB-marked cortex include kinesin motors and actin itself (Cleary et al. 1992; Vanstraelen et al. 2006; Müller et al. 2006; Van Damme et al. 2007; Miki et al. 2014). The identification of various other molecules that tune phragmoplast expansion will help to unravel the signaling network through which a flagged cortical domain can guide cell plate formation (Cleary and Smith 1998; Müller et al. 2006; Walker et al. 2007; Xu et al. 2008).
Outlook
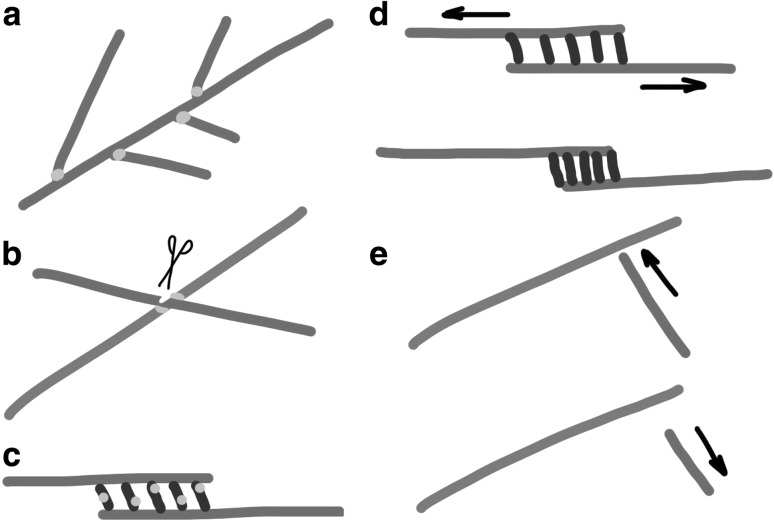
Microtubules in eukaryotic cells exhibit very diverse network geometries all involving a recurring set of activities: nucleation, dynamic instability, bundling, severing and sliding. Recent work on microtubule networks has provided insight into how feedback between these activities is organized through adapter proteins (Fig. 3a–c). Existing microtubules for example trigger nucleation of new microtubules through augmin-mediated recruitment of microtubule nucleation complexes leading to local microtubule amplification (Clausen and Ribbeck 2007; Petry et al. 2013). Directionally biased microtubule-dependent nucleation can reinforce an existing polarity (Smertenko et al. 2011; Kamasaki et al. 2013). Another example of feedback involves the dynamic instability of microtubules in bundles, which is adapted locally through recruitment of CLASP or KIF4 by bundling proteins (Bratman and Chang 2007; Bieling et al. 2010). Bundling proteins were also reported to bind motors, generating crosstalk between bundling and sliding action (Fu et al. 2009). Microtubule cross-overs may furthermore trigger microtubule severing through SPIRAL2-mediated modulation of katanin activity (Wightman et al. 2013). In general, adapter proteins may sense the local configuration of microtubules to provide feedback on microtubule network layout. Examples are MAP65/Ase1/PRC1, which bind to antiparallel-oriented microtubules, and SPIRAL2, which is recruited to sites of microtubule cross-over (Janson et al. 2007; Subramanian et al. 2013; Wightman et al. 2013). The use of such adapter proteins may enable a conserved set of proteins to be efficiently used in various network layouts and may facilitate fast switching between network architectures as observed in plant cell division. Other forms of feedback may be more physical in origin (Fig. 3d, e), such as the packing of bundling proteins within the overlaps of sliding microtubules or the induction of catastrophes through collisions with neighbouring microtubules (Allard et al. 2010a; Braun et al. 2011). Cell geometry plays an additional role as shown by local cell wall curvature which may invoke microtubule catastrophes (Ambrose and Wasteneys 2012). Work on diverse microtubule networks in animal, yeast, and plant cells, and insights gained from reconstituted in vitro systems highlight that the organization of networks can only be understood if multiple microtubule associated activities are integrated into our models.
Fig. 3.
Feedback between microtubule organization and microtubule activities a Augmin-mediated branching of new microtubules from the sides of existing microtubules. b Points of microtubule crossover in plants are sites of microtubule severing by katanin, possibly regulated by SPIRAL2. c Crosslinking proteins in between overlapping microtubules recruit proteins that locally regulate dynamic instability or drive microtubule sliding. d Crosslinkers confined in between sliding microtubules are driven together by convening microtubule ends. e Existing microtubules obstruct the growth of incoming microtubules triggering shrinkage in the near two-dimensional geometry of the plant cortex
Future insights into the organization and functioning of plant microtubule networks for cell division will be aided by new developments on model systems and light microscopy. In particular the continuing development of plane-illumination microscopy will allow for the long-term visualization of 3 dimensional microtubule networks within developing plant tissues (Maizel et al. 2011). Cell division in higher plants occurs in complex meristematic tissues in which microscopic observations of cell division are often hindered by several layers of overlaying cells. Lower plants exhibit simpler body plans and model organisms like the liverwort Marchantia polymorpha and the moss Physcomitrella patens enable the observation of cell division without interference of other cells. Moreover, these organisms have a prominent haploid phase in their life cycle and efficient homologous recombination, which both greatly simplify the generation of controlled gene knockouts, gene alterations, and fluorescent reporter gene fusions (Ishizaki et al. 2013; Miki et al. 2014). These assets allow for large-scale studies into gene functioning and gene product localization that will greatly facilitate mechanistic studies. Moreover, they allow for an evolutionary view on the conservation and diversification of microtubule activities used for network construction. It emerges that some protein functions cannot be generalized from metazoans to plants, possibly reflecting the different challenges faced during cell division (Miki et al. 2014).
Microtubule arrays organized throughout cell division allow plants to organize a fascinating variety of tissues, which are intensely utilized by mankind as food, construction material, and source of renewable energy. Moreover, plants provide us with an intriguing diversity of microtubule network layouts that may teach us fundamental lessons about self-organization in cell biology.
Acknowledgments
We thank Gohta Goshima for helpful discussions and the GFP-tubulin / histone-mRFP moss line. The work of BM is part of the research programme of the Foundation for Fundamental Research on Matter (FOM), which is part of the Netherlands Organisation for Scientific Research (NWO). JK and MEJ are supported by the Human Frontier Science Program.
References
- Al-Bassam J, Kim H, Brouhard G, et al. CLASP promotes microtubule rescue by recruiting tubulin dimers to the microtubule. Dev Cell. 2010;19:245–258. doi: 10.1016/j.devcel.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard JF, Ambrose JC, Wasteneys GO, Cytrynbaum EN. A mechanochemical model explains interactions between cortical microtubules in plants. Biophys J. 2010;99:1082–1090. doi: 10.1016/j.bpj.2010.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard JF, Wasteneys GO, Cytrynbaum EN. Mechanisms of self-organization of cortical microtubules in plants revealed by computational simulations. Mol Biol Cell. 2010;21:278–286. doi: 10.1091/mbc.E09-07-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose C, Wasteneys GO. Nanoscale and geometric influences on the microtubule cytoskeleton in plants: thinking inside and outside the box. Protoplasma. 2012;249(Suppl):S69–S76. doi: 10.1007/s00709-011-0334-x. [DOI] [PubMed] [Google Scholar]
- Ambrose C, Allard JF, Cytrynbaum EN, Wasteneys GO. A CLASP-modulated cell edge barrier mechanism drives cell-wide cortical microtubule organization in Arabidopsis. Nat Commun. 2011;2:430. doi: 10.1038/ncomms1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada T, Sonobe S, Shibaoka H. Microtubule translocation in the cytokinetic apparatus of cultured tobacco cells. Nature. 1991;350:238–241. doi: 10.1038/350238a0. [DOI] [Google Scholar]
- Bieling P, Telley I, Surrey T. A minimal midzone protein module controls formation and length of antiparallel microtubule overlaps. Cell. 2010;142:420–432. doi: 10.1016/j.cell.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Bratman SV, Chang F. Stabilization of overlapping microtubules by fission yeast CLASP. Dev Cell. 2007;13:812–827. doi: 10.1016/j.devcel.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M, Lansky Z, Fink G, et al. Adaptive braking by Ase1 prevents overlapping microtubules from sliding completely apart. Nat Cell Biol. 2011;13:1259–1264. doi: 10.1038/ncb2323. [DOI] [PubMed] [Google Scholar]
- Chan J, Sambade A, Calder G, Lloyd C. Arabidopsis cortical microtubules are initiated along, as well as branching from, existing microtubules. Plant Cell. 2009;12:2298–2306. doi: 10.1105/tpc.109.069716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Ribbeck K. Self-organization of anastral spindles by synergy of dynamic instability, autocatalytic microtubule production, and a spatial signaling gradient. PLoS ONE. 2007;2:e244. doi: 10.1371/journal.pone.0000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary AL, Smith LG. The Tangled1 gene is required for spatial control of cytoskeletal arrays associated with cell division during maize leaf development. Plant Cell. 1998;10:1875–1888. doi: 10.1105/tpc.10.11.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary AL, Gunning BES, Wasteneys GO, Hepler PK. Microtubule and F-actin dynamics at the division site in living Tradescantia stamen hair cells. J Cell Sci. 1992;103:977–988. [Google Scholar]
- De Mey J, Lambert AM, Bajer AS, et al. Visualization of microtubules in interphase and mitotic plant cells of Haemanthus endosperm with the immuno-gold staining method. Proc Natl Acad Sci U S A. 1982;79:1898–1902. doi: 10.1073/pnas.79.6.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N, Geelen D. Cytokinesis in plant male meiosis. Plant Signal Behav. 2013;8:e23394. doi: 10.4161/psb.23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinum EE (2013) Simple models for complex questions on plant development (Chapter 9). Dissertion, Wageningen University
- Deinum EE, Mulder BM. Modelling the role of microtubules in plant cell morphology. Curr Opin Plant Biol. 2013;16:688–692. doi: 10.1016/j.pbi.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Vischer N, Gadella TWJ. Contribution of microtubule growth polarity and flux to spindle assembly and functioning in plant cells. J Cell Sci. 2006;119:3193–3205. doi: 10.1242/jcs.03048. [DOI] [PubMed] [Google Scholar]
- Dixit R, Cyr R. Encounters between dynamic cortical microtubules promote ordering of the cortical array through angle-dependent modifications of microtubule behavior. Plant Cell. 2004;16:3274–3284. doi: 10.1105/tpc.104.026930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt DW, Shaw SL. Microtubule dynamics and organization in the plant cortical array. Annu Rev Plant Biol. 2006;57:859–875. doi: 10.1146/annurev.arplant.57.032905.105329. [DOI] [PubMed] [Google Scholar]
- Eren EC, Dixit R, Gautam N. A 3D computer simulation model reveals the mechanisms for self-organization of plant cortical microtubules into oblique arrays. Mol Biol Cell. 2010;21:2674–2684. doi: 10.1091/mbc.E10-02-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euteneuer U, Mcintosh JR. Polarity of midbody and phragmoplast microtubules. J Cell Biol. 1980;87:509–515. doi: 10.1083/jcb.87.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C, Ward JJ, Loiodice I, et al. Phospho-regulated interaction between kinesin-6 Klp9p and microtubule bundler Ase1p promotes spindle elongation. Dev Cell. 2009;17:257–267. doi: 10.1016/j.devcel.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller BG, Lampson M, Foley E, et al. Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature. 2008;453:1132–1136. doi: 10.1038/nature06923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher K, Smith LG. Discordia mutations specifically misorient asymmetric cell divisions during development of the maize leaf epidermis. Development. 1999;126:4623–4633. doi: 10.1242/dev.126.20.4623. [DOI] [PubMed] [Google Scholar]
- Gardner MK, Zanic M, Howard J. Microtubule catastrophe and rescue. Curr Opin Cell Biol. 2013;25:14–22. doi: 10.1016/j.ceb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M. The 3Ms of central spindle assembly: microtubules, motors and MAPs. Nat Rev Mol Cell Biol. 2009;10:9–20. doi: 10.1038/nrm2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R, Tindemans S, Mulder B. Model for the orientational ordering of the plant microtubule cortical array. Phys Rev E. 2010 doi: 10.1103/PhysRevE.82.011911. [DOI] [PubMed] [Google Scholar]
- Hiwatashi Y, Obara M, Sato Y, et al. Kinesins are indispensable for interdigitation of phragmoplast microtubules in the moss Physcomitrella patens. Plant Cell. 2008;20:3094–3106. doi: 10.1105/tpc.108.061705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C-MK, Hotta T, Guo F, et al. Interaction of antiparallel microtubules in the phragmoplast is mediated by the microtubule-associated protein MAP65-3 in Arabidopsis. Plant Cell. 2011;23:2909–2923. doi: 10.1105/tpc.110.078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C-MK, Hotta T, Kong Z, et al. Augmin plays a critical role in organizing the spindle and phragmoplast microtubule arrays in Arabidopsis. Plant Cell. 2011;23:2606–2618. doi: 10.1105/tpc.110.078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta T, Kong Z, Ho C-MK, et al. Characterization of the Arabidopsis augmin complex uncovers its critical function in the assembly of the acentrosomal spindle and phragmoplast microtubule arrays. Plant Cell. 2012;24:1494–1509. doi: 10.1105/tpc.112.096610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C-K, Coughlin M, Field CM, Mitchison TJ. KIF4 regulates midzone length during cytokinesis. Curr Biol. 2011;21:815–824. doi: 10.1016/j.cub.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki K, Johzuka-Hisatomi Y, Ishida S, et al. Homologous recombination-mediated gene targeting in the liverwort Marchantia polymorpha L. Sci Rep. 2013;3:1532. doi: 10.1038/srep01532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson ME, Loughlin R, Loïodice I, et al. Crosslinkers and motors organize dynamic microtubules to form stable bipolar arrays in fission yeast. Cell. 2007;128:357–368. doi: 10.1016/j.cell.2006.12.030. [DOI] [PubMed] [Google Scholar]
- Jensen C, Bajer A. Spindle dynamics and arrangement of microtubules. Chromosoma. 1973;44:73–89. doi: 10.1007/BF00372574. [DOI] [Google Scholar]
- Kamasaki T, O’Toole E, Kita S, et al. Augmin-dependent microtubule nucleation at microtubule walls in the spindle. J Cell Biol. 2013;202:25–33. doi: 10.1083/jcb.201304031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, Peterman EJG, Kwok BH, et al. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature. 2005;435:114–118. doi: 10.1038/nature03503. [DOI] [PubMed] [Google Scholar]
- Kosetsu K, de Keijzer J, Janson ME, Goshima G (2013) MICROTUBULE-ASSOCIATED PROTEIN65 is essential for maintenance of phragmoplast bipolarity and formation of the cell plate in Physcomitrella patens. Plant Cell 1–15. doi:10.1105/tpc.113.117432 [DOI] [PMC free article] [PubMed]
- Lee YR, Liu B. Identification of a phragmoplast-associated kinesin-related protein in higher plants. Curr Biol. 2000;10:797–800. doi: 10.1016/S0960-9822(00)00564-9. [DOI] [PubMed] [Google Scholar]
- Lee Y-RJ, Liu B. The rise and fall of the phragmoplast microtubule array. Curr Opin Plant Biol. 2013;16:757–763. doi: 10.1016/j.pbi.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Giang HM, Liu B. A novel plant kinesin-related protein specifically associates with the phragmoplast organelles. Plant Cell. 2001;13:2427–2439. doi: 10.1105/tpc.13.11.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-RJ, Li Y, Liu B. Two Arabidopsis phragmoplast-associated kinesins play a critical role in cytokinesis during male gametogenesis. Plant Cell. 2007;19:2595–2605. doi: 10.1105/tpc.107.050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeboom JJ, Nakamura M, Hibbel A, et al. A mechanism for reorientation of cortical microtubule arrays driven by microtubule severing. Science. 2013;342(6163):1245533. doi: 10.1126/science.1245533. [DOI] [PubMed] [Google Scholar]
- Liu B, Marc J, Joshi HC, Palevitz BA. A gamma-tubulin-related protein associated with the microtubule arrays of higher plants in a cell cycle-dependent manner. J Cell Sci. 1993;104(Pt 4):1217–1228. doi: 10.1242/jcs.104.4.1217. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang Z, Jiang K, et al. PRC1 cooperates with CLASP1 to organize central spindle plasticity in mitosis. J Biol Chem. 2009;284:23059–23071. doi: 10.1074/jbc.M109.009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CW, Traas JA. The role of F-actin in determining the division plane of carrot suspension cells. Drug studies. Development. 1988;102:211–221. [Google Scholar]
- Maizel A, von Wangenheim D, Federici F, et al. High-resolution live imaging of plant growth in near physiological bright conditions using light sheet fluorescence microscopy. Plant J. 2011;68:377–385. doi: 10.1111/j.1365-313X.2011.04692.x. [DOI] [PubMed] [Google Scholar]
- Masoud K, Herzog E, Chabouté M-E, Schmit A-C. Microtubule nucleation and establishment of the mitotic spindle in vascular plant cells. Plant J. 2013;75:245–257. doi: 10.1111/tpj.12179. [DOI] [PubMed] [Google Scholar]
- Mcmichael CM, Bednarek SY. Cytoskeletal and membrane dynamics during higher plant cytokinesis. New Phytol. 2013;197:1039–1057. doi: 10.1111/nph.12122. [DOI] [PubMed] [Google Scholar]
- Miki T, Naito H, Nishina M, Goshima G. Endogenous localizome identifies 43 mitotic kinesins in a plant cell. Proc Natl Acad Sci U S A. 2014;111(11):E1053–E1061. doi: 10.1073/pnas.1311243111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineyuki Y, Gunning BES. A role for preprophase bands of microtubules in maturation of new cell walls, and a general proposal on the function of preprophase band sites in cell division in higher plants. J Cell Sci. 1990;97:527–537. [Google Scholar]
- Müller S, Smertenko A, Wagner V, et al. The plant microtubule-associated protein AtMAP65-3/PLE is essential for cytokinetic phragmoplast function. Curr Biol. 2004;14:412–417. doi: 10.1016/j.cub.2004.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S, Han S, Smith LG. Two kinesins are involved in the spatial control of cytokinesis in Arabidopsis thaliana. Curr Biol. 2006;16:888–894. doi: 10.1016/j.cub.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Müller S, Wright AJ, Smith LG. Division plane control in plants: new players in the band. Trends Cell Biol. 2009;19:180–188. doi: 10.1016/j.tcb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Murata T, Hasebe M. Microtubule-dependent microtubule nucleation in plant cells. J Plant Res. 2007;120:73–78. doi: 10.1007/s10265-006-0054-z. [DOI] [PubMed] [Google Scholar]
- Murata T, Sonobe S, Baskin TI, et al. Microtubule-dependent microtubule nucleation based on recruitment of gamma-tubulin in higher plants. Nat Cell Biol. 2005;7:961–968. doi: 10.1038/ncb1306. [DOI] [PubMed] [Google Scholar]
- Murata T, Tanahashi T, Nishiyama T, et al. How do plants organize microtubules without a centrosome? J Integr Plant Biol. 2007;49:1154–1163. doi: 10.1111/j.1672-9072.2007.00545.x. [DOI] [Google Scholar]
- Murata T, Sano T, Sasabe M. Mechanism of microtubule array expansion in the cytokinetic phragmoplast. Nature. 2013;4:1967. doi: 10.1038/ncomms2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaoka Y, Miki T, Fujioka R, et al. An inducible RNA interference system in Physcomitrella patens reveals a dominant role of augmin in phragmoplast microtubule generation. Plant Cell. 2012;24:1478–1493. doi: 10.1105/tpc.112.098509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nislow C, Lombillo V, Kuriyama R, Mclntosh J. A plus-end-directed motor enzyme that moves antiparallel microtubules in vitro localizes to the interzone of mitotic spindles. Nature. 1992;359:543–547. doi: 10.1038/359543a0. [DOI] [PubMed] [Google Scholar]
- Nunes Bastos R, Gandhi SR, Baron RD, et al. Aurora B suppresses microtubule dynamics and limits central spindle size by locally activating KIF4A. J Cell Biol. 2013;202:605–621. doi: 10.1083/jcb.201301094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota T. The role of cytoplasm in cytokinesis of plant cells. Cytologia (Tokyo) 1961;26:428–447. doi: 10.1508/cytologia.26.428. [DOI] [Google Scholar]
- Otegui M, Staehelin L. Cytokinesis in flowering plants: more than one way to divide a cell. Curr Opin Plant Biol. 2000;3:493–502. doi: 10.1016/S1369-5266(00)00119-9. [DOI] [PubMed] [Google Scholar]
- Otegui MS, Verbrugghe KJ, Skop AR. Midbodies and phragmoplasts: analogous structures involved in cytokinesis. Trends Cell Biol. 2005;15:404–413. doi: 10.1016/j.tcb.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman EJG, Scholey JM. Mitotic microtubule crosslinkers: insights from mechanistic studies. Curr Biol. 2009;19:R1089–R1094. doi: 10.1016/j.cub.2009.10.047. [DOI] [PubMed] [Google Scholar]
- Petry S, Groen AC, Ishihara K, et al. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell. 2013;152:768–777. doi: 10.1016/j.cell.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett-heaps AJD, Gunning BES, Brown RC, et al. The cytoplast concept in dividing plant cells: cytoplasmic domains and the evolution of spatially organized cell division. Am J Bot. 1999;86:153–172. doi: 10.2307/2656933. [DOI] [PubMed] [Google Scholar]
- Portran D, Zoccoler M, Gaillard J, et al. MAP65/Ase1 promote microtubule flexibility. Mol Biol Cell. 2013;24:1964–1973. doi: 10.1091/mbc.E13-03-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen CG, Wright AJ, Müller S. The role of the cytoskeleton and associated proteins in determination of the plant cell division plane. Plant J. 2013;75:258–269. doi: 10.1111/tpj.12177. [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A, McNally FJ. Microtubule-severing enzymes. Curr Opin Cell Biol. 2010;22:96–103. doi: 10.1016/j.ceb.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T, Higaki T, Oda Y, et al. Appearance of actin microfilament “twin peaks” in mitosis and their function in cell plate formation, as visualized in tobacco BY-2 cells expressing GFP–fimbrin. Plant J. 2005;44:595–605. doi: 10.1111/j.1365-313X.2005.02558.x. [DOI] [PubMed] [Google Scholar]
- Sasabe M, Machida Y. Regulation of organization and function of microtubules by the mitogen-activated protein kinase cascade during plant cytokinesis. Cytoskeleton (Hoboken) 2012;69:913–918. doi: 10.1002/cm.21072. [DOI] [PubMed] [Google Scholar]
- Sasabe M, Machida Y, Yuji T, et al. MAP65: a bridge linking a MAP kinase to microtubule turnover. Curr Opin Plant Biol. 2006;9:563–570. doi: 10.1016/j.pbi.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Sasabe M, Soyano T, Takahashi Y, et al. Phosphorylation of NtMAP65-1 by a MAP kinase down-regulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes Dev. 2006;20:1004–1014. doi: 10.1101/gad.1408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smertenko AP, Piette B, Hussey PJ. The origin of phragmoplast asymmetry. Curr Biol. 2011;21:1924–1930. doi: 10.1016/j.cub.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Smith LG. Plant cell division: building walls in the right places. Nat Rev Mol Cell Biol. 2001;2:33–39. doi: 10.1038/35048050. [DOI] [PubMed] [Google Scholar]
- Subramanian R, Kapoor TM. Building complexity: insights into self-organized assembly of microtubule-based architectures. Dev Cell. 2012;23:874–885. doi: 10.1016/j.devcel.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian R, Ti S-C, Tan L, et al. Marking and measuring single microtubules by PRC1 and kinesin-4. Cell. 2013;154:377–390. doi: 10.1016/j.cell.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixidó-Travesa N, Roig J, Lüders J. The where, when and how of microtubule nucleation—one ring to rule them all. J Cell Sci. 2012;125:4445–4456. doi: 10.1242/jcs.106971. [DOI] [PubMed] [Google Scholar]
- Tindemans SH, Hawkins RJ, Mulder BM. Survival of the aligned: ordering of the plant cortical microtubule array. Phys Rev Lett. 2010 doi: 10.1103/PhysRevLett.104.058103. [DOI] [PubMed] [Google Scholar]
- Uehara R, Goshima G. Functional central spindle assembly requires de novo microtubule generation in the interchromosomal region during anaphase. J Cell Biol. 2010;191:259–267. doi: 10.1083/jcb.201004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme D. Division plane determination during plant somatic cytokinesis. Curr Opin Plant Biol. 2009;12:745–751. doi: 10.1016/j.pbi.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Van Damme D, Vanstraelen M, Geelen D. Cortical division zone establishment in plant cells. Trends Plant Sci. 2007;12:458–464. doi: 10.1016/j.tplants.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Van Lammeren AA, Keijzer CJ, Willemse MT, Kieft H. Structure and function of the microtubular cytoskeleton during pollen development in Gasteria verrucosa (Mill.) H. Duval. Planta. 1985;165:1–11. doi: 10.1007/BF00392205. [DOI] [PubMed] [Google Scholar]
- Vanstraelen M, Van Damme D, De Rycke R, et al. Cell cycle-dependent targeting of a kinesin at the plasma membrane demarcates the division site in plant cells. Curr Biol. 2006;16:308–314. doi: 10.1016/j.cub.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Vos JW, Dogterom M, Emons AMC. Microtubules become more dynamic but not shorter during preprophase band formation: a possible “search-and-capture” mechanism for microtubule translocation. Cell Motil Cytoskelet. 2004;57:246–258. doi: 10.1002/cm.10169. [DOI] [PubMed] [Google Scholar]
- Vos JW, Pieuchot L, Evrard J-L, et al. The plant TPX2 protein regulates prospindle assembly before nuclear envelope breakdown. Plant Cell. 2008;20:2783–2797. doi: 10.1105/tpc.107.056796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainman A, Buster DW, Duncan T, et al. A new Augmin subunit, Msd1, demonstrates the importance of mitotic spindle-templated microtubule nucleation in the absence of functioning centrosomes. Genes Dev. 2009;23:1876–1881. doi: 10.1101/gad.532209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KL, Müller S, Moss D, et al. Arabidopsis TANGLED identifies the division plane throughout mitosis and cytokinesis. Curr Biol. 2007;17:1827–1836. doi: 10.1016/j.cub.2007.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteneys GO. Microtubule organization in the green kingdom: chaos or self-order? J Cell Sci. 2002;115:1345–1354. doi: 10.1242/jcs.115.7.1345. [DOI] [PubMed] [Google Scholar]
- Wightman R, Turner SR. Severing at sites of microtubule crossover contributes to microtubule alignment in cortical arrays. Plant J. 2007;52:742–751. doi: 10.1111/j.1365-313X.2007.03271.x. [DOI] [PubMed] [Google Scholar]
- Wightman R, Chomicki G, Kumar M, et al. SPIRAL2 determines plant microtubule organization by modulating microtubule severing. Curr Biol. 2013;23:1902–1907. doi: 10.1016/j.cub.2013.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Zhao Q, Rodrigo-Peiris T, et al. RanGAP1 is a continuous marker of the Arabidopsis cell division plane. Proc Natl Acad Sci U S A. 2008;105:18637–18642. doi: 10.1073/pnas.0806157105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara H, Shibaoka H. Inhibition of cell-plate formation by brefeldin A inhibited the depolymerization of microtubules in the central region of the phragmoplast. Plant Cell Physiol. 2000;41:300–310. doi: 10.1093/pcp/41.3.300. [DOI] [PubMed] [Google Scholar]
- Yasuhara H, Sonobe S, Shibaoka H. Effects of taxol on the development of the cell plate and of the phragmoplast in tobacco BY-2 cells. Plant Cell Physiol. 1993;34:21–29. [Google Scholar]
- Zeng CJT, Lee Y-RJ, Liu B. The WD40 repeat protein NEDD1 functions in microtubule organization during cell division in Arabidopsis thaliana. Plant Cell. 2009;21:1129–1140. doi: 10.1105/tpc.109.065953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Fishel E, Bertroche T, Dixit R. Microtubule severing at crossover sites by katanin generates ordered cortical microtubule arrays in arabidopsis. Curr Biol. 2013;23:2191–2195. doi: 10.1016/j.cub.2013.09.018. [DOI] [PubMed] [Google Scholar]