Abstract
The construction of an irreducible minimal cell having all essential attributes of a living system is one of the biggest challenges facing synthetic biology. One ubiquitous task accomplished by any living systems is the division of the cell envelope. Hence, the assembly of an elementary, albeit sufficient, molecular machinery that supports compartment division, is a crucial step towards the realization of self-reproducing artificial cells. Looking backward to the molecular nature of possible ancestral, supposedly more rudimentary, cell division systems may help to identify a minimal divisome. In light of a possible evolutionary pathway of division mechanisms from simple lipid vesicles toward modern life, we define two approaches for recapitulating division in primitive cells: the membrane deforming protein route and the lipid biosynthesis route. Having identified possible proteins and working mechanisms participating in membrane shape alteration, we then discuss how they could be integrated into the construction framework of a programmable minimal cell relying on gene expression inside liposomes. The protein synthesis using recombinant elements (PURE) system, a reconstituted minimal gene expression system, is conceivably the most versatile synthesis platform. As a first step towards the de novo synthesis of a divisome, we showed that the N-BAR domain protein produced from its gene could assemble onto the outer surface of liposomes and sculpt the membrane into tubular structures. We finally discuss the remaining challenges for building up a self-reproducing minimal cell, in particular the coupling of the division machinery with volume expansion and genome replication.
Keywords: Minimal cell, Artificial cell, Cell division, Liposome, Gene expression
Introduction
How simple is the simplest cell? Given the extraordinary complexity of even the simplest existing organism, one can question if such a complexity is really essential for life, or whether instead cellular life might be possible with far less components than we see in nature. This question can be addressed using two complementary research directions. In the top-down approach the extant genome of simple modern organisms is reduced in order to infer a minimal genome size or to define the vital cellular functions (Moya et al. 2009). In the alternative bottom-up direction—or constructive approach—essential cellular functions are assembled using a minimal set of purified constituents under well-controlled conditions (Luisi 2002; Luisi et al. 2006; Noireaux et al. 2011; Schwille 2011). Ultimately, both in vivo reduction and in vitro construction approaches to develop an elementary (or minimal) irreducible cell will arguably unveil new insights about the design principles of life as well as about the nature of the last universal common ancestor (LUCA). The term LUCA originally referred to an antique organism from which all living systems that ever existed on Earth descended (Darwin 1859). The picture of such a rudimentary living entity that ultimately developed into the three primary branches of the universal phylogenetic tree, namely bacteria, archaea and eukarya, is, however, arguably questionable. An attractive hypothesis has been formulated, in which a universal ancestor should rather be apprehended as a diversified population of primitive cells (Woese 1998).
Possible roadmaps for the realization of a minimal cell have been suggested and will not be reviewed here (Luisi et al. 2006; Murtas 2009; Noireaux et al. 2011; Forster and Church 2006). A distinction has to be made between the bottom-up synthetic biology approach to a minimal cell and the truly synthetic approach to protocell models (Morowitz et al. 1988; Szostak et al. 2001; Loakes and Holliger 2009; Walde 2010). In the former, extant molecules provided to us by evolution, such as proteins and DNA, are used as building blocks. Hence, the produced minimal cell should formally be referred as semi-synthetic. In the latter, researchers attempt to create primitive cells from scratch by using merely organic and inorganic materials and natural processes presumably available on the early Earth. Note also the difference with the semi-synthetic cell of Craig Venter’s group, obtained by transplantation of a synthetic genome into a recipient living bacterium (Gibson et al. 2010).
Though the definition of life itself is controversial, one can reasonably say that a molecular assembly should be considered alive if it is capable of self-maintenance, self-replication and evolvability. An essential trait of a living cellular system, even in its simplest representation, is the compartment: a continuous membrane that acts as a functional interface to regulate the ionic and molecular exchanges with the environment. Self-reproduction of a cell implies that both the compartment and the genetic material supporting life self-replicate in a coordinated manner.
Here, inspired by the different modes of cellular division encountered in nature, we try to paint the picture of the simplest mechanisms of the compartment division with the intentions to better understand the self-replication of early cells and to recapitulate a minimal divisome in vitro.
Possible evolutionary scenario of cell division systems: from simple vesicles to modern cells
Cell division in contemporary organisms
Cellular division in modern cells, a process also known as cytokinesis, relies on elaborate multi-protein machineries. Although division mechanisms in eukaryotes, bacteria and archaea have fundamental differences, in particular regarding the coupling between membrane remodeling and genome replication, a number of homologous proteins have been identified. In most prokaryotes the highly conserved tubulin homolog FtsZ assembles at the cell center into a ring structure that serves as a scaffold to recruit the division apparatus (Adams and Errington 2009). The Z-ring then shrinks exerting an inward force on the membrane (Erickson et al. 2010). The parental cell eventually splits into two daughter cells that inherit a similar cell-sized body. However, the FtsZ-centered system is not a universal division mechanism in prokaryotes (Bernander and Ettema 2010). Archaea adopt more diverse and unrelated membrane remodeling systems, including an eukaryotic-type of cytokinesis based on the ESCRT-III (endosomal sorting complexes required for transport) apparatus (Lindas et al. 2008; Samson and Bell 2009; Makarova et al. 2010; Samson et al. 2008, 2011). Besides the wide assortments of proteins related to cell division in archaea, but not in bacteria, the chemical composition of their cell walls and lipids are drastically different as well (Fig. 1, right). This dichotomy suggests distinct evolutionary histories from a primitive simple precursor (Koonin and Mulkidjanian 2013).
Fig. 1.
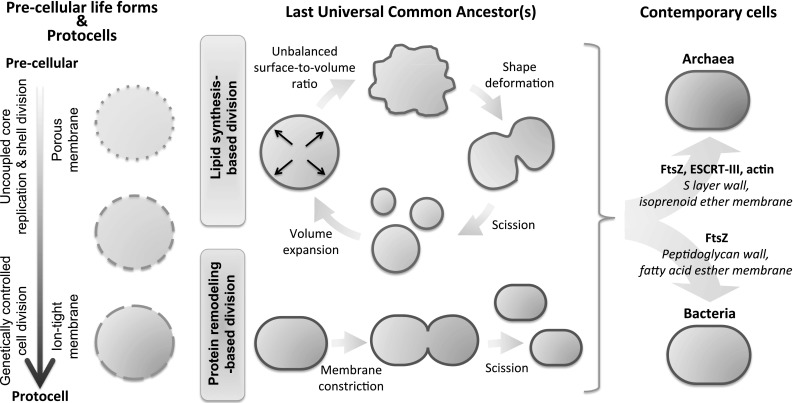
Possible transition of cell division mechanisms and associated factors from simple lipid vesicles to modern cells. (left) Lipid vesicles have long been acknowledged as a plausible compartment for protocells. The transition from pre-cellular to protocell systems might have been driven by the evolution of the vesicle membrane permeability. In pre-cellular life forms, replication of a genetic polymer and fission of short-lived porous liposomes could have occurred independently. Progression to cellular life with genetic control of growth and division further implies that selective ionic and molecular exchanges across the membrane were established. (middle) The putative LUCA should not necessarily be perceived as one single individual from which any species emanated. Instead, it could be envisaged as a diverse population of primitive cells using lateral gene transfer as an innovation mechanism (Woese 1998). Two main division models of the ancestor(s) have been postulated. One in which shape deformation is mediated by an excess of membrane through lipid biosynthesis (see text for details). This part of the figure is adapted from Mercier et al. (2013). The other is based on membrane constriction at midcell, a process assisted by membrane deforming proteins. (right) Bacteria and archaea have separately evolved as suggested by their distinct protein cell walls, lipidic compositions and division mechanisms
Division mechanisms in early cells
A possible evolutionary scenario of cell division machineries from simple lipid vesicles to modern prokaryotic cells is depicted in Fig. 1. Any attempts to reconstruct the events that occurred during the evolution of cellular functions have to deal with the notion of a universal ancestor. In the context of cell division, two main theories have been formulated (Fig. 1, middle). In the first model, “a physical pinching of the cell into two approximately equal halves” is seen as the simplest way to divide (Woese 1998). Though constriction might be achieved by mechanical extrusion through mineral pores (Hanczyc et al. 2003), we favor a protein-based fission mechanism in early cells. This process would have rudimentary capacity, relying on a very few elements and lacking the level of control and integration with other subsystems, in particular genome replication. The nature of the early membrane deforming system is debated. It is still unclear whether FtsZ was ancestral in both bacteria and archaea or whether it was acquired in extant archaea by horizontal gene transfer from bacteria (Koonin and Mulkidjanian 2013). However, this ancestor had probably no stabilizing protein-based cell wall. In the second theory, primitive cell division would proceed through a lipid biosynthesis route (Murtas 2010), in analogy to a mechanism recently observed in wall-less forms of bacteria, called L forms (Leaver et al. 2009; Allan et al. 2009). These L form cells are known to bypass the canonical FtsZ-centered division system and to proliferate by increased fatty acid synthesis, which leads to spontaneous cell shape deformation and scission into smaller progeny cells (Fig. 1, middle) (Mercier et al. 2013). Under this scenario, early cell division would occur without intervention of membrane-remodeling proteins.
Self-assembled lipid vesicles are considered as best candidates for primordial cellular compartment. Simple mechanisms of liposome growth and/or division have extensively been documented (for reviews, see e.g. Hanczyc and Szostak 2004; Mansy and Szostak 2009; Stano and Luisi 2010; Murtas 2013; Caspi and Dekker 2014); most of them imply a morphological change of the vesicle upon phase transition crossing or lipid uptake from the external aqueous medium (Svetina 2009; Peterlin et al. 2009) or from a lipophilic reservoir (Takakura et al. 2003). The resulting unbalanced surface-to-volume ratio is eventually released by division. In the early stages of cellular life evolution, lateral exchange of genetic and metabolic materials through imperfect (leaking) division or through transient membrane defects would conceivably dominate the evolutionary dynamics in a population of vesicle protocells. Only once cell division became genetically controlled, a transition that may have been driven by the co-evolution of more impermeable membrane, would vertical inheritance become prevalent over lateral gene transfer (Fig. 1, left).
Mimicking cell division in its most simple form
Our lab is engaged to the long-term effort to build up a minimal cell. The construction of an elementary cell division system, like the assembly of a minimal cell, is not limited to the use of natural compounds, i.e. recombinant engineered proteins equipped with novel functionalities could for instance be employed. However, we purposely focus on components directly participating to cellular division or involved in membrane remodeling, and discuss how they could be implemented into the minimal cell construction framework to stimulate compartment division. An important question is whether reconstituted systems released from the high organizational level inherent to the cellular context can retain their functions.
Cell-free gene expression inside liposomes as a platform for the construction of a minimal cell
The expression of genes into proteins is a fundamental and ubiquitous cellular process. The transfer of the DNA sequence information to proteins occurs in two steps forming the central dogma in molecular biology: the transcription of the gene in RNA and the subsequent translation of that RNA into a protein. During transcription the double stranded DNA is copied by an RNA polymerase protein into a single stranded messenger RNA (mRNA). Translation is a more complex process involving many different components, with the ribosome as a key player. The synthesis of proteins from DNA templates inside lipid vesicles offers an elegant and unavoidable alternative to the exclusive use of purified constituents. By regulating the timely synthesis of the multiple proteins, the gene expression machinery is arguably at the heart of a programmable, genetically controlled, artificial cell.
Cell-free protein synthesis inside liposomes has first been performed using cellular extracts derived from cytoplasmic parts of Escherichia coli bacteria (Nomura et al. 2003; Noireaux and Libchaber 2004). Despite many advantages, including reduced costs (Jewett and Forster 2010), high yield of protein expression (Caschera and Noireaux 2014) and the possibility to use the endogenous bacterial RNA polymerases (Shin and Noireaux 2010), crude extracts are poorly characterized mixtures containing plenty of undesired components that can interfere with the intended reactions. Alternatively, the protein synthesis using recombinant elements (PURE) system developed by the group of Takuya Ueda (Tokyo University) (Shimizu et al. 2001, 2005), is a minimal gene expression system reconstituted solely from purified enzymes and cofactors (36 different proteins) for transcription, translation, tRNA aminoacylation, energy regeneration and pyrophosphate hydrolysis. All components are from E. coli with the exception of the RNA polymerase that is from the T7 bacteriophage. Since the PURE system is made of well-defined elements with known concentrations and intended functions, it is the preferred core synthesis apparatus of a minimal cell. Moreover, it provides a unique platform for reliable modeling and quantitative understanding of gene expression dynamics, which is essential for high-level control of protein production. Hence, the simplest representation of a living cell can be envisioned as a reconstituted biosynthesis machinery carrying out the expression of a minimal genome inside a self-assembled lipid vesicle.
We recently developed a method to trigger gene expression inside surface-tethered liposomes (Nourian et al. 2012). The enzymatic apparatus of the PURE system and the DNA templates are co-encapsulated inside (sub) micrometer-sized vesicles. Exchanging or diluting the external medium with the feeding solution leads to an osmotic pressure-driven membrane permeabilization, which temporarily enables selective uptake of all necessary nutrients and tRNAs, and initiates the internal synthesis of mRNA and proteins. The stochastic nature of the component entrapment and of the progression of transcription and translation could be evidenced and quantified (Nourian and Danelon 2013; van Nies et al. 2013).
With regards to implementing compartment division of a vesicle-based minimal cell, all necessary enzymes and protein cofactors should be internally produced from their corresponding genes. We envision two main directions for an elementary cell to undergo division: the membrane deforming protein route and the lipid biosynthesis route (Fig. 2).
Fig. 2.
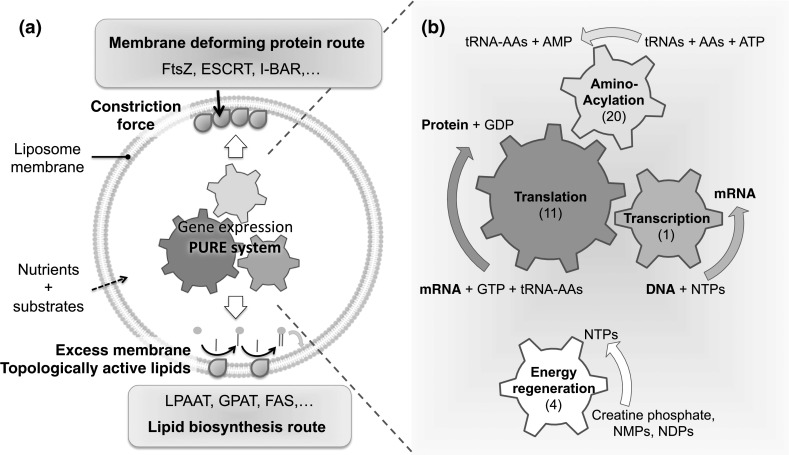
Vesicle division routes in a PURE system-based minimal cell. A programmable, genetically controlled minimal cell can be represented as a gene expression apparatus, the PURE system, encapsulated inside a semi-permeable liposome. a The two pathways envisioned for division are based on the production of membrane deforming proteins or of enzymes involved in lipid biosynthesis. b The PURE system can be broken down in four functional modules: transcription, translation, aminoacylation and energy regeneration. The numbers of purified proteins involved to execute the specific reactions are indicated in parentheses. Abbreviations: AAs, amino acids; NMPs, nucleotide monophosphates; NDPs, nucleotide diphosphates; NTPs, nucleotide triphosphates
The membrane deforming protein route for compartment division
Expression of membrane proteins directly involved in cell division
As discussed above in the context of early cell division, a possible minimal divisome architecture inspired from bacterial cytokinesis would consist of FtsZ protofilaments anchored to the liposome membrane by native auxiliary proteins such as the actin homologue FtsA or ZipA (Osawa et al. 2008, 2009; Osawa and Erickson 2013; Jimenez et al. 2011; Cabre et al. 2013). In the absence of a cell wall mimicry, FtsZ assisted by FtsA was suggested as the simplest machinery to recapitulate all essential steps of cellular division in lipid vesicles: Z ring formation, force generation to distort the liposome membrane, progressive constriction of the Z ring and completed division (Osawa and Erickson 2013).
The ESCRT protein system is involved in many membrane invagination processes in eukaryotic cells (Makarova et al. 2010). In archaea, a cell division mechanism based on the ESCRT-III has recently been discovered (Samson et al. 2008; Lindas et al. 2008). Lipid vesicle fission could be reconstituted in vitro using a minimal ESCRT apparatus based on six different proteins (Saksena et al. 2009; Wollert et al. 2009; Wollert and Hurley 2010).
All experiments so far have been performed using purified proteins mixed with liposomes. Following the minimal cell paradigm, we propose that the PURE system-operating synthesis of both FtsZ and FtsA or of a minimal protein assortment of the ESCRT apparatus inside liposomes constitutes a highly attractive route toward the fission of a genetically controlled artificial cell. The ESCRT-based approach seems more challenging due to the large number of components involved and the possible requirement of posttranslational modifications that are not supported in the E. coli-based PURE system.
Expression of non-canonical membrane remodeling proteins for vesicle division
Though not directly involved in cell division, other types of protein-assisted membrane remodeling processes could be considered in order to drive liposome division of a minimal cell. Below, we identify some potential protein candidates whose role in generating membrane deformation has been demonstrated (Zimmerberg and Kozlov 2006).
In eukaryotes, the plasma membrane undergoes dynamic morphological rearrangements during several cellular processes including membrane trafficking, endocytosis and morphogenesis (McMahon and Gallop 2005). Generation of membrane deformation in the forms of protrusions or invaginations occurs through coordinated effects of actin cytoskeleton and membrane-associated proteins. Examples include the Bin-amphiphysin-Rvs (BAR) domain family proteins and the epsin N-terminal homology (ENTH) domain proteins. Due to their concave geometry canonical BAR domains and most F-BAR domains sense and drive the formation of positive membrane curvature resulting in tubular and vesicular membrane structures both in vitro and in vivo (Peter et al. 2004; Bhatia et al. 2009; Mim and Unger 2012). In contrast, inverse BAR (I-BAR) domains induce negative membrane curvature by interacting with lipids through their convex surface (Saarikangas et al. 2009; Zhao et al. 2011). For global membrane curvature to arise a model has been suggested in which multiple BAR domains arrange on the membrane surface in a lattice through protein–protein interaction (Frost et al. 2008; Yin et al. 2009). Though less documented, several prokaryotic proteins also display membrane tubulation activities in vitro: MinE (Shih et al. 2011), MinD (Hu et al. 2002; Suefuji et al. 2002) and bacterial dynamin-like protein (Low and Löwe 2006; Low et al. 2009).
Besides, viral membranous replication complexes induce membrane shape rearrangements essential for genomic RNA replication of positive-strand RNA viruses (Ahlquist 2006; Diaz and Ahlquist 2012). Reticulon proteins involved in the endoplasmic reticulum membrane sculpting have been identified as essential players to remodel target cell membranes into viral replication vesicles (Diaz and Ahlquist 2012). An appealing approach to achieve coupled core–shell self-replication in an RNA genome-based minimal cell, is to reconstitute the retrovirus or bromovirus membrane-associated RNA-replication factors that comprise both a polymerase domain for RNA synthesis and a membrane-targeting domain.
In order to make these non-canonical proteins more apt to promote vesicle division, mutations might be introduced through protein engineering to change some of their properties. For instance, the high membrane curvature prompted by native BAR family proteins or viral membrane-bound RNA replication proteins is probably incompatible with symmetrical fission of liposomes.
Experimental evidence of genetically controlled deformation of liposomes using in situ expression of the N-BAR domain protein
Studies using the conventional reconstitution approach based on purified proteins have revealed important insights on the mechanisms of liposome deformation evoked, for instance, by MinE (Shih et al. 2011), amphiphysin 1 (Sorre et al. 2012), I-BAR domain proteins (Saarikangas et al. 2009), clathrin (Dannhauser and Ungewickell 2012), and FtsZ with its associated membrane-binding proteins (Osawa et al. 2008; Cabre et al. 2013). However, it has not been reported yet that in situ expressed proteins, i.e. synthesized from a DNA template in the presence of liposomes, could interact with the membrane and elicit curvature. Different membrane binding or transmembrane proteins have previously been synthesized using cell-free expression systems (Noireaux et al. 2005; Kuruma et al. 2012; Maeda et al. 2012; Liu et al. 2013) but, to our knowledge, none of them could direct membrane deformation.
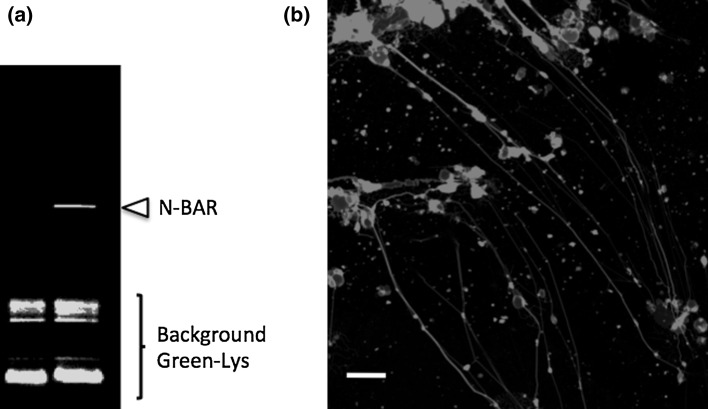
Here, we assessed the ability of a prototypical membrane deformer generated in situ to sculpt liposome membrane: the N-terminal amphipathic helix and BAR domain (N-BAR) of the rat amphiphysin 1. Lipid vesicles containing dioleoyl phospholipids and a small fraction of biotin-PEG (PEG = polyethylene glycol) lipids were loaded with the PUREfrex (Shimizu et al. 2014) enzyme mix and immobilized on the surface of a microscope coverslip as described in the methodology section. The external medium was supplemented with DNA template encoding for the N-BAR domain protein along with the PUREfrex enzyme mix and the feeding solution. In these conditions protein synthesis can only occur outside the vesicles. After 1.5 h incubation at 37 °C the liposomes were imaged using a fluorescent membrane dye. As shown in Fig. 3b, protein synthesis outside the liposomes results in the formation of tubular lipidic structures, some extending over more than 50 μm. This demonstrates the ability of the cell-free produced N-BAR protein to bind to the membrane, form a protein scaffold and exert a force inducing high curvature. The most visible tubular structures under the fluorescence confocal microscope are those tethered to the surface (through the biotin-PEG-lipid anchor) since they are positioned within the same confocal plane throughout their length. Tilted filaments are less visible on single snapshots but could be imaged at multiple heights while scanning. We also noted that not all vesicles (or lipid aggregates) could elongate into tubular structures.
Fig. 3.
In vitro synthesis and activity of the N-BAR membrane deformer. a Protein synthesis was performed in the presence of BODIPY-Lys-tRNALys and the sample was loaded on an SDS-PAGE. The gel was imaged on a fluorescence gel imager. The arrowhead indicates the synthesized N-BAR amphiphysin 1 domain protein, whereas the lower bands correspond to unreacted BODIPY-Lys-tRNALys. The lane on the left is a control sample, where the N-BAR gene is absent. b Fluorescently labeled liposomes immobilized on a glass surface were incubated with the PUREfrex reaction mixture and the linear DNA template encoding for the N-BAR protein. In situ synthesized proteins were able to sculpt liposome membrane into tubular structures. Scale bar is 10 μm
We sought to express the N-BAR genes from the inside of the vesicles, following our well-established protocol (Nourian et al. 2012). However, we failed observing membrane bending and filaments (not shown), presumably because the N-BAR protein cannot bind to the negatively curved membrane exposed from the liposome interior. Alternatively, a lower amount of synthesized N-BAR proteins inside the vesicles compared to that in the external medium may prevent protein pattern and tubular structures from forming. In order to realize compartment deformation from within liposomes, transient exposure of entrapped BAR proteins to positive curvature could be accomplished by generating membrane fluctuations through imbalanced surface-to-volume ratio (see next section). Moreover, I-BAR proteins with convex geometry could be synthesized to induce protrusions by binding from the interior of the filaments. Further optimization is clearly required to pinch off tubular structures into vesicles. Auxiliary factors that would facilitate vesicle release include dynamin that assists amphiphysin in clathrin-mediated endocytosis (Takei et al. 1999; Dannhauser and Ungewickell 2012) and dynamin-related protein 1 (Drp1), known to promote mitochondrial fission (Takamura et al. 2012). The lipid composition could also be adjusted to enhance penetration of the protein domain into the lipid matrix and thus the bending properties (Stahelin et al. 2003; Mattila et al. 2007).
The lipid biosynthesis route for compartment division
The alternative route to minimal cell proliferation consists in implementing a system for lipid biosynthesis and volume expansion. Synthesized lipids would incorporate into the growing membrane and change its equilibrium state (Fig. 2a). In light of the recently unveiled mechanism of L form cell reproduction (Mercier et al. 2013), and of in vitro (Kurihara et al. 2011; Briers et al. 2012) and in silico (Macia and Solé 2007; Almendro-Vedia et al. 2013) liposome deformation studies, we envisage two possible pathways, both relying on the intravesicular production of lipid synthesizing enzymes using the PURE system.
The first approach consists in creating excess membrane by phospholipids or fatty acids synthesis. The natural place to start is with phosphatidic acid (PA) (Schmidli et al. 1991; Wick and Luisi 1996), the universal precursor of phospholipids in bacteria. The pathway for PA synthesis in E. coli entails the genes plsB and plsC. The plsB gene product, the enzyme glycerol-3-phosphate (G3P) acyl transferase (GPAT), uses G3P and either acyl-CoA (CoA, coenzyme A) or acyl-ACP (ACP, acyl carrier protein) substrates to generate lysophosphatidic acid (LPA). In a subsequent enzymatic reaction the LPA and another acyl-CoA/acyl-ACP are converted into PA by the LPA acyl transferase (LPAAT), the plsC gene product. In the cellular context of E. coli, these two enzymes are complemented by a few others to produce phosphatidylglycerol (PG), a bilayer-forming anionic lipid, and phosphatidylethanolamine (PE), a zwitterionic lipid, both representing the largest fraction of the E. coli inner membrane lipidome. We predict that synthesis of PA lipids could be sufficient to induce shape deformation and eventually liposome division, a manifestation of the excess surface area at the internal leaflet of the membrane. The cell-free production of functional GPAT and LPAAT enzymes has been demonstrated (Kuruma et al. 2009). However, their synergetic activity driving vesicle expansion or morphological changes remains to be achieved. One limitation that has been invoked is the poor membrane permeability of the reaction substrates, which precludes higher yield of synthesized enzymes and catalytic products (Kuruma et al. 2009). A simple mode of uptake of exogenous precursors would be to exploit the osmotic pressure-mediated transport across the lipid bilayer, which we employed to fuel the gene expression apparatus with all necessary nutrients and cofactors supplied in the external environment (Nourian et al. 2012). Alternatively, ion channels or acyl transporters could be incorporated in the vesicle membrane to facilitate the diffusion of lipid precursors. Like phospholipid synthesis, internal production of fatty acids by fatty acid synthetases (FAS) could be considered (Murtas 2009, 2010). A point of caution is that the high concentration of Mg2+ contained in the PURE system (around 10 mM) may cause fatty acids to precipitate.
Another way to change the equilibrium state of a membrane is to modify the lipidic composition to encourage topological transitions (Zimmerberg and Kozlov 2006). This could be achieved through synthesis of topologically active lipids like PE (Sakuma and Imai 2011), or via enzyme-assisted modifications of lipids (acyl chain or head group) in the membranes inducing local curvature stresses and further vesicle destabilization. In giant unilamellar vesicles containing mixed lipids of different shapes, dynamical morphology changes driving repetitive division can be controlled by cycling temperature across the bilayer phase transition temperature (Sakuma and Imai 2011). Tuning the fraction of inverse-cone-shaped PE lipids causes liposomes to divide either by budding or translocation of an inclusion vesicle. Once coupled with lipid biosynthesis this mode of boundary self-reproduction holds some promise for continuous growth and division of a minimal cell. It will be important to adjust the lipid composition and annealing temperatures since gene expression in the PURE system becomes short-lived above 37 °C (Nourian et al. 2012).
Concluding remarks
We have discussed a number of potentially simple division mechanisms that could be reconstituted in lipid vesicles in order to create a programmable, genetically controlled, minimal cell. Here, the proteins acting on the membrane are synthesized from a DNA template within liposomes, which radically differs from the conventional approach consisting in encapsulating purified proteins of fixed concentration. In order to feed the biosynthesis machinery with externally supplied nutrients, liposomes with enabled molecular exchange across the lipid bilayer could be prepared (Noireaux and Libchaber 2004; Nourian et al. 2012). Notably, internal production of the membrane binding proteins ensures native vectorial properties, such as force generation, across the lipid bilayer, which is sometimes difficult to control when mixing purified proteins with liposomes.
Several proteins have been recognized as key players in cellular division and are natural candidates for being expressed within liposomes. These include the bacterial FtsZ and its assisting membrane anchoring proteins, and the archaeal ESCRT-III apparatus. We also proposed to extend the repertoire of membrane remodeling proteins whose primary functions are not directly related to cell division, such as BAR domains and viral membranous RNA replication complexes, and to divert them to execute division in artificial cells. Following a lipid biosynthesis route, an excess of lipids in the inner leaflet of the liposome bilayer could be generated or topologically active lipids could be produced to direct shape deformation, which may lead to vesicle fission.
With regard to the cell-inherent complexity that manifests itself at every organizational levels of biological functions it is however legitimate to question whether reconstituted elements and systems will still be active when extracted from their cellular context. Cell division is intimately related to growth (Hill et al. 2013) and genome replication. Mechanisms ensuring that cells grow to the right size and the genome duplicates work in concert with the division machinery. While reconstituting a functional vesicle fission apparatus under genetic control seems reasonably reachable in the short-term future, its integration to other modules of the minimal cell will certainly increase the level of complexity through intervention of metabolic and genetic regulatory networks.
Materials and methods
Preparation of the DNA templates
The plasmid pGEX6P2 coding for the N-BAR from the rat amphiphysin-1 (Bhatia et al. 2009) was kindly provided by Dimitrios Stamou (University of Copenhagen, Denmark). The N-BAR gene was further cloned into the pRSET-B vector (Invitrogen). First, the gene was amplified from the pGEX6P2 plasmid template by polymerase chain reaction (PCR) using the forward 5′-AAGGATCCGATGGCCGACATCAAGACGGGCATCTTC-3′ and reverse 5′-AAGAATTCTTAGGCAGAGGTGGGACAGG-3′ primers (Biolegio) and cloned into pREST-B using the BamHI and EcoRI sites. The obtained pRSET-B clones were checked by digestion patterns. The linear DNA template used for cell-free gene expression was generated by PCR based on the pRSET-B plasmids as previously described (Nourian et al. 2012). The linear DNA encoding for the cyan fluorescent protein (CFP) was prepared as described in our previous work (Nourian and Danelon 2013).
Bulk expression of the N-BAR domain protein
Bulk protein synthesis was performed as described previously (van Nies et al. 2013), except that the PUREfrex reaction mixture was supplemented with BODIPY-Lys-tRNALys (FluoroTect™ GreenLys, Promega), an in vitro translation labeling system. The sample was incubated for 3 h at 37 °C, denatured for 2 min at 70 °C and analyzed on a 12 % SDS-PAGE using a fluorescence gel imager (Typhoon, Amersham Biosciences).
Preparation of lipid film-coated beads
Lipid vesicles were produced essentially as described previously (Nourian et al. 2012; van Nies et al. 2013), with some modifications. Briefly, 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC, Avanti Polar Lipids), N-(6-tetramethylrhodaminethiocarbamoyl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (TRITC-DHPE, Invitrogen), and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[biotinyl(polyethylene glycol)-2000] (DSPE-PEG-biotin, Avanti Polar Lipids), all dissolved in chloroform, were mixed at a molar ratio DOPC:TRITC-DHPE:DSPE-PEG-biotin = 100:0.5:0.1 and added to 212–300-μm glass beads (Sigma-Aldrich) in a round bottom glass flask. The solvent was evaporated under flask rotation at about 400 mbar overnight to obtain lipid film-coated beads.
Membrane deformation assay
The PUREfrex (GeneFrontier Corporation, Japan) expression system was used. Around 10 μL of freshly prepared lipid film-coated beads were poured in a reaction tube and immersed in 11.5 μL swelling solution consisting of 3.5 μL of PUREfrex enzyme mix, 3.5 μL of PUREfrex ribosome, 1.0 μL of the CFP DNA template (260 ng), 0.5 μL of Superase inhibitor (10 units final, SUPERase.In™, Ambion) and 3 μL of nuclease-free water. Liposomes were formed by swelling the lipid film at 30 °C for 2 hours and then subjected to four freeze–thaw cycles. Next, 1 μL of the liposome-containing solution was carefully harvested and the vesicles were immobilized at the bottom of a poly(dimethylsiloxane) chamber bound onto a microscope coverslip functionalized with BSA-biotin and neutravidin (Nourian et al. 2012). Gene expression was triggered by diluting the surface-tethered vesicles with 0.25 μL of PUREfrex enzyme mix, 0.25 μL of PUREfrex ribosome, 2.5 μL of PUREfrex feeding mix, 0.2 μL of the N-BAR linear DNA template (30 nM final concentration), 0.25 μL of Superase inhibitor and 1.4 μL of nuclease-free water. The samples were incubated at 37 °C for 1.5 h and imaged by fluorescence confocal microscopy.
Fluorescence microscopy
A laser scanning confocal microscope (LSM710, Zeiss) equipped with a ×40 oil immersion objective and a 543-nm laser line was used to image TRITC fluorescence at wavelengths >553 nm. All measurements were performed at room temperature (20 °C). Fluorescence images were analyzed with the software ImageJ (Schneider et al. 2012).
Acknowledgments
We thank Dr. Vadym Tkach and Prof. Dimitrios Stamou from the University of Copenhagen for providing us with the plasmid pGEX6P2. We are grateful to Ilja Westerlaken from our lab for cloning the N-BAR gene into the pRSET-B vector, to Mona Mohseni Kabir for her help with the membrane deformation assays and to Pauline van Nies for critical reading of the manuscript. This work was supported by the Netherlands Organization for Scientific Research (NWO) through a VIDI grant and an ALW Open Programma grant to C.D., and by a Natural Sciences and Engineering Research Council of Canada (NSERC) fellowship to A.S.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Adams DW, Errington J. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol. 2009;7(9):642–653. doi: 10.1038/nrmicro2198. [DOI] [PubMed] [Google Scholar]
- Ahlquist P. Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses. Nat Rev Microbiol. 2006;4(5):371–382. doi: 10.1038/nrmicro1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan EJ, Hoischen C, Gumpert J. Bacterial L-forms. Adv Appl Microbiol. 2009;68:1–39. doi: 10.1016/S0065-2164(09)01201-5. [DOI] [PubMed] [Google Scholar]
- Almendro-Vedia VG, Monroy F, Cao FJ. Mechanics of constriction during cell division: a variational approach. PLoS One. 2013;8(8):e69750. doi: 10.1371/journal.pone.0069750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernander R, Ettema TJ. FtsZ-less cell division in archaea and bacteria. Curr Opin Microbiol. 2010;13(6):747–752. doi: 10.1016/j.mib.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Bhatia VK, Madsen KL, Bolinger PY, Kunding A, Hedegård P, Gether U, Stamou D. Amphipathic motifs in BAR domains are essential for membrane curvature sensing. EMBO J. 2009;28(21):3303–3314. doi: 10.1038/emboj.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briers Y, Walde P, Schuppler M, Loessner MJ. How did bacterial ancestors reproduce? Lessons from L-form cells and giant lipid vesicles: multiplication similarities between lipid vesicles and L-form bacteria. Bioessays. 2012;34(12):1078–1084. doi: 10.1002/bies.201200080. [DOI] [PubMed] [Google Scholar]
- Cabre EJ, Sanchez-Gorostiaga A, Carrara P, Ropero N, Casanova M, Palacios P, Stano P, Jimenez M, Rivas G, Vicente M. Bacterial division proteins FtsZ and ZipA induce vesicle shrinkage and cell membrane invagination. J Biol Chem. 2013;288(37):26625–26634. doi: 10.1074/jbc.M113.491688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caschera F, Noireaux V. Synthesis of 2.3 mg/ml of protein with an all Escherichia coli cell-free transcription-translation system. Biochimie. 2014;99:162–168. doi: 10.1016/j.biochi.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Caspi Y, Dekker C (2014) Divided we stand: splitting synthetic cells for their proliferation. Syst Synth Biol [DOI] [PMC free article] [PubMed]
- Dannhauser PN, Ungewickell EJ. Reconstitution of clathrin-coated bud and vesicle formation with minimal components. Nat Cell Biol. 2012;14(6):634–639. doi: 10.1038/ncb2478. [DOI] [PubMed] [Google Scholar]
- Darwin CC. The origins of the species by means of natural selection or the preservation of favoured races in the struggle for life. London: John Murray; 1859. [Google Scholar]
- Diaz A, Ahlquist P. Role of host reticulon proteins in rearranging membranes for positive-strand RNA virus replication. Curr Opin Microbiol. 2012;15(4):519–524. doi: 10.1016/j.mib.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson HP, Anderson DE, Osawa M. FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one. Microbiol Mol Biol Rev. 2010;74(4):504–528. doi: 10.1128/MMBR.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster AC, Church GM. Towards synthesis of a minimal cell. Mol Syst Biol. 2006;2:45. doi: 10.1038/msb4100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost A, Perera R, Roux A, Spasov K, Destaing O, Egelman EH, De Camilli P, Unger VM. Structural basis of membrane invagination by F-BAR domains. Cell. 2008;132(5):807–817. doi: 10.1016/j.cell.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, Merryman C, Vashee S, Krishnakumar R, Assad-Garcia N, Andrews-Pfannkoch C, Denisova EA, Young L, Qi ZQ, Segall-Shapiro TH, Calvey CH, Parmar PP, Hutchison CA, 3rd, Smith HO, Venter JC. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010;329(5987):52–56. doi: 10.1126/science.1190719. [DOI] [PubMed] [Google Scholar]
- Hanczyc MM, Szostak JW. Replicating vesicles as models of primitive cell growth and division. Curr Opin Chem Biol. 2004;8(6):660–664. doi: 10.1016/j.cbpa.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Hanczyc MM, Fujikawa SM, Szostak JW. Experimental models of primitive cellular compartments: encapsulation, growth, and division. Science. 2003;302(5645):618–622. doi: 10.1126/science.1089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill NS, Buske PJ, Shi Y, Levin PA. A moonlighting enzyme links Escherichia coli cell size with central metabolism. PLoS Genet. 2013;9(7):e1003663. doi: 10.1371/journal.pgen.1003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Gogol EP, Lutkenhaus J. Dynamic assembly of MinD on phospholipid vesicles regulated by ATP and MinE. Proc Natl Acad Sci USA. 2002;99(10):6761–6766. doi: 10.1073/pnas.102059099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett MC, Forster AC. Update on designing and building minimal cells. Curr Opin Biotechnol. 2010;21(5):697–703. doi: 10.1016/j.copbio.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez M, Martos A, Vicente M, Rivas G. Reconstitution and organization of Escherichia coli proto-ring elements (FtsZ and FtsA) inside giant unilamellar vesicles obtained from bacterial inner membranes. J Biol Chem. 2011;286(13):11236–11241. doi: 10.1074/jbc.M110.194365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin EV, Mulkidjanian AY. Evolution of cell division: from shear mechanics to complex molecular machineries. Cell. 2013;152(5):942–944. doi: 10.1016/j.cell.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Kurihara K, Tamura M, Shohda KI, Toyota T, Suzuki K, Sugawara T. Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA. Nat Chem. 2011;3(10):775–781. doi: 10.1038/nchem.1127. [DOI] [PubMed] [Google Scholar]
- Kuruma Y, Stano P, Ueda T, Luisi PL. A synthetic biology approach to the construction of membrane proteins in semi-synthetic minimal cells. Biochim Biophys Acta Biomembr. 2009;1788(2):567–574. doi: 10.1016/j.bbamem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Kuruma Y, Suzuki T, Ono S, Yoshida M, Ueda T. Functional analysis of membranous Fo-a subunit of F1Fo-ATP synthase by in vitro protein synthesis. Biochem J. 2012;442(3):631–638. doi: 10.1042/BJ20111284. [DOI] [PubMed] [Google Scholar]
- Leaver M, Dominguez-Cuevas P, Coxhead JM, Daniel RA, Errington J. Life without a wall or division machine in Bacillus subtilis. Nature. 2009;457(7231):849–853. doi: 10.1038/nature07742. [DOI] [PubMed] [Google Scholar]
- Lindas AC, Karlsson EA, Lindgren MT, Ettema TJ, Bernander RA. A unique cell division machinery in the archaea. Proc Natl Acad Sci USA. 2008;105:18942–18946. doi: 10.1073/pnas.0809467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Hansen GP, Venancio-Marques A, Baigl D. Cell-free preparation of functional and triggerable giant proteoliposomes. ChemBioChem. 2013;14(17):2243–2247. doi: 10.1002/cbic.201300501. [DOI] [PubMed] [Google Scholar]
- Loakes D, Holliger P. Darwinian chemistry: towards the synthesis of a simple cell. Mol BioSyst. 2009;5(7):686–694. doi: 10.1039/b904024b. [DOI] [PubMed] [Google Scholar]
- Low HH, Löwe J. A bacterial dynamin-like protein. Nature. 2006;444(7120):766–769. doi: 10.1038/nature05312. [DOI] [PubMed] [Google Scholar]
- Low HH, Sachse C, Amos LA, Löwe J. Structure of a bacterial dynamin-like protein lipid tube provides a mechanism for assembly and membrane curving. Cell. 2009;139(7):1342–1352. doi: 10.1016/j.cell.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi PL. Toward the engineering of minimal living cells. Anat Rec. 2002;268(3):208–214. doi: 10.1002/ar.10155. [DOI] [PubMed] [Google Scholar]
- Luisi PL, Ferri F, Stano P. Approaches to semi-synthetic minimal cells: a review. Naturwissenschaften. 2006;93(1):1–13. doi: 10.1007/s00114-005-0056-z. [DOI] [PubMed] [Google Scholar]
- Macia J, Solé RV. Synthetic turing protocells: vesicle self-reproduction through symmetry-breaking instabilities. Philos Trans R Soc Lond B Biol Sci. 2007;362(1486):1821–1829. doi: 10.1098/rstb.2007.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda YT, Nakadai T, Shin J, Uryu K, Noireaux V, Libchaber A. Assembly of MreB filaments on liposome membranes: a synthetic biology approach. ACS Synth Biol. 2012;1(2):53–59. doi: 10.1021/sb200003v. [DOI] [PubMed] [Google Scholar]
- Makarova KS, Yutin N, Bell SD, Koonin EV. Evolution of diverse cell division and vesicle formation systems in archaea. Nat Rev Microbiol. 2010;8(10):731–741. doi: 10.1038/nrmicro2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansy SS, Szostak JW. Reconstructing the emergence of cellular life through the synthesis of model protocells. Cold Spring Harb Symp Quant Biol. 2009;74:47–54. doi: 10.1101/sqb.2009.74.014. [DOI] [PubMed] [Google Scholar]
- Mattila PK, Pykäläinen A, Saarikangas J, Paavilainen VO, Vihinen H, Jokitalo E, Lappalainen P. Missing-in-metastasis and IRSp53 deform PI(4,5)P2-rich membranes by an inverse BAR domain-like mechanism. J Cell Biol. 2007;176(7):953–964. doi: 10.1083/jcb.200609176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodeling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- Mercier R, Kawai Y, Errington J. Excess membrane synthesis drives a primitive mode of cell proliferation. Cell. 2013;152(5):997–1007. doi: 10.1016/j.cell.2013.01.043. [DOI] [PubMed] [Google Scholar]
- Mim C, Unger VM. Membrane curvature and its generation by BAR proteins. Trends Biochem Sci. 2012;37:526–533. doi: 10.1016/j.tibs.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morowitz HJ, Heinz B, Deamer DW. The chemical logic of a minimum protocell. Orig Life Evol Biosph. 1988;18(3):281–287. doi: 10.1007/BF01804674. [DOI] [PubMed] [Google Scholar]
- Moya A, Gil R, Latorre A, Peretó J, Pilar Garcillán-Barcia M, de la Cruz F. Toward minimal bacterial cells: evolution vs. design. FEMS Microbiol Rev. 2009;33(1):225–235. doi: 10.1111/j.1574-6976.2008.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtas G. Artificial assembly of a minimal cell. Mol BioSyst. 2009;5(11):1292–1297. doi: 10.1039/b906541e. [DOI] [PubMed] [Google Scholar]
- Murtas G. Internal lipid synthesis and vesicle growth as a step toward self-reproduction of the minimal cell. Syst Synth Biol. 2010;4(2):85–93. doi: 10.1007/s11693-009-9048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtas G. Early self-reproduction, the emergence of division mechanisms in protocells. Mol BioSyst. 2013;9(2):195–204. doi: 10.1039/c2mb25375e. [DOI] [PubMed] [Google Scholar]
- Noireaux V, Libchaber A. A vesicle bioreactor as a step toward an artificial cell assembly. Proc Natl Acad Sci USA. 2004;101(51):17669–17674. doi: 10.1073/pnas.0408236101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noireaux V, Bar-Ziv R, Godefroy J, Salman H, Libchaber A. Toward an artificial cell based on gene expression in vesicles. Phys Biol. 2005;2(3):P1–P8. doi: 10.1088/1478-3975/2/3/P01. [DOI] [PubMed] [Google Scholar]
- Noireaux V, Maeda YT, Libchaber A. Development of an artificial cell, from self-organization to computation and self-reproduction. Proc Natl Acad Sci USA. 2011;108(9):3473–3480. doi: 10.1073/pnas.1017075108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura SM, Tsumoto K, Hamada T, Akiyoshi K, Nakatani Y, Yoshikawa K. Gene expression within cell-sized lipid vesicles. ChemBioChem. 2003;4(11):1172–1175. doi: 10.1002/cbic.200300630. [DOI] [PubMed] [Google Scholar]
- Nourian Z, Danelon C. Linking genotype and phenotype in protein synthesizing liposomes with external supply of resources. ACS Synth Biol. 2013;2(4):186–193. doi: 10.1021/sb300125z. [DOI] [PubMed] [Google Scholar]
- Nourian Z, Roelofsen W, Danelon C. Triggered gene expression in fed-vesicle microreactors with a multifunctional membrane. Angew Chem Int Ed. 2012;51(13):3114–3118. doi: 10.1002/anie.201107123. [DOI] [PubMed] [Google Scholar]
- Osawa M, Erickson HP. Liposome division by a simple bacterial division machinery. Proc Natl Acad Sci USA. 2013;110(27):11000–11004. doi: 10.1073/pnas.1222254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Anderson DE, Erickson HP. Reconstitution of contractile FtsZ rings in liposomes. Science. 2008;320(5877):792–794. doi: 10.1126/science.1154520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa M, Anderson DE, Erickson HP. Curved FtsZ protofilaments generate bending forces on liposome membranes. EMBO J. 2009;28(22):3476–3484. doi: 10.1038/emboj.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303(5657):495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- Peterlin P, Arrigler V, Kogej K, Svetina S, Walde P. Growth and shape transformations of giant phospholipid vesicles upon interaction with an aqueous oleic acid suspension. Chem Phys Lipids. 2009;159(2):67–76. doi: 10.1016/j.chemphyslip.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Saarikangas J, Zhao H, Pykäläinen A, Laurinmäki P, Mattila PK, Kinnunen PK, Butcher SJ, Lappalainen P. Molecular mechanisms of membrane deformation by I-BAR domain proteins. Curr Biol. 2009;19(2):95–107. doi: 10.1016/j.cub.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Saksena S, Wahlman J, Teis D, Johnson AE, Emr SD. Functional reconstitution of ESCRT-III assembly and disassembly. Cell. 2009;136:97–109. doi: 10.1016/j.cell.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma Y, Imai M. Model system of self-reproducing vesicles. Phys Rev Lett. 2011;107(19):198101. doi: 10.1103/PhysRevLett.107.198101. [DOI] [PubMed] [Google Scholar]
- Samson RY, Bell SD. Ancient ESCRTs and the evolution of binary fission. Trends Microbiol. 2009;17(11):507–513. doi: 10.1016/j.tim.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Samson RY, Obita T, Freund SM, Williams RL, Bell SD. A role for the ESCRT system in cell division in archaea. Science. 2008;322(5908):1710–1713. doi: 10.1126/science.1165322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson RY, Obita T, Hodgson B, Shaw MK, Chong PLG, Williams RL, Bell SD. Molecular and structural basis of ESCRT-III recruitment to membranes during archaeal cell division. Mol Cell. 2011;41(2):186–196. doi: 10.1016/j.molcel.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidli PK, Schurtenberger P, Luisi PL. Liposome-mediated enzymatic synthesis of phosphatidylcholine as an approach to self-replicating liposomes. J Am Chem Soc. 1991;113(21):8127–8130. doi: 10.1021/ja00021a043. [DOI] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwille P. Bottom-up synthetic biology: engineering in a tinkerer’s world. Science. 2011;333(6047):1252–1254. doi: 10.1126/science.1211701. [DOI] [PubMed] [Google Scholar]
- Shih YL, Huang KF, Lai HM, Liao JH, Lee CS, Chang CM, Mak HM, Hsieh CW, Lin CC. The N-terminal amphipathic helix of the topological specificity factor MinE is associated with shaping membrane curvature. PLoS One. 2011;6(6):e21425. doi: 10.1371/journal.pone.0021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y, Inoue A, Tomari Y, Suzuki T, Yokogawa T, Nishikawa K, Ueda T. Cell-free translation reconstituted with purified components. Nat Biotechnol. 2001;19(8):751–755. doi: 10.1038/90802. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Kanamori T, Ueda T. Protein synthesis by pure translation systems. Methods. 2005;36(3):299–304. doi: 10.1016/j.ymeth.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Kuruma Y, Kanamori T, Ueda T. The PURE system for protein production. Methods Mol Biol. 2014;1118:275–284. doi: 10.1007/978-1-62703-782-2_19. [DOI] [PubMed] [Google Scholar]
- Shin J, Noireaux V. Efficient cell-free expression with the endogenous E. Coli RNA polymerase and sigma factor 70. J Biol Eng. 2010;4:8. doi: 10.1186/1754-1611-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorre B, Callan-Jones A, Manzi J, Goud B, Prost J, Bassereau P, Roux A. Nature of curvature coupling of amphiphysin with membranes depends on its bound density. Proc Natl Acad Sci USA. 2012;109(1):173–178. doi: 10.1073/pnas.1103594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahelin RV, Long F, Peter BJ, Murray D, De Camilli P, McMahon HT, Cho W. Contrasting membrane interaction mechanisms of AP180 N-terminal homology (ANTH) and epsin N-terminal homology (ENTH) domains. J Biol Chem. 2003;278(31):28993–28999. doi: 10.1074/jbc.M302865200. [DOI] [PubMed] [Google Scholar]
- Stano P, Luisi PL. Achievements and open questions in the self-reproduction of vesicles and synthetic minimal cells. Chem Commun. 2010;46(21):3639–3653. doi: 10.1039/b913997d. [DOI] [PubMed] [Google Scholar]
- Suefuji K, Valluzzi R, RayChaudhuri D. Dynamic assembly of MinD into filament bundles modulated by ATP, phospholipids, and MinE. Proc Natl Acad Sci USA. 2002;99(26):16776–16781. doi: 10.1073/pnas.262671699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetina S. Vesicle budding and the origin of cellular life. ChemPhysChem. 2009;10(16):2769–2776. doi: 10.1002/cphc.200900577. [DOI] [PubMed] [Google Scholar]
- Szostak JW, Bartel DP, Luisi PL. Synthesizing life. Nature. 2001;409(6818):387–390. doi: 10.1038/35053176. [DOI] [PubMed] [Google Scholar]
- Takakura K, Toyota T, Sugawara T. A novel system of self-reproducing giant vesicles. J Am Chem Soc. 2003;125(27):8134–8140. doi: 10.1021/ja029379a. [DOI] [PubMed] [Google Scholar]
- Takamura H, Koyama Y, Matsuzaki S, Yamada K, Hattori T, Miyata S, Takemoto K, Tohyama M, Katayama T. TRAP1 controls mitochondrial fusion/fission balance through Drp1 and Mff expression. PLoS One. 2012;7:e51912. doi: 10.1371/journal.pone.0051912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K, Slepnev VI, Haucke V, De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol. 1999;1(1):33–39. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- van Nies P, Nourian Z, Kok M, van Wijk R, Moeskops J, Westerlaken I, Poolman JM, Eelkema R, van Esch JH, Kuruma Y, Ueda T, Danelon C. Unbiased tracking of the progression of mRNA and protein synthesis in bulk and inside lipid vesicles. ChemBioChem. 2013;14(15):1963–1966. doi: 10.1002/cbic.201300449. [DOI] [PubMed] [Google Scholar]
- Walde P. Building artificial cells and protocell models: experimental approaches with lipid vesicles. Bioessays. 2010;32(4):296–303. doi: 10.1002/bies.200900141. [DOI] [PubMed] [Google Scholar]
- Wick R, Luisi PL. Enzyme-containing liposomes can endogenously produce membrane-constituting lipids. Chem Biol. 1996;3(4):277–285. doi: 10.1016/S1074-5521(96)90107-6. [DOI] [PubMed] [Google Scholar]
- Woese C. The universal ancestor. Proc Natl Acad Sci USA. 1998;95(12):6854–6859. doi: 10.1073/pnas.95.12.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464(7290):864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009;458(7235):172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Arkhipov A, Schulten K. Simulations of membrane tubulation by lattices of amphiphysin N-BAR domains. Structure. 2009;17(6):882–892. doi: 10.1016/j.str.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Pykäläinen A, Lappalainen P. I-BAR domain proteins: linking actin and plasma membrane dynamics. Curr Opin Cell Biol. 2011;23(1):14–21. doi: 10.1016/j.ceb.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J, Kozlov MM. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol. 2006;7(1):9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]