Abstract
Along with their well known role in nicotine addiction and autonomic physiology, neuronal nicotinic receptors (nAChRs) also have profound analgesic effects in animal models and humans. This is not a new idea, even in the early 1500s, soon after tobacco was introduced to the new world, its proponents listed pain relief among the beneficial properties of smoking. In recent years, analgesics that target specific nAChR subtypes have shown highly efficacious antinociceptive properties in acute and chronic pain models. To date, the side effects of these drugs have precluded their advancement to the clinic. This review summarizes the recent efforts to identify novel analgesics that target nAChRs, and outlines some of the key neural substrates that contribute to these physiological effects. There remain many unanswered mechanistic questions in this field, and there are still compelling reasons to explore neuronal nAChRs as targets for the relief of pain.
Keywords: Nicotinic, Pain, Nociception, Analgesia
1. Introduction
The last two decades have seen an increase in the use of opioid drugs for the treatment of chronic non-cancer pain in the United States. Although opioid analgesics are highly efficacious [1], the associated side effects limit their usefulness [2]. In addition, opioids have high abuse liability [3]. Although the majority of chronic pain patients do not abuse prescription opioids [3], as noted by Compton and Volkow, the increases in opioid pain prescriptions and opioid abuse have been correlated [4]. Thus, there is a strong need for effective chronic pain treatments that lack the side effects of opioids.
1.1. Nicotine and analgesia
Nicotinic acetylcholine receptors (nAChRs) may represent viable targets for novel analgesics. Although the pain-relieving properties of nicotinic acetylcholine receptors (nAChRs) are relatively recent findings, the link between nicotine and analgesia is not new. Oviedo y Valdes, a Spanish historian, first reported that tobacco relieved pain caused by syphilis in the early 16th century [5]. However, nicotine, a major constituent of tobacco, was not linked with analgesia until the 20th century when nicotine application was found to produce antinociception in cats [6]. More recent research has revealed that nicotine microinjection into various brainstem regions could be either pronociceptive or antinociceptive [7]. In addition, nicotine-induced antinociception can be blocked by mecamylamine, a nonselective neuronal nAChR antagonist [8]. The analgesic effect of nicotine has also been replicated in humans, as nicotine reduces acute pain experienced from a cold pressor test in both smokers and ex-smokers [9]. Although these findings supported developing nicotine as an analgesic, evidence showing its addictive effects raised concern [10,11]. Furthermore, repeated administration of nicotine induces tolerance to antinociceptive effects in rats [12]. Despite these concerns, the focus on nAChRs as analgesic targets has become an active area of investigation.
1.2. nAChR-mediated antinociception
In 1974, while conducting experiments in Ecuador, John Daly and Charles Myers found that a frog skin extract from Phyllobates anthonyi induces a Straub tail response, indicative of opioid expression in the extract [13,14]. However, Daly later showed naloxone, an opioid antagonist, did not block the extract-induced Straub-tail response [13,15]. Eventually the active compound, known as epibatidine [15], was isolated and subsequently shown to induce nAChR-mediated antinociception in rodents [16,17]. Epibatidine is a nAChR agonist that binds to several nAChR subtypes, including α7, α4β2, and the neuromuscular α1β1δγ subtype [18]. Antinociceptive efficacy of epibatidine is similar to morphine with 100-fold higher potency in the hot-plate test [15]. Although epibatidine displays promising antinociceptive effects, it also induces adverse side effects, including dose-dependent decreases in body temperature and locomotor activity [16]. In addition, epibatidine increases blood pressure in rodents, which may show sensitization, as repeated administration can transform nonlethal doses into lethal doses [18]. The nonselective nAChR agonist properties of epibatidine raise the possibility that distinct subsets of nAChRs mediate its antinociceptive vs. adverse effects [19].
2. nAChRs as analgesic targets
2.1. α4β2 nAChRs
Researchers at Abbott labs initially focused on α4β2 nAChR agonists with the goal of developing treatments for Alzheimer’s disease [13,14]. However, they discovered structural similarities between their candidate drugs and epibatidine [13]. This lead to the development of ABT-594 [13], a compound closely related to epitabidine, with higher selectivity for α4β2 nAChRs [19]. ABT-594 produces antinociception that is more potent than either nicotine or morphine, with equal or higher efficacy in multiple preclinical pain models (acute and chronic) [19–21]. Similar to the anitinociceptive effects of nicotine and epibatidine, ABT-594-induced antinociception is also blocked by mecamylamine [19,20]. Furthermore, ABT-594 does not perturb the cardiovascular system, particularly when compared to epibatidine [22], and does not cause signs of physical dependence in mice. Thus, the preclinical data supported the view that ABT-594 could be a promising therapeutic drug for chronic pain patients. However, drug development was hindered by phase II clinical trial findings. Low doses of ABT-594, which were well-tolerated by the patients, did not have significant analgesic effects [23]. Higher ABT-594 doses (150 to 300 µg) significantly reduced pain, however this was accompanied by significant adverse effects (e.g. nausea, dizziness, vomiting) relative to placebo among patients in three different treatment groups for neuropathic pain [23]. Furthermore, patient withdrawal frequency directly correlated with increasing dose [23]. Despite analgesic efficacy comparable to opioid drugs, the prevalence and severity of adverse effects precluded further ABT-594 drug development.
Despite this setback, targeting a specific nAChR subtype may retain the analgesic properties of ABT-594 or epibatidine without the problematic side effects. Two approaches come to mind, if we focus on the major nAChR subtypes: either target non- α4β2 nAChR subtypes, or optimize α4β2 nAChR activation to minimize the adverse effects. A recent study reported that intra-cerebroventricular (i.c.v.) administration of a selective α4β2 agonist reduced the allodynic effects of splanchnic nerve ligation, which was attributed to nAChR binding in thalamus [24]. Antiallodynic effects are seen with a 5 nmol injection, but not a 50 nmol injection, which supports the idea that receptor desensitization can reverse therapeutic effects at high agonist doses.
2.2. α5-containing and α3β4 nAChRs
Residual nAChR-mediated analgesia occurs in mice lacking the α4, or β2 nAChR subunit [25]. Therefore, other nAChR subtypes likely contribute to this behavior. Potential candidates include α5-containing nAChRs, α3β4, and α7 homomeric receptors. The α5 subunits do not form functional receptors alone or in heteromeric complexes with beta subunits. They can be incorporated with α4β2, α3β4, or α3β2 nAChR subtypes, where they alter both pharmacology and physiology of these receptor complexes [26–28]. Mice that lack the α5 subunit display lower antinociceptive potency and efficacy of nicotine relative to wildtype animals [29]. Furthermore, epibatidine-induced antinociception is completely absent in α5-KO mice [29]. However, more work is needed to evaluate the contribution of the α5 nAChR subunit to antinociception in other preclinical models of pain. The α3β4 nAChR subtype is highly expressed in autonomic ganglia, and a recent study demonstrated that the selective antagonist conotoxin AuIB, has no effect on nicotine-mediated antinociception [30].
2.3. α7 nAChRs
In addition to α4β2, the α7 homomeric nAChR subtype is another target for analgesia. Systemic and intrathecal injection of choline, an α7 nAChR selective agonist, produces antinociception [31–33]. There is also evidence for an analgesic role for supraspinal α7 containing nAChRs as injection of a choline analog i.c.v. produces antinociception [34,35]. Potential sites of action for α7-mediated analgesia are discussed in more detail below.
Despite these encouraging results, there are mixed reports on α7 nAChR-mediated analgesia. In recent findings by Gao et al. [36] administration of some α7 agonists result in antinociception, while others have no effect. Animals treated with Compound Q ((S)-5-(pyridin-2-yl)-N-(quinuclidin-3-yl)thiophene-2-carboxamide), a selective α7 nAChR agonist, display antinociception at 50 mg/kg, which is intriguing as Compound Q does not penetrate the blood brain barrier [36]. Thus, peripheral α7 nAChRs could contribute to α7-mediated analgesia.
A subset of α7 nAChR agonists also antagonize spinal 5-HT3 receptor activity [37,38]. After inflammation or injury, 5-HT3 receptor activation contributes to nociceptive facilitation via supraspinal descending pathways [39,40]. Considering that tropisetron, an α7 agonist in this subset, has shown clinical efficacy in fibromyalgia patients [41], the activation of α7 nAChRs along with inhibition of 5-HT3Rs, could lead to a synergistic antinociceptive effect. In addition, α7 activation inhibits serotonin release in the dorsal spinal cord [42], which could further reduce 5-HT signaling and potentially enhance antinociception [43].
2.4. nAChR positive allosteric modulators
An alternative approach to activating nAChRs is to utilize positive allosteric modulators (PAMs), molecules that enhance agonist-evoked response by reducing energy required for a receptor to move from resting to active state [44]. PAMs can be classified into two categories: Type I that enhance receptor function but do not affect desensitization properties, and Type II that reduce receptor desensitization, thereby prolonging the receptor activation [44]. PNU-120596, a Type II α7 PAM, reduces nociceptive behavior more effectively than NS-1738, a Type I α7 PAM, in a variety of preclinical nociceptive tests 6 hours post-injury [45]. This observation suggests that endogenous cholinergic signaling induces significant receptor desensitization and that limiting the transition to that state can inhibit nociceptive signaling. Not surprisingly, co-application of PNU-120596 with an α7 nAChR agonist enhanced the antinociceptive effects relative to either the PAM or α7 agonist alone [32]. In addition, systemic injection of MLA, an α7-selective antagonist reverses α7 PAM – mediated antinociception [45]. These data suggest that combined α7 Type II PAM and agonist application could be an effective treatment for inflammatory pain.
Another useful approach involves developing novel drugs that target α4β2 nAChRs more selectively, to potentially minimize adverse effects caused by ABT-594. The advantage with this approach is the analgesic effects of α4β2 receptors has already been demonstrated in humans [23]. A-366833 is a recently developed α4β2-selective agonist that produces substantial antinociceptive effects in acute, neuropathic and inflammatory models of pain [46,47]. Furthermore, it does not induce emesis in ferrets at concentrations well above the therapeutic dose (ferrets are excellent animal models for emesis) [47]. These findings contrast with observations of Gao et al. [36] who found no antinociceptive effects of systemic administration of the selective α4β2 agonist, ispronicline in preclinical pain models.
PAMs provide an alternate strategy for activating α4β2 nAChRs. Recent findings demonstrate the therapeutic potential of a combined administration of a Type 1 PAM, NS9283 with ABT-594 [48]. NS9283 increases acetylcholine currents without affecting desensitization kinetics [49]. In preclinical pain models, NS9283 enhances ABT-594 potency [48,50]. In addition, NS9283 does not shift emetic threshold [48]. This is important, as it shows that the combination of ABT-594 and NS9283 increases the therapeutic window for analgesic effects of both drugs. Gao et al. [36] tested a different PAM, Compound 2B, and found a decrease in nociceptive behaviors, albeit at extremely high doses [36]. Combined with the observation that NS9283 administration does not reduce mechanical allodynia in the spinal nerve ligation model [48], these results raise concerns about the therapeutic efficacy and potency for PAM-only administration. However, a PAM-plus-agonist approach could be therapeutically effective for multiple nAChR subtypes, including in α7 and α4β2 nAChRs.
3. Neural substrates for nAChR-dependent analgesia
Despite the pharmacological and behavioral evidence supporting nAChR-mediated analgesia, remarkably little is known about its underlying cellular mechanisms. Nicotinic receptors are found along ascending as well as descending nociceptive pathways and have been especially well characterized in the spinal cord dorsal horn and in sensory nerve afferents. In addition to the spinal cord, supraspinal areas are also critical sites of action for nicotinic analgesics, yet we know even less about the contribution and underlying mechanisms of nAChRs in these areas.
3.1. Peripheral nAChRs
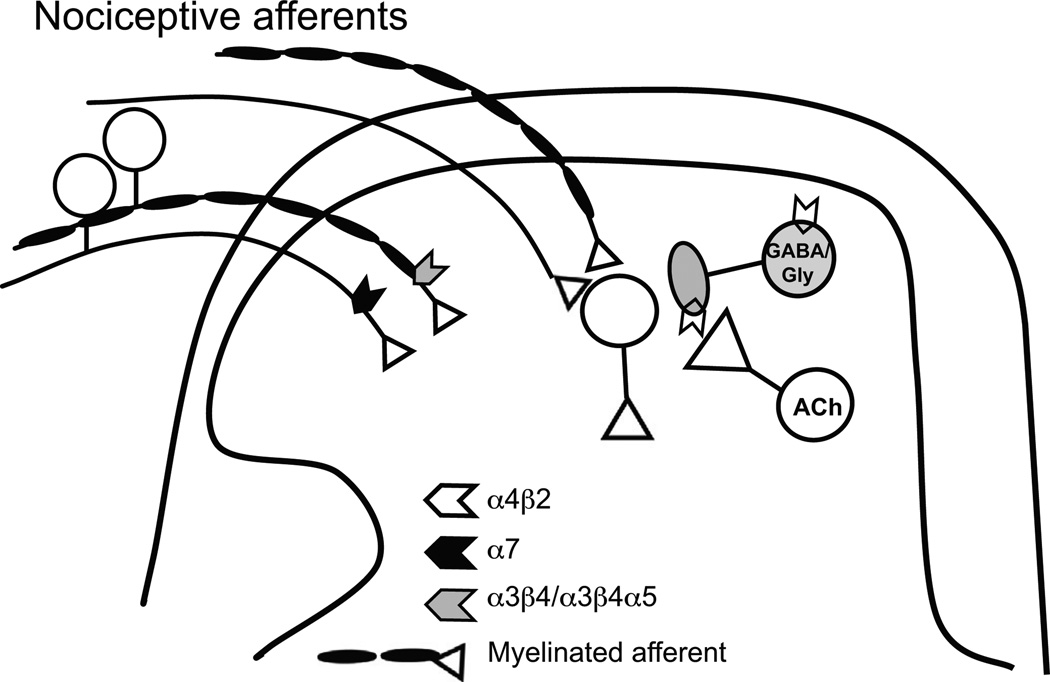
Nicotinic receptors are at the periphery at sites of injury where inflammation is initiated. Dorsal root ganglia (DRG) neurons predominantly express α7 nAChRs, which are especially prevalent in large diameter cells in neonates. This likely includes both Aδ, Aβ populations carrying nociceptive and non-nociceptive sensory information [51,52]. Here, α7 nAChRs enhance glutamate release probability and increase the likelihood of LTP in the dorsal horn [53]. Compared to neonates, α7 nAChR-expressing DRGs in older rats are exclusively found in unmyelinated (C fiber) nociceptors, although these likely include multiple nociceptive modalities [54]. Among these are both peptidergic and nonpeptidergic neurons, including some that bind IB4 and co-express α3β4α5 [54] (Fig. 1).
Fig. 1.
Nicotinic receptors modulate ascending nociceptive information. Myelinated nociceptive inputs to the spinal cord dorsal horn express α3β4/α3β4α5 nAChRs, while unmyelinated nociceptive afferents express α7 nAChRs [54,55]. Both may act to enhance nociception. By contrast, tonic cholinergic modulation of GABA and glycine transmission via α4β2 nAChRs is important for setting baseline inhibitory tone and the influence of incoming nociceptive activity.
In contrast to C fibers, myelinated nociceptors do not express α7, but rather contain α3β4α5 or α3β4 nAChR subtypes [54]. Human unmyelinated nociceptor axons contain nAChRs that increase excitability with pharmacology suggesting the α3β4α5 subtype. These nAChRs are pronociceptive because nAChR antagonist mecamylamine and amitrptyline, a drug used to treat neuropathic pain, both inhibit nicotine-induced enhancement of excitability in a similar manner [55,56]. These α3β4α5 expressing sensory neurons are likely to underlie the reported pronociceptive effects of α5 subunit [57] (Fig. 1).
Peripheral (intraplantar) application of nicotinic agonists selective for the α4β2 or α7 subtype is antinociceptive [19,58,59]. Nicotinic receptors on peripheral terminals may contribute to these effects, although direct evidence for this is lacking. This antinociceptive effect is surprising, given the excitatory effects of activating α7, α3β4α5 and α3β4α nAChRs on nociceptive DRG neurons that support a pronociceptive role. The mechanism for this disparity is not clear and requires further study.
Especially with respect to α7 antinociception, non neuronal cells at the periphery are also likely to contribute to antinociception after injury or inflammation. In nerve injury or inflammatory pain models, α7-mediated antihyperalgesia is associated with a decrease in the immune response at the periphery [60–62]. Cytokine release is inhibited by α7 activation on macrophages [63]. In addition to macrophages, other non neuronal peripheral cells such as keratinocytes and lymphocytes also express α7 and their anti-inflammatory effects likely contribute to reported antinociception [64]. These anti-inflammatory effects would further support therapeutic strategies aimed at α7 nAChRs, but further investigation is needed as to the extent of immune suppression in the long term.
Another nAChR subtype expressed in the periphery is the α9/ α10 subtype. DRG neurons as well as lymphocytes express α9/α10 [65,66]. Alpha-conotoxins RgIA and Vc1.1 are highly selective antagonists to this subtype compared to other nAChRs. Although efficacy in neuropathic pain models is demonstrated, clinical drug development is hindered by controversy over mechanism of action. Activation of presynaptic GABAB receptors on sensory afferents leads to N-type Ca2+ inhibition, which also contributes to the analgesic mechanism [67]. Interestingly, RgIA is 300-fold less potent for human α9/α10 nAChRs than those of the rat [68]. Nevertheless, RgIA and Vc1.1 still hold promise as analgesics due to their independent effects on GABAB receptors and anti-inflammatory effects [69].
3.2. Spinal cord dorsal horn
Intrathecal nicotine or epibatidine produce dose-dependent antinociception in the tail flick test [31,70]. These findings are contradicted by other reports either demonstrating no effect on acute nociception with intrathecal nicotine or epibatidine [71,72] algogenic responses [73],or antinociception only after nerve injury [74]. These mixed results from intrathecal administration reflect the diversity of nAChR subtypes and their effects among incoming sensory afferents and within spinal circuitry.
In the neonate, a subset of dorsal horn (DH) neurons contain somatic α7 nAChRs, and an equal number contain non-α7 nAChRs, with very little overlap of these nAChR subtypes. Local excitatory DH neurons as well as NK1 receptor-positive projection neurons have transcripts for α3, β2, and α7 with associations between the three transcripts in the mouse [75], which supports a pronociceptive role for spinal α7 in the neonate. However, these findings have not been confirmed in the adult. In fact, in adult mice, intrathecal agonists to α7 nAChRs are antinociceptive in both acute and persistent inflammatory models [32,76]. Furthermore, α7 antinociception is demonstrated in a model of colitis [77]. Thus, the preponderance of evidence supports the idea that α7 nAChRs on spinal neurons are antinociceptive, not pronociceptive (Fig. 1).
In contrast to sensory afferents, local inhibitory interneurons in the spinal cord dorsal horn contain transcripts for α4β2 nAChRs [75], and express functional α4β2 nAChRs at synaptic terminals where they enhance GABAergic drive onto DH neurons, possibly contributing to analgesic effects of nicotine [78]. Activation of these α4β2 nAChRs also reduces stimulus-driven LTD of inhibitory inputs, thereby preserving normal balance of inhibitory drive to prevent pathological excitation [78].
In the spinal cord glycinergic transmission is another key inhibitory regulator of nociception. Both GABA and glycinergic neurons contain transcripts for α4β2 nAChRs [75]. Glycinergic transmission is enhanced by presynaptic α4β2 nAChRs in spinal slices and in mechanically dissociated spinal lamina II neurons [79]. However, spinal α4β2 nAChRs are not merely analgesic targets, but actually play an important role in maintaining normal sensory thresholds. GABAergic and glycinergic drive is tonically enhanced by endogenous acetylcholine [80] (Fig. 1). Furthermore, mechanical and thermal thresholds are lower in β2 knockout mice [81].
It is possible that other nAChR subtypes exist in spinal circuitry that are yet to be identified. In the adult rat, nAChRs have been demonstrated on inhibitory terminals in the superficial dorsal horn that are not sensitive to selective antagonists of α4β2 or α7 subtypes [82]. Other subunits found among spinal inhibitory neurons whose nociceptive impact is not well-understood include α6 or α5, which may co-assemble with α4β2 nAChRs [75]. In addition, a subset of inhibitory neurons contain transcripts for the α2 subunit, a potentially promising therapeutic target, since these may be rare in supraspinal areas.
3.3. Supraspinal nAChRs
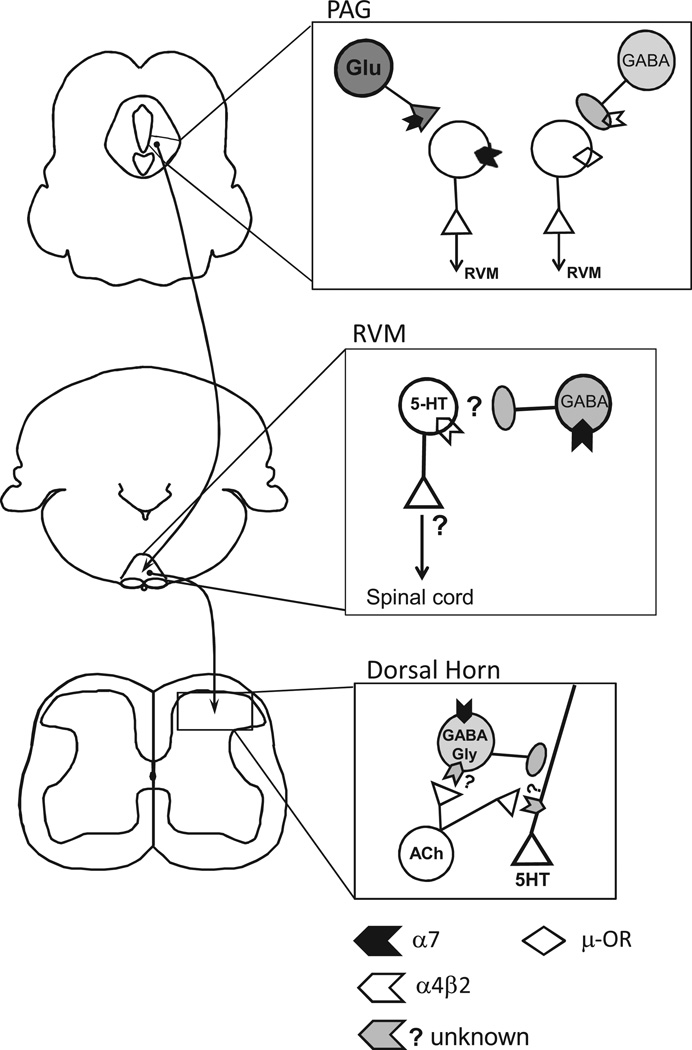
In addition to local spinal inhibitory influences, rostral (supraspinal) areas also exert strong influences on nociception and some of these areas contain nAChRs. The brainstem rostral ventromedial medulla (RVM) is the proposed site of action for the analgesic effects of the selective α4β2 agonist, ABT-594, as the effects are nearly abolished in a4 or β2 null mice [25] and are accompanied by increased c-fos expression in RVM neurons [19,83]. It is the activation of α4β2 in the RVM, not its desensitization that produces antinociception, as the effect is blocked by pretreatment with the nAChR anatagonist mecamylamine, but the antagonist alone had no effect [19].
Despite these compelling findings, the cellular mechanisms of nAChR analgesia in the RVM are not well understood. Serotonin signaling is implicated in these effects, as α4 immunostaining overlaps with markers for serotonin in the RVM, and α4 staining is reduced after selective ablation of serotonergic neurons [83,84]. We do not know how serotonergic RVM neurons exert these effects. In addition, it is clear that α4β2 on serotonergic neurons in the dorsal raphe as well as noradrenergic cell groups also contribute to systemic effects of ABT-594 [83,84] (Fig. 2). Finally, a spinal α4β2 contribution after nerve injury cannot be ruled out [74,80,81,85].
Fig. 2.
Nicotinic receptors modulate descending pain modulatory pathways. PAG:Our recent finding sindicate that a subset of ventrolateral PAG(vlPAG) neurons express α7 nAChRs on their somata (α7),and that this population was distinct from neurons that express µ-opioid receptors (µ-OR). Specifically, 50% (33/69) of vlPAG projection neurons responded to acetylcholine (ACh). Responses were blocked by the α7-selective antagonist MLA (n = 15). In a separate experiment, we observed 4 response classes among vlPAG projection neurons: nAChR-only responses (8/41). µ-OR– response accompanied by weak/no nAChR response (13/41), both nAChR and µ-OR responses (4/41), or no response of either type(16/41) (unpublished observations). In addition, activation of presynaptic α7 nAChRs increased glutamatergic drive to nAChR+neurons that project to the RVM. By contrast, nicotine-induced modulation of GABAergic inputs to nAChR+vlPAG projection neurons was rare. In the right panel, we hypothesize that presynaptic nAChRs modulate inhibitory drive to a majority of vlPAG projection neurons that lack the somatic nAChR. The presynaptic nAChR that mediates this phenomenon has not been characterized but is likely to be α4β2, based on previous work in the PAG [92]. We propose that presynaptic and somatic α7 nAChRs contribute to excitation of putative pain-inhibiting neurons in the vlPAG. This could be a supraspinal mechanism for α7 nAChR-mediated analgesia. RVM: Although the RVM is a well-documented site for supraspinal analgesia, the underlying circuitry is still not well-understood. Immunohistochemistry studies show that α4β2 nAChR subtype and 5-HT markers are co-localized [83]. However, electrophysiological approaches have not verified this finding. In addition, the specific projection targets of α4β2-containing 5HT RVM neurons are not known, particularly within the context of descending pain modulatory pathways. α7 nAChRs are expressed on GABA interneurons in the RVM [86]. Although the RVM is implicated in nicotinic analgesia via behavioral studies, more work is needed to investigate the cellular mechanisms at the circuit level. Spinal Dorsal Horn: Tonic endogenous cholinergic tone modulates serotonin release from descending inputs, which is mediated by nAChRs that are neither α4β2 nor α7. These receptors are located on descending serotonergic inputs, as well as on neighboring GABAergic interneurons that make axo-axonic contacts with serotonergic terminals. Additional modulation of 5HT release via a non-tonic mechanism is mediated by α7 nAChRs located on the somata of GABAergic interneurons that make axo-axonic contacts with descending serotonergic input. The impact of this modulation on nociception in normal or pathological states needs further investigation (modified from [42]).
In addition to α4β2 nAChRs, local inhibitory RVM neurons can express α7 nAChRs and the role these play in nociceptive modulation is not known [86]. The RVM exerts complex influences on nociception and can actually facilitate nociception, in part via serotonergic projections [40,87,88]. Spinal serotonin release via descending projections is also modulated by multiple subtypes of nAChRs residing on serotonergic terminals and their axo-axonal inputs from local interneurons [42]. Further study is needed to determine exactly how brainstem nAChRs fit into nociceptive facilitatory vs. inhibitory pathways and how this changes after injury.
Intra-cerebroventricular administration of choline, an α7 nAChR selective agonist, is antinociceptive in acute, inflammatory and neuropathic pain and an α7-selective antagonist blocks these effects [33–35,89]. Thus supraspinal α7 nAChRs are antinociceptive, but the sites of action are not known. A strong candidate is the midbrain periaqueductal gray (PAG), which relays information from limbic and homeostatic centers (hypothalamus and amygdala) to the spinal cord via the RVM. The PAG contains nAChRs including α7 and locally administered nicotinic agonists produce antinociception [90,91]. Interestingly, our recent findings suggest that α7 nAChRs are expressed on a distinct population of PAG-RVM projection neurons from those expressing somatic µ opioid receptors (unpublished material). In dissociated PAG neurons α4β2 nAChRs on GABAergic terminals enhance release [92]. It will be important to confirm this in older animals and identify the neurons affected. In addition, it is unknown whether excitatory synaptic transmission is also modulated by nicotinic receptors in this area. Although there is evidence for α2 nAChR subunit mRNA in the PAG a nociceptive role for these has not been demonstrated [93]. We know surprisingly little about the distribution of nicotinic subtypes in the PAG, and whether they function to modulate descending facilitatory or inhibitory nociceptive pathways.
4. Conclusions
In summary, nAChRs mediate antinociception via various receptor subtypes distributed among multiple central and peripheral nociceptive areas. This diversity of neural substrates and nAChR subtypes provides a rich environment for analgesic drug development. This richness also carries cost, as unwanted side effects have sidelined some of the strongest candidate nicotinic analgesic drugs. Perhaps the combination of agonists with selective positive allosteric modulators will provide an efficacious strategy for targeting specific receptor populations and optimizing their physiological impact. Although our knowledge base has improved in recent years, the limits of our understanding about the sites of action and cellular mechanisms involved in nicotinic analgesia is remarkable, particularly in supraspinal areas. Successful analgesic drug development will require better understanding of these neural circuits, how they are modified by chronic pain, and how nAChRs can be exploited to suppress pain signaling.
Acknowledgments
This work was supported by grants from the Frank Family Fellowship (I.U.) and the NIH DA07255 (C.D.), DA015918, and DA019695 (D.S.M.)
References
- 1.Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: a review of the evidence. Clinical Journal of Pain. 2008;24:469–478. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- 2.Baldini A, Von Korff M, Lin EH. A review of potential adverse effects of long-term opioid therapy: a practitioner’s guide. Primary Care Companion for CNS Disorders. 2012:14. doi: 10.4088/PCC.11m01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on problems of drug dependence taskforce on prescription opioid non-medical use and abuse: position statement. Drug and Alcohol Dependence. 2003;69:215–232. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 4.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug and Alcohol Dependence. 2006;81:103–108. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Corti C. Trans: Kessinger Publishing; 2007. A history of smoking. [Google Scholar]
- 6.Davis L, Pollock LJ, Stone T. Visceral pain. Surgery Gynecology and Obstetrics. 1932;55:418–427. [Google Scholar]
- 7.Hamann SR, Martin WR. Opioid and nicotinic analgesic and hyperalgesic loci in the rat brain stem. Journal of Pharmacology and Experimental Therapeutics. 1992;261:707–715. [PubMed] [Google Scholar]
- 8.Sahley TL, Berntson GG. Antinociceptive effects of central and systemic administrations of nicotine in the rat. Psychopharmacology. 1979;65:279–283. doi: 10.1007/BF00492216. [DOI] [PubMed] [Google Scholar]
- 9.Fertig JB, Pomerleau OF, Sanders B. Nicotine-produced antinociception in minimally deprived smokers and ex-smokers. Addictive Behaviors. 1986;11:239–248. doi: 10.1016/0306-4603(86)90052-3. [DOI] [PubMed] [Google Scholar]
- 10.Henningfield JE, Miyasato K, Jasinski DR. Abuse liability and pharmacodynamic characteristics of intravenous and inhaled nicotine. Journal of Pharmacology and Experimental Therapeutics. 1985;234:1–12. [PubMed] [Google Scholar]
- 11.Kottke TE, Brekke ML, Solberg LI, Hughes JR. A randomized trial to increase smoking intervention by physicians. Doctors helping smokers, Round I. JAMA. 1989;261:2101–2106. [PubMed] [Google Scholar]
- 12.Cepeda-Benito A, Reynoso J, McDaniel EH. Associative tolerance to nicotine analgesia in the rat: tail-flick and hot-plate tests. Experimental and Clinical Psychopharmacology. 1998;6:248–254. doi: 10.1037//1064-1297.6.3.248. [DOI] [PubMed] [Google Scholar]
- 13.Garraffo HMS, Thomas F, Williams Michael. Epibatidine: from frog alkaloid to analgesic clinical candidates. A Testimonial to true grit! Heterocycles. 2009;79:207–217. [Google Scholar]
- 14.Daly JW, Garraffo HM, Spande TF, Decker MW, Sullivan JP, Williams M. Alkaloids from frog skin: the discovery of epibatidine and the potential for developing novel non-opioid analgesics. Natural Products Reports. 2000;17:131–135. doi: 10.1039/a900728h. [DOI] [PubMed] [Google Scholar]
- 15.Spande TF, Garraffo HM, Edwards MW, Yeh HJ, Pannell L, Daly JW. Epibatidine: a novel (chloropyridyl) azabicycloheptane with potent analgesic activity from an Ecuadoran poison frog. Journal of the American Chemical Society. 1992;114:3475–3478. [Google Scholar]
- 16.Sullivan JP, Decker MW, Brioni JD, Donnelly-Roberts D, Anderson DJ, Bannon AW, et al. (+/−)-Epibatidine elicits a diversity of in vitro and in vivo effects mediated by nicotinic acetylcholine receptors. Journal of Pharmacology and Experimental Therapeutics. 1994;271:624–631. [PubMed] [Google Scholar]
- 17.Rupniak NM, Patel S, Marwood R, Webb J, Traynor JR, Elliott J, et al. Antinociceptive and toxic effects of (+)-epibatidine oxalate attributable to nicotinic agonist activity. British Journal of Pharmacology. 1994;113:1487–1493. doi: 10.1111/j.1476-5381.1994.tb17164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan J, Briggs C, Donnelly-Roberts D, Brioni J, Radek R, McKenna D, et al. (+/−)-Epibatidine can differentially evoke responses mediated by putative subtypes of nicotinic acetylcholine receptors (nAChRs) Medicinal Chemistry Research. 1994;4:502–516. [Google Scholar]
- 19.Bannon AW, Decker MW, Holladay MW, Curzon P, Donnelly-Roberts D, Puttfarcken PS, et al. Broad-spectrum, non-opioid analgesic activity by selective modulation of neuronal nicotinic acetylcholine receptors. Science. 1998;279:77–81. doi: 10.1126/science.279.5347.77. [DOI] [PubMed] [Google Scholar]
- 20.Lynch JJ, 3rd, Wade CL, Mikusa JP, Decker MW, Honore P. ABT-594 (a nicotinic acetylcholine agonist): anti-allodynia in a rat chemotherapy-induced pain model. European Journal of Pharmacology. 2005;509:43–48. doi: 10.1016/j.ejphar.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 21.Kesingland AC, Gentry CT, Panesar MS, Bowes MA, Vernier JM, Cube R, et al. Analgesic profile of the nicotinic acetylcholine receptor agonists, (+)-epibatidine and ABT-594 in models of persistent inflammatory and neuropathic pain. Pain. 2000;86:113–118. doi: 10.1016/s0304-3959(00)00233-5. [DOI] [PubMed] [Google Scholar]
- 22.Holladay MW, Wasicak JT, Lin NH, He Y, Ryther KB, Bannon AW, et al. Identification and initial structure-activity relationships of (R)-5-(2-azetidinylmethoxy)-2-chloropyridine (ABT-594), a potent, orally active, non-opiate analgesic agent acting via neuronal nicotinic acetylcholine receptors. Journal of Medicinal Chemistry. 1998;41:407–412. doi: 10.1021/jm9706224. [DOI] [PubMed] [Google Scholar]
- 23.Rowbotham MC, Duan WR, Thomas J, Nothaft W, Backonja MM. A randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of ABT-594 in patients with diabetic peripheral neuropathic pain. Pain. 2009;146:245–252. doi: 10.1016/j.pain.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Ueda M, Iida Y, Tominaga A, Yoneyama T, Ogawa M, Magata Y, et al. Nicotinic acetylcholine receptors expressed in the ventralposterolateral thalamic nucleus play an important role in anti-allodynic effects. British Journal of Pharmacology. 2010;159:1201–1210. doi: 10.1111/j.1476-5381.2009.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Lena C, Le Novere N, de Kerchove d’Exaerde A, et al. Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature. 1999;398:805–810. doi: 10.1038/19756. [DOI] [PubMed] [Google Scholar]
- 26.Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. Functional contributions of α5 subunit to neuronal acetylcholine receptor channels. Nature. 1996;380:347–351. doi: 10.1038/380347a0. [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, et al. Assembly of human neuronal nicotinic receptor α5 subunits with α3, β2, and β4 subunits. Journal of Biological Chemistry. 1996;271:17656–17665. doi: 10.1074/jbc.271.30.17656. [DOI] [PubMed] [Google Scholar]
- 28.Gerzanich V, Wang F, Kuryatov A, Lindstrom J. α5 Subunit alters desensitiza-tion, pharmacology, Ca2+ permeability and Ca2+ modulation of human neuronal α3 nicotinic receptors. Journal of Pharmacology and Experimental Therapeutics. 1998;286:311–320. [PubMed] [Google Scholar]
- 29.Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, et al. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. Journal of Pharmacology and Experimental Therapeutics. 2010;334:137–146. doi: 10.1124/jpet.110.165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson KJ, Sanjakdar SS, Muldoon PP, McIntosh JM, The Damaj MI. α3β4* nicotinic acetylcholine receptor subtype mediates nicotine reward and physical nicotine withdrawal signs independently of the α5 subunit in the mouse. Neuropharmacology. 2013;70:228–235. doi: 10.1016/j.neuropharm.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damaj MI, Fei-Yin M, Dukat M, Glassco W, Glennon RA, Martin BR. Antinociceptive responses to nicotinic acetylcholine receptor ligands after systemic and intrathecal administration in mice. Journal of Pharmacology and Experimental Therapeutics. 1998;284:1058–1065. [PubMed] [Google Scholar]
- 32.Freitas K, Negus SS, Carroll FI, Damaj MI. In vivo pharmacological interactions between a type II positive allosteric modulator of α7 nicotinic ACh receptors and nicotinic agonists in a murine tonic pain model. British Journal of Pharmacology. 2013;169:567–579. doi: 10.1111/j.1476-5381.2012.02226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Su DM, Wang RH, Liu Y, Wang H. Antinociceptive effects of choline against acute and inflammatory pain. Neuroscience. 2005;132:49–56. doi: 10.1016/j.neuroscience.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 34.Bagdas D, Sonat FA, Hamurtekin E, Sonal S, Gurun MS. The antihyperalgesic effect of cytidine-5’-diphosphate-choline in neuropathic and inflammatory pain models. Behavioural Pharmacology. 2011;22:589–598. doi: 10.1097/FBP.0b013e32834a1efb. [DOI] [PubMed] [Google Scholar]
- 35.Hamurtekin E, Gurun MS. The antinociceptive effects of centrally administered CDP-choline on acute pain models in rats: the involvement of cholinergic system. Brain Research. 2006;1117:92–100. doi: 10.1016/j.brainres.2006.07.118. [DOI] [PubMed] [Google Scholar]
- 36.Gao B, Hierl M, Clarkin K, Juan T, Nguyen H, Valk M, et al. Pharmacological effects of nonselective and subtype-selective nicotinic acetylcholine receptor agonists in animal models of persistent pain. Pain. 2010;149:33–49. doi: 10.1016/j.pain.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Macor JE, Gurley D, Lanthorn T, Loch J, Mack RA, Mullen G, et al. The 5-HT3 antagonist tropisetron (ICS 205–930) is a potent and selective α7 nicotinic receptor partial agonist. Bioorganic and Medicinal Chemistry Letters. 2001;11:319–321. doi: 10.1016/s0960-894x(00)00670-3. [DOI] [PubMed] [Google Scholar]
- 38.Broad LM, Felthouse C, Zwart R, McPhie GI, Pearson KH, Craig PJ, et al. PSAB-OFP, a selective α7 nicotinic receptor agonist, is also a potent agonist of the 5-HT3 receptor. European Journal of Pharmacology. 2002;452:137–144. doi: 10.1016/s0014-2999(02)02273-2. [DOI] [PubMed] [Google Scholar]
- 39.Oatway MA, Chen Y, Weaver LC. The 5-HT3 receptor facilitates at-level mechanical allodynia following spinal cord injury. Pain. 2004;110:259–268. doi: 10.1016/j.pain.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nature Neuroscience. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- 41.Farber L, Stratz T, Bruckle W, Spath M, Pongratz D, Lautenschlager J, et al. Efficacy and tolerability of tropisetron in primary fibromyalgia—a highly selective and competitive 5-HT3 receptor antagonist. Scandinavian Journal of Rheumatology. 2000;113(Suppl.):49–54. doi: 10.1080/030097400446643. [DOI] [PubMed] [Google Scholar]
- 42.Cordero-Erausquin M, Changeux JP. Tonic nicotinic modulation of serotoninergic transmission in the spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2803–2807. doi: 10.1073/pnas.041600698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furst S. Transmitters involved in antinociception in the spinal cord. Brain Research Bulletin. 1999;48:129–141. doi: 10.1016/s0361-9230(98)00159-2. [DOI] [PubMed] [Google Scholar]
- 44.Bertrand D, Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochemical Pharmacology. 2007;74:1155–1163. doi: 10.1016/j.bcp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Freitas K, Ghosh S, Ivy Carroll F, Lichtman AH, Imad Damaj M. Effects of alpha 7 positive allosteric modulators in murine inflammatory and chronic neuropathic pain models. Neuropharmacology. 2013;65:156–164. doi: 10.1016/j.neuropharm.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nirogi R, Jabaris SL, Jayarajan P, Abraham R, Shanmuganathan D, Rasheed MA, et al. Antinociceptive activity of α4β2* neuronal nicotinic receptor agonist A-366833 in experimental models of neuropathic and inflammatory pain. European Journal of Pharmacology. 2011;668:155–162. doi: 10.1016/j.ejphar.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 47.Ji J, Bunnelle WH, Anderson DJ, Faltynek C, Dyhring T, Ahring PK, et al. A-366833: a novel nicotinonitrile-substituted 3,6-diazabicyclo[3.2.0]-heptane α4β2 nicotinic acetylcholine receptor selective agonist: Synthesis, analgesic efficacy tolerability profile in animal models. Biochem. Pharmacology. 2007;74:1253–1262. doi: 10.1016/j.bcp.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 48.Lee CH, Zhu C, Malysz J, Campbell T, Shaughnessy T, Honore P, et al. α4β2 neuronal nicotinic receptor positive allosteric modulation: an approach for improving the therapeutic index of α4β2 nAChR agonists in pain. Biochemical Pharmacology. 2011;82:959–966. doi: 10.1016/j.bcp.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 49.Grupe M, Jensen AA, Ahring PK, Christensen JK, Grunnet M. Unravelling the mechanism of action of NS9283, a positive allosteric modulator of (alpha4)3(-beta2)2 nicotinic ACh receptors. British Journal of Pharmacology. 2013;168:2000–2010. doi: 10.1111/bph.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu CZ, Chin CL, Rustay NR, Zhong C, Mikusa J, Chandran P, et al. Potentiation of analgesic efficacy but not side effects: co-administration of an alpha4beta2 neuronal nicotinic acetylcholine receptor agonist and its positive allosteric modulator in experimental models of pain in rats. Biochemical Pharmacology. 2011;82:967–976. doi: 10.1016/j.bcp.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Dube GR, Kohlhaas KL, Rueter LE, Surowy CS, Meyer MD, Briggs CA. Loss of functional neuronal nicotinic receptors in dorsal root ganglion neurons in a rat model of neuropathic pain. Neuroscience Letters. 2005;376:29–34. doi: 10.1016/j.neulet.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 52.Genzen JR, Van Cleve W, McGehee DS. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. Journal of Neurophysiology. 2001;86:1773–1782. doi: 10.1152/jn.2001.86.4.1773. [DOI] [PubMed] [Google Scholar]
- 53.Genzen JR, McGehee DS. Short- and long-term enhancement of excitatory transmission in the spinal cord dorsal horn by nicotinic acetylcholine receptors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6807–6812. doi: 10.1073/pnas.1131709100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rau KK, Johnson RD, Cooper BY. Nicotinic AChR in subclassified capsaicin-sensitive and -insensitive nociceptors of the rat DRG. Journal of Neurophysiology. 2005;93:1358–1371. doi: 10.1152/jn.00591.2004. [DOI] [PubMed] [Google Scholar]
- 55.Lang PM, Burgstahler R, Sippel W, Irnich D, Schlotter-Weigel B, Grafe P. Characterization of neuronal nicotinic acetylcholine receptors in the membrane of unmyelinated human C-fiber axons by in vitro studies. Journal of Neurophysiology. 2003;90:3295–3303. doi: 10.1152/jn.00512.2003. [DOI] [PubMed] [Google Scholar]
- 56.Freysoldt A, Fleckenstein J, Lang PM, Irnich D, Grafe P, Carr RW. Low concentrations of amitriptyline inhibit nicotinic receptors in unmyelinated axons of human peripheral nerve. British Journal of Pharmacology. 2009;158:797–805. doi: 10.1111/j.1476-5381.2009.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vincler MA, Eisenach JC. Knock down of the α5 nicotinic acetylcholine receptor in spinal nerve-ligated rats alleviates mechanical allodynia. Pharmacology Biochemistry and Behavior. 2005;80:135–143. doi: 10.1016/j.pbb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 58.Rueter LE, Kohlhaas KL, Curzon P, Surowy CS, Meyer MD. Peripheral and central sites of action for A-85380 in the spinal nerve ligation model of neuropathic pain. Pain. 2003;103:269–276. doi: 10.1016/s0304-3959(02)00455-4. [DOI] [PubMed] [Google Scholar]
- 59.Gurun MS, Parker R, Eisenach JC, Vincler M. The effect of peripherally administered CDP-choline in an acute inflammatory pain model: the role of α7 nicotinic acetylcholine receptor. Anesthesia and Analgesia. 2009;108:1680–1687. doi: 10.1213/ane.0b013e31819dcd08. [DOI] [PubMed] [Google Scholar]
- 60.Gahring LC, Osborne AV, Reed M, Rogers SW. Neuronal nicotinic alpha7 receptors modulate early neutrophil infiltration to sites of skin inflammation. Journal of Neuroinflammation. 2010;7:38. doi: 10.1186/1742-2094-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Osborne-Hereford AV, Rogers SW, Gahring LC. Neuronal nicotinic alpha7 receptors modulate inflammatory cytokine production in the skin following ultraviolet radiation. Journal of Neuroimmunology. 2008;193:130–139. doi: 10.1016/j.jneuroim.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rowley TJ, McKinstry A, Greenidge E, Smith W, Flood P. Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. British Journal of Anaesthesia. 2010;105:201–207. doi: 10.1093/bja/aeq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 64.Gahring LC, Rogers SW. Neuronal nicotinic acetylcholine receptor expression and function on nonneuronal cells. AAPS Journals. 2005;7:E885–E894. doi: 10.1208/aapsj070486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lips KS, Pfeil U, Kummer W. Coexpression of α9 and α10 nicotinic acetylcholine receptors in rat dorsal root ganglion neurons. Neuroscience. 2002;115:1–5. doi: 10.1016/s0306-4522(02)00274-9. [DOI] [PubMed] [Google Scholar]
- 66.Peng H, Ferris RL, Matthews T, Hiel H, Lopez-Albaitero A, Lustig LR. Characterization of the human nicotinic acetylcholine receptor subunit alpha (α) 9 (CHRNA9) and alpha (α) 10 (CHRNA10) in lymphocytes. Life Sciences. 2004;76:263–280. doi: 10.1016/j.lfs.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 67.Callaghan B, Haythornthwaite A, Berecki G, Clark RJ, Craik DJ, Analgesic Adams DJ. α-conotoxins Vc1.1 and Rg1A inhibit N-type calcium channels in rat sensory neurons via GABAB receptor activation. J. Neuroscience. 2008;28:10943–10951. doi: 10.1523/JNEUROSCI.3594-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azam L, McIntosh JM. Molecular basis for the differential sensitivity of rat and human α9α10 nAChRs to α-conotoxin RgIA. Journal of Neurochemistry. 2012;122:1137–1144. doi: 10.1111/j.1471-4159.2012.07867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vincler M, Wittenauer S, Parker R, Ellison M, Olivera BM, McIntosh JM. Molecular mechanism for analgesia involving specific antagonism of α9α10 nicotinic acetylcholine receptors. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17880–17884. doi: 10.1073/pnas.0608715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jurna I, Krauss P, Baldauf J. Depression by nicotine of pain-related nociceptive activity in the rat thalamus and spinal cord. Clinical Investigator. 1993;72:65–73. doi: 10.1007/BF00231121. [DOI] [PubMed] [Google Scholar]
- 71.Hama AT, Lloyd GK, Menzaghi F. The antinociceptive effect of intrathecal administration of epibatidine with clonidine or neostigmine in the formalin test in rats. Pain. 2001;91:131–138. doi: 10.1016/s0304-3959(00)00425-5. [DOI] [PubMed] [Google Scholar]
- 72.Rogers DT, Iwamoto ET. Multiple spinal mediators in parenteral nicotine-induced antinociception. Journal of Pharmacology and Experimental Therapeutics. 1993;267:341–349. [PubMed] [Google Scholar]
- 73.Khan IM, Buerkle H, Taylor P, Yaksh TL. Nociceptive and antinociceptive responses to intrathecally administered nicotinic agonists. Neuropharmacology. 1998;37:1515–1525. doi: 10.1016/s0028-3908(98)00143-9. [DOI] [PubMed] [Google Scholar]
- 74.Rashid MH, Ueda H. Neuropathy-specific analgesic action of intrathecal nicotinic agonists and its spinal GABA-mediated mechanism. Brain Research. 2002;953:53–62. doi: 10.1016/s0006-8993(02)03270-5. [DOI] [PubMed] [Google Scholar]
- 75.Cordero-Erausquin M, Pons S, Faure P, Changeux JP. Nicotine differentially activates inhibitory and excitatory neurons in the dorsal spinal cord. Pain. 2004;109:308–318. doi: 10.1016/j.pain.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 76.Damaj MI, Meyer EM, Martin BR. The antinociceptive effects α7 nicotinic agonists in an acute pain model. Neuropharmacology. 2000;39:2785–2791. doi: 10.1016/s0028-3908(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 77.Abdrakhmanova GR, AlSharari S, Kang M, Damaj MI, Akbarali HI. α7-nAChR-mediated suppression of hyperexcitability of colonic dorsal root ganglia neurons in experimental colitis. American Journal of Physiology Gastrointestinal and Liver Physiology. 2010;299:G761–G768. doi: 10.1152/ajpgi.00175.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Genzen JR, McGehee DS. Nicotinic modulation of GABAergic synaptic transmission in the spinal cord dorsal horn. Brain Research. 2005;1031:229–237. doi: 10.1016/j.brainres.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 79.Kiyosawa A, Katsurabayashi S, Akaike N, Pang ZP, Akaike N. Nicotine facilitates glycine release in the rat spinal dorsal horn. Journal of Physiology. 2001;536:101–110. doi: 10.1111/j.1469-7793.2001.t01-1-00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rashid MH, Furue H, Yoshimura M, Ueda H. Tonic inhibitory role of α4β2 subtype of nicotinic acetylcholine receptors on nociceptive transmission in the spinal cord in mice. Pain. 2006;125:125–135. doi: 10.1016/j.pain.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 81.Yalcin I, Charlet A, Cordero-Erausquin M, Tessier LH, Picciotto MR, Schlichter R, et al. Nociceptive thresholds are controlled through spinal β2-subunit-containing nicotinic acetylcholine receptors. Pain. 2011;152:2131–2137. doi: 10.1016/j.pain.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 82.Takeda D, Nakatsuka T, Papke R, Gu JG. Modulation of inhibitory synaptic activity by a non-α4β2, non-α7 subtype of nicotinic receptors in the substantia gelatinosa of adult rat spinal cord. Pain. 2003;101:13–23. doi: 10.1016/s0304-3959(02)00074-x. [DOI] [PubMed] [Google Scholar]
- 83.Bitner RS, Nikkel AL, Curzon P, Arneric SP, Bannon AW, Decker MW. Role of the nucleus raphe magnus in antinociception produced by ABT-594: immediate early gene responses possibly linked to neuronal nicotinic acetylcholine receptors on serotonergic neurons. Journal of Neuroscience. 1998;18:5426–5432. doi: 10.1523/JNEUROSCI.18-14-05426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cucchiaro G, Commons KG. α4 nicotinic acetylcholine receptor subunit links cholinergic to brainstem monoaminergic neurotransmission. Synapse. 2003;49:195–205. doi: 10.1002/syn.10218. [DOI] [PubMed] [Google Scholar]
- 85.Matsumoto M, Xie W, Inoue M, Ueda H. Evidence for the tonic inhibition of spinal pain by nicotinic cholinergic transmission through primary afferents. Molecular Pain. 2007;3:41. doi: 10.1186/1744-8069-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dehkordi O, Millis RM, Dennis GC, Jazini E, Williams C, Hussain D, et al. Expression of alpha-7 and alpha-4 nicotinic acetylcholine receptors by GABAergic neurons of rostral ventral medulla and caudal pons. Brain Research. 2007;1185:95–102. doi: 10.1016/j.brainres.2007.09.027. [DOI] [PubMed] [Google Scholar]
- 87.Godinez-Chaparro B, Lopez-Santillan FJ, Orduna P, Granados-Soto V. Secondary mechanical allodynia and hyperalgesia depend on descending facilitation mediated by spinal 5-HT(4), 5-HT(6) and 5-HT(7) receptors. Neuroscience. 2012;222:379–391. doi: 10.1016/j.neuroscience.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 88.Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Ossipov MH, et al. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. Journal of Neuroscience. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hamurtekin E, Bagdas D, Gurun MS. Possible involvement of supraspinal opioid and GABA receptors in CDP-choline-induced antinociception in acute pain models in rats. Neuroscience Letters. 2007;420:116–121. doi: 10.1016/j.neulet.2007.04.058. [DOI] [PubMed] [Google Scholar]
- 90.Baddick CG, Marks MJ. An autoradiographic survey of mouse brain nicotinic acetylcholine receptors defined by null mutants. Biochemical Pharmacology. 2011;82:828–841. doi: 10.1016/j.bcp.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guimaraes AP, Prado WA. Antinociceptive effects of carbachol microinjected into different portions of the mesencephalic periaqueductal gray matter of the rat. Brain Research. 1994;647:220–230. doi: 10.1016/0006-8993(94)91321-8. [DOI] [PubMed] [Google Scholar]
- 92.Nakamura M, Jang IS. Presynaptic nicotinic acetylcholine receptors enhance GABAergic synaptic transmission in rat periaqueductal gray neurons. European Journal of Pharmacology. 2010;640:178–184. doi: 10.1016/j.ejphar.2010.04.057. [DOI] [PubMed] [Google Scholar]
- 93.Ishii K, Wong JK, Sumikawa K. Comparison of α2 nicotinic acetylcholine receptor subunit mRNA expression in the central nervous system of rats and mice. Journal of Comparative Neurology. 2005;493:241–260. doi: 10.1002/cne.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]