Abstract
Objective
Utilizing United Network for Organ Sharing(UNOS) data, we developed a validated 50-point recipient risk index predicting short term mortality in orthotopic heart transplantation(OHT). We examined the relationship between institutional volume and recipient risk on post-OHT mortality.
Methods
We used UNOS data to identify primary OHT recipients between 1/2000–4/2010. Centers were stratified by mean annual volume. Preoperative IMPACT risk scores (Index for Mortality Prediction After Cardiac Transplantation) were calculated for each patient using our validated 50-point system. Primary outcomes were 30-day(d) and 1-year(yr)survival. Odds ratios(OR) were determined using multivariable logistic regression analysis, including interaction terms to examine effect modification of risk and volume on mortality.
Results
18,226 patients underwent OHT at 141 centers. 1,173(6.4%) recipients had OHT at low volume centers(<7 OHT/yr); 5,353(29.4%) at medium volume centers(7–15 OHT/yr); and 11,700(64.2%) at high volume centers(>15 OHT/yr). Low center volume was associated with worse 1-yr mortality(OR 1.58[1.30–1.92], p<0.001). For 1-yr survival, there was a significant positive interaction between center volume and recipient risk score(1-yr: OR 1.04[1.01–1.07],p=0.02), indicating the effect of risk on mortality is greater at low volume centers than would be expected by either variable analyzed individually. Among high-risk recipients(score≥10), 1-yr survival was improved at high volume centers (79% high; 75% medium; 64% low).
Conclusions
Using UNOS data and our validated recipient risk index, institutional volume acts as an effect modifier on the association between risk and mortality. High-risk patients experience higher mortality when transplanted at low volume centers. These differences dissipate among lower risk recipients. These data support a mandate for transplantation of high-risk recipients at higher volume centers.
Keywords: Orthotopic Heart Transplantation, IMPACT, risk score, center volume, UNOS
Introduction
Over thirty years have elapsed since the early observation that outcomes for a broad array of surgical procedures improve with increasing hospital center volume.1 Many subsequent studies have documented this relationship for a spectrum of surgical procedures.2–10 Specifically, in the field of solid organ transplantation, volume-outcomes associations have been demonstrated in population-based studies for kidney, liver, lung, and heart transplantation(OHT).11–15 In the absence of prospective data, these previous studies can only identify associations. These associations likely represent the different systems or processes of care among centers with different levels of procedural volume that are difficult to measure objectively.
It is possible that certain centers may only have the institutional experience and support framework to treat patients within a given risk profile. Recipient risk and center volume have been examined in OHT patients, however the assignment of recipient risk was not fully characterized.14 We recently developed a novel, quantitative recipient risk index for mortality prediction after cardiac transplantation(IMPACT) using the UNOS registry.16 The impact of center volume was not included in the original IMPACT study, because the aim of that initial study was to define a recipient risk index that was broadly applicable based on only recipient characteristics. In this study, we incorporated this risk index to test the hypothesis that the effect of recipient risk on short-term mortality after OHT varies by different levels of center volume. This interaction, or effect modification, has not been previously studied in OHT. Using a validated risk index, these findings may begin to explain the previously observed outcomes differences based on center volume.
Methods
Data Source
UNOS provided Standard Transplant Analysis and Research files with donor specific and follow-up data from October 1st, 1987 to December 31st, 2010. The registry consists of a prospectively collected database of unique demographic, donor, operative, and postoperative information for all thoracic transplantation patients in the United States. No patient or center identifiers were included, and this study was granted exemption status by the institutional review board.
Study Design
This was a retrospective review involving all primary, adult(≥18yrs) OHT recipients from January 2000 to April 2010, to ensure adequate time for follow-up. All patients who received simultaneous liver transplant(n=69), and those with total artificial hearts(n=71) were excluded. All patients included in the analysis were assigned their appropriate IMPACT risk score. The IMPACT risk score is a quantitative risk index derived and cross-validated using UNOS data, and has been shown to be highly predictive of 1-year mortality for adult patients receiving first time OHT.16 The risk index incorporates twelve recipient specific preoperative variables (age, gender, race, diagnosis, creatinine clearance, pre-operative dialysis, serum bilirubin, pre-operative infection, need for mechanical ventilation, intra-aortic balloon pump, temporary circulatory support, and ventricular assist device) to assign relative points out of a possible maximum of 50 (Table - supplement). No patients were missing data necessary to compose their respective score.
Center Volume
Center code was provided as a random encrypted alphanumeric six character code that is unique for each individual center. Center and patient level data are de-identified in this dataset. The variable of average annual center volume for each center was derived from existing data. Accordingly, each patient outcome was linked with the corresponding hospital volume calculation. Years in which a center was clearly not performing OHTs were subtracted for purposes of average annual volume determinations. Annual center volume was then ranked in thirds with each center counting as an individual observation, and categorized as low (≤7 OHT/year), medium (>7 – ≤15 OHT/year), or high (>15 OHT/year). Categorization in this fashion prevented skewing the cut-points upward due to a greater overall number of patients transplanted at higher volume centers. For ease of interpreting regression coefficients, center volume was reported as a categorical variable in regression analysis, however we examined center volume as a continuous variable also for identifying inflection points regarding risk of 1-year mortality.
Analysis
The primary outcomes of interest were all-cause 30-day and 1-year mortality. Additional postoperative complications examined were drug-treated rejection in the first year after transplant, postoperative renal replacement therapy, and need for cardiac reoperation. Multivariable logistic regression analysis incorporated recipient risk (IMPACT) score, allograft ischemic time, donor age, and annual center volume (low, medium, and high). Allograft ischemic time and center volume were examined as both continuous and categorical variables with cut-offs with Akaike Information Criterion used to identify models with highest explanatory power in a nested model approach. Based on the Akaike Information Criterion, the multivariable regression models using these two variables with cut-points as categorical variables increased the explanatory power of the models. Separate regressions were performed for 30-day mortality and 1-year mortality. The Pearson chi-square goodness of fit test and area under the receiver operative curves were used to confirm the explanatory power of the regression models.
To test for an effect modification of center volume on recipient risk and short-term mortality, an interaction term was constructed. This interaction term consisted of the product of recipient risk score and the inverse of annual center volume. In this way, risk estimates of the individual components of the interaction term are unidirectional, facilitating interpretation of the coefficients. When added to the multivariable regression models, this interaction term allowed the odds of early mortality to vary by level of center volume.
One-year survival was modeled according to the Kaplan-Meier method, with censoring for patients lost to follow-up or administrative purposes(alive at the end of the study). A subgroup survival analysis was performed among patients in the highest quartile of risk(IMPACT score >10). Means are displayed with standard deviations(±SD), medians with interquartile ranges(IQR), and odds ratios(OR) with 95% confidence intervals(CI). Biostatistical analysis was performed using STATA software(v11 SE, StataCorp LP, College Station, TX).
Results
Cohort Statistics
There were 18,226 OHT recipients who satisfied inclusion criteria, and these transplants were performed at a total of 141 unique centers. The mean age was 52±12 years(yrs)and 4,311(23.7%) were women. Recipient race distribution was: 73.1% Caucasian (n=13,328); 16.4% African-American (n=2,988); 7.2% Hispanic (n=1,311); and 3.3% Other (n=599). Donor race distribution was: 69.0% Caucasian (n=12,573); 13.2% African-American (n=2,405); 15.3% Hispanic (n=2,793); and 2.5% Other (n=455). During the study period, 4,892 patients died (incidence rate 6.6 deaths/100 person-yrs). The Kaplan-Meier cumulative incidence of 1-year mortality was 13.6% (n=2,223). Median follow-up was 45 months (IQR:15–75).
Center Volume
Mean annual institutional volume ranged from 0.2 OHT/year to 76 OHT/year with a median of 10 OHT/year. Primary stratification revealed that 1,173(6.4%) patients were transplanted at centers with low volume(≤7 OHT/year, n=47); 5,353(29.4%) at medium volume(>7 – ≤15 OHT/year, n=47); and 11,700(64.2%) at high volume(>15 OHT/year, n=47). Seventy-four(52.4%) centers performed >10 OHT/year, and 28(19.8%) performed more than 20 OHT/year.
IMPACT Score Generation
After assigning all patients their respective risk score index, the average score for the entire cohort was 6.24 ± 3.77. A histogram and test for skewness revealed that the pattern of scores followed a normal distribution, with values ranging from 0–33. When divided into quartiles(Q), the breakdown of scores was: Q1, 0 to <3 (n=4,528, 24.8%); Q2, ≥3 to <6 (n=3,908, 21.4%); Q3, ≥6 to <9 (n=5,760, 31.6%); and Q4, ≥9 (n=4,030, 22.1%). There were 2,129(11.6%) patients with a risk score greater than 10, and only 92(0.5%) with a risk score >20.
Baseline Characteristics
Baseline characteristics were compared among the three center volume groups (Table 1). For post-hoc comparisons the high volume group served as the reference. Although there were several differences in baseline characteristics, patients were evenly matched among the three groups when examining overall recipient risk as assessed by the IMPACT risk score(p=0.8). Patients at high volume centers tended to be Caucasian and older. The presence of diabetes mellitus, renal dysfunction, intra-aortic balloon pump, and prolonged ischemic time were also more common among recipients at high volume centers. Patients at low volume centers tended to have congenital cardiac disease, higher serum bilirubin levels, and were more likely to be African-American. At medium volume centers, there were more patients with ischemic cardiomyopathy, history of hypertension, need for mechanical ventilation, and use of early generation ventricular assist devices.
Table 1.
Patient characteristics and postoperative outcomes stratified by center volume
| Variable | Low Volume (N=1,173) |
Medium Volume (N=5,353) |
High Volume (N=11,700) |
P-Valuea |
|---|---|---|---|---|
| Demographics | ||||
| IMPACT score (±SD) | 6.3 (4.0) | 6.2 (3.7) | 6.2 (3.8) | 0.8 |
| Age, years (±SD) | 48.9 (13.9)b | 51.7 (11.9)b | 52.5 (12.1) | <0.01 |
| Female, n (%) | 262 (22.4%) | 1,318 (24.6%) | 2,731 (23.3%) | 0.1 |
| Caucasian, n (%) | 839 (71.5%)b | 3,792 (70.8%)b | 8,697 (74.3%) | <0.01 |
| African-American, n (%) | 226 (19.3%)b | 952 (17.8%)b | 1,810 (15.5%) | <0.001 |
| Diagnosis, n (%) | <0.01 | |||
| Idiopathic | 452 (38.5%) | 2,288 (42.7%) | 4,988 (42.6%) | |
| Ischemic | 535 (45.6%) | 2,508 (46.9%)b | 5,165 (44.2%) | |
| Congenital | 89 (7.6%)b | 95 (1.8%)b | 285 (2.4%) | |
| Other | 97 (8.3%) | 462 (8.6%) | 1,262 (10.8%) | |
| Acuity | ||||
| HTN, n (%) | 373 (39.7%) | 1,593 (44.0%)b | 3,200 (39.0%) | <0.01 |
| Diabetes Mellitus, n (%) | 220 (18.9%)b | 1,301 (24.5%) | 2,677 (23.2%) | <0.01 |
| Creatinine Clearanceb, ml/min (±SD) | 68.8 (27.1)b | 67.3 (27.1)b | 65.5 (25.5) | <0.01 |
| Serum bilirubin, mg/ml (±SD) | 1.4 (3.1)b | 1.2 (2.2) | 1.3 (2.3) | 0.02 |
| Preop Mechanical Ventilation | 34 (2.9%) | 172 (3.2%)b | 277 (2.4%) | <0.01 |
| Ischemic time, hours (±SD) | 3.0 (1.1)b | 3.1 (1.0)b | 3.3 (1.0) | <0.01 |
| Temporary Circulatory Supportd, n (%) | 26 (2.2%)b | 62 (1.2%) | 165 (1.4%) | 0.1 |
| Ventricular Assist Device | ||||
| Early Generatione, n (%) | 188 (16.0%) | 891 (16.6%)b | 1,724 (14.7%) | <0.01 |
| Late Generationf, n (%) | 5 (0.4%) | 34 (0.6%) | 45 (0.4%) | 0.08 |
| Heartmate II, n (%) | 24 (2.1%)b | 307 (5.7%)b | 571 (4.9%) | <0.01 |
| IABP, n (%) | 43 (3.7%)b | 267 (5.0%) | 656 (5.6%) | <0.01 |
| Postoperative Outcomes | ||||
| Need for cardiac reoperation, n (%) | 120 (13.0%) | 409 (11.7%) | 881 (11.6%) | 0.4 |
| Stroke, n (%) | 26 (2.2%) | 140 (2.6%) | 250 (2.2%) | 0.2 |
| New onset dialysis, n (%) | 113 (9.9%)b | 423 (8.1%)b | 799 (7.2%) | <0.01 |
| Treated rejection in first year, n (%) | 355 (40.3%)b | 1,457 (34.4%)b | 2,598 (28.8%) | <0.01 |
P-value based on Analysis of Variance(continuous variables) or chi-square test (categorical variables)
Post-hoc pairwise comparison p<0.05 (reference = high volume) by Tukey-Kramer method for continuous variables or univariate logistic regression for categorical variables
Based on Cockcroft-Gault Calculation
Includes ECMO and/or extracorporeal VADs i.e.Abiomed BVS5000 (Abiomed, Inc. Danvers, MA), Bio-Medicus(Medtronic Inc. Eden Prairie, MN), TandemHeart (Cardiac Assist, Inc., Pittsburg PA) and Levitronix/Centrimag(Levitronix, Waltham, MA)
Early generation includes para and intracorporeal pulsatile VADs including Abiomed AB5000, Heartmate I, XE and XVE, ThortecIVAD(Thoratec Corp, Pleasanton, CA), Toyobo(Toybo Osaka, Japan), Novacor(World Heart Inc, Oakland CA), Medos(Medos Stolberg, Germany) and LionHeart(Arrow International Inc, Reading PA).
Later generation continuous VADs including Jarvik(Javik Heart Inc, New York, NY), Micromed, Debakey(MicroMed Technology Inc. Houston, TX) and VentrAssist(Ventracor, Sydney, Australia), excluding Heartmate II
Abbreviations: IMPACT=index for mortality prediction after cardiac transplantation; HTN=hypertension; PVR=pulmonary vascular resistance; hrs=hours; IABP=intra-aortic balloon pump.
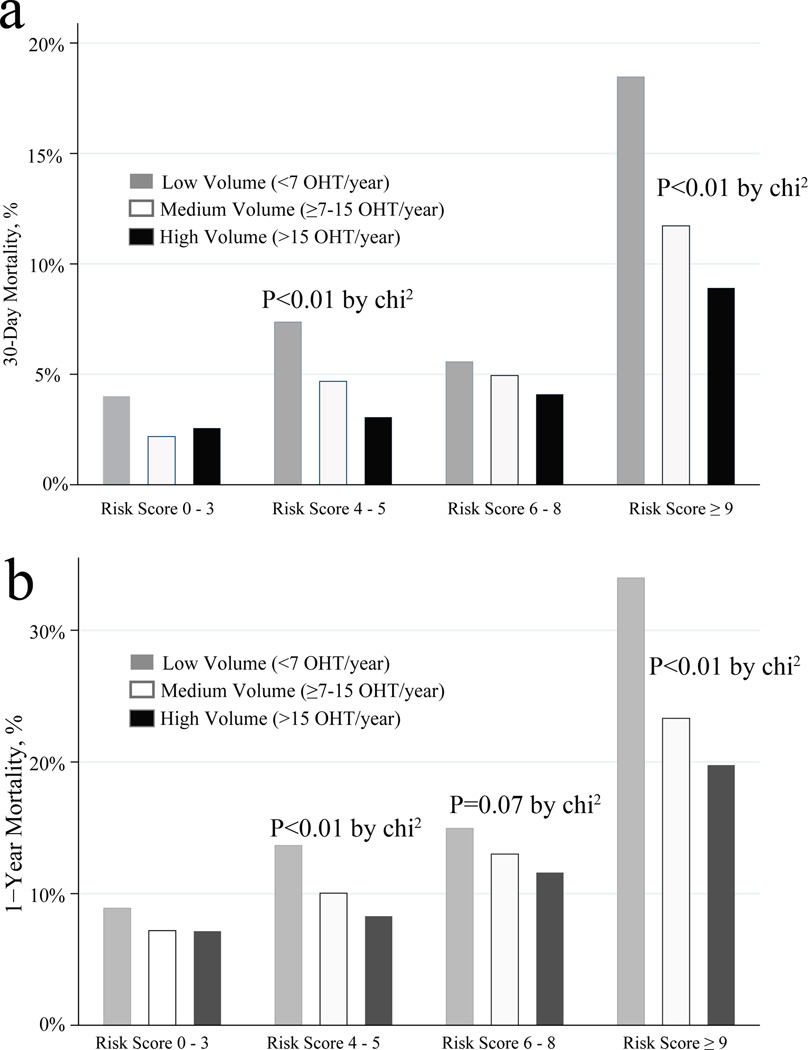
Mortality and Risk-Adjusted Regression Analysis
At thirty days, the unadjusted observed mortality rates were 4.6%(high volume), 5.6%(medium volume) and 9.3%(low volume). Observed 1-year mortality rates, unadjusted for recipient risk, were 11.6%(high volume), 13.5%(medium volume) and 18.1%(low volume). Differences in observed mortality by center volume were more pronounced when examined across increasing risk strata(Figure 1), with significant differences at thirty days and 1-year. In the adjusted multivariable logistic regression analysis, each 1pt increase in recipient risk score corresponded to a 14% increase in the odds of 1-year mortality(OR 1.14[1.13–1.15], p<0.001) (Table 2). Increasing donor age(OR 1.01[1.00–1.02], p<0.001) and prolonged allograft ischemic time(OR 1.75[1.42–2.15], p<0.001) also increased the odds of 1-year mortality. For the 1-year mortality logistic regression model, the area under the receiver operating characteristic curve was 0.67, and the Pearson chi-square statistic was 5180(P=0.25), indicating acceptable model fit.
Figure 1.
1a. Bar graph depicting actual 30-day mortality by center volume (low, medium, and high) according to increasing risk strata. P-values non-significant except where explicitly noted on the plot region.
1b. Bar graph depicting actual 1-Year mortality by center volume (low, medium, and high) according to increasing risk strata. P-values non-significant except where explicitly noted on the plot region.
Table 2.
Odds Ratio Estimates for 30-day Mortality and 1-Year Mortality
| 30-Day Mortality | 1-Year Mortality | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P-Value | OR Ratio | 95% CI | P-Value |
| Without Volume-Risk Interaction Effect | ||||||
| IMPACT score | 1.15 | 1.14–1.17 | <0.001 | 1.14 | 1.13–1.15 | <0.001 |
| Center Volume | ||||||
| High | --- | Reference | --- | --- | Reference | --- |
| Medium | 1.28 | 1.10–1.49 | 0.001 | 1.22 | 1.10–1.35 | <0.001 |
| Low | 2.25 | 1.80–2.82 | <0.001 | 1.76 | 1.50–2.09 | <0.001 |
| Allograft ischemic time | ||||||
| < 2 hours | --- | Reference | --- | --- | Reference | --- |
| ≥ 2 hours to < 4 hours | 1.20 | 0.93–1.54 | 0.2 | 1.06 | 0.90–1.25 | 0.4 |
| ≥ 4 hours to < 6 hours | 1.91 | 1.46–2.49 | <0.001 | 1.58 | 1.33–1.89 | <0.001 |
| > 6 hours | 2.54 | 1.88–3.43 | <0.001 | 1.75 | 1.42–2.15 | <0.001 |
| Donor Age | 1.01 | 1.01–1.02 | <0.001 | 1.01 | 1.01–1.02 | <0.001 |
| With Volume-Risk Interaction Effect | ||||||
| IMPACT score | 1.15 | 1.13–1.16 | <0.001 | 1.13 | 1.12–1.15 | <0.001 |
| Center Volume | ||||||
| High | --- | Reference | --- | --- | Reference | --- |
| Medium | 1.29 | 1.12–1.49 | 0.001 | 1.20 | 1.09–1.33 | <0.001 |
| Low | 1.86 | 1.46–2.38 | <0.001 | 1.58 | 1.30–1.92 | <0.001 |
| Center Volume-Risk Interaction Effect | 1.03 | 1.01–1.06 | 0.04 | 1.04 | 1.01–1.07 | 0.02 |
| Allograft ischemic time | ||||||
| < 2 hours | --- | Reference | --- | --- | Reference | --- |
| ≥ 2 hours to < 4 hours | 1.20 | 0.93–1.55 | 0.2 | 1.06 | 0.91–1.25 | 0.4 |
| ≥ 4 hours to < 6 hours | 1.92 | 1.46–2.51 | <0.001 | 1.59 | 1.33–1.89 | <0.001 |
| > 6 hours | 2.51 | 1.86–3.39 | <0.001 | 1.75 | 1.42–2.15 | <0.001 |
| Donor Age | 1.01 | 1.01–1.02 | <0.001 | 1.01 | 1.01–1.02 | <0.001 |
Abbreviations: IMPACT=Index for Mortality Predication After Cardiac Transplantation; OR=Odds ratio; CI=Confidence Interval
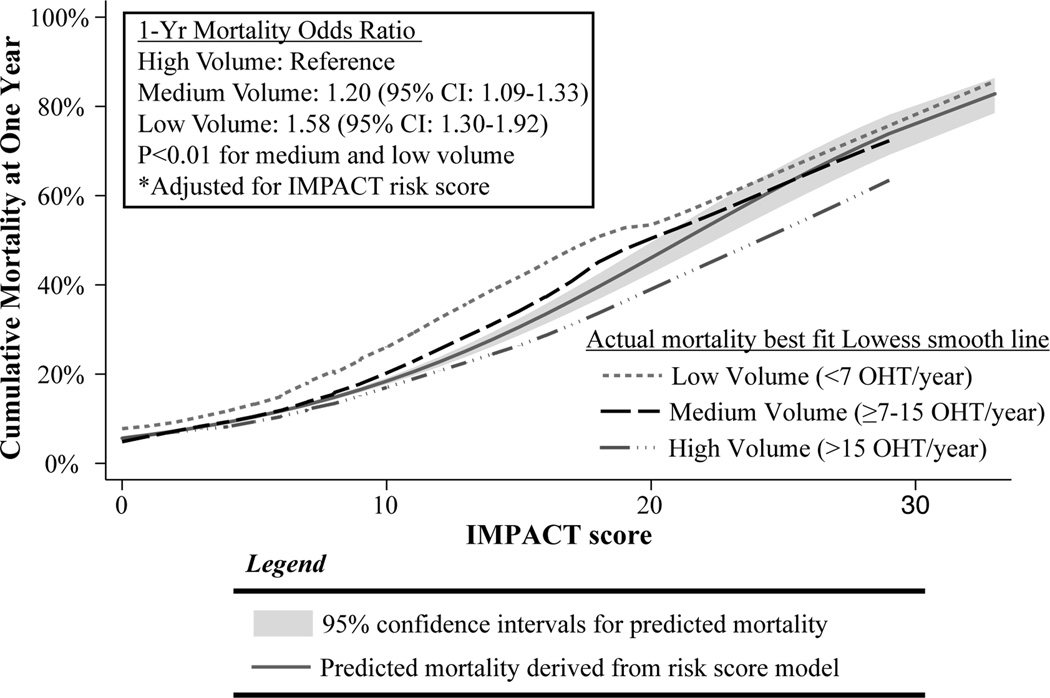
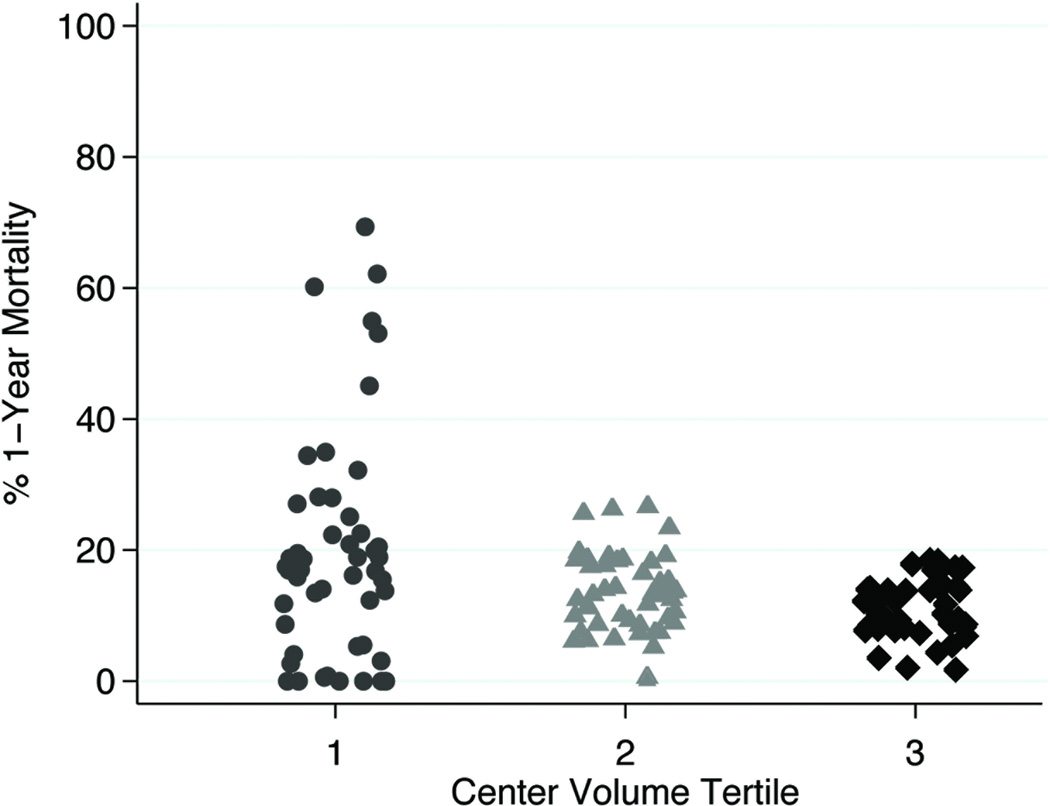
When allowing the effect of recipient risk on 1-year mortality to vary by center volume, there was a positive and significant test of interaction(OR 1.04[1.01–1.07], p<0.001). This interaction signifies that high volume centers minimize the effect of recipient risk, whereas low volume centers amplify the odds of 1-year mortality associated with higher recipient risk. This effect modification is depicted graphically in Figure 2. As recipient risk scores increase, the difference in observed 1-year mortality for each tier of center volume becomes more pronounced, with observed mortality exceeding predicted mortality for low and medium volume centers. Conversely, the risk of death for high volume centers is lower than would be predicted from the model, implying protection for high-risk patients at higher volume centers. It is important to note that there were many low volume centers that also achieved low 1-year mortality results as shown in Figure 3. Since low volume centers had a higher prevalence of congenital heart disease, the entire analysis was performed without congenital heart disease patients to ensure that this variable was not confounding the results. The magnitude and significance levels of the odds ratios for center volume, IMPACT risk score, and the interaction effect remained similar (test of interaction OR:1.06, 95% CI 1.02–1.10, p=0.004).
Figure 2.
Best fit Lowess smoothed line for observed 1-year mortality by level of center volume (low, medium, and high) plotted againstpredicted1-year mortality according to IMPACT score. 95% confidence interval for predicted mortality in shaded area.
Figure 3.
Scatter Plot of Center-Level 1-Year Mortality According to Three Tiers of Center Volume
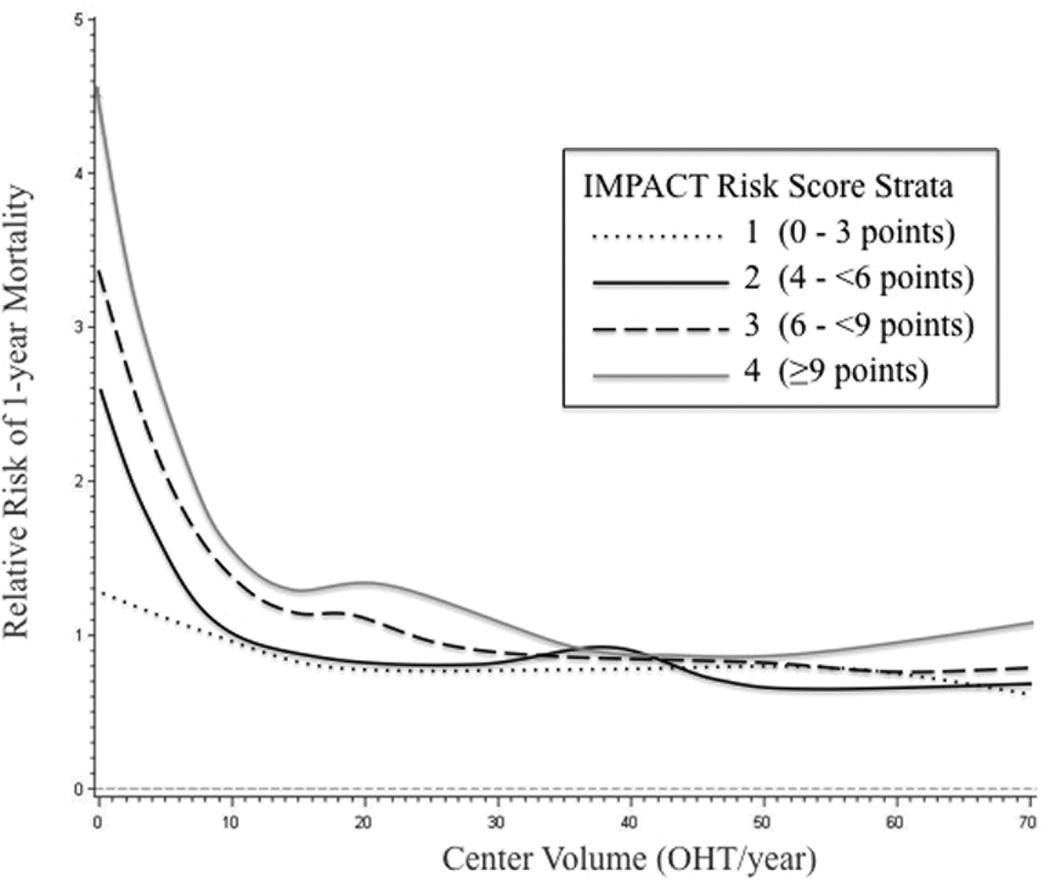
Incorporating four quartiles of recipient risk according to the IMPACT risk score, relative risk of 1-year mortality is plotted against center volume as a continuous variable in Figure 4. As center volume increases from 0 to 10 OHT/year, there is a steep decline in risk of 1-year mortality for all four tiers of recipient risk, although the decline is least pronounced in the lowest risk recipients. In the two lower quartiles of recipient risk, the relative risk of 1-year mortality crosses a threshold of one at a center volume of approximately 10 OHT/year. In contrast, for patients in the upper two quartiles according to IMPACT risk score, the inflection point for relative risk of 1-year mortality occurs at a center volume of approximately 35 OHT/year, with an observed plateau beyond 35–40 OHT/year. Consistent with this relative ratio plot, when center volume was examined as a continuous variable in the regression, increasing center volume was associated with lower odds of 1-year mortality (OR: 0.99, 95% CI 0.98–0.99, p<0.01).
Figure 4.
Relative risk of 1-year mortality plotted against center volume as a continuous variable on the abscissa. Stratification was performed according to four quartiles of recipient risk based on the IMPACT risk index. Relative risk crosses one for the lower two quartiles at an annual center volume of approximately 10 OHT/year, whereas for the two higher quartiles of recipient risk, the risk of 1-year mortality crosses one at an annual center volume of approximately 35–40 OHT/year.
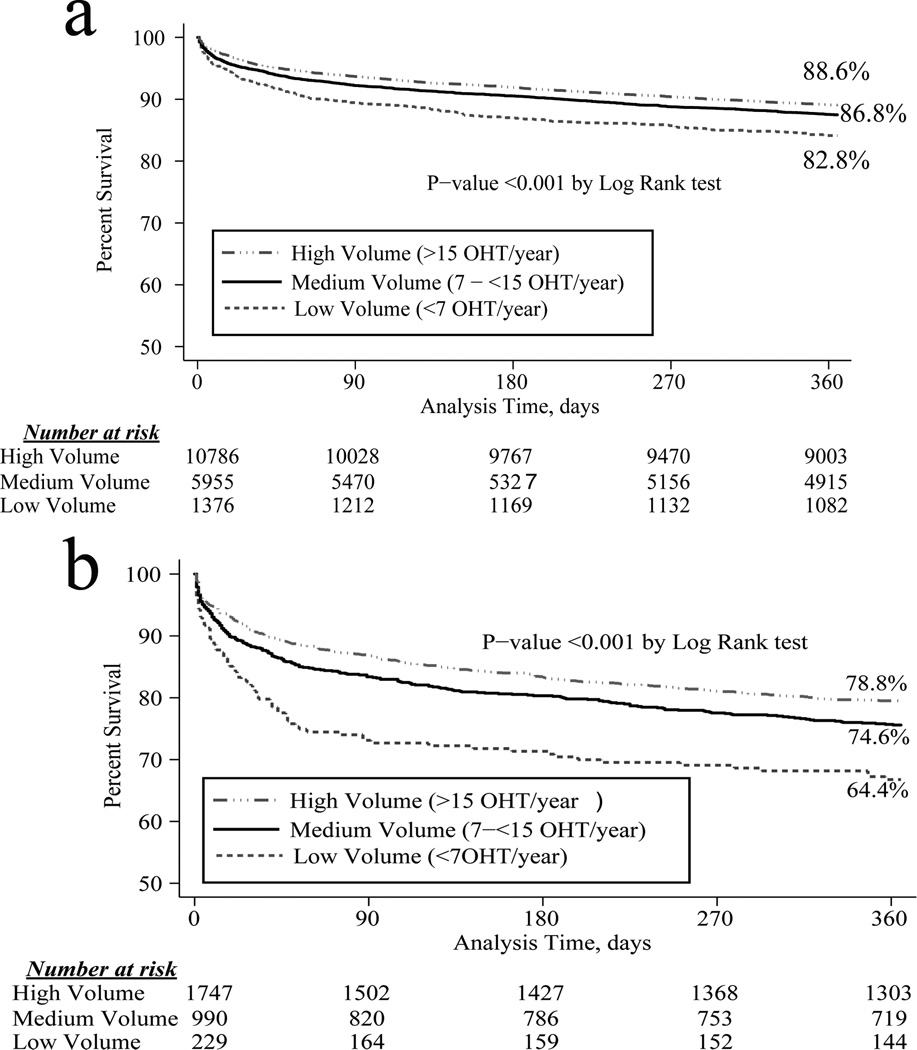
Survival
When comparing survival by center volume using the Cox-Mantel log-rank test, there was improved 1-year survival at high volume centers(p<0.001)(Figure 5a). Post-hoc comparisons revealed a significant difference between high volume and medium volume centers for the cohort overall (p=0.04). High risk patients only(IMPACT score ≥10) were examined in a subgroup analysis, and the significant survival differences persisted(p<0.001)(Figure 5b). In this high risk sub-group, post-hoc testing revealed a trend toward a difference in 1-year survival between high volume and medium volume centers (p=0.056).
Figure 5.
5a. Kaplan-Meier cumulative 1-year survival of recipients in the cohort overall, stratified by center volume.
5b. Kaplan-Meier cumulative 1-year survival of high risk recipients (IMPACT score >10), stratified by center volume.
Postoperative Complications
Commonly encountered postoperative complications are shown at the bottom of Table 1, according to center volume groups. Rates of cardiac re-operation and postoperative stroke were similar among the three groups. However, new onset dialysis was more common at low and medium volume centers(low, 9.9%; medium, 8.1%; and high, 7.2%; p<0.01). In addition, drug treated rejection in the first year after transplant was more prevalent among low and medium volume centers(low, 40.3%; medium, 34.4%; and high, 28.8%; p<0.01).
Discussion
We used UNOS data to evaluate the effect of recipient risk (as assessed by the IMPACT risk score) on short-term post-OHT mortality across different levels of transplant center volume. Since 2000, in the United States the majority of heart transplants have been performed at high volume centers(64%) compared with low volume centers(6%). Accounting for recipient risk, we observed improved 1-year survival rates among higher volume centers which were most pronounced in the highest quartile of recipient risk. Incorporating tests of interaction in multivariable analysis, institutional volume modifies the effect between recipient risk and mortality.
As recipient risk increases, decreasing center volume amplifies the risk of early mortality after OHT. This amplification of risk is most pronounced in the range of recipient scores from 10 to 20. Beyond a risk score of 20 the best fit lines for observed mortality migrate back within the bounds of 95% confidence estimates for predicted mortality, likely related to outliers and small numbers of patients in extreme risk categories(Figure 2). In fact, less than 1% of all OHT in this study were performed on OHT recipients with scores >20, and therefore it is difficult to draw meaningful conclusions for this subset of patients. Nonetheless, it is clear that high-risk patients survive with greater frequency than predicted by the IMPACT model at higher volume centers; and conversely they die with greater frequency than predicted at low volume centers.
There were several differences in baseline characteristics among the three groups of center volume, with high volume centers tending to transplant patients with advanced age, diabetes mellitus, worse renal function, and more frequent use of intra-aortic balloon pumps. Although centers varied in these individual characteristics, it is important to emphasize that overall risk as assessed by the composite risk index was equivalent in the three groups. High volume centers tended to transplant organs with slightly longer ischemic times, and this factor was adjusted for in the multivariable analysis. Low volume centers were more likely to transplant African-American recipients, patients with congenital heart disease, and higher bilirubin levels. Due to the size of the database, several of these statistically significant differences may not have clinical relevance. However, congenital heart disease can be a risk factor for adverse outcomes after OHT, and the prevalence of congenital heart disease was threefold higher at low volume centers. Therefore, we performed a sensitivity analysis excluding congenital heart disease patients from the entire analysis. The results remain the same as with congenital heart disease included, suggesting that this variable was not confounding the results.
Analysis of postoperative complications may partially explain the observed survival differences. Postoperative stroke and cardiac re-operation rates were equivalent in the three center volume groups. However, drug-treated rejection in the first year after OHT was more common in low and medium volume centers, when compared to high volume centers, with an absolute difference between low and high volume centers of 12.5%. Although difficult to determine from the UNOS database, these differences in drug-treated rejection rates may be due to inadequately delivered immunosuppression with the consequence of more rejection-related deaths. Furthermore, postoperative dialysis was more common in low and medium volume centers. The independent effect of postoperative renal dysfunction on mortality in cardiac surgery patients is well known.17 The UNOS variables for these postoperative complications are not reliably coded in the UNOS database, and thus warrant caution in generating strong conclusions from these data fields.
There are numerous causes of death reported in the UNOS database, with the most common being multi-system organ failure, primary graft failure/cardiac arrest, bacterial septicemia, and respiratory failure. However, nearly 20% of this cohort was missing cause of death information, thus preventing reliable use of this variable. Nevertheless, we speculate the higher rates of dialysis and rejection likely contribute to the differences in observed 30-day and 1-year mortality. As well, there were significant differences in 30-day mortality rates among the different tertiles of center volume, and this suggests that early perioperative deaths may in part account for the observed differences in 1-year mortality.
Processes of Care
The principal aim of this study was to better understand at the national level the way in which heart transplant centers interface with OHT patients across the spectrum of recipient risk. These findings should not necessarily be utilized to impugn low volume centers, as there are certain low volume centers that achieve excellent outcomes across the spectrum of recipient risk. Although individual surgeon and provider experience may plausibly account for these results, this issue is clearly quite complex and these data likely underscore variability in processes of care that may be inherently different across different tiers of center volume. Recent studies have demonstrated that dedicated intensive care unit staff, development of patient safety measures, and integration of critical care pathways may significantly affect outcomes.8,18,19 The results of this study should foster further inquiry into systems-based processes of care that may account for these differences in early survival.
Though the specific drivers of the volume effect remain undefined, these data strongly suggest that high-risk OHT recipients are best suited at higher volume centers. However, before advocating such a position, it is important to consider risks associated with such referral patterns. For example, having a high-risk patient travel a greater distance to undergo transplantation at a high volume center may affect post-OHT compliance, clinic visits, and routine surveillance, not to mention the obvious risks incurred from physical transport of a critically ill patient. Furthermore, from a societal standpoint, transport costs for both recipients and organs may nullify any potential benefits of regionalization. Further studies are necessary into the specific processes of care that impart improved outcomes at higher volume centers are. Ideally, such studies could elucidate the most important factors present at higher volume centers and attempts could be made to incorporate them into low volume centers. Ongoing monitoring of outcomes and access to OHT is also paramount to ensure that this center effect persists and that patients are not denied OHT because of an inability to access a higher volume center.
Given these caveats, however, these data begin to shed light on why higher volume centers attain superior results. We now know that it is specifically because they succeed with high-risk patients. These are the patients where low volume centers tend not to achieve equivalent results. As a result, we support a paradigm shift toward transplanting high-risk patients at higher volume centers exclusively.
Previous Work
While the relationship between hospital volume and outcomes for surgical procedures was initially described in the general surgery population, these associations have been applied to cardiac surgery patients.1,20,21 Previous studies have identified this relationship specifically in the context of cardiac transplantation as well.12,14,15 Though one cannot prove causation using a retrospective study, these studies were performed using a nationwide dataset. Hosenpud et al. conducted the first large registry study using UNOS data in OHT during the years 1987–1991, and perioperative care has evolved since that time.15 They reported a 33% increase in the risk of 1-year mortality among centers performing fewer than nine OHT per year. Our group has examined the effect of center volume on post-OHT mortality in a more contemporary cohort (1999–2006), and found centers performing fewer than ten OHT per year had a 35% increase in the hazard of 1-year mortality.12 Though recipient variables were incorporated into a multivariable analysis, that study was limited in its ability to discriminate the effect of recipient risk and center volume on short-term mortality. More recently, Russo and colleagues investigated recipient risk and center volume in the context of post-OHT mortality using UNOS data.14 While corroborating these previous observations, we believe our current analysis expands on these earlier studies in several important ways.
Our current study not only corroborates previous findings, but also expands our understanding of this issue. Specifically, there has previously been no standardized recipient risk index for predicting short-term mortality in cardiac transplantation. The study by Russo et al. incorporated recipient risk, but that study did not derive a composite risk score in a test group and validate the findings in a separate cohort.14 Without a composite index, it is difficult to show generalizability from analysis to analysis. Standardization of risk is important as we attempt to analyze what factors lead to improved outcomes for high-risk patients at higher volume centers. The twelve variables contributing to the 50-point IMPACT risk score (online Table supplement) represent those most significantly affecting 1-year mortality for first time OHT recipients. Furthermore, because of the rigorous statistical methodologies employed to derive the risk score, we believe the present findings are robust and accurately reflect how an individual OHT recipient’s risk profile affects their risk of early mortality at different levels of center volume. Additionally, although interaction effects have been examined in liver transplantation, this is the first study in cardiac transplantation to incorporate interaction terms to assess the varying effect of recipient risk across different strata of center volume.22
Furthermore, the assignment of center volume for OHT has varied in the literature. Russo et al. defined high volume as greater than 47 OHT/year, while the Center for Medicare and Medicaid Services(CMS) currently stipulates that centers perform at least 10 OHT/year in order to maintain certification.14,23 To assign center volume tertiles, we considered each center (with its respective average annual volume) as a single observation, rather than considering each patient as an individual observation. We believe deriving cut-points as was done in this study more accurately reflects a center’s annual OHT experience. This avoids cut-point biases with cut-points that are skewed in the upward direction due to a greater overall number of patients receiving OHT at higher volume centers. In our analysis, only six centers perform more than 47 OHT/year, which is too few to be performing all of the high risk OHT in the United States.
We also treated center volume as a continuous variable. A visual inspection of relative risk for 1-year mortality suggests that the optimal cut-point for low risk OHT recipients may be a center volume around 10 OHT/year. Higher volume centers performing approximately 35 OHT/year may be best suited to handle the highest risk recipients. There are only ninc centers that average 35 OHT/year, and although these data support regionalization of high risk recipients, nine centers may be too few to accommodate all high risk OHT recipients in the United States. Improved means for identifying lower volume centers that achieve excellent outcomes in high risk recipients will ensure continued access to OHT for the most critically ill patients awaiting cardiac transplantation.
Limitations
Given the retrospective nature of this study design, there are certain important limitations. Large national clinical registry studies inherently assume accurate coding of data. It is impossible to determine coding errors, although we have assumed that errors of this nature are likely to be distributed throughout the dataset randomly. If this is not the case, these potential errors may introduce bias in our results. An added limitation of this study is the inability to make strong conclusions about outcomes at centers with very low volume. Even though the UNOS registry represents a large sample size, centers that perform on average less than one OHT per year will have variable outcomes. In the absence of more low volume centers to examine, it is difficult to ascertain whether high mortality rates at a low volume center represent clear trends or natural variability. Additionally, despite the large patient cohort, the event rate of 1-year mortality occurred infrequently in low risk recipients. Thus, this study may have been underpowered to detect a true survival difference according to center volume among low risk recipients.
These findings may not fully account for the interaction between donor characteristics and recipient risk. We incorporated donor age and allograft ischemic time, as these variables were significantly associated with postoperative mortality on univariate analysis. When combined in our risk-adjusted multivariable model, the test of interaction between recipient risk and center volume remained significant, suggesting an effect due to center volume independent of these donor characteristics. Given the limited donor information in the UNOS registry, there may be donor selection factors that vary by center or organ procurement organization and impact mortality, which we have not been able to consider.
Furthermore, the UNOS database includes limited information regarding immunosuppression management, type of rejection, processes of care, and postoperative complications. The strength of the UNOS dataset is derived from its relatively complete mortality information. Therefore, the focus of this study was on 1-year mortality, with reporting of various postoperative complications to supplement the mortality findings. Additionally, retrospective studies cannot control for unrecognized differences in recipient characteristics. We attempted to account for these potential differences by incorporating a validated recipient risk index, yet there may be other variables not accounted for in our risk index that affect risk of short-term mortality. In developing scoring systems, validation is a vital feature. In our original score determinations, we employed cross-validation by deriving the score from a random subset and using the remainder of the sample for validation. This methodology was unlikely to bias the validation, however we acknowledge that our recipient index will benefit from external validation in an independent sample.
Conclusions
Using UNOS data and our novel recipient risk index, institutional volume acts as an effect modifier on the association between risk and mortality. High-risk patients experience higher mortality when transplanted at low volume centers and lower mortality when transplanted at higher volume centers. These differences dissipate among lower risk recipients. These data support transplantation of high-risk recipients at higher volume centers.
Supplementary Material
Acknowledgements
Drs. Arnaoutakis and Weiss are Irene Piccinini Investigators in Cardiac Surgery. Drs. Allen and George are Hugh R. Sharp Cardiac Surgery Research Fellows. This research was supported in part by the National Institutes of Health Grant 1T32CA126607-01A2 (GJA), and also supported by a Health Resources and Services Administration contract 231-00-0115. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Presentation: Plenary session at the 91st annual meeting of the American Association for Thoracic Surgery, May 7–11, 2011, Philadelphia, PA.
Disclosures: Dr. Conte receives research support from Medtronic and Thoratec
References
- 1.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364–1369. doi: 10.1056/NEJM197912203012503. [DOI] [PubMed] [Google Scholar]
- 2.Gordon TA, Burleyson GP, Tielsch JM, Cameron JL. The effects of regionalization on cost and outcome for one general high-risk surgical procedure. Annals of surgery. 1995;221:43–49. doi: 10.1097/00000658-199501000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meguid RA, Weiss ES, Chang DC, Brock MV, Yang SC. The effect of volume on esophageal cancer resections: what constitutes acceptable resection volumes for centers of excellence? The Journal of thoracic and cardiovascular surgery. 2009;137:23–29. doi: 10.1016/j.jtcvs.2008.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen NT, Paya M, Stevens CM, Mavandadi S, Zainabadi K, Wilson SE. The relationship between hospital volume and outcome in bariatric surgery at academic medical centers. Ann Surg. 2004;240:586–593. doi: 10.1097/01.sla.0000140752.74893.24. discussion 593-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138:721–725. doi: 10.1001/archsurg.138.7.721. discussion 726. [DOI] [PubMed] [Google Scholar]
- 6.Harmon JW, Tang DG, Gordon TA, Bowman HM, Choti MA, Kaufman HS, et al. Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Annals of surgery. 1999;230:404–411. doi: 10.1097/00000658-199909000-00013. discussion 411-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieberman MD, Kilburn H, Lindsey M, Brennan MF. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg. 1995;222:638–645. doi: 10.1097/00000658-199511000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimick JB, Cattaneo SM, Lipsett PA, Pronovost PJ, Heitmiller RF. Hospital volume is related to clinical and economic outcomes of esophageal resection in Maryland. Ann Thorac Surg. 2001;72:334–339. doi: 10.1016/s0003-4975(01)02781-3. discussion 339-341. [DOI] [PubMed] [Google Scholar]
- 9.Diaz-Montes TP, Zahurak ML, Giuntoli RL, 2nd, Gardner GJ, Bristow RE. Uterine cancer in Maryland: impact of surgeon case volume and other prognostic factors on short-term mortality. Gynecol Oncol. 2006;103:1043–1047. doi: 10.1016/j.ygyno.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Billingsley KG, Morris AM, Dominitz JA, Matthews B, Dobie S, Barlow W, et al. Surgeon and hospital characteristics as predictors of major adverse outcomes following colon cancer surgery: understanding the volume-outcome relationship. Arch Surg. 2007;142:23–31. doi: 10.1001/archsurg.142.1.23. discussion 32. [DOI] [PubMed] [Google Scholar]
- 11.Axelrod DA, Guidinger MK, McCullough KP, Leichtman AB, Punch JD, Merion RM. Association of center volume with outcome after liver and kidney transplantation. Am J Transplant. 2004;4:920–927. doi: 10.1111/j.1600-6143.2004.00462.x. [DOI] [PubMed] [Google Scholar]
- 12.Weiss ES, Meguid RA, Patel ND, Russell SD, Shah AS, Baumgartner WA, et al. Increased mortality at low-volume orthotopic heart transplantation centers: should current standards change? Ann Thorac Surg. 2008;86:1250–1259. doi: 10.1016/j.athoracsur.2008.06.071. discussion 1259-1260. [DOI] [PubMed] [Google Scholar]
- 13.Weiss ES, Allen JG, Meguid RA, Patel ND, Merlo CA, Orens JB, et al. The impact of center volume on survival in lung transplantation: an analysis of more than 10,000 cases. Ann Thorac Surg. 2009;88:1062–1070. doi: 10.1016/j.athoracsur.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Russo MJ, Iribarne A, Easterwood R, Ibrahimiye AN, Davies R, Hong KN, et al. Post-heart transplant survival is inferior at low-volume centers across all risk strata. Circulation. 2010;122:S85–S91. doi: 10.1161/CIRCULATIONAHA.109.926659. [DOI] [PubMed] [Google Scholar]
- 15.Hosenpud JD, Breen TJ, Edwards EB, Daily OP, Hunsicker LG. The effect of transplant center volume on cardiac transplant outcome. A report of the United Network for Organ Sharing Scientific Registry. JAMA. 1994;271:1844–1849. [PubMed] [Google Scholar]
- 16.Weiss ES, Allen JG, Arnaoutakis GJ, George TG, Russell DA, Shah AS, et al. Creation of a Quantitative Recipient Risk Index for Mortality Prediction After Cardiac Transplantation (IMPACT) Ann Thorac Surg. 2011 doi: 10.1016/j.athoracsur.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 18.Zehr KJ, Dawson PB, Yang SC, Heitmiller RF. Standardized clinical care pathways for major thoracic cases reduce hospital costs. The Annals of thoracic surgery. 1998;66:914–919. doi: 10.1016/s0003-4975(98)00662-6. [DOI] [PubMed] [Google Scholar]
- 19.Makary MA, Sexton JB, Freischlag JA, Millman EA, Pryor D, Holzmueller C, et al. Patient safety in surgery. Annals of surgery. 2006;243:628–632. doi: 10.1097/01.sla.0000216410.74062.0f. discussion 632-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sollano JA, Gelijns AC, Moskowitz AJ, Heitjan DF, Cullinane S, Saha T, et al. Volume-outcome relationships in cardiovascular operations: New York State, 1990–1995. J Thorac Cardiovasc Surg. 1999;117:419–428. doi: 10.1016/s0022-5223(99)70320-x. discussion 428-430. [DOI] [PubMed] [Google Scholar]
- 21.Peterson ED, Coombs LP, DeLong ER, Haan CK, Ferguson TB. Procedural volume as a marker of quality for CABG surgery. JAMA. 2004;291:195–201. doi: 10.1001/jama.291.2.195. [DOI] [PubMed] [Google Scholar]
- 22.Segev DL, Kucirka LM, Nguyen GC, Cameron AM, Locke JE, Simpkins CE, et al. Effect modification in liver allografts with prolonged cold ischemic time. Am J Transplant. 2008;8:658–666. doi: 10.1111/j.1600-6143.2007.02108.x. [DOI] [PubMed] [Google Scholar]
- 23.The Center for Medicare and Medicaid Services Medicare program; hospital conditions of participation: requirements for approval and re-approval of transplant centers to perform organ transplants. Federal Register. 2007:15197–15280. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.