Abstract
Effective screening for the development of early onset preeclampsia (PE) can be provided in the first-trimester of pregnancy. Screening by a combination of maternal risk factors, uterine artery Doppler, mean arterial pressure, maternal serum pregnancy-associated plasma protein-A, and placental growth factor can identify about 95% of cases of early onset PE for a false-positive rate of 10%.
1. Introduction
Preeclampsia (PE) is a major cause of maternal and perinatal morbidity and mortality [1–3] and is thought to be predominantly as the consequence of impaired placentation. Evidence suggests that PE can be subdivided into early onset PE, requiring delivery before 34 weeks' gestation and late onset PE, with delivery at or after 34 weeks, because the former is associated with a higher incidence of adverse outcome [4–7]. A major challenge in modern obstetrics is early identification of pregnancies at high-risk of early onset PE and undertaking the necessary measures to improve placentation and reduce the prevalence of the disease.
The prophylactic use of low-dose aspirin for prevention of PE has been an important research question in obstetrics for the last three decades. In 1979, Crandon and Isherwood observed that nulliparous women who had taken aspirin regularly during pregnancy were less likely to have PE than women who did not. Subsequently, more than 50 trials have been carried out throughout the world and a meta-analysis of these studies reported that the administration of low-dose aspirin in high-risk pregnancies is associated with a decrease in the rate of PE by approximately 10% [8]. In most studies that evaluated aspirin for the prevention of PE the onset of treatment was after 16 weeks' gestation. However, recent meta-analyses reported that the prevalence of PE can potentially be halved by the administration of low-dose aspirin started at 16 weeks or earlier [9–11].
Extensive research in the last 20 years, mainly as a consequence of the shift in screening for aneuploidies from the second- to the first-trimester of pregnancy, has identified a series of early biophysical and biochemical markers of impaired placentation [12, 13]. Using a novel Bayes-based method that combines prior information from maternal characteristics and medical history, uterine artery pulsatility index (PI), mean arterial pressure (MAP), and maternal serum pregnancy-associated plasma protein-A (PAPP-A) and placental growth factor (PlGF) at 11–13 weeks' gestation can identify a high proportion of pregnancies at high-risk for early onset PE [12, 13]. The performance of the different methods of screening for PE is summarized in Table 1.
Table 1.
Estimated detection rates of preeclampsia (PE) requiring delivery before 34, 37, and 42 weeks' gestation, at false positive rates (FPR) of 5% and 10%.
| Screening test | FPR (%) | Detection rate (%) | ||
|---|---|---|---|---|
| PE < 34 weeks | PE < 37 weeks | PE < 42 weeks | ||
| Maternal characteristics | 5.0 | 36 | 33 | 29 |
| 10.0 | 51 | 43 | 40 | |
| Uterine artery pulsatility index (Ut-PI) | 5.0 | 59 | 40 | 31 |
| 10.0 | 75 | 55 | 42 | |
| Mean arterial pressure (MAP) | 5.0 | 58 | 44 | 37 |
| 10.0 | 73 | 59 | 54 | |
| Pregnancy associated plasma protein-A (PAPP-A) | 5.0 | 44 | 37 | 32 |
| 10.0 | 55 | 48 | 42 | |
| Placental growth factor (PlGF) | 5.0 | 59 | 41 | 29 |
| 10.0 | 72 | 54 | 40 | |
| MAP and Ut-PI | 5.0 | 80 | 55 | 35 |
| 10.0 | 90 | 72 | 57 | |
| PAPP-A and PlGF | 5.0 | 60 | 43 | 30 |
| 10.0 | 74 | 56 | 41 | |
| Ut-PI, MAP, and PAPP-A | 5.0 | 82 | 53 | 36 |
| 10.0 | 93 | 75 | 60 | |
| Ut-PI, MAP, and PlGF | 5.0 | 87 | 61 | 38 |
| 10.0 | 96 | 77 | 53 | |
| Ut-PI, MAP, PAPP-A, and PlGF | 5.0 | 93 | 61 | 38 |
| 10.0 | 96 | 77 | 54 | |
2. Screening by Maternal History
Several professional bodies have issued guidelines on routine antenatal care recommending that, at the booking visit, a woman's level of risk for PE, based on factors in her history, should be determined and women at high-risk are advised to take low-dose aspirin daily from early pregnancy until the birth of the baby (Table 2) [14–17]. However, the performance of screening by the recommended method and the effectiveness of intervention have not been formally evaluated.
Table 2.
| (i) Previous preeclampsia (PE) | |
| (ii) Previous early onset PE and preterm delivery at <34 weeks' gestation | |
| (iii) PE in more than one prior pregnancy | |
| (iv) Chronic kidney disease | |
| (v) Autoimmune disease such as systemic lupus erythematosis or antiphospholipid syndrome | |
| (vi) Heritable thrombophilias | |
| (vii) Type 1 or type 2 diabetes | |
| (viii) Chronic hypertension | |
| (ix) First pregnancy | |
| (x) Pregnancy interval of more than 10 years | |
| (xi) New partner | |
| (xii) Reproductive technologies | |
| (xiii) Family history of PE (mother or sister) | |
| (xiv) Excessive weight gain in pregnancy | |
| (xv) Infection during pregnancy | |
| (xvi) Gestational trophoblastic disease | |
| (xvii) Multiple pregnancies | |
| (xviii) Age 40 years or older | |
| (xix) Ethnicity: Nordic, Black, South Asian, or Pacific Island | |
| (xx) Body mass index of 35 kg/m2 or more at first visit | |
| (xxi) Booking systolic blood pressure >130 mmHg or diastolic blood pressure >80 mmHg | |
| (xxii) Increased prepregnancy triglycerides | |
| (xxiii) Family history of early onset cardiovascular disease | |
| (xxiv) Lower socioeconomic status | |
| (xxv) Cocaine and methamphetamine use | |
| (xxvi) Nonsmoking |
The majority of the studies that have reported on the maternal risk factors for the development of PE do not quantify the risk, although some studies do provide relative risks. Most of the available literature is based on retrospective, epidemiological, cohort, or case-control studies though few prospective cohort studies are also reported. Only a few studies have reported on maternal risk factors according to the severity of the disease, that is, early onset PE versus late onset PE.
It has been demonstrated that maternal demographic characteristics, including medical and obstetric history (Table 2), are potentially useful in screening for PE only when the various factors are incorporated into a combined algorithm derived by multivariate analysis [18]. With this approach to screening the effects of variables are expressed as odds ratios for early onset, late onset, or total PE. In general, the maternal risk factor profiles vary between early onset PE and late onset PE. This has led to the view that early and late PE may be different diseases. An alternative view is that PE is a spectrum disorder the degree of which is reflected in gestational age at the time of delivery. Multivariate screening for PE with maternal risk factors has since evolved into a new approach in which the gestation at the time of delivery for PE is treated as a continuous rather than a categorical variable. This approach, which is based on a survival time model, assumes that if the pregnancy was to continue indefinitely, all women would develop PE and whether they do so or not before a specified gestational age depends on a competition between delivery before or after development of PE [12]. In this new approach the effect of various risk factors is to modify the mean of the distribution of gestational age at delivery with PE. In pregnancies at low-risk for PE the gestational age distribution is shifted to the right with the implication that in most pregnancies delivery will actually occur before the development of PE. In high-risk pregnancies the distribution is shifted to the left and the smaller the mean gestational age, the higher the risk for PE (Figure 1).
Figure 1.
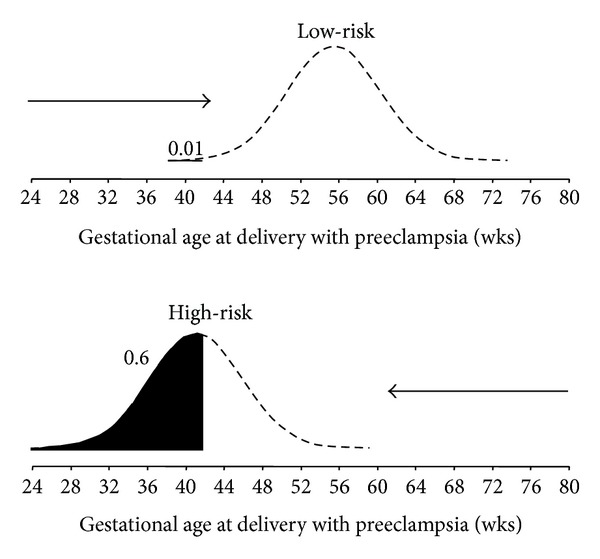
Distribution of gestational age at delivery for preeclampsia (PE). In pregnancies at low-risk for PE the gestational age distribution is shifted to the right and in most pregnancies delivery will occur before the development of PE. In pregnancies at high-risk for PE the distribution is shifted to the left. The risk of PE occurring at or before a specified gestational age is given by the area under the distribution curve (black). In the low-risk group the risk of PE at or before 34 weeks' gestation is 0.01 or 1% and in the high-risk group the risk is 0.6 or 60%.
In this competing risk model the mean gestational age for delivery with PE is 54 weeks with estimated standard deviation of 6.9 weeks. Certain variables, including advancing maternal age over 35 years, increasing weight, Afro-Caribbean and South Asian racial origin, previous pregnancy with PE, conception by in vitro fertilization (IVF) and a medical history of chronic hypertension, preexisting diabetes mellitus, and systemic lupus erythematosus or antiphospholipid syndrome, increase the risk for development of PE. The consequence of this increased risk is a shift to the left of the Gaussian distribution of the gestational age at delivery with PE (Figure 2). Estimated detection rates of PE requiring delivery before 34, 37, and 42 weeks' gestation in screening by maternal factors are about 36%, 33%, and 29%, respectively, at false-positive rate of 5%, and 51%, 43%, and 40%, respectively, at false-positive rate of 10% (Table 1) [12].
Figure 2.
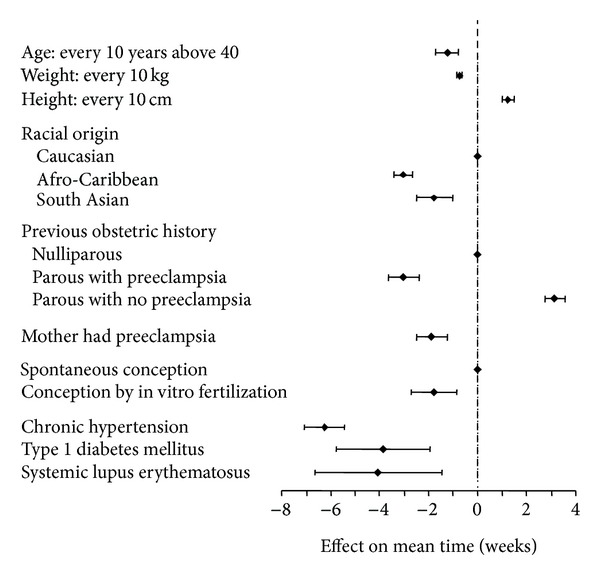
Effects of maternal characteristics (with 95% confidence intervals) on the gestational age at delivery for preeclampsia. This effect is expressed as gestational weeks by which the expected gestational age at delivery for preeclampsia is altered.
3. Screening by Maternal Biophysical Markers
3.1. Uterine Artery Doppler
The most promising screening test for PE is uterine artery Doppler velocimetry. The spiral arteries undergo a series of morphological changes during normal pregnancy [19, 20]. The vessels are firstly invaded by trophoblast, which then becomes incorporated into the vessel wall and replaces the endothelium and muscular layer. This results in the conversion of the small spiral arteries into vessels of greater diameter with low resistance and high compliance, in absence of maternal vasomotor control. This vascular transformation in the uterus is necessary to ensure a dramatic increase in blood supply to the intervillous space. The underlying mechanism for the development of PE is thought to be impaired trophoblastic invasion of the maternal spiral arteries and their conversion from narrow muscular vessels to wide nonmuscular channels [21–25]. Doppler ultrasound provides a noninvasive method for the assessment of the uteroplacental circulation. The finding that impaired placental perfusion, reflected in increased uterine artery PI, is associated with the development of PE is compatible with the hypothesis that PE is the consequence of impaired placentation and the results of previous first- and second-trimester Doppler studies as well as histological studies of the maternal spiral arteries [26–29]. Pathological studies have demonstrated that the prevalence of placental lesions in women with PE is inversely related to the gestation at delivery [30, 31].
The ability to achieve a reliable measurement of uterine artery PI is dependent on appropriate training of sonographers, adherence to a standard ultrasound technique in order to achieve uniformity of results among different operators. Using transabdominal ultrasonography, a sagittal section of the uterus should be obtained and the cervical canal and internal cervical os are identified. Subsequently, the transducer is gently tilted from side to side and color flow mapping is used to identify each uterine artery along the side of the cervix and uterus at the level of the internal os. Pulsed wave Doppler is then used with the sampling gate set at 2 mm to cover the whole vessel and care should be taken to ensure that the angle of insonation is less than 30°. When three similar consecutive waveforms are obtained the PI is measured and the mean PI of the left and right arteries is calculated. It is important to ensure that the peak systolic velocity is greater than 60 cm/s to ensure that the arcuate artery is not being sampled instead of the uterine artery [29].
First-trimester uterine artery PI has been shown to be affected by gestational age at screening, maternal weight, racial origin, and history of preexisting diabetes mellitus, and consequently it should be expressed as multiple of median (MoM) after adjustment for these factors. The MoM value of uterine artery PI is significantly increased at 11–13 weeks' gestation in women who subsequently develop PE and there is a significant negative linear correlation between the uterine artery PI MoM with gestational age at delivery [12]. Estimated detection rates of PE, at false-positive rate of 5% and 10% in screening by maternal factors with uterine artery PI, are given in Table 1. The addition of uterine artery PI to maternal factors improves the detection rates from 36% to 59% and from 33% to 40%, at false-positive rate of 5%, and from 51% to 75% and from 43% to 55%, at false-positive rate of 10%, for PE requiring delivery before 34 and 37 weeks' gestation, respectively, but not for PE delivering before 42 weeks.
3.2. Blood Pressure
In PE, hypertension develops as a result of vasoconstriction and reduced peripheral vascular compliance [32]. Although hypertension is only a secondary sign of PE, it is an important sign as it is an early indication of the disease. This highlights the importance of accurate monitoring of blood pressure during antenatal care. Accurate assessment of blood pressure has been hindered by the considerable variability that blood pressure exhibits within each individual. During blood pressure measurement at rest the first recording is often the highest recording, which decreases as the patients become more familiar with the procedure [33]. It is therefore recommended by professional bodies that a series of blood pressure measurements should be made until a prespecified level of stability is achieved [34, 35]. In current clinical practice, the use of mercury sphygmomanometers remains the gold standard for noninvasive blood pressure monitoring, but there are concerns for both the clinical performance and safety of these instruments [36–38]. Observer error is a major limitation of the auscultatory method [39] and terminal digit preference is perhaps the most common manifestation of suboptimal blood pressure determination. Other considerations include the rate of cuff deflation, the use of correct size cuff, the interarm difference in blood pressure, and the arm position and posture that are recognized to have significant effects on blood pressure determination.
The introduction of automated blood pressure monitoring allows simple, standardized, and repeated measurements to be taken. It also addresses many of the errors associated with the conventional sphygmomanometer but their use still requires the selection of the correct cuff size and proper patient positioning if accurate blood pressure is to be obtained. It has therefore been proposed that MAP should be measured by validated automated devices [40], with women in sitting position with back supported and legs uncrossed, that two measurements should be taken from each arm simultaneously with each arm supported at the level of the heart, and that the average of the four measurements should be used [33].
There is substantial evidence demonstrating that an increase in blood pressure in women destined to develop PE can be observed in the first- and second-trimesters of pregnancy [41–75]. Previous studies, including a mixture of prospective and retrospective and cohort and case-control studies and randomized controlled trials, reported widely contradictory results in the performance of screening (detection rate, median 43%, range 5–100%; false-positive rate, median 16%, range 0–66%) as a consequence of major methodological differences. The data from these studies, including more than 60,000 women with 3,300 cases of PE, were compiled into a systematic review, which concluded that the MAP is significantly better than systolic blood pressure or diastolic blood pressure in predicting PE [76].
First-trimester MAP has been shown to be affected by maternal weight, height, age, racial origin, cigarette smoking, family and prior history of PE, and history of chronic hypertension, and consequently it should be expressed as MoM after adjustment for these factors. Similar to the findings with uterine artery PI, the MoM value of MAP is significantly increased at 11–13 weeks' gestation in women who subsequently develop PE and there is a significant negative linear correlation between the MAP MoM with gestational age at delivery [12]. Estimated detection rates of PE, at false-positive rate of 5% and 10% in screening by maternal factors with MAP, are given in Table 1. The addition of MAP to maternal factors improves the detection rates from 36% to 58%, from 33% to 44%, and from 29% to 37%, at false-positive rate of 5%, and from 51% to 73%, from 43% to 59%, and from 40% to 54%, at false-positive rate of 10%, for PE requiring delivery before 34, 37, and 42 weeks' gestation, respectively.
There is a significant association between uterine artery PI and MAP in PE and unaffected pregnancies and therefore when combining the two biophysical markers in calculating the patient specific risk for PE the correlation factors must be taken into consideration to avoid overestimating the contributions from each marker in order to provide accurate risk assessment for PE. Estimated detection rates of PE requiring delivery before 34, 37, and 42 weeks' gestation in screening by maternal factors are 80%, 55%, and 35%, respectively, at false-positive rate of 5% and 90%, 72%, and 57%, respectively, at false-positive rate of 10% (Table 1).
4. Screening by Maternal Biochemical Markers
A large number of biochemical markers have been investigated for the prediction of PE (Table 3). Many such markers represent measurable manifestations of impaired placentation due to inadequate trophoblastic invasion of the maternal spiral arteries and reduced placental perfusion leading to placental ischemia related damage with the release of inflammatory factors, platelet activation, endothelial dysfunction, maternal renal dysfunction, or abnormal oxidative stress [19, 21–25]. Maternal serum PAPP-A and PlGF are two biochemical markers that have been investigated extensively and have shown promising results in the early prediction of PE. They have both been shown to be useful in screening for aneuploidies in combination with maternal age, fetal nuchal translucency thickness, and maternal serum free β-human chorionic gonadotropin at 11–13 weeks' gestation [77] and they are now part of the platform of automated machines that provide reproducible results within 30–40 minutes of sampling.
Table 3.
Proposed maternal biochemical markers for the prediction of preeclampsia.
| A disintegrin and metalloprotease 12 (ADAM12) | L-Arginine |
| Activin-A | L-Homoarginine |
| Adiponectin | Leptin |
| Adrenomedullin | Magnesium |
| Alpha fetoprotein | Matrix metalloproteinase-9 |
| Alpha-1-microglobulin | Microalbuminuria |
| Ang-2 angiopoietin-2 | Microtransferrinuria |
| Antiphospholipid antibodies | N-Acetyl-β-glucosaminidase |
| Antithrombin III | Neurokinin B |
| Atrial natriuretic peptide | Neuropeptide Y |
| Beta2-microglobulin | Neutrophil gelatinase-associated lipocalin |
| C-reactive protein | P-Selectin |
| Calcium | Pentraxin 3 |
| Cellular adhesion molecules | Placenta growth factor |
| Circulating trophoblast | Placental protein 13 |
| Corticotropin release hormone | Plasminogen activator inhibitor-2 |
| Cytokines | Platelet activation |
| Dimethylarginine (ADMA) | Platelet count |
| Endothelin | Pregnancy associated plasma protein-A |
| Estriol | Prostacyclin |
| Ferritin | Relaxin |
| Fetal DNA | Resistin |
| Fetal RNA | Serum lipids |
| Free fetal hemoglobin | Soluble endoglin |
| Fibronectin | Soluble fms-like tyrosine kinase |
| Genetic markers | Thromboxane |
| Haptoglobin | Thyroid function |
| Hematocrit | Total proteins |
| Homocysteine | Transferrin |
| Human chorionic gonadotropin | Tumor necrosis factor receptor-1 |
| Human placental growth hormone | Uric acid |
| Inhibin A | Urinary calcium to creatinine ratio |
| Insulin-like growth factor | Urinary kallikrein |
| Insulin-like growth factor binding protein | Vascular endothelial growth factor |
| Insulin resistance | Visfatin |
| Isoprostanes | Vitamin D |
PAPP-A is a syncytiotrophoblast-derived metalloproteinase, which enhances the mitogenic function of the insulin-like growth factors by cleaving the complex formed between such growth factors and their binding proteins [78, 79]. The insulin-like growth factor system is believed to play an important role in placental growth and development; it is therefore not surprising that low serum PAPP-A is associated with a higher incidence of PE. Increased level of maternal serum PAPP-A has been observed in established PE [80–82]. In chromosomally normal pregnancies there is evidence that low maternal serum PAPP-A in the first- and second-trimesters is associated with increased risk for subsequent development of PE. However, measurement of PAPP-A alone is not an effective method of screening for PE because only 8–23% of affected cases have serum levels below the 5th percentile, which is about 0.4 MoM. At the 5th percentile of normal for PAPP-A the reported odds ratios for PE varied between 1.5 and 4.6 [83–89].
PlGF, a glycosylated dimeric glycoprotein, is a member of the vascular endothelial growth factor subfamily. It binds to vascular endothelial growth factor receptor-1 which has been shown to rise in pregnancy. PlGF is synthesized in villous and extravillous cytotrophoblast and has both vasculogenetic and angiogenetic functions. It is believed to contribute a change in angiogenesis from a branching to a nonbranching phenotype controlling the expansion of the capillary network. Its angiogenetic abilities have been speculated to play a role in normal pregnancy and changes in the levels of PlGF or its inhibitory receptor have been implicated in the development of PE [90–93]. PE is associated with reduced placental production of PlGF and several studies reported that during the clinical phase of PE the maternal serum PlGF concentration is reduced. These reduced levels of serum PlGF precede the clinical onset of the disease and are evident from both the first- and second-trimesters of pregnancy [94–102].
In biochemical testing it is necessary to make adjustments in the measured maternal serum metabolite concentration to correct for certain maternal and pregnancy characteristics as well as the machine and reagents used for the assays and is then expressed in MoM of the normal [103]. First-trimester maternal serum concentrations of PAPP-A and PlGF have been shown to be affected by gestational age at screening, maternal weight, racial origin, cigarette smoking, conception by IVF, nulliparity, and preexisting diabetes mellitus [103, 104]. In addition, serum PlGF is also affected by maternal age [104]. Consequently, the measured concentrations of PAPP-A and PlGF must be adjusted for these variables before comparing results with pathological pregnancies. Contrary to the findings with biophysical markers, the MoM values of PAPP-A and PlGF are significantly reduced at 11–13 weeks' gestation in women who subsequently develop PE. There is a significant positive linear correlation between the MoM values of these biochemical markers with gestational age at delivery [13]. This observation further confirms that PE is a single pathophysiological entity with a wide spectrum of severity manifested in gestational age at which delivery becomes necessary for maternal and/or fetal indications.
Estimated detection rates of PE, at false-positive rate of 5% and 10% in screening by maternal factors with biochemical markers, are given in Table 1. The addition of maternal serum PAPP-A and PlGF to maternal factors improves the detection rates from 36% to 60% and from 33% to 43%, at false-positive rate of 5%, and from 51% to 74% and from 43% to 56%, at false-positive rate of 10%, for PE requiring delivery before 34 and 37 weeks' gestation, respectively, but not for PE delivering before 42 weeks.
5. Screening by Maternal Biochemical and Biophysical Markers
Analogous to the effective first-trimester combined screening for aneuploidies, effective screening for PE can also be achieved by a combination of maternal factors and biochemical and biophysical markers. Using the competing risk model, the gestational age at the time of delivery for PE is treated as a continuous variable. Bayes theorem is then used to combine prior information from maternal characteristics and medical history with the MoM values of uterine artery PI, MAP, serum PAPP-A, and PlGF. The major advantage of this model, compared to the other published models [105–107], is that it offers the option to clinicians and researchers to select their own gestational age cut-off to define the high-risk group that could potentially benefit from therapeutic interventions starting from the first-trimester of pregnancy [9–11].
It is important to recognize that there are significant associations between all biophysical and biochemical markers in PE and unaffected pregnancies and therefore when combining the four markers in calculating the patient specific risk for PE the correlation factors are taken into account to provide accurate risk assessment for PE. Estimated detection rates of PE requiring delivery before 34, 37, and 42 weeks' gestation in screening by maternal factors are 93%, 61%, and 38%, respectively, at false-positive rate of 5% and 96%, 77%, and 54%, respectively, at false-positive rate of 10% (Table 1).
6. First-Trimester Screening Followed by Third-Trimester Risk Assessment
Effective screening for early onset PE can be achieved in the first-trimester of pregnancy but late onset PE requiring delivery after 34 weeks' gestation accounting for two-thirds of all PE remains a significant challenge for effective early screening. We have therefore proposed a two-stage strategy for identification of pregnancies at risk of PE. The first stage, at 11–13 weeks, should be primarily aimed at effective prediction of early onset PE, because the prevalence of this condition can be potentially reduced substantially by the prophylactic use of low-dose aspirin started before 16 weeks' gestation [9–11]. The second stage, at 30–33 weeks, should be aimed at effective prediction of PE requiring delivery at or after 34 weeks because close monitoring of such pregnancies for earlier diagnosis of the clinical signs of the disease could potentially improve perinatal outcome through such interventions as the administration of antihypertensive medication and early delivery [108].
A competing risk model, using Bayes theorem, has been developed that combines maternal characteristics and history with biophysical and biochemical markers at 30–33 weeks' gestation to estimate the risk of developing PE requiring delivery within selected intervals from the time of screening. Preliminary results to date confirm that the a priori risk for PE depends on maternal characteristics and is increased with increasing maternal age and weight and in women of Afro-Caribbean and South Asian racial origin, in those with personal or family history of PE, and in women with preexisting chronic hypertension, diabetes mellitus, and systemic lupus erythematosus or antiphospholipid syndrome [109]. The third-trimester uterine artery PI and MAP are affected by maternal characteristics and history and the corrected measurements as expressed in MoM values are inversely related to the severity of the disease reflected in the gestational age at delivery. At risk cut-off of 1 : 100, the estimated false-positive and detection rates for PE requiring delivery within the subsequent four weeks were 6% and 91% in screening by a combination of maternal factors, uterine artery PI, and MAP [109].
PE is thought to be the consequence of an imbalance in angiogenic and antiangiogenic proteins [110]. Recent studies have focused on the investigation of pregnancies presenting to specialist clinics with signs of hypertensive disorders with the aim of identifying the subgroup that will develop severe PE requiring delivery within the subsequent 1–4 weeks. In such high-risk pregnancies, measurement of serum PlGF or soluble fms-like tyrosine kinase-1 (sFlt-1) to PlGF ratio is highly accurate in identifying the target group [111–116]. We have demonstrated that serum PlGF decreases with gestational age and maternal weight and is higher in women of Afro-Caribbean and South Asian racial origin than in Caucasians, in parous than nulliparous women, and in smokers than in nonsmokers. Serum sFlt-1 increases with gestational age and maternal age, decreases with maternal weight, is increased in women of Afro-Caribbean racial origin and in pregnancies conceived by IVF, and is lower in parous than nulliparous women [117]. In pregnancies complicated by PE, compared to normal pregnancies, serum PlGF MoM is decreased and sFlt-1 MoM is increased. At risk cut-off of 1 : 100, the estimated false-positive and detection rates for PE requiring delivery within the subsequent four weeks were 4% and 93% in screening by maternal factors, serum PlGF, and sFlt-1 [83] and the false-positive and detection rates improved to 2% and 95% in screening by maternal factors with all biomarkers [118].
7. Conclusion
Effective screening for early onset PE can be achieved in the first-trimester of pregnancy with a detection rate of about 95% and a false-positive rate of 10%. In a proposed new approach to prenatal care the potential value of an integrated clinic at 11–13 weeks' gestation in which maternal characteristics and history are combined with the results of a series of biophysical and biochemical markers to assess the risk for a wide range of pregnancy complications has been extensively documented [119]. In the context of PE the primary aim of such clinic is to identify those cases that would potentially benefit from prophylactic pharmacological interventions to improve placentation; the value of early screening and treatment of the high-risk group with low-dose aspirin is the subject of an ongoing randomized multicentre European study.
It is likely that a similar integrated clinic at 30–33 weeks will emerge for effective prediction of pregnancy complications that develop during the third-trimester. The potential value of such a clinic is to improve perinatal outcome by rationalizing and individualizing the timing and content of subsequent visits for selection of the best time for delivery.
Acknowledgment
This study was supported by a Grant from the Fetal Medicine Foundation (Charity no. 1037116).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.World Health Organization. Make Every Mother and Child Count. Geneva, Switzerland: World Health Organization; 2005. (World Health Report, 2005). [Google Scholar]
- 2.Confidential Enquiry into Maternal and Child Health (CEMACH) Perinatal Mortality 2006. England, Wales and Northern Ireland. London, UK: CEMACH; 2008. [Google Scholar]
- 3.Duley L. The global impact of pre-eclampsia and eclampsia. Seminars in Perinatology. 2009;33(3):130–137. doi: 10.1053/j.semperi.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Yu CKH, Khouri O, Onwudiwe N, Spiliopoulos Y, Nicolaides KH. Prediction of pre-eclampsia by uterine artery Doppler imaging: relationship to gestational age at delivery and small-for-gestational age. Ultrasound in Obstetrics & Gynecology. 2008;31(3):310–313. doi: 10.1002/uog.5252. [DOI] [PubMed] [Google Scholar]
- 5.Witlin AG, Saade GR, Mattar F, Sibai BM. Predictors of neonatal outcome in women with severe preeclampsia or eclampsia between 24 and 33 weeks' gestation. The American Journal of Obstetrics and Gynecology. 2000;182(3):607–611. doi: 10.1067/mob.2000.104224. [DOI] [PubMed] [Google Scholar]
- 6.Irgens HU, Reisæter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. British Medical Journal. 2001;323(7323):1213–1216. doi: 10.1136/bmj.323.7323.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Dadelszen P, Magee LA, Roberts JM. Subclassification of Preeclampsia. Hypertension in Pregnancy. 2003;22(2):143–148. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 8.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. The Lancet. 2007;369(9575):1791–1798. doi: 10.1016/S0140-6736(07)60712-0. [DOI] [PubMed] [Google Scholar]
- 9.Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstetrics and Gynecology. 2010;116(2):402–414. doi: 10.1097/AOG.0b013e3181e9322a. [DOI] [PubMed] [Google Scholar]
- 10.Roberge S, Villa P, Nicolaides K, et al. Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis. Fetal Diagnosis and Therapy. 2012;31(3):141–146. doi: 10.1159/000336662. [DOI] [PubMed] [Google Scholar]
- 11.Roberge S, Giguère Y, Villa P, et al. Early administration of low-dose aspirin for the prevention of severe and mild preeclampsia: a systematic review and meta-analysis. American Journal of Perinatology. 2012;29(7):551–556. doi: 10.1055/s-0032-1310527. [DOI] [PubMed] [Google Scholar]
- 12.Wright D, Akolekar R, Syngelaki A, Poon LC, Nicolaides KH. A competing risks model in early screening for preeclampsia. Fetal Diagnosis and Therapy. 2012;32:171–178. doi: 10.1159/000338470. [DOI] [PubMed] [Google Scholar]
- 13.Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagnosis and Therapy. 2013;33(1):8–15. doi: 10.1159/000341264. [DOI] [PubMed] [Google Scholar]
- 14.National Collaborating Centre for Women's and Children's Health (UK) Hypertension in Pregnancy: The Management of Hypertensive Disorders During Pregnancy. London, UK: RCOG Press; 2010. [PubMed] [Google Scholar]
- 15.World Health Organization. WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia. World Health Organization; 2011. [PubMed] [Google Scholar]
- 16.Magee LA, Helewa M, Moutquin JM, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Journal of Obstetrics & Gynaecology. 2008;30(3, supplement):S1–S48. [Google Scholar]
- 17.American College of Obstetricians and Gynecologists, Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstetrics and Gynecology. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 18.Poon LC, Kametas NA, Chelemen T, Leal A, Nicolaides KH. Maternal risk factors for hypertensive disorders in pregnancy: a multivariate approach. Journal of Human Hypertension. 2010;24:104–110. doi: 10.1038/jhh.2009.45. [DOI] [PubMed] [Google Scholar]
- 19.Pijnenborg R. The placental bed. Hypertension in Pregnancy. 1996;15(1):7–23. [Google Scholar]
- 20.Brosens I, Robertson WB, Dixon HG. The physiological response of the vessels of the placental bed to normal pregnancy. The Journal of Pathology and Bacteriology. 1967;93(2):569–579. doi: 10.1002/path.1700930218. [DOI] [PubMed] [Google Scholar]
- 21.de Wolf F, Robertson WB, Brosens I. The ultrastructure of acute atherosis in hypertensive pregnancy. The American Journal of Obstetrics and Gynecology. 1975;123(2):164–174. doi: 10.1016/0002-9378(75)90522-0. [DOI] [PubMed] [Google Scholar]
- 22.Khong TY, de Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. British Journal of Obstetrics & Gynaecology. 1986;93(10):1049–1059. doi: 10.1111/j.1471-0528.1986.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 23.Redman CWG. Pre-eclampsia and the placenta. Placenta. 1991;12(4):301–308. doi: 10.1016/0143-4004(91)90339-h. [DOI] [PubMed] [Google Scholar]
- 24.Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. The British Journal of Obstetrics and Gynaecology. 1994;101(8):669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 25.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38(3):718–722. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- 26.Olofsson P, Laurini RN, Marsal K. A high uterine artery pulsatility index reflects a defective development of placental bed spiral arteries in pregnancies complicated by hypertension and fetal growth retardation. European Journal of Obstetrics Gynecology and Reproductive Biology. 1993;49(3):161–168. doi: 10.1016/0028-2243(93)90265-e. [DOI] [PubMed] [Google Scholar]
- 27.Papageorghiou AT, Yu CKH, Cicero S, Bower S, Nicolaides KH. Second-trimester uterine artery Doppler screening in unselected populations: a review. Journal of Maternal-Fetal and Neonatal Medicine. 2002;12(2):78–88. doi: 10.1080/jmf.12.2.78.88. [DOI] [PubMed] [Google Scholar]
- 28.Martin AM, Bindra R, Curcio P, Cicero S, Nicolaides KH. Screening for pre-eclampsia and fetal growth restriction by uterine artery Doppler at 11–14 weeks of gestation. Ultrasound in Obstetrics and Gynecology. 2001;18(6):583–586. doi: 10.1046/j.0960-7692.2001.00594.x. [DOI] [PubMed] [Google Scholar]
- 29.Plasencia W, Maiz N, Bonino S, Kaihura C, Nicolaides KH. Uterine artery Doppler at 11 + 0 to 13 + 6 weeks in the prediction of pre-eclampsia. Ultrasound in Obstetrics and Gynecology. 2007;30(5):742–749. doi: 10.1002/uog.5157. [DOI] [PubMed] [Google Scholar]
- 30.Moldenhauer JS, Stanek J, Warshak C, Khoury J, Sibai B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. The American Journal of Obstetrics and Gynecology. 2003;189(4):1173–1177. doi: 10.1067/s0002-9378(03)00576-3. [DOI] [PubMed] [Google Scholar]
- 31.Egbor M, Ansari T, Morris N, Green CJ, Sibbons PD. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. British Journal of Obstetrics and Gynaecology. 2006;113(5):580–589. doi: 10.1111/j.1471-0528.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 32.Salas SP. What causes pre-eclampsia? Bailliere's Best Practice and Research in Clinical Obstetrics and Gynaecology. 1999;13(1):41–57. doi: 10.1053/beog.1999.0005. [DOI] [PubMed] [Google Scholar]
- 33.Poon LCY, Zymeri NA, Zamprakou A, Syngelaki A, Nicolaides KH. Protocol for measurement of mean arterial pressure at 11-13 weeks' estation. Fetal Diagnosis and Therapy. 2012;31(1):42–48. doi: 10.1159/000335366. [DOI] [PubMed] [Google Scholar]
- 34.National Heart Foundation of Australia. Hypertension Management Guide for Doctors. 2004, http://www.heartfoundation.org.au.
- 35.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the American heart association council on high blood pressure research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 36.US Environmental Protection Agency. EPA-452/R-97-003. Washington, Wash, USA: Environmental Protection Agency; 1997. Mercury Study Report to Congress. Volume 1: Executive Summary. [Google Scholar]
- 37.Mion D, Pierin AMG. How accurate are sphygmomanometers? Journal of Human Hypertension. 1998;12(4):245–248. doi: 10.1038/sj.jhh.1000589. [DOI] [PubMed] [Google Scholar]
- 38.Markandu ND, Whitcher F, Arnold A, Carney C. The mercury sphygmomanometer should be abandoned before it is proscribed. Journal of Human Hypertension. 2000;14(1):31–36. doi: 10.1038/sj.jhh.1000932. [DOI] [PubMed] [Google Scholar]
- 39.Rose G. Standardisation of observers in blood pressure measurement. The Lancet. 1965;285(7387):673–674. doi: 10.1016/s0140-6736(65)91827-1. [DOI] [PubMed] [Google Scholar]
- 40.Reinders A, Cuckson AC, Lee JTM, Shennan AH. An accurate automated blood pressure device for use in pregnancy and pre-eclampsia: The Microlife 3BTO-A. BJOG: An International Journal of Obstetrics and Gynaecology. 2005;112(7):915–920. doi: 10.1111/j.1471-0528.2005.00617.x. [DOI] [PubMed] [Google Scholar]
- 41.Fallis NE, Langford HG. Relation of second trimester blood pressure to toxemia of pregnancy in the primigravid patient. The American journal of obstetrics and gynecology. 1963;87:123–125. doi: 10.1016/s0002-9378(16)35153-5. [DOI] [PubMed] [Google Scholar]
- 42.Page EW, Christianson R. The impact of mean arterial pressure in the middle trimester upon the outcome of pregnancy. The American Journal of Obstetrics and Gynecology. 1976;125(6):740–746. doi: 10.1016/0002-9378(76)90839-5. [DOI] [PubMed] [Google Scholar]
- 43.Friedman EA, Neff RK. Systolic and mean arterial blood pressure. In: Friedman EA, Neff RK, editors. Pregnancy Hypertension. A Systematic Evaluation of Clinical Diagnostic Criteria. Littleton, Mass, USA: PSG Publishing; 1977. pp. 212–219. [Google Scholar]
- 44.Robrecht D, Schriever M, Rasenack R. The mean blood pressure in the second trimester (MAP-2) as a valuable aid in the early recognition of the pregnancies with a risk of hypertension. Geburtshilfe und Frauenheilkunde. 1980;40(2):121–124. doi: 10.1055/s-2008-1037205. [DOI] [PubMed] [Google Scholar]
- 45.Moutquin JM, Bilodeau R, Raynault P, et al. A prospective study of blood pressure in pregnancy. Prediction of the complications of hypertension. Journal de Gynecologie Obstetrique et Biologie de la Reproduction. 1982;11(7):833–837. [PubMed] [Google Scholar]
- 46.Oeney T, Balogh A, Kaulhausen H. The predictive value of blood pressure and weight gain during pregnancy for the early diagnosis of gestosis/preeclampsia. Preliminary report. Fortschritte der Medizin. 1982;100(7):277–280. [PubMed] [Google Scholar]
- 47.Mahanna I, Algeri T, Cigarini C, Zinelli G. Arterial pressure, MAP and dynamic tests in the monitoring of pregnancy. Annali di Ostetricia, Ginecologia, Medicina Perinatale. 1983;104(4):248–255. [PubMed] [Google Scholar]
- 48.Oney T, Kaulhausen H. The value of the mean arterial blood pressure in the second trimester (MAP-2 value) as a predictor of pregnancy-induced hypertension and preeclampsia. A preliminary report. Clinical and Experimental Hypertension B. 1983;2(2):211–216. doi: 10.3109/10641958309006081. [DOI] [PubMed] [Google Scholar]
- 49.Moutquin JM, Rainville C, Giroux L, et al. A prospective study of blood pressure in pregnancy: prediction of preeclampsia. American Journal of Obstetrics & Gynecology. 1985;151(2):191–196. doi: 10.1016/0002-9378(85)90010-9. [DOI] [PubMed] [Google Scholar]
- 50.Reiss RE, O'Shaughnessy RW, Quilligan TJ, Zuspan FP. Retrospective comparison of blood pressure course during preeclamptic and matched control pregnancies. The American Journal of Obstetrics and Gynecology. 1987;156(4):894–898. doi: 10.1016/0002-9378(87)90347-4. [DOI] [PubMed] [Google Scholar]
- 51.Ales KL, Norton ME, Druzin ML. Early prediction of antepartum hypertension. Obstetrics and Gynecology. 1989;73(6):928–933. doi: 10.1097/00006250-198906000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Villar LMA, Sibai BM. Clinical significance of elevated mean arterial blood pressure in second trimester and threshold increase in systolic or diastolic blood pressure during third trimester. The American Journal of Obstetrics and Gynecology. 1989;160(2):419–423. doi: 10.1016/0002-9378(89)90463-8. [DOI] [PubMed] [Google Scholar]
- 53.Moutquin JM, Rainville C, Giroux L, et al. Is a threshold increase in blood pressure predictive of preeclampsia? A prospective cohort study. Clinical and Experimental Hypertension B. 1990;9(2):225–235. [Google Scholar]
- 54.Conde-Agudelo A, Belizan JM, Lede R, Bergel EF. What does and elevated mean arterial pressure in the second half of pregnancy predict—gestational hypertension or preeclampsia? The American Journal of Obstetrics and Gynecology. 1993;169(3):509–514. doi: 10.1016/0002-9378(93)90609-m. [DOI] [PubMed] [Google Scholar]
- 55.Kyle PM, Clark SJ, Buckley D, et al. Second trimester ambulatory blood pressure in nulliparous pregnancy: a useful screening test for pre-eclampsia? The British Journal of Obstetrics and Gynaecology. 1993;100(10):914–919. doi: 10.1111/j.1471-0528.1993.tb15106.x. [DOI] [PubMed] [Google Scholar]
- 56.Lopez MC, Belizan JM, Villar J, Bergel E. The measurement of diastolic blood pressure during pregnancy: which Korotkoff phase should be used? American Journal of Obstetrics and Gynecology. 1994;170(2):574–578. doi: 10.1016/s0002-9378(94)70230-6. [DOI] [PubMed] [Google Scholar]
- 57.Rogers MS, Chung T, Baldwin S. A reappraisal of second trimester mean arterial pressure as a predictor of pregnancy induced hypertension. Journal of Obstetrics & Gynaecology. 1994;14(4):232–236. [Google Scholar]
- 58.Valensise H, Tranquilli AL, Arduini D, Garzetti GG, Romanini C. Screening pregnant women at 22–24 weeks for gestational hypertension or intrauterine growth retardation by Doppler ultrasound followed by 24-hour blood pressure recording. Hypertension in Pregnancy. 1995;14(3):351–359. [Google Scholar]
- 59.Atterbury JL, Groome LJ, Baker SL. Elevated midtrimester mean arterial blood pressure in women with severe preeclampsia. Applied Nursing Research. 1996;9(4):161–166. doi: 10.1016/s0897-1897(96)80015-2. [DOI] [PubMed] [Google Scholar]
- 60.Higgins JR, Walshe JJ, Halligan A, O’Brien E, Conroy R, Darling MRN. Can 24-hour ambulatory blood pressure measurement predict the development of hypertension in primigravidae? British Journal of Obstetrics and Gynaecology. 1997;104(3):356–362. doi: 10.1111/j.1471-0528.1997.tb11468.x. [DOI] [PubMed] [Google Scholar]
- 61.Konijnenberg A, Van der Post JAM, Mol BW, et al. Can flow cytometric detection of platelet activation early in pregnancy predict the occurrence of preeclampsia? A prospective study. The American Journal of Obstetrics and Gynecology. 1997;177(2):434–442. doi: 10.1016/s0002-9378(97)70212-6. [DOI] [PubMed] [Google Scholar]
- 62.Sibai BM, Ewell M, Levine RJ, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The American Journal of Obstetrics and Gynecology. 1997;177(5):1003–1010. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 63.Caritis S, Sibai B, Hauth J, et al. Predictors of pre-eclampsia in women at high risk. American Journal of Obstetrics & Gynecology. 1998;179(4):946–951. doi: 10.1016/s0002-9378(98)70194-2. [DOI] [PubMed] [Google Scholar]
- 64.Knuist M, Bonsel GJ, Zondervan HA, Treffers PE. Risk factors for preeclampsia in nulliparous women in distinct ethnic groups: a prospective cohort study. Obstetrics and Gynecology. 1998;92(2):174–178. doi: 10.1016/s0029-7844(98)00143-4. [DOI] [PubMed] [Google Scholar]
- 65.Penny JA, Halligan AWF, Shennan AH, et al. Automated, ambulatory, or conventional blood pressure measurement in pregnancy: which is the better predictor of severe hypertension? The American Journal of Obstetrics and Gynecology. 1998;178(3):521–526. doi: 10.1016/s0002-9378(98)70432-6. [DOI] [PubMed] [Google Scholar]
- 66.Bar J, Maymon R, Padoa A, et al. White coat hypertension and pregnancy outcome. Journal of Human Hypertension. 1999;13(8):541–545. doi: 10.1038/sj.jhh.1000865. [DOI] [PubMed] [Google Scholar]
- 67.Odegard RA, Vatten LJ, Nilsen ST, Salvesen KA, Austgulen R. Risk factors and clinical manifestations of pre-eclampsia. British Journal of Obstetrics and Gynaecology. 2000;107(11):1410–1416. doi: 10.1111/j.1471-0528.2000.tb11657.x. [DOI] [PubMed] [Google Scholar]
- 68.Shaarawy M, Abdel-Magid A-MA. Plasma endothelin-1 and mean arterial pressure in the prediction of pre- eclampsia. International Journal of Gynecology & Obstetrics. 2000;68(2):105–111. doi: 10.1016/s0020-7292(99)00180-0. [DOI] [PubMed] [Google Scholar]
- 69.Stamilio DM, Sehdev HM, Morgan MA, Propert K, Macones GA. Can antenatal clinical and biochemical markers predict the development of severe preeclampsia? American Journal of Obstetrics and Gynecology. 2000;182(3):589–594. doi: 10.1067/mob.2000.103890. [DOI] [PubMed] [Google Scholar]
- 70.Tranquilli AL, Bezzeccheri V, Giannubilo SR, Garbati E. The “relative weight” ofDoppler velocimetry of uterine artery and ambulatory blood pressure monitoring in prediction of gestational hypertension and prccclanipsia. Acta Biomedica de l'Ateneo Parmense. 2000;71(supplement 1):351–355. [PubMed] [Google Scholar]
- 71.Lauszus FF, Rasmussen OW, Lousen T, Klebe TM, Klebe JG. Ambulatory blood pressure as predictor of preeclampsia in diabetic pregnancies with respect to urinary albumin excretion rate and glycemic regulation. Acta Obstetricia et Gynecologica Scandinavica. 2001;80(12):1096–1103. doi: 10.1034/j.1600-0412.2001.801204.x. [DOI] [PubMed] [Google Scholar]
- 72.Iwasaki R, Ohkuchi A, Furuta I, et al. Relationship between blood pressure level in early pregnancy and subsequent changes in blood pressure during pregnancy. Acta Obstetricia et Gynecologica Scandinavica. 2002;81(10):918–925. doi: 10.1034/j.1600-0412.2002.811004.x. [DOI] [PubMed] [Google Scholar]
- 73.Ohkuchi A, Iwasaki R, Ojima T, et al. Increase in systolic blood pressure of ≥30 mm Hg and/or diastolic blood pressure of ≥15 mm Hg during pregnancy: is it pathologic? Hypertension in Pregnancy. 2003;22(3):275–285. doi: 10.1081/PRG-120024031. [DOI] [PubMed] [Google Scholar]
- 74.Perini R, Fisogni C, Bonera R, et al. Role of Doppler velocimetry of uterine arteries and ambulatory blood pressure monitoring in detecting pregnancies at risk for preeclampsia. Minerva Ginecologica. 2004;56(2):117–123. [PubMed] [Google Scholar]
- 75.Poon LC, Kametas NA, Valencia C, Chelemen T, Nicolaides KH. Systolic diastolic and mean arterial pressure at 11-13 weeks in the prediction of hypertensive disorders in pregnancy: a prospective screening study. Hypertens Pregnancy. 2011;30:93–107. doi: 10.3109/10641955.2010.484086. [DOI] [PubMed] [Google Scholar]
- 76.Cnossen JS, Vollebregt KC, de Vrieze N, et al. Accuracy of mean arterial pressure and blood pressure measurements in predicting pre-eclampsia: systematic review and meta-analysis. British Medical Journal. 2008;336(7653):1117–1120. doi: 10.1136/bmj.39540.522049.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wright D, Syngelaki A, Bradbury I, Akolekar R, Nicolaides KH. First-trimester screening for trisomies 21, 18 and 13 by ultrasound and biochemical testing. Fetal Diagnosis and Therapy. 2014;35(2):118–126. doi: 10.1159/000357430. [DOI] [PubMed] [Google Scholar]
- 78.Bonno M, Oxvig C, Kephart GM, et al. Localization of pregnancy-associated plasma protein-a and colocalization of pregnancy-associated plasma protein-a messenger ribonucleic acid and eosinophil granule major basic protein messenger ribonucleic acid in placenta. Laboratory Investigation. 1994;71(4):560–566. [PubMed] [Google Scholar]
- 79.Lawrence JB, Oxvig C, Overgaard MT, et al. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(6):3149–3153. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bersinger NA, Smárason AK, Muttukrishna S, Groome NP, Redman CW. Women with preeclampsia have increased serum levels of pregnancy-associated plasma protein A (PAPP-A), inhibin A, activin A, and soluble E-selectin. Hypertension in Pregnancy. 2003;22(1):45–55. doi: 10.1081/PRG-120016794. [DOI] [PubMed] [Google Scholar]
- 81.Bersinger NA, Ødegård RA. Second- and third-trimester serum levels of placental proteins in preeclampsia and small-for-gestational age pregnancies. Acta Obstetricia et Gynecologica Scandinavica. 2004;83(1):37–45. [PubMed] [Google Scholar]
- 82.Deveci K, Sogut E, Evliyaoglu O, Duras N. Pregnancy-associated plasma protein-A and C-reactive protein levels in pre-eclamptic and normotensive pregnant women at third trimester. Journal of Obstetrics and Gynaecology Research. 2009;35:94–98. doi: 10.1111/j.1447-0756.2008.00835.x. [DOI] [PubMed] [Google Scholar]
- 83.Ong CYT, Liao AW, Spencer K, Munim S, Nicolaides KH. First trimester maternal serum free β human chorionic gonadotrophin and pregnancy associated plasma protein a as predictors of pregnancy complications. British Journal of Obstetrics and Gynaecology. 2000;107(10):1265–1270. doi: 10.1111/j.1471-0528.2000.tb11618.x. [DOI] [PubMed] [Google Scholar]
- 84.Smith GCS, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early pregnancy levels of pregnancy-associated plasma protein A and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. The Journal of Clinical Endocrinology & Metabolism. 2002;87(4):1762–1767. doi: 10.1210/jcem.87.4.8430. [DOI] [PubMed] [Google Scholar]
- 85.Yaron Y, Heifetz S, Ochshorn Y, Lehavi O, Orr-Urtreger A. Decreased first trimester PAPP-A is a predictor of adverse pregnancy outcome. Prenatal Diagnosis. 2002;22(9):778–782. doi: 10.1002/pd.407. [DOI] [PubMed] [Google Scholar]
- 86.Dugoff L, Hobbins JC, Malone FD, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (The FASTER Trial) American Journal of Obstetrics & Gynecology. 2004;191(4):1446–1451. doi: 10.1016/j.ajog.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 87.Spencer K, Yu CKH, Cowans NJ, Otigbah C, Nicolaides KH. Prediction of pregnancy complications by first-trimester maternal serum PAPP-A and free β-hCG and with second-trimester uterine artery Doppler. Prenatal Diagnosis. 2005;25(10):949–953. doi: 10.1002/pd.1251. [DOI] [PubMed] [Google Scholar]
- 88.Pilalis A, Souka AP, Antsaklis P, et al. Screening for pre-eclampsia and fetal growth restriction by uterine artery Doppler and PAPP-A at 11-14 weeks gestation. Ultrasound in Obstetrics and Gynecology. 2007;29(2):135–140. doi: 10.1002/uog.3881. [DOI] [PubMed] [Google Scholar]
- 89.Spencer K, Cowans NJ, Chefetz I, Tal J, Meiri H. First-trimester maternal serum PP-13, PAPP-A and second-trimester uterine artery Doppler pulsatility index as markers of pre-eclampsia. Ultrasound in Obstetrics and Gynecology. 2007;29(2):128–134. doi: 10.1002/uog.3876. [DOI] [PubMed] [Google Scholar]
- 90.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction hypertension, and proteinuria in preeclampsia. The Journal of Clinical Investigation. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circulation Research. 2004;95(9):884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 92.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. The New England Journal of Medicine. 2004;350(7):672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 93.Stepan H, Unversucht A, Wessel N, Faber R. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension. 2007;49(4):818–824. doi: 10.1161/01.HYP.0000258404.21552.a3. [DOI] [PubMed] [Google Scholar]
- 94.Su YN, Lee CN, Cheng WF, Shau WY, Chow SN, Hsieh FJ. Decreased maternal serum placenta growth factor in early second trimester and preeclampsia. Obstetrics and Gynecology. 2001;97(6):898–904. doi: 10.1016/s0029-7844(01)01341-2. [DOI] [PubMed] [Google Scholar]
- 95.Tidwell SC, Ho H, Chiu W, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. American Journal of Obstetrics and Gynecology. 2001;184(6):1267–1272. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 96.Tjoa ML, van Vugt JMG, Mulders MAM, Schutgens RBH, Oudejans CBM, van Wijk IJ. Plasma placenta growth factor levels in midtrimester pregnancies. Obstetrics and Gynecology. 2001;98(4):600–607. doi: 10.1016/s0029-7844(01)01497-1. [DOI] [PubMed] [Google Scholar]
- 97.Polliotti BM, Fry AG, Saller DN, Jr., Mooney RA, Cox C, Miller RK. Second-trimester maternal serum placental growth factor and vascular endothelial growth factor for predicting severe, early-onset preeclampsia. Obstetrics and Gynecology. 2003;101(6):1266–1274. doi: 10.1016/s0029-7844(03)00338-7. [DOI] [PubMed] [Google Scholar]
- 98.Krauss T, Pauer H, Augustin HG. Prospective analysis of placenta growth factor ( PlGF ) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertension in Pregnancy. 2004;23(1):101–111. doi: 10.1081/PRG-120028286. [DOI] [PubMed] [Google Scholar]
- 99.Thadhani R, Mutter WP, Wolf M, et al. First trimester placental growth factor and soluble fms -like tyrosine kinase 1 and risk for preeclampsia. Journal of Clinical Endocrinology and Metabolism. 2004;89(2):770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 100.Akolekar R, Zaragoza E, Poon LCY, Pepes S, Nicolaides KH. Maternal serum placental growth factor at 11 + 0 to 13 + 6 weeks of gestation in the prediction of pre-eclampsia. Ultrasound in Obstetrics and Gynecology. 2008;32(6):732–739. doi: 10.1002/uog.6244. [DOI] [PubMed] [Google Scholar]
- 101.Crispi F, Llurba E, Domínguez C, Martín-Gallán P, Cabero L, Gratacós E. Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound in Obstetrics and Gynecology. 2008;31(3):303–309. doi: 10.1002/uog.5184. [DOI] [PubMed] [Google Scholar]
- 102.Erez O, Romero R, Espinoza J, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. Journal of Maternal-Fetal and Neonatal Medicine. 2008;21(5):279–287. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kagan KO, Wright D, Spencer K, Molina FS, Nicolaides KH. First-trimester screening for trisomy 21 by free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A: impact of maternal and pregnancy characteristics. Ultrasound in Obstetrics & Gynecology. 2008;31(5):493–502. doi: 10.1002/uog.5332. [DOI] [PubMed] [Google Scholar]
- 104.Pandya P, Wright D, Syngelaki A, Akolekar R, Nicolaides KH. Maternal serum placental growth factor in prospective screening for aneuploidies at 8–13 weeks' gestation. Fetal Diagnosis and Therapy. 2012;31(2):87–93. doi: 10.1159/000335684. [DOI] [PubMed] [Google Scholar]
- 105.Poon LC, Kametas NA, Maiz N, Akolekar R, Nicolaides KH. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension. 2009;53:812–818. doi: 10.1161/HYPERTENSIONAHA.108.127977. [DOI] [PubMed] [Google Scholar]
- 106.Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11–13 weeks. Prenatal Diagnosis. 2011;31(1):66–74. doi: 10.1002/pd.2660. [DOI] [PubMed] [Google Scholar]
- 107.Scazzocchio E, Figueras F, Crispi F, et al. Performance of a first-trimester screening of preeclampsia in a routine care low-risk setting. The American Journal of Obstetrics and Gynecology. 2013;208(3):203.e1–203.e10. doi: 10.1016/j.ajog.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 108.Koopmans CM, Bijlenga D, Groen H, et al. Induction of labour versus expectant monitoring for gestational hypertension or mild pre-eclampsia after 36 weeks' gestation (HYPITAT): a multicentre, open-label randomised controlled trial. The Lancet. 2009;374(9694):979–988. doi: 10.1016/S0140-6736(09)60736-4. [DOI] [PubMed] [Google Scholar]
- 109.Tayyar A, Garcia-Tizon Larroca S, Poon LC, Wright D, Nicolaides KH. Competing risks model in screening for preeclampsia by mean arterial pressure and uterine artery pulsatility index at 30–33 weeks’ gestation. Fetal Diagnosis and Therapy. 2014;36(1):18–27. doi: 10.1159/000360792. [DOI] [PubMed] [Google Scholar]
- 110.Bdolah Y, Sukhatme VP, Karumanchi SA. Angiogenic imbalance in the pathophysiology of preeclampsia: newer insights. Seminars in Nephrology. 2004;24(6):548–556. doi: 10.1016/s0270-9295(04)00125-1. [DOI] [PubMed] [Google Scholar]
- 111.Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. The American Journal of Obstetrics and Gynecology. 2012;206(1):58.e1–e8.e1. doi: 10.1016/j.ajog.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 112.Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125(7):911–919. doi: 10.1161/CIRCULATIONAHA.111.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. Journal of Maternal-Fetal and Neonatal Medicine. 2011;24(10):1187–1207. doi: 10.3109/14767058.2011.589932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sibiude J, Guibourdenche J, Dionne M, et al. Placental growth factor for the prediction of adverse outcomes in patients with suspected preeclampsia or intrauterine growth restriction. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0050208.e50208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chappell LC, Duckworth S, Seed PT, et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation. 2013;128(19):2121–2131. doi: 10.1161/CIRCULATIONAHA.113.003215. [DOI] [PubMed] [Google Scholar]
- 116.Ohkuchi A, Hirashima C, Takahashi K, Suzuki H, Matsubara S, Suzuki M. Onset threshold of the plasma levels of soluble fms-like tyrosine kinase 1/placental growth factor ratio for predicting the imminent onset of preeclampsia within 4 weeks after blood sampling at 19–31 weeks of gestation. Hypertension Research. 2013;36(12):1073–1038. doi: 10.1038/hr.2013.95. [DOI] [PubMed] [Google Scholar]
- 117.Lai J, Garcia-Tizon Larroca S, Peeva G, Poon LC, Wright D, Nicolaides KH. Competing risks model in screening for preeclampsia by serum placental growth factor and soluble fms-like tyrosine kinase-1 at 30-33 weeks gestation. Fetal Diagnosis and Therapy. 2014;35(4):240–248. doi: 10.1159/000359968. [DOI] [PubMed] [Google Scholar]
- 118.Garcia-Tizon Larroca S, Tayyar A, Poon LC, Wright D, Nicolaides KH. Competing risks model in screening for preeclampsia by biophysical and biochemical markers at 30–33 weeks’ gestation. Fetal Diagnosis and Therapy. 2014;36(1):9–17. doi: 10.1159/000362518. [DOI] [PubMed] [Google Scholar]
- 119.Nicolaides KH. Turning the pyramid of prenatal care. Fetal Diagnosis and Therapy. 2011;29(3):183–196. doi: 10.1159/000324320. [DOI] [PubMed] [Google Scholar]


