Abstract
Ease of dosing and simplicity of monitoring make new oral anticoagulants an attractive therapy in a growing range of clinical conditions. However, newer oral anticoagulants interact with the coagulation cascade in different ways than traditional warfarin therapy. Replacement of clotting factors will not reverse the effects of dabigatran, rivaroxaban, or apixaban. Currently, antidotes for these drugs are not widely available. Fortunately, withholding the anticoagulant and dialysis are freqnently effective treatments, particularly with rivaroxaban and dabigatran. Emergent bleeding, however, requires utilization of Prothrombin Complex Concentrates (PCCs). PCCs, in addition to recombinant factor VIIa, are used to activate the clotting system to reverse the effects of the new oral anticoagulants. In cases of refractory or emergent bleeding, the recommended factor concentrate in our protocols differs between the new oral anticoagulants. In patients taking dabigatran, we administer an activated PCC (aPCC) [FELBA] due to reported benefit in human in vitro studies. Based on human clinical trial evidence, the 4-factor PCC (Kcentra) is suggested for patients with refractory rivaroxaban- or apixaban-associated hemorrhage. If bleeding continues, recombinant factor VIIa may be employed. With all of these new procoagulant agents, the risk of thrombosis associated with administration of factor concentrates must be weighed against the relative risk of hemorrhage.
1. Introduction
Hemorrhage is the major cause of early mortality after injury and a leading risk in any operative procedure. Recently developed target-specific oral anticoagulants defy traditional reversal protocols previously used with warfarin. In the face of new oral anticoagulants, we have developed additional approaches to management of bleeding. Patients with life threatening hemorrhage may benefit from use of a new 4-factor prothrombin complex concentrate (PCC) (Kcentra, CSL Behring Gmbh, Marburg, Germany). Recent data suggest that there may be a role for factor concentrates including PCCs, activated PCCs (aPCC), and recombinant factor VIIa (rfVIIa, NovoSeven, Novo Nordisk, Bagsvaerd, Denmark) in dabigatran, rivaroxaban, and apixaban-associated bleeding. This report will briefly review the mechanism of action of the oral anticoagulants, present our bleeding management protocols, and discuss the rationale for our use of prothrombin complex concentrates and rfVIIa in refractory hemorrhage.
2. Mechanism of Action
Understanding the mechanism of action of oral anticoagulants provides a biologic foundation for use of PCCs, aPCCs, and rfVIIa in treatment of uncontrolled bleeding. Warfarin creates a deficiency of factors II, VII, IX, and X through inhibition of vitamin K-dependent carboxylation of the clotting proteins [1]. Replacement of functional coagulation factors can reverse the anticoagulant effect of warfarin. The newer oral anticoagulants interact with the coagulation cascade in different ways. Dabigatran directly inhibits thrombin (activated factor II), which limits the formation of fibrin [2]. Rivaroxaban and apixaban directly inhibit activated factor X [3, 4]. Replacement of clotting factors will not reverse the effects of dabigatran, rivaroxaban, and apixaban and we currently do not have antidotes for these drugs.
Rates of bleeding seen with the new oral anticoagulants in real world patients treated with dabigatran are consistent with those seen in clinical trials and less than those seen with warfarin. Most of the data available at this time comes from clinical trials. Gastrointestinal and intracranial hemorrhage are the two most important bleeding complications reported with the new oral anticoagulants. In postmarketing surveillance, there did not appear to be higher bleeding rates associated with dabigatran as opposed to warfarin. Some of the largest trials are in patients with atrial fibrillation. In these studies, reduced rates of intracranial hemorrhage are seen with dabigatran in comparison to warfarin as well as a reduced death rate. The rate of gastrointestinal hemorrhage is similar between dabigatran and warfarin-treated patients. Factor Xa inhibitors are also associated with reduced risk of mortality and intracranial hemorrhage compared to warfarin in trials conducted in patients with atrial fibrillation and may be used in ablation procedures [5–8].
PCCs are plasma-derived products containing factors II, VII, IX, and X. The 3-factor PCCs contain a minimal amount of factor VII. Table 1 shows the PCCs available in the United States. FEIBA (Baxter AG, Vienna, Austria) is unique because it contains factor VII primarily in the activated form (activated PCC (aPCC)) [9]. Kcentra contains all of the vitamin K-dependent proteins (factors II, VII, IX, and X and proteins C and S) [10]. Kcentra and Bebulin (Baxter AG, Vienna, Austria) contain small amounts of heparin which is insufficient to cause anticoagulation but contraindicates use of these products in patients with a history of heparin-induced thrombocytopenia. Recombinant factor VIIa initiates coagulation independent of tissue factor, factor VIII and factor IX, and is approved for use in patients with factor VII deficiency and hemophilia with factor inhibitors [11]. PCCs provide replacement of functional vitamin K-dependent proteins to more rapidly reverse the anticoagulant effect of warfarin [12]. In contrast, for patients with uncontrolled bleeding while taking dabigatran, rivaroxaban, or apixaban, PCCs, aPCCs, and rVIIa can be used to activate the coagulation system.
Table 1.
Prothrombin complex concentrates (PCC) available in the United States.
| Product | Coagulation factors in product | FDA approved indications to manage hemorrhage | Heparin in product? |
|---|---|---|---|
| Nonactivated | |||
| 3-factor PCC | |||
| Profilnine | Nonactivated II, IX, X, and small amounts of VII | Hemophilia B | No |
| Bebulin | Nonactivated II, IX, X, and small amounts of VII | Hemophilia B | Yes |
| 4-factor PCC | |||
| Kcentra | Nonactivated II, VII, IX, X, protein C, and protein S | Warfarin | Yes |
| Activated PCC | |||
| Feiba | Activated VII, nonactivated II, IX, and X |
Hemophilia A & B with inhibitors | No |
3. Animal and Human Data for Management of Dabigatran, Rivaroxaban, and Apixaban-Associated Hemorrhage
The PCC selection in our protocols for dabigatran, rivaroxaban, and apixaban-associated hemorrhage is based upon the available human and animal data presented in detail in Table 2 and summarized in Table 3. Human in vitro and animal studies have shown improved thrombin generation after administration of aPCCs in dabigatran treated animals [13, 14]. In a prospective crossover study, 4-factor PCCs failed to correct coagulation times or thrombin generation in humans taking dabigatran [15] suggesting that 4-factor PCCs are not useful for dabigatran-associated hemorrhage. rfVIIa could be considered in dabigatran-associated bleeding based on corrected time to thrombin generation from in vitro studies and decreased bleeding time in rats [13, 14, 16]. However, one case report suggested decreased bleeding after administration of rfVIIa in a patient taking dabigatran [17] and two reports noted continued bleeding [18–20]. Due to the mixed efficacy reported in case reports of rfVIIa, we use the aPCC (Feiba) prior to rfVIIa in refractory bleeding associated with dabigatran [21, 22]. In contrast to dabigatran, 4-factor PCCs improved rivaroxaban-induced coagulation testing abnormalities in a prospective crossover study in healthy volunteers [15], informing our decision to use a 4-factor PCC (Kcentra) in the setting of rivaroxaban-associated refractory bleeding. Given the mechanistic similarity and positive in vitro data, we also use a 4-factor PCC (Kcentra) in patients with refractory apixaban-associated bleeding [23]. Animal studies suggest that correction of coagulation testing does not always correlate to improvement in bleeding outcomes [14]. Outcomes of patients receiving factor concentrates via these protocols must be closely monitored.
Table 2.
Animal and human studies using factor concentrates to reverse anticoagulant effect of dabigatran, rivaroxaban, and apixaban.
| Author | Reversal agent | Test system | Coagulation test effect | Bleeding outcome effect |
|---|---|---|---|---|
| Dabigatran | ||||
| Van Ryn et al. [16] | aPCC | Rat | aPTT unchanged | Decreased bleeding time |
| rfVIIa | Rat | aPTT decreased | Decreased bleeding time | |
| Van Ryn et al. [14] | 4-Factor PCC | Rat | PT decreased, aPTT unchanged | Decreased bleeding time |
| aPCC | Rat | PT decreased, aPTT unchanged, increased thrombin generation | Decreased bleeding time | |
| rfVIIa | PT and aPTT decreased | Decreased bleeding time | ||
| Lambourne et al. [38] | 4-Factor PCC | Mouse | No effect | No effect |
| rfVIIa | Mouse | aPTT decreased | No effect | |
| 4-Factor PCC + rfVIIa | Mouse | TT and aPTT decreased | No effect | |
| aPCC | Mouse | No effect | No effect | |
| Pragst et al. [39] | 4-Factor PCC | Rabbit | PT decreased, aPTT unchanged | Normalized blood loss |
| Zhou et al. [40] | 4-Factor PCC | Mouse ICH | Prevented hematoma expansion; control level mortality | |
| rfVIIa | Mouse ICH | Ineffective control of hematoma expansion | ||
| Marlu et al. [13] | 4-Factor PCC | Human in vitro | Increased amount of thrombin generation | |
| rfVIIa | Human in vitro | Corrected time to thrombin generation | ||
| aPCC | Human in vitro | Corrected time to thrombin generation | ||
| Eerenberg et al. [15] | 4-Factor PCC | Human | No effect on aPTT, TT, or ECT | |
| Khoo et al. [41] | aPCC | Human in vitro | Increased thrombin generation and corrected time to thrombin generation | |
| Warkentin et al. [17] | rfVIIa | Human case report | Decreased aPTT and PT | Decreased blood loss |
| Garber et al. [18] | rfVIIa | Human case report | Worsening ICH | |
| Truumees et al. [19] | rfVIIa | Human case report | Continued blood loss | |
| Lillo-Le Louët et al. [20] | 4-Factor PCC + rfVIIa | Human case report | PT and aPTT unchanged | Continued bleeding |
| 4-Factor PCC + rfVIIa | Human case report | PT and aPTT unchanged | Bleeding stopped with dialysis | |
| Wychowski and Kouides [42] | 3-Factor PCC | Human case report | TT and aPTT unchanged, PT decreased | No further bleeding |
| Dumkow et al. [43] | 3-Factor PCC | Human case report | Clinical bleeding and hemoglobin stabilized | |
| Rivaroxaban | ||||
| Perzborn and Tinel [44] | 4-factor PCC | Rat | PT decreased | Bleeding time decreased |
| Godier et al. [45] | 4-factor PCC | Rabbit | aPTT normalized, PT decreased | No effect on blood loss |
| rfVIIa | Rabbit | aPTT normalized, PT decreased | Decreased bleeding time, no effect on blood loss | |
| Perzborn et al. [46] | 4-Factor PCC | Rat | Decreased PT, normalized TAT concentration | Reduced bleeding time |
| aPCC | Rat | Decreased PT | Reduced bleeding time | |
| Primate | Reduced PT | Normalized bleeding time | ||
| rVIIa | Rat | Decreased PT | Reduced bleeding time | |
| Primate | Decreased PT | Bleeding time unchanged | ||
| Marlu et al. [13] | 4-Factor PCC | Human in vitro | Increased amount of thrombin generation | |
| rfVIIa | Human in vitro | Corrected time to thrombin generation | ||
| aPCC | Human in vitro | Corrected all thrombin generation parameters | ||
| Eerenberg et al. [15] | 4-Factor PCC | Human in vivo | Normalized PT and thrombin generation | |
| Dinkelaar et al. [47] | 4-Factor PCC | Human in vitro | No effect on PT or time to thrombin generation Normalized amount of thrombin generation | |
| Körber et al. [48] | 4-Factor PCC | Human in vitro | No effect on aPTT, PT | |
| rfVIIa | Human in vitro | Decreased clotting time, no effect on aPTT, PT | ||
| Apixaban | ||||
| Escolar et al. [23] | PCC | Human in vitro | Increased thrombin generation | |
| rfVIIa | Human in vitro | Increased thrombin generation | ||
| aPCC | Human in vitro | Increased thrombin generation |
aPCC: activated prothrombin complex concentrate; rfVIIa: recombinant factor VIIa.
Table 3.
Summary of animal and human data for reversal of dabigatran, rivaroxaban, and apixaban using factor concentrates.
| Dabigatran | Rivaroxaban | Apixaban | ||||
|---|---|---|---|---|---|---|
| Animal | Human | Animal | Human | Animal | Human | |
| 3-factor PCC | Case report +/− | |||||
| 4-factor PCC | Rats +/− Rabbits + Mice − Mice ICH + |
In vitro + In vivo − Case report − |
Rats + Rabbits +/− |
In vitro +/− In vivo + |
In vitro + | |
| aPCC | Rats +/− Mice − |
In vitro + | Rat + Primate + |
In vitro + | In vitro + | |
| rfVIIa | Rats +/− Mice +/− Mice ICH − |
In vitro + Case report +/− |
Rat + Rabbits +/− Primate +/− |
In vitro +/− | In vitro + | |
+: effective; −: ineffective; +/−: mixed results between studies or between coagulation testing and bleeding outcomes; PCC: prothrombin complex concentrate; aPCC: activated prothrombin complex concentrate; rfVIIa: recombinant factor VIIa.
4. Protocols
Our institution has created protocols to help direct the use of factor concentrates in the treatment of life threatening bleeding in patients taking new oral anticoagulants (Figure 1). The initial measures are the same for any bleeding patient, with local intervention and supportive care. In addition, confirmation of medication, dosing, and duration since the last dose guides further therapy. Renal and hepatic function are evaluated to determine patient metabolism of medication. Transfusion of packed RBCs and a transfusion protocol featuring a balance of packed red blood cells, plasma, and platelets may be utilized depending on the severity of hemorrhage. Based largely on retrospective data, the optimal ratio of plasma to packed RBCs administered is thought to be 1 : 1 or 1 : 2 [24]. The effects of antiplatelet agents are reversed by transfusion of 2 apheresis units of platelet concentrates if needed (see below) [25, 26].
Figure 1.
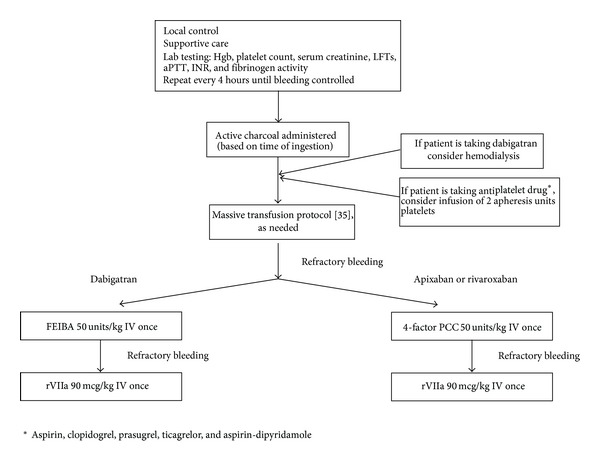
Management protocol for hemorrhage in patients taking dabigatran, rivaroxaban, or apixaban.
Our management protocols in the setting of life threatening bleeding in the patient taking dabigatran, rivaroxaban, or apixaban are summarized in Figure 1. Activated charcoal may be administered if the last dose of anticoagulant was ingested less than 2 hours (dabigatran [2] or rivaroxaban [27]) or 3 hours (apixaban [28]) before presentation. Hemodialysis removes dabigatran and should be considered based on clinical status and need for surgical intervention. In a survey of nonmalignant hematologists, withholding the anticoagulant and dialysis were the most effective treatment used in 80% of bleeding episodes associated with rivaroxaban and dabigatran, respectively [29]. Thus, the use of specialized resuscitation protocols is often not required.
After transfusion of 4 units of PRBCs, we transfuse PRBCs, plasma, and platelets in a 1 : 1 : 1 ratio with a goal of hemoglobin of 9-10 g/dL [24]. We employ this transfusion strategy to avoid additional mechanisms of coagulopathy as the new oral anticoagulants prolong the PTT and INR and, thus, interfere with laboratory monitoring of other coagulation abnormalities. For patients on antiplatelet drugs we consider administration of two apheresis units of platelets.
In cases of refractory bleeding, the recommended factor concentrate in our protocols differs between the new oral anticoagulants. In patients taking dabigatran, we administer 50 units/kg IV of aPCC (Feiba) due to suggested benefit in human in vitro studies [13]. Based on human clinical trial evidence, 50 units/kg IV of the 4-factor PCC (Kcentra) is suggested for patients with refractory rivaroxaban or apixaban-associated hemorrhage. If bleeding continues, rfVIIa may be employed. Thrombosis has been reported with administration of all of the factor concentrates and the relative risk of hemorrhage and thrombosis must be considered.
Platelets are an essential but poorly understood component of hemostasis after injury. Previous evidence identifies admission platelet counts as inversely correlated with early mortality and supports transfusion of platelets with critical injury and trauma, even for platelet counts in the normal reference range. Quantitative platelet deficits have predicted progression of intracranial hemorrhage and mortality after traumatic brain injury. Study of platelet dysfunction has been hindered by technical complexity of existing platelet assays [30, 31].
Given the controversy which continues in the trauma and acute surgery literature, a number of observations can be made [30, 32–37]. Patients receiving antiplatelet agents have an increased number of comorbidities. While recent retrospective work suggests limited impact of antiplatelet medications on outcome with trauma, limited prospective work suggests that the elderly and patients with intracranial hemorrhage are at greater risk for poor outcomes if taking antiplatelet agents. A normal platelet count is not reassuring in this setting. Normal clotting studies also do not predict good outcome. Despite this, a specific role for empiric platelet therapy has not been identified. Finally, while a variety of assays for platelet function have been reported, consensus regarding the optimal assay and the standard for acute management of injury has not been reached. Of assays which can be performed at the bedside, thromboelastography is the most promising bedside assay [31]. Our recommendation favoring consideration of platelet administration in the setting of life threatening bleeding must be understood in the context of the quality of studies which are available.
5. Conclusion
Our institution developed management protocols in an effort to standardize treatment of severe bleeding associated with use of new oral anticoagulants and approval of new factor concentrates. These protocols are based on limited human data but serve as a tool to guide therapy as providers gain experience with these anticoagulants and PCCs. Standardizing therapy allows the collection of clinical data, which can guide further trials. We anticipate continued modification of these protocols as laboratory and clinical experience expand. In particular, thrombosis risk with powerful newer agents designed to enhance coagulation must be monitored. At this time, data related to thrombosis risk is limited to anecdotes [5].
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Lisa Baumann Kreuziger and Joseph Keenan completed a literature search, primary paper writing, and revisions. Colleen Morton assisted with literature search, content review, and revisions and developed protocols. David Dries, MSE, MD, initiated the project, developed protocols, and completed content review and revisions.
References
- 1. Kazmier F, Spittel JA, Thompson JJ, Owen CA. Effect of oral anticoagulation on factors VII, IX, X, and II. Archives of Internal Medicine. 1965;115:667–673. doi: 10.1001/archinte.1960.03860180039007. [DOI] [PubMed] [Google Scholar]
- 2.van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thrombosis & Haemostasis. 2010;103(6):1116–1127. doi: 10.1160/TH09-11-0758. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clinical Pharmacokinetics. 2009;48:1–22. doi: 10.2165/0003088-200948010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Wong PC, Pinto DJP, Zhang D. Preclinical discovery of apixaban, a direct and orally bioavailable factor Xa inhibitor. Journal of Thrombosis and Thrombolysis. 2011;31(4):478–492. doi: 10.1007/s11239-011-0551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegal DM, Garcia DA, Crowther MA. How I treat target-specific oral anticoagulant-associated homorrhage. Blood. 2014;123:1152–1158. doi: 10.1182/blood-2013-09-529784. [DOI] [PubMed] [Google Scholar]
- 6.Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. The New England Journal of Medicine. 2013;368(14):1272–1274. doi: 10.1056/NEJMp1302834. [DOI] [PubMed] [Google Scholar]
- 7.Bruins Slot KM, Berge E. Factor Xa inhibitors vs warfarin for preventing stroke and thromboembolism in pateints with atrial fibrillation. JAMA. 2014;311:1150–1151. doi: 10.1001/jama.2014.1403. [DOI] [PubMed] [Google Scholar]
- 8.Stepanyan G, Badhwar N, Lee RJ, et al. Safety of new oral anticoagulants for patients undergoing atrial fibrillation ablation. Journal of Interventional Cardiac Electrophysiology. 2014;40:33–38. doi: 10.1007/s10840-014-9888-9. [DOI] [PubMed] [Google Scholar]
- 9.Baxter. FEIBA prescribing information. 2011 [Google Scholar]
- 10.CSL Behring GmbH. Kcentra prescribing information. http://labeling.cslbehring.com/PI/US/Kcentra/EN/Kcentra-Prescribing-Information.pdf.
- 11.Novo Nordisk Inc. NovoSeven RT Coagulation Factor VIIa Prescribing Information. 1999. [Google Scholar]
- 12.Meehan R, Tavares M, Sweeney J. Clinical experience with oral versus intravenous vitamin K for warfarin reversal (CME) Transfusion. 2013;53(3):491–498. doi: 10.1111/j.1537-2995.2012.03755.x. [DOI] [PubMed] [Google Scholar]
- 13.Marlu R, Hodaj E, Paris A, Albaladejo P, Cracowski JL, Pernod G. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thrombosis and Haemostasis. 2012;108(2):217–224. doi: 10.1160/TH12-03-0179. [DOI] [PubMed] [Google Scholar]
- 14.van Ryn J, Schurer J, Kink-Eiband M, Clemens A. The successful reversal of dabigatran-induced bleeding by coagulation factor concentrates in a rat tail bleeding model do not correlate with ex vivo markers of anticoagulation. Blood. 2011;118:p. 2316. [Google Scholar]
- 15.Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573–1579. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 16.van Ryn J, Ruehl D, Priepke H, Hauel H, Wienan W. Reversibility of the anticoagulant effect of high doses of the direct thrombin inhibitor dabigatran, by recombinant factor VIIa or activated prothrombin complex concentrate. Haematologica. 2008;93:p. 148. [Google Scholar]
- 17.Warkentin T, Margetts P, Connolly S, Lamy A, Ricci C, Eikelboom J. Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood. 2012;119:2172–2174. doi: 10.1182/blood-2011-11-393587. [DOI] [PubMed] [Google Scholar]
- 18.Garber ST, Sivakumar W, Schmidt RH. Neurosurgical complications of direct thrombin inhibitors—catastrophic hemorrhage after mild traumatic brain injury in a patient receiving dabigatran. Journal of Neurosurgery. 2012;116(5):1093–1096. doi: 10.3171/2012.2.JNS112132. [DOI] [PubMed] [Google Scholar]
- 19.Truumees E, Gaudu T, Dieterichs C, Geck M, Stokes J. Epidural hematoma and intraoperative hemorrhage in a spine trauma patient on pradaxa (dabigatran) Spine. 2012;37:E863–E865. doi: 10.1097/BRS.0b013e31824ee320. [DOI] [PubMed] [Google Scholar]
- 20.Lillo-Le Louët A, Wolf M, Soufir L, et al. Life-threatening bleeding in four patients with an unusual excessive response to dabigatran: implications for emergency surgery and resuscitation. Thrombosis and Haemostasis. 2012;108(3):583–585. doi: 10.1160/TH12-03-0149. [DOI] [PubMed] [Google Scholar]
- 21.Schulman S, Ritchie B, Goy JK, Nahirniak S, Almutawa M, Ghanny S. Activated prothrombin complex concentrate for dabigatran-associated bleeding. British Journal of Haematology. 2013;164:296–310. doi: 10.1111/bjh.12620. [DOI] [PubMed] [Google Scholar]
- 22.Dickneite G, Hoffman M. Reversing the new oral anticoagulants with prothrombin comlpex concentrates (PCCs): what is the evidence? Thrombosis and Haemostasis. 2014;111(2):189–198. doi: 10.1160/TH13-05-0431. [DOI] [PubMed] [Google Scholar]
- 23.Escolar G, Reverter J, Villalta J, Sanz V, Molina P, Diaz-Ricart M. Reversal of apixaban induced alterations of hemostasis by different coagulation factor concentrates: Studies in vitro with circulating human blood. Circulation. 2012;126:p. 521. [Google Scholar]
- 24.Dries DJ. The contemporary role of blood products and components used in trauma resuscitation. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine. 2010;18(1, article 63) doi: 10.1186/1757-7241-18-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Hirsh J, Xie C, Johnston MA, Eikelboom JW. Reversal of the anti-platelet effects of aspirin and clopidogrel. Journal of Thrombosis and Haemostasis. 2012;10(4):521–528. doi: 10.1111/j.1538-7836.2012.04641.x. [DOI] [PubMed] [Google Scholar]
- 26.Thiele T, Sümnig A, Hron G, et al. Platelet transfusion for reversal of dual antiplatelet therapy in patients requiring urgent surgery: a pilot study. Journal of Thrombosis and Haemostasis. 2012;10:968–971. doi: 10.1111/j.1538-7836.2012.04699.x. [DOI] [PubMed] [Google Scholar]
- 27.Janssen Pharmaceuticals Inc. Xarelto prescribing information. 2011.
- 28.Bristol-Myers Squibb. Eliquis (apixiban) prescribing information. 2012, http://packageinserts.bms.com/pi/pi_eliquis.pdf.
- 29.Baumann Kreuziger LM, Reding MT. Management of bleeding associated with dabigatran and rivaroxaban: a survey of current practices. Thrombosis Research. 2013;132(2):e161–e163. doi: 10.1016/j.thromres.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kutcher ME, Redick BJ, McCreery RC, et al. Characterization of platelet dysfunction after trauma. Journal of Trauma and Acute Care Surgery. 2012;73:13–19. doi: 10.1097/TA.0b013e318256deab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolliger D, Seeberger MD, Tanaka KA. Principles and practice of thromboelastography in Clinical Coagulation Management and Transfusion Practice. Transfusion Medicine Reviews. 2012;26(1):1–13. doi: 10.1016/j.tmrv.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Ferraris VA, Bernard AC, Hyde B, Kearney PA. The impact of antiplatelet drugs on trauma outcomes. Journal of Trauma and Acute Care Surgery. 2012;73(2):492–497. doi: 10.1097/ta.0b013e31825b85f1. [DOI] [PubMed] [Google Scholar]
- 33.Joseph B, Pandit V, Sadoun M, et al. A prospective evaluation of platelet function in patients on antiplatelet therapy with traumatic intracranial hemorrhage. Journal of Trauma and Acute Care Surgery. 2013;75:900–994. doi: 10.1097/TA.0b013e3182a96591. [DOI] [PubMed] [Google Scholar]
- 34.Nishijima DK, Zehtabchi S, Berrong J, Legome E. Utility of platelet transfusion in adult patients with traumatic intracranial hemorrhage and preinjury antiplatelet use: a systematic review. Journal of Trauma and Acute Care Surgery. 2012;72:1658–1663. doi: 10.1097/TA.0b013e318256dfc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harr JN, Moore EE, Johnson J, et al. Antiplatelet therapy is not associated with decreased transfusion-associated risk of lung dysfunction, multiple organ failure, and mortality in trauma patients. Critical Care Medicine. 2013;41:399–404. doi: 10.1097/CCM.0b013e31826ab38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peck KA, Calvo RY, Schechter MS, et al. The impact of preinjury anticoagulants and prescription antiplatelet agents on outcomes in older patients with traumatic brain injury. Journal of Trauma and Acute Care Surgery. 2014;76:431–436. doi: 10.1097/TA.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 37.Gordon JL, Fabian TC, Lee MD, Dugdale M. Anticoagulant and antiplatelet medications encountered in emergency surgery patients: a review of reversal strategies. The Journal of Trauma and Acute Care Surgery. 2013;75(3):475–486. doi: 10.1097/TA.0b013e3182a07391. [DOI] [PubMed] [Google Scholar]
- 38.Lambourne MD, Eltringham Smith LJ, Gataiance S, Arnold DM, Crowther MA, Sheffield WP. Prothrombin complex concentrates reduce blood loss in murine coagulopathy induced by warfarin, but not in that induced by dabigatran etexilate. Journal of Thrombosis and Haemostasis. 2012;10(9):1830–1840. doi: 10.1111/j.1538-7836.2012.04863.x. [DOI] [PubMed] [Google Scholar]
- 39.Pragst I, Zeitler SH, Doerr B, et al. Reversal of dabigatran anticoagulation by prothrombin complex concentrate (beriplex P/N) in a rabbit model. Journal of Thrombosis and Haemostasis. 2012;10(9):1841–1848. doi: 10.1111/j.1538-7836.2012.04859.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhou W, Schwarting S, Illanes S, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke. 2011;42:3594–3599. doi: 10.1161/STROKEAHA.111.624650. [DOI] [PubMed] [Google Scholar]
- 41.Khoo T-L, Weatherburn C, Kershaw G, Reddel CJ, Curnow J, Dunkley S. The use of FEIBA in the correction of coagulation abnormalities induced by dabigatran. International Journal of Laboratory Hematology. 2013;35(2):222–224. doi: 10.1111/ijlh.12005. [DOI] [PubMed] [Google Scholar]
- 42.Wychowski MK, Kouides P. Dabigatran-induced gastrointestinal bleeding in an elderly patient with moderate renal impairment. Annals of Pharmacotherapy. 2012;46(4, article e10) doi: 10.1345/aph.1Q747. [DOI] [PubMed] [Google Scholar]
- 43.Dumkow LE, Voss JR, Peters M, Jennings DL. Reversal of dabigatran-induced bleeding with a prothrombin complex concentrate and fresh frozen plasma. American Journal of Health-System Pharmacy. 2012;69(19):1646–1650. doi: 10.2146/ajhp120055. [DOI] [PubMed] [Google Scholar]
- 44.Perzborn E, Tinel H. Prothrombin complex concentrate reverses the effects of high-dose rivaroxaban in rats. Journal of Thrombosis and Haemostasis. 2009;7, article 183 [Google Scholar]
- 45.Godier A, Miclot A, Le Bonniec B, et al. Evaluation of prothrombin complex concentrate and recombinant activated factor VII to reverse rivaroxaban in a rabbit model. Anesthesiology. 2012;116:94–102. doi: 10.1097/ALN.0b013e318238c036. [DOI] [PubMed] [Google Scholar]
- 46.Perzborn E, Gruber A, Tinel H, et al. Reversal of rivaroxaban anticoagulation by haemostatic agents in rats and primates. Thrombosis and Haemostasis. 2013;110(1):162–172. doi: 10.1160/TH12-12-0907. [DOI] [PubMed] [Google Scholar]
- 47.Dinkelaar J, Molenaar PJ, Ninivaggi M, de Laat B, Brinkman HJM, Leyte A. In vitro assessment, using thrombin generation, of the applicability of prothrombin complex concentrate as an antidote for rivaroxaban. Journal of Thrombosis and Haemostasis. 2013;11(6):1111–1118. doi: 10.1111/jth.12236. [DOI] [PubMed] [Google Scholar]
- 48.Körber M, Langer E, Ziemer S, Perzborn E, Gericke C, von Heymann C. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: an in vitro study. Clinical and Applied Thrombosis/Hemostasis. 2013 doi: 10.1177/1076029613494468. [DOI] [PubMed] [Google Scholar]


